Abstract
Aim
The present study is to compare the effectiveness of iliac crest graft and medpor implant, for repairing traumatic orbital floor defects.
Materials and methods
A total of 20 patients were included in the study. Autogenous iliac crest graft and medpor implant was used in 10 patients of the each group. Patients were evaluated for the presence or absence of diplopia, enophthalmos, infraorbital nerve paresthesia, and ocular motility disorders. Surgical indications for orbital exploration included entrapment of orbital tissues, large orbital defect (greater than 50% of the orbital floor or more than 8 mm), or orbital floor defects with involvement of other zygomaticofrontal complex fractures.
Results
All patients were successfully treated by restoration of the orbital wall continuity. Follow-up was done at 1–12 weeks. One patient had postoperative infection. There was no graft extrusion.
Conclusions
Both the groups showed satisfactory results, but group II was better than group I, as there was no donor site morbidity. Porous polyethylene (Medpor) is a biocompatible and high-density polyethylene implant. It is well tolerated by surrounding tissue, and its porous structure is rapidly infiltrated by host tissue. It is a highly stable and somewhat flexible porous alloplast that has rapid tissue in growth into its pores.
Keywords: Medpor, Orbit, Iliac crest, Trauma
Introduction
Orbital wall fracture is a common outcome of orbital injuries. Fracture of orbit may lead to enophthalmos, limitation of orbital movement, diplopia and anaesthesia or paresthesia of the infraorbital nerve [1, 2]. CT is recognized to be the best imaging technique to evaluate orbital fractures [3]. Coronal CT scan offers excellent diagnostic information for assessing blowout fractures of the orbital floor. The extent and location of a blowout fracture in the CT scan were noted to have an effect on the clinical outcome [4]. Indications for surgical intervention are entrapment of the inferior rectus muscle leading to diplopia, significant enophthalmos and large fractures involving more than 50% of the orbital floor [5]. Management of orbital floor fracture has been controversial. There are four pre-requisites for successful repair of fractures of the orbital complex: a thorough understanding of the regional anatomy; an accurate diagnosis; unimpeded exposure and in some cases, rigid fixation of the fracture. The goal of surgery is to reposition herniated orbital fat and tissue within the orbit, and repair of the post traumatic defect [6–8]. Orbital wall defects have been repaired with several types of autogenous grafts [9] or alloplastic [10–13] or allogenic implants [14, 15] to lift the eyeball into its correct position and avoid enophthalmos. The ideal orbital implant should be strong enough to support the orbital contents, easily anchored in position, reshaped to fit the orbital defect, and biocompatible. Autogenous bone grafts have been the preferred material for reconstruction of the orbital walls [10, 13], although there is unpredictable resorption, displacement problems, and donor-site morbidity [15]. In addition, contouring to the appropriate shape and placement of the graft in the orbital floor is extremely difficult. The use of alloplasts can be associated with complications of infection, extrusion of the implant, tissue reaction to the material, and residual diplopia [12, 16] and variable foreign body reactions [17]. Homografts give equally good results as autogenous materials, and well tolerated by the host. Duramater has also been used for repair in different clinical situations [18]. The incision placement and design are guided by good intraoperative visibility and minimal postoperative scar formation [19]. Porous high-density polyethylene (Medpor) is also used for the repair which is well tolerated by surrounding tissue, and its porous structure is rapidly infiltrated by host tissue [20].
As material science advances, these materials are acquiring more and more of the properties mentioned above. It is the responsibility of the surgeon to recognize the diversity of the materials available and to apply them selectively to the appropriate clinical setting.
Materials and Methods
The present study comprised of 20 patients having orbital floor fractures associated with other maxillomandibular fracture, attending the outpatient department and emergency services of Department of Oral and Maxillofacial Surgery, C.S.M. Medical University, Lucknow, India. The patients were selected randomly irrespective of age, sex, and other social categories. Patients with debilitating diseases, e.g., diabetes, acute infection, cardiac insufficiency, metabolic bone disorder, patient taking immuno-suppressive drugs and patient with compromised immunity were excluded.
Preoperative medical and dental history was recorded and informed consent was taken. Patients were diagnosed on the basis of clinical and radiographic examination. Plain radiographs (occipitomental and submentovertex view) and CT scan (axial, coronal and 3-D reconstruction) of midface were done to confirm the clinical diagnosis.
Preoperative evaluations of the patients were done by (1) Lees charting, (2) Enophthalmos measurement by Hertel’s exophthalmometer, (3) Diplopia charting by red green glass, (4) Routine Blood investigation, (5) Urine analysis, (6) X-ray of chest P.A. view, (7) E.C.G, (8) Liver function test, (9) Pulmonary function test (Figs. 1, 2).
Fig. 1.

Preoperative photograph group I
Fig. 2.
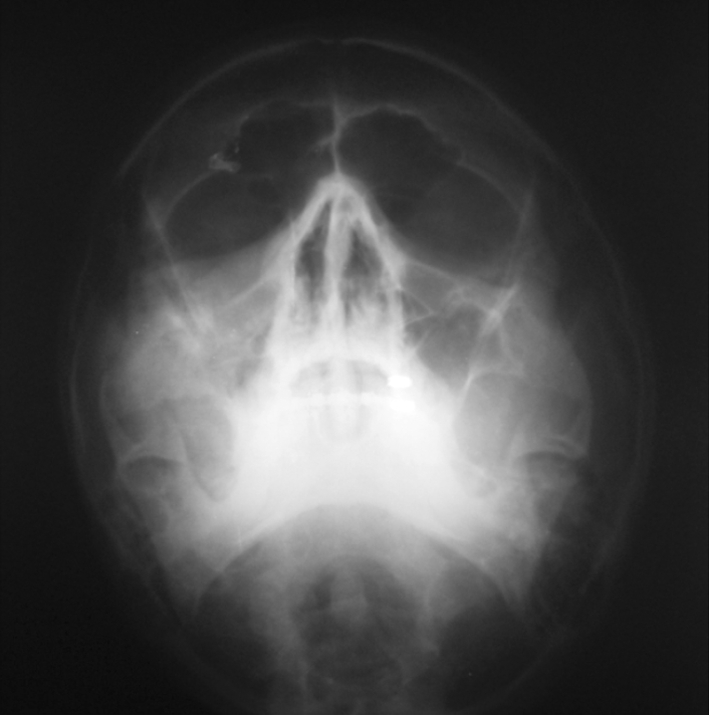
Preoperative radiograph group I
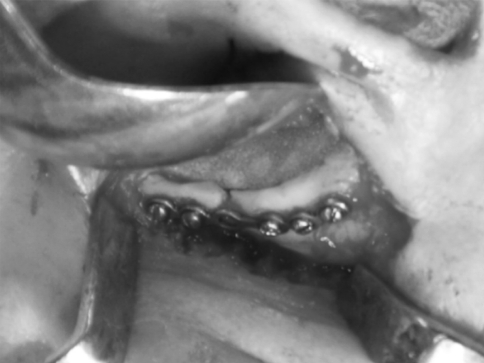
Patients were randomly divided into two equal groups of 10 each: group I patients underwent orbital floor reconstruction with iliac crest bone graft, and group II patients underwent orbital floor reconstruction with medpor (porous polyethylene) orbital implant. Patients were operated under general anaesthesia. Operating sites were exposed through an infra orbital approach or pre-existing laceration or scar mark. After adequate exposure, entrapped fatty-muscular tissues were released. The desired graft either iliac crest or medpor was placed. Antibiotic and analgesics were given for 5 days postoperatively (Fig. 3).
Fig. 3.
Iliac crest graph group I
Postoperative assessment of the patients was done under following parameters:
Pain: Visual Analogue Scale (VAS) (0–10).
Swelling: Present/Absent.
Sub-conjunctival haemorrhage: Present/Absent.
Diplopia charting with red green glass.
Clinical enophthalmos—measured by Hertel’s exophthalmometer.
Lees charting-was done by Lees screen.
Infraorbital paresthesia-Present/Absent.
Implant/Autograft failure-Present/Absent.
Infection: Present/Absent.
Follow-up was done at 1 week, 3 week, 6th week and 3 months after surgery.
The statistical analysis was done using SPSS Version 15.0. The values were represented in number (%) and Mean ± SD (Figs. 4, 5).
Fig. 4.

Postoperative photograph of patient after 12 month group I
Fig. 5.
Postoperative radiograph group I
Results
Out of 20 patients included in the study. The mean age of the subjects was 29.88 ± 9.53 years with a minimum age of 12 years and maximum age of 50 year (Table 1). On preoperative ophthalmic evaluation, 3 patients had diminished or no vision (2 patients in group II and 1 patient in group I) so Lees charting and diplopia charting could not be done in these patients.
Table 1.
Age wise distribution of cases (n = 20)
| S. no. | Age group (years) | No. of cases | % |
|---|---|---|---|
| 1. | 11–20 | 4 | 20 |
| 2. | 21–30 | 7 | 35 |
| 3. | 31–40 | 5 | 25 |
| 4. | 41–50 | 4 | 20 |
Preoperative subconjunctival haemorrhage was present in 5 patients of group I and 5 patients of group II. Preoperatively diplopia was present in 5 patients of group I and 3 patients of group II. Overall, no significant difference could be seen between the two groups (P = 0.457) (Table 2).
Table 2.
Preoperative diploma
| S. no. | Finding | Group I (n = 10) | Group II (n = 10) | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| 1 | Present | 5 | 55.5 | 3 | 37.5 |
| 2 | Absent | 4 | 44.5 | 5 | 62.5 |
Preoperative lees charting revealed presence of external ocular muscle dysfunction in 77.7% cases of group I and 87.5% cases of group II. However, statistically, this difference was not significant (P = 0.600) (Table 3).
Table 3.
Preoperative LEES Charting results
| S. no. | Muscle motility disturbance | Group I (n = 10) | Group II (n = 10) | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| 1 | Absent | 2 | 22.2 | 1 | 12.5 |
| 2 | Present | 7 | 77.7 | 7 | 87.5 |
Mean preoperative enophthalmos size was 4.50 ± 2.17 mm in group I with a range of 2–10 mm whereas in group II it was 3.30 ± 0.95 with a range of 2–5 mm. Statistically, there was no significant difference between two groups (P = 0.143; Table 4; Figs. 6, 7).
Table 4.
Preoperative exophthalmoses size
| S. no. | Case no. | Group I (n = 10) | Group II (n = 10) |
|---|---|---|---|
| 1 | 1 | 5 | 4 |
| 2 | 2 | 3 | 4 |
| 3 | 3 | 3 | 5 |
| 4 | 4 | 5 | 3 |
| 5 | 5 | 4 | 3 |
| 6 | 6 | 2 | 2 |
| 7 | 7 | 10 | 3 |
| 8 | 8 | 4 | 2 |
| 9 | 9 | 4 | 3 |
| 10 | 10 | 5 | 4 |
| Mean + SD | 4.50 + 2.17 | 3.30 + 0.95 | |
| “Z” | 1.562 | ||
| “p” | 0.143 | ||
Fig. 6.
Preoperative photograph group II
Fig. 7.
Preoperative radiograph group II
Infraorbital paresthesia was present in 7 of group I patients and 6 of the group II. Statistically no significant difference was seen between the two groups (P = 0.639). Postoperative pain at different time intervals revealed a mean pain score of 3.40 ± 1.51, 1.60 ± 1.51, 0.10 ± 0.32 and 0, respectively, at 1st, 2nd, 6th and 12th week in group I and 2.90 ± 1.66, 0.70 ± 0.82, and 0, respectively, at the corresponding time intervals in group II.
At first week in both the groups all the patients had swelling. At 2nd week, 9 cases in group I and 8 in group II had swelling. At 6th week, only 2 patients in group I had swelling while none of the group II patients had swelling. At 12th week swelling was resolved in all the patients in both groups.
In group I, at 1st, 2nd, 6th and 12th week time intervals, there were 5, 4, 1 and 0 patients showing subconjunctival hemorrhage; whereas in group II there were 4, 2 patients, respectively, showing subconjunctival hemorrhage. Statistically no significant difference between two groups was seen at any time (P > 0.05).
At 1st and 2nd week time intervals, 5 patients of group I and 3 patients of group II had diplopia. At 6th week, diplopia was resolved in all the cases of group I and situation remained same till 12th week. However, in group II at 6th week time interval 2 patients had diplopia but on 12th week, none of the patients of group II had diplopia. Statistically, there was no significant difference between the two groups at any time interval (P > 0.05) (Table 5).
Table 5.
Postoperative diploma
| S. no. | Time interval (week) | Group I (n = 10) | Group II (n = 10) | X2 | P | ||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | ||||
| 1 | 1 | 5 | 55.5 | 3 | 37.5 | 0.554 | 0.457 |
| 2 | 2 | 5 | 55.5 | 3 | 37.5 | 0.554 | 0.457 |
| 3 | 6 | 0 | 0 | 2 | 25 | 2.55 | 0.110 |
| 4 | 12 | 0 | 0 | 0 | 0 | – | – |
Lees charting showed presence of extraocular muscle motility disturbances in 66.6, 44.4, 22.2 and 11.1% subjects of group I at 1st, 2nd, 6th and 12th week time interval whereas in group II it was seen to be present in 75, 50, 12.5 and 0% subjects, respectively. No significant difference between the two groups was at any time interval (Table 6). The mean enophthalmos was 1.00 ± 1.33 in group I, whereas in group II it was 0.60 ± 0.70 mm showing no significant difference throughout (P = 0.684) (Table 7).
Table 6.
LEES charting at different time intervals
| S. no. | Time interval (week) | Group I (n = 10) | Group II (n = 10) | X2 | P | ||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | ||||
| 1 | 1 | 6 | 66.6 | 6 | 75.0 | 0.142 | 0.707 |
| 2 | 2 | 4 | 44.4 | 4 | 50.0 | 0.052 | 0.819 |
| 3 | 6 | 2 | 22.4 | 1 | 25.5 | 0.275 | 0.600 |
| 4 | 12 | 1 | 11.1 | 0 | 0 | 0.944 | 0.331 |
Table 7.
Comparison of postoperative exophthalmoses at different time intervals in two groups
| S. no. | Time interval (week) | Postoperative exophthalmoses (Mean + SD) | X2 | P | |
|---|---|---|---|---|---|
| Group I (n = 10) | Group II (n = 10) | ||||
| 1 | 1 | 1.00 + 1.33 | 0.6. + 0.70 | 0.452 | 0.684 |
| 2 | 2 | 1.00 + 1.33 | 0.6. + 0.70 | 0.452 | 0.684 |
| 3 | 6 | 1.00 + 1.33 | 0.6. + 0.70 | 0.452 | 0.684 |
| 4 | 12 | 1.00 + 1.33 | 0.6. + 0.70 | 0.452 | 0.684 |
In group I paresthesia was noted in 7, 5, 1 and 1 patients showing postoperatively at 1st, 2nd, 6th and 12th week. In group II, only 5 patients showed paresthesia till 2nd week, on 6th week it was seen in 2 patients but at 12th week, none of the patients showed paresthesia. In group I, infection was seen in 1 case and nil in group II. Statistically no significant difference was seen between two groups (P = 0.305).
Discussion
The rationale of using iliac crest bone grafts (autogenous bone) is its relative resistance to infection, incorporation by the host into new bone, lack of host response against the graft, and lack of concern for late extrusion. Although there are multiple sites for autogenous grafts, the anterior iliac crest remains the most common site. Graft from the anterior iliac crest is a favourable reconstruction material, bearing in mind that enough bone is always available and bone can be harvested simultaneously with orbital exploration. Autogenous bone grafting has been the gold standard to provide framework for the facial skeleton and orbital walls [21].
Porous polyethylene implants (medpor) were used for orbital reconstruction as they are easy to handle, shape contour, position, fix and can be used with other autogenous and alloplastic implants. The material is well tolerated, resists infection, non antigenic and promotes tissue ingrowth. Its porous structure allows fibrovascularization which not only protects the implant from infection but also prevents its migration [20, 22–25].
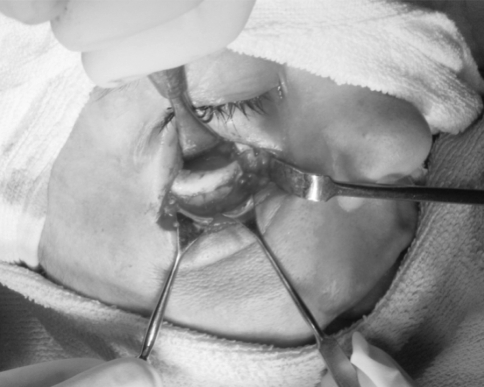
Post traumatic oedema often masks the symptoms of orbital injuries and may cause delayed diagnosis and inadequate treatment. Computed tomography (CT) has now replaced plane radiograph. Coronal CT scans are recognized to be the best imaging techniques for diagnosing orbital floor fracture [26, 27] (Fig. 8).
Fig. 8.
Exposed fracture site
Different surgical approaches like transcutaneous (subciliary, subtarsal and infraorbital) and transconjuctival, are widely used for exposure, evaluation and orbital trauma. In general, lower the incision is made, lower the risk of scleral flow and/or ectropion. The infra orbital approach provides the quickest and most direct route to the orbital rim and floor used in this study [13]. Postoperatively, all patients were given short courses of steroid (dexamethasone) to reduce postoperative swelling and pain. Post-operative use of steroid reduces pain and swelling [28]. In our study, the number of male (85%) patients was higher than females (15%), which is in accordance with the previous studies [25, 29–31].
Most commonly affected age group was 21–40 years (60%), which also corresponds with the study of Polly et al. [10]. This can be attributed to more socially active life, led by this group of individuals [32]. Road traffic accidents were found to be responsible for majority of the fractures. According to the estimates of Association of Automobile Manufacturers of India (AIAMI)—the number of automobiles on road has grown more than 10-fold during the last 10 years (Source: AIAMI website). Apart from this our centre is also the tertiary care referral centre, where patients come from as far as neighbouring districts. This growing number of automobiles on the road has led to increase in number of accidents which often end up in oral and maxillofacial injuries. That is why in our study all the patients received trauma due to road traffic accidents, which also correlates with the previous studies [25, 32, 33].
Pain was assessed using VAS scale on 1st, 2nd, 6th and 12th week. The postoperative pain decreased gradually and was 0 at 6th week in group II while it decreased gradually to 0 in 12th week in group I. Subconjunctival haemorrhage is commonly seen in midfacial injuries. Preoperative sub-conjunctival haemorrhage was seen in 5 patients of group I and 5 patients of group II. Resolution of sub-conjunctival haemorrhage started postoperatively at 1st week. At 6th week follow-up, sub-conjunctival haemorrhage was present in 1 patient of group I and no patient in group II. At 12 week, none of the patient had sub-conjunctival haemorrhage in either group.
Diplopia after orbital injuries is the result of many factors. Oedema or haemorrhage within the orbit or muscles may limit ocular rotation, although this usually resolves soon after operation. Preoperatively, diplopia was present in 8 subjects, corresponds with the study of Chan et al. [27]. Postoperatively resolution of diplopia was better in 1st group. At 6th week, 2 patients of group II had diplopia while no patient of group I had diplopia. On 12th week, diplopia resolved in group II as well.
To rule out extraocular muscle motility dysfunction in our patients Lees charting was performed. Limitation of ocular motility is the result of entrapment, muscle injury or paresis, and incarceration of the soft tissues adjacent to the muscle. Severe motility problems were caused by a dysfunction of the entire motility apparatus of the connective tissue septa surrounding the muscles and intra orbital contents [34]. Preoperative (85%) charting revealed presence of extra ocular muscle dysfunction in 71.4% cases of group I and all the cases of group II. However, statistically, this difference was not significant (P = 0.155). In 3 cases (1 in group I and 2 in group II) Lees charting could not be assessed as the patients had diminished vision. Postoperatively at 1 week follow up Lees charting showed improvement in ocular muscle action in 1 patient of the each group. At 2nd week follow up in both the groups, 3 patients showed improvement in extra-ocular muscle dysfunction while at 12th week follow up only one patient (11.1%) in group I had extraocular muscle dysfunction. However, these differences were not statistically significant at any time interval. Lees screen was also being used by Amrith et al. [32].
Enophthalmos was measured in this study by Hertel exopthalmometer. Hertel exopthalmometry is not easily reproducible and results can vary by up to 2 mm [33]. It is recognized that preoperative evidence of enophthalmos is an indication for orbital exploration, because this will not improve with observation and delayed repair can be more difficult [35].
Mean preoperative enophthalmos size was 4.50 ± 2.17 mm in group I with a range of 2–10 mm whereas in group II it was 3.30 ± 0.95 with a range of 2–5 mm, which was comparable to the study of Chan et al. [27] with median of enophthalmos 4.0 mm (range 1.5–6 mm), Siddique et al. [31], averaged 4.11 mm and 5.06 mm in cranial and iliac crest groups. Postoperatively enophthalmos correction was done in 12 patients. In 7 patients enophthalmos of 1–2 mm persisted without aesthetic defects, while 1 patient had residual enophthalmos of 4 mm which is comparable to the previous studies [22, 29].
Injury to infraorbital nerve leads to anaesthesia or paresthesia of the skin of the cheek and upper lip of the affected side. Recovery of sensation depends on the type of nerve injury. Infraorbital paresthesia was present in 6 of group II and 7 of the group I patients. Postoperatively, infraorbital paresthesia was present up to 1st week. In 2nd week follow-up 2 patients in group I and 1 patient in group II showed complete remission of infraorbital paresthesia. In group I, postoperatively, at 6 and 12 week time interval there was only 1 patient showing paresthesia. In group II, on 6 week, paresthesia was seen to be present in 2 patients but at 12 week time interval, none of the patients showed paresthesia [25, 29, 30, 36].
In group I, only one patient reported with infection at 2nd week follow-up who presented with pus discharge near the suture site. After pus culture, sensitivity test and antibiotic therapy; infection subsided. No graft failure or rejection was seen in both the groups. These findings correlate with the findings of Roncevic et al. [4] Bartkowski et al. [7], Siddique et al. [31], Krishnan et al. [19], Lee et al. [21] and Yilmaz et al. [37].
From the iliac crest, good aesthetics and functional results can be achieved. Despite a large volume of autogenous iliac crest grafts being used, donor site morbidity is very low. Patients having enophthalmos of mild or moderate degree, either of the two materials can be used but patients having severe enophthalmos should be treated by iliac crest graft as the larger volume can be obtained by this modality which is also cost-effective but accompanies the donor site morbidity (Figs. 9, 10).
Fig. 9.
Medpor in position
Fig. 10.

Postoperative photograph of patient after 12 month group II
Conclusion
The study was aimed at investigating the comparison between iliac crest graft and medpor in patients having orbital fractures. Based on the finding of our study, following conclusions were derived.
Road traffic accident was the cause of orbital bone fracture in all our patients, commonest age group was 21–40 years and males were more frequently involved.
Porous polythene implants (medpor) are easy to handle, shape contour, position and fix. Use of medpor was found to be simple, and it offers advantages over iliac crest bone graft by not having donor site morbidity.
Patients having mild to moderate enophthalmos either iliac crest or medpor can be used but patients having severe enophthalmos should be treated by iliac crest as required thickness of graft can be harvested.
Results of the present study were almost similar in both the methods of orbital reconstructions, i.e., reconstruction with iliac crest or by medpor implant. However, medpor is considered to be good option in patients where taking of graft, etc. is not feasible, in these situations medpor gives equally good treatment outcome.
References
- 1.Smith B, Regan WF., Jr Blowout fracture of orbit; mechanism and correction of internal orbit fracture. Am J Ophthalmol. 1957;44(6):733–739. doi: 10.1016/0002-9394(76)90774-1. [DOI] [PubMed] [Google Scholar]
- 2.Kroll M, Wolper J. Orbital blowout fractures. Am J Ophthalmol. 1967;64(6):1169–1172. doi: 10.1016/0002-9394(67)93077-2. [DOI] [PubMed] [Google Scholar]
- 3.Sacks AC, Friedland JA. Orbital floor fractures: should they be explored early? Plastic Reconstr Surg. 1979;64(2):190–193. doi: 10.1097/00006534-197908000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Roncevic R, Malinger B. Experience with various procedures in the treatment of orbital floor fractures. J Maxillofac Surg. 1981;9(2):81–84. doi: 10.1016/S0301-0503(81)80020-3. [DOI] [PubMed] [Google Scholar]
- 5.Wolfe SA. Correction of a lower eyelid deformity caused by multiple extrusions of alloplastic orbital floor implants. Plast Reconstr Surg. 1981;68(3):429–432. doi: 10.1097/00006534-198100000-00037. [DOI] [PubMed] [Google Scholar]
- 6.Rodgers BM, Maher JW, Talbert JL. The use of preserved human dura for closure of abdominal wall and diaphragmatic defects. Ann Surg. 1981;193(5):606–611. doi: 10.1097/00000658-198105000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartkowski SB, Krzystkowa KM. Blow-out fracture of the orbit. Diagnostic and therapeutic considerations, and results in 90 patients treated. J Maxillofac Surg. 1982;10(3):155–164. doi: 10.1016/S0301-0503(82)80033-7. [DOI] [PubMed] [Google Scholar]
- 8.Koornneef L. Current concepts on the management of orbital blowout fractures. Ann Plast Surg. 1982;9(3):185–200. doi: 10.1097/00000637-198209000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Mathog RH. Reconstruction of the orbit following trauma. Otolaryngol Clin North Am. 1983;16(3):585–607. [PubMed] [Google Scholar]
- 10.Polley JW, Ringler SL. The use of Teflon in orbital floor reconstruction following blunt facial trauma: a 20-year experience. Plast Reconstr Surg. 1987;79(1):39–43. doi: 10.1097/00006534-198701000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Waite PD, Clanton JT. Orbital floor reconstruction with lyophilized dura. J Oral Maxillofac Surg. 1988;46(9):727–730. doi: 10.1016/0278-2391(88)90180-2. [DOI] [PubMed] [Google Scholar]
- 12.Scapini DA, Mathog RH. Repair of orbital floor fractures with Marlex mesh. Laryngoscope. 1989;99:697–701. doi: 10.1288/00005537-198907000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Sargent LA, Fulks KD. Reconstruction of internal orbital fractures with Vitallium mesh. Plast Reconstr Surg. 1991;88(1):31–38. doi: 10.1097/00006534-199107000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Bains RA, Rubin PA. Blunt orbital trauma. Int Ophthalmol Clin. 1995;35(1):37–46. doi: 10.1097/00004397-199503510-00005. [DOI] [PubMed] [Google Scholar]
- 15.Friesenecker J, Dammer R, Moritz M, Niederdellmann H. Long-term results after primary restoration of the orbital floor. J Craniomaxillofac Surg. 1995;23(1):31–33. doi: 10.1016/s1010-5182(05)80251-3. [DOI] [PubMed] [Google Scholar]
- 16.Biesman BS, Hornblass A, Lisman R, Kazlas M. Diplopia after surgical repair of orbital floor fractures. Ophthal Plast Constr Surg. 1996;12(1):9–17. doi: 10.1097/00002341-199603000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Cordewener FW, Bos RR, Rozema FR, Houtman WA. Poly (l-lactide) implants for repair of human orbital floor defects: clinical and magnetic resonance imaging evaluation of long-term results. J Oral Maxillofac Surg. 1996;54(1):9–14. doi: 10.1016/S0278-2391(96)90292-X. [DOI] [PubMed] [Google Scholar]
- 18.Celikoz B, Duman H, Selmanpakoglu N. Reconstruction of the orbital floor with lyophilized tensor fascia lata. J Oral Maxillofac Surg. 1997;55(5):240–244. doi: 10.1016/S0278-2391(97)90533-4. [DOI] [PubMed] [Google Scholar]
- 19.Krishnan V, Johnson JV. Orbital floor reconstruction with autogenous mandibular symphyseal bone grafts. J Oral Maxillofac Surg. 1997;55(4):327–332. doi: 10.1016/S0278-2391(97)90117-8. [DOI] [PubMed] [Google Scholar]
- 20.Shumrich KA, Kersten RC, Kulwin DR, Smith CP. Criteria for selective management of the orbital rim and floor in zygomatic complex and midface fractures. Arch Otolaryngol Head Neck Surg. 1997;123(4):378–384. doi: 10.1001/archotol.1997.01900040020003. [DOI] [PubMed] [Google Scholar]
- 21.Lee HH, Alcaraz N, Reino A, Lawson W. Reconstruction of orbital floor fractures with maxillary bone. Arch Otolaryngol Head Neck Surg. 1998;124(1):56–59. doi: 10.1001/archotol.124.1.56. [DOI] [PubMed] [Google Scholar]
- 22.Goldberg RA. Correlation of preoperative computed tomography and postoperative ocular motility in orbital blowout fractures. Arch Facial Plast Surg. 2002;4(1):61–62. doi: 10.1001/archfaci.4.1.61. [DOI] [PubMed] [Google Scholar]
- 23.Flood TR, McManners J, El-Attar A, Moos KF. Randomized prospective study of the influence of steroids on postoperative eyeopening after exploration of the orbital floor. Br J Oral Maxillofac Surg. 1999;37(6):510–511. doi: 10.1054/bjom.1999.0244. [DOI] [PubMed] [Google Scholar]
- 24.Choi JC, Fleming JC, Aitken PA, Shore JW. Porous polyethylene channel implants: a modified porous polyethylene sheet implant designed for repairs of large and complex orbital wall fractures. Ophthal Plast Reconstr Surg. 1999;15(1):56–66. doi: 10.1097/00002341-199901000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Amrith S, Saw SM, Lim TC, Lee TK. Ophthalmic involvement in cranio-facial trauma. J Craniomaxillofac Surg. 2000;28(3):140–147. doi: 10.1054/jcms.2000.0138. [DOI] [PubMed] [Google Scholar]
- 26.Sevin K, Askar I, Saray A, Yormuk E. Exposure of high-density porous polyethylene (Medpor) used for contour restoration and treatment. Br J Oral Maxillofac Surg. 2000;38(1):44–49. doi: 10.1054/bjom.1998.0038. [DOI] [PubMed] [Google Scholar]
- 27.Chan CH, Splaton DJ. Quantitative volume replacement in the correction of post-traumatic enophthalmos. Br J Oral Maxillofac Surg. 2000;38(5):437–440. doi: 10.1054/bjom.2000.0337. [DOI] [PubMed] [Google Scholar]
- 28.Aitasalo K, Kinnunen I, Palmgren J, Varpula M. Repair of orbital floor fractures with bioactive glass implants. J Oral Maxillofac Surg. 2001;59(12):1390–1395. doi: 10.1053/joms.2001.27524. [DOI] [PubMed] [Google Scholar]
- 29.Baumann A, Ewers R. Use of the preseptal transconjunctival approach in orbit reconstruction surgery. J Oral Maxillofac Surg. 2001;59(3):287–291. doi: 10.1053/joms.2001.20997. [DOI] [PubMed] [Google Scholar]
- 30.Ploder O, Klug C, Voracek M, Burggasser G, Czerny C. Evaluation of computer-based area and volume measurement from coronal computed tomography scans in isolated blowout fractures of the orbital floor. J Oral Maxillofac Surg. 2002;60(11):1267–1272. doi: 10.1053/joms.2002.35722. [DOI] [PubMed] [Google Scholar]
- 31.Siddique SA, Mathog RH. A comparison of parietal and iliac crest bone grafts for orbital reconstruction. J Oral Maxillofac Surg. 2002;60(1):44–50. doi: 10.1053/joms.2002.29072. [DOI] [PubMed] [Google Scholar]
- 32.Ellis E, III, Tan Y. Assessment of internal orbital reconstructions for pure blowout fractures: cranial bone grafts versus titanium mesh. J Oral Maxillofac Surg. 2003;61(4):442–453. doi: 10.1053/joms.2003.50085. [DOI] [PubMed] [Google Scholar]
- 33.Ploder O, Klug C, Voracek M, Backfrieder W, Tschabitscher M, Czerny C, Baumann A. A computer-based method for calculation of orbital floor fractures from coronal computed tomography scans. J Oral Maxillofac Surg. 2001;59(12):1437–1442. doi: 10.1053/joms.2001.28278. [DOI] [PubMed] [Google Scholar]
- 34.Kontio R. Treatment of orbital fractures: the case for reconstruction with autogenous bone. J Oral Maxillofac Surg. 2004;62(7):863–868. doi: 10.1016/j.joms.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Ellis E, III, Messo E. Use of nonresorbable alloplastic implants for internal orbital reconstruction. J Oral Maxillofac Surg. 2004;62(7):873–881. doi: 10.1016/j.joms.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 36.Grover AK, Chaudhuri Z, Vidya B (2005) Orbital floor repair: experiences with different materials. In: AIOC 2005 proceedings, pp 359–360
- 37.Yilmaz M, Vayvada H, Aydin E, Menderes A, Atabey A. Repair of fractures of the orbital floor with porous polyethylene implants. Br J Oral Maxillofac Surg. 2007;45(8):640–644. doi: 10.1016/j.bjoms.2007.06.004. [DOI] [PubMed] [Google Scholar]