Abstract
Based on preclinical data available in the RIP1-Tag2 transgenic mouse model, sunitinib is an inhibitor of angiogenesis in pancreatic neuroendocrine tumors blocking vascular endothelial growth factor receptors and platelet-derived growth factor receptors in endothelial cells and pericytes, respectively. Evidence of objective response in phase I trials justified the initiation of a large phase II/III program using sunitinib in patients with advanced/metastatic well-differentiated pancreatic neuroendocrine tumors. In the phase II study, sunitinib showed potent antitumor activity and a safe toxicity profile. In a recent double-blind placebo-controlled randomized phase III trial, sunitinib doubled the progression-free survival of patients, induced objective responses, and reduced the risk of death of patients with advanced/metastatic well-differentiated tumors. These data allowed the approval of sunitinib in several countries including Europe and the United States of America. These recent data provide hope for patients with well-differentiated pancreatic neuroendocrine tumors and will change standards of care in this disease.
Keywords: analogs, angiogenesis, endocrine tumors, mTOR inhibitor, PDGFR, somatostatin combinations, VEGFR
Introduction
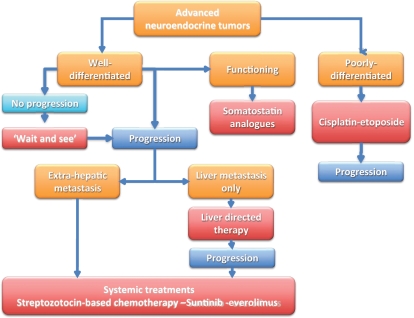
Pancreatic neuroendocrine tumors (PNETs) are uncommon malignancies that arise from endocrine cells located in the endocrine tissues of the pancreas [Arnold, 2005]. These tumors express synaptophysin and chromogranin, detected by immunohistochemistry. PNETs are heterogeneous and are classified according to their degree of differentiation (well-differentiated/low-grade versus poorly differentiated/high-grade) [Kloppel and Anlauf, 2005; Ballian et al. 2009]. Depending on the degree of differentiation, these tumors display different biological features [Couvelard et al. 2009] and clinical behavior (Figure 1). The degree of differentiation plays an important role in the therapeutic approaches of PNETs. The chemosensitivity of grade 1–2 tumors is different from that of grade 3 tumors. High-grade PNETs have a high proliferation rate as reflected by the high expression of Ki67 (MIBI). Treatment of poorly-differentiated/high-grade PNETs consists mainly of cisplatin–etoposide (VePeside) combination chemotherapy [Moertel et al. 1991]. High-grade tumors are initially chemosensitive; however, they almost always display further acquired resistance to chemotherapy, yielding an unfavorable outcome. Conversely, sensitivity of advanced/metastatic well-differentiated PNETs to cytotoxic systemic chemotherapy appears to be unpredictable. Thus, the treatment of advanced well-differentiated PNETs has not been very well defined and no standard active treatment has been really satisfactory.
Figure 1.
Therapeutic algorithm for patients with well-differentiated and poorly differentiated pancreatic neuroendocrine tumors.
Well-differentiated PNETs, with a more indolent clinical behavior, seem to primarily depend on tumor vascularization, as demonstrated by their high level of microvessel density [Couvelard et al. 2005]. These tumors are highly vascularized and show high expression of vascular endothelial growth factor (VEGF), vascular endothelial growth factor receptor 1 and 2 (VEGFR-1, VEGFR-2), and platelet-derived growth factor receptors (PDGFR-a and PDGFR-b). To date, long-acting somatostatin analogs (octreotide or lanreotide) have been the best treatment to achieve symptomatic benefit. There is evidence of tumor control in carcinoid midgut tumors in the recent placebo-controlled, randomized PROMID trial with octreotide [Rinke et al. 2009]; however, no patients with PNETs were included in the PROMID trial so it is unknown whether somatostatin analogs have antitumor activity in this disease.
For localized disease, surgery remains the mainstay of curative treatment for patients with resectable well-differentiated PNETs [Eriksson et al. 2009]. However, most patients with PNETs are not amenable to curative resection because of multiple hepatic, lung, or bone metastasis at the time of primary diagnosis or recurrences. Chemoembolization, radiofrequency ablation, or percutaneous ethanol injection are locoregional treatments that may offer palliative benefits for patients with metastasis limited to the liver [Yao et al. 2001; Knigge et al. 2008]. For patients with bulky liver involvement, rapid tumor progression, or extrahepatic spread, chemotherapy has been offered as a palliative approach to delay tumor-related symptoms [Vilar et al. 2007].
Cancer cell proliferation is highly variable in well-differentiated PNETs as reflected by variable mitotic indexes and various levels of Ki67 expression. Variability in cancer cell proliferation may have accounted for the inconsistent results using chemotherapy in well-differentiated PNETs. A trend towards a better benefit from using chemotherapy was observed in patients with tumors with high mitotic index. So far, the only chemotherapy approved for use in PNETs is streptozotocin, a DNA alkylating agent with high toxicity for islet cells. Other drugs approved in the treatment of PNET are doxorubicin and 5-fluorouracil, which have been combined with streptozotocin, and demonstrated encouraging results in clinical trials [Moertel et al. 1980, 1992; Kouvaraki et al. 2004]. The initially reported response rates of up to 69% with doxorubicin–streptozotocin combination have recently been challenged in clinical trials using modern imaging techniques and efficacy criteria, suggesting that the magnitude of clinical benefit using streptozotocin-based chemotherapy may have been previously overestimated [Heng and Saltz, 1999; McCollum et al. 2004]. Thus, new treatment options were urgently needed for unresectable, advanced and highly metastatic well-differentiated PNETs.
In this review, we focus on the mechanism of action of sunitinib on PNET angiogenesis and the recent clinical evidence of benefit of sunitinib in patients with advanced/metastatic PNET (Box 1).
Box 1. Search terms used to compile information for this review article.
Information for this review was compiled by searching PubMed and MEDLINE databases for articles published until May 2011. Only articles published in English were considered. The search terms used included ‘pancreatic neuroendocrine tumor’ in association with the search terms: ‘angiogenesis’, ‘VEGFR’, ‘PDGFR’, ‘sunitinib’, ‘everolimus’, ‘bevacizumab’, ‘mTOR inhibitors’, ‘rapamycin’, ‘rapalogues’, ‘temozolomide’, ‘streptozotocin’, ‘somatostatin analogs’, ‘IGF1-R inhibitor’, ‘natural product’, ‘metastatic’, ‘clinical trial’, ‘islet cell carcinomas’, ‘carcinoid tumors’, ‘targeted therapy’, ‘cytotoxic therapy’, ‘and ‘prognosis’. Full articles were obtained and references were checked for additional material and references when appropriate. Selected articles from a personal collection were also considered.
Role of angiogenesis in PNETs
As mentioned above, vascularization has been shown to play a role in the development of PNETs [Couvelard et al. 2005]. These tumors have a well-developed vasculature, mainly dependent on VEGF endocrine and paracrine secretion, suggesting a possible role for angiogenesis inhibitors in their treatment [Casanovas et al. 2005; Inoue et al. 2002]. Well-differentiated PNETs seem to express higher levels of hypoxia inducible factor (HIF-1a), VEGF, and microvessel density than poorly differentiated PNETs. HIF-1a is mainly responsible of inducing VEGF in hypoxic condition. These data suggest a major role of tumor angiogenesis in the development and maintenance of PNETs [Couvelard et al. 2005]. Biological data suggest that higher levels of angiogenesis can be observed in tumors with a low level of cancer cell proliferation, as measured by Ki67 expression [Couvelard et al. 2005]. Furthermore, immunohistochemical analysis of tissue from malignant well-differentiated PNETs, shows widespread expression of PDGFR-α, PDGFR-β, stem cell factor receptor (c-KIT), VEGFR-2 and VEGFR-3, and epidermal growth factor receptor (EGFR) [Couvelard et al. 2005; Casanovas et al. 2005; Inoue et al. 2002]. Considering that VEGFR plays a major role in endothelial cell survival and that PDGFR plays a similar function in pericytes, both VEGFR and PDGFR were considered as potential targets for therapeutic interventions in well-differentiated PNETs.
Clinical trials in PNETs
During the last decade, many targeted therapies blocking biological targets involved in the development of malignancy have been developed, and showed significant clinical improvements in the treatment of various malignancies. Consistent preclinical data on cell signaling pathways involved in endocrine tumors led to the identification of several drug targets, providing a rationale for clinical investigations of targeted therapies in well-differentiated PNETs. Owing to the rarity of these tumors, until recently clinical experiences with novel anticancer agents in PNETs derived primarily from noncontrolled phase II studies with limited number of patients. The lack of control group and selection bias that are frequently associated with small phase II trials often led to inconsistent results, and made it difficult to assess the benefit derived from novel therapies in patients with PNETs.
Efforts have been made recently to design cooperative trials, allowing large randomized studies to be performed in these rare tumors. This approach has generated more robust data and allowed for encouraging progress. For example, Zhang and colleagues demonstrated that in patients with PNETs, elevated expression of VEGF correlates with decreased progression-free survival (PFS) and the proportion of patients developing metastasis [Zhang et al. 2007].
Sunitinib
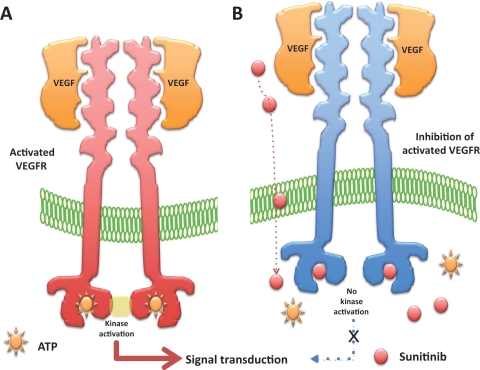
Sunitinib is an anti-angiogenic agent approved for the treatment of advanced renal cell carcinoma (RCC) and imatinib-resistant or intolerant gastrointestinal stromal tumors (GISTs). Sunitinib (sunitinib malate; SU11248; SUTENT; Pfizer Inc, New York, NY) is an indol derivative: C22H27FN4O2 that displays stability and solubility. Sunitinib is as an oral multi-targeted tyrosine kinase inhibitor (TKI) with antitumor and anti-angiogenic activities. It has been identified as a potent inhibitor of VEGFR-1, VEGFR-2, VEGFR-3, KIT (stem-cell factor [SCF] receptor), PDGFR-a and PDGFR-b in both biochemical and cellular assays (Figure 2) [Abrams et al. 2003a; Mendel et al. 2003]. All of these receptors are involved in the angiogenesis of well-differentiated PNETs. In vitro, sunitinib inhibited growth of cell lines driven by VEGF, SCF, and PDGF and induced apoptosis of human umbilical vein endothelial cells. In addition sunitinib inhibits fms-like tyrosine kinase 3, colony-stimulating factor-1 receptor (CSF-1R), and glial cell line derived neurotrophic factor receptor (rearranged during transfection RET), providing rationale for evaluation of sunitinib in PNETs [Mendel et al. 2003; Faivre et al. 2007].
Figure 2.
Mechanism of action of sunitinib in endothelial cells expressing the vascular endothelial growth factor receptors (VEGFRs). (A) The binding of vascular endothelial growth factors (VEGFs) to VEGFR leads to the dimerization of VEGFR and the activation of the intracellular kinase domain of VEGFR. The activation of VEGFR requires the presence of adenosine triphosphate (ATP). (B) Sunitinib penetrates into the cytoplasm of cells and enters into competition with ATP for the VEGFR ATP-binding pocket. In the presence of sunitinib, the activated VEGFR can no longer activate its intracellular kinase domain, preventing further downstream cell signaling.
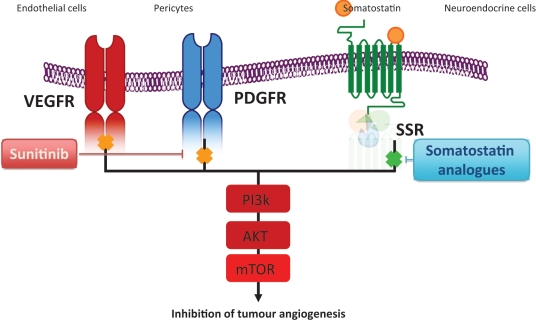
Additional data on sunitinib in the RIP1-Tag2 transgenic mouse model of pancreatic islet cell tumors demonstrated a 75% reduction in the density of endothelial cells and a 63% reduction in pericyte coverage of tumor vessels as a result of the inhibition of VEGFR and PDGFR, respectively (Figure 3) [Pietras and Hanahan, 2005; Couvelard et al. 2005]. The magnitude of the effect was greater than when VEGFR or PDGFR was inhibited alone, suggesting a potential for greater therapeutic benefit when both of the receptor families are inhibited concurrently [Yao et al. 2006]. In the RIP1-Tag2 transgenic mouse model of pancreatic islet cell carcinoma, sunitinib reduced tumor burden and increased animal survival by inhibiting the proliferation of VEGFR-dependent endothelial cell and by reducing the PDGFR-dependent pericyte coverage [Pietras and Hanahan, 2005].
Figure 3.
Sunitinib inhibits vascular endothelial growth factor receptor (VEGFR) in endothelial cells and platelet-derived growth factor receptor (PDGFR) in pericytes resulting in the inhibition of angiogenesis in pancreatic neuroendocrine tumors (PNETs). Somatosatin receptors that act mainly in tumor cells can be used with sunitinib. AKT, protein kinase B; mTOR, mammalian target of rapamycin; PI3K, phosphoinositide 3-kinase; SSR, somatostatin receptor.
Biological and pharmacological effects of sunitinib
Sunitinib exhibited dose- and time-dependent antitumor activity in mice, potently repressing the growth of a broad variety of human tumor xenografts [Mendel et al. 2003; Murray et al. 2003; Abrams et al. 2003b; Morimoto et al. 2004; Yee et al. 2004]. In vivo, sunitinib caused bone marrow depletion and effects in the pancreas in rats and monkeys, as well as adrenal toxicity in rat (microhemorrhages). In monkeys, a slight increase in arterial blood pressure and QT interval were reported at higher doses. In vitro metabolism studies demonstrated that sunitinib was primarily metabolized by cytochrome CYP3A4, resulting in the formation of a major, pharmacologically active N-desethyl metabolite, SU012662. This metabolite was shown to be equipotent to the parent compound in biochemical tyrosine kinase and cellular proliferation assays, acting toward VEGFR, PDGFR, and KIT [Baratte et al. 2004]. Radiolabeled orally administrated sunitinib in preclinical species was primarily excreted in the feces (rat >71% and monkey >84%).
Pharmacokinetic/pharmacodynamic data from animal studies showed that target plasma concentrations of sunitinib plus SU012662 capable of inhibiting PDGFR-ß and VEGFR-2 phosphorylation were established in the range 50–100 ηg/ml [Mendel et al. 2003; Murray et al. 2003; Abrams et al. 2003b]. Interestingly, those data were consistent with those observed in patients with acute myeloid leukemia in whom exposure to sunitinib led to a sustained inhibition of FLT3 phosphorylation in blast cells [O’Farrell et al. 2003]. Although initial studies were planned to provide continuous administration, the 4 weeks on, 2 weeks off schedule was selected at the request of the health authorities to allow patients to recover from potential bone marrow and adrenal toxicity observed in animal models. As a result of this requirement, the clinical development of sunitinib was made primarily using the discontinued 4 weeks on, 2 weeks off schedule. Further clinical development using continuous daily administration showed a safe toxicity profile. Since preclinical data in the RIP-TAG model suggested that increased invasiveness was possible when VEGFR2 inhibition was interrupted, the continuous schedule rather than the intermittent schedule was selected in the phase III trial that led to the approval of sunitinib in PNETs [Pàez-Ribes et al. 2009].
Safety of sunitinib
Most of the past experiences of sunitinib used the discontinued schedule. The phase I study showed that the maximum tolerated dose (MTD) of sunitinib was 75 mg and that the recommended dose was 50 mg/day, 4 weeks on, 2 weeks off schedule [Faivre et al. 2006]. Hypertension and asthenia appear to be the most common adverse effects with sunitinib (as well as other TKIs) [Faivre et al. 2006; Willett et al. 2004; Yang et al. 2003; Ahmad and Eisen, 2004]. Hypertension usually requires standard antihypertensive therapy (or dose reduction), and treatment discontinuation is less frequently necessary. A decrease in left ventricular ejection fraction is a rare but potentially life-threatening complication. A phase I study with sunitinib 2 weeks on, 1 week off in 12 patients with solid tumors showed that five patients presented asymptomatic grade 2 decreases in the left ventricular ejection fraction [Britten et al. 2008]. Other adverse events are skin toxicity, consisting of dry skin, edema, and hand–foot syndrome with/without bullous lesions, observed only at doses ≥75 mg/day that may require treatment discontinuation for a few days and/or dose reduction [Faivre et al. 2006]. The most consistent pathological changes are dermal vascular modifications with slight endothelial changes in grade 1–2 hand–foot syndrome and more pronounced vascular alterations with scattered keratinocyte necrosis and intraepidermal cleavage in grade 3 hand–foot syndrome and peribullous lesions [Faivre et al. 2006].
Sunitinib induces endothelial cell apoptosis in vitro and in animal tumor models and pathologic changes observed in the skin toxicity suggest that dermal vessel alteration and apoptosis might be due to direct anti-VEGFR and/or anti-PDGFR effects of sunitinib on dermal endothelial cells. Consistent with the effects of sunitinib on dermal endothelial cells, asymptomatic sublingual splinter hemorrhages was observed in several patients associated in one case with thrombocytopenia suggesting microangiopathy [Rouffiac et al. 2004; Robert et al. 2005]. Thrombosis has been reported in several other trials of drugs that target VEGF and VEGFRs, and was also observed with sunitinib [Osusky et al. 2004; Marx et al. 2002].
A recent study showed that exposure to SU5416, another multitargeted TKI blocking VEGFR, increased the susceptibility of endothelial cells to be damaged by cisplatin and gemcitabine [Kuenen et al. 2003]. Taken together, these results reinforced the hypothesis suggesting that VEGF plays a protector role for endothelial cells. Reversible hair depigmentation was observed with sunitinib at ≥25 mg/day. Persistence of melanocytes associated with hair follicles indicated that sunitinib did not affect the migration and survival of melanocytes. It is known that hair pigmentation is dependent on the modulation of tyrosinase-related protein 1 genes and tyrosinase, related to the KIT signaling pathway [Kitamura et al. 2004; Hemesath et al. 1998; Botchkareva et al. 2001]. Blocking KIT/PDGFR with imatinib mesylate induced skin depigmentation but paradoxically caused hair repigmentation [Tsao et al. 2003; Etienne et al. 2002]. Therefore, the graying effects of sunitinib may be related to multiple signaling pathways inhibition, including KIT, PDGFR, and VEGFR [Moss et al. 2003; Robert et al. 2003]. Interestingly, gray hair might be regarded as a potential surrogate marker to monitor sunitinib observance and biologic effects.
Diarrhea, anorexia, dysgeusia, stomatitis and edema are other clinically relevant toxicities. Fatigue may in part be related to the development of hypothyroidism during sunitinib therapy. Thyroid hormone levels should be monitored during treatment with sunitinib. The occurrence of clinical signs of hypothyroidism might need treatment with levothyroxine sodium [Theou-Anton et al. 2009]. Hematologic toxicity, consisting mostly of mild thrombocytopenia and neutropenia usually requires no intervention: Faivre and colleagues showed that it resolved during the rest period with the 4 weeks on, 2 weeks off schedule [Faivre et al. 2006].
Although usually well tolerated, sunitinib needs to be administered cautiously with medical follow up in patients with cancer to prevent, avoid and treat adverse effects in order to improve patient compliance. Its established antitumor activity requires attempting to maintain the highest tolerable dose in individual patients. Current oral formulations allow physicians to modulate dosages (between 25 and 50 mg/day) and/or schedules (4 weeks on, 2 weeks off or continuous administration schedule) to optimize the benefit–risk profile of sunitinib in individual patients.
Safety of sunitinib and efficacy in PNETs
During the course of the phase I trial, a strong antitumor activity was noticed, reflected by the unusually high number of objective responses in several tumor types [Faivre et al. 2006]. Tumors that responded to sunitinib were mainly those where the aforementioned kinases play a major role in cell proliferation, tumor development, and had highly angiogenic activity. These tumors are mostly resistant to classical cytotoxic chemotherapy agents. First responses were observed in RCC, and in imatinib-resistant GIST, leading to phase II/III trials that subsequently demonstrated the efficacy of sunitinib in those two indications [Motzer et al. 2007; Demetri et al. 2006]. As a consequence, sunitinib has been approved for the first-line treatment of patients with advanced RCC and for the treatment of patients with GIST after disease progression or intolerance to imatinib therapy and demonstrated a high level of efficacy with acceptable tolerability using either the 50 mg/day oral 4-weeks-on, 2-weeks-off schedule or the continuous daily administration schedule at a lower dose of 37.5 mg/day.
Based on preclinical data strongly suggesting the dramatic effects of sunitinib in PNETs and angiogenesis and encouraging evidence of objective responses in patients with neuroendocrine tumors (NETs) in phase I trials, a multicenter phase II trial was launched with sunitinib (50 mg/day, 4 weeks on and 2 weeks off) in patients with NETs, including carcinoids and PNETs [Kulke et al. 2008]. In this trial, among 66 patients with advanced PNETs, the objective response rate was 16.7%, with 56.1% of the patients experiencing tumor stabilization for more than 6 months, leading to a median time to tumor progression of 7.7 months [Kulke et al. 2008].
These encouraging results set the basis for a large international double-blind randomized phase III trial comparing 37.5 mg sunitinib continuous daily dosing with placebo in patients with well-differentiated PNETs progressing within 12 months before the study entry [Raymond et al. 2009, 2011]. The study was discontinued early, after the independent data and safety monitoring committee observed more serious adverse events and deaths in the placebo group as well as a difference in PFS favoring sunitinib. Median PFS was 11.4 months in the sunitinib group as compared with 5.5 months in the placebo group (hazard ratio [HR] for progression or death, 0.42; 95% confidence interval [CI], 0.26–0.66; p < 0.001) [Raymond et al. 2011]. A Cox proportional-hazards analysis of PFS according to baseline characteristics favored sunitinib in all subgroups studied. The objective response rate was 9.3% in the sunitinib group versus 0% in the placebo group. At the data cutoff point, 9 deaths were reported in the sunitinib group (10%) versus 21 deaths in the placebo group (25%) (HR for death, 0.41; 95% CI 0.19–0.89; p = 0.02). The magnitude of benefit in this trial seems to be independent of the bulk of the liver involvement by the tumor, previous treatments, prior or concurrent use of somatostatin analogs, and the rate of expression of Ki67.
The most frequent adverse events in the sunitinib group were diarrhea, nausea, vomiting, asthenia, and fatigue as in the other trials [Raymond et al. 2011]. Results from this trial suggest that continuous daily administration of sunitinib at a dose of 37.5 mg/day improved PFS, overall survival, and the objective response rate as compared with placebo among patients with well-differentiated PNETs. In addition, sunitinib has a good safety profile using this continuous daily dosing schedule. In this trial, the safety profile (the frequency and magnitude of adverse events) was consistent with that previously reported in other trials in patients with RCC and GIST. Interestingly, the quality of life of patients was not affected by the use of sunitinib treatment in this phase III trial.
Based on these data, approvals from the US Food and Drug Administration and European Medicines Agency have been decided for sunitinib in advanced well-differentiated PNETs. It is likely that the approval of sunitinib in PNETs provides new opportunities for the treatment of this patient population and will change the standard of care of patients with advanced PNETs. Furthermore, it is also likely that sunitinib will be used in future clinical trials as a control arm for novel anticancer agents that will be developed in patients with advanced well-differentiated PNETs.
Conclusions
Sunitinib, demonstrating a good safety profile and important efficacy in PNETs, has paved the way for further trials in other neuroendocrine type tumors, such as carcinoids, more poorly differentiated neuroendocrine diseases, and several other endocrine tumors that depend on VEGF/VEGFR for angiogenesis. Furthermore, based on these data, combinations of sunitinib with somatostatin analogs or chemotherapy may be further evaluated in future clinical trials. Other options may be using sunitinib in the neo- adjuvant or adjuvant setting in patients with resectable PNETs at earlier stages of tumor development. Interestingly, another targeted agent, the mTOR (mammalian target of rapamycin) inhibitor, everolimus, also showed promising activity in patients with well-differentiated PNETs [Yao et al. 2011]. These trials are the first examples demonstrating that targeted agents may have activity in patients with PNETs.
Footnotes
This work was supported by the Foundation Nelia & Amadeo Barleta (FNAB) and by the Association d’Aide à la Recherche et l’Enseignement en Cancérologie (AAREC).
Eric Raymond and Sandrine Faivre are consultants for Pfizer and Novartis. Eric Raymond and Sandrine Faivre received grants from Pfizer and Novartis to support research in their institution.
References
- Abrams T.J., Lee L.B., Murray L.J., Pryer N.K., Cherrington J.M. (2003a) SU11248 inhibits KIT and platelet-derived growth factor receptor beta in preclinical models of human small cell lung cancer. Mol Cancer Ther 2: 471–478 [PubMed] [Google Scholar]
- Abrams T.J., Murray L.J., Pesenti E., Holway V.W., Colombo T., Lee L.B., et al. (2003b) Preclinical evaluation of the tyrosine kinase inhibitor SU11248 as a single agent and in combination with "standard of care" therapeutic agents for the treatment of breast cancer. Mol Cancer Ther 2: 1011–1021 [PubMed] [Google Scholar]
- Ahmad T., Eisen T. (2004) Kinase inhibition with BAY 43-9006 in renal cell carcinoma. Clin Cancer Res 10: 6388S–6392S [DOI] [PubMed] [Google Scholar]
- Arnold R. (2005) Endocrine tumours of the gastrointestinal tract. Introduction: definition, historical aspects, classification, staging, prognosis and therapeutic options. Best Pract Res Clin Gastroenterol 19: 491–505 [DOI] [PubMed] [Google Scholar]
- Ballian N., Loeffler A.G., Rajamanickam V., Norstedt P.A., Weber S.M., Cho C.S. (2009) A simplified prognostic system for resected pancreatic neuroendocrine neoplasms. HPB (Oxford) 11: 422–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratte S., Sarati S., Frigerio E., James C.A., Ye C., Zhang Q. (2004) Quantitation of SU11248, an oral multi-target tyrosine kinase inhibitor, and its metabolite in monkey tissues by liquid chromatograph with tandem mass spectrometry following semi-automated liquid-liquid extraction. J Chromatogr 1024: 87–94 [DOI] [PubMed] [Google Scholar]
- Botchkareva N.V., Khlgatian M., Longley B.J., Botchkarev V.A., Gilchrest B.A. (2001) SCF/c-kit signaling is required for cyclic regeneration of the hair pigmentation unit. FASEB J 15: 645–658 [DOI] [PubMed] [Google Scholar]
- Britten C.D., Kabbinavar F., Hecht J.R., Bello C.L., Li J., Baum C., et al. (2008) A phase I and pharmacokinetic study of sunitinib administered daily for 2 weeks, followed by a 1-week off period. Cancer Chemother Pharmacol 61: 515–524 [DOI] [PubMed] [Google Scholar]
- Casanovas O., Hicklin D.J., Bergers G., Hanahan D. (2005) Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell 8: 299–309 [DOI] [PubMed] [Google Scholar]
- Couvelard A., Deschamps L., Ravaud P., Baron G., Sauvanet A., Hentic O., et al. (2009) Heterogeneity of tumor prognostic markers: a reproducibility study applied to liver metastases of pancreatic endocrine tumors. Mod Pathol 22: 273–281 [DOI] [PubMed] [Google Scholar]
- Couvelard A., O’Toole D., Turley H., Leek R., Sauvanet A., Degott C., et al. (2005) Microvascular density and hypoxia-inducible factor pathway in pancreatic endocrine tumours: negative correlation of microvascular density and VEGF expression with tumour progression. Br J Cancer 17(92): 94–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetri G.D., van Oosterom A.T., Garrett C.R., Blackstein M.E., Shah M.H., Verweij J., et al. (2006) Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomized controlled trial. Lancet 368: 1329–1338 [DOI] [PubMed] [Google Scholar]
- Eriksson B., Annibale B., Bajetta E., Mitry E., Pavel M., Platania M., et al. (2009) ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: chemotherapy in patients with neuroendocrine tumors. Neuroendocrinology 90: 214–219 [DOI] [PubMed] [Google Scholar]
- Etienne G., Cony-Makhoul P., Mahon F.X. (2002) Imatinib mesylate and gray hair. N Engl J Med 347: 446. [DOI] [PubMed] [Google Scholar]
- Faivre S., Delbaldo C., Vera K., Robert C., Lozahic S., Lassau N., et al. (2006) Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol 24: 25–35 [DOI] [PubMed] [Google Scholar]
- Faivre S., Demetri G., Sargent W., Raymond E. (2007) Molecular basis for sunitinib efficacy and future clinical development. Nat Rev Drug Discov 6: 734–745 [DOI] [PubMed] [Google Scholar]
- Hemesath T.J., Price E.R., Takemoto C., Badalian T., Fisher D.E. (1998) MAP kinase links the transcription factor Microphthalmia to c-Kit signaling in melanocytes. Nature 391: 298–301 [DOI] [PubMed] [Google Scholar]
- Heng P.N., Saltz L.B. (1999) Failure to confirm major objective antitumor activity for streptozocin and doxorubicin in the treatment of patients with advanced islet cell carcinoma. Cancer 86: 944–948 [PubMed] [Google Scholar]
- Inoue M., Hager J.H., Ferrara N., Gerber H.P., Hanahan D. (2002) VEGF-A has a critical, nonredundant role in angiogenic switching and pancreatic beta cell carcinogenesis. Cancer Cell 1: 193–202 [DOI] [PubMed] [Google Scholar]
- Kitamura R., Tsukamoto K., Harada K., Shimizu A., Shimada S., Kobayashi T., et al. (2004) Mechanisms underlying the dysfunction of melanocytes in vitiligo epidermis: Role of SCF/KIT protein interactions and the downstream effector, MITF-M. J Pathol 202: 463–475 [DOI] [PubMed] [Google Scholar]
- Kloppel G., Anlauf M. (2005) Epidemiology, tumour biology and histopathological classification of neuroendocrine tumours of the gastrointestinal tract. Best Pract Res Clin Gastroenterol 19: 507–517 [DOI] [PubMed] [Google Scholar]
- Knigge U., Hansen C.P, Stadil F. (2008) Interventional treatment of neuroendocrine liver metastases. Surgeon 6: 232–239 [DOI] [PubMed] [Google Scholar]
- Kouvaraki M.A., Ajani J.A., Hoff P., Wolff R., Evans D.B., Lozano R., et al. (2004) Fluorouracil, doxorubicin, and streptozocin in the treatment of patients with locally advanced and metastatic pancreatic endocrine carcinomas. J Clin Oncol 22: 4762–4771 [DOI] [PubMed] [Google Scholar]
- Kuenen B.C., Levi M., Meijers J.C., van Hinsbergh V.W., Berkhof J., Kakkar A.K., et al. (2003) Potential role of platelets in endothelial damage observed during treatment with cisplatin, gemcitabine, and the angiogenesis inhibitor SU5416. J Clin Oncol 21: 2192–2198 [DOI] [PubMed] [Google Scholar]
- Kulke M.H., Lenz H.J., Meropol N.J., Posey J., Ryan D.P., Picus J., et al. (2008) Activity of sunitinib in patients with advanced neuroendocrine tumors. J Clin Oncol 26: 3403–3410 [DOI] [PubMed] [Google Scholar]
- Marx G.M., Steer C.B., Harper P., Pavlakis N., Rixe O., Khayat D. (2002) Unexpected serious toxicity with chemotherapy and antiangiogenic combinations: Time to take stock! J Clin Oncol 20: 1446–1448 [DOI] [PubMed] [Google Scholar]
- McCollum A.D., Kulke M.H., Ryan D.P., Clark J.W., Shulman L.N., Mayer R.J., et al. (2004) Lack of efficacy of streptozocin and doxorubicin in patients with advanced pancreatic endocrine tumors. Am J Clin Oncol 27: 485–488 [DOI] [PubMed] [Google Scholar]
- Mendel D.B., Laird A.D., Xin X., Louie S.G., Christensen J.G., Li G., et al. (2003) In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: Determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res 9: 327–337 [PubMed] [Google Scholar]
- Moertel C.G., Hanley J.A., Johnson L.A. (1980) Streptozocin alone compared with streptozocin plus fluorouracil in the treatment of advanced islet-cell carcinoma. N Engl J Med 303: 1189–1194 [DOI] [PubMed] [Google Scholar]
- Moertel C.G., Kvols L.K., O’Connell M.J., Rubin J. (1991) Treatment of neuroendocrine carcinomas with combined etoposide and cisplatin. Evidence of major therapeutic activity in the anaplastic variants of these neoplasms. Cancer 68: 227–232 [DOI] [PubMed] [Google Scholar]
- Moertel C.G., Lefkopoulo M., Lipsitz S., Hahn R.G., Klaassen D. (1992) Streptozocin-doxorubicin, streptozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med 326: 519–523 [DOI] [PubMed] [Google Scholar]
- Morimoto A.M., Tan N., West K., McArthur G., Toner G.C., Manning W.C., et al. (2004) Gene expression profiling of human colon xenograft tumors following treatment with SU11248, a multitargeted tyrosine kinase inhibitor. Oncogene 23: 1618–1626 [DOI] [PubMed] [Google Scholar]
- Moss K.G., Toner G.C., Cherrington J.M., Mendel D.B., Laird A.D. (2003) Hair depigmentation is a biological readout for pharmacological inhibition of KIT in mice and humans. J Pharmacol Exp Ther 307: 476–480 [DOI] [PubMed] [Google Scholar]
- Motzer R.J., Hutson T.E., Tomczak P., Michaelson M.D., Bukowski R.M., Rixe, et al. (2007) Sunitinib versus interferon α in metastatic renal-cell carcinoma. N Eng J Med 356: 115–124 [DOI] [PubMed] [Google Scholar]
- Murray L.J., Abrams T.J., Long K.R., Ngai T.J., Olson L.M., Hong W., et al. (2003) SU11248 inhibits tumor growth and CSF-1R-dependent osteolysis in an experimental breast cancer bone metastasis model. Clin Exp Metastasis 20: 757–766 [DOI] [PubMed] [Google Scholar]
- O’Farrell A.M., Foran J.M., Fiedler W., Serve H., Paquette R.L., Cooper M.A., et al. (2003) An innovative phase I clinical study demonstrates inhibition of FLT3 phosphorylation by SU11248 in acute myeloid leukemia patients. Clin Cancer Res 9: 5465–5476 [PubMed] [Google Scholar]
- Osusky K.L., Hallahan D.E., Fu A., Ye F., Shyr Y., Geng L. (2004) The receptor tyrosine kinase inhibitor SU11248 impedes endothelial cell migration, tubule formation, and blood vessel formation in vivo, but has little effects on existing tumor vessels. Angiogenesis 7: 225–233 [DOI] [PubMed] [Google Scholar]
- Pàez-Ribes M., Allen E., Hudock J., Takeda T., Okuyama H., Viñals F., et al. (2009) Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell 15: 220–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietras K., Hanahan D. (2005) A multitargeted, metronomic, and maximum-tolerated dose “chemo-switch” regimen is antiangiogenic, producing objective responses and survival benefit in a mouse model of cancer. J Clin Oncol 23: 939–952 [DOI] [PubMed] [Google Scholar]
- Raymond E., Dahan L., Raoul J.L., Bang Y.J., Borbath I., Lombard-Bohas C., et al. (2011) Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 364: 501–513 [DOI] [PubMed] [Google Scholar]
- Raymond E., Faivre S., Hammel P., Ruszniewski P. (2009) Sunitinib paves the way for targeted therapies in neuroendocrine tumors. Target Oncol 4: 253–254 [DOI] [PubMed] [Google Scholar]
- Rinke A., Muller H.H., Schade-Brittinger C., Klose K.J., Barth P., Wied M., et al. (2009) Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID study group. J Clin Oncol 27: 4656–4663 [DOI] [PubMed] [Google Scholar]
- Robert C., Faivre S., Raymond E., Armand J.P., Escudier B. (2005) Sublingual splinter hemorrhages: A clinical window to inhibition of vascular endothelial growth factor receptors? Ann Intern Med 143: 313–314 [DOI] [PubMed] [Google Scholar]
- Robert C., Spatz A., Faivre S., Armand J.P., Raymond E. (2003) Tyrosine kinase inhibition and grey hair. Lancet 361: 1056. [DOI] [PubMed] [Google Scholar]
- Rouffiac V., Bouquet C., Lassau N., Opolon P., Koscielny S., Peronneau P., et al. (2004) Validation of a new method for quantifying in vivo murine tumor necrosis by sonography. Invest Radiol 39: 350–356 [DOI] [PubMed] [Google Scholar]
- Theou-Anton N., Faivre S., Dreyer C., Raymond E. (2009) Benefit-risk assessment of sunitinib in gastrointestinal stromal tumours and renal cancer. Drug Safety 32: 717–734 [DOI] [PubMed] [Google Scholar]
- Tsao A.S., Kantarjian H., Cortes J., O’Brien S., Talpaz M. (2003) Imatinib mesylate causes hypopigmentation in the skin. Cancer 98: 2483–2487 [DOI] [PubMed] [Google Scholar]
- Vilar E., Salazar R., Perez-Garcıa J., Cortes J., Oberg K., Tabernero J. (2007) Chemotherapy and role of the proliferation marker Ki-67 in digestive neuroendocrine tumors. Endocr Relat Cancer 14: 221–232 [DOI] [PubMed] [Google Scholar]
- Willett C.G., Boucher Y., di Tomaso E., Duda D.G., Munn L.L., Tong R.T., et al. (2004) Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med 10: 145–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.C., Haworth L., Sherry R.M., Hwu P., Schwartzentruber D.J., Topalian S.L., et al. (2003). A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 349: 427–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J.C., Shah M.H., Ito T., Bohas C.L., Wolin E.M, Van Cutsem E., et al. (2011) RAD001 in Advanced Neuroendocrine Tumors, Third Trial (RADIANT-3) Study Group. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 364: 514–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao K.A., Talamonti M.S., Nemcek A., Angelos P., Chrisman H., Skarda J., et al. (2001) Indications and results of liver resection and hepatic chemoembolization for metastatic gastrointestinal neuroendocrine tumors. Surgery 130: 677–682 [DOI] [PubMed] [Google Scholar]
- Yao V.J., Sennino B., Davis R.B. (2006) Combined anti-VEGFR and anti-PDGFR actions of sunitinib on blood vessels in preclinical tumor models. Eur J Cancer 4(27): 8 [Google Scholar]
- Yee K.W., Schittenhelm M., O’Farrell A.M., Town A.R., McGreevey L., Bainbridge T., et al. (2004) Synergistic effect of SU11248 with cytarabine or daunorubicin on FLT3-ITD positive leukemic cells. Blood 104: 4202–4209 [DOI] [PubMed] [Google Scholar]
- Zhang J., Jia Z., Li Q., Wang L., Rashid A., Zhu Z., et al. (2007) Elevated expression of vascular endothelial growth factor VEGF correlates with increased angiogenesis and decreased progression-free survival among patients with low-grade neuroendocrine tumors. Cancer 109: 1478–1486 [DOI] [PubMed] [Google Scholar]