Epidermal LCs but not dermal DCs take up skin surface protein through intact tight junctions and mediate IgG1 antibody responses to bacterial antigen, conferring protective immunization.
Abstract
Epidermal Langerhans cells (LCs) extend dendrites through tight junctions (TJs) to survey the skin surface, but their immunological contribution in vivo remains elusive. We show that LCs were essential for inducing IgG1 responses to patch-immunized ovalbumin in mice that lacked skin dendritic cell subsets. The significance of LC-induced humoral responses was demonstrated in a mouse model of staphylococcal scalded skin syndrome (SSSS), a severe blistering disease in which the desmosomal protein Dsg1 (desmoglein1) is cleaved by Staphylococcus aureus–derived exfoliative toxin (ET). Importantly, ET did not penetrate TJs, and patch immunization did not alter epidermal integrity. Nevertheless, neutralizing anti-ET IgG1 was induced after patch immunization and abolished upon LC depletion, indicating that antigen capture through TJs by LCs induced humoral immunity. Strikingly, the ET-patched mice were protected from developing SSSS after intraperitoneal ET challenge, whereas LC-depleted mice were susceptible to SSSS, demonstrating a vital role for LC-induced IgG1 in systemic defense against circulating toxin in vivo. Therefore, LCs elicit humoral immunity to antigens that have not yet violated the epidermal barrier, providing preemptive immunity against potentially pathogenic skin microbes. Targeting this immunological process confers protection with minimal invasiveness and should have a marked impact on future strategies for development of percutaneous vaccines.
The longstanding Langerhans cell (LC) paradigm (Schuler and Steinman, 1985; Wilson and Villadangos, 2004) holds that LCs acquire skin-associated antigens and present them to T cells upon migrating to skin-draining lymph nodes. However, a series of recent studies using transgenic mouse models has failed to clearly demonstrate a critical contribution of LCs in the induction of immunity to percutaneous antigens in vivo.
LCs were dispensable for antiviral immunity in a herpes simplex virus skin scarification model (Allan et al., 2003), and mouse models in which LCs were transiently or constitutively depleted showed that they were not essential for eliciting hapten-induced contact hypersensitivity (Kaplan et al., 2005; Kissenpfennig et al., 2005; Bennett et al., 2007). It is possible that LCs are tolerogenic, but this may depend on the nature of the antigen (e.g., foreign or self), route of antigen exposure (e.g., through the epidermis or directly into the dermis), and presence or absence of environmental cues, including interactions with pathogens (Kautz-Neu et al., 2011).
We recently reported that LCs induced IgG1 (Th2) responses to gene gun–immunized bacterial antigens and that skin DC subsets had distinct roles in inducing humoral responses (Nagao et al., 2009). Although this experimental procedure represented in vivo data with antigen specificity, it circumvented the epidermal barrier system and antigen uptake processes. Our subsequent study demonstrated that LCs extend dendrites through the tight junctions (TJs) to the stratum corneum and capture protein antigens without disturbing barrier integrity (Kubo et al., 2009). These findings indicate that LCs survey not only within the skin but also on the skin surface outside of the TJs. In this study, we focused on antigen capture through TJs (ACT) by LCs to determine whether these enigmatic skin DCs are capable of inducing immune responses to percutaneous protein antigens. We used a model of experimental staphylococcal scalded skin syndrome (SSSS), a severe blistering disease caused by exfoliative toxin (ET)–producing Staphylococcus aureus (Stanley and Amagai, 2006), to further demonstrate the critical role of LCs in protective immunity in vivo.
RESULTS AND DISCUSSION
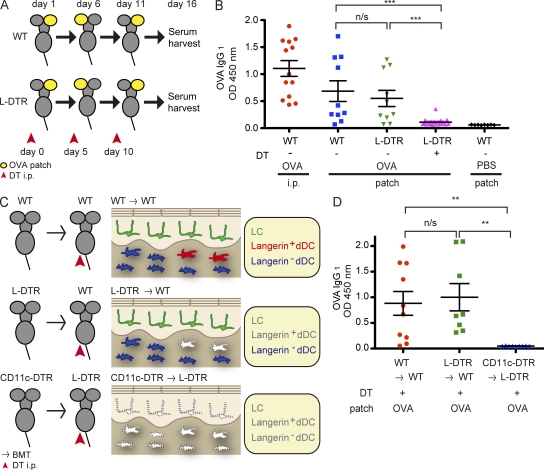
To test whether ACT by LCs leads to antigen-specific IgG1 responses against protein antigens, we patch-immunized WT C57BL/6 mice with OVA three times at 5-d intervals and determined serum anti-OVA antibody levels by ELISA (Fig. 1 A). TJs regulate paracellular trafficking of ions and do not allow passage of soluble proteins (Vermeer et al., 2003). Therefore, OVA does not pass through TJs but can be taken up by LCs across the TJ barrier, as we have recently shown (Kubo et al., 2009).
Figure 1.
LCs are essential for the induction of IgG1 responses to OVA captured through TJs. (A) Patch immunization protocol. 4 mg/ml OVA in PBS was used to patch immunize mice via an occlusive dressing on the gently tape-stripped ears (yellow); the patch was removed after 24 h. In DC depletion experiments, DT was administered i.p. to mice 24 h before each patch immunization. (B) OVA-specific IgG1 responses were determined by ELISA of sera (1:500 dilution) from mice that received OVA i.p. (n = 13), OVA-patched WT mice (n = 10), Langerin-DTR mice without DT i.p. (n = 10), Langerin-DTR mice with DT i.p. (n = 17), and PBS-patched (n = 8) WT mice. ***, P = 0.0006 (second lane vs. fourth lane) and P = 0.0008 (third lane vs. fourth lane) by Student’s t test. Shown are results from a single experiment representative of three experiments. (C) Lethally irradiated recipient mice were reconstituted with BM from the indicated donor mice. After complete chimerism, the mice were treated with DT i.p. to generate mice that harbored (top; WT → WT) or lacked (bottom; CD11c-DTR → L-DTR) all skin DCs and mice that lacked only langerin+ dDCs (middle; L-DTR → WT). BMT, BM transfer. (D) OVA-specific IgG1 responses in sera (1:500 dilution) of mice from C. n = 10 WT → WT, n = 8 L-DTR → WT, and n = 9 CD11c-DTR → L-DTR mice from a single experiment that was reproduced in a similar, independent experiment. **, P = 0.0035 (first lane vs. third lane) and P = 0.0017 (second lane vs. third lane) by Student’s t test. (B and D) Error bars represent mean value ± SEM.
Consistent with our previous results using gene gun immunization (Nagao et al., 2009), WT mice produced OVA-specific antibodies restricted to the IgG1 subclass (Fig. 1 B). To determine the contribution of LCs, we used Langerin-DTR (diphtheria toxin [DT] receptor) mice (Bennett et al., 2005), in which langerin-expressing cells are selectively and transiently depleted upon DT treatment. Langerin+ dermal DCs (dDCs), a recently described dDC subset (Bursch et al., 2007; Ginhoux et al., 2007; Poulin et al., 2007), do not repopulate the dermis for 3–7 d, and LCs do not repopulate the epidermis for at least 2 wk during steady-state after conditional depletion (Nagao et al., 2009; Noordegraaf et al., 2010). Langerin-DTR mice received i.p. DT 1 d before each patch immunization (Fig. 1 A). In contrast to the robust IgG1 responses obtained in WT and DT-untreated Langerin-DTR mice, the responses were abolished in DT-treated Langerin-DTR mice, demonstrating that langerin-expressing DCs were required for humoral responses to patch-immunized protein antigens (Fig. 1 B).
Given that langerin+ dDCs are also depleted in this experimental setting (Nagao et al., 2009), we combined conditional depletion and BM transplantation to generate mice in which only langerin+ dDCs (Langerin-DTR BM → WT) or all DCs (CD11c-DTR BM → Langerin-DTR) were depleted, as well as mice that harbored all DCs (WT BM → WT; Fig. 1 C and Fig. S1). As LCs are radio resistant (Merad et al., 2002), lethally irradiated WT mice chimerized with Langerin-DTR BM could be depleted of langerin+ dDCs upon DT treatment, whereas the LCs of host origin remained unaffected in the epidermis.
Robust anti-OVA responses were observed in mice that harbored all DCs, in contrast to mice that lacked all DCs (Fig. 1 D). Of note, mice that lacked langerin+ dDCs responded with anti-OVA IgG1 levels similar to those of mice harboring all DCs (Fig. 1 D). These results demonstrate an essential role for LCs but not dDCs in inducing antigen-specific IgG1 responses subsequent to ACT.
The aforementioned results led us to wonder whether LCs performed ACT to survey for antigens from bacterial flora that could exert pathogenicity under certain conditions. S. aureus is a clinically relevant bacterium that is capable of colonizing and/or infecting skin, and SSSS is a severe blistering disease that occurs during childhood, caused by skin infection with S. aureus that produces ET (Stanley and Amagai, 2006). ET circulates systemically via the bloodstream to distal skin sites (Nishioka et al., 1977b; Ladhani et al., 1999), where it cleaves Dsg1 (desmoglein1), a desmosomal adhesion molecule of the cadherin family which is critical for maintaining cell–cell adhesion in the upper epidermis (Amagai et al., 2000; Stanley and Amagai, 2006).
Anti-ET antibodies can be detected in healthy individuals with no history of SSSS (Yamasaki et al., 2005), and it has been suggested that the presence or absence of circulating anti-ET antibodies may affect disease severity (Nishioka et al., 1977b; Ladhani et al., 1999). Therefore, we sought to determine whether LCs were capable of capturing S. aureus–derived ET through intact TJs and subsequently prepare a repertoire of antibodies that conferred protection from SSSS development.
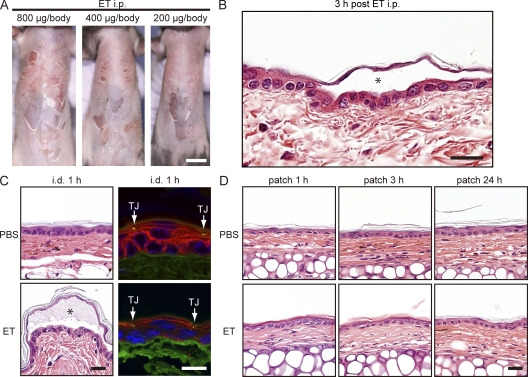
We modified a mouse model of experimental SSSS (Nishioka et al., 1977a) by using recombinant ETA, which is a major isoform of ET along with ETB, in adult mice. A single i.p. injection of ETA induced skin erosions with dose-dependent severity as soon as 3 h after treatment (Fig. 2 A). Epidermal detachment in the granular layer, identical to that seen in human SSSS, was confirmed by histology (Fig. 2 B; Amagai et al., 2000; Stanley and Amagai, 2006). We then determined that TJs do not allow penetration of ETA. When ETA was injected into the dermis, it cleaved Dsg1 on keratinocytes located below but not above the TJ barrier (as visualized by ZO-1; Fig. 2 C). Moreover, tape-stripped skin to which ETA was patch immunized for 1, 3, or 24 h remained intact, with no clinical or histological sign of acantholysis (Fig. 2 D). These findings showed that ETA did not penetrate the epidermal barrier system to reach the viable layers, further suggesting that any immune responses achieved after ETA patch immunization are likely attributable to ACT by LCs.
Figure 2.
Patch-immunized ETA does not alter epidermal integrity. (A) Development of SSSS 3 h after i.p. injection of ETA. (B) Histological features of superficial acantholysis in experimental SSSS. The asterisk denotes blister formation. (C) Visualization of Dsg1 and TJs in ear skin after intradermal (i.d.) injection of PBS or ETA. Dsg1 (red) and TJs (shown via ZO-1 staining in green; arrows) were visualized in the periphery of the blister in ear skin denoted by the asterisk (bottom) that received ETA intradermally (right). (D) Histological features of ear skin that received PBS or ETA patch immunization for 1, 3, or 24 h (right). Each experiment was performed three times with n = 3. Bars: (A) 1 cm; (B, C [left], and D) 25 µm; (C, right) 10 µm.
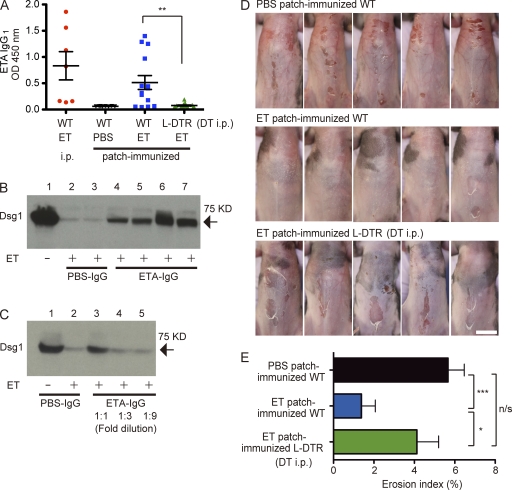
Consistent with the data in Fig. 1, patch immunization with ETA led to anti-ETA IgG1 production (Fig. 3 A). Immunization with OVA i.p. resulted predominantly in IgG1 production, whereas immunization with ETA i.p. induced robust IgG1, IgG2b, and IgG2c responses, in contrast to the IgG1-restricted responses induced by ETA patch immunization (Fig. 3 A and Fig. S2). This further supports a role for LCs in driving the Th2 response (Nagao et al., 2009). Importantly, Langerin-DTR mice that were DT treated before each patch immunization failed to mount ETA-specific IgG1 responses (Fig. 3 A).
Figure 3.
ACT by LCs confers protective and systemic humoral immunity against experimental SSSS. (A) The anti-ETA IgG1 response in WT mice that received ETA i.p., PBS patch, or ETA patch, as well as Langerin-DTR mice that were treated with DT 1 d before each ETA patch immunization. Sera were diluted 1:500. **, P = 0.0024. (B) Cleavage analysis of hrDsg1. ETA was preincubated with purified IgG from mice patch immunized with either PBS (PBS-IgG) or ETA (ETA-IgG) and then further incubated with hrDsg1. Each lane represents IgG purified from a single mouse. The arrow denotes hrDsg1, ∼75 kD in size. (C) Dose dependency of the neutralizing activity of ETA-IgG. (D) PBS patch-immunized WT mice (n = 16), ETA patch-immunized WT mice (n = 14), and DT-treated, ETA patch-immunized Langerin-DTR mice (n = 15) from A were challenged i.p. with 200 µg ETA, and images were taken 3 h later. Shown are representative data from a single experiment. Bar, 1 cm. Experiments in A–C were performed in two other independent experiments and D in one other experiment with similar results. (E) Erosion area index of ventral trunk skin in mice from D. *, P = 0.0432; ***, P = 0.0003. (A and E) Error bars represent mean value ± SEM.
To functionally characterize LC-induced anti-ETA IgG1, we purified IgG from PBS (PBS-IgG) or ETA (ETA-IgG) patch-immunized mice and determined its effect on the Dsg1-cleaving activity of ETA in vitro. Incubation of soluble human recombinant Dsg1 (hrDsg1) with ETA leads to efficient cleavage in vitro (Amagai et al., 2000). Although preincubation of PBS-IgG with ETA had no effect on hrDsg1 cleavage by ETA, the coincubation of ETA-IgG and ETA resulted in a marked decrease in the amount of cleaved hrDsg1 (Fig. 3 B), in a dose-dependent manner (Fig. 3 C), demonstrating that ETA-IgG exerts neutralizing activity toward ETA.
Finally, to directly demonstrate the in vivo role of LC-induced IgG1, we challenged patch-immunized mice with a single i.p. injection of ETA (200 µg/body). The PBS-immunized mice developed skin erosions (Fig. 3 D, top), but the majority of ETA patch-immunized mice were protected from developing SSSS (Fig. 3 D, middle). In stark contrast, Langerin-DTR mice treated with DT before each immunization developed SSSS (Fig. 3 D, bottom), with skin erosion indices significantly higher than those of ETA patch-immunized mice (Fig. 3 E). These results established the essential role of LCs in the induction of humoral responses that conferred systemic protection against circulating toxin in this model of SSSS.
Collectively, ACT by LCs led to the induction of humoral immunity against ETA in the presence of an intact TJ barrier, and this immunity protected mice from developing experimental SSSS upon systemic ETA challenge. This study reveals a novel preemptive role for LCs in protective humoral immunity in vivo against bacteria-derived pathogenic components that exist on the skin surface but have not yet breached the epidermal barrier. The protein antigens used in this study do not reach the viable epidermal layers, and how B cells in the draining lymph nodes recognize them is an interesting question to be clarified in future studies.
Vaccine development to date has focused on overcoming the epidermal barrier, using a variety of physical forces such as a gene gun and microneedles (Prausnitz et al., 2009; Yager et al., 2009). The current work shows that this may not be essential. Targeting ACT by LCs can be extended to other toxins or pathogens and should have a marked impact on the strategies by which topical vaccines are designed to achieve defined systemic humoral immunity, with minimal invasiveness and stress.
MATERIALS AND METHODS
Mice.
Mice were bred in specific pathogen–free facilities at the Keio University School of Medicine. C57BL/6J mice (CLEA), 6–8 wk old, were used for immunization experiments. Heterozygous C57BL/6J Langerin-DTR mice were obtained by breeding homozygous Langerin-DTR mice with C57BL/6J WT mice. CD11c-DTR mice were a gift from D.R. Littman (New York University School of Medicine, New York, NY). All animal procedures and study protocols were approved by the Keio University Ethics Committee for Animal Experiments.
Genomic PCR of Langerin-DTR mice.
The langerin WT allele was detected using the primers LangWTf1 (5′-TGCTTCTGCCCACAACTGCTCTT-3′) and LangWTb1 (5′-GACACCAAGGACTGTAGCCAAAAGG-3′). The langerin-DTR allele was detected by using LangWTf1 and LangDTR2 (5′-TCAGTGGGAATTAGTCATGCC-3′).
In vivo depletion of langerin+ DCs.
For in vivo depletion of langerin+ DCs, heterozygous Langerin-DTR mice were injected i.p. with 500 ng DT (Sigma-Aldrich) in sterile PBS.
Patch immunization.
The dorsal mouse ears were gently tape-stripped five times with Scotch tape (3M), and double-layered filter paper wetted with 50 µl of 4 mg/ml OVA or ETA in PBS was used to patch immunize the mice, using a patch device (Finn Chamber; SmartPractice). The mice were fitted with plastic collars to avoid scratching of the immunization sites. The Finn Chambers were removed after 24 h, but the collars were kept on for 2 or 3 d to prevent the mice scratching their ears. Immunization was performed three times at 5-d intervals, and the ear that received patch immunization was switched each time from right to left or vice versa to prevent any inadvertent damage to the immunization site. Sera were collected 5 d after the final immunization.
ELISA.
The wells of MaxiSorp ELISA plates (Thermo Fisher Scientific) were coated with OVA or ET (50 ng/well) at 4°C overnight. After blocking, sera were serially diluted 1:500 in PBS containing 3% skim milk and added to the wells. The plates were incubated for 2 h at room temperature. After washing, the plates were incubated with horseradish peroxidase–conjugated goat anti–mouse IgG1/2b/2c/3 antibodies (Bethyl Laboratories, Inc.) for 2 h. Immunoreactivity was detected with TMB substrate solution (MBL), and enzymatic reactions were halted with 1 M NH2SO4 before measuring the optical density at 450 nm.
Generation of BM chimeric mice.
8-wk-old recipient C57BL/6 and/or Langerin-DTR mice were lethally irradiated (9.5 Gy) and reconstituted with 2 × 106 BM cells from C57BL/6, Langerin-DTR, or CD11c-DTR mice.
Antibodies.
Rat anti–mouse EpCAM mAb (clone G8.8; Developmental Studies Hybridoma Bank) labeled with Alexa Fluor 568 (Invitrogen), rat anti–mouse langerin mAb (clone L31; provided by C.-G. Park and R. Steinman, The Rockefeller University, New York, NY) labeled with Alexa Fluor 647 (Invitrogen), and FITC-conjugated rat anti–mouse I-A/I-E mAb (clone M5/114.15.2; BioLegend) for detection of MHC class II were used for immunohistochemistry of epidermal and dermal sheets. Anti–mouse CD16/32 mAb (clone 93; BioLegend) was routinely used to block Fc receptors before staining, and cell nuclei were stained with Hoechst 33258 (Invitrogen). Mouse anti–ZO-1 mAb (clone T8-754 [Itoh et al., 1991]; provided by M. Furuse, Kobe University, Kobe, Japan), anti-Dsg1 scFVs mAb with a hemagglutinin tag (provided by K. Ishii, Teikyo University Chiba Medical Center, Ichihara, Japan; Ishii et al., 2008), and an antihemagglutinin mAb were used for cryosection staining (3F10; Roche). Goat anti–E tag polyclonal antibody (Abcam) and horseradish peroxidase–conjugated donkey anti–goat IgG polyclonal antibody were used for immunoblotting.
Preparation and staining of epidermal and dermal sheets and cryosections.
Epidermal and dermal sheets were prepared with 3.8% ammonium thiocyanate (Wako) in 100 mM sodium phosphate (Nagao et al., 2009). Sheets were fixed in cold acetone and blocked with 3% skim milk (Morinaga), anti-CD16/32 mAb, and goat serum (Jackson ImmunoResearch Laboratories, Inc.) in PBS before incubation with primary antibody overnight at 4°C. Cryosections were fixed with 95% ethanol and postfixed with acetone. Primary antibodies were detected with appropriate Alexa Fluor–labeled secondary antibodies (Invitrogen). All immunofluorescence images were collected and visualized with a laser-scanning confocal microscope (TCS-SP5; Leica) equipped with a 63× objective, using optical slices of 0.4–0.5 µm. Levels of images were linearly adjusted using Photoshop CS5 (Adobe).
Experimental SSSS.
ETA was prepared as described previously (Hanakawa et al., 2002). Abdominal hair was removed using a depilating cream 24–48 h before the experiment. 200 µg ETA diluted in 100 µl of sterile PBS was used to challenge via i.p. injection. Abdominal skin, divided into three parts, was gently rubbed once with the right index finger 3 h after ETA challenge to observe epidermal detachment (Nikolsky’s sign). The skin erosion area was quantified as several pixels, using ImagePro version 6.3 (Media Cybernetics), and this number was divided by the total pixel number of the entire abdominal area.
In vitro Dsg1 cleavage assay.
A Melon Gel IgG Purification System (Thermo Fisher Scientific) was used to obtain purified IgG from sera of mice that were patch immunized with ETA or PBS. The entire extracellular domain of hrDsg1 with an E tag on the carboxyl terminus was produced as a secreted protein, using the baculovirus system as described previously (Amagai et al., 2000; Ishii et al., 1997). 20 µl of recombinant ET (4 µg/ml) was preincubated with 20 µl of purified IgG for 1 h at 37°C and then with 1 µg hrDsg1 in 10 µl PBS containing 1 mM CaCl2 for 1 h. The digested samples were subjected to SDS-PAGE and transferred to polyvinylidene fluoride membranes (Bio-Rad Laboratories). Bands were detected using anti–E tag antibody and visualized with a chemiluminescent substrate (ECL Plus; GE Healthcare), followed by exposure to Kodak BioMax film (VWR international).
Statistical analysis.
Statistical analysis was performed with the Student’s t test using Prism version 5 (GraphPad Software), and p-values ≤0.05 were regarded as significant.
Online supplemental material.
Fig. S1 shows the generation of mice that lack langerin+ dDCs. Fig. S2 shows that the humoral response induced by LCs subsequent to ACT is restricted to IgG1. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20111718/DC1.
Acknowledgments
We thank Showbu Sato, Hiromi Ito, and Kayoko Eguchi for invaluable technical assistance.
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, Research on Measures against Intractable Diseases, the Ministry of Health, Labour and Welfare of Japan, and Keio Gijuku Academic Development Funds. B.E. Clausen is a fellow of The Netherlands Organization for Scientific Research.
S. Koyasu is a consultant for Medical and Biological Laboratories Co., Ltd. The authors otherwise have no financial conflicts of interest.
Author contributions: T. Ouchi performed the majority of the experiments with assistance from A. Kubo, M. Yokouchi, T. Adachi, T. Kobayashi, D.Y. Kitashima, and H. Fujii, overseen by M. Amagai and K. Nagao. B.E. Clausen provided Langerin-DTR mice. K. Nagao conceived the experiments, and H. Fujii and S. Koyasu assisted with the BM transfer experiments. T. Ouchi and K. Nagao wrote the manuscript.
Footnotes
Abbreviations used:
- ACT
- antigen capture through TJs
- dDC
- dermal DC
- DT
- diphtheria toxin
- DTR
- DT receptor
- ET
- exfoliative toxin
- hrDsg1
- human recombinant Dsg1
- LC
- Langerhans cell
- SSSS
- staphylococcal scalded skin syndrome
- TJ
- tight junction
References
- Allan R.S., Smith C.M., Belz G.T., van Lint A.L., Wakim L.M., Heath W.R., Carbone F.R. 2003. Epidermal viral immunity induced by CD8alpha+ dendritic cells but not by Langerhans cells. Science. 301:1925–1928 10.1126/science.1087576 [DOI] [PubMed] [Google Scholar]
- Amagai M., Matsuyoshi N., Wang Z.H., Andl C., Stanley J.R. 2000. Toxin in bullous impetigo and staphylococcal scalded-skin syndrome targets desmoglein 1. Nat. Med. 6:1275–1277 10.1038/81385 [DOI] [PubMed] [Google Scholar]
- Bennett C.L., van Rijn E., Jung S., Inaba K., Steinman R.M., Kapsenberg M.L., Clausen B.E. 2005. Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J. Cell Biol. 169:569–576 10.1083/jcb.200501071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett C.L., Noordegraaf M., Martina C.A., Clausen B.E. 2007. Langerhans cells are required for efficient presentation of topically applied hapten to T cells. J. Immunol. 179:6830–6835 [DOI] [PubMed] [Google Scholar]
- Bursch L.S., Wang L., Igyarto B., Kissenpfennig A., Malissen B., Kaplan D.H., Hogquist K.A. 2007. Identification of a novel population of Langerin+ dendritic cells. J. Exp. Med. 204:3147–3156 10.1084/jem.20071966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F., Collin M.P., Bogunovic M., Abel M., Leboeuf M., Helft J., Ochando J., Kissenpfennig A., Malissen B., Grisotto M., et al. 2007. Blood-derived dermal langerin+ dendritic cells survey the skin in the steady state. J. Exp. Med. 204:3133–3146 10.1084/jem.20071733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanakawa Y., Schechter N.M., Lin C., Garza L., Li H., Yamaguchi T., Fudaba Y., Nishifuji K., Sugai M., Amagai M., Stanley J.R. 2002. Molecular mechanisms of blister formation in bullous impetigo and staphylococcal scalded skin syndrome. J. Clin. Invest. 110:53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K., Amagai M., Hall R.P., Hashimoto T., Takayanagi A., Gamou S., Shimizu N., Nishikawa T. 1997. Characterization of autoantibodies in pemphigus using antigen-specific enzyme-linked immunosorbent assays with baculovirus-expressed recombinant desmogleins. J. Immunol. 159:2010–2017 [PubMed] [Google Scholar]
- Ishii K., Lin C., Siegel D.L., Stanley J.R. 2008. Isolation of pathogenic monoclonal anti-desmoglein 1 human antibodies by phage display of pemphigus foliaceus autoantibodies. J. Invest. Dermatol. 128:939–948 10.1038/sj.jid.5701132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M., Yonemura S., Nagafuchi A., Tsukita S., Tsukita S. 1991. A 220-kD undercoat-constitutive protein: its specific localization at cadherin-based cell-cell adhesion sites. J. Cell Biol. 115:1449–1462 10.1083/jcb.115.5.1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan D.H., Jenison M.C., Saeland S., Shlomchik W.D., Shlomchik M.J. 2005. Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 23:611–620 10.1016/j.immuni.2005.10.008 [DOI] [PubMed] [Google Scholar]
- Kautz-Neu K., Noordegraaf M., Dinges S., Bennett C.L., John D., Clausen B.E., von Stebut E. 2011. Langerhans cells are negative regulators of the anti-Leishmania response. J. Exp. Med. 208:885–891 10.1084/jem.20102318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissenpfennig A., Henri S., Dubois B., Laplace-Builhé C., Perrin P., Romani N., Tripp C.H., Douillard P., Leserman L., Kaiserlian D., et al. 2005. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 22:643–654 10.1016/j.immuni.2005.04.004 [DOI] [PubMed] [Google Scholar]
- Kubo A., Nagao K., Yokouchi M., Sasaki H., Amagai M. 2009. External antigen uptake by Langerhans cells with reorganization of epidermal tight junction barriers. J. Exp. Med. 206:2937–2946 10.1084/jem.20091527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladhani S., Joannou C.L., Lochrie D.P., Evans R.W., Poston S.M. 1999. Clinical, microbial, and biochemical aspects of the exfoliative toxins causing staphylococcal scalded-skin syndrome. Clin. Microbiol. Rev. 12:224–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merad M., Manz M.G., Karsunky H., Wagers A., Peters W., Charo I., Weissman I.L., Cyster J.G., Engleman E.G. 2002. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat. Immunol. 3:1135–1141 10.1038/ni852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao K., Ginhoux F., Leitner W.W., Motegi S., Bennett C.L., Clausen B.E., Merad M., Udey M.C. 2009. Murine epidermal Langerhans cells and langerin-expressing dermal dendritic cells are unrelated and exhibit distinct functions. Proc. Natl. Acad. Sci. USA. 106:3312–3317 10.1073/pnas.0807126106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka K., Nakano T., Hirao N., Asada Y. 1977a. Staphylococcal scalded skin syndrome I. Purification of exfoliatin and maternal transmission of neutralizing ability against exfoliatin. J. Dermatol. 4:13–18 [DOI] [PubMed] [Google Scholar]
- Nishioka K., Nakano T., Hirao N., Teranishi H., Asada Y. 1977b. Staphylococcal scalded skin syndrome. II. Serum level of anti exfoliatin and anti alpha-toxin in patients with staphylococcal scalded skin syndrome or bullous impetigo. J. Dermatol. 4:65–68 [DOI] [PubMed] [Google Scholar]
- Noordegraaf M., Flacher V., Stoitzner P., Clausen B.E. 2010. Functional redundancy of Langerhans cells and Langerin+ dermal dendritic cells in contact hypersensitivity. J. Invest. Dermatol. 130:2752–2759 10.1038/jid.2010.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin L.F., Henri S., de Bovis B., Devilard E., Kissenpfennig A., Malissen B. 2007. The dermis contains langerin+ dendritic cells that develop and function independently of epidermal Langerhans cells. J. Exp. Med. 204:3119–3131 10.1084/jem.20071724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prausnitz M.R., Mikszta J.A., Cormier M., Andrianov A.K. 2009. Microneedle-based vaccines. Curr. Top. Microbiol. Immunol. 333:369–393 10.1007/978-3-540-92165-3_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler G., Steinman R.M. 1985. Murine epidermal Langerhans cells mature into potent immunostimulatory dendritic cells in vitro. J. Exp. Med. 161:526–546 10.1084/jem.161.3.526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley J.R., Amagai M. 2006. Pemphigus, bullous impetigo, and the staphylococcal scalded-skin syndrome. N. Engl. J. Med. 355:1800–1810 10.1056/NEJMra061111 [DOI] [PubMed] [Google Scholar]
- Vermeer P.D., Einwalter L.A., Moninger T.O., Rokhlina T., Kern J.A., Zabner J., Welsh M.J. 2003. Segregation of receptor and ligand regulates activation of epithelial growth factor receptor. Nature. 422:322–326 10.1038/nature01440 [DOI] [PubMed] [Google Scholar]
- Wilson N.S., Villadangos J.A. 2004. Lymphoid organ dendritic cells: beyond the Langerhans cells paradigm. Immunol. Cell Biol. 82:91–98 10.1111/j.1440-1711.2004.01216.x [DOI] [PubMed] [Google Scholar]
- Yager E.J., Dean H.J., Fuller D.H. 2009. Prospects for developing an effective particle-mediated DNA vaccine against influenza. Expert Rev. Vaccines. 8:1205–1220 10.1586/erv.09.82 [DOI] [PubMed] [Google Scholar]
- Yamasaki O., Yamaguchi T., Sugai M., Chapuis-Cellier C., Arnaud F., Vandenesch F., Etienne J., Lina G. 2005. Clinical manifestations of staphylococcal scalded-skin syndrome depend on serotypes of exfoliative toxins. J. Clin. Microbiol. 43:1890–1893 10.1128/JCM.43.4.1890-1893.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]