Abstract
Simultaneous and accurate measurement of vitamin D and 25-hydroxyvitamin D in biological samples is a barrier limiting our ability to define “optimal” vitamin D status. Thus, our goal was to optimize conditions and evaluate an LC-MS method for simultaneous detection and quantification of vitamin D2, vitamin D3, 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3 in serum. Extraction and separation of vitamin D forms were achieved using acetone liquid–liquid extraction and by a reversed phase C8 column, respectively. Detection was performed on a triple quadrupole tandem mass spectrometer (QQQ-MS/MS) equipped with atmospheric pressure photo ionization source. The LOQs for all analytes tested were 1 ng/mL for hydroxylated molecules and 2 ng/mL for the parent vitamin Ds. RSD at lower LOQ (2 ng/mL) and in medium (80 ng/mL) and high (200 ng/mL) quality control samples did not exceed 20 and 15% CV, respectively. Accuracy of the method for determination of hydroxylated molecules was also validated using National Institutes of Standards and Technology standard samples and found to be in the range of 90.9–111.2%. In summary, a sensitive and reproducible method is reported for simultaneous quantification of vitamin D2, vitamin D3, 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3 molecules in biological samples.
1 Introduction
Vitamin D status and its relationship to health and chronic disease is an active research area in nutrition and medicine 1, 2. In addition to its known role in bone health, emerging evidence suggests that vitamin D may play a role in the risk of several chronic diseases including cancer, autoimmune diseases, cardiovascular disease and type II diabetes 1. However, progress in our understanding of the relationship between vitamin D status and health is dependent on our ability to accurately measure vitamin D status. Many recent reports have assessed the incidence of vitamin D deficiency or insufficiency around the world 1. Vitamin D status is largely indicated by the 25(OH)D level in serum since it is the main storage metabolite of vitamin D representing its accumulation both from diet and cutaneous synthesis. In serum, 25(OH)D is preferentially bound to vitamin D binding protein and its half-life in serum is longer than either vitamin D itself or the vitamin D hormone, 1,25(OH)2D. However, there are barriers to the accurate analysis of 25(OH)D that impede accurate and reproducible assessment of vitamin D status in individuals and populations 3. The analytical uncertainties in measuring serum 25(OH)D was recently emphasized at the NIH workshop “Nutrient Biomarkers Analytical Methodology: Vitamin D Workshop” held on December 16, 2009.
The analytical challenges associated with measurement of vitamin D and its metabolites extend to both foods and biological tissues. In addition, vitamin D exists as vitamin D2 (D2) and vitamin D3 (D3). While the D2 vitamer is found in plants, D3 is produced in the skin from 7-dehydrocholesterol after UV irradiation. They are present in low concentrations in both foods and biological tissues. Further vitamin D is stored as 25(OH)D and converted to the biologically active form, 1,25(OH)2D, which in turn is catabolized to a variety of compounds that may appear in measurable amounts in tissues 4. The tissue distribution, apart from blood of vitamin D forms and metabolites, is largely unexplored due to inadequate methods to measure these metabolites and the ability to distinguish the two forms of vitamin D and their metabolites in small quantities.
Current vitamin D analysis methods have strengths and weaknesses; they often lack sensitivity and specificity needed to address questions concerning tissue distribution of the many forms of vitamin D. Commercially available kit assays provide high-throughput analysis of 25(OH)D, but not of vitamin D, and interlaboratory performance is poor 5, 6. The kits rely on an extraction method from serum based on acetonitrile and a short C18 column to separate 25(OH)D from other metabolites. Additionally, these kits are unable to accurately and separately measure 25-hydroxyvitamin D3 (25(OH)D3) and 25-hydroxyvitamin D2 (25(OH)D2), as they are confounded by cross-reactivity with catabolic 24,25(OH)2D metabolites. Therefore, these methods may underestimate 25(OH)D2 due to lower affinity of the antibodies used for 25(OH)D2.
HPLC can resolve D3 and D2, as well as their 25(OH)D metabolites 7. Although HPLC coupled with UV/VIS detector is typically used to measure vitamin D in food 8, 9 it lacks the desired level of specificity due to spectral interferences for complex biological matrices. On the other hand, HPLC coupled with MS (LC-MS) offers both increased sensitivity and selectivity 10 and minimizes interferences commonly seen from complex food matrices 11. Several isotope dilution LC-MS/MS methods have been developed for measuring 25(OH)D2 and 25(OH)D3 in plasma that reported LOQs range of 1.7–6.5 ng/mL 12–18. Recently, Duan et al. and Eyles et al. have published methods for determination of vitamin D metabolites with LODs 1–2 pg/mL 19, 20. However, to achieve these LOQs they rely on pre-concentration steps before injection, derivatization and the use of microflow chromatography. Methods are also designed for analysis of structurally similar vitamin D forms and metabolites (D2 and D3 or 25(OH)D2 and 25(OH)D3). While promising, no method determining all four metabolites in single chromatographic run has been published. Our aim was to develop an improved LC-MS method for simultaneous determination of D2, D3, 25(OH)D2, and 25(OH)D3 in plasma using an alternative ionization technique known as atmospheric pressure photo ionization (APPI) to provide additional sensitivity for analysis without pre-concentration. Additionally, the method was validated using the newly available National Institutes of Standards and Technology (NIST, Gaithersburg, MD) serum standards for 25(OH)D2 and 25(OH)D321.
2 Materials and methods
2.1 Chemicals and reagents
Analytical standards for D2, D3, 25(OH)D2, 25(OH)D3, as well as pooled serum, toluene (≥99.9%), and ethanol (200 proofs) were purchased from Sigma Aldrich (St. Louis, MO). The deuterated [2H6]-D3 (26,26,26,27,27,27-d6), [2H6]- D2 (26,26,26,27,27,27-d6), [2H6]-25(OH)D3 (26,26,26,27,27,27-d6) and [2H6]-25(OH)D2 (26,26,26,27,27,27-d6) were obtained from Medical Isotopes (Pelham, NH). Standard reference materials (SRMs; Levels 1–4) for 25(OH)D2 and 25(OH)D3 in human serum were obtained from the NIST. Acetonitrile (≥99.8%), acetone (≥99.5%), methanol (≥99.9%), isopropanol (≥99.5%) and hexane (≥99.5%) were purchased from Mallinckrodt Chemicals (Phillipsburg, NJ). Double-deionized water was produced by a Milli-Q gradient A10 system from Millipore (Bedford, MA).
2.2 Stock and working standard solutions
Stock solutions of D2, D3, 25(OH)D2, 25(OH)D3, [2H6]-D2, [2H6]-D3, [2H6]-25(OH)D2 and [2H6]-25(OH)D3 were prepared at a concentration of 1 mg/mL in ethanol and stored at −70°C until use. Working standard mix solutions were generated by mixing equal volumes of D2, D3, 25(OH)D2 and 25(OH)D3 stock solutions and serially diluted to concentrations of 20, 40, 80, 160, 400, 800, 2000, 3000 and 6000 ng/mL (each) with ethanol. Internal standard (IS) solution was prepared by mixing equal volumes of [2H6]-D2, [2H6]-D3, [2H6]-25(OH)D2 and [2H6]-25(OH)D3 stock solution and diluted to 400 ng/mL (each) with ethanol.
2.3 Preparation of calibration curves, quality control and serum samples
Calibration standards were prepared freshly at concentrations of 1, 2, 4, 8, 20, 40, 100, 150, and 300 ng/mL (each) by adding 10 μL of individual calibration working solutions with 180 μL of PBSA solution (10 mM phosphate buffer, pH 7.5; 140 mM NaCl; 8% BSA) and 10 μL of IS (20 ng/mL final concentration).
Quality control (QC) samples were prepared independently in a similar way at concentrations of 2 ng/mL (lower LOQ – LLOQ), 80 ng/mL (medium quality control – MQC) and 200 ng/mL (high QC – HQC) for 25(OH)D2 and 25(OH)D3, and at 4 ng/mL (LLOQ), 80 ng/mL (MQC) and 200 ng/mL (HQC) for D2 and D3. In addition, NIST standard reference samples of vitamin D in human serum (Levels 1, 2, 3 and 4) were used for QC. In this case, 95 μL of serum was spiked with 5 μL of IS solution at 20 ng/mL (final concentration).
2.4 Sample extraction
To evaluate extraction efficacy, common extraction solvents, including acetonitrile, acetone, methanol, isopropanol and hexane, were examined. For each extraction, 200 μL of individual solvent was added to 100 μL of serum spiked with deuterated standards at a concentration of 100 ng/mL (each) and mixtures were vortexed for 5 min, sonicated for 10 min and centrifuged at 15.500×g for 5 min. Supernatants (200 μL) were directly transferred to an auto-sampler vial, analyzed using LC-MS/MS and corresponding signal intensities compared.
The extraction of the samples for method validation was achieved by mixing 200 μL of ice-cold acetone with 100 μL of calibration standards or QC samples in PBSA, mixture was vortexed and sonicated for 5 and 10 min, respectively, and centrifuged at 15.500×g for 5 min. Supernatant (250 μL) was directly transferred to an auto-sampler vial and analyzed using LC-MS/MS.
The NIST samples were extracted with ice-cold acetone as described above except the serum was spiked with deuterated standards at 20 ng/mL (each, final concentration).
2.5 LC-MS
The LC-MS/MS analysis was performed with an Agilent Rapid Res 1200 LC system consisting of a binary pump, degasser and auto sampler. Column ACE3C8 (3 μm; 4.2 mm×75 mm; VWR, Batavia, IL) was used for reverse phase separation. Solvent A was water and solvent B was methanol containing 1% toluene. The mobile phase flow rate was 0.3 mL/min and injection volume was 40 μL. The gradient was as follows: t=0 min: 50% B; t=0.5 min: 50% B; t=1.5 min: 95% B; t=21.5 min: 95% B; t=23 min: 50% B; t=25 min: 50% B. Following separation, column effluent was connected to an Agilent 6460 triple quadruple mass spectrometer (Agilent Technologies, Santa Clara, CA) equipped with APPI source. Vitamin D forms and metabolites were detected in positive mode using multiple reaction monitoring (MRM) technique. The source conditions were as follows: gas temperature, 250°C; vaporizer temperature, 200°C; gas flow rate, 7 L/min; nebulizer pressure, 40 psi; capillary voltage, +3500 V; electron multiplier voltage, 500 eV. The fragmentor voltage was 50 V and dwell time 50 ms. The MS was set up to allow the [MH+] ions of Vitamin D forms and metabolites to pass the first quadrupole (Q1) into the collision cell (Q2), fragmented and corresponding fragments were monitored through third quadrupole (Q3). A total of three transitions were monitored for each metabolite and corresponding deuterated IS (Table 1).
Table I.
Transitions and corresponding fragmentor and collision energies.
| Compound | Prec. Ion [m/z] | Prod. Ion [m/z] | FE [V] | CE [V] |
|---|---|---|---|---|
| D2 | 397.3 | 379.2 | 100 | 5 |
| 397.3 | 271.2 | 100 | 10 | |
| 397.3 | 255.2 | 100 | 10 | |
| d6-D2 | 403.3 | 385.2 | 100 | 5 |
| 403.3 | 277.3 | 100 | 10 | |
| 403.3 | 255.2 | 100 | 10 | |
| D3 | 385.3 | 367.2 | 100 | 5 |
| 385.3 | 259.2 | 100 | 5 | |
| 385.3 | 247.2 | 100 | 10 | |
| d6-D3 | 391.3 | 373.2 | 100 | 5 |
| 391.3 | 265.3 | 100 | 5 | |
| 391.3 | 253.2 | 100 | 10 | |
| 25OHD2 | 413.3 | 395.2 | 50 | 5 |
| 413.3 | 355.1 | 50 | 5 | |
| 413.3 | 337.2 | 50 | 5 | |
| d6-25OHD2 | 419.3 | 401.2 | 50 | 5 |
| 419.3 | 355.2 | 50 | 5 | |
| 419.3 | 337.2 | 50 | 5 | |
| 25OHD3 | 401.2 | 383.2 | 50 | 5 |
| 401.2 | 365.3 | 50 | 5 | |
| 401.2 | 257.2 | 50 | 10 | |
| d6-25OHD3 | 407.3 | 389.2 | 50 | 5 |
| 407.3 | 371.3 | 50 | 5 | |
| 407.3 | 263.2 | 50 | 10 |
To evaluate the effect of dopant on photo-ionization efficiency, three modification of mobile phase B were assessed including mobile phase B containing no dopant, mobile phase B with 1% toluene and mobile phase B containing both 1% toluene and 1% acetone. Chromatographic and MS/MS conditions were the same as described above.
2.6 Method validation
Calibration curves of individual vitamin D forms and metabolites were generated by plotting the peak-area ratios of target and IS versus nominal concentrations. The linearity was determined using correlation coefficient (R2) of the curves by means of weighted least-squares linear regression method. The LOQ of D2, D3, 25(OH)D2 and 25(OH)D3 were determined using S/N ratio of 10:1.
Precision and accuracy were calculated using extracts of QC samples prepared in PBSA at concentrations of 2, 80 and 200 ng/mL for 25(OH)D2 and 25(OH)D3, and at 4, 80 and 200 ng/mL for D2 and D3, respectively. For intraday precision, samples were reanalyzed for four times on a single day and for interday precision once on four consecutive days, respectively. Concentrations were calculated form calibration curves prepared on the same day as described above. To evaluate the method using Levels 1–4 of NIST standard reference samples of vitamin D in human serum, concentrations were determined from the area ratio between non-deuterated (unknown amount in sample) and deuterated (known amount of IS) forms of corresponding analytes. The accuracy was calculated by the equation: (mean of determined concentration/nominal concentration)×100, and expressed in percentage (%). The precision was evaluated by the relative CV (%CV).
3 Results and discussion
3.1 Optimization of MS/MS conditions
One of the most challenging aspects in vitamin D analysis is establishing the ionization efficiencies for low (fmole) levels of specific analytes expected in biological matrices. ESI is commonly used in conjunction with LC-MS for analysis of several vitamins. However, the effectiveness of this method of ionization is analyte-dependent. The structure of vitamin D-related molecules (Fig. 1) suggests they would not readily protonate during ESI. As such, atmospheric pressure chemical ionization (APCI) has been used in combination with a MRM techniques for sensitive detection of vitamin D forms and metabolites in human plasma 12, 13, 16, 22, 23. While promising, these methods have relatively high LOQs. In addition, all methods include a concentration step that increases variability of the assay. Recently, APPI has been increasingly and successfully used for the detection of a wide range of biologically relevant molecules at very low concentrations 24, 25. Comparison of APPI and APCI revealed that APPI offers generally higher signal intensities, lower detection limits and better S/N ratios 24. Therefore APPI-MRM-MS/MS may represent a useful method for analysis of vitamin D-related compounds in biological samples.
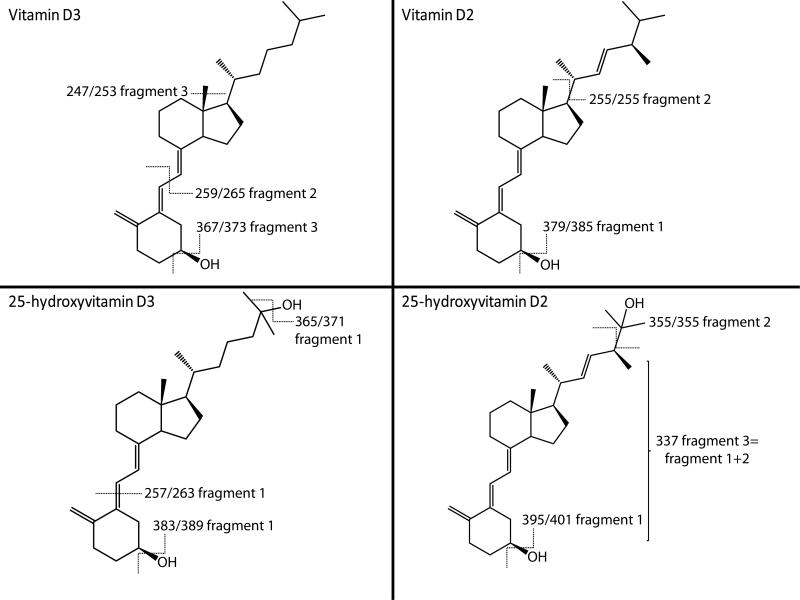
Figure 1.
In order to assess the usefulness of APPI-MRM-MS/MS techniques for vitamin D forms and their metabolite analysis, experiments were first conducted to establish the most efficient transition for MRM. Traditionally [M−H2O+H]+ ions are used for monitoring and quantification of all vitamin D-related compounds. Although these fragments seem to be the most abundant, they suffer from poor reproducibility and selectivity as the simple loss of one water molecule requires very low fragmentation energy and only limited structural information is gained to distinguish between similar molecules. To evaluate ionization sources and MRM transitions, the three most intense fragments for each vitamin D form and metabolite were selected (Fig. 1 and Table 1) using a screen of corresponding standards at 20 ng/mL. To further improve MRM conditions, the effect of gas temperature, and collision and fragmentor energies were investigated over the range of 200–300°C, 1–20 eV and 25–150 V, respectively. While optimal gas temperature was determined at 250°C for all studied analytes, values for fragmentor energy and collision energy varied for each vitamin D form and metabolite (Table 1).
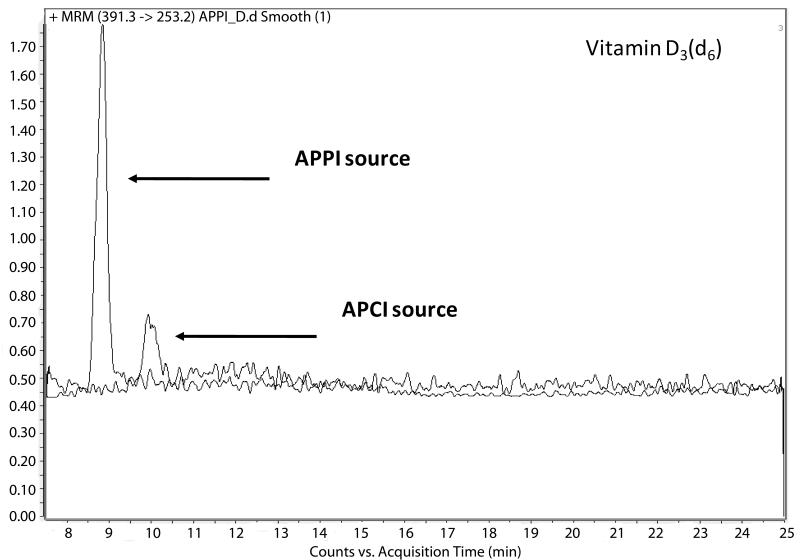
The response of individual vitamin D-related compounds to APCI and APPI sources was evaluated by monitoring the intensities of corresponding fragments in MRM transitions using standards at 20 ng/mL. As expected, the most predominant transitions for both ionization sources were obtained for single water loss. Although the rest of the monitoring ions exhibited lower peak intensities they could be still considered as good targets for quantitative analysis in complex samples (Table 2). The use of APPI source resulted in more intense signals for all tested fragments. Detailed comparison of both ionization sources revealed that improvement in ionization by APPI depends on the individual structures of the vitamin D-related molecules studied. The signal intensities of D2 and D3 increased four- to sevenfold compared with APCI while 25(OH)D3 and 25(OH)D2 signal intensities increased eight- to elevenfold (Fig. 2) and 16–27-fold, respectively (Table 2). These results suggest that implementation of APPI source may significantly increase method sensitivity for vitamin D determination through enhanced ionization efficiency.
Table II.
Comparison of APPI and APCI ionization sources.
| Compound | Prec. Ion [m/z] | Prod. Ion [m/z] | APPI [Intensity] | APCI [Intensity] | APPI/APCI |
|---|---|---|---|---|---|
| d6-D2 | 403.3 | 385.2 | 1051 | 225 | 5 |
| 403.3 | 277.3 | 1509 | 222 | 7 | |
| 403.3 | 255.2 | 2960 | 669 | 4 | |
| d6-D3 | 391.3 | 373.2 | 2201 | 412 | 5 |
| 391.3 | 265.3 | 3606 | 817 | 4 | |
| 391.3 | 253.2 | 4133 | 821 | 5 | |
| d6-25OHD2 | 419.3 | 401.2 | 1181 | 65 | 18 |
| 419.3 | 355.2 | 1099 | 67 | 16 | |
| 419.3 | 337.2 | 2955 | 108 | 27 | |
| d6-25OHD3 | 407.3 | 389.2 | 1785 | 230 | 8 |
| 407.3 | 371.3 | 3212 | 310 | 10 | |
| 407.3 | 263.2 | 11489 | 1031 | 11 |
Figure 2.
3.2 Optimization of LC conditions
The choice of the composition of organic solvents is crucial for any reversed phase LC separation as this has great impact on the retention time of analytes, peak shapes, sensitivity and therefore on the overall separation efficiency. The majority of current methods are based on separation of vitamin D forms and metabolites using reversed phase chromatography and isocratic elution with high concentration of organic solvent such as methanol, acetonitrile or hexane. However, these methods typically target structurally similar vitamin D-related molecules (D2 and D3 or 25(OH)D2 and 25(OH)D3) that elute under similar conditions. Simultaneous analysis of all four metabolites requires either a two-step isocratic or linear gradient elution to accommodate broad differences in hydrophobicity of the individual vitamers and their hydroxylated forms. In this study, separation of these molecules was achieved using C8 reverse phase column and by steep linear gradient from 50% methanol to 95% methanol in 1 min followed by 20 min isocratic elution with 95% methanol. Although these conditions were not able to fully resolve all of the standards (retention times of 25(OH)D2 and 25(OH)D3 are 6.5 and 6.6 min, respectively, and retention times of D2 and D3 are 9.3 and 9.4 min, respectively) differentiation of these unresolved molecules was easily accomplished at the MS level by following different selective transitions in MRM mode.
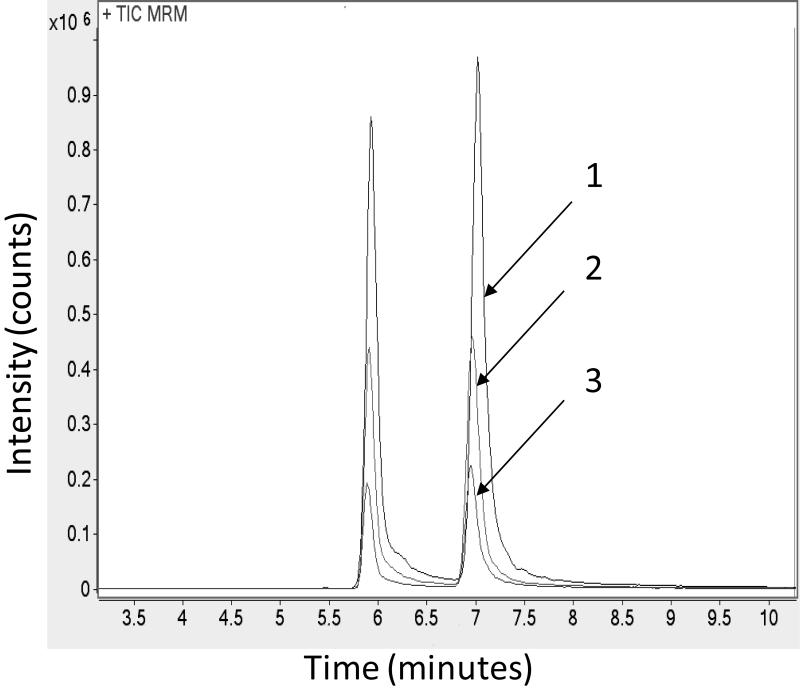
The photo-ionization efficiency is highly dependent on the concentration of dopant such as anisole, acetone or toluene in mobile phase. The effect of dopant on the signal intensities of vitamin D forms and metabolites was investigated using three mobile phases containing no dopant, 1% toluene, or 1% toluene and 1% acetone. The best ionization efficiency was achieved with 1% toluene in mobile phase B enhancing the signal of these compounds by approximately fourfold (Fig. 3).
Figure 3.
3.3 Evaluation of the extraction methods
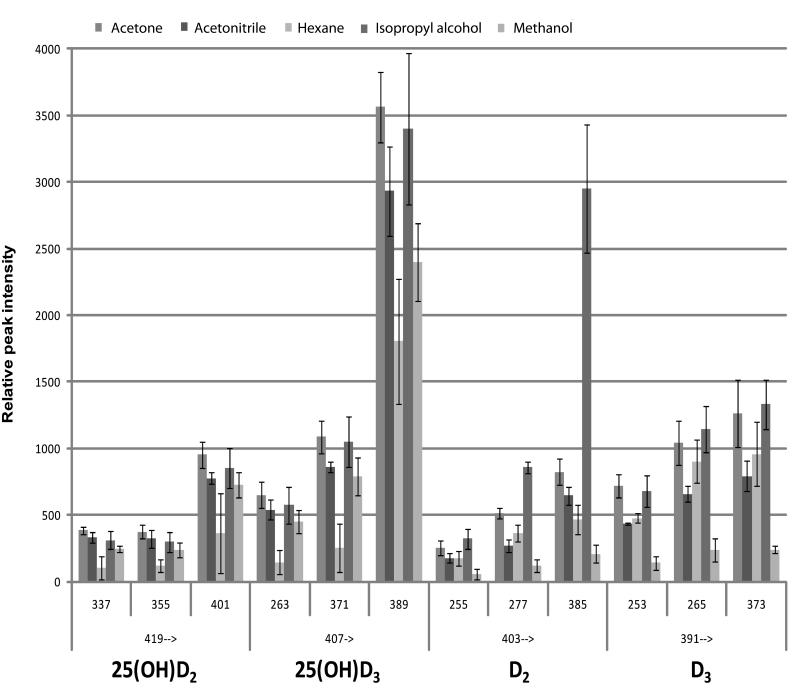
To optimize extraction several liquid–liquid extraction (LLE) methods were evaluated on serum samples spiked with deuterated standards at a concentration of 100 ng/mL. The use of LLE is attractive as this procedure is simple and economical. Compared with more complex multistep techniques such as SPE, LLE often suffers from general extraction conditions resulting in presence of interfering compounds and the existence of sample matrix effects 26. On the other hand, APPI is less susceptible to these matrix effects 24 and allows for use of simplified and reproducible solvent extractions. Solvents used in this study included acetone, methanol, acetonitrile, isopropanol and hexane. Methanol, acetonitrile and hexane have been routinely used for LLE of vitamin D forms or their metabolites with extraction efficiencies of more than 90% 12, 13, 22, 27. Acetone, however, is more efficient at effectively precipitating and removing lipids from proteins in complex matrixes 28 and therefore may be a better extraction solvent for vitamin D-related molecules that are usually strongly associated with carrier or binding proteins.
To evaluate the efficiency of extraction with these solvents, LLE samples were analyzed by MRM using three transitions per individual metabolite. The results were assessed on the overall intensity of the response calculated from the peak area as well as the reproducibility expressed as standard deviation. Acetone and isopropanol extraction provided the highest peak intensities followed by acetonitrile, methanol and hexane (Fig. 4). However, RSD of the methods using isopropyl alcohol and hexane exceed 25% CV. For these reasons, LLE based on acetone was chosen for further analysis.
Figure 4.
3.4 Method validation
Optimized LC-MS/MS conditions were validated using purified standards and their deuterated forms for three transitions per analyte as indicated in Table 1.
The LOQ and linearity of detector response were investigated using nine calibration standards ranging in concentration from 1 to 300 ng/mL in a 40 μL-injection volume. A standard curve was generated by plotting calculated peak area ratios of individual vitamin D metabolites to the corresponding IS versus their nominal concentrations. LOQs (estimated from the standard curve) were determined as 1 ng/mL for hydroxylated molecules, and 2 ng/mL for the parent vitamin D compounds (based on S/N ratios ≥10). The best fit of individual standard curves was achieved using a first-order linear regression (Table 3). Except for 397/271 transition of D2, all other analytes showed good linearity within the tested ranges (R2≥0.995).
Table III.
Linearity ranges, correlation coefficient and LOQs of APPI method.
| Compound | Transition | Linearity range [ng/mL] |
R2 | LOQ [ng/mL] |
Inj. amount at LOQ [pg] |
|---|---|---|---|---|---|
| D2 | 397.3→379.2 | 4 - 300 | 0.9987 | 2 | 26 |
| 397.3→271.2 | NA | NA | NA | NA | |
| 397.3→255.2 | 4 - 300 | 0.9934 | 2 | 26 | |
| D3 | 385.3→367.2 | 2 - 300 | 0.9977 | 2 | 26 |
| 385.3→259.2 | 2 - 300 | 0.9992 | 2 | 26 | |
| 385.3→247.2 | 2 - 300 | 0.9952 | 2 | 26 | |
| 25OHD2 | 413.3→395.2 | 1 - 300 | 0.9941 | 1 | 13 |
| 413.3→355.1 | 1 - 300 | 0.9969 | 2 | 26 | |
| 413.3→337.2 | 1 - 300 | 0.9975 | 1 | 13 | |
| 25OHD3 | 401.2→383.2 | 1 - 300 | 0.9994 | 1 | 13 |
| 401.2→365.3 | 1 - 300 | 0.9963 | 1 | 13 | |
| 401.2→257.2 | 1 - 300 | 0.9978 | 1 | 13 |
Precision was determined by analyzing samples prepared in PBSA at concentrations of 2 ng/mL (LLOQ), 80 ng/mL (MQC) and 200 ng/mL (HQC) for 25(OH)D2 and 25(OH)D3 and at 4 ng/mL (LLOQ), 80 ng/mL (MQC) and 200 ng/mL (HQC) for D2 and D3. For interday, four independently prepared sample sets were analyzed. Intraday precision was calculated from the samples prepared and analyzed in the same day. Concentrations were obtained from the calibration curves, and the accuracy and precision were evaluated by relative error and coefficient of variation, respectively (Table 4). Both precision and accuracy were determined within or slightly exceed 15% (transitions for water loss in D2 and D3, and at LLOQ for transitions of 413 to 337 and 401 to 257 for 25(OH)D2 and 25(OH)D3, respectively) and those of previously published methods 12, 13, 16, 22, 23. In other methods the reported LOQs range from 1.7 to 6.5 ng/mL compared with the 1–2 ng/mL LOQs obtained by using APPI. However to achieve these LOQs the other methods concentrate the samples before injection. Therefore the APPI method described here increases the sensitivity limits by five- to tenfold compared with non-concentrated samples. Implementation of drying step into the APPI was also investigated. Although this reduces the LOQs to 0.5 ng/mL the increased sensitivity was accompanied by increase in the RSD (RSD≥20%).
Table IV.
Intra- and Inter-day accuracy and precision
| D2 Intra day | ||||||
|---|---|---|---|---|---|---|
| Nominal Conc. [ng/mL] |
Transition | |||||
| 397/255 | 397/271 | 397/792 | ||||
| Accuracy [%] | %CV | Accuracy [%] | %CV | Accuracy [%] | %CV | |
| 4 | 103.9 | 12.8 | ND | ND | 117.5 | 17.3 |
| 80 | 88.0 | 6.3 | ND | ND | 87.8 | 5.7 |
| 200 | 99.4 | 6.4 | ND | ND | 101.7 | 4.8 |
| D2 Inter day | ||||||
|---|---|---|---|---|---|---|
| Nominal Conc. [ng/mL] |
Transition | |||||
| 397/255 | 397/271 | 397/792 | ||||
| Accuracy [%] | %CV | Accuracy [%] | %CV | Accuracy [%] | %CV | |
| 4 | 103.8 | 13.7 | ND | ND | 118.5 | 14 |
| 80 | 105.6 | 7.5 | ND | ND | 107.0 | 6.7 |
| 200 | 101.7 | 6.1 | ND | ND | 100.0 | 4.3 |
| D3 Intra day | ||||||
|---|---|---|---|---|---|---|
| Nominal Conc. [ng/mL] |
Transition | |||||
| 385/247 | 385/259 | 385/367 | ||||
| Accuracy [%] | %CV | Accuracy [%] | %CV | Accuracy [%] | %CV | |
| 4 | 103.8 | 2.6 | 98.1 | 5.6 | 111.4 | 7.0 |
| 80 | 83.5 | 5.2 | 84.3 | 2.1 | 82.7 | 2.1 |
| 200 | 89.4 | 2.5 | 89.3 | 0.3 | 94.6 | 2.4 |
| D3 Inter day | ||||||
|---|---|---|---|---|---|---|
| Nominal Conc. [ng/mL] |
Transition | |||||
| 385/247 | 385/259 | 385/367 | ||||
| Accuracy [%] | %CV | Accuracy [%] | %CV | Accuracy [%] | %CV | |
| 4 | 101.5 | 10.3 | 105.9 | 10.7 | 105.0 | 10.1 |
| 80 | 106.9 | 10.3 | 100.0 | 13.1 | 108.8 | 7.9 |
| 200 | 98.4 | 12.7 | 94.6 | 17.8 | 99.5 | 16.9 |
| 25OHD2 Intra day | ||||||
|---|---|---|---|---|---|---|
| Nominal Conc. [ng/mL] |
Transition | |||||
| 413/337 | 413/355 | 413/395 | ||||
| Accuracy [%] | %CV | Accuracy [%] | %CV | Accuracy [%] | %CV | |
| 2 | 101.1 | 16.7 | 107.6 | 10.3 | 108.3 | 2.9 |
| 80 | 77.6 | 5.8 | 91.8 | 5.1 | 87.8 | 3.0 |
| 200 | 84.9 | 2.7 | 107.7 | 1.1 | 98.0 | 2.3 |
| 25OHD2 Inter day | ||||||
|---|---|---|---|---|---|---|
| Nominal Conc. [ng/mL] |
Transition | |||||
| 413/337 | 413/355 | 413/395 | ||||
| Accuracy [%] | %CV | Accuracy [%] | %CV | Accuracy [%] | %CV | |
| 2 | 106.5 | 14.0 | 104.8 | 12.9 | 105.5 | 11.5 |
| 80 | 103.0 | 10.0 | 107.0 | 14.2 | 110.8 | 9.0 |
| 200 | 86.6 | 5.1 | 103.0 | 7.0 | 101.7 | 6.0 |
| 25OHD3 Intra day | ||||||
|---|---|---|---|---|---|---|
| Nominal Conc. [ng/mL] |
Transition | |||||
| 401/257 | 401/265 | 401/383 | ||||
| Accuracy [%] | %CV | Accuracy [%] | %CV | Accuracy [%] | %CV | |
| 2 | 113.7 | 3.7 | 99.5 | 18.4 | 104.1 | 12.0 |
| 80 | 86.3 | 3.5 | 83.9 | 5.5 | 72.8 | 1.3 |
| 200 | 95.3 | 2.3 | 89.0 | 3.3 | 87.0 | 2.6 |
| 25OHD3 Inter day | ||||||
|---|---|---|---|---|---|---|
| Nominal Conc. [ng/mL] |
Transition | |||||
| 401/257 | 401/265 | 401/383 | ||||
| Accuracy [%] | %CV | Accuracy [%] | %CV | Accuracy [%] | %CV | |
| 2 | 102.7 | 15.4 | 104.9 | 14.5 | 105.6 | 9.5 |
| 80 | 103.8 | 6.8 | 105.3 | 3.9 | 94.8 | 9.9 |
| 200 | 95.0 | 4.2 | 91.8 | 6.7 | 93.4 | 9.9 |
3.5 Determination of 25(OH)D2 and 25(OH)D3 in NIST samples
The APPI method was further validated using the SRM 972 prepared by NIST 21 (Table 5). SRM 972 was developed as standard for determining the accuracy of a new vitamin D analysis method. It includes four serum vials with various concentrations (levels 1–4) lowest to highest of 25(OH)D2 and 25(OH)D3. Concentrations of these two compounds were determined in the four standard samples using the most reliable transitions for 25(OH)D2 (m/z 413 to 337) and 25(OH)D3 (m/z 401 to 257). The Level 1 sample contains 25(OH)D3 at a certified value of 23.9 ng/mL. The concentration determined by APPI method was 26.6±0.5 ng/mL (n=3; 111.2% accuracy and 2.0% CV). Level 2 and 3 samples contain 1.71 and 26.4 ng/mL of 25(OH)D2, and 12.3 and 18.5 ng/mL of 25(OH)D3, respectively. Our method measured 1.74 ng/mL (102.0% accuracy; 23.7% CV) and 24.0 ng/mL (90.9% accuracy; 1.5% CV) of 25(OH)D2, and 12.3 ng/mL (100.2% accuracy; 2.7% CV) and 19.4 ng/mL (104.7% accuracy; 0.6% CV) of 25(OH)D3, respectively. The NIST sample Level 4 contains 25(OH)D2, 25(OH)D3 and its epimer 3-epi-25(OH)D3 at certified concentrations of 2.4, 33.0 and 37.7 ng/mL, respectively.
Table V.
Determination of 25(OH)D2 and 25(OH)D3 levels in NIST samples.
| NIST -Level 1 | Certified conc. [ng/mL] |
Measured conc. [ng/mL] |
Accuracy [%] |
RSD [%] |
|---|---|---|---|---|
| 25OH-D2 | ND | ND | ND | ND |
| 25OH-D3 | 23.9 | 26.6 | 111.2 | 2.0 |
| NIST -Level 2 | ||||
| 25OH-D2 | 1.7 | 1.7 | 102.0 | 23.7 |
| 25OH-D3 | 12.3 | 12.3 | 100.2 | 2.7 |
| NIST -Level 3 | ||||
| 25OH-D2 | 26.4 | 24.0 | 90.9 | 1.5 |
| 25OH-D3 | 18.5 | 19.4 | 104.7 | 0.6 |
| NIST -Level 4 | ||||
| 25OH-D2 | 2.4 | 2.4 | 98.5 | 5.4 |
| 25OH-D3 | 70.7a | 65.9a | 93.5 | 5.5 |
Value represents the sum of 25(OH)D3 and 3-epi-25(OH)D3
Interestingly while the measured 25(OH)D2 concentration in this sample matched the certified value well (2.4 ng/mL; 98.5% accuracy; 5.4% CV), the 25(OH)D3 level was found much higher at 65.9 ng/mL. However evaluation of the LC-MS/MS chromatograms revealed that our method is unable to separate 25(OH)D3 and 3-epi-25(OH)D3 and therefore the value we obtained represents their sum. In this case accuracy was determined at 93.5% with 5.5% CV.
4 Concluding remarks
A method using LC coupled with APPI-MS/MS has been developed to determine vitamin D forms and metabolites in serum. The implementation of APPI source resulted in more intense signals for all tested compounds and comparison to APCI sources revealed that improvement in ionization by APPI depends on the structures of the vitamin D molecules studied. The signal intensities of D2 and D3 increased four- to sevenfold compared with APCI while 25(OH)D3 and 25(OH)D2 signal intensities increased eight- to elevenfold. Evaluation of extraction efficiency showed that acetone extraction provided the highest extraction efficiency and reproducibility, followed by acetonitrile, methanol and hexane. LOQs were determined as 1 ng/mL for hydroxylated molecules, and 2 ng/mL for their parent molecules (based on S/N ratios ≥10). The best fit of individual standard curves was achieved using a first-order linear regression and showed good linearity within the tested ranges (R2≥0.995). Compared with other methods, the reported LOQs range from 1.7 to 6.5 ng/mL; however, to achieve these LOQs these methods concentrate the samples before injection. Therefore, the APPI method described here provides an increase in sensitivity limits by five- to tenfold compared with non-concentrated samples and is suitable for high-throughput rapid screening of major vitamin D forms in serum. The extendibility of this method to other biological tissues and foods remains to be established.
Acknowledgements
This project was supported by a Purdue University Discovery Park seed grant and Lallemand/American Yeast.
Footnotes
The authors have declared no conflict of interest.
5 References
- 1.Holick MF. Nutr. Rev. 2008;66:S182–S194. doi: 10.1111/j.1753-4887.2008.00104.x. [DOI] [PubMed] [Google Scholar]
- 2.Jenab M, Bueno-de-Mesquita HB, Ferrari P, van Duijnhoven FJ, Norat T, Pischon T, Jansen EH, Slimani N, Byrnes G, Rinaldi S, Tjonneland A, Olsen A, Overvad K, Boutron-Ruault MC, Clavel-Chapelon F, Morois S, Kaaks R, Linseisen J, Boeing H, Bergmann MM, Trichopoulou A, Misirli G, Trichopoulos D, Berrino F, Vineis P, Panico S, Palli D, Tumino R, Ros MM, van Gils CH, Peeters PH, Brustad M, Lund E, Tormo MJ, Ardanaz E, Rodriguez L, Sanchez MJ, Dorronsoro M, Gonzalez CA, Hallmans G, Palmqvist R, Roddam A, Key TJ, Khaw KT, Autier P, Hainaut P, Riboli E. BMJ. 2010:340. doi: 10.1136/bmj.b5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carter GD. Clin. Chem. 2009;55:1300–1302. doi: 10.1373/clinchem.2009.125906. [DOI] [PubMed] [Google Scholar]
- 4.Goltzman D. Ann. N. Y. Acad. Sci. 2010;1192:145–152. doi: 10.1111/j.1749-6632.2009.05226.x. [DOI] [PubMed] [Google Scholar]
- 5.Binkley N, Krueger D, Cowgill CS, Plum L, Lake E, Hansen KE, DeLuca HF, Drezner MK. J. Clin. Endocrinol. Metab. 2004;89:3152–3157. doi: 10.1210/jc.2003-031979. [DOI] [PubMed] [Google Scholar]
- 6.Hollis BW. J. Clin. Endocrinol. Metab. 2004;89:3149–3151. doi: 10.1210/jc.2004-0682. [DOI] [PubMed] [Google Scholar]
- 7.Lensmeyer GL, Wiebe DA, Binkley N, Drezner MK. Clin. Chem. 2006;52:1120–1126. doi: 10.1373/clinchem.2005.064956. [DOI] [PubMed] [Google Scholar]
- 8.Blake CJ. J. AOAC Int. 2007;90:897–910. [PubMed] [Google Scholar]
- 9.Byrdwell WC, Devries J, Exler J, Harnly JM, Holden JM, Holick MF, Hollis BW, Horst RL, Lada M, Lemar LE, Patterson KY, Philips KM, Tarrago-Trani MT, Wolf WR. Am. J. Clin. Nutr. 2008;88:554S–557S. doi: 10.1093/ajcn/88.2.554S. [DOI] [PubMed] [Google Scholar]
- 10.Yeung B, Vouros P, Siu-Caldera ML, Reddy GS. Biochem. Pharmacol. 1995;49:1099–1710. doi: 10.1016/0006-2952(95)98507-6. [DOI] [PubMed] [Google Scholar]
- 11.Huang M, LaLuzerne P, Winters D, Sullivan D. J. AOAC Int. 2009;92:1327–1335. [PubMed] [Google Scholar]
- 12.Chen H, McCoy LF, Schleicher RL, Pfeiffer CM. Clin. Chim. Acta. 2008;391:6–12. doi: 10.1016/j.cca.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 13.Maunsell Z, Wright DJ, Rainbow SJ. Clin. Chem. 2005;51:1683–1690. doi: 10.1373/clinchem.2005.052936. [DOI] [PubMed] [Google Scholar]
- 14.Saenger AK, Laha TJ, Bremner DE, Sadrzadeh SM. Am. J. Clin. Pathol. 2006;125:914–920. doi: 10.1309/J32U-F7GT-QPWN-25AP. [DOI] [PubMed] [Google Scholar]
- 15.Singh RJ, Taylor RL, Reddy GS, Grebe SK. J. Clin. Endocrinol. Metab. 2006;91:3055–3061. doi: 10.1210/jc.2006-0710. [DOI] [PubMed] [Google Scholar]
- 16.Taylor RL, Grebe SK, Singh RJ. Clin. Chem. 2005;51:A231–A232. [Google Scholar]
- 17.Tsugawa N, Suhara Y, Kamao M, Okano T. Anal. Chem. 2005;77:3001–3007. doi: 10.1021/ac048249c. [DOI] [PubMed] [Google Scholar]
- 18.Vogeser M, Kyriatsoulis A, Huber E, Kobold U. Clin. Chem. 2004;50:1415–1417. doi: 10.1373/clinchem.2004.031831. [DOI] [PubMed] [Google Scholar]
- 19.Duan X, Weinstock-Guttman B, Wang H, Bang E, Li J, Ramanathan M, Qu J. Anal. Chem. 2010;82:2488–2497. doi: 10.1021/ac902869y. [DOI] [PubMed] [Google Scholar]
- 20.Eyles D, Anderson C, Ko P, Jones A, Thomas A, Burne T, Mortensen PB, Norgaad-Pedersen B, Hougaard DM, McGrath J. Clin. Chim. Acta. 2009;403:145–151. doi: 10.1016/j.cca.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Phinney KW. Am. J. Clin. Nutr. 2008;88:511S–512S. doi: 10.1093/ajcn/88.2.511S. [DOI] [PubMed] [Google Scholar]
- 22.Dimartino G. J. AOAC Int. 2009;92:511–517. [PubMed] [Google Scholar]
- 23.Knox S, Harris J, Calton L, Wallace AM. Ann. Clin. Biochem. 2009;46:226–230. doi: 10.1258/acb.2009.008206. [DOI] [PubMed] [Google Scholar]
- 24.Cai SS, Syage JA. Anal. Chem. 2006;78:1191–1199. doi: 10.1021/ac0515834. [DOI] [PubMed] [Google Scholar]
- 25.Cai SS, Syage JA. J. Chromatogr. A. 2006;1110:15–26. doi: 10.1016/j.chroma.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 26.Nerin C, Salafranca J, Aznar M, Batlle R. Anal. Bioanal. Chem. 2009;393:809–833. doi: 10.1007/s00216-008-2437-6. [DOI] [PubMed] [Google Scholar]
- 27.Higashi T, Shibayama Y, Fuji M, Shimada K. Anal. Bioanal. Chem. 2008;391:229–238. doi: 10.1007/s00216-007-1780-3. [DOI] [PubMed] [Google Scholar]
- 28.Jiang L, He L, Fountoulakis M. J. Chromatogr. A. 2004;1023:317–320. doi: 10.1016/j.chroma.2003.10.029. [DOI] [PubMed] [Google Scholar]