Abstract
Aim
Inherent fundamental difference exists among arteries of different sizes. The purpose of this study was to evaluate the relation between regional difference of wall shear stress (WSS) in various sizes arteries and contents of nitrite and NO synthase (NOS) isoforms.
Methods
Five different conduit arteries in a wide range of diameter (1–8 mm) were examined in the hind limbs of 13 pigs. Blood flow rate and outer diameter were measured in vivo to determine WSS. Arterial tissues were harvested for the measurement of nitrite and NOS protein contents. The concentration of nitrite, a product of NO synthesis, was determined by high-performance liquid chromatography method. Western blot analysis was used to assess the protein contents of endothelial NOS (eNOS), inducible NOS (iNOS) and neuronal NOS (nNOS).
Results
Our data show that WSS increases with a decrease in artery diameter. Nitrite level increases with increasing WSS and hence decreases with artery diameter. The eNOS protein contents decrease with an increase in diameter. No significant difference for iNOS and nNOS protein contents was found with different artery diameter. A significant positive correlation between tissue nitrite and eNOS protein contents was also observed. Finally, the WSS-normalized eNOS is not significantly different in various size vessels.
Conclusion
Regional difference in blood flow has no effect on iNOS and nNOS protein contents in these conduit arteries. Regional difference in eNOS expression and nitrite contents may be related to the WSS-induced NO by the endothelium under normal physiological conditions.
Keywords: arteries, diameter, nitrite, NOS, shear stress
Mechanical forces elicited by the flow of blood (shear stress) and pressure (strain) cause acute releases of nitric oxide (NO) (Sessa et al. 1994, Awolesi et al. 1995). NO is implicated in the regulation of numerous cellular functions throughout the systemic circulation including the maintenance of low arterial tone at rest, inhibition of leucocyte–endothelial interactions, attenuation of platelet aggregation and inhibition of smooth muscle cell proliferation (Celermajer 1997, Rudic et al. 1998).
There is evidence suggesting that heterogeneity of function exists within different size vessels (Kuo et al. 1995). The vascular wall consists of three different functional and structural compartments. Smooth muscle cells constitute the media layer, whereas endothelial cells constitute the intima and physiologically perceive the wall shear stress (WSS) at the interface between the flowing blood and the arterial wall. It has been shown that both endothelium and vascular smooth muscle can produce NO (Pollock et al. 1993, Boulanger et al. 1998). Although the vasculature constantly produces NO, and endothelial-derived NO is now recognized as a major mechanism regulating vascular tone and local blood flow (Chandran et al. 1998), a direct relation between the size of vessels, blood flow and the WSS-induced NO production under physiological conditions is unavailable.
Nitric oxide synthase (NOS) is a family of isozymes catalysing the production of NO. NO produced by endothelial NOS (eNOS) acts as a vasodilator in the cardiovascular system, NO produced by neuronal NOS (nNOS) is known to act as a neurotransmitter/neuromodulator in both the central and the peripheral nervous systems, and the NO produced by inducible NOS (iNOS) acts as a cytotoxic effector in the immune system (Chandran et al. 1998). The eNOS expression has been thought to be responsible for shear stress-induced NO generation in endothelial cells (Cooke et al. 1990, Nishida et al. 1992). A low-level expression of iNOS has been recently found to occur, however, constitutively in the blood vessels, heart and other tissues under normal conditions (Park et al. 1996). The expression of nNOS, on the other hand, was first observed in the brain (Bredt et al.1990) and has since been found in vascular smooth muscle cells (Boulanger et al. 1998). Although these studies demonstrate that NOS is expressed in blood vessels, the potential role of the different NOS isoforms in the regulation of blood flow-induced NO in normal vessel wall remains to be determined.
Here, we quantified the relation between shear stress, nitrite contents and NOS protein contents in different sizes of arteries. We chose segments of five conduit arteries from the hind limb of pig: abdominal aorta, common iliac artery, femoral artery, superficial femoral artery and a small branch of the femoral artery. The main goal of the study was to: (1) determine the level of shear stress in different sizes of arteries in vivo, (2) quantify tissue contents of nitrite in relation to arterial diameter, (3) determine the correlation between protein contents of eNOS, iNOS and nNOS and NO production, and (4) evaluate the role of WSS on protein contents of eNOS, iNOS and nNOS and NO production. We found that nitrite contents increase with decrease in artery diameter under physiological conditions and the regional difference in nitrite largely associated with eNOS and eNOS content is largely determined by WSS. Our findings underscore the relation between NO, NOS and dimensions of arteries.
Materials and methods
Animal preparation
Thirteen normal Yorkshire swine of either sex with age of 13–16 weeks and body weight of 32–35 kg were used in this study. The animals were anaesthetized with ketamine (33 mg kg−1) and atropine (0.05 mg kg−1), and anaesthesia was maintained with isoflurane (1–2%). Ventilation with 100% O2 was provided with a respiratory pump. All animal experiments were performed in accordance with nationaland local ethical guidelines, including the Institute of Laboratory Animal Research guidelines, Public Health Service policy, the Animal Welfare Act, and an approved University of Indiana-Purdue University, Indianapolis protocol regarding the use of animals in research.
Flow rate and arterial size
The superficial femoral artery, femoral artery and a small branch of the femoral artery were carefully exposed. Water-resistant carbon particles were used to mark each artery segment to measure axial changes as described in a previous publication (Guo et al. 2006). The rate of blood flow was measured by use of appropriate size flow probes, which were connected to a flow-meter (Transonic Systems, Ithaca, NY, USA). The pigs were then subjected to a midline abdominal incision. The common iliac artery and abdominal aorta close to the common iliac bifurcation was exposed, and the rate of blood flow was measured. The external geometry of each artery was photographed to obtain the outer diameter and in vivo axial length with the aid of a dissecting microscope. After the pig was killed by an injection of a saturated KCl solution through the jugular vein to arrest the heart, the segments of these arteries were isolated immediately. The length and cross section of each segment was photographed. The morphological measurements of the in vitro axial length, inner and outer circumference, wall thickness and wall area were made from the images by using a morphometric analysis system (Sigma Scan; Aspire Software International, Ashburn, VA, USA).
The loaded inner radius of the vessel was determined from the incompressibility assumption. The incompressibility condition for a cylindrical vessel can be expressed as:
| (1) |
where ro and ri are the outer and inner radii at the loaded state respectively. λz = l/lo is the stretch ratio in the axial direction where l and lo are the vessel length in the loaded and no-load state, respectively, and Ao is the wall area in the no-load state.
Wall shear stress
The wall shear stress, WSS, can be evaluated if the diameter and flow rate are known, assuming a laminar, incompressible Newtonian flow through a rigid cylindrical vessel as given by the following equation:
| (2) |
where Q and D represent the volumetric flow rate and inner diameter of the vessel and μ denotes the viscosity of blood which was assumed to be a constant value of 4 cP.
Nitrite/nitrate measurements
A portion of each isolated artery segment was coarsely grounded in methanol in ice bath. A homogenate (approx. 100–300 μL in total volume) was then centrifuged at 800 g at 4 °C for 10 min and the supernatant was assayed for nitrite (NO2−) and nitrate (NO3−). The nitrite and nitrate ion concentrations in solution were measured by the combination of a diazo coupling method and high-performance liquid chromatography (ENO-20 NOx Analyser; EiCom, Kyoto, Japan). The method for NOx analysis has been described in detail previously (Zeballos et al. 1995). Briefly, the peaks of detected voltage for nitrite and nitrate were converted into nitrite and nitrate concentrations by use of calibration solutions. The signals were corrected for contamination by subtraction of the control nitrite concentration. Endogenous NO production was evaluated as the concentration of nitrite or nitrate per unit volume of arterial wall (nmol mm−3).
Western blot analysis
The remaining artery segments were homogenized in a lysis buffer containing 50 mmol L−1 β-glycerophosphate, 100 μmol L−1 sodium orthovanadate, 2 mmol L−1 magnesium chloride, 1 mmol L−1 EGTA, 0.5% Triton X-100, 1 mmol L−1 DL-dithiothreitol, 20 μmol L−1 pepstatin, 20 μmol L−1 leupeptin, 0.1 U mL−1 aprotinin and 1 mmol L−1 phenylmethyl-sulfonyl fluoride, and then incubated on ice for 1 h. The sample was centrifuged at 1000 g for 15 min at 1 °C and the supernatant was collected. The total protein was measured using the BCA kit (Bio-Rad Laboratories, Hercules, CA, USA). Equal amounts of protein (25 μg for eNOS, 40 μg for iNOS and nNOS) were loaded and electrophoresed in 10% SDS-PAGE gel and transferred onto a ployvinylidene difluoride membrane. After being blocked for 2 h in 6% dried milk in TBS-Tween buffer, the membrane was incubated overnight at 4 °C with specific primary antibody [1 : 1000 dilution for eNOS, 1 : 500 dilution for iNOS and nNOS in blocking buffer, provided by BD Transduction Laboratory, San Jose, CA, USA (catalogue numbers 610296, 610431 and 610308 respectively)]. The membrane was then rinsed, incubated with horseradish peroxidase-conjugated secondary antibody for 2 h (1 : 3000 dilution in blocking buffer, Bio-Rad). The protein of specific NOS isoforms was detected by enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ, USA) and evaluated by densitometry (SigmaScan; Aspire Software International). All samples from each group were simultaneously probed with anti-β-actin, a mouse monoclonal antibody (primary antibody 1 : 1000 dilution in blocking buffer, Santa Cruz Biotechnology, Santa Cruz, CA, USA) to correct for sample loading.
Statistical analysis
All values for morphological and shear stress calculation, quantitative analysis for Western blot and NO metabolite (nitrite) measurements were expressed as mean ± SE. Significance of the differences between the different groups was evaluated using the two-way anova or t-test. The results were considered statistically significant when P < 0.05 (two-tailed).
Results
Wall shear stress and artery diameter
The blood flow and outer diameter of differently sized arteries were directly measured in vivo in anaesthetized pig. The mean systemic blood pressure measured at the femoral artery was 88 ± 12 (mean ± SD) mmHg. The mean outer diameter of segments from five different arteries were:7.8 ± 0.4 mm (mean ± SD) for abdominal aorta, 5.4 ± 0.7 mm for the the common iliac artery, 3.7 ± 0.5mm for the femoral artery, 2.5 ± 0.2 mm for the superficial femoral artery and 1.1 ± 0.3 mm for a branch of the femoral artery. The inner diameter and WSS were calculated according to Eqns 1 and 2 respectively. Figure 1 shows the variation of WSS as function of inner diameter of the porcine artery. The WSS significantly decreased with an increase in diameter (P < 0.01). The curve was fitted by a linear least-square fit.
Figure 1.

Relationship between wall shear stress (WSS) and inner diameter of porcine hind limb arteries. The change in WSS with inner diameter (D) is statistically significant (P < 0.05). The solid line is a least-square fit of the following form: WSS = −1.04D + 16.6 (R2 = 0.98).
Nitrite and artery diameter
In the present study, the measurement of nitrate was not very stable. Hence, the NO concentration was only measured as nitrite, a more reliable NO marker than nitrate by using the current method. The measured concentration of nitrite by the ENO-20 NOx Analyzer ranged from approx. 200 to 1000 nm (minimum detectable concentrations are <5 nm). The variation of nitrite concentrations was normalized to arterial wall volume, as shown in Figure 2, in relation to arterial diameter and WSS. The nitrite contents per unit volume of arterial wall showed a significant decrease with an increase in diameter (P < 0.01) and a significant increase with an increase in WSS (P < 0.01). The curves were fitted by second-order polynomials.
Figure 2.
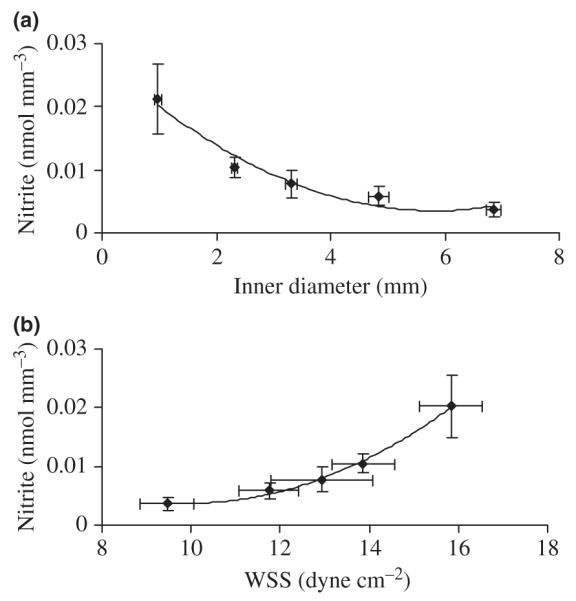
Relationship between nitrite concentration per unit volume of arterial wall and (a) inner diameter and (b) wall shear stress (WSS). The change in nitrite concentration (N) with inner diameter (D) and WSS are statistically significant (P < 0.05). The solid lines are polynomial regression curves of the forms: N = 7.1E−04D2−8.3E−03D + 2.8E−02 (R2 = 0.96) and N = 5.2E−04WSS2−1.1E−02WSS + 5.7E−02 (R2 = 0.99).
NOS and artery diameter
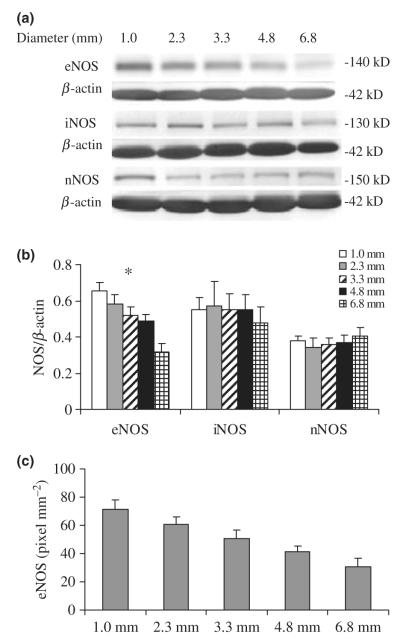
Figure 3 shows Western blot and quantitative analysis of eNOS, iNOS and nNOS protein contents in five different sizes of arteries. The band at 140 kDa for eNOS, 130 kDa for iNOS and 150 kDa for nNOS was detected. The final value for three NOS isoforms densitometry was computed as the ratio of NOS to β-actin. The anti-β-actin antibody reacted with a 42 kDa protein corresponding to the size of β-actin. The eNOS protein contents relative to total soluble protein significantly decreased with an increase in diameter (P < 0.05) as shown in Figure 3b. No significant difference for iNOS and nNOS protein contents relative to total soluble protein was found with a change in artery diameter. Figure 3c shows the relation between eNOS protein contents normalized to the surface area of the arterial endothelium and inner diameter. The relative densitometric unit of protein contents was expressed as pixel. Luminal surface area was calculated as the product of arterial luminal circumference and segment length for each arterial segment. The eNOS protein contents per unit endothelial surface area decreased with an increase in diameter (P < 0.05). As expected, a significant increase for eNOS protein contents was observed with increasing WSS (P < 0.05, data not shown). Figure 4 shows the variation of eNOS protein contents per unit WSS with inner diameter. The eNOS contents relative to total soluble protein per unit WSS and eNOS contents normalized to the surface area of the arterial endothelium per unit WSS were both found to have no significant dependence on diameter (P > 0.05).
Figure 3.
(a) Western blots of nitric oxide synthase (NOS) isoform protein expression for different diameters of arteries. (b) Quantitative analysis of NOS protein contents relative to total soluble protein for various diameter arteries. Bar graph shows mean data of protein densitometries computed as the ratio of protein to β-actin. *Statistically significant differences among the five groups of vessels (P < 0.05). (c) Quantitative analysis of endothelial NOS (eNOS) protein contents normalized to the surface area of the arterial endothelium. Protein densitometries are expressed as pixel, a relative densitometric unit. The differences are statistically significant among the five groups of vessels (P < 0.05).
Figure 4.

(a) A quantitative analysis of endothelial nitric oxide synthase (eNOS) protein content per unit wall shear stress (WSS) for different diameter vessels. The variation is not significantly different among five groups of vessels (P = 0.647). (b) Quantitative analysis of eNOS protein contents normalized to the surface area of the arterial endothelium per unit WSS for different diameter vessels (P = 0.417).
Nitrite and NOS
A positive correlation between eNOS protein contents and nitrite contents (P < 0.05) is shown in Figure 5. The data were fitted with a linear least-square fit.
Figure 5.
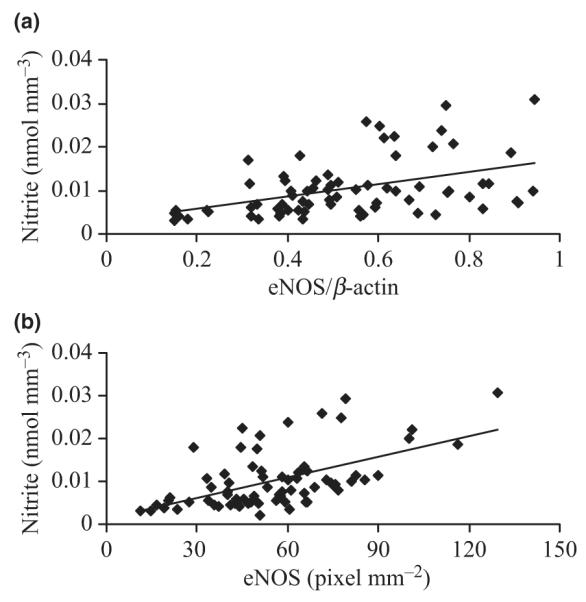
Relationship between (a) endothelial nitric oxide synthase (eNOS) protein contents (eNOS/β-actin), (b) eNOS protein contents normalized to the surface area of the arterial endothelium (eNOS mm−2) and nitrite concentration (N). The solid lines are least-square fit of the following forms: eNOS/β-actin = 1.4E−02N + 2.8E−03 (R2 = 0.21, P < 0.05) and eNOS mm−2 = 2.0E−04N + 1.4E−03 (R2 = 0.30, P < 0.05).
Discussion
We quantified the tissue contents of nitrite and NOS protein contents in different sizes of arteries from porcine hind limb under normal physiological conditions. The major findings are that (1) shear stress is inversely related to vessel diameter; (2) nitrite and eNOS contents are inversely related to diameter; (3) iNOS and nNOS protein contents do not vary with diameter; (4) eNOS protein content shows a positive correlation with tissue nitrite contents; and (5) the WSS-normalized eNOS content and eNOS/endothelial surface area did not vary with vessel diameter. Our results demonstrate regional difference in eNOS expression and nitrite contents may be related to the WSS-induced NO by the endothelium under normal physiological conditions.
Shear stress and artery diameter
Wall shear stress is an important regulator of vascular diameter, vascular wall remodelling, homeostasis and inflammatory responses (Ibrahim et al. 2003). Accurate measurement of WSS is very difficult in vivo due to the pulsatility of flow. In conduit arteries where the blood flow is generally laminar and unidirectional, however, WSS is thought to be directly related to the velocity and the viscosity of the blood but inversely related to the vessel diameter (Gnasso et al. 2001). WSS, an important determinant of endothelial function and gene expression, has been previously assumed to be constant along the arterial tree (Giddens et al. 1993). In the present study, in vivo measurements have shown a decrease in WSS as the diameter of artery increases (Fig. 1). Our data show that a sevenfold increase in diameter causes a 66% decrease in WSS implying a non-uniform distribution of wall shear rates in the arterial system similar to recent reports (Wu et al. 2004, Stroev et al. 2007).
Nitrite and artery diameter
Nitric oxide is an endogenous auto-regulator of blood flow. An increase in blood flow results in an increase in NO production and dilation in heart and blood vessels (Lamontagne et al. 1992, Ueeda et al. 1992). Nitrite is a stable metabolite of NO. The presence of nitrite in an artery reflects NO synthesis and release in the blood vessel. As NO can be generated physiologically from vascular smooth muscle cells (Boulanger et al. 1998) and endothelial cells (Pollock et al. 1993), the nitrite contents was normalized relative to the arterial wall volume in the study. Our data show that the nitrite content per unit of arterial wall volume decreases with increasing arterial diameter, but increases with increasing WSS (Fig. 2). This result implies that the observed nitrite content gradient may be associated with regional differences in WSS caused by blood flow.
eNOS and artery diameter
Vascular endothelial cells are constantly exposed to shear stress due to blood flow, and NO is known to be mainly released from the endothelial cell (Cooke et al. 1990). Some studies have reported that mechanical deformation of the endothelium by well-defined flow-induced shear stress increases eNOS mRNA, protein and enzymatic activity in vitro (Noris et al. 1995, Ranjan et al. 1995). Furthermore, a previous study in our group has verified that the expression of eNOS is restricted to the endothelium of the mouse aorta (Guo et al. 2006). In the present study, Western blot analysis suggests that eNOS protein content is greater in small arteries than in larger arteries (Fig. 3b). As the endothelium is a single cell layer, we normalized eNOS protein contents to the surface area of the arterial endothelium. A similar increase in eNOS protein content per unit area of arterial endothelium was observed as the diameter of artery increases (Fig. 3c). Our data demonstrate that protein contents of eNOS increase with an increase in WSS due to the variation of artery diameter. Our data also show that there are no differences in eNOS content in various conduit vessels when eNOS content is normalized with respect to WSS (Fig. 4). This implies that WSS may be responsible, at least in part, for the regional difference in eNOS content in various diameter vessels under physiological conditions.
Contrary to our findings, Laughlin et al. (2003) found that eNOS protein content is greater in the endothelial cells of larger coronary arterioles than in the endothelial cells of small coronary arterioles. This difference may be explained by the different distribution of blood flow-induced shear stress in resistance vasculature and in large conduit arteries. It is also possible that different mechanisms independent of haemodynamics are involved in the regulation of vascular eNOS protein expression in peripheral large arteries and resistance small arteries.
nNOS/iNOS and artery diameter
It is well accepted that shear stress is a powerful stimulus for the modulation of eNOS protein expression (Woodman et al. 1999), which is the primary source of NO in vessels (Nishida et al. 1992). It was recently reported that shear stress, however, can induce iNOS in vascular smooth muscle cells (Gosgnach et al. 2000) and endothelium in vitro (Ozawa et al. 2004). In addition, positive immunostaining for nNOS has been observed in pulmonary artery and vein endothelial cells and smooth muscle cells of human cerebral blood vessels (Tomimoto et al. 1994). In the present study, the Western blot measurement confirms both iNOS and nNOS protein expression along the arterial tree of pig hind limb. Moreover, when normalized to the total cellular protein of vessel segments, no significant difference was found in iNOS and nNOS protein contents with an increase in arterial diameter (Fig. 3b). Our findings suggest that a regional difference in blood flow has no effect on iNOS and nNOS protein contents in porcine arteries.
NO production and NOS
Wall shear stress is known to increase NO production by endothelial cells (Cooke et al. 1990), which is generally thought to be ascribed to eNOS up-regulation (Nishida et al. 1992, Noris et al. 1995). An in vitro study by Papadaki et al. (1998) demonstrated that nNOS protein content in human aortic smooth muscular cells is regulated by fluid flow, and flow-induced NO production is independent of iNOS induction. In the present study, we found that the concentration of nitrite (an indicator of NO synthesis) within blood vessels changes similar to the eNOS protein contents with a decrease in artery diameter. Nitrite contents are strongly correlated with eNOS protein expression. Our data show a significant linear relationship between the nitrite and the eNOS protein contents (Fig. 5). No correlation was observed between nitrite and iNOS or nNOS protein contents (data not shown). The NO formation derived from eNOS in conduit arteries could be involved in several mechanisms, including: (1) increased eNOS protein expression; (2) increased eNOS activity without altered eNOS content; and (3) increased activity of pathways responsible for stimulation of eNOS activity. It is reported that eNOS can be rapidly phosphorylated by shear stress and enhances the production of NO via activation of the protein kinase B/Akt pathway (Dimmeler et al. 1999, Fulton et al. 1999). Our finding indicates that eNOS protein expression may play an important role in the release of WSS-induced NO production under normal physiological conditions. The evaluation of the contribution of NOS activity to WSS-induced NO formation remains a task for the future.
Acknowledgments
This research was supported in part by the National Heart, Lung, and Blood Institute Grant HL084529 and HL055554-11.
Footnotes
Conflict of interest There is no conflict of interest.
References
- Awolesi MA, Sessa WC, Sumpio BE. Cyclic strain upregulates nitric oxide synthase in cultured bovine aortic endothelial cells. J Clin Invest. 1995;96:1449–1454. doi: 10.1172/JCI118181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger CM, Heymes C, Benessiano J, Geske RS, Levy BI, Vanhoutte PM. Neuronal nitric oxide synthase is expressed in rat vascular smooth muscle cells: activation by angiotensin II in hypertension. Circ Res. 1998;83:1271–1278. doi: 10.1161/01.res.83.12.1271. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Hwang PM, Snyder SH. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990;347:768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- Celermajer DS. Endothelial dysfunction: does it matter? Is it reversible? J Am Coll Cardiol. 1997;30:325–333. doi: 10.1016/s0735-1097(97)00189-7. [DOI] [PubMed] [Google Scholar]
- Chandran S, Sridhar N, Veeranjaneyulu A. Nitric oxide: concepts, current perspectives and future therapeutic implications. Indian J Pharmacol. 1998;30:351–366. [Google Scholar]
- Cooke JP, Stamler J, Andon N, Davies PF, McKinley G, Loscalzo J. Flow stimulates endothelial cells to release a nitrovasodilator that is potentiated by reduced thiol. Am J Physiol. 1990;259:H804–H812. doi: 10.1152/ajpheart.1990.259.3.H804. [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giddens D, Zarins C, Glagov S. The role of fluid mechanics in the localization and detection of atherosclerosis. J Biomech Eng. 1993;115:588–594. doi: 10.1115/1.2895545. [DOI] [PubMed] [Google Scholar]
- Gnasso A, Carallo C, Irace C, De Franceschi MS, Mattioli PL, Motti C, Cortese C. Association between wall shear stress and flow-mediated vasodilation in healthy men. Atherosclerosis. 2001;156:171–176. doi: 10.1016/s0021-9150(00)00617-1. [DOI] [PubMed] [Google Scholar]
- Gosgnach W, Messika-Zeitoun D, Gonzalez W, Philipe M, Michel JB. Shear stress induces iNOS expression in cultured smooth muscle cells: role of oxidative stress. Am J Physiol. 2000;279:C1880–C1888. doi: 10.1152/ajpcell.2000.279.6.C1880. [DOI] [PubMed] [Google Scholar]
- Guo X, Lu X, Ren H, Levin ER, Kassab GS. Estrogen modulates the mechanical homeostasis of mouse arterial vessels through nitric oxide. Am J Physiol Heart Circ Physiol. 2006;290:H1788–H1797. doi: 10.1152/ajpheart.01070.2005. [DOI] [PubMed] [Google Scholar]
- Ibrahim J, Miyashiro JK, Berk BC. Shear stress is differentially regulated among inbred rat strains. Circ Res. 2003;92:1001–1009. doi: 10.1161/01.RES.0000069687.54486.B1. [DOI] [PubMed] [Google Scholar]
- Kuo L, Davis MJ, Chilian WM. Longitudinal gradients for endothelium-dependent and -independent vascular responses in the coronary microcirculation. Circulation. 1995;92:518–525. doi: 10.1161/01.cir.92.3.518. [DOI] [PubMed] [Google Scholar]
- Lamontagne D, Pohl U, Busse R. Mechanical deformation of vessel wall and shear stress determine the basal release of endothelium-derived relaxing factor in the intact rabbit coronary vascular bed. Circ Res. 1992;70:123–130. doi: 10.1161/01.res.70.1.123. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Turk JR, Schrage WG, Woodman CR, Price EM. Influence of coronary artery diameter on eNOS protein content. Am J Physiol Heart Circ Physiol. 2003;284:H1307–H1312. doi: 10.1152/ajpheart.00792.2002. [DOI] [PubMed] [Google Scholar]
- Nishida K, Harrison DG, Navas J, Fisher AA, Dockery SP, Uematsu M, Nerem RM, Alexander RW, Murphy TJ. Molecular cloning and characterization of the constitutive bovine aortic endothelial cell nitric oxide synthase. J Clin Invest. 1992;90:2092–2096. doi: 10.1172/JCI116092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noris M, Morigi M, Donadelli R, Aiello S, Foppol M, Todeschini M, Orisio S, Remuzzi G, Remuzzi A. Nitric oxide synthesis by cultured endothelial cells is modulated by flow conditions. Circ Res. 1995;76:536–543. doi: 10.1161/01.res.76.4.536. [DOI] [PubMed] [Google Scholar]
- Ozawa N, Shichiri M, Iwashina M, Fukai N, Yoshimoto T, Hirata Y. Laminar shear stress up-regulates inducible nitric oxide synthase in the endothelium. Hypertens Res. 2004;27:93–99. doi: 10.1291/hypres.27.93. [DOI] [PubMed] [Google Scholar]
- Papadaki M, Tilton RG, Eskin SG, McIntire LV. Nitric oxide production by cultured human aortic smooth muscle cells: stimulation by fluid flow. Am J Physiol. 1998;274(2 Pt 2):H616–H626. doi: 10.1152/ajpheart.1998.274.2.h616. [DOI] [PubMed] [Google Scholar]
- Park CS, Park R, Krishna G. Constitutive expression and structural diversity of inducible isoform of nitric oxide synthase in human tissues. Life Sci. 1996;59:219–225. doi: 10.1016/0024-3205(96)00287-1. [DOI] [PubMed] [Google Scholar]
- Pollock JS, Nakane M, Buttery LDK, Martinez A, Springall D, Polak JM, Forstermann U, Murad F. Characterization and localization of endothelial nitric oxide synthase using specific monoclonal antibodies. Am J Physiol. 1993;265:C1379–C1387. doi: 10.1152/ajpcell.1993.265.5.C1379. [DOI] [PubMed] [Google Scholar]
- Ranjan V, Xiao Z, Diamond SL. Constitutive NOS expression in cultured endothelial cells is elevated by fluid shear stress. Am J Physiol. 1995;269:H550–H555. doi: 10.1152/ajpheart.1995.269.2.H550. [DOI] [PubMed] [Google Scholar]
- Rudic RD, Shesely EG, Maeda N, Smithies O, Segal SS, Sessa WC. Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J Clin Invest. 1998;101:731–736. doi: 10.1172/JCI1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessa WC, Pritchard K, Seyedi N, Wang J, Hintze TH. Chronic exercise in dogs increases coronary vascular nitric oxide production and endothelial cell nitric oxide synthase gene expression. Circ Res. 1994;74:349–353. doi: 10.1161/01.res.74.2.349. [DOI] [PubMed] [Google Scholar]
- Stroev PV, Hoskins PR, Easson WJ. Distribution of wall shear rate throughout the arterial tree: a case study. Atherosclerosis. 2007;191:276–280. doi: 10.1016/j.atherosclerosis.2006.05.029. [DOI] [PubMed] [Google Scholar]
- Tomimoto H, Nishimura M, Suenaga T, Nakamura S, Akiguchi I, Wakita H, Kimura J, Mayer B. Distribution of nitric oxide synthase in the human cerebral blood vessels and brain tissues. J Cereb Blood Flow Metab. 1994;14:930–938. doi: 10.1038/jcbfm.1994.124. [DOI] [PubMed] [Google Scholar]
- Ueeda M, Silvia SK, Olsson RA. Nitric oxide modulates coronary autoregulation in the guinea pig. Circ Res. 1992;70:1296–1303. doi: 10.1161/01.res.70.6.1296. [DOI] [PubMed] [Google Scholar]
- Woodman CR, Muller JM, Rush JW, Laughlin MH, Price EM. Flow regulation of eNOS and Cu/Zn SOD mRNA expression in porcine coronary arterioles. Am J Physiol Heart Circ Physiol. 1999;276:H1058–H1063. doi: 10.1152/ajpheart.1999.276.3.H1058. [DOI] [PubMed] [Google Scholar]
- Wu SP, Ringgaard S, Oyre S, Hansen MS, Rasmus S, Pedersen EM. Wall shear rates differ between the normal carotid, femoral, and brachial arteries: an in vivo MRI study. J Magn Reson Imaging. 2004;19:188–193. doi: 10.1002/jmri.10441. [DOI] [PubMed] [Google Scholar]
- Zeballos GA, Bernstein RD, Thompson CI, Forfia PR, Seyedi N, Shen W, Kaminiski PM, Wolin MS, Hintze TH. Pharmacodynamics of plasma nitrate/nitrite as an indication of nitric oxide formation in conscious dogs. Circulation. 1995;91:2982–2988. doi: 10.1161/01.cir.91.12.2982. [DOI] [PubMed] [Google Scholar]