Abstract
We have previously reported that a 30 s ethanol (10 and 100 mM) pre-exposure significantly enhanced EtOH inhibition of N-methyl-d-aspartate-induced peak currents (INMDA) in primary cultured cerebellar granule cells (CGC)s. The purpose of this study was to determine if intracellular factors play a role in ethanol pre-exposure-enhanced inhibition of INMDA and if so, to identify the intracellular target(s) mediating this effect. Ethanol pre-exposure-enhanced inhibition was reduced when ethanol was present intracellularly prior to the initiation of the pretreatment protocol. Similar to results acquired with the whole-cell configuration, ethanol pre-exposure-enhanced inhibition of INMDA was also observed in the perforated patch (PP)-clamp mode. Collectively, these results suggest an intracellular target not easily dialyzed from the cell. That perturbation of the actin cytoskeleton was responsible for the ethanol pre-exposure-enhanced inhibition of INMDA was supported by the observation that the intracellular presence of the actin stabilizer phalloidin prevented ethanol pre-exposure-enhanced inhibition. Similar to the effects of ethanol, the depolymerizing agent latrunculin A inhibited INMDA after a 30 s pretreatment exposure with full recovery of receptor function after washout of the drug. Furthermore, latrunculin A occluded the enhanced inhibition of INMDA by ethanol pre-exposure for both 10 and 100 mM ethanol. The microtubule depolymerizing agent taxol had no affect on ethanol pretreatment-enhanced inhibition of INMDA. Confocal microscopy with phalloidin-FITC indicated that F-actin filaments in neurites were depolymerized after a 30 s treatment of either latrunculin A or 100 mM ethanol. Our observations indicate that ethanol inhibition of NMDAR function may involve perturbation of the actin cytoskeleton.
Keywords: NMDA receptor, Cerebellar granule cells, Cytoskeleton, Ethanol pretreatment-enhanced inhibition, F-actin depolymerization, Confocal microscopy
Introduction
The N-methyl-d-aspartate receptor (NMDAR) is a ligand gated cation channel that is activated by endogenous excitatory amino acids and the co-agonist glycine. The function of this receptor is modified by many endogenous and exogenous substances (for review see McBain and Mayer, 1994) such as ethanol (Lima-Landman and Albuquerque, 1989; Lovinger et al, 1989; Simson et al., 1991). Ethanol readily traverses the cell membrane and affects ethanol-sensitive intracellular targets that also modulate NMDAR function. Protein kinase C (PKC) (Snell et al., 1994), Fyn tyrosine kinase (Miyakawa et al., 1997) and tyrosine phosphatases (Alvestad et al., 2003) have been implicated in affecting the ethanol sensitivity of native NMDARs. Scaffolding proteins such as RACK1, (Yaka et al., 2003); cytoskeletal-associated proteins such as α-Actinin-2 (Anders et al., 2000) and filamentous actin (F-actin) (Offenhauser et al., 2006) can also alter the ethanol sensitivity of recombinant and native NMDARs.
We have previously reported a phenomenon observed in primary cultured cerebellar granule cells (CGC)s in which ethanol inhibition of NMDAR function is enhanced following a 30 s ethanol pre-exposure prior to the co-application of ethanol and agonists (Popp et al., 1999a). Pronounced inhibition was observed at ethanol concentrations previously reported to result in only a small degree of NMDAR inhibition (5 and 10 mM). The effects by ethanol at extracellular NMDAR sites should equilibrate within micro- to milliseconds. Therefore, it is possible that enhanced inhibition due to ethanol pre-exposure could be attributed to actions on an intracellular target that then modulates NMDAR function in a manner that enhances receptor inhibition.
We observed that intracellular application of ethanol prior to ethanol pretreatment attenuated enhanced inhibition of NMDAR function. Pretreatment-enhanced inhibition was observed during perforated patch-clamp (PP) recording. Results from these experiments suggested that the enhanced inhibition of NMDAR function could be due to an intracellular ethanol-sensitive target that is not readily dialyzed from the cell. It is well documented that actin depolymerization results in attenuation of NMDAR function (Rosenmund and Westbrook, 1993). Furthermore, destabilization of the actin cytoskeleton by ethanol has been observed in astroglial cells (Allansson et al., 2001; Guasch et al., 2003) and decreases in the intoxicating affects by ethanol as well as decreases in the inhibitory actions by ethanol on neuronal NMDAR function have been reported in knockout mice with altered actin dynamics (Offenhauser et al., 2006). Therefore, we hypothesized that an intracellular component was responsible for ethanol pre-exposure-enhanced inhibition of NMDAR function and that this target could involve the actin cytoskeleton. Results obtained with electrophysiological recordings and confocal microscopy indicate that actin depolymerizing and stabilizing agents could mimic or occlude ethanol pretreatment-enhanced inhibition of NMDAR function. Data from these studies are the first to show that physiologically relevant concentrations of ethanol (10 mM) result in F-actin depolymerization in primary cultured neurons. Furthermore, our results strongly suggest that ethanol-induced actin depolymerization is responsible for the enhanced inhibition observed with the ethanol pretreatment protocol. The significantly increased ethanol sensitivity of the NMDAR we observe due to ethanol pretreatment could be a method of intoxication because this paradigm mimics steady-state ethanol levels in vivo.
Materials and Methods
Preparation and maintenance of CGC cultures
Ethanol (190 proof) was purchased from AAper Alcohol and Chemical Company (Shelbyville, KY). All drugs were purchased from Sigma-Aldrich Company (St. Louis, MO) unless otherwise noted. CGC cultures were prepared from 6-8 day-old Sprague Dawley rat cerebellum. This method has been previously published in detail (Popp et al., 1999a) and all procedures were approved by the institutional Animal Care and Use Committee and conducted in accordance to “Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council, National Academy Press, 2003). We have previously reported that the ethanol sensitivity (10, 25, 50 and 100 mM) of NMDARs contained in CGCs between 6 days in vitro (DIV) and 6 weeks in vitro is unaffected by age in culture with the simultaneous ethanol application protocol as well as with the ethanol pretreatment application protocol (10 and 100 mM ethanol) (Popp et al., 1999a). Therefore, for the current experiments neurons 6-40 DIV were used.
Labeling the Actin Cytoskeleton
CGCs were seeded at 550,000 cells / ml in 35 mm culture dishes containing glass coverslips. Individual dishes of CGCs ranging between 9 and 19 DIV were exposed to the following drug exposures: Control, 30 s 100 mM ethanol, 30 s 20 nM latrunculin A, 30 s 0.002% DMSO, 2.5 min 100 mM ethanol followed by a 30 s wash with external medium and 2.5 min 20 nM latrunculin A followed by a 30 s wash with external medium. Drug treatment was terminated by aspiration of the medium. The Cells were rinsed with 1 x PBS with calcium and magnesium and fixed in 3.7% Formaldehyde (Polysciences Inc., Warrington, PA) for 5 min. Coverslips were removed from the culture dishes and stored in PBS at 4°C for later use.
Stock solutions of Phalloidin-FITC and 4′,6-Diamidino-2-phenylindole (DAPI) (Molecular Probes, Eugene, OR) were made in DMSO and water, respectively. Nuclei were labeled with the nuclear stain DAPI (300 nM in PBS) to facilitate identification of CGC fields, but immunofluorescence for the nucleus was not included in the figures. F-actin was labeled with the fluorescent phallotoxin derivative Phalloidin-FITC [5 μg / ml]. Coverslips were mounted on glass slides in the mounting medium Mowiol 40-88 that contained the anti-fade agent DABCO. Cells were examined with an Olympus IX-71 inverted confocal microscope (Olympus America, Inc., Center Valley, PA) equipped with argon/krypton and blue diode lasers and Fluoview FV300 (Olympus America, Inc.) software (version 5.0). Images were obtained with a 100 × /1.35 oil-immersion objective lens using the Argon 488 laser to excite FITC fluorescence. Single optical sections through individual cells are presented. Images were prepared with Adobe Photoshop 7.0 software (Adobe Systems Incorporated, San Jose, CA).
Characterization and quantification of depolymerized F-actin in CGCs
To assess depolymerization of F-actin, images of multiple fields (3 - 8) from each coverslip were acquired as described in the preceding section. All images were saved as 8-bit grayscale images and imported into MetaMorph (version 6.3) software (Molecular Devices, Downingtown, PA) for Integrated Morphometry Analysis (IMA). Each image was thresholded to select and highlight pixels representing the phalloidin-FITC labeled cytoskeleton. Unlike primary cultured hippocampal neurons, cultured CGCs do not contain an abundance of dendritic spines (Palay and Chan-Palay, 1974). Therefore, we could not assess alterations in the actin cytoskeleton by changes in spine number as indicated by decreases in punctuated fluorescent intensity. However, others have characterized actin depolymerization in cultured cells lacking spines (Bar-Ziv et al., 1999). Actin depolymerization leads to the development of “pearls” along spineous processes, so named because the appearance resembles a string of pearls (Bar-Ziv et al., 1999). Pearls were initially characterized from image fields from a latrunculin A treated cover slip. The teach mode was used to identify pearls as regions of interest (ROI) and were characterized by width and breadth (μm / pixel). This maneuver was performed five to six times and a range of width (1.3 – 3.75) and length (2.25 – 7.0) values was determined for individual pearls. It has been noted that pearl number increases with F-actin depolymerization (Bar-Ziv et al., 1999). Individual neurites were traced to create an ROI and with the length and breadth filters set to the above criteria, pearls could be identified within the ROI. Only neurites attached to somas were measured. Using the IMA module, the length and breadth of each ROI was measured and data was logged into an excel file. Both the image acquisition and the pearl analysis were performed such that the experimenter was blind to the treatment group. Data was obtained from nine culture batches.
Whole-cell patch-clamp recordings
Detailed methodology has been previously published (Popp et al., 1999a). Briefly, culture dishes were placed on the stage of an inverted microscope with epifluorescent capabilities (NIKON Inc., Melville, NY) and superfused at 1-2 ml / min with external medium (150 mM NaCl, 5 mM KCl, 2.5 mM CaCl, 10 mM HEPES, 10 mM d-glucose and 200 nM tetrodotoxin (Alomone Labs Ltd., Jerusalem, ISRAEL), pH adjusted to 7.4 with NaOH and osmolality adjusted to 333-336 mmol/kg with sucrose. All recordings were performed at room temperature using the Axopatch 200 patch-clamp or Axopatch 200B amplifier (Axon Instruments, Union City, CA). The internal patch electrode solution consisted of: 100 mM N-methyl-d-glucamine, 100 mM MeSO3, 40 mM CsF, 10 mM HEPES, 1 mM MgCl2, 3 mM Lidocaine N-ethyl bromide and 5 mM EGTA; pH adjusted to 7.4 with CsOH and osmolality adjusted to 314-317 mmol / kg with sucrose. For experiments in which 10 or 100 mM ethanol was included in the internal recording solution, both pH and osmolality were adjusted after addition of the appropriate concentration of ethanol. A similar strategy was used for adjusting the pH and osmolality of the internal solution in the experiments in which taxol (Molecular Probes) or phalloidin were included in the patch electrode. A 1.2 mM stock solution of phalloidin was made with DMSO, aliquoted and stored at −20° C. The working concentration of phalloidin in the patch electrode was 1 μM (Rosenmund and Westbrook, 1993) and 0.1% for DMSO. Stock taxol (5 mM) aliquots and latrunculin A (1 and 10 mM) were similarly made and stored. The working concentration in the patch electrode was 5 μM for taxol and 0.1 % for DMSO (Rosenmund and Westbrook, 1993). For experiments requiring latrunculin A, the working concentration in external media was 20 ng/ml and 0.005 % for DMSO.
Perforated patch-clamp recordings
These methods have been previously described in detail (Popp et al., 1999a). Briefly, in PP experiments both the patch electrode and external solutions were the same as those used in the whole-cell (WC) patch-clamp experiments with the exception that amphotericin B was added to the internal from a stock solution of 5 mg / 100 μl in DMSO. The stock solution was prepared fresh and diluted 100 fold into the patch-electrode solution so as to permeabilize the membrane patch at the tip of the electrode. The holding potential in all WC or PP experiments was −60 mV.
Drug solutions and solution application
In all experiments drugs were dissolved in the external medium and delivered by gravity from solution-containing reservoirs placed above the preparation, gated by plastic stopcocks and connected to a linear array of microcapillary tubes (0.32 mm inner diameter). The array was moved manually to apply different solutions to cells. We have measured the solution exchange rate by switching agonist application with the external medium described above to one containing an impermeant ion, N-methyl-d-glutamine (Zhou et al., 1998). With this system, our solution exchange rate was 168.2 ± 11.8 ms and this was faster than the time to reach peak current amplitudes (230.2 ± 7.8 ms). Data was low pass filtered at 1 kHz with a 3-pole Bessel filter (Axopatch 200 amplifier) or an 8-pole Bessel filter (Axopatch 200B amplifier) at a sampling rate of 10 kHz with DigiData 1200A and pClamp software 5.5 or 9.2 (Axon Instruments).
The agonists used in all experiments were 100 μM NMDA and 10 μM glycine dissolved in external medium. Two types of ethanol application protocols were used: simultaneous ethanol application (simultaneous protocol) and pretreatment ethanol application (pretreatment protocol). In the simultaneous protocol, ethanol was co-applied with the agonists for five seconds, whereas in the pretreatment protocol, the neuron being patch-clamped was bathed in ethanol for 30 s prior to the co-application of an identical concentration of ethanol and agonists (five seconds). Identical drug application protocols were used for latrunculin A in the absence of ethanol: simultaneous latrunculin A, pre-treatment latrunculin A. In experiments designed to assess the affect of latrunculin A on the inhibition of INMDA with different ethanol application protocols, 20 ng / ml of latrunculin A was present in the external medium during the entire experiment. Changes in peak current amplitudes (indicated as INMDA) in the presence of ethanol were compared against control values (no ethanol present). Values were the mean peak current amplitude obtained from several ethanol applications that had been normalized to mean NMDA peak current amplitudes obtained before and after a specific ethanol application. Peak current amplitudes were normalized to capacitance (pA / pF) for experiments characterizing the affects of latrunculin A on INMDA in the absence of ethanol and all statistical analyses were performed on the normalized currents. Only current traces in which a stable baseline was maintained throughout the experiment or there was no change in series resistance were analyzed. Cells were patch-clamped for no more than 40 min and in most instances experiments were completed within 30 min.
Statistics for Electrophysiological Experiments
Data values are expressed as mean ± s.e.m. Since it is well established that an increase in ethanol concentration results in increasing inhibition of NMDAR function, this variable was not included in any of the subsequent analyses. All statistical analyses were done within each ethanol concentration (10 or 100 mM). Paired t test was used to assess the affect of ethanol application protocol (simultaneous or pretreatment) on percent inhibition of control NMDA-induced peak currents (INMDA) by ethanol for data from the internal ethanol, PP, taxol and phalloidin experiments. Paired t test was also used to determine the effect of latrunculin A pre-exposure on INMDA as well as the effect of latrunculin A on ethanol (10 or 100 mM) inhibition of INMDA with the simultaneous protocol. One-way ANOVA ascertained the effect of simultaneous application of latrunculin A on pre- and post-INMDA. The effect of application protocol (simultaneous or pretreatment) and drug treatment (with or without latrunculin A) on ethanol inhibition of INMDA was determined by a two-way ANOVA for two ethanol concentrations (10 and 100 mM). The Multiple t Test was the post hoc analysis used to determine individual group differences when F values resulted in P ≤ 0.05.
Statistics for Confocal Experiments
Data values are expressed as mean ± s.e.m. One-way ANOVA determined significant differences in total pearl number, pearl number per neurite, pearl width and pearl breadth attributable to drug treatment: Control, 30 s 100 mM ethanol, 30 s 20 nM latrunculin A, 30 s 0.002% DMSO, 2.5 min 100 mM ethanol followed by a 30 s wash with external medium and 2.5 min 20 nM latrunculin A followed by a 30 s wash with external medium. The Multiple t Test was the post hoc analysis used to determine individual group differences when F values resulted in P ≤ 0.05.
Results
Intracellular locus of non-diffusible factors involved in ethanol pretreatment-enhanced inhibition of INMDA
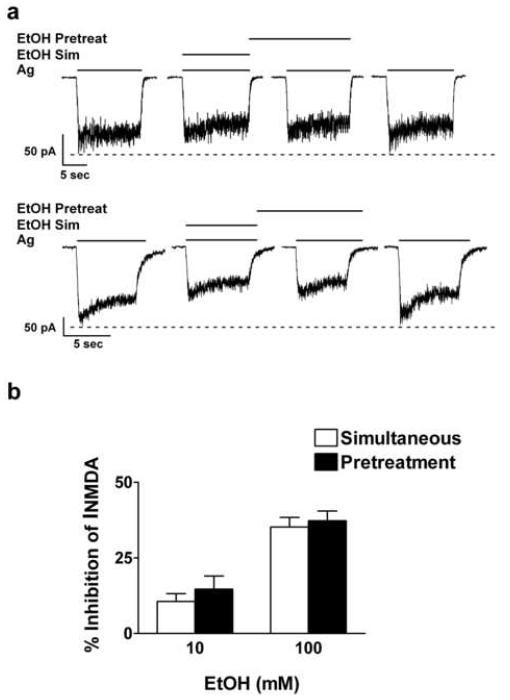
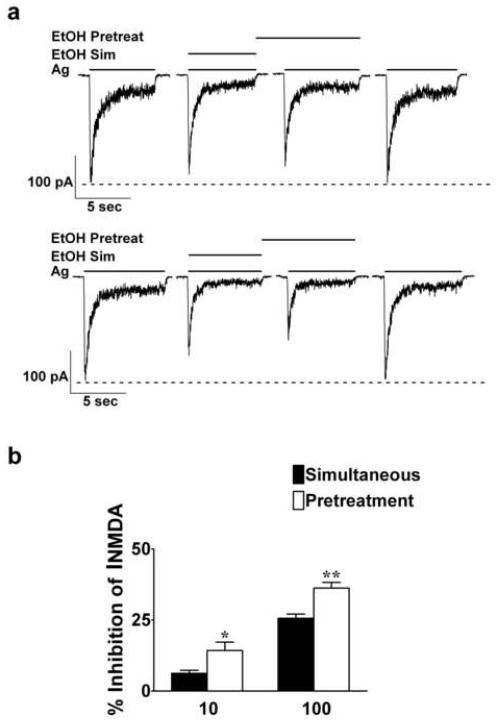
The on / off rate of ethanol is on the order of milliseconds (Wirkner et al., 2000), as expected for an interaction with low affinity. Thus, it is unlikely that the ethanol pretreatment effect could be attributed to a difference in ethanol binding affinities for the NMDAR or slow equilibration at an extracellular site of action. Furthermore, it has been reported that a 30 s 100 mM ethanol exposure prior to agonist application without ethanol results in no attenuation of agonist-induced currents (Wirkner et al., 2000). Therefore, to test our hypothesis that ethanol pretreatment-enhanced inhibition of INMDA is due to the activation of an intracellular target, we introduced ethanol into the cell prior to the application of ethanol and agonists. Under normal recording conditions (no ethanol in the patch solution), ethanol (10 and 100 mM) inhibition of INMDA with the pretreatment protocol was similar to previously published values (Popp et al., 1999a) (Figure 1). Inhibition of INMDA increased from 10.3 ± 1.9 to 22.8 ± 2.1 % (t = 7.9, df = 43; P ≤ 0.001) and from 28.4 ± 1.5 to 38.2 ± 2.0 % (t = 4.4, df = 43; P ≤ 0.001) for 10 and 100 mM ethanol, respectively. However, application of ethanol intracellularly prior to the initiation of the pretreatment protocol blocked the enhanced inhibition of INMDA normally observed with this protocol (Figures 2a and b). Statistical analysis using the paired t test indicated that when intracellular ethanol was present, inhibition of INMDA did not differ between the two ethanol application protocols: inhibition of INMDA was 10.6 ± 2.6 and 14.7 ± 4.3; t = 1.57, df = 9; P ≥ 0.1 for 10 mM ethanol simultaneous and pretreatment, respectively. Similar results were observed with 100 mM ethanol in that inhibition of INMDA with the simultaneous protocol was 35.2 ± 3.2 and 36.9 ± 3.1 for the pretreatment protocol; t = 0.57, df = 10; P ≥ 0.6. The intracellular presence of ethanol did not alter the direct inhibitory actions by ethanol on INMDA with the simultaneous protocol, an observation previously reported by Peoples and Stewart (2000). The results from these experiments support the premise that ethanol pretreatment-enhanced inhibition of INMDA is due to actions at an intracellular target in addition to the direct action by ethanol on NMDAR function.
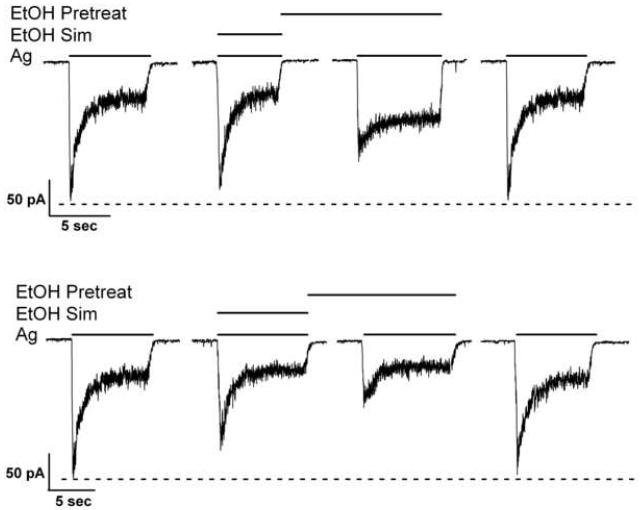
Fig. 1.
Ethanol pretreatment-enhanced inhibition of NMDA-induced currents. Representative current traces depicting enhanced inhibition of NMDA-induced currents by 10 and 100 mM ethanol pre-exposure. Both current traces are from two different 7 days in vitro cerebellar granule cell neurons. Results were summarized from 44 cells from 10 and 8 different culture batches for 10 and 100 mM ethanol, respectively.
Fig. 2.
The affect of intracellular ethanol on pretreatment-enhanced inhibition of NMDA-induced currents. (a) Representative current traces depicting inhibition by 10 and 100 mM ethanol with either the simultaneous or pretreatment ethanol application protocols. Current traces were from a 28 and 16 days in vitro cerebellar granule cell for 10 and 100 mM ethanol, respectively. (b) Values depicted in the graphs are mean ± s.e.m. percent ethanol (10 or 100 mM) inhibition of NMDA-induced currents. Results were summarized from 10 cells for 10 mM ethanol and 11 cells for 100 mM ethanol obtained from three different culture batches.
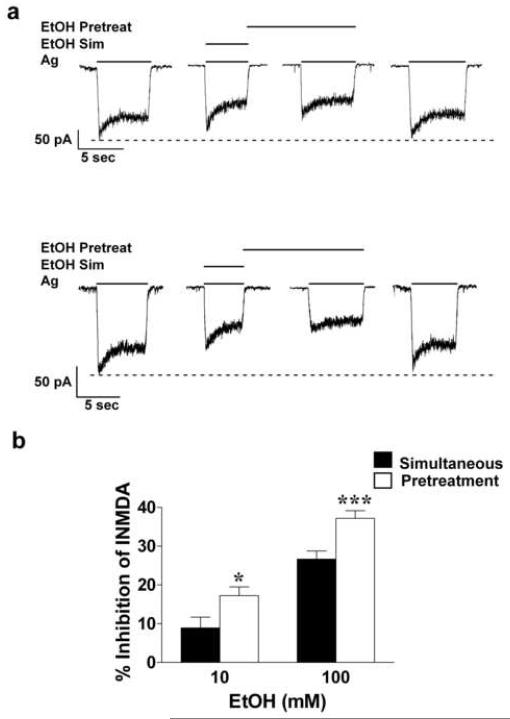
It has been suggested that during WC patch-clamp electrophysiological experiments, intracellular dialysis and / or a disruption among the interconnected scaffolding of intracellular proteins and the membrane bound NMDAR may occur. To determine if ethanol pretreatment-enhanced inhibition is sensitive to these effects of WC recording, we examined the affect of ethanol application protocol on inhibition of INMDA under PP-clamp conditions. Recording configuration did not affect ethanol inhibition of INMDA since the degree of ethanol inhibition observed with the PP-clamp technique was identical to values previously reported during WC recordings (Popp et al., 1999a). Furthermore, results obtained with the pretreatment protocol in the PP configuration were similar to those obtained with the WC patch-clamp method reported above. A 30 s ethanol pre-exposure resulted in an enhanced ethanol inhibition of INMDA from 8.9 ± 2.8 % to 17.2 ± 2.3 % for 10 mM ethanol (t = 3.1, df = 13; P ≤ 0.05) and an enhanced inhibition from 26.7 ± 2.1 to 37.2 ± 2 % for 100 mM ethanol (t = 5.9, df = 18; P ≤ 0.001). Representative current traces and summarized data are show in Figures 3a and b, respectively. These findings suggest that the intracellular factors involved in ethanol pretreatment-enhanced inhibition of INMDA are not readily diffusible from the interior of the cell and not otherwise affected during WC recordings.
Fig. 3.
Affect of ethanol pre-exposure-enhanced inhibition of NMDA-induced currents measured with the perforated patch-clamp method. (a) Representative current traces in this figure were taken from 13 days in vitro cerebellar granule cells for both ethanol concentrations. (b) Results were summarized from 14 (10 mM ethanol) and 19 (100 mM ethanol) different cerebellar granule cells obtained from three different culture batches. Ethanol inhibition of NMDA-induced currents was enhanced with the pretreatment protocol. Results from paired t test indicate significant differences: P* ≤ 0.05 and P*** ≤ 0.001.
Phalloidin prevents ethanol pre-exposure-enhanced inhibition of INMDA but taxol does not
Results from previous studies have implicated the importance of the actin cytoskeleton in regulating NMDAR channel activity. In the initial findings of Rosenmund and Westbrook (1993), time-dependent attenuation of NMDA-induced peak current amplitude (rundown) was attributed to calcium-mediated depolymerization of F-actin. In turn, F-actin depolymerization by latrunculin A results in a selective attenuation of NMDAR-mediated synaptic activation (Sattler et al., 2000). Ethanol has been reported to alter the order of F-actin in astroglial primary cultures (Allansson et al., 2001; Guasch et al., 2003). A high, but pharmacologically relevant concentration of ethanol (100 mM) resulted in F-actin reorganization in astroglial cells, and changes were observed within five (Allansson et al., 2001) and two minutes of ethanol exposure (Guasch et al., 2003). These findings along with the results from our internal ethanol and PP-clamp experiments provided the rationale for our hypothesis that enhanced-inhibition of INMDA by ethanol pre-exposure is due to a disruption of the actin cytoskeleton. If this premise were true, then the actin stabilizer phalloidin should prevent enhanced inhibition of INMDA by ethanol pre-exposure.
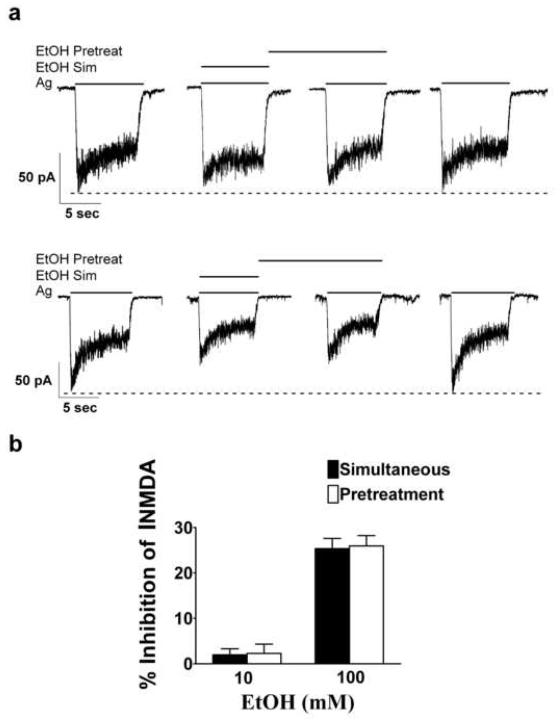
Indeed, we observed that inclusion of 1 μM phalloidin in the patch electrode prevented the enhanced inhibition of INMDA normally observed with the ethanol pretreatment protocol (Figures 4a and b). Ethanol (10 mM) inhibited INMDA by 1.96 ± 1.4 % and by 2.3 ± 2.0 %, for simultaneous and pre-treatment protocols, respectively (t = 0.16, df = 8, P ≥ 0.9), and inhibition of INMDA by 100 mM ethanol was 25.34 ± 2.3 % with the simultaneous and 25.9 ± 2.3 % with the ethanol pretreatment protocol (t = 0.45, df = 8, P ≥ 0.66). Ethanol pre-exposure enhanced-inhibition is specific for the actin cytoskeleton since the microtubule stabilizer taxol did not attenuate ethanol pretreatment-enhanced inhibition of INMDA. When taxol (5 μM) was introduced into the patch electrode, ethanol pretreatment-enhanced inhibition of INMDA was still observed. Ethanol (10mM) inhibited INMDA by 6.23 ± 1.08 and by 14.28 ± 2.94 %, for simultaneous and pre-treatment protocols, respectively (t = 2.18, df = 12, P ≤ 0.05), and inhibition of INMDA by 100 mM ethanol was 25.63 ± 1.49 % with the simultaneous protocol and 36.28 ± 1.97 % with the ethanol pretreatment protocol (t = 2.18, df = 12, P ≤ 0.01). Representative current traces and summarized data are shown in Figures 5a and 5b, respectively. The vehicle (0.1 % DMSO) for both phalloidin and taxol had no affect on INMDA (data not shown).
Fig. 4.
Affect of phalloidin on ethanol inhibition of NMDA-induced currents. (a) Representative current traces taken from two separate cerebellar granule cell neurons, both 16 days in vitro during recordings in which phalloidin was present in the patch solution. (b) Statistical analysis of data summarized from experiments involving nine cerebellar granule cells obtained from two different culture batches indicated that the presence of phalloidin in the patch electrode prevented ethanol pretreatment-enhanced inhibition of NMDA-induced currents.
Figure 5.
Inclusion of 5 μM taxol in the patch solution does not inhibit EtOH pretreatment-enhanced inhibition of NMDA-induced currents. (a) Representative current traces from a 6 DIV cerebellar granule cell depicting enhanced inhibition of NMDA-induced currents by 10 and 100 mM EtOH after the cell was permeablized with taxol. (b) Summary graph from 13 CGCs for both 10 and 100 mM EtOH obtained from nine different culture batches. EtOH pre-exposure significantly enhanced inhibition of IPk; P* ≤ 0.05 and P** ≤ 0.01.
Latrunculin A pre-exposure is required to acutely inhibit INMDA
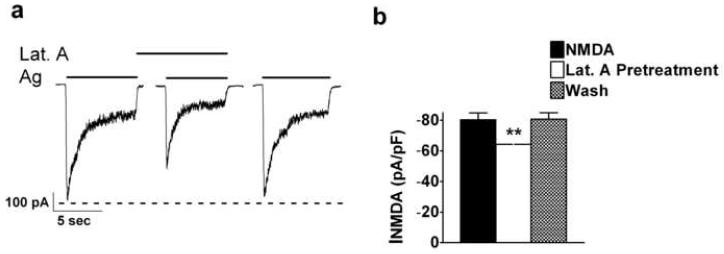
If ethanol pretreatment-enhanced inhibition was due to F-actin depolymerization, then an agent that perturbed the actin cytoskeleton should prevent enhanced-inhibition of INMDA by ethanol pre-exposure. The depolymerizing agent latrunculin A is commonly used to assess the relationship between NMDARs and the actin cytoskeleton (Allison et al., 1998; Sattler et al., 2000; Zhou et al., 2001). However effects by this cell-permeant agent reported in these studies were at concentrations that ranged between 1-20 μM and with exposure times between 10 - 20 min. In order to mimic our ethanol pretreatment effect on INMDA, it was necessary to observe effects by latrunculin A in the range of 30 s to approximately 2.5 min. Elegant experiments conducted by Fisher and co-workers (1998) with time-lapsed recordings indicated that primary cultured hippocampal neurons exposed to 20 ng / ml of cytochalasin D (an F-actin depolymerizing agent) resulted in changes in actin-filled dendritic spine shape as well as a decrease in spine motility within seconds to minutes. These effects were completely reversible upon washout of cytochalasin D. We therefore selected a concentration of 20 ng / ml (47 nM) latrunculin A for our experiments. At this concentration, co-application of latrunculin A with NMDA and glycine (agonist) for five seconds did not alter INMDA. INMDA was −55.75 ± 10.9 pA / pF and −55.59 ± 11.75 pA / pF for agonist-induced currents before and after latrunculin A, respectively and did not significantly differ from agonist-induced currents in the presence of latrunculin A, (−54.11 ± 11.1 pA / pF; F = 0.78, df = 2/9, P ≥ 0.05).
We then assessed the effects of latrunculin A and agonists on INMDA after a brief, 30 s application of latrunculin A alone (latrunculin A pretreatment) (Figures 6a and b). A 30 s exposure of 20 ng / ml of latrunculin A prior to the exposure of latrunculin A with agonists significantly decreased INMDA from −80.6 ± 9.55 pA /pF to −64.2 ± 6.33 pA /pF (t = 5.0. df = 4, P ≤ 0.01). There was no significant lasting effect by latrunculin A on INMDA, in that post latrunculin A agonist-induced current amplitudes did not differ from pre-latrunculin A agonist-induced current values (t = 0.27, df = 4, P ≥ 0.05).
Fig. 6.
A 30 s latrunculin A pretreatment significantly attenuates NMDA-induced currents. (a) Representative current traces taken from a 14 days in vitro cerebellar granule cell. (b) Summary graph of data from five cerebellar granule cells obtained from three different culture batches indicating that a brief 30 s pre-exposure of latrunculin A inhibits NMDA-induced currents. P** ≤ 0.01.
Disruption of the actin cytoskeleton by latrunculin A occludes ethanol pretreatment-enhanced inhibition of INMDA
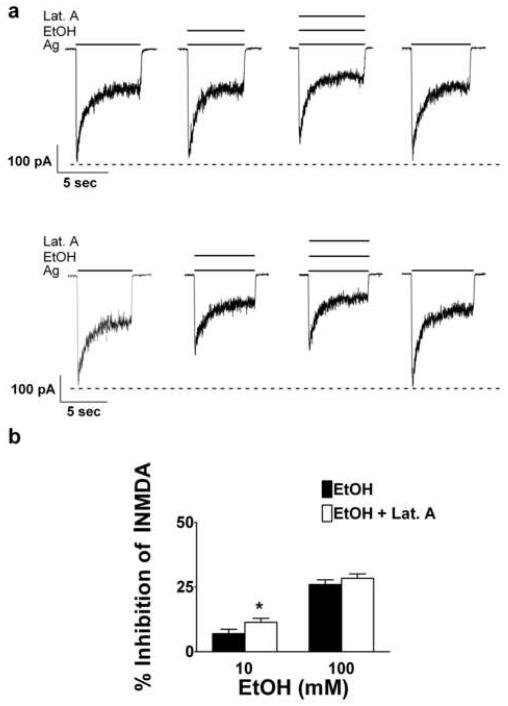
Results from the above experiments suggested that we had identified a concentration of an F-actin depolymerizing agent that inhibited INMDA in a manner similar to that observed with the ethanol pretreatment protocol. However, we needed to determine if the actin cytoskeleton might also play a role in ethanol inhibition of INMDA during simultaneous application. We therefore decided to assess the effect of latrunculin A on ethanol inhibition of INMDA with this application protocol. When the simultaneous application of latrunculin A and agonist was combined with a low concentration of ethanol (10 mM), inhibition of INMDA was significantly increased compared to the effects of ethanol alone (Figures 7a and b). The combination of latrunculin A with ethanol and agonists significantly increased percent inhibition by 10 mM ethanol on INMDA from 6.15 ± 1.6 % to 11.36 ± 1.5 % (t = 2.45, df = 9, P ≤ 0.05).
Fig. 7.
Latrunculin A and a low ethanol concentration, enhances ethanol inhibition of NMDA-induced currents with the simultaneous protocol. (a) Representative current traces taken from an 18 days in vitro cerebellar granule cell. (b) Summary graph of data from 10 cerebellar granule cells for both 10 and 100 mM ethanol obtained from five different culture batches indicating that latrunculin A significantly enhanced inhibition of NMDA-induced currents for 10 but not 100 mM ethanol; P* ≤ 0.05.
With the simultaneous protocol, latrunculin A did not further increase inhibition by 100 mM ethanol on INMDA. Percent inhibition of INMDA was 25.9 ± 1.8 % for ethanol combined with latrunculin A and 28.4 ± 1.68 % for ethanol alone (t = 1.14, df = 9, P ≥ 0.28). The observation that latrunculin A exposure for greater than 30 s could inhibit INMDA (Figures 7a and b) similar in magnitude to that observed with ethanol pretreatment suggested that F-actin depolymerization could be involved in ethanol inhibition of NMDAR function and specifically that ethanol pretreatment could enhance inhibition of INMDA via an action involving F-actin depolymerization. Therefore, we predicted that ethanol pretreatment would be ineffective in enhancing inhibition of NMDAR function when F-actin depolymerization was produced by prior latrunculin A exposure.
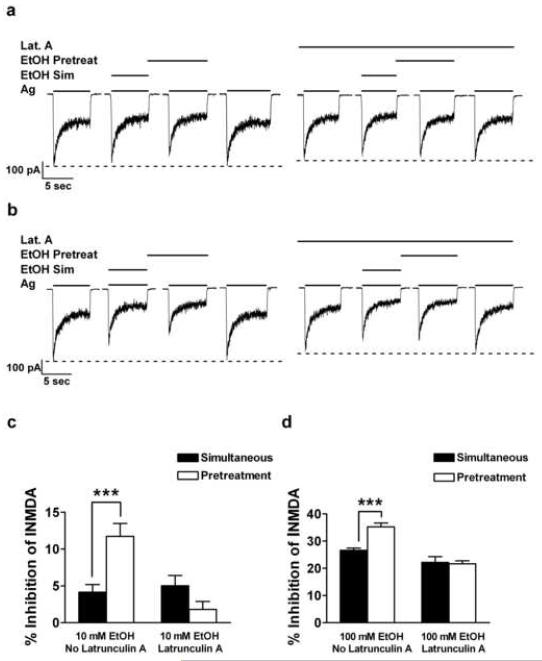
The effect of latrunculin A on ethanol (10 and 100 mM) inhibition of INMDA was assessed with each application protocol (simultaneous and pretreatment) and compared with ethanol inhibition in the absence of latrunculin A. For these experiments latrunculin A (20 ng / ml) was present throughout all drug applications, agonists and agonists plus ethanol, and all drug combinations were compared within the same cell. In the absence of latrunculin A, pretreatment-enhanced inhibition of INMDA by 10 mM ethanol was observed. Percent inhibition of NMDA-induced currents by 10 mM ethanol significantly increased (t = 4.2, df = 32, P ≤ 0.001) from 4.2 ± 1.03 % with the simultaneous protocol to 11.73 ± 1.74 % with the pretreatment protocol in the absence of latrunculin A. Yet, when latrunculin A was present, pretreatment-enhanced inhibition of INMDA by 10 mM ethanol was not observed: ethanol inhibition was 5.03 ± 1.37 % and 1.81 ± 1.08 % with the simultaneous and pretreatment protocols, respectively, t = 1.8, df = 32, P ≥ 0.08. Furthermore, the effects of latrunculin A on ethanol inhibition of INMDA were specific for the pretreatment protocol (Finteraction = 18.3, df = 32, P ≤ 0.001) in that percent inhibition of INMDA by a simultaneous application of 10 mM ethanol did not differ in the presence or absence of latrunculin A: percent inhibition was 4.15 ± 1.0 % and 5.03 ± 1.37 % in the absence and presence of latrunculin A, respectively (t = 0.5, df = 32, P ≥ 0.6). Representative current traces are shown in Figure 8a with summarized data shown in Figure 8c.
Fig. 8.
Latrunculin A occludes ethanol pre-exposure-enhanced inhibition of NMDA-induced currents. (a) Representative current traces taken from a 13 days in vitro cerebellar granule cell indicating that the presence of the depolymerizing agent latrunculin A occludes ethanol inhibition by 10 mM ethanol with the pretreatment protocol. Summary graphs of data from a 2-Way ANOVA and post-hoc analysis from 9 CGCs obtained from five different culture batches for 10 (c) and 100 mM ethanol (d); P*** ≤ 0.001).
Similar results were observed with 100 mM ethanol in that latrunculin A affects on INMDA were dependent upon the ethanol application protocol used (Finteraction = 11.4, df = 32, P ≤ 0.01). In the absence of latrunculin A, 35.24 ± 1.4 % compared to 26.6 ± 0.8 % inhibition was observed with the pretreatment and simultaneous protocol, respectively (t = 4.51, df = 32, P ≤ 0.001). In the presence of latrunculin A pretreatment-enhanced inhibition of INMDA by 100 mM ethanol did not differ: 22.2 ± 2.1 % and 21.7 ± 1.1 % for simultaneous and pretreatment protocols, respectively (t = 0.3, df = 32, P ≥ 0.8). Latrunculin A did not affect inhibition of INMDA by simultaneous application of 100 mM ethanol: inhibition of INMDA was 26.6 ± 0.8 and 22.2 ± 2.1 with and without latrunculin A, respectively (t = 2.4, df = 32, P ≥ 0.05). Representative current traces and summarized data are shown in figures 8b and 8d, respectively. In summary, the percent inhibition almost doubles with the ethanol pretreatment protocol for a low concentration of ethanol and this is similar to the enhanced inhibition by 10 mM ethanol and latrunculin A when co-applied for 5 s. Second, continuous application of latrunculin A results in a decrease of INMDA of approximately 25% which is similar in magnitude to the percent inhibition observed with 100 mM ethanol pretreatment. Lastly, the continuous presence of latrunculin A completely blocks the additional inhibition of INMDA normally observed by ethanol pre-exposure. These data strongly suggest that disruption of the actin cytoskeleton prior to the ethanol pretreatment protocol does not result in further inhibition of INMDA normally observed with this ethanol application protocol.
Acute latrunculin A and ethanol exposure disrupts the actin cytoskeleton of primary cultured CGCs
Actin depolymerizing agents have been used extensively with confocal microscopy to assess disruption of the actin cytoskeleton in primary cultured neurons. While most of the studies have been conducted with primary cultured hippocampal neurons, Sattler et al., 2000 also assessed the effects of actin depolymerizing agents in primary cultured cortical neurons. As explained in the methods section of this paper we defined the criteria by which to identify pearls with Metamorph 6.3 and then used these criteria to identify pearls resultant of drug treatment. We noticed that pearls were present within an image field devoid of CGCs. Conformational changes in F-actin described as “ring formations” have been observed in astroglial primary cultures after ethanol exposure (Allansson et al., 2001). To ensure that pearling was specific to the CGCs, data was acquired only from processes associated with a soma. Table 1 summarizes the data from these experiments and Figure 9 contains representative confocal images. Drug treatment had no effect on mean neurite length (F = 0.35, df = 5/20, P ≥ 0.9) or mean neurite breadth (F = 0.38, df = 5/20, P ≥ 0.8) but did significantly increase the number of pearls per neurite (F = 3.27, df = 5/20, P ≤ 0.05) as well as the overall number of pearls (F = 10.7, df = 5/20, P ≤ 0.001). Post-hoc analysis revealed that both a 30 s 20 ng / ml latrunculin A (Figure 9b) and a 30 s 100 mM ethanol (Figure 9c) treatment significantly increased the number of pearls per neurite compared to non-treated (control; Figure 9a) CGCs (t = 2.8, df = 20, P ≤ 0.01 for latrunculin A and t = 3.2, df = 20, P ≤ 0.01 for ethanol. There was no significant difference between control CGCs (Figure 9A) or CGCs treated with vehicle (0.002% DMSO; Figure 9d). While there was a trend for an increase in overall number of pearls after latrunculin A or ethanol or DMSO treatment, only the latrunculin A treated CGCs exhibited a statistically significant increase in pearls compared to control values (t = 6.7, df = 20, P ≤ 0.001; Table 1). Results from these experiments indicate that disruption of the actin cytoskeleton as seen by increases in overall pearl number as well as increases in pearl number per neurite could be observed with 20 ng / ml latrunculin A in a time frame identical to that responsible for changes in INMDA. Furthermore, the disruption of the actin cytoskeleton by a brief 100 mM ethanol exposure was similar to that observed with an F-actin depolymerizing agent.
TABLE 1.
Drug-induced F-actin depolymerization in primary cultured cerebellar granule cells. (Values are mean ± s.e.m.)
| Pearl Number | Pearl / Neurite | Fiber Length (μm / pixel) |
Fiber Breadth (μm / pixel) |
|
|---|---|---|---|---|
| Control | 28.0±7.1 | 2.5±0.4 | 3.9±0.25 | 2.3±0.1 |
| Ethanol with wash | 33.3±11.1 | 2.7±0.4 | 3.8±0.2 | 2.5±0.15 |
| Latrunculin A with wash | 30.3±8.8 | 2.9±0.9 | 4.0±0.4 | 2.3±0.3 |
| Ethanol | 50.0±7.1 | 5.2±2.4**a | 4.0±0.6 | 2.3±0.1 |
| Latrunculin A | 101±10.4***b | 4.2±0.4** | 3.8±0.05 | 2.4±0.1 |
| DMSO | 46.0±11.2 | 3.3±0.5 | 3.8±0.15 | 1.4±0.1 |
Control, no drug treatment; Ethanol with wash, removal of 100 mM ethanol after a 2.5 min exposure time by a 30 s wash with normal external; Latrunculin A with wash, removal of 20 ng / ml Latrunculin A after a 2.5 min exposure time by a 30 s wash with normal external; ethanol, 30 s exposure of 100 mM ethanol; Latrunculin A, 30 s exposure of 20 ng / ml latrunculin A; DMSO, 30 s exposure of 0.002 % DMSO (vehicle control). Significant values are indicated: P ≤ 0.01** or P ≤ 0.001*** compared to control values and P ≤ 0.05a compared to ethanol with wash and P ≤ 0.001b compared to latrunculin A with wash.
Fig. 9.
Treatment conditions that alter NMDA-induced currents result in F-actin depolymerization in cerebellar granule cells. F-actin contained in primary cultured cerebellar granule cells was stained by phalloidin-FITC. Scale bar is 20.0 μm for all images. (a) Image taken from 19 days in vitro cerebellar granule cells that did not receive any drug treatment. Note the absence of pearls. (b, c) Brief (30 s) exposures to 20 nM latrunculin A (b) or 100mM ethanol (c) resulted in a significant increase in pearls associated with neurites (arrowheads). Images were taken from 17 days in vitro cerebellar granule cells for images b and c. The amount of pearling observed in the DMSO-treated days in vitro (d) did not statistically differ from control cerebellar granule cells, yet more pearls are present compared to control cells and are indicated by arrowheads. Image was taken from 14 days in vitro cerebellar granule cells. (e, f) Despite a longer drug exposure, 16 days in vitro cerebellar granule cells that were washed for 30 s with external medium after a 2.5 min 100mM ethanol treatment (e) or 19 days in vitro cerebellar granule cells that were washed for 30 s in external medium after a 2.5 min 20 nM latrunculin A (f) indicated a reversal of the depolymerizing actions of these two agents as seen by a decrease to absence of pearls. Note in both the latrunculin A (b) and ethanol (c) treated cerebellar granule cells that pearls are present that appear not to be associated with neurons (arrows). This may be due to F-actin depolymerization in glial cells.
To mimic conditions similar to our electrophysiological experiments we assessed if the depolymerizing actions of latrunculin A and ethanol could be reversed upon washout of the drugs. Ethanol pretreatment-enhanced inhibition (a total ethanol exposure of 2.5 to 3 min) of INMDA is completely reversible (Popp et al., 1999a). The degree of F-actin depolymerization as indicated by pearling of neurites was for the most part reversed after a 30 s washout of a 2.5 min 100 mM ethanol exposure (Figure 9e). While both neurite length and breadth did not differ from control values, pearl per neurite values were significantly lower after ethanol washout compared to values resultant of a 30 s ethanol exposure (t = 2.7, df = 20, P ≤ 0.05; Table 1). Similar to ethanol, washout of a 2.5 min latrunculin A treatment resulted in number of pearls per neurite as well as overall number of pearls that did not differ significantly from control values. Also, the 30 s washout significantly decreased the total number of pearls compared to the 30 s latrunculin A treated cells (t = 5.2, df = 20, P ≤ 0.001; Figure 9f and Table 1). In summary these data suggest that neurite F-actin depolymerization is partially reversible under conditions that result in the full recovery from decreases in NMDAR function due to ethanol and latrunculin A pre-exposure.
Discussion
The purpose of this study was to identify a mechanism for ethanol pre-exposure-enhanced inhibition of NMDAR function (Popp et al., 1999a). We report that ethanol (10 and 100 mM) pretreatment enhanced-inhibition of INMDA was prevented by the intracellular presence of the F-actin stabilizing agent, phalloidin. We observed that brief exposure (30 s) to a low concentration (20 ng / ml) of the depolymerizing agent Latrunculin A attenuated INMDA and the magnitude of this inhibition was similar to the extra inhibition observed with the ethanol pretreatment protocol. Brief exposure (5 s) of latrunculin A can enhance inhibition by a low but not a high concentration of ethanol and continuous exposure of latrunculin A can occlude further enhancement of INMDA by ethanol pre-exposure. Results from these electrophysiological experiments provide strong evidence for the premise that ethanol pretreatment-enhanced inhibition of INMDA is due to destabilization of the actin cytoskeleton by ethanol. The observation that a short exposure of latrunculin A worked in a synergistic manner with low ethanol concentrations suggests that the organization of the actin cytoskeleton can also affect alterations in NMDAR function by a very brief (5 s) exposure to low concentrations of ethanol. Results from our imaging experiments also indicate that acute ethanol exposure results in reorganization of the actin cytoskeleton. Under conditions similar to those that resulted in altered INMDA, ethanol-exposed CGCs labeled with phalloidin-FITC resembled CGCs that had been exposed to the depolymerizing agent latrunculin A. The imaging results indicate that a brief, 30 s ethanol exposure results in F-actin reorganization in neurons, a result not previously reported. This study is also the first to show that acute ethanol exposure at concentrations observed after one drink result in transient F-actin depolymerization and this leads to an attenuation of NMDA-induced macroscopic currents.
While our data suggest that the ethanol-sensitive target responsible for pretreatment-enhanced inhibition of NMDAR function is the actin cytoskeleton, it is possible that other proteins connected to the actin cytoskeleton may be involved. A protein that cross-links with actin filaments, alpha-actinin-2, has been implicated in altering the ethanol sensitivity of NMDARs and this phenomenon appears to be specific for NR2A-containing receptors (Anders et al., 2000). We have reported ethanol pretreatment enhanced-inhibition in primary cultured CGCs that express NR2A / NR2B NMDARs (Popp et al., 1999a) as well as in primary cultured cortical neurons (Popp et al., 1999b). Experiments conducted on the cultured cortical cells were done between 6 and 14 DIV. The NR2B is the predominant NR2 subunit present in this age of cultured cortical neurons as determined pharmacologically (Lovinger, 1995) and by western blot analysis (unpublished data). Thus, while alpha-actinin-2 may contribute to the ethanol sensitivity of recombinant NMDARs expressed in HEK cells, this protein may not be responsible for ethanol pretreatment-enhanced inhibition of NMDARs expressed in neurons. Eps8, another actin-associated protein, is involved in the regulation of actin dynamics (Disanza et al., 2004). Eps8 can influence behavioral responses to ethanol as well as the ethanol sensitivity of NMDARs (Offenhauser et al., 2006). Relevant to our study are the findings that while the immediate inhibition of INMDA produced by 100 mM ethanol did not differ between the wild type (WT) and Eps8 knockout (KO) mice, longer ethanol exposure at a higher concentration (400 mM) resulted in inhibition of NMDA-mediated EPSCs in only WT mice. Based on results from the Offenhauser study (2006) it is possible that ethanol pretreatment enhanced-inhibition may be due to alterations in actin dynamics via Eps8.
Another mechanism by which actin depolymerization modulates NMDAR function is through activation of myosin light chain kinase (MLCK) (Lei et al., 2001). Inhibition of constituitively activated MLCK results in an attenuation of INMDA in acutely dissociated hippocampal neurons and this effect was dependent upon an intact cytoskeleton. In brain (Haorah et al., 2005) and intestinal epithelial cells (Ma et al., 1999) acute ethanol exposure, at pharmacologically relevant concentrations, reorganizes the actin cytoskeleton in such a manner that results in enhanced permeability through tight junctions (TJ). This effect on TJ integrity is believed to be mediated by ethanol activation of MLCK (Haorah et al., 2005; Ma et al., 1999). Given that MLCK activity is increased by acute ethanol exposure, ethanol pretreatment-enhanced inhibition of INMDA would not be the predicted result if mediated through MLCK. However, in addition to changes in MLKC activity and phosphorylated products, Ma and coworkers (1999) also reported ethanol-induced “disorganization” of myosin filaments. Therefore in epithelial cells, multiple ethanol sensitive targets exist that contribute to alterations in TJ integrity. Thus it is possible that multiple ethanol-sensitive targets that supplement the direct actions by ethanol on NMDAR function may also exist in neurons.
It appears that the ethanol sensitivity of the receptor or the degree of inhibition of INMDA is resultant of two actions by ethanol: a direct action on receptor gating (Ronald et al., 2001; Honse et al., 2004) and the culmination of effects by ethanol-sensitive targets. The present findings indicate that one of these effects involves ethanol interactions with the organization of the actin cytoskeleton and these in turn influence NMDAR function. However, myriad intracellular components associate with the actin cytoskeleton. Furthermore, ethanol alone may modulate a diverse set of intracellular mechanisms that converge to alter NMDAR function. The ethanol pretreatment application protocol is an excellent paradigm to use in identifying these intracellular targets as well as elucidating the mechanism by which they affect NMDAR function. During drinking and intoxication, ethanol is present throughout the brain for long periods of time. Therefore, the ethanol pretreatment application protocol may more accurately mimic acute ethanol affects on NMDAR function.
Acknowledgements
This work was supported by grants from the National Institute on Alcohol Abuse and Alcoholism (NIAAA): F32AA05458 and RO1AA13436 (R. L. P.). Experiments contained in this manuscript were initiated under the guidance of Dr. David Lovinger. Dr. Lovinger’s insightful comments on the initial data were instrumental in determining subsequent experiments. I also thank Dr. Lovinger for his support and strong encouragement that I continue this project after departing from his laboratory and his valuable comments on the final version of this manuscript. I would also like to thank Jason Reneau and Emmanuel Duran for technical assistance and Yaminiben Bhakta for her help with figure preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allansson L, Khatibi S, Olsson T, Hansson E. Acute ethanol exposure induces [Ca2+]i transients, cell swelling and transformation of actin cytoskeleton in astroglial primary cultures. J. Neurochem. 2001;76:472–479. doi: 10.1046/j.1471-4159.2001.00097.x. [DOI] [PubMed] [Google Scholar]
- Allison DW, Gelfand VI, Spector I, Craig AM. Role of actin in anchoring postsynaptic receptors in cultured hippocampal neurons: Differential attachment of NMDA versus AMPA receptors. J. Neurosci. 1998;18:2423–2436. doi: 10.1523/JNEUROSCI.18-07-02423.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvestad RM, Grosshans DR, Coultrap SJ, Nakazawa T, Yamamoto T, Browning MD. Tyrosine dephosphorylation and ethanol inhibition of N-Methyl-d-Aspartate receptor function. J. Biol. Chem. 2003;278:11020–11025. doi: 10.1074/jbc.M210167200. [DOI] [PubMed] [Google Scholar]
- Anders DL, Blevins T, Smothers CT, Woodward JJ. Reduced ethanol inhibition of N-methyl-d-aspartate receptors by deletion of the NR1 C0 domain or overexpression of α-actinin-2 proteins. J. Biol. Chem. 2000;275:15019–15024. doi: 10.1074/jbc.275.20.15019. [DOI] [PubMed] [Google Scholar]
- Bar-Ziv R, Tlusty T, Moses E, Safran SA, Bershadsky A. Pearling in cells: A clue to understanding cell shape. Proc. Natl. Acad. Sci. 1999;96:10140–10145. doi: 10.1073/pnas.96.18.10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disanza A, Carlier M, Stradel TE, Didry D, Frittoli E, Confalonieri S, Croce A, Wehland J, Di Fiore PP, Scita G. Eps8 controls actin-based motility by capping the barbed ends of actin filaments. Nat. Cell. Biol. 2004;6:1180–1188. doi: 10.1038/ncb1199. [DOI] [PubMed] [Google Scholar]
- Fischer M, Kaech S, Knutti D, Matus A. Rapid actin-based plasticity in dendritic spines. Neuron. 1998;20:847–854. doi: 10.1016/s0896-6273(00)80467-5. [DOI] [PubMed] [Google Scholar]
- Guasch RM, Tomas M, Minambres R, Valles S, Renau-Piqueras J, Guerri C. RhoA and lysophosphatidic acid are involved in the actin cytoskeleton reorganization of astrocytes exposed to ethanol. J. Neurosci. 2003;72:487–502. doi: 10.1002/jnr.10594. [DOI] [PubMed] [Google Scholar]
- Haorah J, Heilman D, Knipe B, Chrastil J, Leibhart J, Ghorpade A, Miller DW, Persidsky Y. Ethanol-induced activation of myosin light chain kinase leads to dysfunction of tight junctions and blood-brain barrier compromise. Alcohol. Clin. Exp. Res. 2005;29:999–1009. doi: 10.1097/01.alc.0000166944.79914.0a. [DOI] [PubMed] [Google Scholar]
- Honse Y, Ren H, Lipsky RH, Peoples RW. Sites in the fourth membrane-associated domain regulate alcohol sensitivity of the NMDA receptor. Neuropharmacol. 2004;46:647–654. doi: 10.1016/j.neuropharm.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Lei A, Czerwinska E, Czerwinski W, Walsh MP, MacDonald JF. Regulation of NMDA receptor activity by F-actin and myosin light chain kinase. J. Neurosci. 2001;21:8464–8472. doi: 10.1523/JNEUROSCI.21-21-08464.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima-Landman MT, Albuquerque EX. Ethanol potentiates and blocks NMDA-activated single-channel currents in rat hippocampal pyramidal cells. FEBS Lett. 1989;247:61–67. doi: 10.1016/0014-5793(89)81241-4. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- Lovinger DM. Developmental decreases in ethanol inhibition of N-methyl-d-aspartate receptors in rat neocortical neurons: relation to the actions of ifenprodil. J. Pharmacol. and. Exp. Therapeutics. 1995;274:164–172. [PubMed] [Google Scholar]
- Ma TY, Nguyen D, Bui V, Nguyen H, Hoa N. Ethanol modulation of intestinal epithelial tight junction barrier. Am. J. Physiol. 1999;276:G965–G974. doi: 10.1152/ajpgi.1999.276.4.G965. [DOI] [PubMed] [Google Scholar]
- McBain CJ, Mayer ML. N-methyl-d-aspartic acid receptor structure and function. Physiological. Rev. 1994;74:723–760. doi: 10.1152/physrev.1994.74.3.723. [DOI] [PubMed] [Google Scholar]
- Miyakawa T, Yagi T, Kitazawa H, Yasuda M, Kawai N, Tsuboi K, Niki H. Fyn-kinase as a determinant of ethanol sensitivity: relation to NMDA-receptor function. Science. 1997;278:698–701. doi: 10.1126/science.278.5338.698. [DOI] [PubMed] [Google Scholar]
- Offenhäuser N, Castelletti D, Mapelli L, Soppo BE, Regondi MC, Rossi P, D’Angelo E, Frassoni C, Amadeo A, Tocchetti A, et al. Increased ethanol resistance and consumption in Eps8 knockout mice correlates with altered actin dynamics. Cell. 2006;127:213–226. doi: 10.1016/j.cell.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Palay SL, Chan-Palay SV. Cerebellar Cortex Cytology and Orientation. Springer-Verlag; New York: 1974. pp. 76–77. [Google Scholar]
- Peoples RW, Stewart RR. Alcohols inhibit N-methyl-d-aspartate via a site exposed to the extracellular surface. Neuropharmacol. 2000;39:1681–1691. doi: 10.1016/s0028-3908(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Popp RL, Lickteig R, Lovinger DM. Factors that enhance ethanol inhibition of N-methyl-d-aspartate receptors in cerebellar granule cells. J. Pharmacol. Exp. Therapeutics. 1999a;289:1564–1574. [PubMed] [Google Scholar]
- Popp RL, Lovinger DM. Acute ethanol pre-exposure enhances inhibition of NMDAR-mediated currents in primary cultured neurons. Alc. Clin. Exp. Res. Suppl. 1999b;23:22. [Google Scholar]
- Pusch M, Neher E. Rates of diffusion exchange between small cells and a measuring patch pipette. Pflugers. Archiv. 1988;411:204–211. doi: 10.1007/BF00582316. [DOI] [PubMed] [Google Scholar]
- Ronald KM, Mirshahi T, Woodward JJ. Ethanol inhibition of N-methyl-d-aspartate receptors is reduced by site directed mutagenesis of a transmembrane domain phenylalanine residue. J. Biol. Chem. 2001;276:44729–44735. doi: 10.1074/jbc.M102800200. [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Westbrook GL. Calcium-induced actin depolymerization reduces NMDA channel activity. Neuron. 1993;10:805–814. doi: 10.1016/0896-6273(93)90197-y. [DOI] [PubMed] [Google Scholar]
- Sattler R, Xiong Z, Lu WY, MacDonald JF, Tymianski M. Distinct roles of synaptic and extrasynaptic NMDA receptors in excitotoxicity. J. Neurosci. 2000;20:22–33. doi: 10.1523/JNEUROSCI.20-01-00022.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simson PE, Criswell HE, Johnson KB, Hicks RE, Breese GE. Ethanol inhibits NMDA-evoked electrophysiological activity in vivo. J. Pharmacol. and Exp. Therapeutics. 1991;257:225–231. [PubMed] [Google Scholar]
- Snell LD, Tabakoff B, Hoffman PL. Involvement of protein kinase C in ethanol-induced inhibition of NMDA receptor function in cerebellar granule cells. Alcohol Clin. Exp. Res. 1994;18:81–85. doi: 10.1111/j.1530-0277.1994.tb00884.x. [DOI] [PubMed] [Google Scholar]
- Wirkner K, Eberts C, Poelchen W, Allgaier C, Illes P. Mechanism of inhibition by ethanol of NMDA and AMPA receptor channel functions in cultured rat cortical neurons. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2000;36:568–576. doi: 10.1007/s002100000262. [DOI] [PubMed] [Google Scholar]
- Yaka R, Phamluong K, Ron D. Scaffolding of fyn kinase to the NMDA receptor determines brain region sensitivity to ethanol. J. Neurosci. 2003;23:3623–3632. doi: 10.1523/JNEUROSCI.23-09-03623.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Xiao MY, Nicoll RA. Contribution of the cytoskeleton to the internalization of AMPA receptors. Proc. Natl. Acad. Sci. 2001;98:1261–1266. doi: 10.1073/pnas.031573798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Verdoorn TA, Lovinger DM. Alcohols potentiate the function of 5-HT3 receptor-channels on NCB-20 neuroblastoma cells by favoring and stabilizing the open channel state. J. Physiol. 1998;507.2:335–352. doi: 10.1111/j.1469-7793.1998.335bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]