Abstract
Pulmonary carcinosarcoma is a rare and aggressive neoplasm that has both epithelial and mesenchymal components. We report on a 63-year-old woman who was found to have a right upper-lobe pulmonary carcinosarcoma with metastases to the liver and gastric fundus. There are currently no published guidelines on the treatment of pulmonary sarcomatoid carcinomas. However, with our expanding knowledge of cancer metastasis, cases of carcinosarcoma illustrate our current understanding of epithelial–mesenchymal transition in action. Here, we discuss the development and treatment of these biphasic tumors and the possible role of epithelial–mesenchymal transition.
Keywords: carcinosarcoma, epithelial–mesenchymal transition, lung neoplasms
Introduction
Pulmonary sarcomatoid carcinomas (PSCs) are poorly differentiated neoplasms containing an admixture of epithelial components of conventional non-small cell lung cancers (NSCLCs) and spindle or giant cell components of sarcomas. Based on the 2004 World Health Organization classification, there are five major variants: pleomorphic carcinoma, spindle cell carcinoma, giant cell carcinoma, carcinosarcoma, and pulmonary blastoma [Travis et al. 2004].
Case report
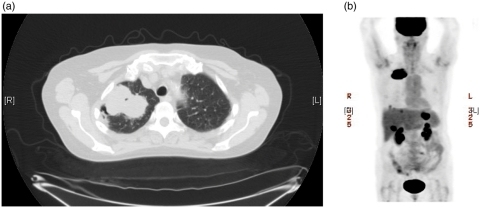
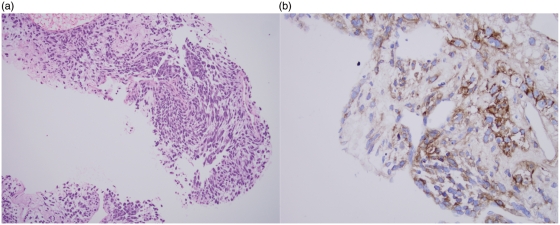
A 63-year-old white woman with a 50 pack-year smoking history presented with complaints of right upper back and shoulder pain. Chest computerized tomography revealed a 6.5 cm × 4.0 cm mass in the right upper lobe (Figure 1(a)). A positron emission tomography scan showed intense fludeoxyglucose 18F uptake in the right lung and in the liver and gastric fundus (Figure 1(b)). A core biopsy of the lung lesion showed a poorly differentiated malignancy with two morphologies. Areas of epithelial differentiation with squamous morphology were identified, along with areas of spindle cell morphology with focal osteoid and chondroid matrix formation (Figure 2(a)). Both components were positive for pan-cytokeratin and cytokeratin 7 (CK7). CK5/6 and p63 were positive in the epithelial component, supporting the histological impression of squamous differentiation. The tumor was negative for CD20, thyroid transcription factor (TTF-1), chromogranin, synaptophysin, and estrogen receptor. Strong membranous staining for epidermal growth factor receptor (EGFR) was noted in the tumor cells (Figure 2(b)), and an antibody against Ki-67 (MIB-1) was noted to be high, focally up to 30%. A subsequent biopsy of the gastric mass showed a similar morphology and staining pattern as the lung lesion confirming the diagnosis of metastatic carcinosarcoma. The patient was started on treatment with cisplatin and gemcitabine. However, her disease progressed quickly on first-line treatment and she died after receiving second-line docetaxel.
Figure 1.
(a) Chest computerized tomography scan showing a 6.5 cm × 4.0 cm mass in the right upper lobe. (b) Positron emission tomography scan showing intense fludeoxyglucose 18F uptake in the right lung mass and in the liver and gastric fundus.
Figure 2.
(a) Pulmonary carcinosarcoma on lung biopsy showing epithelioid and spindle cell morphology (H&E stain; 40×). (b) Carcinosarcoma demonstrating strong, membranous immunoreactivity for epidermal growth factor receptor (EGFR) (immunohistochemical stain for EGFR; 40×).
Discussion
PSCs make up approximately 0.2–1.3% of all lung malignancies [Travis et al. 2004; Davis et al. 1984]. The male to female ratio is approximately 4:1, the average age of presentation is 60 years and the disease is prevalent in people who smoke [Travis et al. 2004; Rossi et al. 2003; Chang et al. 2001; Davis et al. 1984]. Pulmonary blastoma, however, affects men and women equally with an average age of presentation of 40 years [Travis et al. 2004; Robert et al. 2002]. PSCs present more commonly in the upper lobes, and diameters up to 19 cm have been noted with a median size of 4.5 cm [Pelosi et al. 2010; Chang et al. 2001]. The location of the tumor is associated with the carcinomatous component. Squamous carcinomas are most often found in central tumors, while glandular histology is seen in peripheral lung lesions. The sarcomatous component, however, appears to be independent of location [Losada et al. 2010; Adachi et al. 1992]. PSCs tend to invade adjacent structures by forming large necrotic and hemorrhagic masses [Pelosi et al. 2010; Travis et al. 2004]. They cannot be distinguished from conventional NSCLCs on the basis of clinical evaluation.
PSCs usually metastasize to the same tissues as conventional NSCLCs, namely brain, bone, adrenals, and liver. Unusual locations have been documented, including skin, esophagus, jejunum, rectum, heart, pancreas, kidney, and gastric metastases, as was noted in our patient [Pelosi et al. 2010; Mochizuki et al. 2008; Travis et al. 2004]. Clinical signs and symptoms are usually locally related to the anatomical structures involved with the tumor itself [Pelosi et al. 2010; Rossi et al. 2003; Koss et al. 1999; Fishback et al. 1994].
On the whole, patients with PSCs have poorer overall survival compared with patients with conventional NSCLCs [Martin et al. 2007; Mochizuki et al. 2008; Rossi et al. 2003; Chang et al. 2001]. In one study of resected pulmonary lesions, 63 PSC patients were matched 1:1 with NSCLC patients. The 5-year survival rate was found to be 24.5% versus 46.5% respectively (p = 0.01) with a median time to recurrence of 11.3 months compared with 61.4 months (p = 0.001) [Martin et al. 2007].
In 1865, Rudolf Virchow first suggested that the biphasic appearance of carcinosarcoma is due to a single ancestor undergoing divergent epithelial and mesenchymal differentiation early during neoplastic transformation [Pelosi et al. 2010; Thompson et al. 1996]. An opposing view suggests that sarcomatoid metaplasia of dedicated carcinoma cells occurs later during cancer progression [Pelosi et al. 2010; Dacic et al. 2002]. Microdissection-based allelotyping supports the evolution of sarcoma from pure carcinoma based on evidence of identical allelic losses shared by both tumor components [Dacic et al. 2002]. Ultrastructural studies of carcinosarcoma have also revealed the presence of tonofibrils and desmosomes (characteristic of epithelial cells), not only in the epithelial component but also in the sarcomatous component, suggesting derivation of the sarcomatous component from the carcinomatous component [Haraguchi et al. 1999]. For these reasons, the current notions of epithelial–mesenchymal transition (EMT) are based on this latter conversion paradigm.
EMT is a complex series of physical and cellular events resulting in the loss of characteristic epithelial traits of carcinoma cells and acquisition of mesenchymal traits. Through EMT, epithelial cells gain motility and invasiveness by undergoing a loss of cell-to-cell contacts, loss of cell polarity, and reorganization of cytoskeletal elements, resulting in decreased expression of epithelial cytokeratins, decreased expression of cell-to-cell adhesion proteins (E-cadherin and plakoglobin), and increased expression of vimentin, smooth-muscle actin, and fibronectin [Acloque et al. 2009; Voulgari and Pintzas, 2009; Turley et al. 2008].
Epithelial cells are formed of cells with apical–basolateral polarity, connected to each other by lateral adherens junctions composed of a complex of E-cadherin, catenins, and actin rings, and anchored to the basement membrane via integrins [Acloque et al. 2009; Voulgari et al. 2009]. They are prevented from undergoing shape, polarity, and motility changes as a result of these adhesive structures and continued expression of adhesion molecules [Voulgari et al. 2009].
Mesenchymal cells are more loosely organized and are not typically in contact with a basal membrane. They form weak, disorganized adhesions to neighboring cells and exhibit spindle-like morphology with front-to-back polarity. Unlike cytokeratin-rich filaments seen in epithelial tissues, mesenchymal cells are vimentin based. They are capable of independent motility and invasion through the basement membrane into surrounding tissue via secretion of extracellular matrix-degrading enzymes [Voulgari et al. 2009].
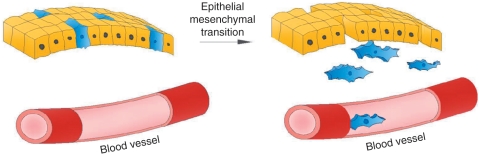
The process of EMT begins with the detachment of epithelial cells from connecting junctions via repression of transcription genes encoding adherens and tight junctions. This results in loss of cell polarity and internalization of E-cadherin, targeting it for degradation. Cytoskeletal remodeling leads to cell delamination, and metalloprotease activation favors migration of these now mesenchymal-type cells (Figure 3) [Acloque et al. 2009].
Figure 3.
The process of epithelial–mesenchymal transition showing detachment of epithelial cells from connecting junctions. Cytoskeleton remodeling leads to cell delamination, and metalloprotease activation favors migration of these now mesenchymal-type cells.
EMT, though physiologically necessary during development and morphogenesis, is usually maintained in a silent state in the adult. However, EMT inducers can be aberrantly activated during tumor progression and invasion [Acloque et al. 2009; Thiery et al. 2009]. Molecular elucidation of EMT in carcinogenesis has revealed dysregulation in cell-signaling networks with significant ‘cross talk’ among these complex pathways [Voulgari et al. 2009; Thiery and Sleeman, 2006].
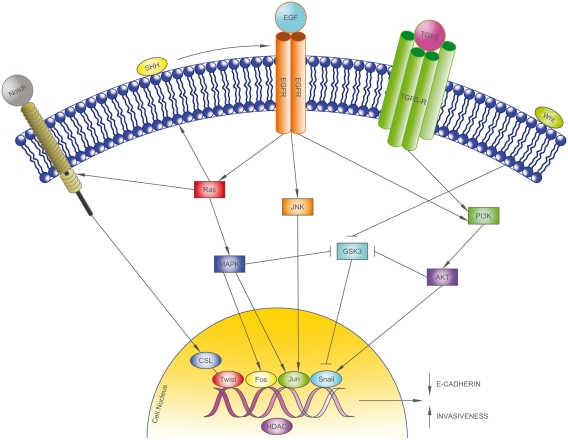
Several EMT induction pathways were studied in 22 cases of pulmonary carcinosarcomas. High nuclear activity in the transcription factor c-Jun was noted along with consecutive overexpression of vimentin and fascin, suggesting that EMT propagation in carcinosarcomas may be through the c-Jun/vimentin signaling pathway [Blaukovitsch et al. 2006]. Novel drug development is now beginning to target these EMT-related pathways by targeting molecular activators of EMT, such as transforming growth factor beta, Notch, and Hedgehog, as well as downstream transcriptional regulators including c-Jun, Twist, and Snail (Figure 4) [Voulgari et al. 2009; Blaukovitsch et al. 2006; Thiery and Sleeman, 2006].
Figure 4.
Epithelial–mesenchymal transition (EMT)-related pathways. EMT propagation in carcinosarcomas may be via the c-Jun/vimentin signaling pathway. Novel drugs are being developed to target molecular activators of EMT, such as transforming growth factor beta (TGFβ), Notch, and Hedgehog, as well as downstream transcriptional regulators including c-Jun, Twist, and Snail. AKT/PKB, protein kinase B; CSL, [CBF-1, Su(H), Lag-1]-type transcription factors; EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; GSK3, glycogen synthase kinase 3; HDAC, histone deacetylase; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; PI3K, phosphoinositide 3-kinase; SHH, sonic hedgehog homolog; TGFβ-R, transforming growth factor beta receptor.
EGFR has been identified as an important therapeutic target in a subset of NSCLCs. Interestingly, a single study evaluating EGFR and Kirsten rat sarcoma viral oncogene homolog (KRAS) status in a series of PSCs revealed a consistent lack of EGFR mutations and a high rate of KRAS mutations [Italiano et al. 2009]. PSCs may, thus, be less likely to benefit from anti-EGFR therapy, but data addressing the use of EGFR inhibitors in this patient population are lacking.
There are a small proportion of patients with EGFR mutations that do not respond to currently available anti-EGFR therapy (erlotinib or gefitinib). Several hypotheses for this differential response have been proposed. As tumor cells dedifferentiate and take on a more mesenchymal phenotype, EMT mechanisms may be involved in modulating EGFR activation and signaling or in activating alternative downstream targets that do not rely exclusively on the EGFR pathway (such as AKT), resulting in a decreased response to EGFR inhibitors (Figure 4) [Voulgari and Pintzas, 2009; Miyanaga et al. 2008; Andle and Rustgi, 2006] Data suggest that E-cadherin expression (a reliable marker of EMT) may be responsible for inhibiting EGFR activity by decreasing receptor mobility and ligand affinity [Andle and Rustgi, 2006; Yauch et al. 2005; Fedor-Chaiken et al. 2003]. Immunohistochemical staining of E-cadherin and vimentin in 62 unselected primary NSCLCs revealed the frequency of the epithelial phenotype (E-cadherin positivity) to be significantly greater in people with EGFR mutations than in the wild-type EGFR tumors [Deng et al. 2009].
E-Cadherin expression has been shown to be downregulated by histone deacetylase (HDAC) [Witta et al. 2006]. HDACs are involved in chromatin remodeling and induce transcriptional repression by deacetylating lysine residues on histone tails. HDAC inhibitors are an emerging class of therapeutic agents that can reactivate gene expression and inhibit the growth and survival of tumor cells [Johnstone, 2002]. A recent clinical study revealed that HDAC inhibitors induce E-cadherin expression and may allow for increased sensitivity of lung cancer to combined HDAC and EGFR-tyrosine kinase inhibition [Jotte et al. 2010].
There is ongoing controversy regarding the application of the term ‘EMT’ to what may, in part, be dedifferentiation events occurring during cancer progression and metastasis. Klymkowky and Savanger suggest that true EMT is defined by absolute transdifferentiation of epithelial cells as is noted during physiologic cellular remodeling and mesoderm and neural crest formation [Klymkowky and Savanger 2009]. The authors propose that the intermediate or partially differentiated phenotype exhibited in the cancer environment should be considered an ‘EMT-like phenotype’. While this may certainly be the case, in vivo gene expression profiling in metastatic disease consistently shows a definitive switch of tumors cells from a proliferative to an invasive phenotype [Hollier et al. 2009]. Traditional chemotherapy based on log kill assays targets cell proliferation pathways and may not be effective in eradicating this less avidly dividing, invasive cell population [Brabletz et al. 2001]. Furthermore, identification of EMT or an EMT-like event may help to better determine the type of antineoplastic therapy likely to be most beneficial. Traditional therapies may not be as efficacious for cancer phenotypes lacking epithelial differentiation and such information can help us look for better targets.
Conclusion
The outlook for patients with pulmonary carcinosarcomas is extremely poor. Current treatment of localized carcinosarcomas relies on definitive surgical resection. Case reports consistently report poor outcomes in patients with metastatic disease without overt benefit from systemic chemotherapy regimens (old or new). However, new insights into EMT induction, such as the c-Jun pathway, can aid in identification of discriminating markers for better prognostication and treatment of these aggressive tumors. Targeting EMT pathways and its modulators may be the key to eliminating surviving cancer cells, with the ultimate goal of preventing recurrence and improving long-term survival.
Footnotes
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
The authors declare no conflict of interest in preparing this article.
References
- Acloque H., Adams M.S., Fishwick K., Bronner-Fraser M., Nieto M.A. (2009) Epithelial–mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest 119: 1438–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi H., Morimura T., Yumoto T., Ikeda M., Fukui H. (1992) True pulmonary carcinosarcoma (squamous cell carcinoma and chondrosarcoma). A case report. Acta Pathol Jpn 42: 751–754 [DOI] [PubMed] [Google Scholar]
- Andl C.D., Rustgi A.K. (2006) No one-way street: cross-talk between E-cadherin and receptor tyrosine kinase (RTK) signaling. A mechanism to regulate RTK activity. Cancer Biol Ther 4: 28–31 [DOI] [PubMed] [Google Scholar]
- Blaukovitsch M., Halbwedl I., Kothmaier H., Gogg-Kammerer M., Popper H.H. (2006) Sarcomatoid carcinomas of the lung: are these histogenetically heterogeneous tumors?. Virchows Arch 449: 455–461 [DOI] [PubMed] [Google Scholar]
- Brabletz T., Jung A., Reu S., Porzner M., Hlubek F., et al. (2001) Variable β-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci U S A 98: 10356–10361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y.L., Lee Y.C., Shih J.Y., Wu C.T. (2001) Pulmonary pleomorphic (spindle) cell carcinoma: peculiar clinicopathologic manifestations different from ordinary nonsmall cell carcinoma. Lung Cancer 34: 91–97 [DOI] [PubMed] [Google Scholar]
- Dacic S., Finkelstein S.D., Sasatomi E., Swalsky P.A., Yousem S.A. (2002) Molecular pathogenesis of pulmonary carcinosarcoma as determined by microdissection based allelotyping. Am J Surg Pathol 26: 510–516 [DOI] [PubMed] [Google Scholar]
- Davis M.P., Eagan R.T., Weiland L.H., Pairolero P.C. (1984) Carcinosarcoma of the lung: Mayo Clinic experience and response to chemotherapy. Mayo Clin Proc 59: 598–603 [abstract]. [DOI] [PubMed] [Google Scholar]
- Deng Q.F., Zhou C.C., Su C.X. (2009) Clinicopathological features and epidermal growth factor receptor mutations associated with epithelial–mesenchymal transition in non-small cell lung cancer. Respirology 14: 371–376 [DOI] [PubMed] [Google Scholar]
- Fedor-Chaiken M., Hein P.W., Stewart J.C., Brackenburv R., Kinch M.S. (2003) E-cadherin binding modulates EGF receptor activation. Cell Commun Adhes 10: 105–118 [PubMed] [Google Scholar]
- Fishback N.F., Travis W.D., Moran C.A., Guinee D.G., Jr, McCarty W.F., Koss M.N. (1994) Pleomorphic (spindle/giant cell) carcinoma of the lung. A clinicopathologic correlation of 78 cases. Cancer 73: 2936–2945 [DOI] [PubMed] [Google Scholar]
- Haraguchi S., Fukuda Y., Sugisaki Y., Yamanaka N. (1999) Pulmonary carcinosarcoma: immunohistochemical and ultrastructural studies. Pathol Int 49: 903–908 [DOI] [PubMed] [Google Scholar]
- Hollier B.G., Evans K., Mani S.A. (2009) The epithelial-to-mesenchymal transition and cancer stem cells: a coalition against cancer therapies. J Mammary Gland Biol Neoplasia 14: 29–43 [DOI] [PubMed] [Google Scholar]
- Italiano A., Cortot A.B., Ilie M., Martel-Planche G., Fabas T., Pop D., et al. (2009) EGFR and KRAS status of primary sarcomatoid carcinomas of the lung: implications for anti-EGFR treatment in rare lung malignancy. Int J Cancer 125: 2479–2482 [DOI] [PubMed] [Google Scholar]
- Johnstone R.W. (2002) Histone-deacetylase inhibitors: novel drugs for the treatment of cancer. Nat Rev Drug Discov 1: 287–299 [DOI] [PubMed] [Google Scholar]
- Jotte R.M., Witta S.E., Spira A.I., Neubauer M.A., Konduri K., Ruxer R.L., et al. (2010) Molecular analysis identifies a subset of non-small cell lung cancer patients with differential sensitivity to histone. Deacetylase inhibitor (HDACI)/epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) treatment. J Thorac Oncol 5(12 Suppl. 7): S557–S563, [abstract] [Google Scholar]
- Klymkowsky M.W., Savagner P. (2009) Epithelial-mesenchymal transition: a cancer researcher’s conceptual friend and foe. Am J Pathol 174: 1588–1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss M.N., Hochholzer L., Frommelt R.A. (1999) Carcinosarcomas of the lung: a clinicopathologic study of 66 patients. Am J Surg Pathol 23: 1514–1526 [DOI] [PubMed] [Google Scholar]
- Losada M.J., Monterde V.B., Arriaga B.A., Perez A.I., Solares S.S., Puyal M.T. (2010) Lung carcinosarcoma. Clin Transl Oncol 12: 303–305 [DOI] [PubMed] [Google Scholar]
- Martin L.W., Correa A.M., Ordonez N.G., Roth J.A., Swisher S.G., Vaporciyan A.A., et al. (2007) Sarcomatoid carcinoma of the lung: a predictor of poor prognosis. Ann Thorac Surg 84: 973–980 [DOI] [PubMed] [Google Scholar]
- Miyanaga A., Gemma A., Ando M., Kosaihira S., Noro R., Minegishi Y., et al. (2008) E-Cadherin expression and epidermal growth factor receptor mutation status predict outcome in non-small cell lung cancer patients treated with gefitinib. Oncol Rep 19: 377–383 [PubMed] [Google Scholar]
- Mochizuki T., Ishii G., Nagai K., Yoshida J., Nishimura M., Mizuno T., et al. (2008) Pleomorphic carcinoma of the lung: clinicopathologic characteristics of 70 cases. Am J Surg Pathol 32: 1727–1735 [DOI] [PubMed] [Google Scholar]
- Pelosi G., Sonzogni A., De Pas T., Galetta D., Veronesi G., Spaggiari L., et al. (2010) Review article: pulmonary sarcomatoid carcinomas: a practical overview. Int J Surg Pathol 18: 103–120 [DOI] [PubMed] [Google Scholar]
- Robert J., Pache J.C., Seium Y., De Perrot M., Spiliopoulos A. (2002) Pulmonary blastoma: report of five cases and identification of clinical features suggestive of the disease. Eur J Cardiothorac Surg 22: 708–711 [DOI] [PubMed] [Google Scholar]
- Rossi G., Cavazza A., Sturm N., Migaldi M., Facciolongo N., Longo L., et al. (2003) Pulmonary carcinomas with pleomorphic, sarcomatoid, or sarcomatous elements. A clinicopathologic and immunohistochemical study of 75 cases. Am J Surg Pathol 27: 311–324 [DOI] [PubMed] [Google Scholar]
- Thiery J.P., Acloque H., Huang R.Y., Nieto M.A. (2009) Epithelial–mesenchymal transitions in development and disease. Cell 139: 871–890 [DOI] [PubMed] [Google Scholar]
- Thiery J.P., Sleeman J.P. (2006) Complex networks orchestrate epithelial–mesenchymal transitions. Nat Rev Mol Cell Biol 7: 131–142 [DOI] [PubMed] [Google Scholar]
- Thompson L., Chang B., Barsky S.H. (1996) Monoclonal origins of malignant mixed tumors (carcinosarcomas). Evidence for a divergent histogenesis. Am J Surg Pathol 20: 277–285 [DOI] [PubMed] [Google Scholar]
- Travis W.D., Brambilla E., Muller-Hermelink H.K., Harris C.C. (eds) (2004) World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart, Lyon: IARC Press [Google Scholar]
- Turley E.A., Veiseh M., Radisky D.C., Bissell M.J. (2008) Mechanisms of disease: epithelial–mesenchymal transition – does cellular plasticity fuel neoplastic progression?. Nat Clin Pract Oncol 5: 280–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voulgari A., Pintzas A. (2009) Epithelial–mesenchymal transition in cancer metastasis: mechanisms, markers, and strategies to overcome drug resistance in the clinic. Biochim Biophys Acta 1796: 75–90 [DOI] [PubMed] [Google Scholar]
- Witta S.E., Gemmill R.M., Hirsch F.R., Coldren C.D., Hedman K., Ravdel L., et al. (2006) Restoring E-cadherin expression increases sensitivity to epidermal growth factor receptor inhibitors in lung cancer cell lines. Cancer Res 66: 944–950 [DOI] [PubMed] [Google Scholar]
- Yauch R.L., Januario T., Eberhard D.A., Cavet G., Zhu W., Fu L., et al. (2005) Epithelial versus mesenchymal phenotype determines in vitro sensitivity and predicts clinical activity of erlotinib in lung cancer patients. Clin Cancer Res 11: 8686–8698 [DOI] [PubMed] [Google Scholar]