Abstract
To study the role of the interfacial properties of ceramides in their interlipid interactions, we synthesized palmitoylceramide (PCer) analogs in which a methyl group was introduced to the amide-nitrogen or the C3-oxygen of the sphingosine backbone. A differential scanning calorimetry analysis of equimolar mixtures of palmitoylsphingomyelin (PSM) and PCer showed that these sphingolipids formed a complex gel phase that melted between 67°C and 74°C. The PCer analogs also formed gel phases with PSM, but they melted at lower temperatures compared with the system with PCer. In complex bilayers composed of an unsaturated glycerophospholipid, PSM, and cholesterol, the 3O-methylated ceramide formed a cholesterol-poor ordered phase with PSM. However, the 2N-methylated and doubly methylated (2N and 3O) PCer analogs failed to displace sterol from interactions with PSM. Like PCer, the analogs reduced sterol affinity for the complex bilayers, but this effect was most pronounced for the 3O-methylated ceramide. Taken together, our results show that 2N-methylation weakened the ceramide-PSM interactions, whereas the 3O-methylated ceramide behaved more like PCer in interactions with PSM. Our findings are compatible with the view that interlipid interactions between the amide-nitrogen and neighboring lipids are important for the cohesive properties of sphingolipids in membranes, and this also appears to be a valid model for ceramide.
Introduction
In the past few decades, ceramides have attracted substantial interest because of their observed biological importance as cellular effectors (1). In addition to playing a major role in the formation of the stratum corneum epidermal layer of animal skin, ceramides are also associated with important cellular signaling pathways such as stress response (2,3), apoptosis (4,5), and cell differentiation (6,7). Ceramides are peculiar membrane lipids because although they are found in cells in only low levels under normal conditions, in certain circumstances their concentration can change remarkably (8–11). The biophysical effects of ceramides on membrane structure and lateral organization have been proposed to contribute significantly to their biological effects (12). Model membrane studies have shown that when ceramides are mixed with phospholipids they increase the molecular order of the phospholipid and induce lateral phase separation and domain formation (13–15). Thus, ceramides have a tendency to self-aggregate and form small, ceramide-enriched gel phase domains that may also contain sphingomyelin (SM) (16–21). Ceramides also have the ability to displace cholesterol from interactions with SM (19,22–24). Treatment of SM-containing bilayers with sphingomyelinase was found to induce phase separation (15,25,26), and generation of ceramide at one side of a bilayer membrane was shown to give rise to transbilayer lipid redistribution (27). In cellular membranes, generation of ceramide has been shown to have different consequences depending on the cell type (18,28), and it has been proposed that ceramide-enriched platforms aid in transmitting and amplifying transmembrane signals in cells (29–31). Furthermore, curvature stress induced by ceramides promotes the formation of hexagonal phases and membrane trafficking mediated by vesicle formation and fusion (32). These findings underscore the importance of understanding the bilayer behavior of ceramides and their intermolecular interactions with other membrane lipids.
The characteristic bilayer behavior of ceramides is dependent on their structure, which also regulates their biophysical properties. The rigid amide group of ceramide has been deduced to have a planar resonance structure and a perpendicular orientation toward the axes of the two hydrocarbon chains (33). The inverted cone-shaped molecular structure, with a small polar function (hydroxyl), and the conformationally ordered acyl chains allow ceramide molecules to pack tightly. In addition to the attractive van der Waals forces between the extended, all-trans acyl chains, the extensive network of hydrogen (H)-bonds at the interfacial region also contributes to the characteristic high gel-to-liquid crystalline phase transition temperatures of ceramides. Furthermore, the C4-C5 double bond in the sphingosine long-chain base is an important structural factor that promotes close packing of the ceramide hydrocarbon chains (34,35).
In an attempt to study how disruption of the interfacial properties of the ceramide molecule (e.g., H-bonding, hydrophobicity and conformation) affects its membrane behavior and interlipid interactions, we synthesized ceramide analogs with structurally modified interfacial functional groups. A methyl group was introduced to the nitrogen (2N) of the amide linkage or the C3-oxygen (3O) in the long-chain base, either separately or simultaneously, yielding 2N(methyl)-palmitoyl-d-erythro-ceramide (NMeCer), 3O(methyl)-N-palmitoyl-ceramide (OMeCer), and 2N(methyl)-3O(methyl)-palmitoyl-d-erythro-ceramide (NMeOMeCer), respectively (Scheme 1). To avoid differences arising from the hydrocarbon chains, each of the analogs had a palmitoyl moiety linked to the sphingosine backbone. In addition to decreasing the hydrophilicity (H replaced with methyl) and increasing the size of the interfacial region (the steric bulk of methyl), the methyl groups should attenuate H-bonding with the amide nitrogen and the C3-oxygen. Therefore, the methyl groups might also affect the conformation of the rigid, planar amide linkage, and the properties of the C4-C5 double bond in the proximity of the C3-oxygen. The analogs were studied in binary mixtures with palmitoylsphingomyelin (PSM) and in multicomponent mixtures composed of palmitoyloleoylphosphocholine (POPC), PSM, and cholesterol. We show that methylation of 2NH dramatically interfered with intermolecular interactions, whereas methylation of 3OH did not markedly alter the ceramide-like behavior of the analog.
Scheme 1.
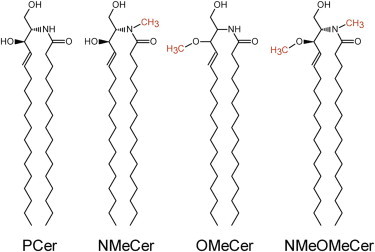
Molecular drawings of the ceramide analogs used in this study. OMeCer was a racemic mixture.
Materials and Methods
PSM was purified from egg yolk SM (Avanti Polar Lipids, Alabaster, AL) as previously described (36). NMeCer, OMeCer, and NMeOMeCer were synthesized as described in the Supporting Material. The synthetic procedures and sources of the commercial chemicals are described in the Supporting Material.
We used differential scanning calorimetry (DSC) to study the thermotropic properties of binary bilayers of PSM and interface methylated ceramides in multilamellar vesicles. We studied the formation of ordered or gel phase domains by the methylated ceramide analogs by measuring the fluorescence quenching of trans-parinaric acid (tPA) by 1-palmitoyl-2-stearoyl-(7-doxyl)-sn-glycero-3-phosphocholine (7SLPC) in multilamellar vesicles as a function of temperature (37). The samples for the tPA-quenching experiment (both quenched (F) and unquenched (F0)) were composed of POPC, PSM, cholesterol, and ceramide. Quenching was determined as the relative fluorescence intensity from the quenched F samples, which contained both 7SLPC (replacing 50% of POPC) and tPA (1 mol %), over the intensity from the unquenched F0 samples with 1 mol % of tPA. From the tPA-quenching curves, the onset temperature for domain melting was determined as the temperature at which the slope of the F/F0 ratio started to decline. The offset for domain melting was determined as the temperature at which the F/F0 ratio started to level off or reached the lowest point. The Tm (temperature at 50% melting of the ordered phase) was determined from the tPA-quenching data as the temperature at the midway between the onset and offset values of F/F0. To obtain additional information about the properties of the ordered or gel phases formed by the methylated PCer analogs in POPC, or in the presence of PSM and cholesterol, we measured the time-resolved fluorescence decays of tPA (0.5 mol %) in multilamellar vesicles at 23°C. We studied the formation of sterol-containing ordered domains, or the possible displacement of sterol from PSM-rich ordered domains, by measuring the 7SLPC-induced fluorescence quenching of cholestatrienol (CTL) in multilamellar vesicles composed of POPC, PSM, cholesterol, and ceramide (38). Quenching was determined as in the tPA-quenching experiment, with the F samples now containing both 7SLPC (replacing 50% of POPC) and CTL (1 mol % replacing 10% of cholesterol) and the F0 samples containing 1 mol % of CTL. We measured the partition of CTL (2 mol %) between unilamellar vesicles and methyl-β-cyclodextrin (mβCD) to determine the affinity of sterol for bilayers containing the interface methylated ceramides (37). The molar fraction partitioning coefficient (Kx) was obtained as described previously (39). All methods are described in more detail in the Supporting Material.
Results
Thermotropic properties of the binary bilayers of PSM and interface methylated ceramides
To study the thermotropic properties of the methylated PCer analogs in equimolar PSM bilayers, we performed heating (Fig. 1 A) and cooling (Fig. 1 B) DSC scans. A previous study of PSM/PCer mixtures showed that PCer is able to interact with PSM when in equimolar amounts (38). Consistent with that finding, the heating thermogram of a 1:1 mixture of PCer and PSM reported a melting transition with the highest temperature peak at 72.2°C (Fig. 1 A5). The heating thermograms of binary mixtures containing the methylated PCer analogs showed that the structural modifications at the interfacial region did not prevent the interaction of these ceramides with PSM (Fig. 1 A2–4), even though the mixtures displayed a lower degree of cooperativity compared with pure PSM (Fig. 1 A1) and a reduction in the highest Tm component compared with the PCer-containing mixture. Compared with PCer, the 3O-methylation (OMeCer) caused a downshift of the highest temperature component of the binary mixture to 61.2°C (Fig. 1 A4). Methylation at 2N resulted in an even lower transition temperature, because the PSM/NMeCer-mixture had the main transition at 57.6°C (Fig. 1 A3). As expected, NMeOMeCer with the two bulky methyl groups interacted more poorly with PSM (Fig. 1 A2). The thermal stabilization of the PSM gel phase by NMeOMeCer was modest compared with the singly methylated analogs, suggesting that the cumulative effect of the two methylations remarkably affected the packing of the lipid acyl chains and/or the formation of H-bonds between the two lipids. However, melting of the PSM/NMeOMeCer mixture seemed surprisingly cooperative compared with the mixtures with the other analogs or PCer. The transitions of all of the mixtures displayed a higher degree of cooperativity in the cooling scans (Fig. 1 B) compared with the heating thermograms. Because the second heating and cooling scans of four consecutive scans are shown, this suggests that the mixtures behaved differently depending on whether they were undergoing a melting or a recrystallization. However, the highest Tm components for the binary mixtures in the cooling scans (Fig. 1 B) were almost identical to those observed in the heating scans.
Figure 1.
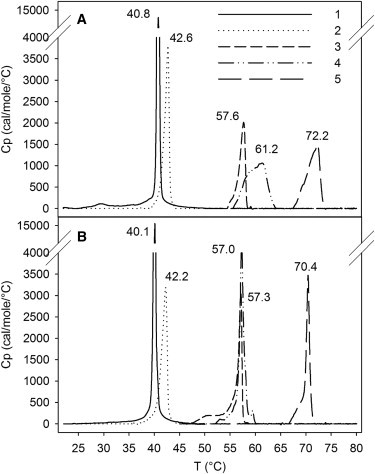
Representative thermograms of pure PSM and PSM/XCer (1:1, by mol) binary mixtures. DSC heating (A) and cooling (B) scans were run at a rate of 1°C/min. Sample identification: 1), PSM; 2), PSM/NMeOMeCer; 3), PSM/NMeCer; 4), PSM/OMeCer; and 5), PSM/PCer. For clarity, only the temperature interval with the peak is shown for each sample, omitting long stretches of straight baseline. The highest Tm peak is given for each mixture. The second heating and cooling scans are shown.
Formation of ordered phases by the interface methylated ceramides in complex bilayers
Because the DSC results showed that the interface methylated ceramides were able to interact with PSM at equimolar ratios in binary mixtures, we sought to determine whether these interlipid interactions would remain in mixed bilayers that also contained POPC and cholesterol. To that end, we performed 7SLPC-induced fluorescence quenching of tPA. tPA is known to partition preferentially into gel and ordered phases (40,41). In a bilayer composed of POPC, PSM, and cholesterol (60/30/10 by mol), tPA reported a clear domain melting with an end melting temperature of ∼43°C (Fig. 2 A). Reducing the ratio of PSM to POPC (e.g., in POPC/PSM/CHL 75:15:10 by mol) also markedly reduced the thermostability of the PSM-rich domain (Fig. 2 B). When half of the PSM was replaced by PCer, the end melting temperature of the domains increased to ∼50°C (Fig. 2 C), clearly showing thermal stabilization of the PSM-rich domains by PCer. The 2N-methylation seemed to prevent such thermal stabilization, as the end melting temperature of the PSM-rich domains was only ∼39°C in the presence of NMeCer (Fig. 2 D). In the presence of OMeCer, the PSM-rich domains showed an end melting at ∼44°C (Fig. 2 E), intermediate between those reported for the PCer- and NMeCer-containing bilayers. For NMeOMeCer-containing bilayers, tPA reported domains with an end melting at ∼32°C (Fig. 2 F), which was at a slightly higher temperature compared with a bilayer system containing only 15 mol % PSM.
Figure 2.
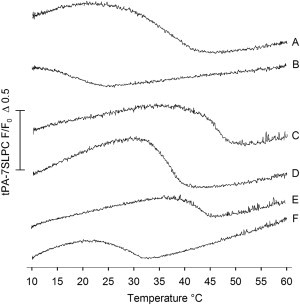
Detection of PSM/XCer ordered domains in laterally heterogeneous bilayers. The ability of the interface methylated ceramides to form ordered or gel phase domains in multicomponent bilayers was deduced from the 7SLPC-induced fluorescence quenching of tPA as a function of temperature. Representative curves, defined as the relative fluorescence intensity from bilayers with both 7SLPC and tPA (F) over the intensity from bilayers with only tPA (F0), are shown. Sample identification: (A) POPC/PSM/CHL 60/30/10 (by mol), (B) POPC/PSM/CHL 75/15/10, (C) POPC/PSM/PCer/CHL 60/15/15/10 (applies for all ceramide-containing samples), (D) POPC/PSM/NMeCer/CHL, (E) POPC/PSM/OMeCer/CHL, and (F) POPC/PSM/NMeOMeCer/CHL.
A more thorough analysis of the tPA-reported melting behavior of the multicomponent bilayers shown in Fig. 2 revealed that in addition to the end melting temperature, the slope of the quenching curves and the length of the temperature range within which the melting occurred also varied for the different compositions. By analyzing the Tm range, we were able to determine the Tm for the ordered phase in the different bilayers. Due to variations in the kinetics of domain melting, the Tm does not necessarily correspond to the end melting temperature. The results from such an analysis are shown in Fig. 3, for which data were collected from the tPA-quenching results. The onset and offset temperatures for domain melting were deduced as the temperature at the highest (onset) and lowest (offset) amplitudes of F/F0 (i.e., where the F/F0 ratio begins to decrease and where it levels off). The analysis showed that the Tm for the different quaternary mixtures mostly followed the same pattern as the end melting temperature values. However, the Tm was ∼6°C higher in the OMeCer-containing bilayers than in the bilayers that contained 30 mol % of PSM (Fig. 3), even though the end melting temperature values for these bilayers were almost equal (Fig. 2, A and E, and Fig. 3). Thus, this analysis revealed that OMeCer was the only analog that was able to induce an increase in the Tm of the PSM-rich domains, suggesting that OMeCer actually behaved like PCer and induced thermal stabilization of the ordered phase.
Figure 3.
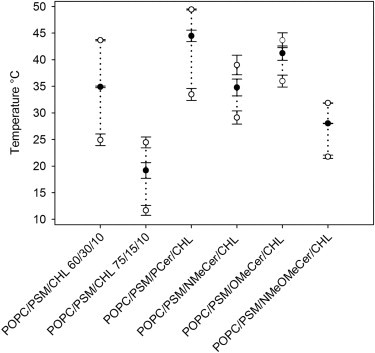
Analysis of the melting of the ordered or gel phase domains detected by tPA in Fig. 2. The onset and offset temperatures ± SD (open circles) for domain melting were determined as the temperatures at the highest (onset) and lowest (offset) amplitudes of F/F0 within the approximate range of domain melting. Tm ± SD (solid circles) for each sample was determined as the corresponding temperature at the midway between onset and offset F/F0-values. The composition of all ceramide-containing samples was 60/15/15/10 (by mol). See Supporting Material for a detailed description of the analysis.
Effects of the interface methylated ceramides on tPA lifetime
To obtain additional information about the properties of the ordered or gel phases formed by the methylated PCer analogs alone in a fluid bilayer, or in the presence of PSM and cholesterol, we measured the lifetime components of tPA in such bilayer systems. tPA lifetimes are sensitively affected by changes in the microenvironment of tPA, being longer in ordered phases and shorter in phases of lower packing density (41–44). Because tPA preferentially partitions into ordered and gel phases, it is likely to give useful information specifically about their properties. The lifetime of tPA in POPC is short because of efficient quenching in the disordered bilayer. We observed two lifetime components for tPA in POPC at 23°C: a dominant 5.8 ns component and a small 1.5 ns component (Table 1). Addition of PCer to POPC at 15 mol % still gave two lifetime components for tPA, but the longer one was now at 47 ns with 81% fractional intensity, and the shorter one was at 5.7 ns (Table 1). OMeCer gave lifetimes for tPA similar to those obtained with PCer, although the longer component was at 56 ns with only 50% fractional intensity. NMeCer and NMeOMeCer caused the longest lifetime components of tPA to become significantly shorter (39 and 8 ns, respectively), and the 91% fractional intensity of the 8 ns component for the doubly methylated analog suggests that no ordered phases were formed by this analog. These data suggest that the methylations affected the properties of the ordered phases. The 2N-methylation reduced the degree of order, whereas in the bilayers with the 3O-methylated analog, only the contribution of the longest lifetime component to the total fluorescence intensity of tPA was reduced, and the lengths of the lifetimes were similar to those measured in the presence of PCer.
Table 1.
Time-resolved tPA-fluorescence decays in varying bilayer compositions at 23°C ± SD
| τ1 | f1 | α1 | τ2 | f2 | α2 | τ3 | f3 | α3 | |
|---|---|---|---|---|---|---|---|---|---|
| POPC | 5.8 ± 0.1 | 88.5 ± 1.8 | 66.5 ± 1.4 | 1.5 ± 0.4 | 11.5 ± 1.8 | 33.5 ± 1.4 | |||
| POPC/PCer | 47.2 ± 1.1 | 81.4 ± 3.2 | 34.8 ± 3.8 | 5.7 ± 0.2 | 18.6 ± 3.2 | 65.2 ± 3.8 | |||
| POPC/NMeCer | 38.6 ± 1.8 | 43.4 ± 4.9 | 11.8 ± 1.8 | 6.7 ± 0.5 | 56.6 ± 4.9 | 88.2 ± 1.8 | |||
| POPC/OMeCer | 55.7 ± 0.8 | 50.1 ± 0.8 | 9.3 ± 0.4 | 5.7 ± 0.1 | 49.9 ± 0.8 | 90.6 ± 0.4 | |||
| POPC/NMeOMeCer | 7.9 ± 0.3 | 90.8 ± 4.9 | 72.8 ± 4.2 | 2.1 ± 0.9 | 9.2 ± 4.9 | 27.2 ± 4.2 | |||
| POPC/PSM/CHL 60/30/10 | 40.6 ± 0.3 | 43.7 ± 0.9 | 19.8 ± 0.3 | 18.2 ± 0.4 | 48.2 ± 0.2 | 48.7 ± 0.2 | 4.7 ± 0.4 | 8.1 ± 0.6 | 31.5 ± 0.5 |
| POPC/PSM/CHL 75/15/10 | 22.8 ± 0.5 | 36.0 ± 1.9 | 17.5 ± 1.0 | 10.8 ± 0.3 | 58.2 ± 1.3 | 59.8 ± 0.7 | 2.8 ± 0.3 | 5.7 ± 0.6 | 22.7 ± 0.3 |
| POPC/PSM/PCer/CHL | 54.8 ± 2.3 | 68.1 ± 4.4 | 34.6 ± 5.1 | 16.7 ± 2.6 | 29.1 ± 2.3 | 49.1 ± 6.6 | 4.4 ± 0.8 | 4.1 ± 1.2 | 24.5 ± 2.6 |
| POPC/PSM/NMeCer/CHL | 47.1 ± 2.3 | 56.2 ± 6.6 | 26.6 ± 3.5 | 17.7 ± 2.0 | 40.5 ± 5.2 | 50.8 ± 3.4 | 3.2 ± 1.5 | 3.3 ± 1.6 | 22.6 ± 3.1 |
| POPC/PSM/OMeCer/CHL | 52.9 ± 2.7 | 38.3 ± 3.5 | 11.0 ± 1.2 | 13.3 ± 1.6 | 56.9 ± 2.2 | 55.8 ± 1.2 | 3.1 ± 1.4 | 7.2 ± 2.6 | 34.0 ± 0.5 |
| POPC/PSM/NMeOMeCer/CHL | 35.4 ± 1.8 | 30.3 ± 6.6 | 14.2 ± 3.5 | 16.6 ± 1.2 | 63.8 ± 3.4 | 63.0 ± 1.3 | 4.1 ± 1.6 | 6.0 ± 3.2 | 22.9 ± 3.9 |
All binary mixtures were 1:1 by mol, and the quaternary mixtures containing ceramides were 60/15/15/10 by mol. τ, lifetimes (ns); ƒ, fractional intensities (%); α, fractional amplitudes (%).
In more-complex bilayers that contained POPC, PSM, ceramide, and cholesterol, at least three lifetime components were found for tPA (Table 1). The longest lifetime component of tPA was again almost equal in the PCer- and OMeCer-containing bilayers (55 and 53 ns, respectively), and shorter in the presence of NMeCer and the doubly methylated analogs (47 and 35 ns, respectively). However, in the presence of OMeCer, the fractional intensity of the longest lifetime component decreased again, whereas at the same time the fractional intensity from the second-longest component increased compared with PCer. These results are consistent with the tPA-quenching data presented in Figs. 2 and 3, which show that OMeCer and PCer gave almost comparably stable ordered domains. 2N-methylation clearly affected the ability of PCer to form ordered domains, as well as the properties of the domains that were formed, more dramatically.
Effect of methylated ceramides on PSM-sterol interaction in complex bilayers
Ceramides and cholesterol are known to have somewhat complex, concentration-dependent effects on each other's lateral distribution in mixed bilayer membranes. Nevertheless, within a certain concentration range, PCer is known to displace cholesterol from interactions with PSM (19,22–24). Therefore, we studied the effect of the methylated ceramide analogs on PSM-sterol interaction in a composition in which a clear displacement of sterol by PCer was observed. This was achieved by means of 7SLPC-induced fluorescence quenching of CTL (Fig. 4). These experiments also served as a cross-check to the tPA-quenching experiment to confirm the possible formation of ceramide-enriched ordered phases. In samples that contain gel or ordered phases other than ceramide-enriched (e.g., the bilayers containing 30 or 15 mol % of PSM), CTL and tPA in general report similar end melting temperature values (Figs. 2, A and B, and 4, A and B). As shown in Fig. 4 C, addition of PCer to a bilayer containing POPC, PSM, and cholesterol/CTL abolished the protection against quenching that CTL experienced in the absence of PCer. This strongly suggests that PCer was able to displace cholesterol and CTL from interactions with PSM (where CTL was protected against quenching), and forced cholesterol and CTL to partition more extensively into the POPC-rich phase where quenching of CTL was more efficient (due to CTL colocalization with 7SLPC). Replacing PCer with OMeCer also changed the quenching susceptibility of CTL (Fig. 4 E), suggesting that OMeCer was able to displace the sterol from interacting with PSM. However, the bilayer presence of NMeCer or NMeOMeCer did not markedly change the CTL quenching susceptibility (Fig. 4 D and F), suggesting that the 2N-methylation rendered PCer unable to displace sterol from interacting with PSM. However, the stability of the CTL-rich domains (and tPA-reported domains in Fig. 2) was slightly different when NMeCer or NMeOMeCer were present in the bilayer system, suggesting that although these ceramides did not displace CTL (or form thermostable ordered phases), they still appeared to affect the stability of the ordered domains indirectly.
Figure 4.
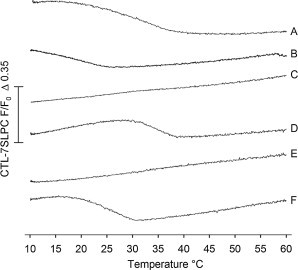
Detection of sterol-enriched domains in mixed bilayers containing interface methylated ceramides. The possible displacement of sterol from PSM-rich domains by the ceramide analogs was determined from the 7SLPC-induced fluorescence quenching of CTL as a function of temperature. Quenching was defined as in Fig. 2. Sample identification: (A) POPC/PSM/CHL 60/30/10 (by mol), (B) POPC/PSM/CHL 75/15/10, (C) POPC/PSM/PCer/CHL 60/15/15/10 (applies for all ceramide-containing samples), (D) POPC/PSM/NMeCer/CHL, (E) POPC/PSM/OMeCer/CHL, and (F) POPC/PSM/NMeOMeCer/CHL.
Effect of methylated ceramides on the bilayer affinity of sterol
The bilayer affinity of cholesterol can provide valuable information about the sterol-lipid interactions that take place in a bilayer (39,45). To calculate the molar fraction partition coefficient of cholesterol, we measured the equilibrium partitioning of CTL between bilayers and cyclodextrin as described previously (46). This method responds well to changes in bilayer composition, especially in the presence of ceramides (37,39). Furthermore, this assay could reveal whether the ceramide analogs had effects on cholesterol distribution independently of their own lateral localization in the bilayers. In agreement with previous observations, sterol affinity was low for pure POPC vesicles at both 23°C and 37°C, but increased dramatically when PSM/cholesterol domains were present (Fig. 5). Replacement of half of the PSM by PCer and the subsequent formation of PSM/PCer ordered domains caused a drop in the sterol partition coefficient to levels close to those of pure POPC, clearly demonstrating the displacement of sterol from the ordered phase to the fluid bulk for which it had lower affinity. All of the interface methylated ceramides were less efficient than PCer in lowering the bilayer affinity of CTL, suggesting that none of them displaced sterol from interactions with the bilayers as efficiently as PCer did. However, at 23°C, OMeCer, which appeared to displace sterol efficiently based on the CTL quenching data (Fig. 4), gave the lowest Kx for cholesterol partitioning of the methylated ceramide analogs (Fig. 5). At 37°C the difference in Kx for the different ceramide analogs became much smaller, and both NMeCer and the doubly methylated analog displayed surprisingly low Kx-values even though, according to the CTL-quenching data (Fig. 4), they were not able to displace sterol to the fluid phase. In those bilayers, the low Kx was probably caused by the fluid state of the bilayers rather than by a specific effect of the ceramides on sterol partitioning, just as in the bilayers that contained 15 mol % PSM. At 37°C the sterol partitioning was also lower throughout than at 23°C, probably due to a larger fraction of the bilayers being in a fluid state, when the overall order of the bilayers was affected by the increased temperature. Nevertheless, the differences between the samples followed more or less the same pattern at 37°C as at 23°C (Fig. 5).
Figure 5.
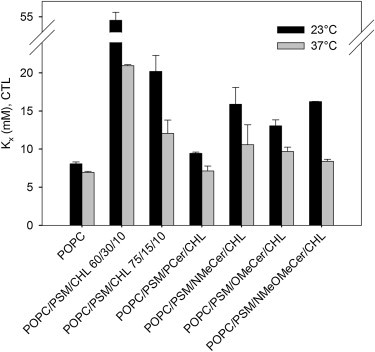
Equilibrium partitioning of CTL between mβCD and unilamellar vesicles containing interface methylated ceramides. The bilayer affinity of sterol was defined as the partition coefficient ± SD at 23°C (black bars) and 37°C (gray bars) as previously described (46). The composition of all ceramide-containing samples was 60/15/15/10 (by mol).
Discussion
In this study, we explored the specific interactions between SM and ceramide by means of targeted structural modification of the ceramide interface region. Thus, the C2-NH or C3-OH, or both, of the sphingosine backbone of PCer were methylated so as to abolish the interfacial H-bonding mediated by 2N and 3O. Of course, the methylations could also have steric and/or hydrophobic effects on interlipid interactions. Nevertheless, the results we obtained with methylated ceramide analogs show that disruption of the interfacial properties of ceramide at 2N or 3O differently affected their interlipid interactions.
PCer has been shown to interact with and induce a thermal stabilization of a PSM gel phase (38). Interestingly, disrupting the PCer interfacial properties at 2N and 3O did not prevent the interaction of PCer with PSM (so as to lead to phase separation), even though the ability to thermally stabilize the PSM gel phase was reduced for the analogs (Fig. 1, A and B). Extensive networks of H-bonding and tight packing of the acyl chains contribute to the ability of ceramides to form thermally stable phases of high molecular order. Accordingly, variations in the length and degree of acyl chain saturation and (as shown in this study) H-bonding ability affect membrane properties such as compressibility, interlipid interactions, and phase behavior. In general, ceramide-rich phases are stabilized by an increasing acyl chain length (47,48) and low degree of unsaturation (except for the C4-C5 trans-double bond of sphingosine) (34,49). In addition to these properties, our results suggest an important role for the amide linkage nitrogen in the tight interlipid interactions that enable the formation of ceramide-enriched phases in multicomponent bilayers (Figs. 2 and 3, Table 1). However, the modification of the C3-oxygen seemed to cause less change in the interfacial behavior of ceramide.
It has been shown that high concentrations of cholesterol can solubilize ceramide-enriched domains (42) and prevent the lateral cosegregation of ceramide and SM (20,50), whereas in the presence of stable SM/ceramide domains, cholesterol is displaced from them (19,23,24). Such displacement has also been shown to occur in cellular plasma membranes as a consequence of SM breakdown to ceramide by enzymatic activity (22,51). Thus, we were also interested in looking at how alterations in the interfacial properties of PCer would affect its ability to form sterol-displacing phases. Again, the amide nitrogen appeared to be fundamental for the sterol-displacing property of ceramide, because structural modification of the nitrogen totally abolished this effect (Fig. 4). This competition between ceramide and cholesterol over interactions with SM is believed to stem from the hydrophobic effect, which attempts to minimize the unfavorable contacts of the small headgroup ceramide and cholesterol with water by accommodation under the large headgroup of SM. Ceramide is usually preferred over cholesterol in such competition because the saturated aliphatic chains of ceramide interact better and more stably with the SM chains than the bulky ring structure of cholesterol. Apparently, a methylation at the 2N affected ceramide's interactions with SM so as to switch the situation in favor of cholesterol. Furthermore, the sterol-displacing ability of ceramide seemed to relate to the degree of thermal stabilization of the SM gel phase by the ceramides, because only ceramides that clearly were able to form a thermally stable SM/ceramide phase were able to displace sterol (i.e., PCer and OMeCer). The DSC data for gel phase stabilization were also corroborated independently by the tPA quenching data (Fig. 2) and the tPA lifetime data (Table 1). In sphingosine-based ceramides, the C4-C5 double bond is also found to have a remarkable role in allowing tight acyl chain packing (49). Therefore, because the gel phase and sterol-displacing properties of PCer were not affected in the OMeCer, the 3O-methylation that resided close to the double bond was unlikely to disturb the local conformation around the double bond.
Megha and co-workers (47) conducted studies similar to those presented here using ceramide analogs in which structural modifications, such as addition of a methyl to the C3-carbon in the sphingosine backbone (preserving the C3-OH), were introduced in or near the headgroup. They demonstrated that the stability of the ordered domains formed by these ceramide analogs, as well as their ability to displace sterol from PSM/cholesterol domains, was largely unchanged compared with unmodified ceramides. However, domain stability was affected by the length of the fatty acyl chain of the ceramide. They concluded that the ability of ceramide molecules to pack tightly is more sensitive to changes in the hydrophobic segments than in the polar interface region. However, the analogs they studied did not involve modification of the amide group, which in our study appeared to be important for the characteristic properties of ceramide. In a comparison of the bilayer properties of 2N- and 3O-methylated PSM analogs, Björkbom et al. (52) demonstrated that the 2N-methyl was buried deeper in the bilayer than the unperturbed 2NH or the 3O-methyl. This could also be one of the factors that contribute to the attenuated bilayer properties of our 2N-methylated ceramide analog. The fact that nitrogen is a part of the rigid planar amide link could also explain why it is more sensitive to structural alterations than the 3-hydroxyl, which sticks out from the molecule and has more conformational flexibility. Thus, in that sense, our results and certainly previous data on methyl-branched ceramides (34,37) seem to agree with the assumption of Megha and co-workers that structural alterations closer to the middle hydrophobic region of the ceramide molecule have greater effects on its membrane properties.
The distribution of H-bonds within ceramides or between ceramide and other lipids, such as SMs, is still largely unknown; however, many models for the H-bond patterns have been presented. The ability of the ceramide hydroxyls, carbonyl oxygen, and amide to serve as both H-bond donors and acceptors distinguishes ceramide from glycerolipids. In the 1970s, Pascher (33) concluded from crystal structures, infrared spectra, and monolayer studies that these chemical moieties participate in lateral H-bonds, thereby increasing the stability of ceramide-containing membranes. The ceramide amide group was found to have a rigid planar resonance structure and a perpendicular orientation toward the axes of the two hydrocarbon chains. The lone pair of electrons on the amide nitrogen was deduced to be delocalized by resonance into the carbonyl of the fatty acid, forming a partial double bond between the nitrogen and the carbonyl carbon. Thus, the nitrogen was concluded to only be able to act as a H-bond donor. In the same study, intermolecular interactions were determined to cause the observed self-condensation of natural ceramides. Later, on the basis of DSC and x-ray diffraction results, Shah et al. (53) suggested that the hydroxyl groups in the sphingosine base will H-bond to the amides of adjacent ceramide molecules. Our results suggest that the amide nitrogen plays an important role in intermolecular interactions and perhaps also H-bonding of ceramides, because a methylation of the nitrogen markedly attenuated the characteristic membrane behavior of the ceramide. Methylation of the 3OH of sphingosine again appeared not to affect the interlipid interactions of ceramide. Altogether, our results suggest a model compatible with that presented by Pascher (33) and Shah et al. (53), in which ceramides were found to form intermolecular H-bonds. However, other models for the H-bonding of ceramide have also been proposed. Based on NMR studies, Li and co-workers (54) introduced a model of an extensive intramolecular network of H-bonds stabilized by two water molecules and the C4-C5 double bond. Together with attractive van der Waals forces, this internally satisfied H-bond network was believed to lead to minimal steric repulsion, allowing tight packing of the ceramide acyl chains. This model, which involves both a network of intramolecular H-bonds and a critical water molecule tethered by the double bond of the sphingosine base, was supported by a computational calculation of potential and probable H-bonds within or between ceramides (55). A partial role of the C4-C5 double bond as an important component of the intramolecular H-bonding network and enhancer of close packing of the ceramide acyl chains was also suggested by Brockman and co-workers (49) based on monolayer studies of a series of synthetic ceramide analogs. More recently, yet another model was deduced from molecular-dynamics simulation of ceramide bilayers, where the 3OH of sphingosine was observed to form an intermolecular H-bond with the carbonyl oxygen of an adjacent ceramide molecule (56). Nonetheless, according to our results, the 2NH seems to play a more crucial role than the 3OH in the interlipid interactions of ceramides. However, our findings cannot solely be interpreted as results of altered H-bonding capability, because steric restrictions or hydrophobic effects mediated by the additional methyl groups were not ruled out.
Conclusions
This study clearly demonstrates that altering the interfacial properties of a long-chain, saturated ceramide by targeted introduction of bulky methyl groups to key positions affected the lateral membrane behavior of ceramide. Methylation of the C3-OH in the sphingosine backbone did not appear to affect the phase behavior and interlipid interactions of PCer, whereas methylation of the amide nitrogen markedly attenuated the interlipid interactions of ceramide. This was observed as a reduced ability of the 2N-methylated ceramide to form highly ordered phases. Not surprisingly, simultaneous methylation of C3-OH and C2-NH appeared to cause a total loss of the characteristic properties of ceramide. Our results suggest that the amide nitrogen of ceramide is an important factor in determining ceramide bilayer behavior, and, as has been suggested for SMs, they support the model of engagement of the amide nitrogen in intermolecular interactions even for ceramides.
Acknowledgments
The authors thank Prof. Robert Bittman for some valuable suggestions.
This work was supported by Medicinska Understödsföreningen Liv och Hälsa, National Doctoral Program in Informational and Structural Biology, Åbo Akademi University, and the Oskar Öflund Foundation (T.M.); Sigrid Juselius Foundation (J.P.S.); and Ministry of Education, Culture, Sports, Science and Technology and Matching Fund Subsidy for a Private University, Japan (S.K.).
Supporting Material
References
- 1.Perry D.K., Hannun Y.A. The role of ceramide in cell signaling. Biochim. Biophys. Acta. 1998;1436:233–243. doi: 10.1016/s0005-2760(98)00145-3. [DOI] [PubMed] [Google Scholar]
- 2.Hannun Y.A., Luberto C. Ceramide in the eukaryotic stress response. Trends Cell Biol. 2000;10:73–80. doi: 10.1016/s0962-8924(99)01694-3. [DOI] [PubMed] [Google Scholar]
- 3.Hannun Y.A., Obeid L.M. The ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J. Biol. Chem. 2002;277:25847–25850. doi: 10.1074/jbc.R200008200. [DOI] [PubMed] [Google Scholar]
- 4.Taha T.A., Mullen T.D., Obeid L.M. A house divided: ceramide, sphingosine, and sphingosine-1-phosphate in programmed cell death. Biochim. Biophys. Acta. 2006;1758:2027–2036. doi: 10.1016/j.bbamem.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heller R.A., Krönke M. Tumor necrosis factor receptor-mediated signaling pathways. J. Cell Biol. 1994;126:5–9. doi: 10.1083/jcb.126.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim M.Y., Linardic C., Hannun Y. Identification of sphingomyelin turnover as an effector mechanism for the action of tumor necrosis factor α and γ-interferon. Specific role in cell differentiation. J. Biol. Chem. 1991;266:484–489. [PubMed] [Google Scholar]
- 7.Okazaki T., Bielawska A., Hannun Y.A. Role of ceramide as a lipid mediator of 1 α,25-dihydroxyvitamin D3-induced HL-60 cell differentiation. J. Biol. Chem. 1990;265:15823–15831. [PubMed] [Google Scholar]
- 8.Van Veldhoven P.P., Matthews T.J., Bell R.M. Changes in bioactive lipids, alkylacylglycerol and ceramide, occur in HIV-infected cells. Biochem. Biophys. Res. Commun. 1992;187:209–216. doi: 10.1016/s0006-291x(05)81480-9. [DOI] [PubMed] [Google Scholar]
- 9.Venable M.E., Lee J.Y., Obeid L.M. Role of ceramide in cellular senescence. J. Biol. Chem. 1995;270:30701–30708. doi: 10.1074/jbc.270.51.30701. [DOI] [PubMed] [Google Scholar]
- 10.Miller C.J., Stein G.H. Human diploid fibroblasts that undergo a senescent-like differentiation have elevated ceramide and diacylglycerol. J. Gerontol. A Biol. Sci. Med. Sci. 2001;56:B8–B19. doi: 10.1093/gerona/56.1.b8. [DOI] [PubMed] [Google Scholar]
- 11.Haimovitz-Friedman A., Kan C.C., Kolesnick R.N. Ionizing radiation acts on cellular membranes to generate ceramide and initiate apoptosis. J. Exp. Med. 1994;180:525–535. doi: 10.1084/jem.180.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cremesti A.E., Goni F.M., Kolesnick R. Role of sphingomyelinase and ceramide in modulating rafts: do biophysical properties determine biologic outcome? FEBS Lett. 2002;531:47–53. doi: 10.1016/s0014-5793(02)03489-0. [DOI] [PubMed] [Google Scholar]
- 13.Hsueh Y.W., Giles R., Thewalt J. The effect of ceramide on phosphatidylcholine membranes: a deuterium NMR study. Biophys. J. 2002;82:3089–3095. doi: 10.1016/S0006-3495(02)75650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holopainen J.M., Lehtonen J.Y., Kinnunen P.K. Lipid microdomains in dimyristoylphosphatidylcholine-ceramide liposomes. Chem. Phys. Lipids. 1997;88:1–13. doi: 10.1016/s0009-3084(97)00040-6. [DOI] [PubMed] [Google Scholar]
- 15.Holopainen J.M., Subramanian M., Kinnunen P.K. Sphingomyelinase induces lipid microdomain formation in a fluid phosphatidylcholine/sphingomyelin membrane. Biochemistry. 1998;37:17562–17570. doi: 10.1021/bi980915e. [DOI] [PubMed] [Google Scholar]
- 16.Grassmé H., Jendrossek V., Gulbins E. Ceramide-rich membrane rafts mediate CD40 clustering. J. Immunol. 2002;168:298–307. doi: 10.4049/jimmunol.168.1.298. [DOI] [PubMed] [Google Scholar]
- 17.Grassmé H., Cremesti A., Gulbins E. Ceramide-mediated clustering is required for CD95-DISC formation. Oncogene. 2003;22:5457–5470. doi: 10.1038/sj.onc.1206540. [DOI] [PubMed] [Google Scholar]
- 18.Kolesnick R.N., Goñi F.M., Alonso A. Compartmentalization of ceramide signaling: physical foundations and biological effects. J. Cell. Physiol. 2000;184:285–300. doi: 10.1002/1097-4652(200009)184:3<285::AID-JCP2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 19.Alanko S.M., Halling K.K., Ramstedt B. Displacement of sterols from sterol/sphingomyelin domains in fluid bilayer membranes by competing molecules. Biochim. Biophys. Acta. 2005;1715:111–121. doi: 10.1016/j.bbamem.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Massey J.B. Interaction of ceramides with phosphatidylcholine, sphingomyelin and sphingomyelin/cholesterol bilayers. Biochim. Biophys. Acta. 2001;1510:167–184. doi: 10.1016/s0005-2736(00)00344-8. [DOI] [PubMed] [Google Scholar]
- 21.Chiantia S., Kahya N., Schwille P. Effects of ceramide on liquid-ordered domains investigated by simultaneous AFM and FCS. Biophys. J. 2006;90:4500–4508. doi: 10.1529/biophysj.106.081026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu C., Alterman M., Dobrowsky R.T. Ceramide displaces cholesterol from lipid rafts and decreases the association of the cholesterol binding protein caveolin-1. J. Lipid Res. 2005;46:1678–1691. doi: 10.1194/jlr.M500060-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Megha, London E. Ceramide selectively displaces cholesterol from ordered lipid domains (rafts): implications for lipid raft structure and function. J. Biol. Chem. 2004;279:9997–10004. doi: 10.1074/jbc.M309992200. [DOI] [PubMed] [Google Scholar]
- 24.Sot J., Ibarguren M., Alonso A. Cholesterol displacement by ceramide in sphingomyelin-containing liquid-ordered domains, and generation of gel regions in giant lipidic vesicles. FEBS Lett. 2008;582:3230–3236. doi: 10.1016/j.febslet.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 25.Fanani M.L., Härtel S., Maggio B. Bidirectional control of sphingomyelinase activity and surface topography in lipid monolayers. Biophys. J. 2002;83:3416–3424. doi: 10.1016/S0006-3495(02)75341-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Härtel S., Fanani M.L., Maggio B. Shape transitions and lattice structuring of ceramide-enriched domains generated by sphingomyelinase in lipid monolayers. Biophys. J. 2005;88:287–304. doi: 10.1529/biophysj.104.048959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Contreras F.X., Basañez G., Goñi F.M. Asymmetric addition of ceramides but not dihydroceramides promotes transbilayer (flip-flop) lipid motion in membranes. Biophys. J. 2005;88:348–359. doi: 10.1529/biophysj.104.050690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kolesnick R.N. Sphingomyelin and derivatives as cellular signals. Prog. Lipid Res. 1991;30:1–38. doi: 10.1016/0163-7827(91)90005-p. [DOI] [PubMed] [Google Scholar]
- 29.Stancevic B., Kolesnick R. Ceramide-rich platforms in transmembrane signaling. FEBS Lett. 2010;584:1728–1740. doi: 10.1016/j.febslet.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dobrowsky R.T. Sphingolipid signalling domains floating on rafts or buried in caves? Cell. Signal. 2000;12:81–90. doi: 10.1016/s0898-6568(99)00072-8. [DOI] [PubMed] [Google Scholar]
- 31.Bollinger C.R., Teichgräber V., Gulbins E. Ceramide-enriched membrane domains. Biochim. Biophys. Acta. 2005;1746:284–294. doi: 10.1016/j.bbamcr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 32.van Blitterswijk W.J., van der Luit A.H., Borst J. Ceramide: second messenger or modulator of membrane structure and dynamics? Biochem. J. 2003;369:199–211. doi: 10.1042/BJ20021528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pascher I. Molecular arrangements in sphingolipids. Conformation and hydrogen bonding of ceramide and their implication on membrane stability and permeability. Biochim. Biophys. Acta. 1976;455:433–451. doi: 10.1016/0005-2736(76)90316-3. [DOI] [PubMed] [Google Scholar]
- 34.Löfgren H., Pascher I. Molecular arrangements of sphingolipids. The monolayer behaviour of ceramides. Chem. Phys. Lipids. 1977;20:273–284. doi: 10.1016/0009-3084(77)90068-8. [DOI] [PubMed] [Google Scholar]
- 35.Rerek M.E., Chen H., Moore D.J. Phytosphingosine and sphingosine ceramide headgroup hydrogen bonding: structural insights through thermotropic hydrogen/deuterium exchange. J. Phys. Chem. B. 2001;105:9355–9362. [Google Scholar]
- 36.Maula T., Westerlund B., Slotte J.P. Differential ability of cholesterol-enriched and gel phase domains to resist benzyl alcohol-induced fluidization in multilamellar lipid vesicles. Biochim. Biophys. Acta. 2009;1788 doi: 10.1016/j.bbamem.2009.08.024. 2454–61. [DOI] [PubMed] [Google Scholar]
- 37.Maula T., Urzelai B., Peter Slotte J. The effects of N-acyl chain methylations on ceramide molecular properties in bilayer membranes. Eur. Biophys. J. 2011;40:857–863. doi: 10.1007/s00249-011-0702-7. [DOI] [PubMed] [Google Scholar]
- 38.Björkqvist Y.J., Nyholm T.K., Ramstedt B. Domain formation and stability in complex lipid bilayers as reported by cholestatrienol. Biophys. J. 2005;88:4054–4063. doi: 10.1529/biophysj.104.054718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nyholm T.K., Grandell P.M., Slotte J.P. Sterol affinity for bilayer membranes is affected by their ceramide content and the ceramide chain length. Biochim. Biophys. Acta. 2010;1798:1008–1013. doi: 10.1016/j.bbamem.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 40.Silva L., de Almeida R.F., Prieto M. Ceramide-platform formation and -induced biophysical changes in a fluid phospholipid membrane. Mol. Membr. Biol. 2006;23:137–148. doi: 10.1080/09687860500439474. [DOI] [PubMed] [Google Scholar]
- 41.Silva L.C., de Almeida R.F., Prieto M. Ceramide-domain formation and collapse in lipid rafts: membrane reorganization by an apoptotic lipid. Biophys. J. 2007;92:502–516. doi: 10.1529/biophysj.106.091876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Castro B.M., Silva L.C., Prieto M. Cholesterol-rich fluid membranes solubilize ceramide domains: implications for the structure and dynamics of mammalian intracellular and plasma membranes. J. Biol. Chem. 2009;284:22978–22987. doi: 10.1074/jbc.M109.026567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Castro B.M., de Almeida R.F., Prieto M. Formation of ceramide/sphingomyelin gel domains in the presence of an unsaturated phospholipid: a quantitative multiprobe approach. Biophys. J. 2007;93:1639–1650. doi: 10.1529/biophysj.107.107714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nyholm T.K., Lindroos D., Slotte J.P. Construction of a DOPC/PSM/cholesterol phase diagram based on the fluorescence properties of trans-parinaric acid. Langmuir. 2011;27:8339–8350. doi: 10.1021/la201427w. [DOI] [PubMed] [Google Scholar]
- 45.Niu S.L., Litman B.J. Determination of membrane cholesterol partition coefficient using a lipid vesicle-cyclodextrin binary system: effect of phospholipid acyl chain unsaturation and headgroup composition. Biophys. J. 2002;83:3408–3415. doi: 10.1016/S0006-3495(02)75340-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nyström J.H., Lönnfors M., Nyholm T.K. Transmembrane peptides influence the affinity of sterols for phospholipid bilayers. Biophys. J. 2010;99:526–533. doi: 10.1016/j.bpj.2010.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Megha, Sawatzki P., Kolter T., Bittman R., London E. Effect of ceramide N-acyl chain and polar headgroup structure on the properties of ordered lipid domains (lipid rafts) Biochim. Biophys. Acta. 2007;1768:2205–2212. doi: 10.1016/j.bbamem.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nybond S., Björkqvist Y.J., Slotte J.P. Acyl chain length affects ceramide action on sterol/sphingomyelin-rich domains. Biochim. Biophys. Acta. 2005;1718:61–66. doi: 10.1016/j.bbamem.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 49.Brockman H.L., Momsen M.M., Bittman R. The 4,5-double bond of ceramide regulates its dipole potential, elastic properties, and packing behavior. Biophys. J. 2004;87:1722–1731. doi: 10.1529/biophysj.104.044529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Busto J.V., Sot J., Alonso A. Cholesterol displaces palmitoylceramide from its tight packing with palmitoylsphingomyelin in the absence of a liquid-disordered phase. Biophys. J. 2010;99:1119–1128. doi: 10.1016/j.bpj.2010.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ridgway N.D., Lagace T.A., Byers D.M. Differential effects of sphingomyelin hydrolysis and cholesterol transport on oxysterol-binding protein phosphorylation and Golgi localization. J. Biol. Chem. 1998;273:31621–31628. doi: 10.1074/jbc.273.47.31621. [DOI] [PubMed] [Google Scholar]
- 52.Björkbom A., Róg T., Slotte J.P. N- and O-methylation of sphingomyelin markedly affects its membrane properties and interactions with cholesterol. Biochim. Biophys. Acta. 2011;1808:1179–1186. doi: 10.1016/j.bbamem.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 53.Shah J., Atienza J.M., Shipley G.G. Structural and thermotropic properties of synthetic C16:0 (palmitoyl) ceramide: effect of hydration. J. Lipid Res. 1995;36:1936–1944. [PubMed] [Google Scholar]
- 54.Li L., Tang X., Yappert M.C. Conformational characterization of ceramides by nuclear magnetic resonance spectroscopy. Biophys. J. 2002;82:2067–2080. doi: 10.1016/S0006-3495(02)75554-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vorobyov I., Yappert M.C., DuPré D.B. Energetic and topological analyses of cooperative σH- and πH-bonding interactions. J. Phys. Chem. A. 2002;106:10691–10699. [Google Scholar]
- 56.Pandit S.A., Scott H.L. Molecular-dynamics simulation of a ceramide bilayer. J. Chem. Phys. 2006;124:14708–14715. doi: 10.1063/1.2140689. [DOI] [PubMed] [Google Scholar]
- 57.Bittman R. Chemical preparation of glycerolipids: a review of recent syntheses. In: Cevec G., editor. Phospholipids Handbook. Marcel Dekker; New York: 1993. 154–155. [Google Scholar]
- 58.Bittman R., Verbicky C.A. Methanolysis of sphingomyelin. Toward an epimerization-free methodology for the preparation of D-erythro-sphingosylphosphocholine. J. Lipid Res. 2000;41:2089–2093. [PubMed] [Google Scholar]
- 59.Cohen R., Barenholz Y., Dagan A. Preparation and characterization of well defined D-erythro sphingomyelins. Chem. Phys. Lipids. 1984;35:371–384. doi: 10.1016/0009-3084(84)90079-3. [DOI] [PubMed] [Google Scholar]
- 60.Fischer R.T., Stephenson F.A., Schroeder F. δ5,7,9(11)-Cholestatrien-3 β-ol: a fluorescent cholesterol analogue. Chem. Phys. Lipids. 1984;36:1–14. doi: 10.1016/0009-3084(84)90086-0. [DOI] [PubMed] [Google Scholar]
- 61.Zhang L., Hellgren L.I., Xu X. Enzymatic production of ceramide from sphingomyelin. J. Biotechnol. 2006;123:93–105. doi: 10.1016/j.jbiotec.2005.10.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


