Abstract
With the intention to modulate gene expression in vascular mural cells of remodeling vessels, we generated and characterized transgenic mouse lines with Cre recombinase under the control of the platelet-derived growth factor receptor-β promoter, referred to as Tg(Pdgfrb-Cre)35Vli. Transgenic mice were crossed with the Gt(ROSA)26Sortm1Sor strain and examined for Cre activation by β-galactosidase activity, which was compared with endogenous Pdgfrb expression. In addition, Pdgfrb-Cre mice were used to drive expression of a conditional myc-tagged Cthrc1 transgene. There was good overlap of β-galactosidase activity with endogenous Pdgfrb immunoreactivity. However, dedifferentiation of vascular mural cells induced by carotid artery ligation revealed a dramatic discrepancy between ROSA26 reporter activity and Pdgfrb promoter driven Cre dependent myc-tagged Cthrc1 transgene expression. Our studies demonstrate the capability of the Pdgfrb-Cre mouse to drive conditional transgene expression as a result of prior Cre mediated recombination in tissues known to express endogenous Pdgfrb. In addition, the study shows that ROSA26 promoter driven reporter mice are not suitable for lineage marking of smooth muscle in remodeling blood vessels.
Keywords: vascular smooth muscle, dedifferentiation, Cthrc1
Introduction
Blood vessel development is guided by receptor ligand interactions that allow for crosstalk between the endothelial cells and the surrounding mesenchyme. The role of platelet-derived growth factor (Pdgf) in recruiting mural cells during blood vessel formation has been demonstrated through gene targeting of the ligand (Pdgfb) (Hellstrom et al., 1999; Leveen et al., 1994) and the platelet-derived growth factor receptor beta (Pdgfrb) (Soriano, 1994); however, major arteries and the heart appeared to develop normally in Pdgfrb deficient embryos (Soriano, 1994). The dependence of pericyte/smooth muscle cell (SMC) recruitment on Pdgfb/Pdgfrb signaling was shown to be variable between different tissues, with vascular mural cells expressing Pdgfrb in most tissues except liver, as determined by in situ hybridization (Hellstrom et al., 1999).
The expression of Pdgf receptors has been well characterized and the expression of the Pdgfrb has been demonstrated in fibroblasts, kidney mesangium (Alpers et al., 1993; Seifert et al., 1998), myoblasts and muscle lineages (Crosby et al., 1998), smooth muscle (Majesky et al., 1990), pericytes (Lindahl et al., 1999), and neurons (Smits et al., 1991). In the developing embryo, Pdgfrb expression was prominent in the developing mesenchyme and in the developing gastrointestinal tract (Shinbrot et al., 1994).
In mice and rats, endothelial cells are typically the only cell type that is found in the tunica intima of arteries. Various models have been developed to induce intimal thickening in carotid arteries and our laboratory developed and characterized the mouse carotid artery wire denudation (Lindner et al., 1993) as well as the carotid artery ligation model (Kumar and Lindner, 1997). Intimal lesion formation in these models is preceded by dedifferentiation and proliferation of SMC in the media with subsequent migration of proliferating SMC into the intima where they give rise to the neointima (Kumar and Lindner, 1997; Lindner et al., 1993; Reidy et al., 1992). The dedifferentiation of SMC in this process is characterized by a reduction in SMC specific markers such as smooth muscle alpha actin, SM22-alpha, and smooth muscle myosin heavy chain (SM-MHC) (Lindner et al., 1993; Regan et al., 2000).
Pdgf signaling also plays important roles in arteries and in the response to arterial injury by promoting migration of SMC from the media into the developing neointima (Buetow et al., 2003; Ferns et al., 1991; Jackson et al., 1993; Jawien et al., 1992; Majesky et al., 1990). Using the rat balloon injury model we previously demonstrated that the activated and dedifferentiated SMC of the neointima expressed elevated levels of Pdgfrb (Lindner et al., 1995; Lindner and Reidy, 1995). In the present study we describe the generation of transgenic mice using a Pdgfrb promoter fragment to drive Cre expression. Pdgfrb-Cre mice were crossed with Gt(ROSA)26Sortm1Sor reporter mice as well as mice carrying a Cre inducible transgene in order to characterize the sites of prior Cre recombinase activity in embryonic tissues, postnatal tissues and in remodeling arteries.
Results
We generated transgenic mice with Cre recombinase under the control of the Pdgfrb promoter and crossed them to the Cre reporter strain B6;129S4-Gt(ROSA)26Sortm1Sor (Soriano, 1999). In this reporter strain LacZ expression is silent until the transcriptional stop, flanked by LoxP sites, is excised by Cre recombinase (Fig. 1). Tg(Pdgfrb-Cre)35Vli mice were also bred with transgenic mice expressing Cre inducible collagen triple helix repeat containing 1 (Cthrc1) with a myc epitope tag fused to its C-terminus, designated strain name Tg(CagGfp-Cthrc1myc)11Vli. In the absence of Cre recombination this transgenic mouse line expresses GFP under the control of the CAG promoter. Upon Cre recombination, the GFP cassette, flanked by LoxP sites, is removed and Cthrc1-myc is expressed (Fig. 1). This allowed for comparison of β-galactosidase staining and myc tag immunoreactivity localization.
Figure 1.
Crossing the Tg(Pdgfrb-Cre)35Vli mice (b) with the Gt(ROSA)26Sortm1Sor reporter mice (a) allows Cre recominbase to excise the stop sequence between the loxP sites resulting in LacZ expression and β-galactosidase activity under the control of the ROSA26 promoter in cells where the Pdgfrb promoter is also active (d). Crossing the Tg(Pdgfrb-Cre)35Vli mice (b) with Tg(CagGfp-Cthrc1-myc)11Vli mice (c) elicits the Cre recominbase protein to remove the GFP cassette flanked by loxP sites. Subsequently, the Cthrc1-myc transgene is expressed under the control of the CAG promoter in cells where the Pdgfrb promoter is active (e). In the absence of Cre recombinase, the Tg(CagGfp-Cthrc1myc)11Vli transgenic mouse line expresses GFP under the control of the CAG promoter. Generation of a myc epitope tag rabbit monoclonal antibody allowed for specific detection of the transgene.
The distribution pattern of β-galactosidase activity in various postnatal tissues seen in the Tg(Pdgfrb-Cre)35Vli/Gt(ROSA)26Sortm1Sor mice was similar to the immunoreactivity of the Cre inducible transgene driven by the Pdgfrb promoter in Tg(Pdgfrb-Cre) 35Vli/Tg(CagGfp-Cthrc1myc)11Vli mice (Fig. 2). Consistent with endogenous Pdgfrb expression, β-galactosidase activity and transgene immunoreactivity were seen in vascular and alimentary tract smooth muscle, kidney mesangium, skeletal muscle, adult myocardium and dermal fibroblasts (Fig. 2). The blood vessels of the liver showed no β-galactosidase activity, transgene expression or Pdgfrb immunoreactivity (not shown). The recombination efficiency in esophageal smooth muscle and skeletal muscle of four adult Tg(Pdgfrb-Cre)35Vli/Gt(ROSA)26Sortm1Sor mice was determined to be 99.5±0.6% and 99±2.0%, respectively (Table 1). In embryonic tissues, β-galactosidase activity was present in the developing dermis, skeletal muscle, mesenchymal condensation/perichondrium, lung tissue, spinal cord/ectoderm, kidney mesangial cells and the myocardium. Serial sections displaying Pdgfrb immunoreactivity showed an analogous staining pattern (Fig. 3).
Figure 2.
Characterization of the Tg(Pdgfrb-Cre)35Vli strain crossed to the ROSA26 reporter strain, Gt(ROSA)26Sortm1Sor, was performed by detection of β-galactosidase activity (left column). The ability to activate a Cre-inducible transgene was determined by crossing the Tg(Pdgfrb-Cre)35Vli strain with the Tg(CagGfp-Cthrc1myc)11Vli strain and transgene expression was analyzed by anti-myc immunohistochemistry (left center column, immunoreactivity in brown). Endogenous expression of Pdgfrb in serial sections was assessed by anti-Pdgfrb immunohistochemistry (right center column). Negative controls for the antibody stains are also shown on serial sections (right column). In both the ROSA26 and myc-tagged Cthrc1 reporter crosses, prior Cre mediated recombination was detected in the areas where endogenous Pdgfrb expression is present: the myocardium (MC), esophageal smooth muscle (ESM), skeletal muscle, fibroblasts of the dermis (DF), mesangial cells of kidney glomeruli (M), and vascular smooth muscle cells (vSMC) of the lung and the brain. All images were taken at the same magnification, scale bar = 50 μm.
Table 1.
Recombination efficiency in skeletal and smooth muscle
| Esophageal Smooth Muscle (%β-gal positive muscle area) | Skeletal Muscle (%β-gal positive muscle area) | |
|---|---|---|
| Pdgfrb-Cre/R26R #1 | 100.0 | 100.0 |
| Pdgfrb-Cre/R26R #2 | 100.0 | 100.0 |
| Pdgfrb-Cre/R26R #3 | 99.0 | 96.0 |
| Pdgfrb-Cre/R26R #4 | 99.0 | 100.0 |
| Average±SD | 99.5±0.6 | 99.0±2.0 |
To assess recombination efficiency in esophageal smooth muscle and skeletal muscle, the percentage of muscle displaying β-galactosidase staining was quantified in 4 three month old Tg(Pdgfrb-Cre)35Vli/Gt(ROSA)26Sortm1Sor mice.
Figure 3.

Embryos were harvested from Tg(Pdgfrb-Cre)35Vli/Gt(ROSA)26Sortm1Sor crosses at E10.5 (a–d), E13.5 (e–j) and E17.5 (k–n). β-galactosidase activity is depicted in blue (right column) and Pdgfrb immunoreactivity is shown in brown (left column). In adjacent sections of E10.5 embryos, β-galactosidase activity and endogenous Pdgfrb expression were detected in the developing dermis (a, b) and developing skeletal muscle of the limb (c, d). In adjacent sections of E13.5 embryos, the mesenchymal condensation/perichondrium (MC/P) (e, f), lung tissue (g, h) but not the airway epithelium (A), and the spinal cord/ectoderm (i, j) showed β-galactosidase activity and endogenous Pdgfrb expression. Mesangial cells of kidney glomeruli (M) (k, l) and the myocardium (MC) (m, n) showed β-galactosidase activity and endogenous Pdgfrb expression in adjacent sections of E17.5 embryos. All images were taken at the same magnification, scale bar = 50 μm.
We analyzed β-galactosidase activity staining on sections of remodeling and normal carotid arteries from Tg(Pdgfrb-Cre)35Vli/Gt(ROSA)26Sortm1Sor mice to assess recombination efficiency in vascular SMC. Activity of β-galactosidase was restricted to medial SMC with no detectable staining observed in the adventitia. The percentage of carotid artery SMC exhibiting β-galactosidase activity in uninjured common carotid arteries was similar for the left and right side (not shown), with a range from 35.5% to 79.0% and an average of 61.9±17.9%. Surprisingly, remodeling carotid arteries responding to ligation revealed very few β-galactosidase positive cells in the neointima (Fig. 4). In addition, the percentage of SMC in the media exhibiting β-galactosidase activity also declined. Quantification of β-galactosidase staining in the ligated (left) and corresponding contralateral (non-injured) carotid artery is shown in Table 2 for five adult mice. The medial SMC and dedifferentiated SMC of the neointima did express endogenous Pdgfrb as well as the Pdgfrb-Cre driven myc-tagged transgene (Fig. 4).
Figure 4.
Remodeling carotid arteries of Tg(Pdgfrb-Cre)35Vli mice crossed to the Gt(ROSA) 26Sortm1Sor reporter strain reveal reduction of β-galactosidase activity in dedifferentiated SMC and medial smooth muscle cells (mSMC) (b) when compared to the high β-galactosidase activity of the mSMC in the contralateral artery control (a). Remodeling arteries of Gt(ROSA)26Sor mice with constitutive expression of LacZ under ROSA26 promoter control show a similar reduction of β-galactosidase activity (d) compared to the contralateral carotid artery (c) indicating that the reduction of ROSA26 reporter activity in dedifferentiated SMC is independent of Cre recombinase activity. Serial sections of remodeling carotid arteries of Tg(Pdgfrb-Cre)35Vli mice crossed with the Tg(CagGfp-Cthrc1-myc)11Vli Cre-inducible transgenic line reveal transgene activity in the neointimal SMC (h) comparable to endogenous Pdgfrb expression (f). The transgene expression in mSMC of the contralateral artery control (g) is also analogous to the endogenous Pdgfrb staining of mSMC (e) in serial sections. All images were taken at the same magnification, scale bar = 50 μm. Medial smooth muscle cells, mSMC; inner elastic lamellae, IEL, indicating the boundary between the mSMC and neointimal lesion; Tg(Pdgfrb-Cre)35Vli × Gt(ROSA) 26Sortm1Sor, Cre35 × R26R; Gt(ROSA)26Sor, ROSALacZ; Tg(Pdgfrb-Cre)35Vli × Tg(CagGfp-Cthrc1-myc)11Vli, Cre35 × Cag13.
Table 2.
Quantification of β-galactosidase activity in normal and remodeling carotid arteries.
| Control Carotid Artery (%β-gal positive medial area) | Ligated Carotid Artery (%β-gal positive medial area) | Ligated Carotid Artery (%β-gal positive intimal area) | |
|---|---|---|---|
| Pdgfrb-Cre/R26R #1 | 79.0 | 3.1 | 6.9 |
| Pdgfrb-Cre/R26R #2 | 51.6 | 23.7 | 11.0 |
| Pdgfrb-Cre/R26R #3 | 35.5 | 1.9 | 1.9 |
| Pdgfrb-Cre/R26R #4 | 72.3 | 5.6 | 6.3 |
| Pdgfrb-Cre/R26R #5 | 71.0 | 3.3 | 0.2 |
| Average±SD | 61.9±17.9 | 7.5±9.1 | 5.3±4.3 |
The percentage of vessel area displaying β-galactosidase staining was quantified for the media of unmanipulated carotid arteries and separately for the media and intima of the remodeling carotid artery of 5 three month old Tg(Pdgfrb-Cre)35Vli/Gt(ROSA)26Sortm1Sor mice.
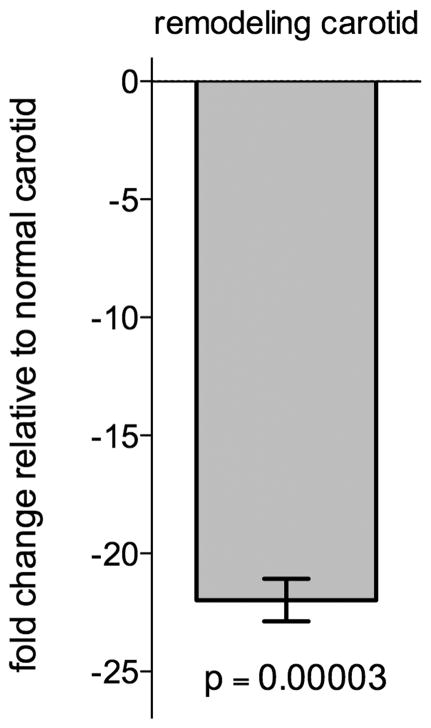
To address the utility of the Gt(ROSA)26Sortm1Sor reporter line for analyzing Cre recombinase activity in remodeling arteries we applied the carotid artery ligation model to B6;129S-Gt(ROSA)26Sor mice with constitutive expression of LacZ under ROSA26 promoter control (Friedrich and Soriano, 1991). β-galactosidase activity localization in these mice also exhibited a significant decrease in the number of β-galactosidase positive SMC in the media as well as in the developing neointima of the remodeling artery (Fig. 4). Quantitative real time reverse transcription PCR (qRT-PCR) data revealed a 21.98 ± 1.56 fold decrease (p=0.00003) in LacZ expression in remodeling carotid arteries when compared to expression in normal carotid arteries (Fig. 5). These findings indicate that the decrease in ROSA26 reporter activity is independent of Cre mediated recombination.
Figure 5.
LacZ transcript levels in remodeling and normal carotid arteries from Gt(ROSA)26Sor mice with constitutive expression of LacZ under ROSA26 promoter control were analyzed using qRT-PCR. LacZ expression in the remodeling carotid arteries was quantified as a fold-change relative to expression in normal carotid arteries (−21.98 ± 1.56 fold change, p=0.00003).
Discussion
One major reason for developing Pdgfrb-Cre transgenic mice was the high activity of the Pdgfrb promoter in dedifferentiated vascular smooth muscle of remodeling and injured arteries (Lindner and Reidy, 1995; Majesky et al., 1990). While a Pdgfrb-Cre transgenic mouse line has been previously developed by others (Abraham et al., 2008; Foo et al., 2006), a detailed analysis of this mouse line has not been published. The purpose of the study conducted here was to characterize a Pdgfrb-Cre transgenic mouse line with emphasis on vascular smooth muscle and mural cells. The Tg(Pdgfrb-Cre)35Vli/Gt(ROSA) 26Sortm1Sor mice displayed β-galactosidase activity in most medial SMC of the carotid arteries, indicating that these cells were indelibly tagged by Cre recombination. With β-galactosidase now under the control of ROSA26 regulatory elements and the ROSA26 promoter thought to be active in most cells and tissues, we anticipated that SMC forming the neointima would display β-galactosidase activity because they originated from cells in the media (Reidy et al., 1992). The low β-galactosidase activity seen in dedifferentiated SMC was therefore surprising and raised the question whether the ROSA26 reporter is suppressed in dedifferentiated SMC.
Using Gt(ROSA)26Sor mice that have constitutive β-galactosidase activity (in the absence of any prior Cre mediated recombination), we found low β-galactosidase activity levels (Fig. 3) and a significant reduction in LacZ expression (Fig. 5) in dedifferentiated SMC. These data indicated a relative reduction of ROSA26 reporter activity in dedifferentiated SMC. We ruled out the possibility that only LacZ negative cells from the media contributed to neointima formation because SMC undergoing dedifferentiation in the media also lost expression of LacZ (Table 2). We could also rule out a potential discrepancy between LacZ expression and β-galactosidase enzyme activity because remodeling and control tissue specimens were meticulously treated in an identical fashion and because β-galactosidase activity was still detectable in some SMC that had not undergone dedifferentiation within the remodeling artery.
We previously discovered Cthrc1 as a gene expressed in remodeling arteries and we generated a transgenic mouse expressing myc-tagged Cthrc1, Tg(CagGfp-Cthrc1myc)11Vli, in the presence of Cre recombinase (Fig. 1). Using myc-tagged Cthrc1 transgene expression as an alternative readout for prior Cre recombinase activity, we clearly demonstrated that Cre recombination had occurred in the SMC that dedifferentiated in response to carotid artery ligation in Tg(Pdgfrb-Cre) 35Vli/Tg(CagGfp-Cthrc1myc)11Vli double mutant mice. We were not able to directly demonstrate Cre protein expression in remodeling arteries by immunohistochemistry due to the lack of suitable antibodies.
We previously generated transgenic mouse lines that constitutively overexpress Cthrc1 under the control of the CMV promoter (Pyagay et al., 2005). When we crossed two different transgenic mouse lines, we observed brittle bones with reduced collagen matrix deposition (Pyagay et al., 2005). Yamamoto et al. (Yamamoto et al., 2008) as well as Kimura et al. (Kimura et al., 2008) reported that targeted disruption of the Cthrc1 in mice did not result in any developmental abnormalities. Here we used a mutant mouse strain with inducible expression of Cthrc1 following Pdgfrb-Cre mediated recombination. We deliberately chose expression of the myc-tagged Cthrc1 transgene as a readout for prior Cre activity for this study because we anticipated no major developmental abnormalities that would have complicated the interpretation of the transgene expression results. Although not investigated in any detail here, we did not observe any obvious developmental abnormalities in Tg(Pdgfrb-Cre)35Vli/Tg(CagGfp-Cthrc1myc)11Vli double mutant mice. Because Cthrc1 is an efficiently secreted protein and Pdgfrb is a cell surface receptor, the distribution of the immunoreactivity for these two proteins is different at the cellular level.
Our results indicate that the Pdgfrb −4.7/+0.1kb regulatory sequence of the Pdgfrb gene used here drives Cre mediated recombination and subsequent transgene expression in most cell types that express endogenous Pdgfrb. The Tg(Pdgfrb-Cre)35Vli strain should be a useful tool for researchers in various fields including vascular and kidney biology. We also show that the Gt(ROSA)26Sortm1Sor reporter mice are not suitable for cell lineage tracking in blood vessels undergoing remodeling because LacZ expression as well β-galactosidase activity under direct control of the ROSA26 locus are decreased in dedifferentiated SMC. These results may be limited to reporter mice driven by the endogenous ROSA26 promoter and may not apply to more recent ROSA26 reporter strains such as those with the CAG promoter knocked into the ROSA26 locus (Yamamoto et al., 2009). The most likely explanation for these results is that the activity of the ROSA26 promoter is decreased in remodeling vasculature. However, in the absence of any in-depth characterization of the ROSA26 promoter we cannot rule out decreased stability of the LacZ transcript as an alternative explanation.
Methods
Construct generation
A −4.7/+0.1 kb fragment of the Pdgfrb promoter including the first exon was amplified from mouse genomic DNA using the forward primer (CGTTGCTTCCTTGATGTTTGCTTGTGA) and the reverse primer (GCTGTCTTCTGTTATTTTGCTGGACCCA). The amplified sequence was fused to a nuclear targeted Cre recombinase sequence.
Animals
All studies involving mice were approved by the Institutional Animal Care and Use Committee of the Maine Medical Center Research Institute, which adheres to all applicable federal laws and rules, and the guidelines and recommendations spelled out in the “Guide for Care and Use of Laboratory Animals.”
Oocytes from an FVB female mated with a homozygous male of the B6;129S4-GT(ROSA)26Sortm1Sor1 strain (JAX #003309) (Soriano, 1999) were injected with the construct using standard methodologies. Founder animals were identified and crossed to Gt(ROSA)26Sortm1Sor mice to analyze Cre recombinase activity by examining β-galactosidase activity in embryonic tissue (E10.5, E13.5 and E17.5), adult organs and after vascular injury. Three month old animals (n=5) were used in the carotid artery flow cessation model as described (Kumar and Lindner, 1997). The carotid arteries, aortae and organs were harvested 4 weeks later for analysis of β-galactosidase activity.
Cre-inducible transgenic mice expressing myc-tagged Cthrc1, Tg(CagGfp-Cthrc1myc)11Vli, were generated (Fig. 1) and bred with Tg(Pdgfrb-Cre)35Vli mice to obtain double transgenic mice. The myc tag allowed for specific detection of the transgene by immunohistochemistry. Three month old animals (n=5) were used in the carotid artery flow cessation model as described (Kumar and Lindner, 1997). The carotid arteries, aortae and organs were harvested 4 weeks later for anti-myc and Pdgfrb immunohistochemistry.
To assess the utility of the ROSA26 promoter driven reporter mice in remodeling arteries more directly, B6; 129S-Gt(ROSA)26Sor mice (JAX # 002073) (Friedrich and Soriano, 1991) with constitutive expression of LacZ under ROSA26 promoter control were subjected to carotid artery ligation to induce arterial remodeling. The carotid arteries, aortae and hearts were harvested 4 weeks later for histological analysis of β-galactosidase activity or one week later for analysis of LacZ expression.
Histological analysis, immunohistochemistry and morphometry
Frozen sections of carotid arteries, aortae and embryonic tissues (20μm thick) were prepared for β-galactosidase activity localization with nuclear Fast Red counterstain as described (Weiler et al., 2001). Immunohistochemistry for Pdgfrb (Epitomics, Inc., #1469-1, 1:250 dilution) and the myc epitope tag were performed with rabbit monoclonal antibodies on serial sections of paraformaldehyde fixed tissues embedded in paraffin following antigen retrieval with heat in citrate buffer pH=6.0 (LeClair et al., 2007). The myc epitope tag specific rabbit monoclonal antibody (clone Vli-1, 10ng/ml dilution) was raised against a synthetic peptide derived from the human c-myc proto-oncogene (EQKLISEEDL). Adjacent sections were also stained without a primary antibody to serve as negative immunohistochemistry controls.
Image analysis was used to quantify β-galactosidase activity on cross-sections of aortae and ligated as well as contralateral carotid arteries as previously described (LeClair et al., 2007). These measurements were also used to assess recombination efficiency. In all cases, recombination efficiency was similar in left and right common carotid arteries from unmanipulated mice. The percentage of medial and neointimal area exhibiting blue β-galactosidase staining in the remodeling artery was determined and expressed as a percentage of the blue area determined for the non-ligated carotid artery. Recombination efficiency for esophageal smooth muscle and skeletal muscle was determined by quantifying the percentage of muscle tissue showing β-galactosidase activity in tissue sections from four adult mice.
Quantitative real time reverse transcription PCR (qRT-PCR)
Levels of LacZ transcript in remodeling and normal carotid arteries from Gt(ROSA)26Sor mice with constitutive expression of LacZ under ROSA26 promoter control were compared by isolating RNA from remodeling (left) and normal (right) carotid arteries (n=7). cDNA was prepared by reverse transcription and transcript levels were determined using qRT-PCR with glyceraldehyde-3-phosphate dehydrogenase (Gapdh) as a reference gene. qRT-PCR was performed on the Bio-Rad iQ5 cycler using the iQ-SYBR Green Supermix (Bio-Rad). Primers amplifying LacZ (GGTAAACTGGCTCGGATTAGGG and GCTGATGTTGAACTGGAAGTCGC) and mGAPDH (ATCACTGCCACCCAGAAGACTG and ATCCACGACGGACACATTGG) were used. A non-template control was included for all qRT-PCR reactions. All samples were run in triplicate. The thermal profile was 95°C for 10 min, 40 cycles of 95°C for 15 sec and 58°C for 1 min. Samples were normalized with respect to Gapdh and expression levels in the remodeling carotid arteries were expressed as a fold change relative to the normal carotid arteries using the comparative Ct method (ΔΔCt = ΔCt sample − ΔCt reference).
Acknowledgments
Supported by NIH Grants HL69182 (V.L.) and P20 RR-15555 from the National Center for Research Resources. The Histopathology Core Facility is supported by P20RR181789 (Director Dr. Don Wojchowski). The Mouse Transgenic Core was supported by P20 RR-15555 (Director Dr. Robert E. Friesel).
The authors thank Anne Harrington for her services related to transgenic animal generation and Kathleen Carrier for excellent histology services.
References
- Abraham S, Kogata N, Fassler R, Adams RH. Integrin beta1 subunit controls mural cell adhesion, spreading, and blood vessel wall stability. Circ Res. 2008;102:562–570. doi: 10.1161/CIRCRESAHA.107.167908. [DOI] [PubMed] [Google Scholar]
- Alpers CE, Seifert RA, Hudkins KL, Johnson RJ, Bowen-Pope DF. PDGF-receptor localizes to mesangial, parietal epithelial, and interstitial cells in human and primate kidneys. Kidney Int. 1993;43:286–294. doi: 10.1038/ki.1993.45. [DOI] [PubMed] [Google Scholar]
- Buetow BS, Tappan KA, Crosby JR, Seifert RA, Bowen-Pope DF. Chimera analysis supports a predominant role of PDGFRbeta in promoting smooth-muscle cell chemotaxis after arterial injury. Am J Pathol. 2003;163:979–984. doi: 10.1016/s0002-9440(10)63457-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby JR, Seifert RA, Soriano P, Bowen-Pope DF. Chimaeric analysis reveals role of Pdgf receptors in all muscle lineages. Nat Genet. 1998;18:385–388. doi: 10.1038/ng0498-385. [DOI] [PubMed] [Google Scholar]
- Ferns GA, Raines EW, Sprugel KH, Motani AS, Reidy MA, Ross R. Inhibition of neointimal smooth muscle accumulation after angioplasty by an antibody to PDGF. Science. 1991;253:1129–1132. doi: 10.1126/science.1653454. [DOI] [PubMed] [Google Scholar]
- Foo SS, Turner CJ, Adams S, Compagni A, Aubyn D, Kogata N, Lindblom P, Shani M, Zicha D, Adams RH. Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell. 2006;124:161–173. doi: 10.1016/j.cell.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Friedrich G, Soriano P. Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev. 1991;5:1513–1523. doi: 10.1101/gad.5.9.1513. [DOI] [PubMed] [Google Scholar]
- Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–3055. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- Jackson CL, Raines EW, Ross R, Reidy MA. Role of endogenous platelet-derived growth factor in arterial smooth muscle cell migration after balloon catheter injury. Arterioscler Thromb. 1993;13:1218–1226. doi: 10.1161/01.atv.13.8.1218. [DOI] [PubMed] [Google Scholar]
- Jawien A, Bowen-Pope DF, Lindner V, Schwartz SM, Clowes AW. Platelet-derived growth factor promotes smooth muscle migration and intimal thickening in a rat model of balloon angioplasty. J Clin Invest. 1992;89:507–511. doi: 10.1172/JCI115613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H, Kwan KM, Zhang Z, Deng JM, Darnay BG, Behringer RR, Nakamura T, de Crombrugghe B, Akiyama H. Cthrc1 is a positive regulator of osteoblastic bone formation. PLoS ONE. 2008;3:e3174. doi: 10.1371/journal.pone.0003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Lindner V. Remodeling with neointima formation in the mouse carotid artery after cessation of blood flow. Arterioscler Thromb Vasc Biol. 1997;17:2238–2244. doi: 10.1161/01.atv.17.10.2238. [DOI] [PubMed] [Google Scholar]
- LeClair RJ, Durmus T, Wang Q, Pyagay P, Terzic A, Lindner V. Cthrc1 is a novel inhibitor of transforming growth factor-beta signaling and neointimal lesion formation. Circ Res. 2007;100:826–833. doi: 10.1161/01.RES.0000260806.99307.72. [DOI] [PubMed] [Google Scholar]
- Leveen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev. 1994;8:1875–1887. doi: 10.1101/gad.8.16.1875. [DOI] [PubMed] [Google Scholar]
- Lindahl P, Bostrom H, Karlsson L, Hellstrom M, Kalen M, Betsholtz C. Role of platelet-derived growth factors in angiogenesis and alveogenesis. Curr Top Pathol. 1999;93:27–33. doi: 10.1007/978-3-642-58456-5_4. [DOI] [PubMed] [Google Scholar]
- Lindner V, Fingerle J, Reidy MA. Mouse model of arterial injury. Circ Res. 1993;73:792–796. doi: 10.1161/01.res.73.5.792. [DOI] [PubMed] [Google Scholar]
- Lindner V, Giachelli CM, Schwartz SM, Reidy MA. A subpopulation of smooth muscle cells in injured rat arteries expresses platelet-derived growth factor-B chain mRNA. Circ Res. 1995;76:951–957. doi: 10.1161/01.res.76.6.951. [DOI] [PubMed] [Google Scholar]
- Lindner V, Reidy MA. Platelet-derived growth factor ligand and receptor expression by large vessel endothelium in vivo. Am J Pathol. 1995;146:1488–1497. [PMC free article] [PubMed] [Google Scholar]
- Majesky MW, Reidy MA, Bowen-Pope DF, Hart CE, Wilcox JN, Schwartz SM. PDGF ligand and receptor gene expression during repair of arterial injury. J Cell Biol. 1990;111:2149–2158. doi: 10.1083/jcb.111.5.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyagay P, Heroult M, Wang Q, Lehnert W, Belden J, Liaw L, Friesel RE, Lindner V. Collagen triple helix repeat containing 1, a novel secreted protein in injured and diseased arteries, inhibits collagen expression and promotes cell migration. Circ Res. 2005;96:261–268. doi: 10.1161/01.RES.0000154262.07264.12. [DOI] [PubMed] [Google Scholar]
- Regan CP, Adam PJ, Madsen CS, Owens GK. Molecular mechanisms of decreased smooth muscle differentiation marker expression after vascular injury. J Clin Invest. 2000;106:1139–1147. doi: 10.1172/JCI10522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidy MA, Fingerle J, Lindner V. Factors controlling the development of arterial lesions after injury. Circulation. 1992;86:III43–46. [PubMed] [Google Scholar]
- Seifert RA, Alpers CE, Bowen-Pope DF. Expression of platelet-derived growth factor and its receptors in the developing and adult mouse kidney. Kidney Int. 1998;54:731–746. doi: 10.1046/j.1523-1755.1998.00046.x. [DOI] [PubMed] [Google Scholar]
- Shinbrot E, Peters KG, Williams LT. Expression of the platelet-derived growth factor beta receptor during organogenesis and tissue differentiation in the mouse embryo. Dev Dyn. 1994;199:169–175. doi: 10.1002/aja.1001990302. [DOI] [PubMed] [Google Scholar]
- Smits A, Kato M, Westermark B, Nister M, Heldin CH, Funa K. Neurotrophic activity of platelet-derived growth factor (PDGF): Rat neuronal cells possess functional PDGF beta-type receptors and respond to PDGF. Proc Natl Acad Sci U S A. 1991;88:8159–8163. doi: 10.1073/pnas.88.18.8159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes Dev. 1994;8:1888–1896. doi: 10.1101/gad.8.16.1888. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Weiler H, Lindner V, Kerlin B, Isermann BH, Hendrickson SB, Cooley BC, Meh DA, Mosesson MW, Shworak NW, Post MJ, Conway EM, Ulfman LH, von Andrian UH, Weitz JI. Characterization of a mouse model for thrombomodulin deficiency. Arterioscler Thromb Vasc Biol. 2001;21:1531–1537. doi: 10.1161/hq0901.094496. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Shook NA, Kanisicak O, Yamamoto S, Wosczyna MN, Camp JR, Goldhamer DJ. A multifunctional reporter mouse line for Cre- and FLP-dependent lineage analysis. Genesis. 2009;47:107–114. doi: 10.1002/dvg.20474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Nishimura O, Misaki K, Nishita M, Minami Y, Yonemura S, Tarui H, Sasaki H. Cthrc1 selectively activates the planar cell polarity pathway of Wnt signaling by stabilizing the Wnt-receptor complex. Dev Cell. 2008;15:23–36. doi: 10.1016/j.devcel.2008.05.007. [DOI] [PubMed] [Google Scholar]