Abstract
Objective
The presence of pulmonary hypertension (PH) historically has been considered a significant risk factor affecting early and late outcomes following valve replacement. Given the number of recent advances in the management of PH following cardiac surgery a better understanding of the impact of PH on outcomes may assist in the clinical management of these patients. The purpose of this study was to determine if pulmonary hypertension remains a risk factor in the modern era for adverse outcomes following aortic valve replacement (AVR) for aortic valve stenosis.
Methods
From January 1996 to June 2009, 1,080 patients underwent AVR for primary aortic valve stenosis, of which 574 (53%) had normal systolic pulmonary artery pressures (sPAP) and 506 (47%) had PH. PH was defined as mild (sPAP 35-44 mmHg), moderate (45-59mmHg), or severe (≥ 60mmHg). In the group of patients with PH, 204 had postoperative echocardiograms.
Results
Operative mortality was significantly higher in patients with PH (47/506, 9% versus 31/574, 5%; p=0.02). The incidence of postoperative stroke was similar (p=0.14), but patients with PH had an increased median hospital LOS (8 versus 7 days, p=0.001) and an increased incidence of prolonged ventilation (26% versus 17%, p<0.001). Preoperative PH was an independent risk factor for decreased long term survival (RR 1.7, p=0.02). Those with persistent PH postoperatively had decreased survival. Five-year survival (Kaplan-Meier) was 78 ± 6% with normal sPAP and 77 ± 7% with mild PH postoperatively, compared to 64 ± 8% with moderate PH and 45 ± 12% with severe PH (p<0.001).
Conclusion
In patients undergoing AVR, preoperative PH increased operative mortality and decreased long-term survival. Patients with persistent moderate or severe PH after AVR had decreased long-term survival. These data suggest that PH had a significant impact on outcomes in patients undergoing AVR and should be considered in preoperative risk assessment.
INTRODUCTION
Historically, patients with aortic valve stenosis and pulmonary hypertension (PH) have had worse left ventricular function, mitral regurgitation, and increased left ventricular diastolic pressure (LVEDP).1-3 Because of associated morbidities and potential for right heart compromise, PH may increase morbidity and mortality when patients with aortic valve stenosis undergo valve replacement. Small observational studies have demonstrated benefit with aortic valve replacement in patients with aortic stenosis and PH in both symptoms and 5-year survival rates when compared to medically managed patients.2-6 Replacement of the aortic valve in these patients may decrease strain on the right heart with left ventricular unloading, thus improving survival. However, there have been no large studies to date characterizing the outcomes of patients with PH in spite of the possible increased risks.
Surgery in the setting of PH may carry an increased risk compared to patients without PH, due to fixed vasculopathy and right heart failure. These comorbid conditions can make postoperative care challenging. There has not yet been sufficient evidence in the literature to define an increase in risk for patients that undergo aortic valve replacement for aortic stenosis with PH. Transcatheter approaches are currently being used to treat high-risk patients and determination of additional risk posed by PH may aid in selection of patients for intervention by surgery, transcatheter approach, or medical treatment alone. We reviewed our database of patients who underwent aortic valve replacement for aortic stenosis from 1996 through 2009 to evaluate long-term survival and to determine whether PH is a risk factor in patients undergoing aortic valve replacement for aortic stenosis.
PATIENTS AND METHODS
This retrospective study was approved by the Institutional Review Board of Washington University School of Medicine, and individual consent was waived. Between January 1, 1996, and June 30, 2009, 1,777 patients underwent aortic valve replacement with or without concomitant procedures (coronary artery bypass graft; mitral, tricuspid, or pulmonary valve repair or replacement; atrial septal defect repair; ventricular septal defect repair; or arrhythmia surgery). A total of 697 patients had a Ross procedure, endocarditis, aneurysm reconstruction, or aortic regurgitation as the primary diagnosis and these were excluded from analysis, leaving 1080 patients for review. Aortic valve replacement was performed in accordance with American College of Cardiology/American Heart Association (ACC/AHA) guidelines and indications for valve replacement in patients with aortic stenosis.7-8 Preoperative demographic information and perioperative events were stored in a computerized database. Fourteen surgeons of the Division of Cardiothoracic Surgery, Washington University in St. Louis, performed the procedures at Barnes-Jewish Hospital. Although approaches varied among surgeons, all procedures were done with cardiopulmonary bypass with mild systemic hypothermia (30°C to 34°C). Myocardial protection was achieved with cold blood cardioplegia. The procedures were performed with either a standard or partial median sternotomy. The selection of valve prosthesis type was at the discretion of the operating surgeon. There has been a strong institutional preference for biological valves in patients older than 65 years and in younger patients who want to avoid coumadin. Coronary artery bypass grafts (CABG) were performed for recognized indications in 428 patients.
Patients were evaluated prospectively for the presence PH [systemic pulmonary artery pressure (sPAP)≥ 35 mm Hg] or absence of PH (sPAP of <35mmHg) by either echocardiography or right heart catheterization. These were recorded and entered prospectively into the database. Forty-seven percent (506/1080) of patients had PH. Patients with PH were further subdivided into groups of mild (sPAP 35-44 mmHg), moderate (sPAP 45-59 mmHg) or severe (sPAP ≥60 mmHg). Mean duration of follow-up was 4.5 ± 3.5 years for patients without PH and 3.4 ± 3.2 years for patients with PH. Overall mean follow-up was 4.0 ± 3.4 years. Echocardiogram follow-up consisted of those patients who survived surgery and had postoperative echocardiograms (284/1008, 28%) at an average of 3.1 ± 2.7 years after surgery which were reviewed for assessment of sPAP.
Operative mortality was defined as death within 30 days or before discharge from the hospital. Preoperative, operative, and postoperative variables including operative mortality was compared between the early and late eras in the study: the early group underwent surgery from January of 1996 through December 31, 2002 (seven years, 172 patients) and the late group of patients had surgery from January 1, 2003 through June 30, 2009 (6.5 years, 334 patients). Continuous data are expressed as mean ± standard deviation and categorical data are expressed as counts and proportions. The Kaplan-Meier estimate was used to depict survival over time among operative survivors. The χ2 or Fisher exact univariate tests were used to analyze differences in proportions in the categorical data. Factors found to trend towards significance (gender, age, PH, isolated AVR, NYHA class 3 or 4, myocardial infarction, stroke, LVEF, systemic hypertension, diabetes, renal failure, dialysis, atrial fibrillation, hypercholesterolemia, COPD, previous CABG, body surface area, intra-aortic balloon pump at the time of surgery) by univariate testing (p ≤ 0.10) were entered into a multivariate analysis. The Cox multivariate proportional hazards regression model was used to identify independent prognostic factors for death. Relative risks (RR) with 95% confidence intervals (CI) were calculated for each of the significant risk factors. All data analysis was performed using SPSS 11.0 (SPSS Inc, Chicago, IL) for Windows (Microsoft Corp, Redmond, WA). All values of p < 0.05 were considered to be statistically significant.
RESULTS
Compared to patients with normal sPAP, patients with PH were significantly older (72 versus 70 years old, p=0.004), more often NYHA class III or IV (77% versus 59%, p<0.001), and had a prior MI(29% versus 19%, p <0.001), diabetes (37% versus 23%, p<0.001), hypertension 74% versus 69%, p=0.01), and lower LVEF (46% versus 52%, p<0.001)(Table 1). More concomitant procedures were done on patients with PH (51.9% versus 43.5%), and subsequently the cardiopulmonary bypass time was slightly longer in these patients (160 minutes versus 153 minutes, p=0.034). Patients with PH had longer ventilation times (26.1 hrs versus 16.7 hrs, p<0.001). Although they had a higher rate of cerebrovascular accident this was not statistically different (4.5% versus 2.8% , p=0.14).
Table 1.
Baseline clinical characteristics. Continuous data are presented as mean ± SD.
| Variable | AVR with PH n = 506 | AVR no PH n = 574 | p-value |
|---|---|---|---|
| Preoperative Variables | (%) | (%) | |
| Age in years | 72.3 ± 10.7 | 70.2 ± 13.5 | 0.004 |
| Male gender | 299 (59.1) | 332 (57.8) | 0.711 |
| NYHA III or IV | 390 (77.1) | 338 (58.9) | <0.001 |
| COPD | 44 (8.7) | 44 (7.7) | 0.578 |
| MI | 147 (29.1) | 111 (19.3) | <0.001 |
| Diabetes | 188 (37.2) | 129 (22.5) | <0.001 |
| Hypertension | 374 (73.9) | 384 (66.9) | 0.014 |
| LVEF | 46 ± 15 | 52 ± 13 | <0.001 |
| Previous CPB surgery | 107 (21) | 88 (15) | 0.014 |
| Operative Variables | |||
| Isolated AVR | 220 (43.5) | 298 (51.9) | 0.003 |
| Concomitant Procedures | |||
| CABG ± other | 193 | 209 | |
| CABG + MVR | 21 | 5 | |
| MV ± other | 38 | 9 | |
| Other | 34 | 52 | |
| CPB time | 160 ± 57 | 153 ± 54 | 0.034 |
| Aortic CC time | 109 ± 45 | 106 ± 40 | 0.297 |
| Postoperative Variables | |||
| Prolonged ventilation | 132 (26.1) | 96 (16.7) | <0.001 |
| CVA | 23 (4.5) | 16 (2.8) | 0.142 |
| Atrial fibrillation/flutter | 180 (36) | 201 (35) | 0.849 |
| Placement of IABP | 56 (11) | 15 (3) | <0.001 |
| Operative mortality | 47 (9.3) | 31 (5.4) | 0.018 |
| Median LOS days | 8 (6-84) | 7 (6-91) | 0.001 |
| Total Follow up (years) | 3.4 ± 3.2 | 4.4 ± 3.5 | <0.001 |
NYHA, New York Heart Association Functional Heart Failure class; COPD, chronic obstructive pulmonary disease; MI, myocardial infarction; LVEF, left ventricular ejection fraction; AVR, aortic valve replacement; CPB, cardiopulmonary bypass; CC, cross-clamp; CVA, cerebrovascular accident; LOS, length of stay. Other surgeries include tricuspid valve, pulmonary valve, atrial septal defect, ventricular septal defect, and surgery for arrhythmia.
Operative mortality was statistically higher in patients with PH (9.3% versus5.4%, p=0.018). Cox regression multivariate analysis demonstrated that mortality was adversely affected by the presence of renal failure (RR=2.5, p<0.001), diabetes (RR 1.7, p<0.001), previous cardiac surgery (RR=1.6, p=0.001), and PH (RR=1.5, p=0.002), as well as age(RR=1.04, p<0.001), and LVEF (RR=0.99, p=0.05)(Table 2).
Table 2.
Multivariate Cox Regression analysis of risk factors for decreased survival following aortic valve replacement for aortic stenosis (n=1080). Factors found to trend towards significance(gender, age, PH, isolated AVR, NYHA class III or IV, myocardial infarction, stroke, LVEF, systemic hypertension, diabetes, renal failure, dialysis, atrial fibrillation, hypercholesterolemia, COPD, previous cardiac surgery requiring cardiopulmonary bypass, body surface area) by univariate testing (p ≤ 0.10) were analyzed.
| Variable | N | Relative Risk | 95% CI | p-Value |
|---|---|---|---|---|
| Pulmonary hypertension | 506 | 1.51 | 1.160 – 1.965 | 0.002 |
| Age (years) | 1080 | 1.036 | 1.020 – 1.051 | <0.001 |
| Diabetes | 317 | 1.697 | 1.120 – 3.875 | <0.001 |
| Renal failure | 93 | 2.505 | 1.613 – 3.890 | <0.001 |
| Dialysis | 40 | 2.083 | 1.308 – 2.202 | 0.020 |
| NYHA Class III or IV | 728 | 1.507 | 1.101 – 2.061 | 0.010 |
| Left ventricular ejection fraction (%) | 876 | 0.991 | 0.983 – 1.000 | 0.049 |
| Previous cardiopulmonary bypass | 195 | 1.631 | 1.210 – 2.198 | 0.001 |
** Gender, isolated AVR, myocardial infarction, stroke, systemic hypertension, atrial fibrillation, hypercholesterolemia, COPD, BSA were not found to be significant independent predictors for lower survival following AVR for aortic stenosis in the MV model.
Evaluation of early versus late era of surgery showed that pre- and intraoperative variables were similar except for LVEF (40% ± 16% versus 48% ± 16%, p<0.001), percentage of patients that underwent isolated AVR [36%(62 patients) versus 47%(158 patients), p=0.02] cardiopulmonary bypass time (181± 56 min. versus 149 ± 57 min., p=<0.001) and cross clamp time (131 ± 47 min. versus 98 ± 47 min., p=<0.001) in the early versus late groups, respectively. Patients who had surgery in the first seven years of the study had an operative mortality of 8.7% (15/172) while those who underwent surgery in the late era it was 9.6% (32/334, p=0.8).
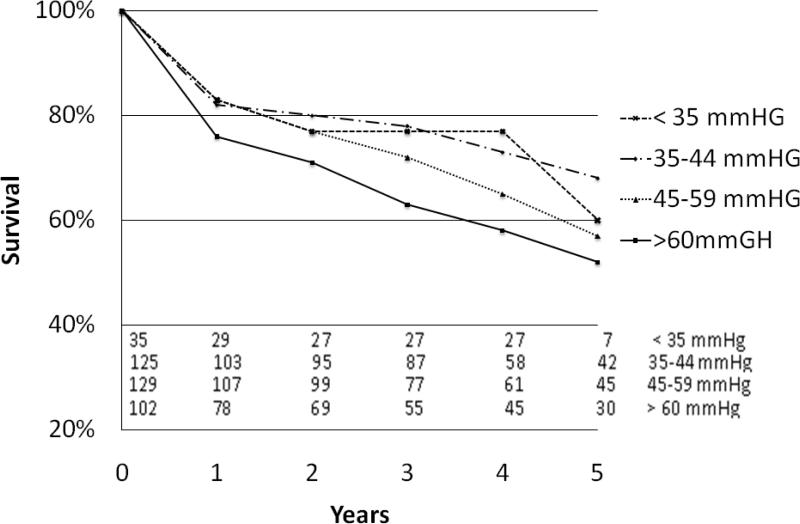
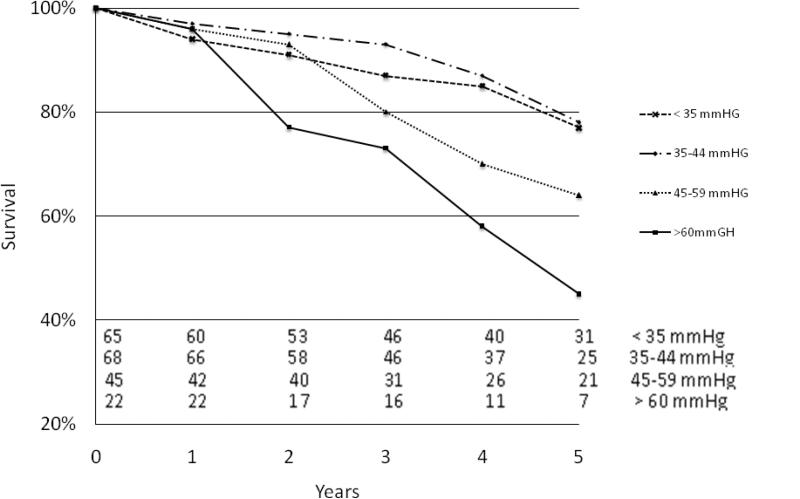
Survival was further evaluated based on severity of PH including four subgroups: no PH (sPAP of <35mm Hg, n=66), mild PH (sPAP 35-44mm Hg n=125), moderate PH (sPAP 45-59 mm Hg, n=129) and severe PH (sPAP ≥60mm Hg, n=102). Kaplan Meier analysis of patients with preoperative PH showed that survival was significantly worse in the more severe PH groups (p=0.020)(Figure 1). Similarly, analysis was completed using measurements of patients’ postoperative sPAP, demonstrating that 5-year survival was diminished in those with more severe PH versus those with mild or no PH (45% versus 78%, p<0.001)(Figure 2).
Figure 1.
Kaplan-Meier survival curve based on preoperative pulmonary artery pressure. Number at each time point depicted at bottom of graph.
Figure 2.
Kaplan-Meier survival curve based on postoperative pulmonary artery pressure. Number at each time point depicted at bottom of graph.
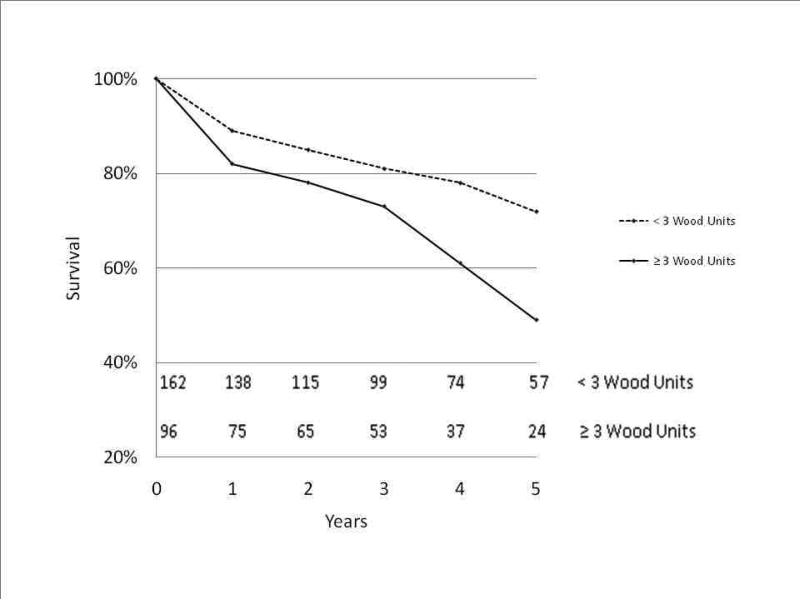
Evaluation of patients with high pulmonary vascular resistance was done by calculating the pulmonary vascular resistance using PAP, left ventricular end diastolic pressure(LVEDP) and cardiac output (CO): (mean PAP – LVEDP)/(CO) and then evaluating survival comparing patients with pulmonary vascular resistance of < 3 Wood units versus those with ≥ 3 Wood units. Kaplan-Meier analysis showed that survival was decreased in those with higher pulmonary vascular resistance (5-year survival 49% versus 72%, p=0.001)(Figure 3).
Figure 3.
Kaplan-Meier survival curve based on preoperative pulmonary artery vascular resistance. Number at each time point depicted at bottom of graph.
Patients with mild PH showed no improvement in sPAP when comparing postoperative measurements to preoperative(p=0.22)(Table 3). In contrast, sPAP fell after aortic valve replacement in patients with moderate PH (51± 4 mm Hg versus 45 ± 16 mm Hg, p=0.01), with and even more substantial fall in patients with severe PH (69 ± 12 mm Hg versus 45 ± 14 mm Hg, p<0.001)(Table 3).
Table 3.
Change in systolic pulmonary artery pressure after aortic valve replacement in patients with mild, moderate, and severe pulmonary hypertension.
| Group | Mean Pre-op sPAP | Mean Post-op sPAP | p |
|---|---|---|---|
| 35-44 mm/Hg (n=61) | 39 ± 3 | 37 ± 11 | 0.218 |
| 45-59 mm/Hg (n=58) | 51 ± 4 | 45 ± 16 | 0.014 |
| ≥60 mm/Hg (n=46) | 69 ± 12 | 45 ± 14 | <0.001 |
| Overall Group | 51 ± 14 | 42 ± 14 | <0.001 |
To eliminate potential conflicting impact of associated imtral valve and other valvular diseases, we repeated the analysis, looking specifically at patients who underwent only AVR or AVR + CABG, excluding all the patients who had concomitant valve surgery. In this group of patients (n=978 patients) we found similar changes: operative mortality was 5.2% (28/542) in those with no pulmonary hypertension, while for those with PH it was 8.0% (35/436, p=0.08). The fall in sPAP for those with PH was similar to that for the overall group as well (preoperative sPAP 50.1mm Hg ± 13.5 mm Hg fell postoperatively to 41.1 mm Hg ±13.5 mm Hg).
DISCUSSION
Pulmonary hypertension is common among patients who undergo aortic valve replacement for stenosis.4, 6, 9 In this study, PH was present in 47% of patients who underwent AVR. In spite of this, there have been no large scale studies which have delineated the risks of this comorbidity or what the outcomes are after AVR. Furthermore, the presence of any PH is not considered in major preoperative risk assessment tools available. The EuroSCORE uses only the presence of severe PH (PAP > 60 mm Hg) and the STS risk calculator does not consider PH at all. 10-12
There have been several small observational studies which have shown improvement in outcome for patients that underwent AVR with PH compared to medically treated patients. Malouf and associates compared 37 patients with severe PH who underwent aortic valve replacement to a group of 10 similar patients who were treated medically. In that study there was improved survival at 5 years (52% versus 20%).3 In another retrospective study by Pai and colleagues, 36 patients with severe aortic stenosis and PH underwent surgery and were compared to 83 who were treated medically.5 After adjustment for comorbidities, the authors found an improvement in 5-year survival (65% versus 20%) compared to the nonoperative group. These studies demonstrated better outcomes in patients undergoing valve replacement versus medical therapy alone, however the number of patients in these studies was small and comparison between groups was difficult because many of the medically treated patients were likely deemed inoperable due to comorbidities. In a study of 92 patients by Johnson and coauthors, thirteen of fifteen patients with severe PH underwent AVR for stenosis and there was no operative mortality.2 In a study by Tracy, thirty-seven of fifty-two (70%) patients undergoing AVR for AS had PH, with an operative mortality of only 1.9%.4 From these small studies it was difficult to draw adequate conclusions about operative mortality in patients with PH. In the current report, operative mortality was nearly twice as high when PH was present (9.3% versus 5.4%), similar to previous reports.10 Because survival is influenced by the presence of PH, and as over 200,000 aortic valve replacements are done annually worldwide, determination of its incremental risk is warranted.11
Historical studies have shown that patients with PH have worse outcomes after AVR for aortic stenosis. Copeland and others showed in an early report of 1,127 patients undergoing isolated AVR that the presence of mean PAP of ≥ 30 mm Hg (n=173) decreased long-term survival compared to those with a mean PAP < 30 (n=492, p=0.006).9 We similarly found that in the presence of preoperative PH, long-term survival is compromised. In this study, the degree of PH affected adversely the long-term survival, as those with moderate and severe PH had only 57% and 52% five-year survival while those with no or mild PH had 68% and 62% five-year survival. In addition, those with persistent postoperative moderate or severe PH also had even worse five-year survival (45%-64%) compared to those without or with mild postoperative PH (77% -78%, Figure 1).
Advances in the treatment of PH such as prostacyclin, intravenous milrinone, inhaled nitric oxide, sildenafil, and other modalities have ameliorated some of the physiologic consequences of PH.12-14 Almost half of patients undergoing surgery for aortic stenosis have PH (in both this and other studies), 4, 6, 9 and therefore continued efforts to improve the treatment of these patients is warranted. Our current first line treatment strategy for patients with PH is typically inhaled epoprostenol with concurrent inotropic support as the intraoperative or postoperative hemodynamics warrant (i.e. elevated pulmonary artery pressures and evidence of or concern for right heart failure). Inhaled nitric oxide is not used as a first line agent despite its efficacy due to cost considerations. Inhaled pulmonary vasodilator therapy is usually continued for the first 24 hours and then weaned as right heart function tolerates. Inotropic therapy is usually maintained until pulmonary vasodilator therapy is weaned substantially or discontinued. Rarely, chronic oral medications are administered (i.e. sildenafil or bosentan) and usually only in patients previously taking these drugs. These treatment strategies are used for management of PH in general and are not unique to patients undergoing AVR as little scientific evidence is available on improvement in outcomes after AVR. A transcatheter approach for the treatment of aortic stenosis is being performed for high risk patients at several centers throughout the world. The mortality rate for this procedure was higher in the presence of PH in one study: in 345 procedures on 339 patients, the relative risk of mortality within 30 days was 2.1 for patients with PH.15 PH has also been found to be a risk factor in octogenarians undergoing cardiac operations.16 Preoperative risk assessment is an important part of the decision-making process of whether patients should undergo a surgical or transcatheter valve implantation procedure for aortic stenosis. Although it has been shown that high risk patients can successfully undergo surgery, including the elderly and patients with other comorbidities,17-19 an accurate risk assessment is critical to an informed discussion between the surgeon and patient so that appropriate decisions can be made as to whether an intervention should be performed and which approach should be undertaken. The current study demonstrated that operative mortality nearly doubled in the presence of PH, and 5-year survival was significantly diminished in those with severe preoperative PH versus those with mild or no PH (52% versus 60-68% respectively).
Multiple factors can contribute to and influence pulmonary artery pressures, including pulmonary vascular resistance, right heart function, and left ventricular systolic and diastolic function. It remains unclear whether some underlying causes or consequences of pulmonary hypertension may contribute more to the adverse outcomes observed. For example, elevated left ventricular end diastolic pressure has also been shown to be an independent risk factor in coronary cardiac surgery.20 In this study it was demonstrated that survival was decreased in the presence of PVR. Pulmonary vascular resistance is another measurement which is often used to describe the severity of the pulmonary vascular disease in patients with PH. The equation which describes it is PVR = (mean PAP – PAWP (LVEDP) / CO) X 80 (this give PVR in dyn-s/cm5) versus the Wood Unit which is (mean PAP – PAWP / CO). Those with PVR more than 3 Wood Units had 49% 5-year survival versus those with normal PVR who had 72% five-year survival. The presence of increased PVR clearly portends a worse outcome. The different causes of PH (e.g. whether it is due to cardiac disease or whether it is from pulmonary pathology) may influence outcomes differently. However, elevated PVR is sometimes due to intrinsic lung vascular disease, but can also be an effect from long-term elevated end diastolic pressures in the left ventricle. This current study evaluated a heterogeneous group of patients with PH from multiple causes, likely including some individuals with PH due to elevated left ventricular end-diastolic pressure as well as other patients who had PH due to lung disease or to intrinsic pulmonary vascular disease. Further studies need to be done to elucidate which preoperative variables associated with PH, including etiology, impact survival. The presence of eleveated PVR likely plays a critical role.
LIMITATIONS
Retrospective studies have inherent limitations. Follow-up was not complete in all 1080 patients. However there were sufficient numbers included in the study to determine outcomes in groups both with and without PH. Several surgeons were involved during this thirteen year period, which could increase the variability of the data. The large number of surgeons would, however, limit the effect that surgeon-dependent factors might have had on outcomes. Echocardiography studies used estimations of PAP while the catheterization methods used direct measurements; the estimation method may have decreased the overall accuracy of the PAP measurements. Furthermore, echocardiography and catheterization data was not collected on every patients due to the large number of patients over the extended study period. This could introduce selection bias for sicker patients who may undergo more extensive studies such as echocardiography or catheterization allowing identification of PH more often in this group of patients with more comorbidities.
Inclusion of patients with mitral valve disease severe enough to warrant surgical intervention may be considered a confounding factor. However, analysis of mortality and major morbidity after excluding this group of patients yielded results very similar to the analysis of those who had mitral valve disease. As the etiologies of PH are likely multifactorial in many cases, inclusion of these patients better represents the real-world scenario of those who present with need for surgery for severe aortic stenosis. Further studies are needed to elucidate which causative factors of PH have the highest impact on outcome.
SUMMARY
In patients undergoing aortic valve replacement for aortic stenosis, PH increased operative and long-term mortality. Earlier intervention—before irreversible changes in the pulmonary circulation can occur—should be considered for asymptomatic or minimally symptomatic patients with significant AS and moderate to severe PH. It is important for surgeons to consider PH in preoperative risk assessment of patients undergoing AVR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Faggiano P, Antonini-Canterin F, Ribichini F, D'Aloia A, Ferrero V, Cervesato E, et al. Pulmonary artery hypertension in adult patients with symptomatic valvular aortic stenosis. Am J Cardiol. 2000 Jan 15;85(2):204–8. doi: 10.1016/s0002-9149(99)00643-8. [DOI] [PubMed] [Google Scholar]
- 2.Johnson LW, Hapanowicz MB, Buonanno C, Bowser MA, Marvasti MA, Parker FB., Jr. Pulmonary hypertension in isolated aortic stenosis. Hemodynamic correlations and follow-up. J Thorac Cardiovasc Surg. 1988 Apr;95(4):603–7. [PubMed] [Google Scholar]
- 3.Malouf JF, Enriquez-Sarano M, Pellikka PA, Oh JK, Bailey KR, Chandrasekaran K, et al. Severe pulmonary hypertension in patients with severe aortic valve stenosis: clinical profile and prognostic implications. J Am Coll Cardiol. 2002 Aug 21;40(4):789–95. doi: 10.1016/s0735-1097(02)02002-8. [DOI] [PubMed] [Google Scholar]
- 4.Tracy GP, Proctor MS, Hizny CS. Reversibility of pulmonary artery hypertension in aortic stenosis after aortic valve replacement. Ann Thorac Surg. 1990 Jul;50(1):89–93. doi: 10.1016/0003-4975(90)90095-n. [DOI] [PubMed] [Google Scholar]
- 5.Pai RG, Varadarajan P, Kapoor N, Bansal RC. Aortic valve replacement improves survival in severe aortic stenosis associated with severe pulmonary hypertension. Ann Thorac Surg. 2007 Jul;84(1):80–5. doi: 10.1016/j.athoracsur.2007.02.094. [DOI] [PubMed] [Google Scholar]
- 6.Snopek G, Pogorzelska H, Zielinski T, Rajecka A, Korewicki J, Biederman A, et al. Valve replacement for aortic stenosis with severe congestive heart failure and pulmonary hypertension. J Heart Valve Dis. 1996 May;5(3):268–72. [PubMed] [Google Scholar]
- 7.Bonow RO, Carabello BA, Kanu C, de Leon AC, Jr., Faxon DP, Freed MD, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 2006 Aug 1;114(5):e84–231. doi: 10.1161/CIRCULATIONAHA.106.176857. [DOI] [PubMed] [Google Scholar]
- 8.Bonow RO, Carabello B, de Leon AC, Jr., Edmunds LH, Jr., Fedderly BJ, Freed MD, et al. Guidelines for the management of patients with valvular heart disease: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients with Valvular Heart Disease). Circulation. 1998 Nov 3;98(18):1949–84. doi: 10.1161/01.cir.98.18.1949. [DOI] [PubMed] [Google Scholar]
- 9.Copeland JG, Griepp RB, Stinson EB, Shumway NE. Long-term follow-up after isolated aortic valve replacement. J Thorac Cardiovasc Surg. 1977 Dec;74(6):875–89. [PubMed] [Google Scholar]
- 10.Copeland JG, Griepp RB, Stinson EB, Shumway NE. Isolated aortic valve replacement in patients older than 65 years. JAMA. 1977 Apr 11;237(15):1578–81. [PubMed] [Google Scholar]
- 11.Brown JM, O'Brien SM, Wu C, Sikora JA, Griffith BP, Gammie JS. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J Thorac Cardiovasc Surg. 2009 Jan;137(1):82–90. doi: 10.1016/j.jtcvs.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 12.De Wet CJ, Affleck DG, Jacobsohn E, Avidan MS, Tymkew H, Hill LL, et al. Inhaled prostacyclin is safe, effective, and affordable in patients with pulmonary hypertension, right heart dysfunction, and refractory hypoxemia after cardiothoracic surgery. J Thorac Cardiovasc Surg. 2004 Apr;127(4):1058–67. doi: 10.1016/j.jtcvs.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 13.Klodell CT, Jr., Morey TE, Lobato EB, Aranda JM, Jr., Staples ED, Schofield RS, et al. Effect of sildenafil on pulmonary artery pressure, systemic pressure, and nitric oxide utilization in patients with left ventricular assist devices. Ann Thorac Surg. 2007 Jan;83(1):68–71. doi: 10.1016/j.athoracsur.2006.08.051. discussion. [DOI] [PubMed] [Google Scholar]
- 14.Trachte AL, Lobato EB, Urdaneta F, Hess PJ, Klodell CT, Martin TD, et al. Oral sildenafil reduces pulmonary hypertension after cardiac surgery. Ann Thorac Surg. 2005 Jan;79(1):194–7. doi: 10.1016/j.athoracsur.2004.06.086. discussion -7. [DOI] [PubMed] [Google Scholar]
- 15.Rodes-Cabau J, Webb JG, Cheung A, Ye J, Dumont E, Feindel CM, et al. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: acute and late outcomes of the multicenter Canadian experience. J Am Coll Cardiol. 2010 Mar 16;55(11):1080–90. doi: 10.1016/j.jacc.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 16.Kirsch M, Guesnier L, LeBesnerais P, Hillion ML, Debauchez M, Seguin J, et al. Cardiac operations in octogenarians: perioperative risk factors for death and impaired autonomy. Ann Thorac Surg. 1998 Jul;66(1):60–7. doi: 10.1016/s0003-4975(98)00360-9. [DOI] [PubMed] [Google Scholar]
- 17.Melby SJ, Zierer A, Kaiser SP, Guthrie TJ, Keune JD, Schuessler RB, et al. Aortic valve replacement in octogenarians: risk factors for early and late mortality. Ann Thorac Surg. 2007 May;83(5):1651–6. doi: 10.1016/j.athoracsur.2006.09.068. discussion 6-7. [DOI] [PubMed] [Google Scholar]
- 18.Florath I, Rosendahl UP, Mortasawi A, Bauer SF, Dalladaku F, Ennker IC, et al. Current determinants of operative mortality in 1400 patients requiring aortic valve replacement. Ann Thorac Surg. 2003 Jul;76(1):75–83. doi: 10.1016/s0003-4975(03)00341-2. [DOI] [PubMed] [Google Scholar]
- 19.Vaquette B, Corbineau H, Laurent M, Lelong B, Langanay T, de Place C, et al. Valve replacement in patients with critical aortic stenosis and depressed left ventricular function: predictors of operative risk, left ventricular function recovery, and long term outcome. Heart. 2005 Oct;91(10):1324–9. doi: 10.1136/hrt.2004.044099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sastry P, Theologou T, Field M, Shaw M, Pullan DM, Fabri BM. Predictive accuracy of EuroSCORE: is end-diastolic dysfunction a missing variable? Eur J Cardiothorac Surg. 2010 Feb;37(2):261–6. doi: 10.1016/j.ejcts.2009.05.059. [DOI] [PubMed] [Google Scholar]