Abstract
Accumulation of cholesterol by macrophage uptake of LDL is a key event in the formation of atherosclerotic plaques. Previous research has shown that granulocyte-macrophage colony-stimulating factor (GM-CSF) is present in atherosclerotic plaques and promotes aortic lipid accumulation. However, it has not been determined whether murine GM-CSF-differentiated macrophages take up LDL to become foam cells. GM-CSF-differentiated macrophages from LDL receptor-null mice were incubated with LDL, resulting in massive macrophage cholesterol accumulation. Incubation of LDL receptor-null or wild-type macrophages with increasing concentrations of 125I-LDL showed nonsaturable macrophage LDL uptake that was linearly related to the amount of LDL added, indicating that LDL uptake was mediated by fluid-phase pinocytosis. Previous studies suggest that phosphoinositide 3-kinases (PI3K) mediate macrophage fluid-phase pinocytosis, although the isoform mediating this process has not been determined. Because PI3Kγ is known to promote aortic lipid accumulation, we investigated its role in mediating macrophage fluid-phase pinocytosis of LDL. Wild-type macrophages incubated with LDL and the PI3Kγ inhibitor AS605240 or PI3Kγ-null macrophages incubated with LDL showed an ∼50% reduction in LDL uptake and cholesterol accumulation compared with wild-type macrophages incubated with LDL only. These results show that GM-CSF-differentiated murine macrophages become foam cells by fluid-phase pinocytosis of LDL and identify PI3Kγ as contributing to this process.
Keywords: atherosclerosis, cholesterol, granulocyte-macrophage colony-stimulating factor, phosphoinositide 3-kinase, low density lipoprotein
Development of atherosclerotic plaques results from the accumulation of cholesterol within arterial vessel walls. Macrophages play a critical role in this process by mediating uptake of the cholesterol-rich particle LDL, resulting in arterial accumulation of cholesterol. LDL uptake by macrophages is thought to be dependent on oxidative modification of LDL allowing for receptor-mediated endocytosis of LDL to generate cholesterol-rich foam cells (1). Recently, we identified an additional mechanism of LDL uptake by macrophages: nonreceptor mediated fluid-phase pinocytosis of LDL (2). In contrast to receptor-mediated endocytosis, macrophage uptake of solute by fluid-phase pinocytosis occurs at levels directly proportional to the amount of solute within the extracellular fluid. LDL is present within the intimal layer of the vessel wall (where foam cells develop) at high levels (0.7–2.7 mg/ml) (3–5). Incubation of human macrophages with these physiological concentrations of LDL promotes foam-cell formation by fluid-phase pinocytosis (6, 7). Macrophage fluid-phase pinocytosis not only occurs in cell culture but also in plaque-resident macrophages of atherosclerosis-prone mice (8).
Fluid-phase pinocytosis can occur by the formation of macropinosomes (>0.2 µm) or micropinosomes (≤0.2 µm) (9). Human macrophage colony-stimulating factor (M-CSF)-differentiated macrophages take up LDL by fluid-phase macropinocytosis and micropinocytosis to become foam cells (10). Macrophages differentiated with human serum have a granulocyte-macrophage colony-stimulating factor (GM-CSF)-differentiated phenotype, and when activated with PMA, primarily take up LDL by macropinocytosis to become foam cells (6, 11). Similar to human macrophages, murine macrophages can be generated in the presence of M-CSF or GM-CSF (12). Murine macrophages differentiated with M-CSF take up solute by macropinocytosis and micropinocytosis (13). In contrast to the many studies characterizing murine macrophages differentiated with M-CSF, there are few studies characterizing murine macrophages differentiated with GM-CSF, and the pinocytotic pathways mediating uptake of solute are unknown. GM-CSF is found within human (14) and murine (15) atherosclerotic plaques and is known to induce accumulation of lipid in the aorta (14). However, it is not known whether murine macrophages differentiated with GM-CSF become lipid-rich foam cells when incubated with LDL.
Previous studies show an important role for phosphoinositide 3-kinase (PI3K) signaling in macropinosome formation, although the PI3K isoform mediating this process has not been identified (13). A major objective of this study was to identify the PI3K isoform mediating macrophage fluid-phase uptake of LDL. Determining this PI3K isoform is important for targeting fluid-phase pinocytosis of LDL by macrophages. In this study, we identify PI3Kγ as a mediator of fluid-phase pinocytosis that contributes to GM-CSF-differentiated murine macrophages foam-cell formation through uptake of LDL.
MATERIALS AND METHODS
Culture of murine bone marrow-derived macrophages
Male C57BL/6 and low-density lipoprotein-null mice (C57BL/6 background) were obtained from Jackson Laboratory. Male PI3Kγ-null (C57BL/6 background) mice were generated as previously described (16, 17). Femurs and tibias were isolated from mice, and muscle was removed. Both ends of bones were cut with scissors and then flushed with 5 ml of RPMI 1640 with a 25-gauge needle. Cells were seeded at a density of 2 × 105 nucleated bone marrow cells/cm2 in RPMI 1640 (catalog number 15-040-CM, MediaTech) containing 10% FBS (catalog number 16000-036, Gibco), 100 U/ml penicillin, 0.1 mg/ml streptomycin, 2 mM L-glutamine (catalog number G6784, Sigma), and 50 ng/ml GM-CSF (catalog number 315-03, Peprotech) in 6-well Cellbind plates containing 3 ml per well (catalog number 3335, Corning). After a 24 h incubation at 37°C with 5% CO2/95% air, cells were rinsed three times with 3 ml RPMI 1640 to remove nonadherent cells, and then cultured further with 3 ml of RPMI 1640 containing 10% FBS, 100 U/ml penicillin, 0.1 mg/ml streptomycin, 2 mM L-glutamine, and 50 ng/ml GM-CSF (hereafter referred to as complete medium). Cell culture medium was subsequently changed every 3 days with fresh complete medium. After 3 weeks in culture, experiments were performed with serum-free RPMI, 50 ng/ml GM-CSF, and the indicated additions. GM-CSF differentiation of murine bone marrow cells required 3 weeks to acquire a near-uniform culture of macrophages with a rounded, heavily vacuolated morphology. A small proportion of cells had a flattened morphology. Culture of M-CSF-differentiated macrophages was identical to the procedure for GM-CSF-differentiated macrophages, except cells were incubated with 50 ng/ml M-CSF (catalog number 315-02, Peprotech) instead of GM-CSF, and experiments were performed after one week of culture.
Quantification of macrophage cholesterol
Macrophages were rinsed three times in Dulbecco's phosphate-buffered saline containing Ca2+ and Mg2+ (DPBS) (catalog number 21-030-CV, Mediatech), lysed with 1 ml of ultrapure water per well, then detached with a cell scraper and collected. Lipid was isolated from the cell lysate using the Folch method (18), and cholesterol was quantified as previously described by Gamble et al. (19). Protein quantification of cell lysates was measured using the Lowry method (20) with a BSA standard.
Visualization of neutral lipid
Macrophages were incubated with 4 mg/ml LDL for 24 h, rinsed three times with DPBS, and then fixed at room temperature for 30 min with 4% paraformaldehyde in DPBS. Macrophages were rinsed three times with 3 ml of DPBS and then stained for 15 min at room temperature with 1 ml of filtered Oil Red O (21). Oil Red O solution was prepared by dissolving Oil Red O (catalog number O0625, Sigma) in isopropanol to obtain a 3% solution (w/v). Oil Red O was diluted by adding two parts water to three parts Oil Red O and then filtered (0.45 µm). After Oil Red O staining, macrophages were rinsed three times with water, stained for 2 min with hematoxylin QS (catalog number H-3403, Vector), rinsed with water, and then coverslipped with glycerol-gelatin mounting media (catalog number GG1, Sigma).
Analysis of 125I-LDL cell association and degradation
Macrophage uptake of 125I-LDL was assessed by measuring cell-associated and degraded 125I-LDL according to the method of Goldstein et al. (22). A portion of culture media was centrifuged at 15,000 g for 10 min, and trichloroacetic acid-soluble organic iodide radioactivity was measured to quantify lipoprotein degradation. Cell-associated 125I-LDL was assessed as follows. Macrophages were rinsed three times with DPBS containing 0.2% BSA, followed by three rinses with DPBS, all at 4°C. Macrophages were dissolved overnight in 0.1 N NaOH at 37°C and then assayed for 125I radioactivity with a γ counter. 125I radioactivity values for wells incubated with 125I-LDL without macrophages were subtracted from 125I radioactivity values obtained for macrophages incubated with 125I-LDL. Values were <1% of cell-associated 125I-LDL. For protein quantification, an aliquot of cell lysate was measured using the Lowry method (20). 125I-LDL uptake is presented as the sum of cell-associated 125I-LDL and degraded 125I-LDL.
Analysis of CD11c expression by cultured macrophages
Macrophages were rinsed three times with DPBS and then fixed 20 min with −20°C methanol. Except for fixation, all procedures were carried out at room temperature. After fixation, macrophages were rinsed three times with DPBS. For identification of CD11c, macrophages were blocked with biotin/streptavidin (catalog number SP-2001, Vector) for 30 min, then rinsed three times with DPBS and incubated with 10 µg/ml biotinylated hamster anti-mouse CD11c (catalog number 553800, BD Pharmingen) or a hamster IgG isotype control (catalog number 553952, BD Pharmingen). Both antibodies were diluted in DPBS. Macrophages were rinsed three times in DPBS and then incubated with 5 µg/ml streptavidin Alexa Fluor 488 (catalog number S32354, Invitrogen) diluted in DPBS. After three washes with DPBS, macrophages were mounted with Vectashield containing DAPI (catalog number H-1500, Vector) and examined by fluorescence microscopy. For identification of CD68, macrophages were incubated with 10% goat serum in DPBS for 30 min, then rinsed three times with DPBS and incubated with 5 µg/ml of either rat anti-mouse CD68 (catalog number MCA1957, AbD Serotec) or rat IgG2 isotype control (catalog number MCA1212, AbD Serotec) for 30 min. Both antibodies were diluted in DPBS. Macrophages were rinsed three times in DPBS and then incubated with 2.5 µg/ml Alexa Fluor 594-conjugated goat anti-rat IgG (catalog number A11007, Invitrogen) diluted in DPBS. After three washes with DPBS, macrophages were mounted with Vectashield containing DAPI and examined by fluorescence microscopy. For fluorescence and phase-contrast microscopic analysis, macrophages were imaged using an Olympus IX81 fluorescence microscope with a 0.4 NA Olympus 20× objective.
Electron microscopic analysis of macrophages
To examine the general ultrastructure of macrophages, cell cultures were rinsed three times with DPBS. Then macrophages were fixed for 1 h with 2.5% glutaraldehyde in 0.1 M cacodylate buffer at room temperature. Next, macrophages were dehydrated by exposure to increasing concentrations of ethanol (50%, 70%, 95%, and 100%), embedded with Epon, and then polymerized overnight at 60°C. After polymerization, thin sections (90 nm) of macrophages were prepared and counterstained with saturated uranyl acetate and 0.4% lead citrate. Macrophage thin sections were then examined using a Joel 1200EX electron microscope.
Ultrastructural analysis of LDL uptake
To assess the fluid-phase pinocytotic pathways mediating LDL uptake, macrophages were pretreated with or without 1 µM AS605240 for 1 h. Control incubations received DMSO vehicle. Macrophages were then incubated with 4 mg/ml LDL with or without 1 µM AS605240 for 10 min, fixed at room temperature with 2% glutaraldehyde for 1 h, embedded in LR White resin, and prepared for immunogold labeling of LDL as described previously (23), except 1% dry skim milk instead of 1% BSA was used to block nonspecific staining. Thin sections were labeled first with 10 µg/ml affinity-purified rabbit anti-human LDL antibody (catalog number BT-905; Biomedical Technologies, Inc.), and then with a 1:10 dilution of 10 nm gold-conjugated goat F(ab)2 anti-rabbit IgG (catalog number EM.GFAR10, Ted Pella, Inc.). Both primary and secondary antibodies were diluted in 0.05 M Tris-HCl buffer containing 0.1% dry skim milk. As a control, macrophages incubated with 4 mg/ml LDL were labeled with 10 µg/ml affinity-purified rabbit anti-GFP antibody (catalog number 14-6774; eBioscience). Alternatively, macrophages were incubated without LDL and subsequently labeled with the anti-human LDL antibody. All sections were counterstained with 0.4% lead citrate and analyzed with a Joel 1200EX electron microscope. Thirty random cells were observed in thin sections for untreated or AS605240-treated macrophages. Immunogold labels within vesicles was counted, and the diameter of each pinosome containing LDL was measured.
Analysis of macrophage cytokine production
Macrophages were cultured in 12-well Cellbind plates in 1.5 ml complete medium per well for 3 weeks. Then each well containing macrophages was incubated with 0.5 ml RPMI 1640 containing 100 U/ml penicillin, 0.1 mg/ml streptomycin, 2 mM L-glutamine, and 50 ng/ml GM-CSF without or with 1 mg/ml LDL. After 24 h of incubation with or without LDL, macrophages were washed three times with DPBS and then incubated with 0.5 ml RPMI 1640 containing 100 U/ ml penicillin, 0.1 mg/ml streptomycin, 2 mM L-glutamine, and 50 ng/ml GM-CSF without LDL or serum. Culture supernatants were removed after 24 h, centrifuged at 1,500 g at 4°C for 10 min, and then 0.4 ml of supernatant was removed and stored at −20°C. Supernatants were thawed, and cytokines were assayed using the Mouse Proteome Profiler kit (catalog number ARY006; R and D Systems) according to the manufacturer's instructions. Luminescence was detected using Pierce ECL Western Blotting Substrate (catalog number 32106; Pierce) and a 5 min exposure to Kodak BioMax MR Film (catalog number 895 2855; Kodak). The resulting film was scanned and analyzed using ImageJ software. Cytokine intensities are presented as mean pixel density of two replicate wells per condition.
Statistical analysis
Data are presented as the mean ± SEM. Means were representative of three replicate wells. A paired, two-tailed t-test was used for statistical comparisons.
RESULTS
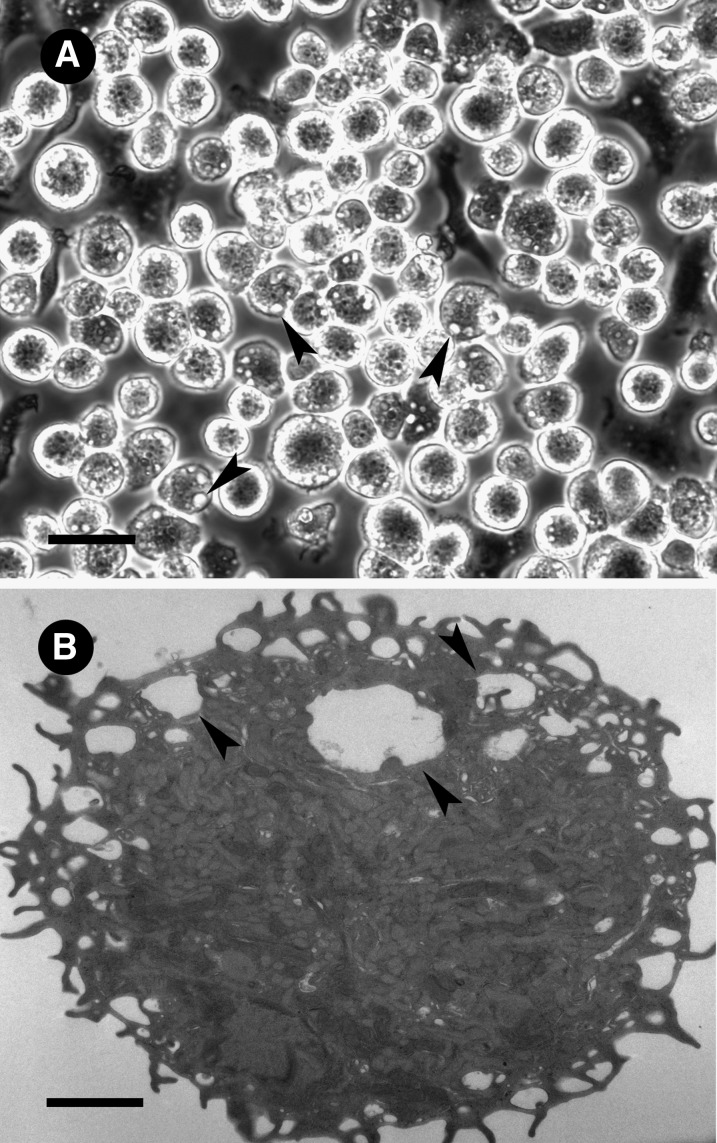
Murine bone marrow-derived macrophages differentiated with GM-CSF were generated from LDL receptor-null mice. The GM-CSF-differentiated macrophages were round with abundant vacuoles resembling macropinosomes, suggestive of high levels of fluid-phase pinocytosis (Fig. 1). GM-CSF bone marrow-derived macrophages generated from wild-type mice were morphologically similar to macrophages generated from LDL receptor-null mice.
Fig. 1.
GM-CSF-differentiated macrophage morphology. LDL receptor-null macrophages were differentiated from bone marrow in the presence of GM-CSF and examined by (A) phase-contrast microscopy or (B) electron microscopy. The scale bar for (A) light and (B) electron microscopic observation is 40 µm and 2 µm, respectively. Arrowheads indicate macropinosomes.
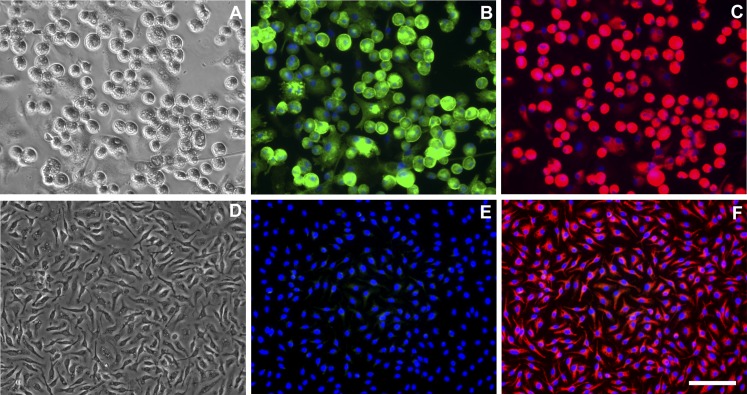
GM-CSF is present in atherosclerotic plaques of mice (15) and humans (14) and is known to stimulate proliferation of CD11c+CD68+ cells in atherosclerotic lesions of mice (24). CD68 is commonly utilized as a marker of macrophages, whereas CD11c+ is usually considered a specific marker of dendritic cells. However, previous studies have demonstrated that CD11c is expressed by some types of macrophages (25–27). The cells we generated from mouse bone marrow with GM-CSF resembled macrophages as described previously by Inaba et al. (28). The round macrophages also resembled the round foam cells that abundantly accumulate in mouse atherosclerotic lesions that show CD11c staining (24). Therefore, we determined whether our cultured GM-CSF-differentiated macrophages expressed CD11c. Because different macrophage phenotypes result from differentiation of macrophage precursors with either with GM-CSF or M-CSF (12), we also assessed CD11c expression by LDL receptor-null bone marrow-derived macrophages that were differentiated with either GM-CSF or M-CSF. In contrast to GM-CSF-differentiated macrophages, M-CSF-differentiated macrophages have an elongated morphology similar to human M-CSF-differentiated macrophages (11). As expected, both macrophage phenotypes expressed the macrophage marker CD68 (Fig. 2C, F). However, CD11c was expressed only by GM-CSF-differentiated macrophages; it was not expressed by M-CSF-differentiated macrophages (Fig. 2B, E). Identical results were obtained for macrophages differentiated from wild-type mice (supplementary Fig. I). These results identify a differential marker for murine GM-CSF- and M-CSF-differentiated macrophages and provide evidence that GM-CSF-differentiated macrophages are present within atherosclerotic lesions of mice.
Fig. 2.
GM-CSF-differentiated macrophages express CD11c. LDL receptor-null bone marrow cells were differentiated into macrophages with (A–C) GM-CSF or (D–F) M-CSF. Macrophages were immunostained for (B and E) CD11c or (C and F) CD68 and counterstained with DAPI. (A and D) Phase-contrast images of GM-CSF- or M-CSF-differentiated macrophages. Scale bar = 50 µm (applies to all micrographs).
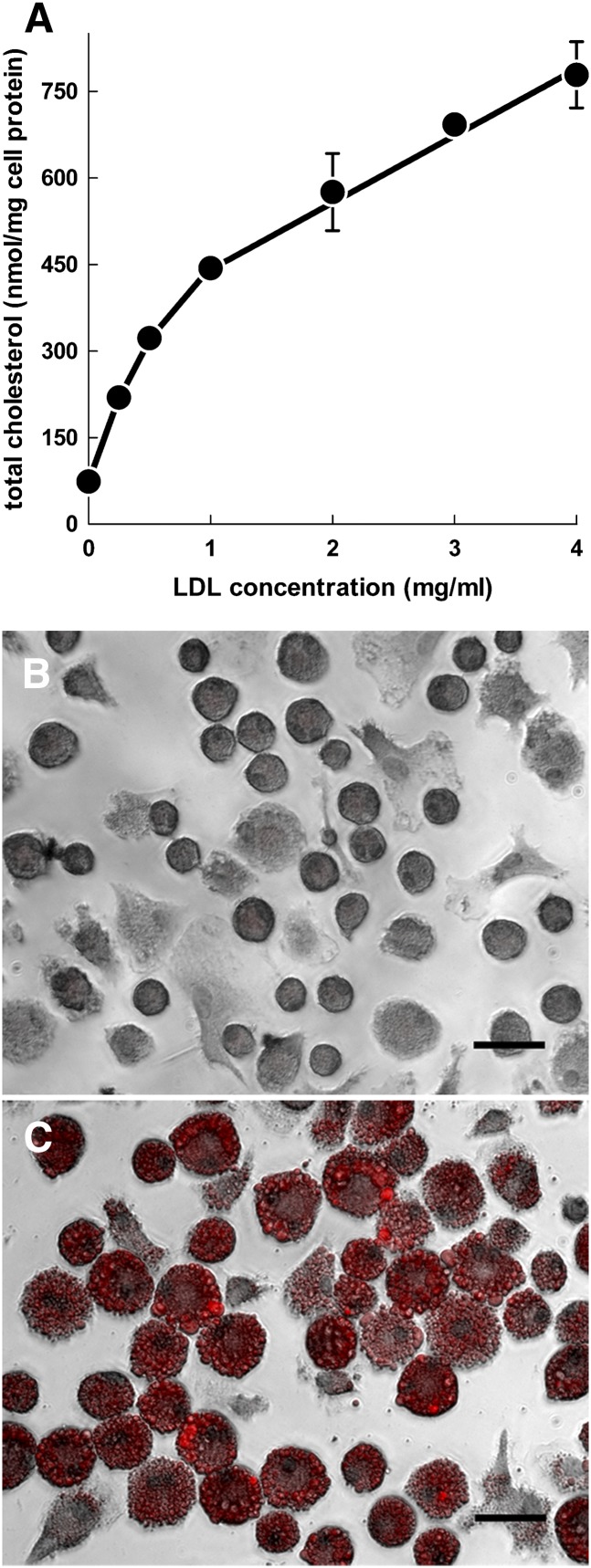
To determine whether GM-CSF-differentiated macrophages become cholesterol-enriched foam cells when incubated with native LDL, we incubated macrophages with 0–4 mg/ml of LDL (Fig. 3A). Macrophages showed massive, progressive accumulation of cholesterol when incubated with LDL concentrations up to 4 mg/ml. Assessment of macrophage-neutral lipid by Oil Red O staining revealed intense staining of macrophages incubated with LDL (Fig. 3C), whereas macrophages incubated without LDL showed no staining (Fig. 3B). This result shows that GM-CSF-differentiated murine macrophages become lipid-enriched foam cells when incubated with native LDL.
Fig. 3.
Macrophage incubation with LDL leads to massive accumulation of cholesterol and lipid droplets. (A) LDL receptor-null macrophages were incubated with increasing concentrations of LDL for 24 h and assessed for cholesterol accumulation. (B and C) LDL receptor-null macrophages were incubated (B) without or (C) with 4 mg/ml LDL and stained with Oil Red O to identify neutral lipid. Scale bars = 40 µm.
We next examined the possibility that macrophages may increase the production of inflammatory cytokines when incubated with LDL. To examine this, macrophages were incubated without or with 1 mg/ml LDL, and extracellular cytokine levels were assayed (supplementary Fig. II). Surprisingly, macrophages incubated with LDL showed no significant difference in cytokine levels compared with macrophages incubated without LDL.
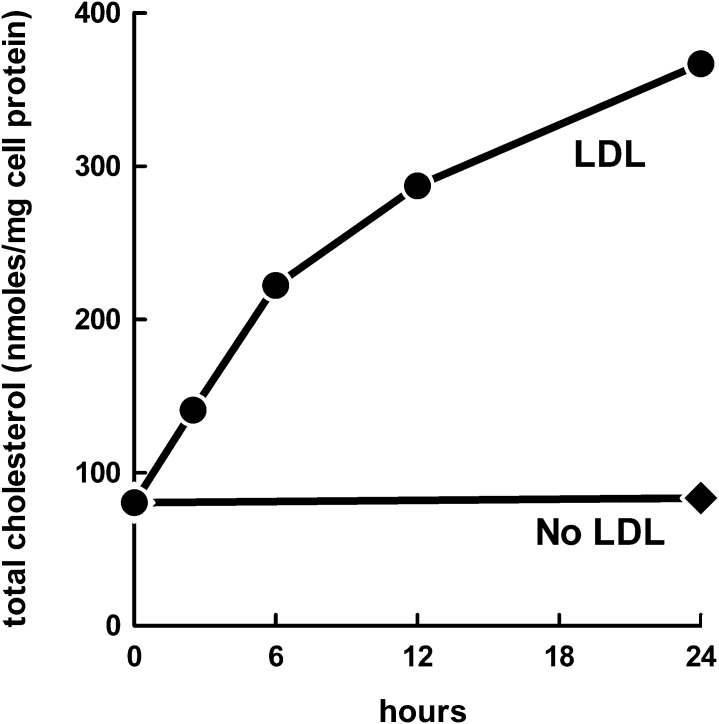
To determine whether macrophages incubated with LDL downregulate cholesterol accumulation with time, we incubated macrophages with 1 mg/ml LDL for 0–24 h and assessed cholesterol accumulation (Fig. 4). Macrophages continued to accumulate LDL-derived cholesterol for the 24 h incubation, showing that downregulation of cholesterol accumulation does not occur within the incubation period examined.
Fig. 4.
Accumulation of LDL-derived cholesterol by macrophages is progressive with time. LDL receptor-null macrophages were incubated with 1 mg/ml LDL for 0–24 h and assessed for cholesterol accumulation.
We also determined whether removal of GM-CSF from the culture medium affects macrophage accumulation of LDL-derived cholesterol, as previous studies have shown that fluid-phase pinocytosis of solute by M-CSF-differentiated, mouse bone marrow-derived macrophages can be stimulated by M-CSF (29). Macrophages were incubated with LDL in the presence or absence of GM-CSF for 6 h, and cholesterol accumulation was assessed. Removal of GM-CSF from the macrophage culture medium did not decrease the amount of cholesterol accumulated (supplementary Fig. III), indicating that macrophage LDL-derived cholesterol accumulation is constitutive.
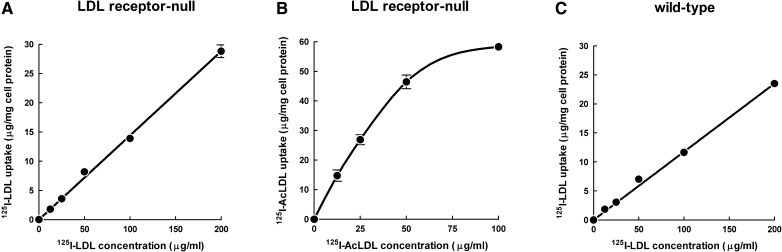
Because the GM-CSF-differentiated macrophages showed morphologic evidence of macropinosomes and because we previously showed that human monocyte-derived macrophages show fluid-phase pinocytosis of LDL mediated by macropinocytosis (6, 7, 10), we anticipated that LDL uptake was mediated by fluid-phase pinocytosis rather than receptor-mediated endocytosis, given that the LDL receptor could not explain the accumulation of cholesterol. To test this idea, GM-CSF-differentiated macrophages were incubated with increasing concentrations of 125I-LDL to assess whether receptors mediate uptake of 125I-LDL (Fig. 5A). Similar to what we previously showed for human macrophages, 125I-LDL uptake by the mouse macrophages was linearly related to the concentration of 125I-LDL added to cells and did not show saturation, indicating that uptake occurred through fluid-phase pinocytosis and not by receptor-mediated endocytosis. Greater than 90% of 125I-LDL taken up by macrophages was degraded. 125I-LDL degradation could not be explained by extracellular degradation of 125I-LDL because conditioned media from macrophages incubated with 200 µg/ml unlabeled LDL did not produce degradation of 125I-LDL added to the conditioned medium (data not shown). In contrast to macrophage nonsaturable uptake of 125I-LDL, macrophages incubated with increasing concentrations of 125I-acetylated LDL (125I-AcLDL) showed saturation of 125I-AcLDL uptake, indicating the expected receptor-mediated uptake of 125I-AcLDL (Fig. 5B). These data show that macrophage uptake of 125I-AcLDL occurs by binding to receptors, whereas native 125I-LDL uptake occurs by fluid-phase pinocytosis independently of receptors.
Fig. 5.
Macrophage uptake of LDL is mediated by fluid-phase pinocytosis. LDL receptor-null macrophages were incubated 24 h with increasing concentrations of (A) 125I-LDL or (B) 125I-AcLDL for 24 h, and total 125I-LDL uptake was assessed. (C) Wild-type macrophages were incubated 24 h with increasing concentrations of 125I-LDL, and total 125I-LDL uptake was assessed. Macrophage uptake of 125I-LDL or 125I-AcLDL represents the sum of cell-associated and degraded 125I-lipoprotein.
We next sought to determine whether the LDL receptor contributes to any significant amount of macrophage uptake of native LDL by mouse bone marrow-derived, GM-CSF-differentiated wild-type macrophages. To test for this possibility, we incubated wild-type macrophages with increasing doses of 125I-LDL (Fig. 5C). Similar to LDL receptor-null macrophages, uptake of 125I-LDL by wild-type macrophages did not saturate, and it was linearly related to the concentration of 125I-LDL added to cells, indicating that the LDL receptor did not contribute to macrophage uptake of 125I-LDL. To confirm that macrophage uptake of LDL occurs independently of receptors, we incubated wild-type macrophages with 125I-LDL alone or 125I-LDL and a 20-fold excess of unlabeled LDL (supplementary Fig. IV). Macrophage uptake of 125I-LDL was not significantly different between groups. In contrast, when macrophages were incubated with 125I-AcLDL and a 20-fold excess of unlabeled AcLDL, 125I-AcLDL uptake levels were significantly reduced compared with macrophages incubated with 125I-AcLDL only. This result confirms that macrophage uptake of unmodified LDL occurs by fluid-phase pinocytosis independently of receptors.
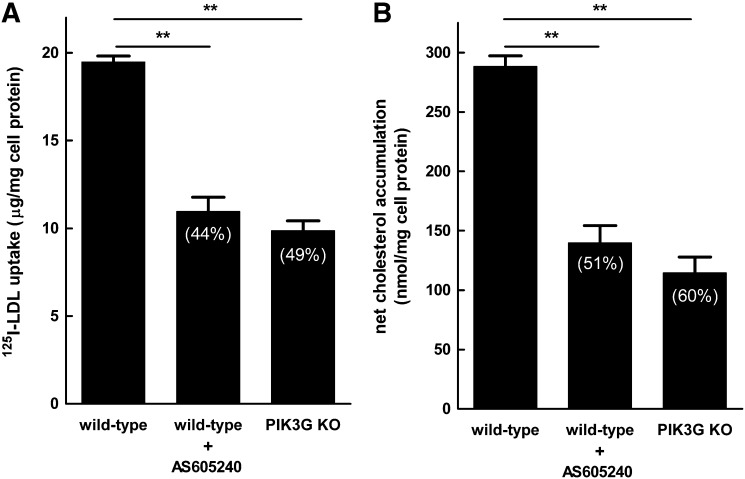
Murine macrophage fluid-phase pinocytosis previously has been shown to be dependent on PI3K signaling pathways (13), and an earlier study showed that inhibition of PI3Kγ with the PI3Kγ-specific inhibitor AS605240 resulted in an approximate 50% reduction in atherosclerosis (30). We therefore tested the effect of AS605240 on macrophage LDL-derived cholesterol accumulation. Wild-type macrophages were incubated without LDL, with 1 mg/ml LDL, or with 1 mg/ml LDL and AS605240 (Fig. 6A). Macrophage net cholesterol accumulation was inhibited 51% by AS605240, suggesting that PI3Kγ is a mediator of LDL-derived cholesterol accumulation. We assessed LDL-derived cholesterol accumulation for GM-CSF-differentiated macrophages from PI3Kγ-null mice to confirm that PI3Kγ mediates LDL-derived cholesterol accumulation (Fig. 6A). PI3Kγ-null macrophages accumulated 60% less cholesterol compared with wild-type macrophages, confirming our result with the PI3Kγ inhibitor. We next sought to determine whether the reduced cholesterol accumulation for AS605240-treated and PI3Kγ-null macrophages was due to reduced fluid-phase pinocytosis of LDL. To assess this, 125I-LDL was incubated with wild-type macrophages in the presence or absence of AS605240, or with macrophages derived from PI3Kγ-null mice (Fig. 6B). Macrophage uptake of 125I-LDL was inhibited 44% by AS605240 compared with untreated macrophages, suggesting that AS605240 inhibition of PI3Kγ was responsible for the reduced uptake of 125I-LDL. Incubation of PI3Kγ-null macrophages with 125I-LDL showed a 49% reduction in LDL uptake compared with wild-type macrophages, confirming that PI3Kγ is a mediator of LDL uptake and foam-cell formation in GM-CSF-differentiated macrophages.
Fig. 6.
PI3Kγ mediates LDL-derived cholesterol accumulation and LDL uptake by macrophages. (A) Wild-type macrophages were incubated for 24 h with 1 mg/ml LDL without or with 1 µM AS605240, PI3Kγ-null macrophages were incubated for 24 h with 1 mg/ml LDL, and then cholesterol accumulation was assessed. (B) Wild-type macrophages were incubated 24 h with 200 µg/ml 125I-LDL without or with1 µM AS605240, PI3Kγ-null macrophages were incubated 24 h with 200 µg/ml 125I-LDL, and then total 125I-LDL uptake was assessed (i.e., sum of cell-associated and degraded 125I-LDL). The percentage inhibition is indicated in parentheses. The basal cholesterol level was 83 nmol/mg cell protein for untreated wild-type macrophages and 78 nmol/mg cell protein for untreated PI3Kγ-null macrophages. **P < 0.01.
Because macropinocytosis requires actin polymerization, we also determined the effect of the actin polymerization inhibitor cytochalasin D on LDL-derived cholesterol accumulation (supplementary Fig. V). Net LDL-derived cholesterol accumulation was inhibited by 93% in the presence of cytochalasin D, whereas no effect of cytochalasin D was observed for net AcLDL-derived cholesterol accumulation. This result shows that macrophage actin polymerization is required for LDL-derived cholesterol accumulation but not AcLDL-derived cholesterol accumulation, and suggests that almost all LDL uptake occurs by macropinocytosis. To confirm that most macrophage uptake of LDL is mediated by macropinocytosis, we incubated macrophages with 4 mg/ml LDL for 10 min and quantified immunogold-labeled LDL in macropinosomes (>0.2 µm) and micropinosomes (≤0.2 µm) by transmission electron microscopy (Table 1). Greater than 97% of LDL particles were found within macropinosomes (supplementary Fig. VI), and thus, essentially all LDL particles were internalized within macropinosomes. Macrophages incubated without LDL showed no anti-LDL immunogold labeling (supplementary Fig. VI), consistent with most LDL being taken up by macropinocytosis. AS605240 treatment of macrophages reduced the number of LDL-containing macropinosomes by 38% compared with vehicle-treated macrophages. This resulted in a similar 41% decrease in the LDL particles internalized by macropinosomes.
TABLE 1.
AS605240 inhibits macrophage macropinocytosis of LDL
| Pinosome Size | Number of Pinosomes Containing
Immunogold-labeled LDL Particles per Cell Section |
Number of Immunogold LDL Particles in
Pinosomes per Cell Section |
Percentage of Immunogold-labeled LDL
Particles in Macro- and Micropinosomes |
|||
| AS605240 | AS605240 | AS605240 | ||||
| − | + | − | + | − | + | |
| macropinosomes | 15.2 | 9.4 | 88 | 52 | 99 | 97 |
| micropinosomes | 0.7 | 0.9 | 1 | 1 | 1 | 3 |
GM-CSF-differentiated macrophages were incubated 10 min with 4 mg/ml LDL with or without AS605240. LDL particles were identified by anti-LDL immunogold labeling and counted. The immunogold-labeled particles in pinosomes were counted in 30 random cells for each condition (i.e., ±AS605240). Macropinosomes are >0.2 μm. Micropinosomes are ≤0.2 μm.
DISCUSSION
It was previously thought that LDL uptake by murine macrophages occurs only by receptor-mediated uptake of modified LDL. In this study, we report that murine GM-CSF-differentiated macrophages take up native LDL by fluid-phase pinocytosis and become lipid-rich foam cells independently of receptors. Macrophage uptake of LDL could not be attributed to receptors because uptake of LDL was linearly related to the concentration of LDL added to macrophages. Furthermore, the LDL receptor did not mediate macrophage uptake of LDL because wild-type macrophages showed linear uptake of LDL, similar to macrophages lacking the LDL receptor. In contrast to macrophage fluid-phase pinocytosis of native LDL, uptake of acetylated LDL showed curvilinear kinetics with respect to the acetylated LDL concentration added to macrophages, indicating saturation of receptors. As acetylated LDL binds to scavenger receptors (31), native LDL uptake by scavenger receptors would be expected to show receptor saturation similar to acetylated LDL. We found no evidence of receptor saturation, indicating that scavenger receptors do not mediate native LDL uptake. Our results show that modification of plasma LDL is not necessary for foam-cell formation in murine GM-CSF-differentiated macrophages.
In this study, murine bone marrow cells were differentiated into macrophages with GM-CSF. Because differentiation of murine bone marrow with GM-CSF can also generate dendritic cells, it is important to note that GM-CSF-differentiated macrophages are clearly distinguishable from dendritic cells. In contrast to GM-CSF-differentiated dendritic cells that are weakly adherent to tissue culture plastic, GM-CSF-differentiated macrophages are strongly adherent (28). During macrophage differentiation, we observed a small number of nonadherent dendritic cells with characteristic dendrites. These cells were removed by thorough washing upon changing the culture medium. The resulting strongly adherent GM-CSF-differentiated macrophages lacked the dendrite processes of the nonadherent dendritic cells and, in contrast to bone marrow-derived dendritic cells, were heavily vacuolated (28). Thus, we show that our culture conditions produce macrophages, not dendritic cells.
CD11c+ cells are present within nascent (24) and advanced (15) atherosclerotic plaques. These cells are thought to be dendritic cells due to the expression of CD11c, a marker regarded as specific for dendritic cells (25). However, CD11c is also expressed on some types of macrophages (25–27), including as we have shown, the bone marrow-derived, GM-CSF-differentiated macrophages described in this study. Most CD11c+ cells within early lesions have a rounded morphology and lack dendrites similar to the GM-CSF-differentiated macrophage phenotype we observed in this study, suggesting that many of these lesion cells are macrophages (24).
Inhibition of PI3Kγ signaling by AS605240 treatment or genetic deletion of PI3Kγ was recently found to prevent the development of atherosclerosis in ApoE−/− and LDLr−/− mice (30). AS605240 is a potent and selective inhibitor of PI3Kγ (32, 33). We show that AS605240 inhibits macrophage uptake of LDL and cholesterol accumulation ∼50% compared with untreated cells. As a similar reduction in LDL uptake and cholesterol accumulation was observed when comparing PI3Kγ-null with wild-type macrophages, this provides further evidence that AS605240 targets PI3Kγ-mediated fluid-phase uptake of LDL. Our findings suggest that the reduction of atherosclerosis by AS605240 treatment or deficiency of PI3Kγ may be contributed to by inhibition of macrophage uptake of LDL.
Although we did not examine other PI3K isoforms in this study, it was previously reported that the murine macrophage cell line RAW264.7 takes up FITC-labeled dextran in a PI3Kα-dependent manner (34). The authors attributed PI3Kα-mediated uptake by RAW264.7 cells to fluid-phase pinocytosis, even though uptake of the fluid-phase pinocytosis tracer Lucifer Yellow was shown to occur independently of PI3Kα. Fluorescently labeled dextrans are commonly used as fluid-phase pinocytosis tracers even though some cells, including certain types of macrophages and dendritic cells, express receptors that bind dextran (35, 36). Receptor-mediated binding of FITC-dextran by RAW264.7 cells may explain why PI3Kα mediates FITC-dextran uptake but not Lucifer Yellow uptake in these cells.
In this study, GM-CSF-differentiated murine macrophages were found to take up LDL and accumulate cholesterol almost exclusively by actin-dependent macropinocytosis, similar to our previous observation of LDL uptake in human macrophages showing the GM-CSF phenotype (6). In contrast to murine macrophages incubated with native LDL, we observed no difference in cholesterol accumulation when murine macrophages were incubated with acetylated LDL in the presence or absence of the actin polymerization inhibitor cytochalasin D, indicating that receptor-mediated endocytosis of acetylated LDL occurs independently of actin polymerization for murine GM-CSF-differentiated macrophages.
Because 99% of macrophage fluid-phase pinocytotic uptake of LDL was mediated by macropinocytosis and AS605240 inhibits macropinocytosis of LDL by ∼50%, our data indicate that at least one additional fluid-phase macropinocytotic pathway exists that is independent of PI3Kγ. Many signaling components have been demonstrated to mediate macropinocytosis in various types of cells (37). Although one or more of these components may mediate PI3Kγ-independent macropinocytosis for murine GM-CSF-differentiated macrophages, these components may mediate macropinocytosis only in certain cell types. For example, previous studies show a role for Src family kinases in mediating macropinocytosis in several cell lines (38, 39), whereas we previously observed that human M-CSF-differentiated macrophages show Src family kinase-independent macropinocytosis (10). Future studies are warranted to identify the PI3Kγ-independent pathways mediating macropinocytosis for murine GM-CSF-differentiated macrophages. Targeting this additional pathway together with PI3Kγ is likely to lead to more complete inhibition of macrophage uptake of LDL and cholesterol accumulation.
LDL uptake and foam-cell formation by murine GM-CSF-differentiated macrophages were found to occur independently of additional stimulation. In contrast, the human GM-CSF macrophage phenotype requires protein kinase C stimulation of fluid-phase macropinocytosis to take up native LDL and become lipid-rich foam cells (11). These differences may be due to the different source of macrophages (bone marrow compared with blood monocytes), or they may represent a species difference between mice and humans. We were unable to determine why these differences exist because the macrophage yield of murine monocytes differentiated with GM-CSF was too low to assess LDL uptake and cholesterol accumulation.
Murine bone marrow-derived M-CSF-differentiated macrophages are dependent on the presence of M-CSF for macropinocytosis (40). In contrast, murine bone marrow-derived GM-CSF-differentiated macrophages showed macropinocytosis of LDL independently of the presence of GM-CSF, as cholesterol accumulation was not decreased by removal of GM-CSF. Thus, macropinocytosis of LDL is constitutive for murine GM-CSF-differentiated macrophages. We did not observe downregulation of macrophage LDL-derived cholesterol accumulation, showing that cholesterol is able to continually accumulate within these macrophages during the 24 h of incubation with LDL.
Murine macrophage uptake of LDL by fluid-phase pinocytosis is consistent with reports showing that genetic knockout of the scavenger receptors SRA and/or CD36, which mediate uptake of oxidized LDL, does not affect lipid accumulation and foam-cell formation in murine atherosclerotic plaques (41, 42). Our results help explain how cholesteryl ester and lipid droplets accumulate within arterial plaque macrophages, as macrophages incubated with native LDL showed massive accumulation of cholesteryl ester and lipid droplets. Furthermore, we have identified PI3Kγ as a signaling intermediate to target for limiting macrophage cholesterol accumulation in atherosclerotic plaques.
Supplementary Material
Acknowledgments
The authors thank the Electron Microscopy Core Facility, National Heart, Lung, and Blood Institute, National Institutes of Health, for the use of its electron microscope.
Footnotes
Abbreviations:
- AcLDL
- acetylated LDL
- DPBS
- Dulbecco's phosphate-buffered saline containing Ca2+ and Mg2+
- GM-CSF
- granulocyte-macrophage colony-stimulating factor
- M-CSF
- macrophage colony-stimulating factor
- PI3K
- phosphoinositide 3-kinase
This work was supported by the Intramural Research Program, National Heart, Lung, and Blood Institute, National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. M.K.B. was supported by a fellowship from the Indo-US Science and Technology Forum and received additional support from the International Atherosclerosis Society.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of six figures.
REFERENCES
- 1.Steinberg D. 2009. The LDL modification hypothesis of atherogenesis: an update. J. Lipid Res. 50(Suppl.): S376–S381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kruth H. S. 2011. Receptor-independent fluid-phase pinocytosis mechanisms for induction of foam cell formation with native low-density lipoprotein particles. Curr. Opin. Lipidol. 22: 386–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoff H. F., Gaubatz J. W., Gotto A. M., Jr 1978. Apo B concentration in the normal human aorta. Biochem. Biophys. Res. Commun. 85: 1424–1430. [DOI] [PubMed] [Google Scholar]
- 4.Smith E. B. 1990. Transport, interactions and retention of plasma proteins in the intima: the barrier function of the internal elastic lamina. Eur. Heart J. 11(Suppl. E): 72–81. [DOI] [PubMed] [Google Scholar]
- 5.Smith E. B., Ashall C. 1983. Low-density lipoprotein concentration in interstitial fluid from human atherosclerotic lesions. Relation to theories of endothelial damage and lipoprotein binding. Biochim. Biophys. Acta. 754: 249–257. [DOI] [PubMed] [Google Scholar]
- 6.Kruth H. S., Jones N. L., Huang W., Zhao B., Ishii I., Chang J., Combs C. A., Malide D., Zhang W. Y. 2005. Macropinocytosis is the endocytic pathway that mediates macrophage foam cell formation with native low density lipoprotein. J. Biol. Chem. 280: 2352–2360. [DOI] [PubMed] [Google Scholar]
- 7.Zhao B., Li Y., Buono C., Waldo S. W., Jones N. L., Mori M., Kruth H. S. 2006. Constitutive receptor-independent low density lipoprotein uptake and cholesterol accumulation by macrophages differentiated from human monocytes with macrophage-colony-stimulating factor (M-CSF). J. Biol. Chem. 281: 15757–15762. [DOI] [PubMed] [Google Scholar]
- 8.Buono C., Anzinger J. J., Amar M., Kruth H. S. 2009. Fluorescent pegylated nanoparticles demonstrate fluid-phase pinocytosis by macrophages in mouse atherosclerotic lesions. J. Clin. Invest. 119: 1373–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swanson J. A., Watts C. 1995. Macropinocytosis. Trends Cell Biol. 5: 424–428. [DOI] [PubMed] [Google Scholar]
- 10.Anzinger J. J., Chang J., Xu Q., Buono C., Li Y., Leyva F. J., Park B. C., Greene L. E., Kruth H. S. 2010. Native low-density lipoprotein uptake by macrophage colony-stimulating factor-differentiated human macrophages is mediated by macropinocytosis and micropinocytosis. Arterioscler. Thromb. Vasc. Biol. 30: 2022–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waldo S. W., Li Y., Buono C., Zhao B., Billings E. M., Chang J., Kruth H. S. 2008. Heterogeneity of human macrophages in culture and in atherosclerotic plaques. Am. J. Pathol. 172: 1112–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falk L. A., Vogel S. N. 1988. Comparison of bone marrow progenitors responsive to granulocyte-macrophage colony stimulating factor and macrophage colony stimulating factor-1. J. Leukoc. Biol. 43: 148–157. [DOI] [PubMed] [Google Scholar]
- 13.Araki N., Johnson M. T., Swanson J. A. 1996. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J. Cell Biol. 135: 1249–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haghighat A., Weiss D., Whalin M. K., Cowan D. P., Taylor W. R. 2007. Granulocyte colony-stimulating factor and granulocyte macrophage colony-stimulating factor exacerbate atherosclerosis in apolipoprotein E-deficient mice. Circulation. 115: 2049–2054. [DOI] [PubMed] [Google Scholar]
- 15.Shaposhnik Z., Wang X., Weinstein M., Bennett B. J., Lusis A. J. 2007. Granulocyte macrophage colony-stimulating factor regulates dendritic cell content of atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 27: 621–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirsch E., Katanaev V. L., Garlanda C., Azzolino O., Pirola L., Silengo L., Sozzani S., Mantovani A., Altruda F., Wymann M. P. 2000. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science. 287: 1049–1053. [DOI] [PubMed] [Google Scholar]
- 17.Patrucco E., Notte A., Barberis L., Selvetella G., Maffei A., Brancaccio M., Marengo S., Russo G., Azzolino O., Rybalkin S. D., et al. 2004. PI3Kgamma modulates the cardiac response to chronic pressure overload by distinct kinase-dependent and -independent effects. Cell. 118: 375–387. [DOI] [PubMed] [Google Scholar]
- 18.Folch J., Lees M., Sloane Stanley G. H. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226: 497–509. [PubMed] [Google Scholar]
- 19.Gamble W., Vaughan M., Kruth H. S., Avigan J. 1978. Procedure for determination of free and total cholesterol in micro- or nanogram amounts suitable for studies with cultured cells. J. Lipid Res. 19: 1068–1070. [PubMed] [Google Scholar]
- 20.Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193: 265–275. [PubMed] [Google Scholar]
- 21.Kruth H. S. 1984. Histochemical detection of esterified cholesterol within human atherosclerotic lesions using the fluorescent probe filipin. Atherosclerosis. 51: 281–292. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein J. L., Basu S. K., Brown M. S. 1983. Receptor-mediated endocytosis of low-density lipoprotein in cultured cells. Methods Enzymol. 98: 241–260. [DOI] [PubMed] [Google Scholar]
- 23.Kruth H. S., Skarlatos S. I., Lilly K., Chang J., Ifrim I. 1995. Sequestration of acetylated LDL and cholesterol crystals by human monocyte-derived macrophages. J. Cell Biol. 129: 133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu S. N., Chen M., Jongstra-Bilen J., Cybulsky M. I. 2009. GM-CSF regulates intimal cell proliferation in nascent atherosclerotic lesions. J. Exp. Med. 206: 2141–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hume D. A. 2008. Macrophages as APC and the dendritic cell myth. J. Immunol. 181: 5829–5835. [DOI] [PubMed] [Google Scholar]
- 26.Li P., Lu M., Nguyen M. T., Bae E. J., Chapman J., Feng D., Hawkins M., Pessin J. E., Sears D. D., Nguyen A. K., et al. 2010. Functional heterogeneity of CD11c-positive adipose tissue macrophages in diet-induced obese mice. J. Biol. Chem. 285: 15333–15345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Rijt L. S., Jung S., Kleinjan A., Vos N., Willart M., Duez C., Hoogsteden H. C., Lambrecht B. N. 2005. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J. Exp. Med. 201: 981–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inaba K., Inaba M., Deguchi M., Hagi K., Yasumizu R., Ikehara S., Muramatsu S., Steinman R. M. 1993. Granulocytes, macrophages, and dendritic cells arise from a common major histocompatibility complex class II-negative progenitor in mouse bone marrow. Proc. Natl. Acad. Sci. USA. 90: 3038–3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Racoosin E. L., Swanson J. A. 1989. Macrophage colony-stimulating factor (rM-CSF) stimulates pinocytosis in bone marrow-derived macrophages. J. Exp. Med. 170: 1635–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fougerat A., Gayral S., Gourdy P., Schambourg A., Ruckle T., Schwarz M. K., Rommel C., Hirsch E., Arnal J. F., Salles J. P., et al. 2008. Genetic and pharmacological targeting of phosphoinositide 3-kinase-gamma reduces atherosclerosis and favors plaque stability by modulating inflammatory processes. Circulation. 117: 1310–1317. [DOI] [PubMed] [Google Scholar]
- 31.Goldstein J. L., Ho Y. K., Basu S. K., Brown M. S. 1979. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc. Natl. Acad. Sci. USA. 76: 333–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barber D. F., Bartolome A., Hernandez C., Flores J. M., Redondo C., Fernandez-Arias C., Camps M., Ruckle T., Schwarz M. K., Rodriguez S., et al. 2005. PI3Kgamma inhibition blocks glomerulonephritis and extends lifespan in a mouse model of systemic lupus. Nat. Med. 11: 933–935. [DOI] [PubMed] [Google Scholar]
- 33.Camps M., Ruckle T., Ji H., Ardissone V., Rintelen F., Shaw J., Ferrandi C., Chabert C., Gillieron C., Francon B., et al. 2005. Blockade of PI3Kgamma suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat. Med. 11: 936–943. [DOI] [PubMed] [Google Scholar]
- 34.Tamura N., Hazeki K., Okazaki N., Kametani Y., Murakami H., Takaba Y., Ishikawa Y., Nigorikawa K., Hazeki O. 2009. Specific role of phosphoinositide 3-kinase p110alpha in the regulation of phagocytosis and pinocytosis in macrophages. Biochem. J. 423: 99–108. [DOI] [PubMed] [Google Scholar]
- 35.Norbury C. C. 2006. Drinking a lot is good for dendritic cells. Immunology. 117: 443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sallusto F., Cella M., Danieli C., Lanzavecchia A. 1995. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J. Exp. Med. 182: 389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lim J. P., Gleeson P. A. Macropinocytosis: an endocytic pathway for internalising large gulps. Immunol. Cell Biol. Epub ahead of print. March 22, 2011; doi: 10.1038/icb.2011.20. [DOI] [PubMed] [Google Scholar]
- 38.Veithen A., Cupers P., Baudhuin P., Courtoy P. J. 1996. v-Src induces constitutive macropinocytosis in rat fibroblasts. J. Cell Sci. 109: 2005–2012. [DOI] [PubMed] [Google Scholar]
- 39.Kasahara K., Nakayama Y., Sato I., Ikeda K., Hoshino M., Endo T., Yamaguchi N. 2007. Role of Src-family kinases in formation and trafficking of macropinosomes. J. Cell. Physiol. 211: 220–232. [DOI] [PubMed] [Google Scholar]
- 40.Racoosin E. L., Swanson J. A. 1992. M-CSF-induced macropinocytosis increases solute endocytosis but not receptor-mediated endocytosis in mouse macrophages. J. Cell Sci. 102: 867–880. [DOI] [PubMed] [Google Scholar]
- 41.Manning-Tobin J. J., Moore K. J., Seimon T. A., Bell S. A., Sharuk M., Alvarez-Leite J. I., de Winther M. P., Tabas I., Freeman M. W. 2009. Loss of SR-A and CD36 activity reduces atherosclerotic lesion complexity without abrogating foam cell formation in hyperlipidemic mice. Arterioscler. Thromb. Vasc. Biol. 29: 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moore K. J., Kunjathoor V. V., Koehn S. L., Manning J. J., Tseng A. A., Silver J. M., McKee M., Freeman M. W. 2005. Loss of receptor-mediated lipid uptake via scavenger receptor A or CD36 pathways does not ameliorate atherosclerosis in hyperlipidemic mice. J. Clin. Invest. 115: 2192–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.