Abstract
Ray, R. and D. Zald. Anatomical insights into the interaction of emotion and cognition in the prefrontal cortex. NEUROSCI BIOBEHAV REV 36(X) XXX-XXX, 2011. -Psychological research increasingly indicates that emotional processes interact with other aspects of cognition. Studies have demonstrated both the ability of emotional stimuli to influence a broad range of cognitive operations, and the ability of humans to use top-down cognitive control mechanisms to regulate emotional responses. Portions of the prefrontal cortex appear to play a significant role in these interactions. However, the manner in which these interactions are implemented remains only partially elucidated. In the present review we describe the anatomical connections between ventral and dorsal prefrontal areas as well as their connections with limbic regions. Only a subset of prefrontal areas are likely to directly influence amygdalar processing, and as such models of prefrontal control of emotions and models of emotional regulation should be constrained to plausible pathways of influence. We also focus on how the specific pattern of feedforward and feedback connections between these regions may dictate the nature of information flow between ventral and dorsal prefrontal areas and the amygdala. These patterns of connections are inconsistent with several commonly expressed assumptions about the nature of communications between emotion and cognition.
Keywords: dorsolateral, ventrolateral, orbitofrontal, functional connectivity, emotion regulation, attention, working memory
1. Introduction
Accumulating research examines how emotion interacts with other aspects of cognition. Such work has elucidated the ways in which emotionally valenced information can direct or bias attention (Ohman et al. 2001; Most et al. 2005; Mathews & Wells, 1999), and influence decision processes (Knutson et al. 2008). At the same time, a growing literature indicates that cognitive processes such as reappraisal can regulate emotional responsiveness (Jackson et al. 2000; Kim & Hamann, 2007; Ochsner et al. 2002; Ochsner, Ray, et al. 2004; Ray, Wilhelm & Gross, 2008). Indeed, the interactions between functions that are traditionally defined as strictly emotional or strictly cognitive are substantial enough to call into question the often artificial divide between these domains (see for instance Pessoa, 2008). However, the divide provides conceptual value in that emotional processing has specific characteristics of operation that can be distinguished from other cognitive domains in the same manner in which processes of attention or memory have differing characteristics and are instantiated in different (albeit sometimes partially overlapping) networks of brain regions.
The manner in which emotion and other cognitive domains interact has become increasingly central to models of psychopathology. For example, conceptualizations of anxiety disorders frequently focus on accentuated attentional biases towards threatening stimuli (Bishop, 2007; Cisler & Koster, 2010; Ouimet, Gawronski & Dozois, 2009; Williams et al. 1996). Similarly, failures to apply top down control over emotion are increasingly viewed as central to psychiatric disorders ranging from major depression (Fales et al. 2008; Johnstone et al. 2007; Almeida et al. 2009; Taylor Tavares et al. 2008), to borderline personality disorder (New et al. 2008).
Prefrontal regions figure prominently in neurobiological models of the interface between emotion and other aspects of cognition. However, the anatomical features of different prefrontal regions are often given only cursory attention in considering the validity of such models. To the extent that anatomy is considered, it usually is discussed only in broad terms of whether the area has any direct afferent or efferent connections with limbic regions, such as the amygdala or hypothalamus. However, the details of these connections are essential to understanding these regional interactions. For instance, a model that posits that the dorsolateral prefrontal cortex (DLPFC) directly inhibits amygdalar activity can only be sound if it is demonstrated that the DLPFC sends sufficient direct projections to the amygdala. If such projections are modest or absent, alternative models that rely on intermediary regions will be necessary to explain a posited DLPFC influence on amygdalar responses.
The structural features of different prefrontal regions and the laminar pattern of their connections may also provide substantial insights into the interactions between emotion and cognitive processes mediated by prefrontal cortex (PFC). Specifically, the cytoarchitectural features of different cortical regions dictate the manner in which they process information and interact with other regions. This second level of analysis has generally not entered into discussions of the neural substrates of emotion-cognitive interactions, although it has substantial implications for understanding these processes.
In the present paper, we attempt to outline several features of interregional communication among different PFC areas, and their interactions with the amygdala. We particularly focus on contrasts between orbital and dorsolateral PFC because of long-standing associations of the orbitofrontal cortex (OFC) to emotional processes (Zald & Kim, 1996) and similarly long-standing association of DLPFC to executive aspects of cognition (Fuster, 1989; Stuss & Benson, 1986). We also describe the role of anterior cingulate (ACC)/medial frontal structures in these interactions, as increasing data indicate that these structures provide a critical interface between emotion and other aspects of cognition.
2. Topography and cytoarchitectural features of the PFC
Topography
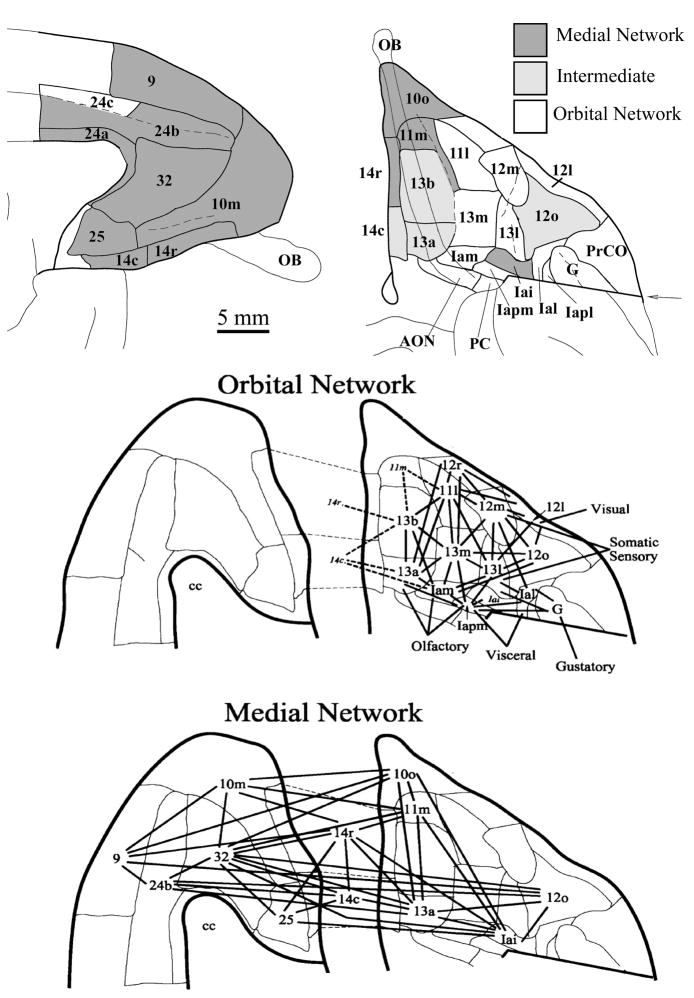
The PFC is frequently divided into 6 broad regions, dorsolateral, ventrolateral (VLPFC), frontopolar (FP), OFC, ventromedial (VMPFC), and dorsomedial (DMPFC) (see Figure 1). The exact topographical boundaries of these regions are variably applied by researchers, but the general nomenclature has proven useful as a broad organizing framework for understanding the anatomy and function of the PFC.
Figure 1.
General regions of the PFC in humans. The colored regions represent rough approximations of the broad zones of PFC. In both the lateral view (left) and the medial view (right), the regions are overlaid on a “partially inflated” hemisphere that allows clear visualization of sulci. Abbreviations: DLPFC dorsolateral prefrontal cortex; VLPFC ventrolateral prefrontal cortex; FP frontopolar cortex; OFC orbitofrontal cortex, DMPFC dorsomedial prefrontal cortex; VMPFC ventromedial prefrontal cortex. Figure adapted with permission from mindblog.dericbownds.net.
Phylogeny and Cytoarchitecture
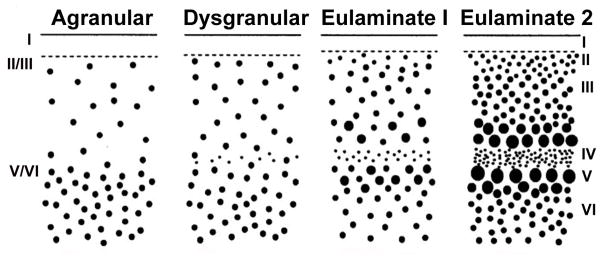
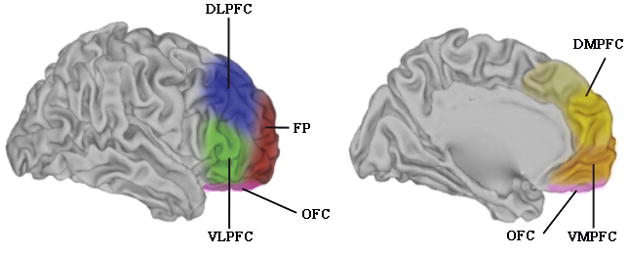
The PFC contains two separable, phylogenetically distinct architectonic trends (Barbas, 1988; Sanides, 1969; Yeterian & Pandya, 1991). The basoventral trend extends from an olfactory (allocortical) core through the OFC and spreads anteriorly to the ventral frontal pole, and laterally to the VLPFC (ending in Brodmann area (BA) V46). In contrast, the mediodorsal trend begins along the corpus collosum, progresses through the medial wall of the frontal lobe and then wraps around the superior edge of the lobe into the DLPFC (ending in BA D46). Each of these trends shows a pattern of successive stages of cortical architecture reflected in the development and widening of granular layer IV. The evolutionarily oldest part of these trends is agranular in nature, whereas the evolutionarily youngest areas have a dense and well-defined granular layer. In the basoventral trend, this cortical progression starts in the posterior OFC (agranular insula using the terminology of Carmichael and Price (Carmichael & Price, 1994)) followed by dysgranular (weakly granular) cortex in the central areas of the OFC, moving to eulaminate I cortex with a distinct granular layer IV as one moves anteriorly or laterally, and eventually reaching eulaminate II cortex with a dense layer IV and strong supragranular layers as one moves towards the frontal pole and ventrolateral regions (Barbas & Pandya, 1989; Carmichael & Price, 1994; Petrides & Mackey, 2006; Price, 2006a). The mediodorsal trend shows a similar cytoarchitectural progression. Starting with periallocortex cortex along the rostral corpus collosum, the trend becomes dysgranular in the cingulate (including subgenual, pregenual, and supragenual regions), eulaminate I as one moves anteriorly along the medial wall or superiorly into the superior frontal gyrus, and eventually becomes elumaniate II in dorsolateral regions (BA 8 and 46).
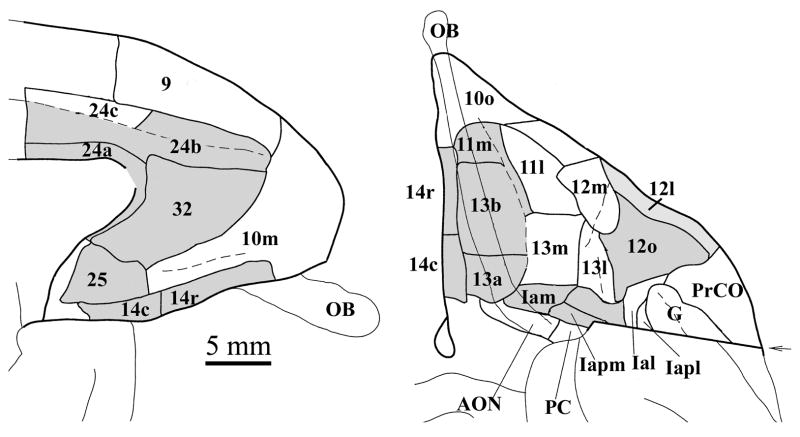
In order to avoid confusion, we note that the use of the term mediodorsal trend should not be confused with the DMPFC region outlined in Figure 1. The mediodorsal trend includes the DMPFC, but also includes VMPFC areas 25 and 32, and portions of BA 10 along the medial wall (area 10m in the nomenclature of Ongur et al. (2003); Figure 2).
Figure 2.
The basoventral and mediodorsal phylogenetic trends. In both trends, the cortex becomes progressively more differentiated. Figure adapted with permission from Barbas and Pandya 1989. Abbreviations: Pro proisocortex; PAII limbic periallocortex; D dorsal; L lateral; M medial; O orbital.
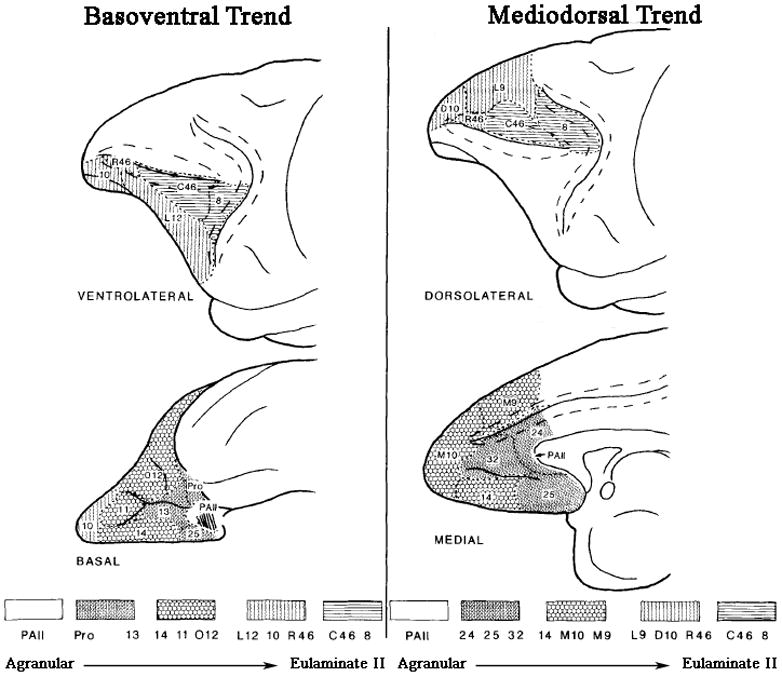
The pattern of cytoarchitectural development as one moves from agranular to eulaminate II cortex is accompanied by increases in the total number of neurons (cell density), the size of pyramidal cells in layers II and V, and level of myelination (Barbas & Pandya, 1989; Dombrowski et al. 2001; Figure 3), which together result in different information processing characteristics across the different regions. Other major differences between prefrontal regions arise in terms of histological staining, often reflecting different interneuron features. Carmichael and Price (Carmichael & Price, 1994) divide the Macaque OFC and medial PFC into multiple subregions based on such features (see figure 4), and many of these features can be identified in humans (Ongur et al., 2003). The differential interneuron features seen across prefrontal subregions impact the specific characteristics of information processing accomplished by prefrontal subregions (Wang et al., 2004; Zald, 2007), but are beyond the scope of this paper. Critically, the structurally defined divisions of PFC possess dramatically different patterns of connectivity both within the PFC and with other cortical and subcortical brain regions.
Figure 3.
Successive levels of differentiation in cortical layers within the PFC. Along with the emergence of granular cortical layer IV, there is an increase in the density of cells, and the size of pyramidal neurons in layers III and V. Figure adapted with permission from Dombrowski, Hilgetag and Barbas, 2001.
Figure 4.
Flat map showing cytoarchitectural divisions of the PFC in the Macaque. In this flat map representation, the cortex is cut at the principle sulcus (bottom and top line of both figures). The figure and labeling scheme is adapted from Carmichael and Price, 1994). Abbreviations: AON anterior olfactory nucleus; D dorsal; I intermediate; G gustatory cortex; l lateral, m medial, p posterior; PrCo precentral operculum; V ventral; Ia agranular insula. Adapted with permission from Carmichael and Price (1996).
Cytoarchitecture in Humans
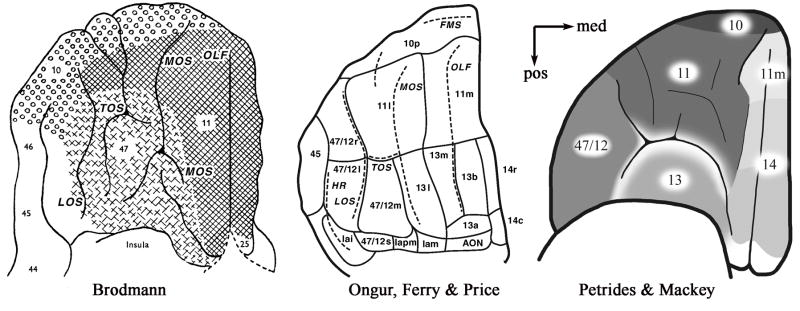
Although there is significant homology in primate and human cytoarchitecture within the frontal lobes, and the general phylogenetic trends are shared across primate species, several difficulties arise in moving between human and animal data. First, human neuroimaging studies often refer to Brodmann areas (Brodmann, 1914), but do not reflect developments in the identification of cytoarchitectural areas and areal boundaries that have occurred since Brodmann’s pioneering work almost a century ago. Second, the application of these area labels are often based on the Talairach atlas (Talairach & Tournoux, 1988), but this atlas is at best an approximation, since cytoarchitectural analysis was not performed on the brain that forms the basis of the atlas. Third, there is a mismatch between animal labels and human labels in the ventral frontal lobe, in that the animal data utilizes variants on the labeling developed by Walker (Walker, 1940), which some authors have now extended to humans (Petrides & Mackey, 2006; Ongur et al., 2003), while most neuroimaging researchers still utilize the Brodmann labels. Unfortunately, it is sometimes not clear which labeling system neuroimaging researchers are referencing when reporting their findings. This produces particular ambiguity in the lateral OFC/VLPFC, where human researchers often refer to BA 47, but the animal literature refers to area 12. The label 47/12 is now adopted by some neuroanatomists to describe this area in humans, although the medial boundary of this region remains disputed by leading neuroanatomists (Petrides & Mackey, 2006; Ongur et al., 2003). Similarly, areas 13 and 14 are clearly demarcated in monkeys, and homologous areas are observed in humans, but are not captured by Brodmann or Talairach, who applied a generic label of area 11 to both posterior and anterior sections of the medial OFC. In describing human neuroimaging data, we generally reference the broad labeling system described by Petrides and Mackey (2006), rather than the Talairach atlas in order to take advantage of data from nonhuman primate studies.
3. Connections
Most existing data on prefrontal connections derives from animal studies. Nevertheless, given the cytoarchitectural homology across primates (Petrides & Mackey, 2006; Ongur et al., 2003), it is generally assumed that the connectivity of these areas is largely conserved across primate species. As such, it is reasonable to use the nonhuman primate literature on connectivity as a basis for evaluating connectivity in humans. We focus on two types of connectivity here: amygdala-PFC connections, and connections between the different PFC regions.
Amygdalar input to PFC
The OFC and medial PFC receive substantial input from the amygdala (Amaral et al., 1992; Carmichael & Price, 1995; Barbas & Zikopoulos, 2006). This stands in sharp contrast to the DLPFC, which receives minimal direct projections from the amygdala. A review of the literature indicates that some of the projections to ventral and medial PFC vary depending upon the nucleus or subnucleus of origin (Amaral & Price, 1984; Barbas & De, 1990; Amaral et al., 1992; Carmichael & Price, 1995). However, these details are beyond the scope of this paper, and a strong enough picture of connectivity emerges across the different nuclei to inform a general discussion of connectivity patterns. Figure 5 displays a general schematic of amygdalar projections (arising from several amygdalar nuclei) into the medial and ventral surface of the macaque brain using the Carmichael and Price nomenclature. The figure makes evident that the orbital surface is not uniform in its afferent connections with the amygdala. Of particular note is the relative absence of substantial input into areas 13m, 13l, 12m, 11l, and 10o on the orbital surface. The medial wall also receives substantial amygdalar input, but again is not uniform, as neither area 10m nor area 9 receives significant amygdalar input.
Figure 5.
Cytoarchitectural maps of the orbital surface. Cytoarchitectural labeling of the frontal lobes adapted from Brodmann (1914) (right), Ongur, Ferry & Price (2003) (middle) and Petrides and Mackey (2006)(left). Note the substantial differences in labeling schemes particularly in regards to more posterior-medial regions. Figures adapted with permission from Brodmann (1914), Ongur, Ferry & Price (2003), and Petrides and Mackey (2006) respectively. Abbreviations, LOS= lateral orbital sulcus, MOS=medial orbital sulcus, TOS= transverse orbital sulcus, Olf= Olfacotry sulcus.
Two conclusions may be drawn from this pattern of input. First, the amygalar input into the PFC is architectonically specific and is concentrated in the least cytoarchitecturally developed regions. This indicates that it would be a mistake to generically treat all of the OFC or medial PFC as if it were heavily connected to the amygdala. Rather, attention to the location within the OFC and medial PFC is advised before drawing inferences about amygdalar connectivity. Second, the DLPFC and FP receive extremely weak direct amygdalar input (indeed only the most sensitive techniques show evidence of an amygdalar input). As a consequence, amygdalar influences on DLPFC and FP processing are likely to be indirect, either being transmitted through the cingulate or posterior OFC regions (or via other more general mechanisms, such as modulation of neurotransmitter systems).
Prefrontal output to the amygdala
The outputs of the PFC to the amygdala are also regionally specific (Price, 2006b; Ghashghaei et al., 2007; Stefanacci & Amaral, 2002; Stefanacci & Amaral, 2000). In general, prefrontal areas that receive projections from the amygdala send projections back to the amygdala, while areas that do not receive substantial amygdalar input (such as the DLPFC and FP) have at best weak projections to the amygdala. The density of projections largely reflects cytoarchitectonics, with a weakening gradation of projection density as one moves from agranular areas to more structurally developed eulaminate isocortex. This pattern indicates that isocortical areas (DLPFC and FP) cannot provide a strong direct influence over the amygdala, and to the extent that they do influence the amygdala, the influence is likely to be indirect. This is not to say there are no direct projections from the DLPFC to the amygdala, as multiple studies have indeed observed direct projections from area 9 and 46 (Stefanacci & Amaral, 2002; Aggleton et al., 1980; Amaral & Insausti, 1992). Rather, the projections are generally too light to provide a broad regulation of amygdalar processing.
Although general cytoarchitectonics provide a strong organizing principal in terms of amygdala-prefrontal connections, the relative regional distribution of inputs and outputs is not symmetric (Ghashghaei et al., 2007). Notably, the highest amygdalar input into the PFC is located in the agranular insular region along the posterior OFC, whereas, the largest output to the amygdala arises from the posterior subgenual cingulate region (BA 25) and portions of the dorsal anterior cingulate (BA 24). In general terms, medial wall areas have higher output to than input from the amygdala, whereas posterior OFC areas have higher input than output. Interestingly, the more sparsely connected lateral PFC, DLPFC regions (BA 8, 9 and dorsal 46) possess greater input from than output to the amygdala, whereas the pattern is reversed within VLPFC. Of note in this regard, the posterior region of area 12l within the VLPFC provides moderate projections to the amygdala, making it the only lateral PFC region with a significant direct input to the amygdala. Indeed, these projections are stronger than what is seen in the anterior orbital regions, which share areas 12l’s greater proportion of output than input, but show generally weaker levels of connectivity than area 12l.
A number of different amygdala subnuclei receive PFC input. The basal and accessory basal and medial nuclei receive the densest projections, as well as receiving projections from the broadest array of PFC regions, while the lateral, central and cortical nuclei receive PFC projections, but at a less dense and widespread level (Stefanacci & Amaral, 2002). BA 25 is notable in that it not only sends the densest projections to the amygdala, it also sends projections to the broadest array of nuclei, as every amygdala subnucleus noted above receives input from BA 25. Although appearing as light in column B of Figure 6, it is worth noting that BA 32 does provide reasonably well-described projections to the amygdala. In many respects BA32 appears homologous to prelimbic cortex in rodents (Price, 2006a). In rodents, prelimbic cortex projects to portions of the basolateral and central nucleus of the amygdala (Vertes, 2004). In nonhuman primates, projections have also been observed from BA 32 to a discrete portion of the accessory basal nucleus (Chiba et al., 2001). Thus, although substantially less dense and widespread than the projections from BA 25, BA 32 appears in a position to interact with selective amygdalar processes.
Figure 6.
Amygdala recipient regions of the PFC. The figure represents a composite from multiple tracing studies following injections in portions of the basal nucleus, accessory basal, medial, and lateral amygdala nuclei. Areas in grey receive significant input from at least one region of the amygdala. The involvement of more rostral (13b, 11m) and lateral regions (12o, 12l) often depend upon the specific subnucleus being studied. Note in the original version of this figure, area 12l is not marked as having significant input, but area 12l does show specific labeling following injection into the dorsal basal nucleus (Carmichael & Price, 1995), and so was included as an amygdala recipient region in this modified figure. Figure adapted with permission from Price (2006).
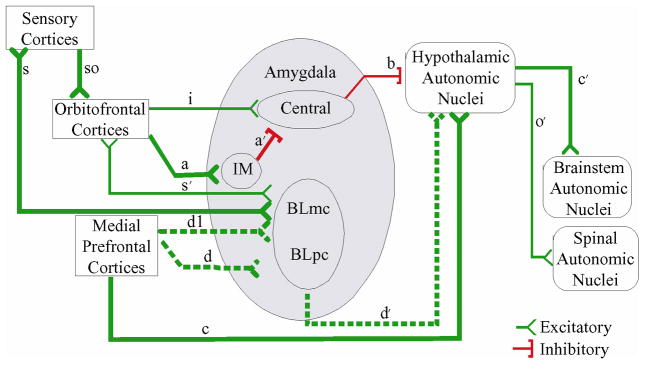
Barbas and Zikopoulos (2006) argue that the medial prefrontal and OFC outputs to the amygdala may have differential impacts on amygdalar functioning. BA25 on the medial surface provides particularly robust excitatory output to basolateral portions of the amygdala, which in turn provides excitatory projections to the hypothalamus. In contrast, the posterior agranular OFC substantially innervates the intercalated masses that surround the basal nucleus (See Fig. 7). The intercalated masses provide an inhibitory input into the central nucleus. When stimulated, the intercalated masses halt a tonic inhibitory pathway from the central nucleus to the hypothalamus, thus causing a disinhibition of the hypothalamus. Lighter excitatory projections also reach the central nucleus directly from the posterior OFC, allowing the OFC to both increase or decrease central nucleus firing.
Figure 7.
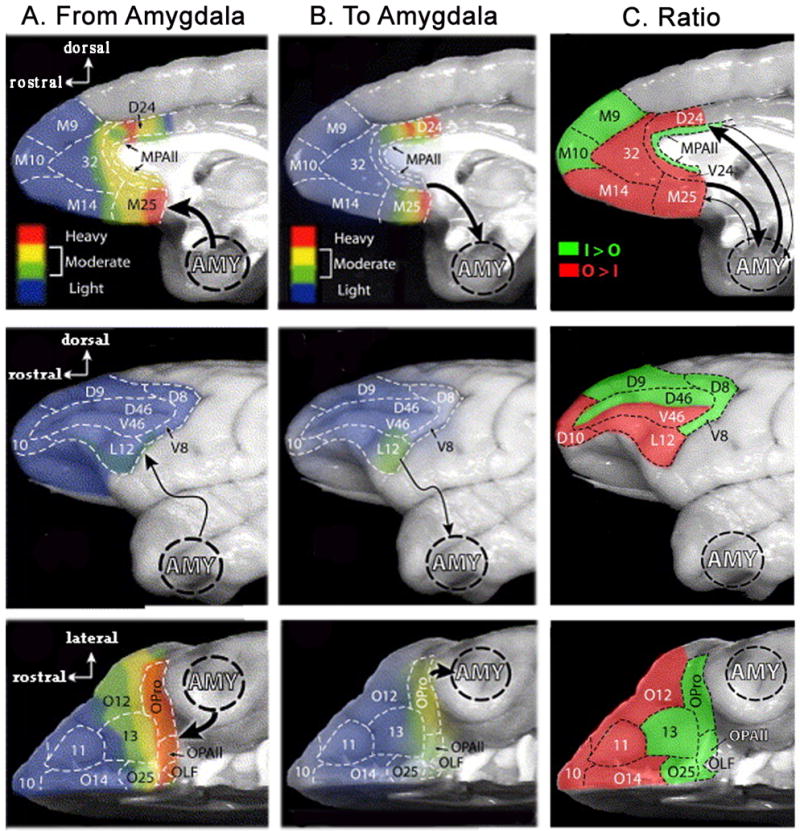
A) Amygdala input into the PFC; B) Prefrontal output to the amygdala; and C) Ratio of input from vs. output to the amygdala. Figures derive from labeling density studies by Ghashghaei et al. (2007). Projection density and ratios are shown on lateral, medial and ventral surfaces of PFC. The color code for density is based on a normalization of anterograge or retrograde labeling in which 1= lowest density and 100 = highest density. Areas marked as light (blue) correspond to normalized values of 1–25, moderate corresponds to 26–50 (green) and 51–75 (yellow), and heavy corresponds to 76–100 (red). Adapted with permission from (Ghashghaei et al., 2007).
Prefrontal projections to the hypothalamus and brainstem
Areas of the OFC and medial PFC possessing projections to the amygdala also typically project to the hypothalamus and autonomic brainstem/periaqueductal gray regions (An et al., 1998; Barbas et al., 2003; Price, 2006b; Rempel-Clower & Barbas, 1998), providing a direct ability to influence autonomic effector regions associated with emotional output (see Figure 8). These projections appear particularly strong from more medial wall structures, but also arise from the crescent like area on the orbital surface where amygdalar input is substantial. As with its lack of direct access to the amygdala, the DLPFC and FP are largely devoid of direct projections to these sites. Additionally, more anterior portions of the OFC show little direct output to these autonomic centers.
Figure 8.
Prefrontal Pathways for modulating amygdalar output to autonomic regions. Adapted with permission from (Barbas & Zikopoulos, 2006). The excitatory OFC projections to the intercalated masses (IM) (path a) leads to disinhibition of the hypothalamus by removing the central nucelus’s tonic inhibition of the hypothalamus (path b). The medial PFC projects both directly to the hypothalamus (path c) and indirectly (paths dl,d′) through the basolateral nucleus (BLmc & BLpc).
Connections within the frontal lobe
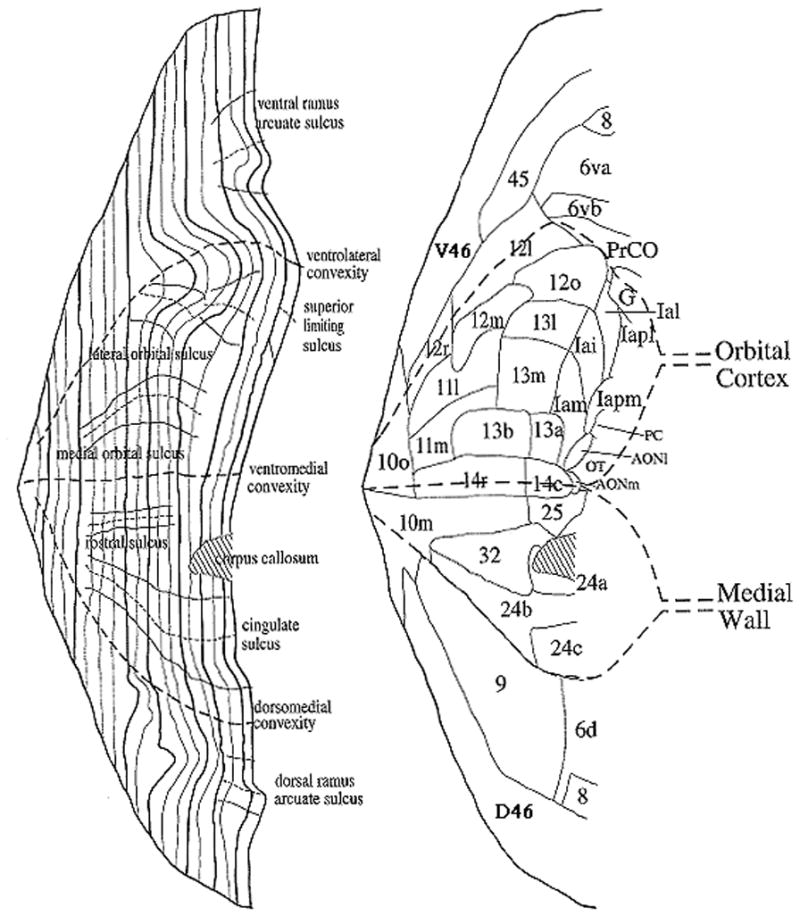
As noted above the PFC can be divided into two major phylogenetic trends. The highest connections of each region are with areas within the same trend, particularly neighboring areas that are no more than one stage of development away from the area in question. Thus, for instance, the agranular insular regions in the posterior OFC have substantial connections to other agranular and dysgranular orbital regions, but are generally devoid of connections to isocortical regions such as ventral area 46 within its own trend, or dorsal area 46 across trends. Where inter-trend connections arise they generally do not jump more than one stage of architectonic development. For instance, isocortical ventral area 46 is strongly connected with isocortical dorsal area 46 in the mediodorsal trend, but does not connect to more poorly developed medial wall areas such as the subgenual cingulate (BA 25). More anterior and lateral OFC areas possess substantial connections with ventral area 46 and neighboring area 45, but connections that jump the principal sulcus to the dorsal part of area 46 are much rarer.
Nevertheless, several OFC areas do appear to possess direct connections with the DLPFC. Specifically areas 11m, 12o, 13a and 14r each possess connections with the DLPFC. The gyrus rectus (which includes area 14r) may be viewed as either part of the mediodorsal trend or as a transitional area between trends, so its connections do not represent an inter-trend jump. However, areas 11m, 12o, and 13a are grouped as part of the basoventral trend, so their links with the DLPFC represent inter-trend connections. To understand the large-scale network position of these areas, it is useful to consider an alternative system for classifying orbital and medial regions. Rather than basing models on phylogeny, Carmichael and Price (1996) divide the OFC and medial wall into an orbital and a medial network based strictly on the strength of connections between regions (See Fig. 9). This sort of categorization scheme shows substantial overlap with the phyolgenetic division between basoventral and mediodorsal trends, which is not surprising given the organization of connections already discussed. However, the two classification systems are not entirely synonymous. Interestingly, all of the orbital areas that connect to DLPFC are either part of Carmichael and Price’s medial network, or are considered intermediary between networks. For instance, area 11m is considered part of a medial network, because it has greater connections with medial wall regions than with the rest of the OFC. Carmichael and Price classify areas 12o and 13a as interface regions because they contain heavy connections to both medial and orbital areas. These differential patterns of connectivity make evident that there will be marked regional, or even subregional, differences in the ability of the OFC to interact with other prefrontal areas. Specifically, the gyrus rectus, as well as 11m, 12o, and 13a are in a position to interact with both medial wall areas (e.g. the cingulate) and DLPFC areas, whereas other orbital areas lack this direct relationship.
Figure 9.
Orbital and Medial connection networks as defined by Price and colleagues. Adapted with permission from (Price, 2006b). Note Price does not include dorsal and ventral area 46 in these networks, although data indicate that more dorsolateral regions show connectivity with the medial network, whereas ventrolateral regions show greater connectivity with the orbital network.
Prefrontal network connections dictate pathways to the amygdala
For areas lacking strong direct output to the amygdala, the ability to influence amygdalar processing must rely on indirect pathways, and these pathways will be largely dictated by their position within the major prefrontal networks. Given the strength of the subgenual cingulate’s (BA 25) projections to the amygdala, it may provide a particularly important relay through which different PFC regions influence the amygdala. As can be seen from Figure 9, BA 25 receives substantial projections from medial network areas and areas on the orbital surface that are associated with the medial network. In contrast, more dorsolateral projections are scarcer. Vogt and Pandya (1987) note that BA 25 receives projections from the DLPFC, and specifically describe input from area 9 in the superior portion of DLPFC. Yet, the strength of this connection appears weak, and has not been clearly seen in some studies (Barbas & Pandya, 1989). Nevertheless, BA 9 is well connected with BA 32 along the medial wall, which in turn is heavily connected with BA 25 (Carmichael & Price, 1996; Barbas & Pandya, 1989), and thus provides a feasible indirect route for DLPFC processing to influence BA 25. Similarly, dorsal BA 46 lacks substantial connections with BA 25, and would likely have to engage BA 32, or perhaps other portions of cingulate cortex, in order to communicate with BA 25.
The dorsal ACC (BA 24) also provides a critical output zone to the amygdala. This region has a rich pattern of inputs from the PFC (Carmichael & Price, 1996; Vogt & Pandya, 1987; Barbas & Pandya, 1989). This includes a substantial input from BA 9, and to a lesser extent BA 46 in the DLPFC, portions of BA 32, and BA 10 on the medial wall, and several OFC regions (particularly medial/intermediate network areas 13a and Iai, and 12o). Thus, the dorsal ACC appears in a particularly strong position to integrate aspects of PFC functioning across multiple regions.
Although more anterior OFC and VLPFC regions appear to have a greater ratio of output to the amygdala than input from the amygdala, because these projections are relatively modest, these anterior regions may additionally utilize indirect pathways to engage the amygdala. For the anterior OFC regions, this would most likely be directed through the posterior agranular OFC regions. In contrast, for ventrolateral regions, posterior area 12l may provide a relatively specific route for engaging the amygdala, given its unique position within intra-prefrontal and prefrontal-amygdala networks.
4. The Structural model
The cytoarchitectural features of a cortical region substantially influence how the region interacts with other brain regions. Specifically, the level of granularity and laminar development impact its level of feedforward and feedback projections (Barbas & Rempel-Clower, 1997; Barbas 2000). Within the model presented by Barbas, feedforward projections are defined structurally as arising from superficial layers and projecting to deep layers of cortex. In sensory systems, early stages of the processing stream provide information to subsequent stages through this type of feedforward projection (Rockland & Pandya, 1979; Pandya, 1995). Within systems possessing a clear hierarchy of information flow, such as sensory systems, feedforward projections may also be described as ascending as they move from a primary region to higher levels in the processing stream (for instance V1 to V2). In terms of cognitive processes, such forwarding of information is consistent with what traditional cognitive theorists label as a bottom-up process (Kastner & Ungerleider, 2000).
By contrast, feedback projections start in deep layers of cortex and project to superficial layers of cortex (see Figure 10). In sensory systems with a clear hierarchical structure, these feedback projections may be described as descending, as they travel from later to earlier stages of the sensory processing stream (e.g., V2 to V1). Feedback projections act to modify or bias the computations being performed in the earlier processing stages (Raizada & Grossberg, 2003). For instance, these feedback projections act to help accentuate the responses of cells coding attended objects or locations, while attenuating or suppressing responses to unattended objects (Mehta et al., 2000; Saalmann et al., 2007). Such feedback aids in basic perceptual processes such as figure-ground discrimination (Domijan & Setic, 2008; Roland et al., 2006), as well as allowing top-down control of what is processed in the information stream (Grossberg, 2007). In cognitive terms, this top-down control allows for modulation of processing based on expectations, current goals and directed attention (Glibert and Sigman 2007).
Figure 10.

Feedback and feedforward connections of the PFC. Based on the dominant patterns of laminar origins and terminations, and the structural model described by Barbas and colleagues, lateral prefrontal (LPFC) “feedforward” projections primarily arise in superficial layers and project to deep layers in the OFC, while the OFC sends “feedback” projections that arise in deep layers and project to superficial layers of the lateral PFC.
For clarity, it is useful to distinguish between the terminology of ascending/descending, feedforward/feedback, and bottom-up/top-down, as they imply different things (see Penny et al. 2004 for a discussion). Ascending and descending projections refer to specific hierarchical features, and the terminology is particularly useful in the context of well-defined processing streams. We use the term bottom-up and top-down to specifically refer to cognitive processes, with bottom-up referring to more automatic processes, such as responses that are driven by the perception of a stimulus, and top-down referring to mechanisms that allow for adaptive modulation of processing congruent with current goals and expectations. The terms feedforward and feedback in this context have dual meanings, as they are defined by the specific laminar properties of projections, but they also imply information processing features.
Because feedback and feedforward projections are defined by laminar features, a concern may be raised regarding the specific laminar criteria used by Barbas and colleagues for characterizing projections as feedforward or feedback outside of sensory processing streams. In models of the visual system, feedforward projections are typically defined in specific relation to laminar IV terminations, with ascending feedforward projections arising in superficial layers and terminating in layer IV (as opposed to deep layers more generally; Felleman & Van Essen, 1991). In contrast, Barbas uses a broader definition that does not specifically distinguish between layer IV and infragranular layers. This extension is on the surface reasonable given the presence of prefrontal regions that lack a strong granular layer, and the more diffuse laminar termination patterns observed in these regions. However, the full functional implications of this extension remain to be elucidated.
A slightly different issue arises in the definition of feedback projections. Barbas’s definition of feedback focuses exclusively on projections arising from deep (infragranular) layers and terminating in superficial layers, consistent with the original work of Rockland and Pandya (1979). However, Felleman and Van Essen (1991) argue that some additional descending feedback projections may have bilaminar origins with a combination of infra- and supra-granular origins. Since, Barbas retains the more conservative definition, acceptance of her critieria is rather straightforward. However, it does lead to the possibility that the proportion of projections characterized as feedback in the PFC might be higher using a more liberal definition.
A critical feature of the structural model is that the level of feedback and feedforward projections between regions is substantially determined by the relative degree of cytoarchitectural development of the regions. Projections from more differentiated cortex (i.e., more differentiated, and denser granular layer) to less cytoarchitecturally developed cortex follow the feedforward pattern, while those from less cytoarchitecturally developed to more cytoarchitecturally developed cortex follow the feedback variety. This pattern is consistent with what is seen in sensory systems, but the pattern appears generalizable to multiple systems. In the PFC, the structural model predicts the balance of feedforward and feedback projections approximately 80% of the time, with the relative balance of feedback and feedforward connections becoming more extreme the greater the difference in cytoarchitectural development between the two regions in question (Barbas & Rempel-Clower, 1997).
The core utility of the structural model to the present topic is that it leads to strong predictions about the nature of communication between brain regions even in the absence of direct functional data. Of course, ultimately, electrophysiological or other techniques capable of examining laminar information flow will be necessary to confirm that laminar projection patterns in the PFC are functionally similar to what is seen in sensory cortices (i.e., that structurally defined feedback and feedforward projections are associated with similar properties of information flow regardless of the system in question). Such electrophysiological studies may also eventually help to refine the criteria for structurally defining feedfoward and feedback projections. In the meantime, the structural model provides the strongest anatomical basis currently available for predicting the nature of information flow in the PFC. If the structural model is accurate in its characterization of information flow within the PFC, it has significant implications for models of emotion-cognition interactions.
Laminar patterns and intrinsic prefrontal connections
Consistent with the structural model, analyses of the laminar patterns of projections indicates that the dysgranular OFC is characterized by strong feedback features in its connections with more cytoarchitecturally developed regions of the PFC (Barbas, 2000). By analogy to sensory systems, this would mean that the OFC projections are geared towards biasing or modifying computations. By contrast, the eulaminate DLPFC has substantially higher levels of feedforward projections, which allow it to feed the results or output of its computations to subsequent brain regions. This general pattern of feedforward and feedback projections also characterizes the specific connections between the OFC and DLPFC. Lateral prefrontal connections to the OFC originate mostly in the upper cortical layers (2–3) and their axons terminate predominantly in the deep layers (4–6), which corresponds to the feedforward pattern (Barbas & Rempel-Clower, 1997). In contrast, the OFC’s projections to the lateral PFC originate predominantly in deep layers (5–6) with their axons terminating mostly in the upper layers (1–3), a pattern characteristic of feedback. This pattern appears to apply to roughly 70–80% of the projections. Thus, information flow from the OFC to granular PFC consists mostly of feedback, whereas information flow in the other direction conforms primarily to a feedforward pattern.
The structural model is provocative in that it proposes that the nature of inter-regional communication can be inferred based on laminar connectivity. If the structural model is correct, it forces us to attend to the feedforward and feedback nature of inter-regional communication. Models that posit that the lateral PFC acts primarily or exclusively through the implementation of top-down mechanisms are difficult to reconcile with its prominent feedforward features. Similarly, models of the OFC that view it as simply conveying the results of a computation (for instance of reward value) to the lateral PFC, fail to capture the region’s potential ability to bias computations being carried out in the lateral PFC. Yet, as described later in this article, existing models of interactions between PFC regions, and between areas involved in “emotional” vs. “cognitive” processing consistently ignore the potential implications of the structural model. Indeed, existing models typically couch lateral PFC functions, particularly DLPFC functions, in terms of top-down control, and rarely consider the possibility that less structurally developed areas like the OFC might provide a top-down influence on more lateral PFC regions.
Laminar patterns of prefrontal-amygdalar connections
The amygdala projections to the posterior OFC enervate all layers of cortex, and therefore may not be strictly limited to feedforward or feedback type projections (Ghashghaei et al., 2007). However, it is clear that there is a strong feedforward component to these projections based on laminar termination. In contrast, the OFC’s projections to the amygdala principally arise from layer 5, indicating their characterization as feedback projections (suggesting that they act to bias amygdalar processing rather than conveying specific information such as the sensory characteristics of the stimuli). Interestingly, the feedforward projections from the lateral PFC are directed to layer 5 of the OFC, which is the primary output layer from which the OFC’s projections to the amygdala arise.
Can anatomical insights inform discussions of prefrontal functions? The last several years have witnessed an explosion of interest in the manner in which different brain areas interact. This interest has in part arisen as a consequence of the emergence of techniques for examining functional connectivity with fMRI, providing for the first time the ability to empirically examine interactions between brain regions in healthy humans. However, discussions of these data, and the models that arise from these data, have not always been constrained by anatomy. As these models have become increasingly influential, we believe it is useful to evaluate how well they fit with the neuroanatomy outlined above. We believe that such models need to be consistent with both the known connectional patterns linking different cortical and subcortical regions and the feedback/feedforward nature of these patterns. When models do not conform to these constraints, they lack plausibility, or at a minimum require an explanation of how they can be viable given their inconsistency with the known connections of the brain.
A growing psychological literature attempts to understand the manner in which “cognitive” processes interact with “emotional” processes. While there are definite limitations to an artificial divide between cognitive and emotional processes (Pessoa, 2008), the distinction has proven useful in characterizing a range of behaviors such as emotion regulation, motivation, economic decision-making and the direction of attentional mechanisms. In the sections that follow, we describe emerging data and models for emotion regulation, working memory and dorsal-ventral prefrontal interactions, with a focus on their consistency with anatomical data. We particularly focus on the emotion regulation literature, as this literature increasingly figures in discussions of psychopathology and psychotherapeutic treatment.
5. Emotion Regulation
Emotion regulation has been defined as those processes involved in changing the onset, duration, intensity or content of an emotional response (Gross, 1998; Gross, 2008). Emotion regulation processes range from actions taken long before an emotion arises, such as situation selection, to those processes engaged either just prior to or once an emotion has begun to emerge, such as attention deployment or cognitive reappraisal (Gross, 1998). It is in these latter types of strategies that investigations into the relationship between regions associated with the cognitive control of emotion and those associated with the emotional response become of greatest interest. These investigations either implicitly or explicitly describe emotion regulation as the deployment of top-down, ‘cold’ cognitive control regions of the PFC to down regulate bottom-up, ‘hot’ reactive processes involving the subcortical limbic regions like the amygdala. Failures in the successful deployment of PFC top-down cognitive control mechanisms or overactive bottom-up amygdala processes have been proposed to contribute to several forms of psychopathology (Rottenberg & Gross, 2003; Rottenberg & Johnson, 2007).
The emotion regulation strategy that has received the most attention in the neuroimaging literature is cognitive reappraisal. This regulation strategy involves cognitively reinterpreting emotional information in order to change an emotional response (Gross, 1998). Reappraisal encompasses a broad class of related processes. For example, a reappraisal can focus on the reinterpretation of the personal meaning of the emotional object to make it more or less self-relevant. Alternatively, a reappraisal can focus on reinterpreting the cause, consequence, or the reality of emotional stimuli without changing one’s relationship to the stimuli. For example, one could reappraise a car accident on the side of the road as probably ending with all parties walking away from the incident safely. A number of functional neuroimaging studies have now been performed during reappraisal tasks, and are listed in Table 1, with the location of PFC activations displayed in Figure 11. Using the key words emotion regulation, distraction and reappraisal, empirical articles measuring voluntary emotion regulation were included. These fMRI studies consisted of instructed cognitive reappraisal, emotion suppression and distraction studies in non-clinical populations. This list of emotion regulation studies is not exhaustive; for example, it does not include related concepts like mood regulation. We note that in all Tables we have retained the nomenclature (applied Brodmann labels, or topographical/regional descriptions) used by the authors of the original papers. There are some cases where questions could be raised about the specific application of labels, but lacking a published “gold standard” coordinate system for most prefrontal regions, we have not generally changed labels, with the exception that in the text we specifically note VLPFC activations that are consistent with the posterior part of BA 47/12. Lacking a clear demarcation of the portion of BA 47/12 with significant amygdala connections in humans, we consider the portion of the region that is posterior to y = 32 as generally representing posterior BA 47/12. We also indicate in text when OFC foci are consistent with the location of BA 13 (regardless of their original designation).
Table 1.
Prefrontal Regions Recruited During Emotion Regulation
| Study | Emotion Regulation Contrast | Regions | BA | X | Y | Z |
|---|---|---|---|---|---|---|
| Beauregard et al. (2001) | Inhibition Erotic >Neutral | R. Superior Prefrontal Gyrus | 10 | 22 | 46 | 13 |
| R. Anterior Cingulate Gyrus | 32 | 9 | 42 | 4 | ||
| Cooney et al. (2007) | Positive recall in sad mood > Positive recall without negative mood | L. Subgenual Cinguate Cortex | 25 | −8 | 15 | −18 |
| R. Subgenual Cingulate Cortex | 25 | 8 | 15 | −18 | ||
| L. Inferior Frontal Gyrus | 47 (47/12) | −41 | 26 | −11 | ||
| R. Orbitofrontal Cortex | 11 | 26 | 34 | −14 | ||
| L. Anterior Cingulate Cortex | 32 | −8 | 34 | −11 | ||
| Delgado et al. (2008a) | Regulate > Attend | L. Middle Frontal Gyrus | 6/9 | −43 | 3 | 37 |
| L. Inferior Frontal Gyrus | 6/44 | −45 | 0 | 32 | ||
| L. Subgenual Cingulate Cortex | 25 | −3 | 12 | −5 | ||
| Delgado et al. (2008b) | Regulate > Attend | L. Middle Frontal Gyrus | 9/46 | −43 | 28 | 30 |
| vmPFC | 32 | 0 | 35 | −8 | ||
| Subgenual Cingulate Cortex | 25 | 0 | 14 | −11 | ||
| Domes et al. (2010) | Reapraise > Maintain | L. Suppl. Motor Area | 6 | −2 | 16 | 54 |
| R. Suppl. Motor Area | 6 | 12 | 7 | 59 | ||
| L. Precentral Gyrus | 6 | −44 | 1 | 47 | ||
| L. Inferior Frontal Gyrus | 38 (47/12) | −52 | 17 | 0 | ||
| L. Inferior Frontal Gyrus | 44 | −58 | 14 | 23 | ||
| R. Inferior Frontal Gyrus | 38 (47/12) | 51 | 24 | −1 | ||
| R. Inferior Frontal Gyrus | 45 | 57 | 22 | 9 | ||
| R. Inferior Frontal Gyrus | 38 (47/12) | 57 | 12 | 1 | ||
| R. Ventrolateral Prefrontal Cortex | 46 | 32 | 42 | 26 | ||
| R. Ventrolateral Prefrontal Cortex | 46 | 32 | 31 | 32 | ||
| L. Ventrolateral Prefrontal Cortex | 46 | −30 | 47 | 25 | ||
| R. Precentral Gyrus | 6 | 50 | 5 | 46 | ||
| Eippert et al. (2007) | Whole Brain Decrease Negative > View Negative | Middle Cingulate Gyrus | 4 | 17 | 38 | |
| Anterior Cingulate Cortex | −7 | 24 | 31 | |||
| ROIs Decrease Negative > View Negative | DLPFC (Inferior Frontal Gyrus) | −43 | 12 | 7 | ||
| DLPFC (Inferior Frontal Gyrus) | −40 | 18 | 5 | |||
| DLPFC (Middle Frontal Gyrus) | −29 | −4 | 55 | |||
| DLPFC (Inferior Frontal Gyrus) | −57 | 20 | 11 | |||
| L. Anterior Cingulate Cortex | −7 | 24 | 31 | |||
| L. Anterior Cingulate Cortex | −4 | 18 | 33 | |||
| OFC (Inferior Orbitofrontal Gyrus) | −43 | 15 | 2 | |||
| Correlation with Self-Reported Emotion Regulation Success for Decrease | R. Medial Orbitofrontal Cortex | 2 | 49 | 6 | ||
| R. Anterior Cingulate Cortex | −1 | 46 | 9 | |||
| Negative Correlation with Self-Reported Emotion Regulation for Decrease | R. Dorsolateral Prefrontal Cortex | 38 | 22 | 23 | ||
| Goldin et al. (2008) | Reappraise > Watch (Early) | Medial Prefrontal Cortex | 10 | −11 | 67 | 18 |
| R. Inferior Frontal Gyrus (DLPFC) | 10/46 | 48 | 42 | 1 | ||
| L. Inferior Frontal Gyrus | 46 | −51 | 41 | 2 | ||
| L. Middle Frontal Gyrus | 6 | −37 | 7 | 41 | ||
| L. Lateral Orbitofrontal Cortex | 11 | −28 | 42 | −6 | ||
| L. Lateral Orbitofrontal Cortex | 11 | −38 | 45 | −10 | ||
| L. Ventrolateral Prefrontal Cortex | 47 | −35 | 38 | −6 | ||
| Hayes et al. (2010) | Reappraisal > View | L. Inferior Frontal Gyrus | (47/12) | −49 | 23 | −9 |
| L. Inferior Frontal Gyrus | (47/12) | 46 | 26 | −8 | ||
| L. Paracingulate Gyrus | −5 | 23 | 40 | |||
| L. Middle Frontal Gyrus | −41 | 21 | 42 | |||
| L. Superior Frontal Gyrus | −25 | 23 | 51 | |||
| Kalisch et al. (2005) | Anxiety Regulation > Anxiety No Regulation | Anterolateral Prefrontal Cortex | 38 | 41 | 25 | |
| Kanske et al. (2011) | Reappraise>View | L. Superior/Medial Frontal | 6/8 | −11 | 16 | 57 |
| R. Superior/Medial Frontal | 6/8 | 12 | 19 | 59 | ||
| L. Middle Frontal | 6/9/46 | −44 | 13 | 42 | ||
| R. Middle Frontal | 6/9/46 | 39 | 38 | 40 | ||
| L. Middle Frontal | 46 | −38 | 43 | 12 | ||
| R. Middle Frontal | 46 | 35 | 45 | 26 | ||
| L. Orbitofrontal | 47 | −35 | 41 | −1 | ||
| R. Orbitofrontal | 47 | 39 | 41 | −5 | ||
| Distraction > View | L. Anterior Cingulate/Dorsomedial Frontal | 6/8/32 | −11 | 14 | 44 | |
| R. Anterior Cingulate/Dorsomedial Frontal | 6/8/32 | 12 | 23 | 41 | ||
| L. Middle Frontal | 6/44/45/46 | −38 | 2 | 31 | ||
| R. Middle Frontal | 9/44/45/46 | 44 | 34 | 27 | ||
| L. Superior Frontal | 6/8 | −21 | 9 | 52 | ||
| R. Superior Frontal | 6/8 | 27 | 9 | 50 | ||
| Kim & Hamann (2007) | Decrease Negative > Watch Negative | L. Inferior Orbitofrontal Cortex | 47 | −40 | 33 | −4 |
| L. Inferior Orbitofrontal Cortex | 47 (47/12) | −45 | 27 | −2 | ||
| L. Middle Frontal Gyrus | 9 | −43 | 9 | 40 | ||
| L. Middle Frontal Gyrus | 46 | −38 | 14 | 43 | ||
| L. Middle Frontal Gyrus | 46 | −26 | 15 | 35 | ||
| L. Middle Frontal Gyrus | 10 | −26 | 48 | 19 | ||
| L. Middle Frontal Gyrus | 46 | −34 | 42 | 21 | ||
| L. Middle Frontal Gyrus | 45 | −40 | 33 | 23 | ||
| R. Middle Frontal Gyrus | 44 | 32 | 3 | 40 | ||
| R. Middle Frontal Gyrus | 46 | 35 | 14 | 47 | ||
| R. Middle Frontal Gyrus | 9 | 37 | 5 | 49 | ||
| R. Middle Frontal Gyrus | 10 | 32 | 53 | 18 | ||
| R. Inferior Frontal Gyrus | 45 | 52 | 22 | 15 | ||
| R. Orbitofrontal Gyrus | 47 (47/12) | 44 | 27 | −3 | ||
| R. Orbitofrontal Gyrus | 47 (47/12) | 55 | 23 | 7 | ||
| L. Inferior Frontal Gyrus | 44 | −51 | 15 | 32 | ||
| L. Inferior Frontal Gyrus | 44 | −51 | 13 | 24 | ||
| L. Inferior Frontal Gyrus | 45 | −48 | 34 | 17 | ||
| Superior Medial Frontal Gyrus | 32 | −1 | 23 | 42 | ||
| Pre-SMA | 6 | −2 | 4 | 61 | ||
| L. Superior Medial Frontal Gyrus | 8 | −4 | 22 | 52 | ||
| R. Superior Medial Frontal Gyrus | 9 | 10 | 51 | 36 | ||
| R. Superior Frontal Gyrus | 10 | 16 | 55 | 29 | ||
| L. Superior Frontal Gyrus | 10 | −18 | 49 | 33 | ||
| L. Superior Frontal Gyrus | 9 | −24 | 43 | 40 | ||
| L. Superior Frontal Gyrus | 9 | −15 | 40 | 38 | ||
| L. Superior Frontal Gyrus | 10 | −26 | 48 | 11 | ||
| R. Anterior Cingulate | 24 | 7 | 16 | 25 | ||
| L. Anterior Cingulate | 32 | −7 | 13 | 30 | ||
| L. Anterior Cingulate | 32 | −12 | 21 | 28 | ||
| Decrease Positive > Watch Positive | L. Inferior Orbitofrontal Gyrus | 47 (47/12) | −54 | 18 | 2 | |
| L. Superior Temporal Pole | 47 (47/12) | −48 | 19 | −6 | ||
| L. Inferior Orbitofrontal Gyrus | 47 | −42 | 36 | −4 | ||
| R. Inferior Orbitofrontal Gyrus | 47 (47/12) | 35 | 32 | 0 | ||
| R. Inferior Orbitofrontal Gyrus | 47 (47/12) | 44 | 18 | −1 | ||
| R. Inferior Orbitofrontal Gyrus | 47 (47/12) | 30 | 24 | −4 | ||
| R. Inferior Orbitofrontal Gyrus | 47 | 49 | 38 | 1 | ||
| R. Inferior Orbitofrontal Gyrus | 47 (47/12) | 38 | 22 | −12 | ||
| R. Superior Frontal Gyrus/Pre-SMA | 6 | 7 | 7 | 62 | ||
| R. Superior Frontal Gyrus | 8 | 12 | 3 | 67 | ||
| R. Superior Frontal Gyrus | 8 | 18 | 7 | 54 | ||
| R. Superior Frontal Gyrus | 10 | 13 | 58 | 29 | ||
| R. Superior Frontal Gyrus | 10 | 18 | 54 | 37 | ||
| R. Middle Frontal Gyrus | 9 | 46 | 5 | 46 | ||
| L. Middle Frontal Gyrus | 9 | −18 | 24 | 33 | ||
| L. Middle Frontal Gyrus | 9 | −24 | 26 | 39 | ||
| R. Middle Frontal Gyrus | 46 | 41 | 39 | −10 | ||
| R. Inferior Frontal Gyrus | 47 (47/12) | 55 | 20 | 7 | ||
| Koenigsberg et al. (2010) | Reappraise (Distancing) Neg. –Neu. > Look Neg. –Neu. | Anterior Cingulate Gyrus/L. Medial Frontal Gyrus | 32/9 | −2 | 28 | 35 |
| L. Inferior Frontal Gyrus/Insula | 45/47/13 | −37 | 15 | 4 | ||
| R. Middle/Superior Frontal Gyrus | 10 | 31 | 53 | 18 | ||
| R. Medial Frontal Gyrus | 10 | 7 | 49 | 0 | ||
| L. Medial/Superior Frontal Gyrus | 10 | −10 | 51 | 8 | ||
| Levesque et al. (2003) | Inhibition of Sadness > Sadness | R. Orbitofrontal Cortex | 11 | 24 | 46 | −17 |
| R. Dorsolateral Prefrontal Cortex | 9 | 36 | 25 | 26 | ||
| Mak et al. (2009) | Reappraise Positive > View | L. Superior Frontal Gyrus | 8 | −4 | 40 | 36 |
| L. Middle Frontal Gyrus | 9 | −35 | 13 | 37 | ||
| Correlation with Self-Report Positive Emotion Change | L. Middle Frontal Gyrus | 9 | −23 | 29 | 37 | |
| Reappraise Negative > View | L. Inferior Orbitofrontal Gyrus | 11 | −20 | 19 | −16 | |
| L. Anterior Cingulate Gyrus | 32 | −12 | 41 | 18 | ||
| L. Superior Frontal Gyrus | 8 | −23 | 22 | 47 | ||
| Correlated Negatively with Decreases in Self- Report Negative Emotion | L. Amygdala | −31 | −10 | −6 | ||
| McRae et al. (2009) | Reappraise Negative > Look Negative | L. Superior Frontal Gyrus | 6 | −7 | 2 | 60 |
| L. Middle Frontal Gyrus | 10 | −34 | 55 | 20 | ||
| L. Middle Frontal Gyrus | 9 | −40 | 16 | 32 | ||
| R. Inferior Frontal Gyrus | 47 (47/12) | 32 | 17 | 3 | ||
| R. Middle Frontal Gyrus | 10 | 34 | 56 | 23 | ||
| R. Middle Frontal Gyrus | 9 | 38 | 23 | 38 | ||
| Reappraise Negative > Look Negative correlated with decreases in Negative Affect | L. Inferior Frontal Gyrus | 47 (47/12) | −42 | 26 | −4 | |
| L. Superior Frontal Gyrus | 6 | −18 | 10 | 65 | ||
| L. Superior Frontal Gyrus | 10 | −26 | 49 | 35 | ||
| L. Inferior Frontal Gyrus | 47 (47/12) | −54 | 23 | −3 | ||
| L. Middle Frontal Gyrus | 8 | −36 | 14 | 47 | ||
| R. Middle Frontal Gyrus | 6 | 53 | −4 | 49 | ||
| R. Middle Frontal Gyrus | 46 | 50 | 21 | 33 | ||
| R. Middle Frontal Gyrus | 8 | 1 | 25 | 47 | ||
| R. Middle Frontal Gyrus | 10 | 30 | 54 | 18 | ||
| R. Middle Frontal Gyrus | 11 | 41 | 47 | −2 | ||
| Distract Negative > Look Negative correlated with decreases in Negative Affect | L. Superior Frontal Gyrus | 6 | −7 | −1 | 71 | |
| Ochsner et al. (2002) | Reappraise Negative > Attend Negative | L. Superior Frontal Gyrus | 6 | −35 | 6 | 57 |
| L. Superior Frontal Gyrus | 6/8 | −24 | −2 | 61 | ||
| L. Middle Frontal Gyrus | 6/8 | −24 | 3 | 55 | ||
| L. Middle Frontal Gyrus | 6/8 | −39 | −5 | 57 | ||
| L. Inferior Frontal Gyrus | 46 | −51 | 37 | 18 | ||
| L. Inferior Frontal Gyrus | 44/10 | −45 | 41 | 11 | ||
| Dorsomedial Prefrontal Cortex | 8 | −13 | 10 | 54 | ||
| Dorsomedial Prefrontal Cortex | 8 | −5 | 12 | 54 | ||
| Dorsomedial Prefrontal Cortex | 8/32 | 6 | 21 | 42 | ||
| Negatively Correlated with Decrease in Negative Affect | R. Anterior Cingulate | 24 | 4 | 8 | 34 | |
| Ochsner et al. (2004) | Decrease > Look | L. Superior Frontal Gyrus | 6 | −11 | 10 | 61 |
| L. Superior Frontal Gyrus | 6 | −4 | 8 | 61 | ||
| R. Superior Frontal Gyrus | 6 | 8 | 11 | 63 | ||
| L. Superior Frontal Gyrus | 6 | −33 | 5 | 55 | ||
| L. Superior Frontal Gyrus | 8/9 | −16 | 38 | 45 | ||
| L. Superior Frontal Gyrus | 8 | −9 | 37 | 51 | ||
| R. Superior Frontal Gyrus | 10 | 19 | 38 | 35 | ||
| L. Middle Frontal Gyrus | 6 | −44 | −1 | 52 | ||
| L. Middle Frontal Gyrus | 8 | −44 | 2 | 43 | ||
| L. Middle Frontal Gyrus | 9 | −53 | 2 | 40 | ||
| L. Middle Frontal Gyrus | 8 | −35 | 15 | 48 | ||
| L. Middle Frontal Gyrus | 8 | −24 | 17 | 47 | ||
| R. Middle Frontal Gyrus | 8 | 47 | 9 | 49 | ||
| R. Middle Frontal Gyrus | 6/8 | 45 | 0 | 46 | ||
| R. Middle Frontal Gyrus | 9 | 37 | 23 | 41 | ||
| R. Middle Frontal Gyrus | 8 | 32 | 15 | 46 | ||
| L. Inferior Frontal Gyrus | 45 | −51 | 16 | 12 | ||
| L. Inferior Frontal Gyrus | 44 | −55 | 10 | 17 | ||
| L. Inferior Frontal Gyrus | 47 (47/12) | −42 | 20 | −2 | ||
| L. Inferior Frontal Gyrus | 47 (47/12) | −29 | 20 | −9 | ||
| R. Inferior Frontal Gyrus | 44 | 53 | 6 | 17 | ||
| R. Inferior Frontal Gyrus | 45 | 54 | 13 | 23 | ||
| R. Inferior Frontal Gyrus | 47 (47/12) | 45 | 15 | 1 | ||
| R. Inferior Frontal Gyrus | 47 (47/12) | 31 | 22 | −8 | ||
| Cingulate Gyrus | 32 | 10 | 18 | 33 | ||
| Ohira et al. (2006) | Suppressing > Attending | R. Orbitofrontal Cortex | 11 | 9 | 23 | −15 |
| L. Orbitofrontal Cortex | 11 | −6 | 57 | −16 | ||
| Phan, et al (2005) | Suppress Negative > Maintain Negative | R. Dorsomedial Prefontal Cortex | 8/9 | 10 | 33 | 56 |
| R. Dorsomedial Prefontal Cortex | 8/9 | 4 | 20 | 53 | ||
| R. Dorsolateral Prefrontal Cortex | 9 | 36 | 15 | 46 | ||
| R. Lateral Orbitofrontal Cortex | 11 | 47 | 29 | −1 | ||
| R. Lateral Orbitofrontal Cortex | 11 | 47 | 27 | 0 | ||
| R. Ventrolateral Prefrontal Cortex | 44/46 | 51 | 20 | 11 | ||
| R. Ventrolateral Prefrontal Cortex | 44/46 | 30 | 47 | 20 | ||
| L. Dorsal Anterior Cingulate | 32 | −9 | 13 | 45 | ||
| R. Dorsal Anterior Cingulate | 32 | 4 | 29 | 39 | ||
| Negatively Correlated with Decrease in Negative Affect | R. Dorsal Anterior Cingulate | 32 | 6 | 21 | 45 | |
| Anterior Insula | 43 | 6 | 36 | |||
| L. Dorsolateral Prefrontal Cortex | 8 | −42 | 12 | 42 | ||
| R. Dorsolateral Prefrontal Cortex | 8 | 49 | 13 | 41 | ||
| L. Ventrolateral Prefrontal Cortex | 46/10 | −41 | 44 | 17 | ||
| R. Ventrolateral Prefrontal Cortex | 46/10 | 32 | 45 | 20 | ||
| Urry et al. (2006) | Increase & Decrease > Attend | L Superior Dorsal Medial Frontal Gyrus | 6 | −3 | −7 | 58 |
| Decrease >Attend | Ventromedial Prefrontal Cortex | 32 | −23 | −43 | −10 | |
| Ventromedial Prefrontal Cortex | 11 | 5 | 37 | −12 | ||
| Van Reekum et al. (2007) | Increase>Decrease> Attend | L. Inferior Frontal Gyrus | 45 | 43 | 19 | 6 |
| R. Inferior Frontal Gyrus | 45/44 | 49 | 25 | 8 | ||
| L. Inferior Frontal Gyrus | 45/44 | 49 | 11 | 18 | ||
| L. Middle Frontal Gyrus | 9 | 43 | 13 | 30 | ||
| L. Middle Frontal Gyrus/Precentral Gyrus | 6 | 35 | 1 | 48 | ||
| Precentral Gyrus | 6/4 | 23 | −17 | 52 | ||
| Medial/Superior Frontal Gyrus | 6 | −3 | −1 | 56 |
Note: All MNI coordinates were transformed into Talairach space using the program GingerAle (Eickhoff et al., 2009).
Figure 11.
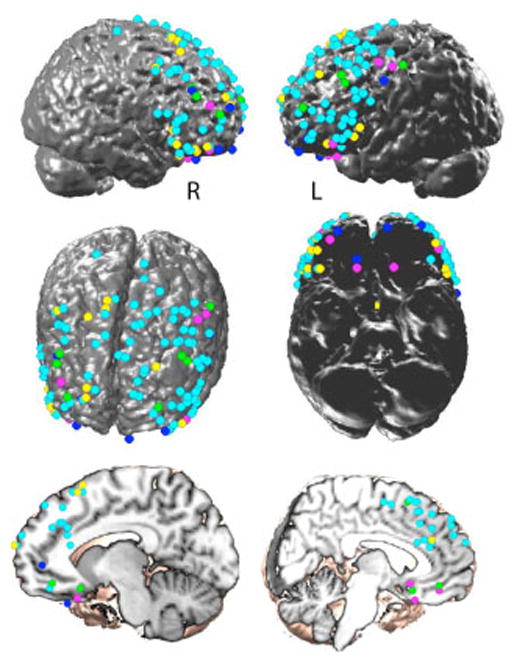
Areas activated during emotional regulation of negative emotions. The cyan markers are surface renderings of coordinates reported as more engaged in reappraisal to decrease negative emotion than a non-regulated condition. The blue markers are coordinates reported as more responsive to inhibition or suppression of negative emotion than a non-regulation condition. The yellow markers are coordinates reported as more active in reappraisal when decreasing positive emotion than in a non-regulated condition. The green markers designate those coordinates reported as increased during distraction over an unregulated condition. The pink markers are coordinates reported as more active during the recall of positive or soothing memories or images to regulate anxiety or sadness. The rendering was made with StudyplotUtility (http://psych.colorado.edu/~tor/). GingerAle was used to convert all coordinates to Talairach space (Eickhoff et al., 2009).
The most common paradigm for studying reappraisal asks participants to view primarily negatively valenced, highly arousing, static images (e.g., mutilation, assault, decay and defecation) and compares neural activation during trials cued for cognitive reappraisal with trials cued for passive viewing (Eippert et al., 2006; Kim et al., 2007; Ochsner et al., 2002; Ochsner et al., 2004; Phan et al., 2005; Urry et al., 2006; Van Reekum et al., 2007). While there are variations in the details of the reappraisal instructions from study to study, they consistently require participants to create a new interpretation of the meaning, cause, consequence or the personal significance of the image during the reappraisal trials. Reappraisal contrasted with unregulated viewing of negative images recruits broad areas of the PFC, including bilateral DLPFC and VLPFC (often more heavily left sided), and regions of the dorsal ACC and/or medial PFC as supporting the cognitive control aspects of reappraisal. Figure 11 displays the location of reappraisal related activations (cyan markers for decreasing negatively valenced stimuli, and yellow for decreasing positively arousing stimuli) from the above cited studies.
A related paradigm uses dynamic movie images instead of static pictures. These studies also demonstrate recruitment of bilateral DLPFC during cognitive reappraisal but vary as to whether regions of ACC and medial PFC are additionally recruited to decrease sadness, disgust or sexual arousal (Beauregard et al., 2001; Goldin et al., 2008; Levesque et al., 2003, 2004).
In several reappraisal studies utilizing either static or dynamic images, amygdala decreases were used as a proxy for change in negative valence and arousal along with decreases in insula recruitment in some studies (Goldin et al., 2008; Levesque et al., 2003; Ochsner et al., 2002; 2004; Phan et al., 2005). We note that a simple equating of amygdalar activity with negative affect is problematic, given that 1) the amygdala becomes active in situations that are not negative, and 2) negative affective experiences involve cortical and subcortical components that extend beyond the amygdala. However, given our interest in regional brain interactions, the down-regulation of the amygdalar activity provides a useful index for measuring prefrontal-limbic interactions regardless of the extent to which its activity correlates with negative affect. Most of the studies find decreases in the left amygdala, and often bilateral amygdalae, when utilizing reappraisal to down regulate negative affect. Only a couple of studies have examined reappraisal of positively valenced stimuli. When asked to reappraise or down regulate positive or sexually arousing stimuli, the level of right amygdala activation to the stimuli decreased (Beauregard et al., 2001; Kim & Hamann, 2007). This may raise speculation as to the laterality of emotion regulation, but in general, studies testing for formal interactions with amygdala laterality are lacking.
Another emotion regulation strategy involves bringing to mind positive or soothing images either from nature or from one’s past either to replace or counteract negative affect. Behavioral experiments demonstrate that recalling mood incongruent memories or images decreases negative affect (Erber & Erber, 1994; Joormann, Seimer & Gotlib, 2007; Parrott & Sabini, 1990; Rusting & DeHart, 2000). Two neuroimaging studies compared regulating one’s affect by calling to mind a calming image or memory to the unregulated anticipation of shock. Kalisch and colleagues (2005) cued trials with tones indicating whether there was a probability of shock on those trials or not. In the regulation trials, participants were instructed to detach from their feelings of anxiety and think of a special place identified earlier. In the non-regulation trials, participants were instructed to engage with their emotional responses. ROI analyses showed that this form of regulation recruited a region of right anterolateral frontal cortex (MNI: 42, 48, 18) and regulation in the presence of anxiety recruited regions of the medial PFC and rostral ACC (−4, 46, 28). In a similar study, Delgado and colleagues (2008b) used colored blocks to designate trials in which shock was possible, and asked participants to regulate their anxiety by calling to mind one of two pre-identified places in nature. Their ROI analyses show that calling to mind nature images when anticipating shock recruits the left middle frontal gyrus (Talairach: −43, 28, 30). The amplitude of which was associated with regulation success. Regulation also resulted in activation in the ventral medial wall and subgenual cingulate (BA 32; −3 36, −8 and BA 25; 0, 14, −11), which the authors point out has been associated with extinction (Phelps et al., 2004) and decreases in left amygdalar activity. While both of these studies employ similar paradigms, their analytic approaches including choice of ROIs and modeling of tonic versus phasic effects may be responsible for some of the differences in regions reported for drawing upon positive or soothing images to counter the anxiety associated with waiting for possible shock.
Similar to the prior emotion regulation strategy, distraction involves holding neutral and irrelevant information in one’s working memory. Behavioral research shows that doing so decreases negative affect in both dysphoric and nondysphoric individuals (Fennell, Teasdale, Jones, & Damle, 1987; Lyubomirsky, Caldwell, & Nolen-Hoeksema, 1998; Teasdale & Rezin, 1978). By taking up working memory capacity with mood incongruent cognitions, mood congruent thoughts are prevented from gaining access to attentional resources (Siemer, 2005). Neuroimaging studies of distraction have utilized two different paradigms. The first, employed by Kalisch et al. (2006), utilized the anticipation of shock paradigm, except instead of having the participant recall a pleasant or safe memory, there was an open distraction instruction in which the participant was encouraged to think of anything other than the possible shock. This paradigm identified a region of the left PFC (MNI: −56, 30, 22) that was more active in trials in which participants were instructed to distract themselves than in the no distraction trials. The second distraction paradigm involved an assigned distraction task (Sternberg working memory task) in which the participant holds a series of letters in working memory while viewing negative or neutral static images and then following the picture offset has to respond to whether a single letter was in the set they were holding in mind. McRae et al. (2009) report that engaging in a working memory task while viewing negative slides as compared to passive viewing increases the BOLD response in left and right superior and middle frontal gyri (MNI: BA6; −6, 10, 62 and −56, −4, 48 and 48, 42, 32; BA 9; −42, 22, 30 and 42, 30, 34; BA 10; −36, 62, 12 and 38, 64, 14) as well as right inferior PFC (BA47/12p; 36, 20, −4).
Many neuroimaging reports of emotion regulation explicitly present DLPFC regions as being engaged in some kind of cognitive control and are cautious about attributing concurrent decreases in amygdala responses to direct connections with the amygdala. In the case of reappraisal and distraction, this caution is particularly warranted since these processes produce foci that are distributed across the PFC (Figure 11). As mentioned earlier, the pattern of anatomical projections from the cortex suggest that direct paths from regions of DLPFC are unlikely to exert strong control of amygdala processing. Areas of the PFC with moderately dense projections in the lateral PFC are only found in a small portion of the VLPFC, specifically in the more posterior regions of BA 47/12. Unfortunately, as mentioned earlier, the nomenclature used to report activations in this region in most studies creates ambiguity when it comes to questions of connectivity with the amygdala. Studies of reappraisal, positive memory or image engagement and distraction commonly report activations in the general regions of VLPFC and medial OFC (Eippert et al., 2007; Goldin et al., 2008; Kim & Hamann, 2007; Lieberman et al., 2007; McRae et al., 2009; Ochsner, Ray et al., 2004). Specifically, many of the reappraisal studies report bilateral activations of BA 47/12 when decreasing negative or positive emotion. As noted above, BA 47/12 is a large and heterogeneous area and only posterior regions of BA 47/12 are sites of significant amygdalar projections. Therefore, strong statements about direct cognitive influence on the amygdala become more plausible in those studies with activations in this specific segment of BA 47/12.
Medial regions of the PFC are often treated as having privileged access to subcortical regions such as the amygdala. However, according to the mapped medial direct connections to the amygdala, only those regions of subgenual cingulate (BA 25) and dorsal ACC (BA 24) have dense direct connections with the amygdala. Only the studies by Delgado and colleagues (2008a, 2008b) report foci on the medial surface that are in regions positioned to broadly impact the amygdala. Given the anatomical data, it may seem surprising that activations of BA25 does not arise more frequently in these studies. However, it is plausible that signal drop out in the posterior VMPFC has prevented studies from demonstrating more consistent activation in this region. More frequently, studies of inhibition/suppression, distraction and reappraisal only report foci in BA 32, which may reflect a more specific modulation of the amygdala, given the more circumscribed nature of BA 32 input to the amygdala.
Correlational Studies of amygdala deactivation
In order to understand in more detail how top-down emotion regulation interacts with the amygdala, a subset of emotion regulation studies have gone further than task versus control contrasts to investigate the specific correlates of decreases in amygdala activity (See Table 2). That is to say, instead of asking what areas are engaged in a task known to down regulate amygdala activity, they explicitly tested the correlation or functional/effective connectivity between the amygdala and the whole brain during emotional regulation performance. Alternatively, some studies correlated amygdala decreases with already identified prefrontal regions from the main regulation contrasts. These studies indicate that amygdalar decreases are negatively correlated with many areas of PFC activity. Of particular note are the activations in the VMPFC, including BA 11m/14r (5, 37, −12; −6, 46, −20: Urry et al., 2006, Ochsner et al., 2002 respectively). Additionally, subgenual and pregenual cingulate regions were observed to be negatively correlated with amygdala activity during regulation. For instance, Urry and colleagues (2006) reported a region of BA 32/10 (maximum at −23, 43, −10) that extended ventrally and medially. Delgado et al. (2008b) also report an inverse correlation between BA 32 (0, 35, −8) activity and amygdala decreases. Posterior (BA 13) areas of the OFC also negatively correlated with amygdala deactivation (−24, 28, −14; 26, 24, −22: Banks et al, 2007: −30, 22, −16; 34, 24, −16: Ochsner, Ray et al., 2004). Less ventral areas of the PFC in BA 47 (34, 54, 12) and BA46 (−54, 12, 12: Urry et al., 2006; −54, 42, 12: Ochsner et al., 2002), also arose in these studies. Two studies statistically linked specific DLPFC regions to medial regions, which then corresponded to decreases in amygdala response. In a study by Urry et al. (2006), a mediation analysis demonstrated the connection between the amygdala, BA 10 (3, 63, 18) and a DLPFC region (−50, 23, 19). Delgado et al. (2008b) alternatively used the medial BA 32 region as the seed for their PPI analysis that then identified a left amygdala region and a DLPFC region. Importantly, these studies identify regions corresponding to amygdala decreases that have also been noted above as projecting to the amygdala such as the dorsal anterior cingulate, subgenual cingulate and posterior orbitofrontal cortex.
Table 2.
Studies that report correlations between decreased amygdala activity and prefrontal region increases during emotion regulation tasks.
| Study | Type of Analysis | Regions | BA | X | Y | Z |
|---|---|---|---|---|---|---|
| Banks et al. (2007) | PPI with Left Amygdala region active for both Reappraisal and Maintain | L. Orbitofrontal Cortex | −21 | 26 | −6 | |
| R. Orbitofrontal Cortex | 23 | 22 | −13 | |||
| L. Dorsolateral Prefrontal Cortex | −11 | 25 | 54 | |||
| Superior PFC | −13 | 14 | 59 | |||
| Superior PFC | 17 | 15 | 60 | |||
| Dorsomedial PFC | 6 | 36 | 37 | |||
| Subgenual PFC | 5 | 21 | 5 | |||
| Kanske et al. (2011) | PPI with Left Amygdala for Reappraisal | L. Superior Medial Frontal | 10 | −6 | 59 | 12 |
| 9 | 0 | 46 | 43 | |||
| R. Superior Frontal | 6 | 22 | −6 | 67 | ||
| L. Inferior Orbitofrontal | 47 (47/12) | −32 | 28 | −8 | ||
| R. Inferior Orbitofrontal | 47 (47/12) | 31 | 31 | −8 | ||
| L. Ventromedial Frontal/Anterior Cingulate | 25/10/11 | −9 | 23 | −3 | ||
| PPI with Left Amygdala for Distraction | R. Anterior Cingulate/Dorsomedial Frontal | 6/8/32 | 6 | 26 | 44 | |
| L. Middle Frontal | 44 | −47 | 27 | 29 | ||
| 6 | −52 | 6 | 34 | |||
| R. Middle Frontal | 44/46 | 47 | 31 | 35 | ||
| Ochsner et al. (2004) | Negative Correlation with right Amygdala decrease during Reappraisal | 47 (47/12) | −39 | 19 | −9 | |
| 47 (47/12) | 31 | 22 | −8 | |||
| 44 | 53 | 6 | 17 | |||
| 32 | −18 | 26 | 26 | |||
| Ochsner et al. (2002) | Negative Correlation with Amygdala decrease during reappraisal | 46/10 | −51 | 37 | 18 | |
| Urry et al. (2006) | PPI with Decrease >Attend Left Amygdala signal | R. Ventromedial Prefrontal Cortex | 11 | 5 | 37 | −12 |
| L. Ventromedial Prefrontal Cortex | 32/10 | −23 | 43 | −10 |
Note: All MNI coordinates were transformed into Talairach space using the program GingerAle (Eickhoff et al., 2009).
Of the regions reported from these correlational analyses or multiple regression analyses, a limited number of them have plausible direct connections into the amygdala. The most common regions that are negatively correlated with amygdala response are regions of the posterior OFC and subgenual cingulate and VLPFC (Figure 12). Of the lateral prefrontal regions only the posterior lateral portion of BA 47/12 has strong projections to the amygdala. Regions of anterior BA 32 are also identified in correlational analyses, which could reflect projections to the assessory and basal lateral nucleus of the amygdala (Cheba et al, 2001).
Figure 12.
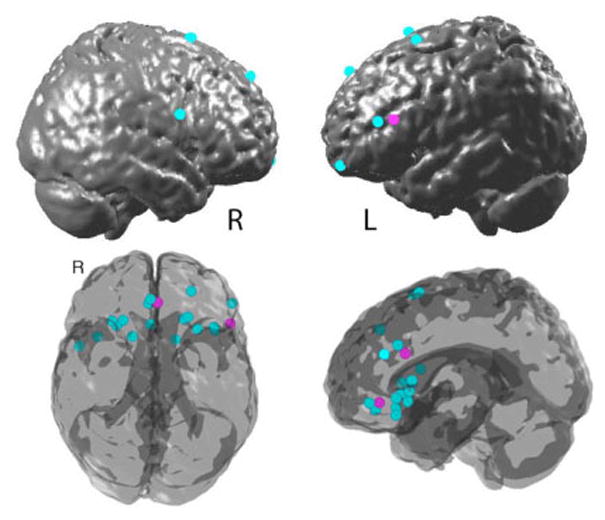
Coordinates identified in Table 2 as correlated with deactivations in the amygdala during emotion regulation plotted on the surface of a template brain (top left and right) and rendered on a glass brain (bottom view and left view). The cyan markers are coordinates reported in studies of reappraisal. The maroon markers are coordinates reported in studies using recall of positive or soothing memories or images to regulate anxiety or sadness. The rendering was made with StudyplotUtility (http://psych.colorado.edu/~tor/) written by Tor Wager. GingerAle (http://brainmap.org/ale/index.html) was used to transform MNI coordinates into Talairach space.
Models of emotion regulation
To date, the most sophisticated data driven model of emotion regulation comes from a study of positive reappraisal by Wager and colleagues (2008). The outcome variable of interest is change in self-reported negative affect. A structural equation methodology was applied to a neuroimaging dataset from a reappraisal paradigm similar to the ones used by Ochsner et al. (2002; 2004). The right VLPFC was chosen as the starting point for the analyses, with coordinates centered in an area that plausibly includes the posterior portion of area 47/12 with projections to the amygdala. The authors first used an ROI approach to test the role of the amygdala and nucleus accumbens as mediators between the right VLPFC and decreased negative affect which was identified as the primary metric of reappraisal success. In this ROI analysis both structures were shown to mediate the relationship between the right VLPFC and self-reported decrease in negative affect (see Figure 13).
Figure 13.

A diagram of the mediation analysis testing the relationship between the right VLPFC and decreases in negative affect mediated by activation in the amygdala and nucleus accumbens. Figure adapted with permission from Wager, Davidson, Hughes, Lindquist, & Ochsner (2008).
The authors then used whole brain cluster analysis and nonparametric inference to identify two networks as possible mediators of the relationship between the VLPFC and changes in self-reported negative affect (see Figure 14). One network has an indirect positive bias towards increasing the change in negative affect. This network includes regions of nucleus accumbens, subgenual cingulate (BA 25), pre-SMA, precuneus, DMPFC (MNI: 24, 41, 40), and superior frontal gyrus (24, 21, 58). Amongst these regions, the nucleus accumbens and subgenual cingulate have the most interconnection with the amygdala. The second network identified has an indirect negative bias towards decreasing the change in negative affect and reducing reappraisal success. This network includes the rostral dorsal ACC, amygdala (bilateral) and posterior-lateral OFC (48, 24, −18). Future work will have to elucidate how the components of the networks interact and whether these networks are specific to this particular type of emotion regulation strategy.
Figure 14.
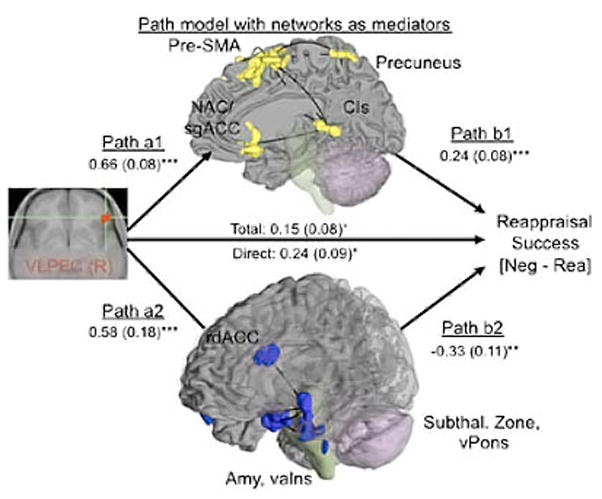
Path model of the positively biased network in yellow and negatively biased network in blue mediating the relationship between the VLPFC and the decrease in self-reported negative affect. Figure adapted with permission from Wager, Davidson, Hughes, Lindquist, & Ochsner (2008).
Several investigators have put forth theoretical models as to the neural mechanisms behind emotion regulation. The simplest of these models proposes that a limited number of areas exert a direct influence on the amygdala. Delgado et al. (2008b), Hansel and von Kanel (2008) and Quirk and Beer (2006) each propose that the ventromedial PFC down regulates regions of the amygdala. These models importantly attempt to ground our understanding of the neuroanatomical bases of human emotion regulation in the extensive animal literature on extinction and the ventromedial PFC’s connections to the intercalcated masses in the basolateral amygdala (Morgan, Romanski & LeDoux, 1993; Likhtik et al., 2005; Quirk et al., 2000). Quirk and Beer (2006) build on the presence of both excitatory and inhibitory effects of the “ventral” medial PFC projections to the amygdala in humans and rats. The subgenual cingulate region, BA 25, is argued to be more inhibitory whereas the more dorsal and anterior BA 32 is proposed to have excitatory connections with the amygdala. Both BA 25 and 32 have connections with the amygdala. BA 32, however, has much more limited connections.
Phillips et al (2008) have developed a circuit model that attempts to explain the neural underpinnings of multiple types of emotion regulation (see Figure 15). The model contains component regions of the DLPFC, OFC, VLPFC, DMPFC and ACC. Of particular interest, the authors distinguish between areas involved in automatic emotion regulation (in subgenual and rostral ACC) and regions that are recruited for voluntary emotion regulation (DLPFC and VLPFC). They characterize these latter regions as phylogenetically newer and providing feedback to the older emotion generation processes. The OFC, DMPFC and ACC, on the other hand are phylogenetically older regions that are described as operating through feedforward processes to relay internal state information to the DLPFC and VLPFC. The authors place the DMPFC as the conduit through which the OFC feeds value information forward to neocortical regions of the brain for decision processes.
Figure 15.
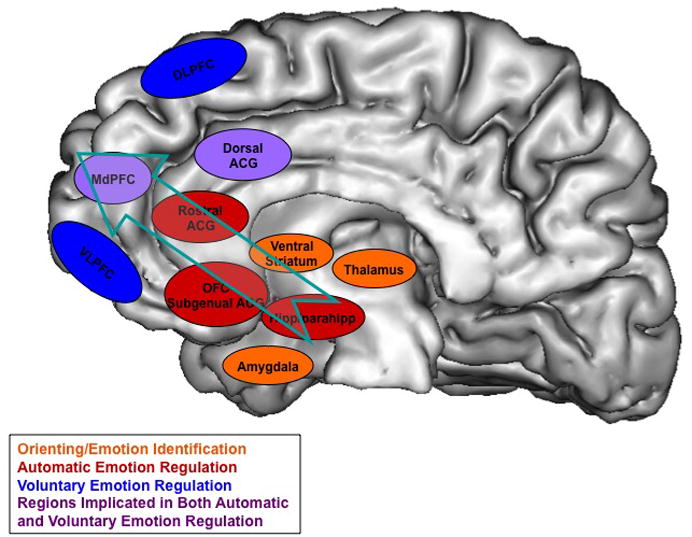
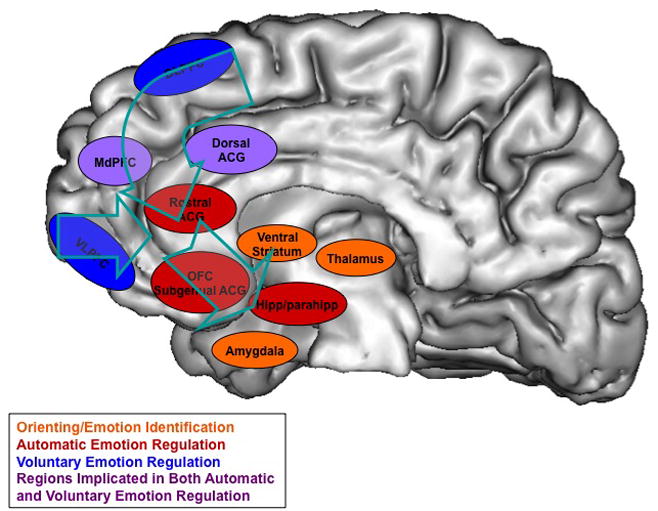
Phillips et al. (2008) model of prefrontal amygdala interactions a) The OFC, subgenual ACG (ACC), and rostral ACG (ACC) feedforward information to the MdPFC and then to the lateral PFC regions for decision and action. B) The feedback processes from the DLPFC and VLPFC to the OFC, rostral ACG (ACC), subgenual ACG (ACC) to the amygdala. Figure adapted with permission from Phillips, Ladouceur & Drevets (2008).
One unique aspect to this model is the explicit articulation of the processes of feedforward and feedback. The model is intuitively appealing and clearly fits with traditional ideas about the DLPFC exerting top-down control over more “emotional” regions. However, it is difficult to reconcile this conceptualization with the structural model, given the laminar distribution of PFC connections (Barbas & Rempel-Clower, 1997; Barbas, 2000). Indeed, the structural model suggests that the information flow between the DLPFC and the OFC is actually in the opposite direction with processes originating in the OFC and going to the DLPFC characterized predominantly as feedback, and those arising in the DLPFC and going to the OFC predominantly characterized as feedforward.
The Phillips et al. model is also notable in its placement of so called “automatic regulation” regions such as the subgenual cingulate and OFC as the primary route through which more phylogenetically newer regions impact limbic areas such as the amygdala. This is largely consistent (particularly the subgenual cingulate region) with the network arrangements described above. It may be speculated, however, that there may be more than one route through which voluntary emotion control areas can impact amygdala processing. In particular, the posterior VLPFC may be able to directly impact amygdala processes without requiring engagement of one of the more medial “automatic regulation” regions, given its direct inputs to amygdala nuclei.
In summary, a wealth of data indicate the engagement of PFC regions during emotion regulation tasks, with activity in a more select group of areas (BA 47/12, BA25 and BA 32) showing associations with the ability to down-regulate amygdala activity. Increasingly sophisticated models have been proposed to explain these data. The emergence of these models is appealing, as is the concern shown by their authors for the plausibility of the proposed connectional networks. We do note, however, that no models to date have explicitly acknowledged the laminar pattern of connections between different PFC regions. For instance, Wager et al (2008) provides the most complicated model for a particular emotion regulation strategy, but does not address the nature of the information flow between the component regions. Phillips et al. more explicitly incorporate the concept of feedforward and feedback information, but do not reconcile these ideas with the observed pattern of feedback and feedforward projections in the regions in question. We believe that reconciling these issues provides one of the key challenges for researchers attempting to understand the neural substrates of emotion regulation.
6. Cognitive control of emotional distraction
While much of our analysis has focused on studies of emotion regulation, many similar issues arise when considering the literature on cognitive control. Broadly, cognitive control refers to the high-level executive processes that promote goal relevant processing, while inhibiting goal irrelevant processing. The term is particularly used to apply to tasks requiring the selective attention to incoming goal-relevant sensory information and the inhibition of goal irrelevant sensory information, and the accompanying selection of goal-promoting responses and suppression of competing goal-inappropriate responses. Such a selection process is often explicitly presented in terms of top-down modulation and biasing of processing pathways. Emotional regulation studies may be viewed as a specific subcategory of cognitive control that focuses on modulating the affective response itself. In contrast, most other types of cognitive control studies involving emotion focus on the ability to overcome the distraction caused by emotional stimuli. Because of their inherent (often automatic) attention capturing qualities (Most et al., 2005; 2007; Pessoa, 2008), emotional stimuli often provoke a strong need for cognitive control in order to maintain appropriate selection of goal relevant information. This need to avoid distraction from emotional stimuli particular occurs in studies where emotional stimuli occur simultaneously with other stimuli, are incongruent with other task demands, or during working memory tasks, where disruption could interfere with the on-line maintenance of information. We briefly review these studies in order to highlight their convergence with the emotion-regulation literature. For a more thorough review of this literature readers are referred to Banich et al. (2009).
Suppression of emotional stimuli during cognitive tasks
Multiple studies have employed paradigms in which participants have to respond to a task-relevant nonemotional feature of a stimulus (such as color) and not be distracted by emotional content (i.e, emotional words), or to attend to a nonemotional stimulus (i.e. a house) while ignoring an emotional stimulus (a fearful face). For instance, rostral (dorsomedial, pregenual and dorsal ACC) regions and both DLPFC and VLPFC regions have all been observed in emotional Stroop paradigms in which subjects must avoid being distracted by the emotional content of words (Whalen et al., 1998; Compton et al., 2003; Herrington et al., 2005; Mohanty et al., 2007). For a more thorough review of how attention control and emotion control may involve the same neurocognitive substrates readers are referred to Blair & Mitchell (2009) and Mitchell (2011).
An interpretational limitation of many of these paradigms arises though in that it is not always clear whether these regions are being engaged because they are exerting cognitive control, monitoring conflict, are engaged because of greater conflict/distraction without necessarily controlling the conflict/distraction, or are simply responding to the emotional nature of the stimuli. For example, Mohanty and colleagues (2007) elegantly demonstrate that the pregenual cingulate region (approximately BA 24/32) shows increased activation during a Stroop task with emotional words, and that this correlates with increased reaction time in the task. This could be interpreted in terms of the rACC becoming engaged in order to exert cognitive control over the emotional distractors. However, given that the activation of this region correlates with greater reaction time, its level of activation does not appear to be related to successful inhibition of the distractors. Moreover, it showed increased functional coupling with the amygdala, which is obviously inconsistent with the hypothesis that the rACC was driving a down-regulation of the amygdala. Indeed it is notable that the authors suggest that rather than reflecting rACC regulation of the amygdala, the heightened connectivity during exposure to the emotional distractors may reflect the amygdalar regulation of or input to the rACC, rather than the other way round.
Among the more striking pieces of evidence for prefrontal cognitive control over emotional processing in the amygdala comes from a study by Etkin et al. (2006), in which participants performed a Stroop-like task in which emotional facial expressions could be congruent or incongruent with words naming an emotion. The design of the study was relatively complicated as the authors focused not on a simple comparison of emotional vs. neutral trials or incongruent vs. congruent trials, but rather examined effects during incongruent trials that specifically followed either a congruent or incongruent trial. Interestingly, the DLPFC, a DMPFC region in the superior frontal gyrus, and the rostral (pregenual) ACC showed activations during incongruent trials that were dependent upon whether the prior trial was congruent or not. The DLPFC (and the DMPFC) responded greater to incongruent trials that followed a congruent trial, whereas the rostral ACC responded greater to trials that followed another incongruent trial. The study is one of the few studies in the cognitive control literature that specifically examined the relationship of prefrontal cortical regions to amygdala activity (using psychophysiological interaction analysis, Friston et al. 1997). Remarkably, greater activity in the rostral ACC was inversely correlated with right amygdala activity. Based on the pattern of amygdala responses, the authors argue that amygdala activity is correlated with the degree of conflict on a given trial, and by suppressing amygdala activity the rostral ACC provides control over this conflict. Support for this idea comes from behavioral data in that those who showed greater inverse functional connectivity on incongruent trials showed greater conflict resolution as measured by reaction times on the task. In a follow-up study Etkin et al. (2010) observed that this suppression of amygdala activity appears weaker in patients with generalized anxiety disorder relative to healthy controls, providing a potential neural correlate of the difficulty controlling emotional distraction or conflict in this patient population.
An important caveat is warranted in regard to this literature. First, studies by Etkin’s group do not suggest the presence of a global tonic inhibition of the amygdala by PFC regions during conflicting emotional information, or a constant engagement of “cognitive control regions,” but rather a task specific inhibition that depends upon the level of conflict between immediately prior stimuli. If correct, the ability to observe inverse associations between the pregenual cingulate (or other PFC regions) and the amygdala may be highly task and analysis specific.
Other lines of evidence also raise the possibility that other prefrontal areas, particularly dorsal ACC, may exert inhibitory control over the amygdala. For instance, in a study using the same paradigm as Etkin et al. (Chechko et al., 2009), patients with panic disorder showed greater slowing than healthy controls during emotionally incongruent trials, as well as higher amygdala, but lower dorsal ACC/DMPFC activity, leading to a suggestion that panic disorder is characterized by insufficient DMPFC/dorsal ACC control. Similarly, Hariri et al., (2003) observed a negative correlation between the amygdala and dorsal ACC (and VLPFC) when subjects had to label vs. match emotional pictures (with amygdala activity increasing for the match condition, and the VLPFC and dorsal ACC increasing activity during the label condition). It has also been suggested that dACC may exert regulatory control over the amygdala even in the absence of specific conflict or emotional distraction of a task. Pezawas et al. (2005) observed significant inverse associations between dACC and amygdala activity during a threat face matching task. It may also be noted that the subgenual ACC in the Pezawas study was positively correlated with amygdala activity, suggesting a unique interplay between different areas of the cingulate and the amygdala, and further suggesting, as in the Monhaty et al. (2007) paper, that the rACC, at least in some situations is positively, rather than negatively, coupled with the amygdala.
Working Memory
Another subclass of cognitive control experiments focuses on the ability to suppress emotional distraction during working memory tasks. Because the amount of information that can be held and manipulated on-line is limited (Cowan, 2010), it is critical that individuals appropriately prioritize which information enters this on-line store. Ideally, we should maintain goal relevant information relative to less important information, but also be able to dump the contents of working memory when more important information supersedes prior goals. As such, working memory provides a potentially useful domain for examining emotion-cognition interactions, especially given the critical role of the DLPFC and VLPFC in working memory processes (Badre et al., 2005; Blumenfeld et al., 2010; Curtis & D’Esposito, 2004; Jonides et al., 2005; Levy & Goldman-Rakic, 2000, Nee & Jonides, 2010; Postle, 2006; Thompson-Schill et al., 2002).
Two reports by Dolcos and colleagues are of note as they particularly link brain activations to successful performance or tap into issues of functional connectivity (Dolcos and McArthy 2006; Dolcos et al., 2006). Both reports analyzed data from a simple face matching delayed response task in which emotional or neutral images were presented during the delay (maintenance) period of the task. In the first study, they demonstrated that the ventrolateral cortex (BA 45/47) activated bilaterally during emotional relative to neutral distractors. Participants who showed greater ventrolateral activity in the presence of emotional distractors rated those distractors as less distracting. In a follow-up study they showed that left BA 45 activity (but not right BA 45) activity differentiated between trials in which the individuals successfully vs. unsuccessfully ignored the distractor (as demonstrated by correct or incorrect delayed response performance). Dolcos et al. (2006) also report on VLPFC- amygdala functional connectivity, with both areas increasing during emotional relative to neutral distractor trials. Importantly this connectivity is in the positive direction and cannot be interpreted as reflecting suppression of amygdalar firing.
The studies by Dolcos and colleagues also provide evidence for dissociable patterns of activation and deactivation across frontal regions. Specifically, ventrolateral areas increased with emotional distractions, while DLPFC (BA 9/46) decreased, suggesting a reciprocal relationship between these regions. This reciprocal relationship echoes an inverse dorsal vs. ventral pattern observed by Perlstein et al. (2002) who had subjects perform a working memory task in which emotionally valenced pictures appeared as task relevant cues and probes [interestingly, the reciprocal relationship was closely linked to valence with DLPFC going up with rewarding stimuli and ventral regions (BA 10/11) showing increased activity for negative stimuli]. The inverse pattern between more dorsal and ventral PFC regions has also been observed in other working memory paradigms, with greater DLPFC relative to ventral frontal activity being associated with greater working memory load (Rypma et al., 2002; Woodward et al., 2006), although the specific ventral PFC regions involved in such studies vary. The apparent inverse pattern of ventral and dorsal regions suggests an oppositional tension between these regions, but do not indicate the causal nature of the relationship. Ranganath (2006) proposes a hierarchical structure to working memory processes in which caudal/ventral PFC regions provide top down control of posterior systems, while the dorsal/rostral PFC provides control of the more caudal ventral frontal regions. Within this perspective, Ranganath states that selection processes implemented by rostral/dorsal PFC involves modulations of activity in caudal/ventral PFC. However, as described below, modulations in the opposite direction also warrant consideration.
7. Affective regulation of cognitive areas
Given the structural model outlined in earlier sections, OFC projections to the lateral PFC, including DLPFC can be categorized as predominantly providing feedback. As such, these projections may provide biasing and regulation of more cytoarchitecturally developed regions. Although seemingly opposed to philosophical views that place rationality over emotion, the idea that an area involved in affective processing might provide feedback type biasing over areas involved in other cognitive interactions fits easily with modern views of emotion that emphasize the ability of emotion to prioritize and bias information processing in order to facilitate biologically and socially significant goals. This view of emotion is elegantly articulated by Gray and colleagues (Gray, 2001, Gray, Braver, Raichle, 2002), who posit that approach and withdrawal states adaptively influence the efficiency of specific cognitive functions, both enhancing and disrupting different cognitive functions in order to meet situational demands more effectively. Evidence for such emotional modulation of cognition is well accepted in decision-making (Delgado et al., 2003; Grabenhorst & Rolls, 2009; Hardin, Pine & Ernst, 2009; Piech et al., 2010), but also can be observed in other prefrontal mediated functions such as working memory. For instance, spatial vs. verbal working memory performance are inversely modulated by induction of positive vs. negative mood states, with spatial working memory being enhanced by withdrawal mood states and impaired by approach states, and verbal working memory showing the opposite effect (Gray, 2001). Additionally, positive and negative emotion information reduces working memory interference compared to neutral information (Levens & Phelps, 2008; 2010). The right OFC (33 24 −8) and the left anterior insula (−32 21 2) respond more in the emotional interference resolution.
Similarly, in a behavioral cognitive set-switching task, the induction of positive affect, as compared with neutral or negative affect, promoted cognitive flexibility and reduced perseveration, but also led to increased distractibility (Dreisbach & Goschke, 2004). Such findings are consistent with a growing body of evidence that positive and negative mood states can broaden or narrow attention depending upon the strength of the approach or withdrawal characteristics of the mood state (Fredrickson & Branigan, 2005; Gable & Harmon-Jones, 2008; 2010; Gasper, & Clore 2002).
Critically, increasing evidence indicates that motivational effects impact BOLD responses in the DLPFC (BA 9) during a working memory task (Gray, Braver, & Raichle, 2002; Savine & Braver, 2010). Indeed, Savine & Braver (2010) demonstrate that within the left DLPFC (BA 9), monetary reward incentives specifically enhanced task–cue-related activations, and this activation predicted whether a trial would be performed optimally. Taken together, such studies require a reframing of unidirectional views of the relationship between cognitive and emotional processes.
Single cell studies provide some additional insights into the time course of communication between ventral and more dorsal regions, in relation to reward. Data from primates suggest that orbital areas code for a purer valuation of rewards than other frontal regions, and that the OFC provides this valuation information to more dorsal prefrontal regions (Hikosaka & Watanabe, 2000; Wallis & Miller, 2003; Rushworth et al, 2005). Importantly, OFC neurons show responses to reward information that precedes reward-related responses in the DLPFC (Wallis & Miller, 2003). This jives with the idea that the OFC first codes the reward value and then feeds this information to areas capable of linking this information with actions or other contextual information necessary to gain access to the reward. We note, however, that it is not clear to what extent this incentive information specifically reaches the DLPFC in terms of feedback type projections, or may be considered feedforward in nature, as some 30% of the OFC, DLPFC projections may be considered feedforward in nature (Barbas & Rempel-Clower, 1997). According to the structural model this distinction would determine whether the reward sensitivity of DLPFC cells reflects a feedback type biasing of DLPFC or reflects a more simple (feedforward type) transmission of information on valuation that the DLPFC can operate upon. Please refer to Mitchell (2011) for a review on how the neural substrates of reward may overlap with those of emotion regulation.
The idea that emotional processing influences cognitive operations also may be useful when considering functional connectivity between the amygdala and prefrontal regions. As noted earlier, positive functional connectivity between PFC regions (particularly pregenual cingulate and VLPFC) has been observed in past studies (Pezawas et al., 2005; Dolcos et al., 2006). We suggest that in these situations the amygdala may be the initiator, in that it would likely compute the salience of the situation first, and communicate that information or attempt to regulate PFC regions based on that information rather than the other way around. However, to date few attempts have been made to model the causal direction of this functional connectivity.
8. Discussion
We believe that the above review illustrates the need to attend to the details of the anatomical connections within the PFC and their relationship to the amygdala when considering emotion-cognition interactions. Failure to do so can lead to models that are difficult to reconcile with anatomy, and are thus likely to prove inaccurate. In contrast, attention to the details of neurocircuitry not only can provide for more plausible models of the interaction between emotional and cognitive processes, but may also reveal functional properties that otherwise would not be attended to.
Insights for Emotion Regulation
Based on the selective nature of neuroanatomical pathways between the PFC and amygdala, plausible models of PFC modulation by necessity must involve modulation of, or relay through, dorsal anterior cingulate, the subgenual region extending into the gyrus rectus, or through the posterior part of area 47/12. At this stage of the field, simple statements that the PFC is involved in emotional regulation provide insufficient detail to be useful, and in many cases may in fact be misleading, as the majority of PFC regions lack strong projections to the amygdala. The emergence of path models that concentrate on the key nodes projecting to the amygdala, such as the models proposed and tested by Wager et al. and Phillips et al. are an encouraging development in this regard. We suspect that for further progress to be made in understanding the PFCs involvement in emotion regulation, the relative roles of the dorsal anterior cingulate, posterior 47/12 and subgenual region in regulating the amygdala will need to be determined.
A key question also remains regarding how the extremely widespread PFC activations that arise during emotion regulation relate to these key nodes, as only a few studies have directly assessed intra-PFC functional connectivity. Anatomically, these PFC areas are not equally connected to the dorsal anterior cingulate, posterior 47/12 or the subgenual region, and therefore are likely to be selectively associated with different pathways to the amygdala. We suspect that a full understanding the PFC’s involvement in emotion regulation will require elucidation of how many of these PFC regions that lack direct limbic projections selectively interact with other PFC regions that do have sufficient projections to modulate limbic processing.
Insights on the directionality of influences
We have argued that dominant models of intra PFC, and PFC-amygdala interactions that articulate a strict unidirectional top-down cognitive control over emotional processes are inconsistent with the laminar characteristics of connections between these regions. Our argument against these traditional top-down models of PFC-amygdala and intra-PFC interactions relies heavily on the structural model described by Barbas and colleagues, in which the laminar pattern of projections dictate whether the projections represent feedback-like biasing of processing, or feedforward conveyance of information. If correct, more emotion related areas appear to provide greater top-down feedback control relative to bottom up feedforward conveyance of information than the more traditionally cognitive areas of the PFC.
We believe that the terminology of top-down regulation has led to a conceptual bias in understanding the relationship between brain regions and cognitive-emotional processes. This bias fits with a philosophical view of the roles of “cognitive” and “emotional” processes that places cognition above the more animalistic emotions. But this bias may interfere with our ability to gain a full understanding of the manner in which the brain processes information. If emotional processes regulate and bias “cognitive” operations, as much as or more than the other way round, the terminology of top-down and bottom-up may be inappropriate in considering emotion-cognition interactions.
Limitations in inferring function from structure
The elegance of the structural model is that it leads to strong predictions about the nature of inter-regional communication. However, several criticisms may be immediately raised in drawing functional conclusions based on anatomical features. First, although the structural model is strongly supported in terms of its predictions of laminar connection patterns based on cytoarchitecture, inferences regarding the functional implications of these laminar connection patterns has not received formal testing in circuits outside of sensory processing streams. Although it seems reasonable to assume that the same functional features characterize laminar patterns of projections throughout the brain, this is not necessarily the case. As such, inferences about the functional properties of connections in the PFC are only valid if the functional characteristics of structural feedforward and feedback projections are proven to hold throughout association cortices.
We have posited a strong linkage between functional feedback and top-down regulation, and a similarly strong link between feedforward and bottom-up processes. The terms feedback and feedforward originate from control theory, which attempts to describe the functioning of dynamic systems. The adoption of these terms by neuroscientists and psychologists is unsurprising as the concept of feedback mechanisms providing a regulatory control and feedforward mechanisms providing the transfer of information to higher areas in a processing stream is intuitive. However, a simple equation of top-down regulation with feedback and bottom-up with feedforward is problematic to the extent that additional features are implied by top-down and bottom-up conceptualizations. Such additional features are rarely made explicit, but could prove critical in conceptualizing information processing pathways. We suspect that some theorists utilize the terms top-down and bottom-up in ways that are inconsistent with feedback and feedforward mechanisms as defined by control theory, but such inconsistencies are rarely made explicit in the literature.
In characterizing the feedback and feedforward projections of the PFC, we note that we are not implying that all projections are of the same kind. Areas have a combination of feedback, feedforward, and lateral connections, but the proportions of these connections differ dramatically across areas. Thus we are characterizing dominant patterns of connections, but this does not mean the remaining connections are not functionally significant. For instance, eulaminate PFC regions certainly have enough feedback projections to help regulate aspects of less granular regions, even if this is not the dominant mode of communication between the areas.
Moreover, feedforward type projections connections could in some cases modulate processing in target regions rather than simply carrying information. Perhaps the best example of this sort of feedforward modulation arises in integrated competition models (Desimone and Duncan 1995; Duncan et al. 1997) in which the gain of one representation results in the suppression of another. In such models, the feeding forward of a given representation can lead to an enhancement of processing of that stimulus, and the mutual suppression of another stimulus (Desimone and Duncan 1995). In this manner, what gets fed forward can act to modulate processing in target regions. In the context of PFC functioning, a DLPFC signal could thus alter the competition between potential representations in the OFC through this sort of feedforward projection. This type of competitive mechanism is intriguing because it would imply specific computational features (Walther & Koch, 2006), that have not generally been incorporated into models of emotional regulation.
In considering the structural model, it is important to reiterate that the criteria used by Barbas and colleagues to define feedforward and feedback connections are not entirely consistent with the criteria that have been used by other investigators examining the hierarchical arrangement of laminar projections. Specifically, definitions of feedback and forward connections are often defined with reference to layer IV, such that feedforward (ascending) projections are defined by their termination in layer IV (or predominately in layer IV), while feedback (descending) projections terminate outside of layer IV. While a strict adherence to a layer IV rule is probably ill advised, as exceptions to this patterns have been observed (Pandya and Rockland, 1979; Felleman and Van Essen, 1991), the impact of broadening criteria to allow projections terminating in infragranular layers V and VI to be treated as feedforward projections is not fully understood. Arguably, studies of the timing of firing in different PFC cortical layers could address this question, but data on this issue are lacking.
The question of criteria causes pause before assuming that the OFC-DLPFC truly has a pattern in which the OFC should be treated as a higher level than the DLPFC, and it is not our intention to argue such. Nevertheless, it can be clearly stated that the patterns of projections certainly do not conform to a hierarchical organization in which the DLPFC is in a hierarchical position above the OFC, in a manner similar to higher level sensory areas sitting above primary sensory areas. As such, models of PFC organization would be wise to avoid the pervasive positioning of the DLPFC as sitting at the top of a hierarchy of PFC regions.
Modeling of feedforward and feedback connections
In evaluating the existing models of emotion-cognition interactions it is notable that few published studies to date have included specific tests of whether projections reflect feedback, feedforward or lateral projections (with the notable exception of Seminowicz et al. 2004). Most neuroimaging studies of course do not provide laminar specific information that could address this issue. However, recent developments in techniques for modeling effective connectivity provide tools that can be used to model the nature and direction of connectivity between regions. For example, dynamic causal modeling (DCM) using family level inference and Bayesian model averaging can be applied to test hypotheses regarding the direction and nature of information flow and causal modulation of different brain regions (Friston et al. 2003; Chen et al. 2009; Daunizeau et al. 2009; Friston & Dolan 2010; Penny et al. 2010). DCM can also test competing models such as providing head to head comparisons of whether the DLPFC down-regulates the amygdala directly or via some intermediary structure. To date, only a few DCM studies related to emotional processing have been published (Ethofer et al. 2006; Smith et al. 2006; Rowe et al. 2008; Almeida et al. 2009), and to our knowledge no studies have been published directly dealing with emotion regulation. However, the application of such techniques is likely to substantially enhance our understanding of emotion-cognition interactions in the coming years.
Direct tests of influence
Perhaps the best way to establish the functional relationships between brain regions is through the examination of one region during the selective physiological up or down regulation of the other area. For instance, if the DLPFC truly works to dampen OFC processing, one would expect exaggerated responses in the OFC when the DLPFC is taken offline. This possibility could be addressed by examining OFC functions with fMRI in patients with DLPFC lesions. Alternatively, transcranial magnetic stimulation (TMS) could be applied over the DLPFC to temporarily alter the influence of the DLPFC on OFC functions. Indeed, Knoch et al. (2006) recently reported that TMS over the right DLPFC produced changes in posterior OFC activity in a frequency dependent manner. Similarly, it would be of interest to know how lesions in one part of the prefrontal cortex affect processing in other parts of the network. For instance, if the OFC is important for calculating a pure reward value, what happens to more dorsal areas when the OFC is removed? Saddoris et al. (2005) have used this type of approach to examine how OFC lesions alter amygdalar firing in rodents, but other studies taking this approach are rare to nonexistent. The growing literature on functional connectivity is similarly likely to increase our understanding of how these critical brain regions interact. However, a full understanding of these interactions will only be reached with careful attention to the specific neuroanatomical features of these circuits.
Research Highlights.
Specific prefrontal connections dictate emotion regulation of the amygdala
Laminar projection patterns determine flow of information in prefrontal cortex
Feedforward and feedback projections challenge prefrontal organization
Acknowledgments
This work was supported by grants T32MH018931-21, T32MH018921-20, & 5R01MH074567-04 from the National Institute of Mental Health. We thank Tawny Richardson for help preparing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Aggleton JP, et al. Cortical and subcortical afferents to the amygdala of the rhesus monkey (Macaca mulatta) Brain Res. 1980;190:347–368. doi: 10.1016/0006-8993(80)90279-6. [DOI] [PubMed] [Google Scholar]
- Almeida JR, et al. Abnormal amygdala-prefrontal effective connectivity to happy faces differentiates bipolar from major depression. Biol Psychiatry. 2009;66:451–459. doi: 10.1016/j.biopsych.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Insausti R. Retrograde transport of D-[3H]-aspartate injected into the monkey amygdaloid complex. Exp Brain Res. 1992;88:375–388. doi: 10.1007/BF02259113. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis) J Comp Neurol. 1984;230:465–496. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- Amaral DG, et al. Anatomical organization of the primate amygdaloid complex. In: Aggleton JP, editor. Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. Wiley-Liss; New York: 1992. pp. 1–66. [Google Scholar]
- An X, et al. Prefrontal cortical projections to longitudinal columns in the midbrain periaqeductal gray in macaque monkeys. J Comp Neurol. 1998;401:455–479. [PubMed] [Google Scholar]
- Badre D, et al. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47:907–918. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Banich MT, et al. Cognitive control mechanisms, emotion and memory: a neural perspective with implications for psychopathology. Neurosci Biobehav Rev. 2009;33:613–630. doi: 10.1016/j.neubiorev.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H. Anatomic organization of basoventral and mediodorsal visual recipient prefrontal regions in the rhesus monkey. J Comp Neurol. 1988;276:313–342. doi: 10.1002/cne.902760302. [DOI] [PubMed] [Google Scholar]
- Barbas H. Connections underlying the synthesis of cognition, memory, and emotion in primate prefrontal cortices. Brain Res Bull. 2000;52:319–330. doi: 10.1016/s0361-9230(99)00245-2. [DOI] [PubMed] [Google Scholar]
- Barbas H, De OJ. Projections from the amygdala to basoventral and mediodorsal prefrontal regions in the rhesus monkey. J Comp Neurol. 1990;300:549–571. doi: 10.1002/cne.903000409. [DOI] [PubMed] [Google Scholar]
- Barbas H, Pandya DN. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1989;286:353–375. doi: 10.1002/cne.902860306. [DOI] [PubMed] [Google Scholar]
- Barbas H, Rempel-Clower N. Cortical structure predicts the pattern of corticocortical connections. Cereb Cortex. 1997;7:635–646. doi: 10.1093/cercor/7.7.635. [DOI] [PubMed] [Google Scholar]
- Barbas H, et al. Serial pathways from primate prefrontal cortex to autonomic areas may influence emotional expression. BMC Neurosci. 2003;4:25. doi: 10.1186/1471-2202-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H, Zikopoulos B. In: Sequential and parallel circuits for emotional processing in primate orbitofrontal cortex. Zald DH, Rauch SL, editors. Orbitofrontal Cortex Oxford University Press; 2006. [Google Scholar]
- Beauregard M, et al. Neural correlates of conscious self-regulation of emotion. The J Neurosci. 2001;21:1–6. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SJ. Neurocognitive mechanisms of anxiety: an integrative account. Trends Cogn Sci. 2007;11:307–316. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Blair RJR, Mitchell DGV. Psychopathy, attention and emotion. Psychological Medicine. 2009;39:543–555. doi: 10.1017/S0033291708003991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld RS, et al. Putting the pieces together: The role of dorsolateral prefrontal cortex in relational memory encoding. J Cogn Neurosci. 2010 doi: 10.1162/jocn.2010.21459. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodmann K. Physiologie des Gehrins. Neue Deutsche Chirugie Neue Deutsche Chirugie. 1914;2:85–426. [Google Scholar]
- Carmichael ST, Price JL. Architectonic subdivision of the orbital and medial prefrontal cortex in the macaque monkey. J Comp Neurol. 1994;346:366–402. doi: 10.1002/cne.903460305. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol. 1996;346:179–207. doi: 10.1002/(SICI)1096-9861(19960722)371:2<179::AID-CNE1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Chechko N, et al. Unstable prefrontal response to emotional conflict and activation of lower limbic structures and brainstem in remitted panic disorder. PLos One. 2009;4:e5537. doi: 10.1371/journal.pone.0005537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, et al. Forward and backward connections in the brain: a DCM study of functional asymmetries. Neuroimage. 2009;45:453–462. doi: 10.1016/j.neuroimage.2008.12.041. [DOI] [PubMed] [Google Scholar]
- Chiba T, et al. Efferent projections of infralimbic and prelimbic areas of the medial prefrontal cortex in the Japanese monkey, Macaca fuscata. Brain Res. 2001;888:83–101. doi: 10.1016/s0006-8993(00)03013-4. [DOI] [PubMed] [Google Scholar]
- Cisler JM, Koster EHW. Mechanisms of attentional biases towards threat in anxiety disorders: An integrative review. Clin Psychol Rev. 2010;30:203–216. doi: 10.1016/j.cpr.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton RJ, et al. Paying attention to emotion: an fMRI investigation of cognitive and emotional stroop tasks. Cogn Affect Behav Neurosci. 2003;3:81–96. doi: 10.3758/cabn.3.2.81. [DOI] [PubMed] [Google Scholar]
- Cooney RE, et al. Remembering the good times: neural correlates of affect regulation. Neuroreport. 2007;18:1771–1774. doi: 10.1097/WNR.0b013e3282f16db4. [DOI] [PubMed] [Google Scholar]
- Cowan N. Magical mystery four: How is working memory capacity limited, and why? Curr Dir Psychol Sci. 2010;19:51–57. doi: 10.1177/0963721409359277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis CE, D’Esposito M. The effects of prefrontal lesions on working memory performance and theory. Cogn Affect Behav Neurosci. 2004;4:528–539. doi: 10.3758/cabn.4.4.528. [DOI] [PubMed] [Google Scholar]
- Daunizeau J, David O, Stephan KE. Dynamic causal modelling: a critical review of the biophysical and statistical foundations. Neuroimage. doi: 10.1016/j.neuroimage.2009.11.062. in press. [DOI] [PubMed] [Google Scholar]
- Delgado MR, et al. Dorsal striatum responses to reward and puishment: effects of valence and magnitude manipulations. Cogn Affect Behav Neurosci. 2003;3:27–38. doi: 10.3758/cabn.3.1.27. [DOI] [PubMed] [Google Scholar]
- Delgado MR, et al. Regulating the expectation of reward via cognitive strategies. Nat Neurosci. 2008a;11:880–881. doi: 10.1038/nn.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, et al. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008b;59:829–838. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Ann Rev Neurosci. 1995;8:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Dolcos F, et al. Role of the inferior frontal cortex in coping with distracting emotions. Neuroreport. 2006;17:1591–1594. doi: 10.1097/01.wnr.0000236860.24081.be. [DOI] [PubMed] [Google Scholar]
- Dolcos F, McCarthy G. Brain systems mediating cognitive interference by emotional distraction. J Neurosci. 2006;26:2072–2079. doi: 10.1523/JNEUROSCI.5042-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrowski SM, et al. Quantitative architecture distinguishes prefrontal cortical systems in the rhesus monkey. Cereb Cortex. 2001;11:975–988. doi: 10.1093/cercor/11.10.975. [DOI] [PubMed] [Google Scholar]
- Domes G, et al. The neural correlates of sex differences in emotional reactivity and emotion regulation. Human Brain Mapping. 2010;31:758–769. doi: 10.1002/hbm.20903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domijan D, Setic M. A feedback model of figure-ground assignment. J Vis. 2008;8:10–27. doi: 10.1167/8.7.10. [DOI] [PubMed] [Google Scholar]
- Dreisbach G, Goschke T. How positive affect modulates cognitive control: Reduced perseveration at the cost of increased distractibility. J Exp Psychol Learn Mem Cogn. 2004;30:343–353. doi: 10.1037/0278-7393.30.2.343. [DOI] [PubMed] [Google Scholar]
- Drevets WC, et al. A functional anatomical study of unipolar depression. J Neurosci. 1992;12:3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J, Humphreys G, Ward R. Competitive brain activity in visual attention. Curr Opin Neurobiol. 1997;7:255–61. doi: 10.1016/s0959-4388(97)80014-1. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, et al. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30:2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eippert F, et al. Regulation of Emotional Responses Elicited by Threat-Related Stimuli. Hum Brain Mapp. 2007;28:409–423. doi: 10.1002/hbm.20291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethofer T, et al. Cerebral pathways in processing of affective prosody: a dynamic causal modeling study. Neuroimage. 2006;30:580–587. doi: 10.1016/j.neuroimage.2005.09.059. [DOI] [PubMed] [Google Scholar]
- Erber R, Erber MW. Beyond mood and social judgment: Mood incongruent recall and mood regulation. Eur J Soc Psychol. 1994;24:79–88. [Google Scholar]
- Etkin A, et al. Resolving emotional conflict: A role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Etkin A, et al. Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. Am J Psychiatry. 2010;167:545–554. doi: 10.1176/appi.ajp.2009.09070931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fales CL, et al. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biol Psychiatry. 2008;63:377–384. doi: 10.1016/j.biopsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed heierarchical processingin the primate cerebral cortex. Cerebral Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Fennell MJ, et al. Distraction in neurotic and endogenous depression: an investigation of negative thinking in major depressive disorder. Psychol Med. 1987;17:441–452. doi: 10.1017/s0033291700025009. [DOI] [PubMed] [Google Scholar]
- Fredrickson BL, Branigan C. Positive emotions broaden the scope of attention and thought-action repertoires. Cognition and Emotion. 2005;19:313–332. doi: 10.1080/02699930441000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, et al. Physiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6:18–29. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W. Dynamic causal modelling. NeuroImage. 2003;19:1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Dolan RJ. Computational and dynamic models in neuroimaging. Neuroimage. 2010;52:752–765. doi: 10.1016/j.neuroimage.2009.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, et al. Modulation of effective connectivity during emotional processing by Delta(9)-tetrahydrocannabinol and cannabidiol. International Journal of Neuropsychopharmacology. 2010;13:421–432. doi: 10.1017/S1461145709990617. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The Prefrontal Cortex. New York: Raven Press; 1989. [Google Scholar]
- Gable PA, Harmon-Jones E. Approach-motivated positive affect reduces breadth of attention. Psychol Sci. 2008;19:476–82. doi: 10.1111/j.1467-9280.2008.02112.x. [DOI] [PubMed] [Google Scholar]
- Gable PA, Harmon-Jones E. The effect of low versus high approach-motivated positive affect on memory for peripherally versus centrally presented information. Emotion. 2010;10:599–603. doi: 10.1037/a0018426. [DOI] [PubMed] [Google Scholar]
- Gasper K, Clore GL. Attending to the big picture: Mood and global versus local processing of visual information. Psychol Sci. 2002;13:34–40. doi: 10.1111/1467-9280.00406. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, et al. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert CD, Sigman M. Brain States: top-down influences in sensory processing. Neuron. 2007;54:677–96. doi: 10.1016/j.neuron.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Goldin PR, et al. The neural bases of emotion regulation: Reappraisal and suppression of negative emotion. Biol Psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabenhorst F, Rolls ET. Different representations of relative and absolute subjective value in the human brain. NeuroImage. 2009;48:258–268. doi: 10.1016/j.neuroimage.2009.06.045. [DOI] [PubMed] [Google Scholar]
- Gray JR. Emotional modulation of cognitive control: Approach-withdrawal states double-dissociate spatial from verbal two-back task performance. J Exp Psychol Gen. 2001;130:436–52. doi: 10.1037//0096-3445.130.3.436. [DOI] [PubMed] [Google Scholar]
- Gray JR, et al. Integration of emotion and cognition in the lateral prefrontal cortex. PNAS. 2002;99:4115–4120. doi: 10.1073/pnas.062381899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ. Antecedent- and response-focused emotion regulation: divergent consequences for experience, expression, and physiology. J Pers Soc Psychol. 1998;74:224–237. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Gross JJ. Emotion regulation. In: Lewis M, Haviland-Jones JM, Barrett LF, editors. Handbook of emotions. 3. Guilford; New York: 2008. pp. 497–512. [Google Scholar]
- Grossberg S. Towards a unified theory of neocortex: Laminar cortical circuits for vision and cognition. Prog Brain Res. 2007;165:79–104. doi: 10.1016/S0079-6123(06)65006-1. [DOI] [PubMed] [Google Scholar]
- Hänsel A, von Känel R. The ventro-medial prefrontal cortex: a major link between the autonomic nervous system, regulation of emotion, and stress reactivity? Biopsychosocial Med. 2008;2:21. doi: 10.1186/1751-0759-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin MG, et al. The influence of context valence in the neural coding of monetary outcomes. NeuroImage. 2009;48:249–257. doi: 10.1016/j.neuroimage.2009.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, et al. The amygdala response to emotional stimuli: A comparison of faces and scenes. NeuroImage. 2003;17:317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- Hayes JP, et al. Staying cool when things get hot: emotion regulation modulates neural mechanisms of memory encoding. Frontiers in Human Neuroscience. 2010;4:1–10. doi: 10.3389/fnhum.2010.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington JD, et al. Emotion-modulated performance and activity in left dorsolateral prefrontal cortex. Emotion. 2005;5:200–207. doi: 10.1037/1528-3542.5.2.200. [DOI] [PubMed] [Google Scholar]
- Hikosaka K, Watanabe M. Delay activity of orbital and lateral prefrontal neurons of the monkey varying with different rewards. Cereb Cortex. 2000;10:263–271. doi: 10.1093/cercor/10.3.263. [DOI] [PubMed] [Google Scholar]
- Jackson DC, et al. Suppression and enhancement of emotional responses to unpleasant pictures. Psychophysiology. 2000;37:515–522. [PubMed] [Google Scholar]
- Johnstone T, et al. Failure to regulate: Counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonides J, et al. Processes of working memory in mind and brain, Curr. Dir Psychol Sci. 2005;14:2–5. [Google Scholar]
- Joormann J, et al. Mood regulation in depression: Differential effects of distraction and recall of happy memories on sad mood. J Abnorm Psychol. 2007;116:484–490. doi: 10.1037/0021-843X.116.3.484. [DOI] [PubMed] [Google Scholar]
- Kalisch R, et al. Anxiety reduction through detachment: Subjective, physiological and neural effects. J Cogn Neurosci. 2005;17:874–883. doi: 10.1162/0898929054021184. [DOI] [PubMed] [Google Scholar]
- Kalisch R, et al. Neural correlates of self-distraction from anxiety and a process model of cognitive emotion regulation. J Cogn Neurosci. 2006;18:1266–1276. doi: 10.1162/jocn.2006.18.8.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanske P, et al. How to regulate emotion? Neural networks for reappraisal and distraction. Cerebral Cortex. 2011;21:1379–1388. doi: 10.1093/cercor/bhq216. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annu Rev Neurosci. 2000;23:315–41. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Kilpatrick LA, et al. Sex-related differences in amygdala functional connectivity during resting conditions. Soc Neurosci Abst. 2003:85.1. doi: 10.1016/j.neuroimage.2005.09.065. [DOI] [PubMed] [Google Scholar]
- Kim SH, Hamann S. Neural correlates of positive and negative emotion regulation. J Cogn Neurosci. 2007;19:776–798. doi: 10.1162/jocn.2007.19.5.776. [DOI] [PubMed] [Google Scholar]
- Knoch D, et al. Lateralized and frequency-dependent effects of prefrontal rTMS on regional cerebral blood flow. Neuroimage. 2006;31:641–648. doi: 10.1016/j.neuroimage.2005.12.025. [DOI] [PubMed] [Google Scholar]
- Knutson B, et al. Nucleus accumbens activation mediates the influence of reward cues on financial risk taking. Neuroreport. 2008;19:509–513. doi: 10.1097/WNR.0b013e3282f85c01. [DOI] [PubMed] [Google Scholar]
- Koenigsberg HW, et al. Neural correlates of using distancing to regulate emotional responses to social situations. Neuropsychologia. 2010;48:1813–1822. doi: 10.1016/j.neuropsychologia.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: Evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72:341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Levens SM, Phelps EA. Emotion processing effects on interference resolution in working memory. Emotion. 2008;8:267–280. doi: 10.1037/1528-3542.8.2.267. [DOI] [PubMed] [Google Scholar]
- Levens SM, Phelps EA. Insula and orbial frontal cortex activity underlying emotion interference resolution in working memory. J Cogn Neurosci. 2010;22:2790–2803. doi: 10.1162/jocn.2010.21428. [DOI] [PubMed] [Google Scholar]
- Levesque J, et al. Neural Circuitry Underlying Voluntary Suppression of Sadness. Biol Psychiatry. 2003;53:502–510. doi: 10.1016/s0006-3223(02)01817-6. [DOI] [PubMed] [Google Scholar]
- Levesque J, et al. Neural basis of emotional self-regulation in childhood. Neuroscience. 2004;129:361–369. doi: 10.1016/j.neuroscience.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Levy R, Goldman-Rakic PS. Segregation of working memory functions within the dorsolateral prefrontal cortex. Exp Brain Res. 2000;133:23–32. doi: 10.1007/s002210000397. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, et al. Putting feelings into words: Affect labeling disrupts amygdala activity in response to affective stimuli. Psychol Sci. 2006;18:421–428. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- Likhtik E, et al. Prefrontal control of the amygdala. J Neurosci. 2005;25:7429–7437. doi: 10.1523/JNEUROSCI.2314-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubomirsky S, et al. Effects of ruminative and distracting responses to depressed mood on retrieval of autobiographical memories. J Pers Soc Psychol. 1998;75:166–177. doi: 10.1037//0022-3514.75.1.166. [DOI] [PubMed] [Google Scholar]
- Mak AKY, et al. Neural correlates of regulation of positive and negative emotions. An fMRI study. 2009;457:101–106. doi: 10.1016/j.neulet.2009.03.094. [DOI] [PubMed] [Google Scholar]
- Mathews G, Wells A. The cognitive science of attention and emotion. In: Dalgleish T, Power MJ, editors. Handbook of cognition and emotion. John Wiley & Sons Ltd; Chichester, England: 1999. pp. 171–192. [Google Scholar]
- Mayberg HS, et al. Regional metabolic effects of fluoxetine in major depression: Serial changes and relationship to clinical response. Biol Psychiatry. 2000;48:830–843. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- McRae K, et al. The Neural Bases of Distraction and Reappraisal. J Cogn Neurosci. 2010;22:248–262. doi: 10.1162/jocn.2009.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta AD, et al. Intermodal selective attention in monkeys. II: Physiological mechanisms of modulation. Cereb Cortex. 2000;10:359–370. doi: 10.1093/cercor/10.4.359. [DOI] [PubMed] [Google Scholar]
- Mitchell DGV. The nexus between decision making and emotion regulation: A review of convergent neurocognitive substrates. Behavioral Brain Research. 2011;217:215–231. doi: 10.1016/j.bbr.2010.10.030. [DOI] [PubMed] [Google Scholar]
- Mohanty A, et al. Neural mechanisms of affective interference in schizotypy. J Abnorm Psychol. 2005;114:16–27. doi: 10.1037/0021-843X.114.1.16. [DOI] [PubMed] [Google Scholar]
- Mohanty A, et al. Differential engagement of anterior cingulate cortex subdivisions for cognitive and emotional function. Psychophysiology. 2007;44:343–351. doi: 10.1111/j.1469-8986.2007.00515.x. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning: contribution of medial prefrontal cortex. Neurosci Lett. 1993;163:109–113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- Most SB, Chun MM, Widders DM, Zald DH. Attentional rubbernecking: Cognitive control and personality in emotion-induced blindness. Psychonom Bull Rev. 2005;12:654–661. doi: 10.3758/bf03196754. [DOI] [PubMed] [Google Scholar]
- Most SB, Smith SD, Cooter AB, Levy BN, Zald DH. The naked truth: Positive, arousing distractors impair rapid target perception. Cognition & Emotion. 2007;21:964–981. [Google Scholar]
- Nee DE, Jonides J. Dissociable contributions of prefrontal cortex and the hippocampus to short-term memory: Evidence for a 3-state model of memory. NeuroImage. 2010 doi: 10.1016/j.neuroimage.2010.09.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New AS, Goodman M, Triebwasser J, Siever LJ. Recent advances in the biological study of personality disorders. Psychiatric Clinics of North Amercia. 2008;31:441–61. doi: 10.1016/j.psc.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: An fMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ. For better or for worse: Neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Ohira H, Nomura M, Ichikawa N, Isowa T, Iidaka T, Sato A, Fukuyama S, Nakajima T, Yamada J. Association of neural and physiological responses during voluntary emotion suppression. NeuroImage. 2006;29:721–733. doi: 10.1016/j.neuroimage.2005.08.047. [DOI] [PubMed] [Google Scholar]
- Ohman A, Flykt A, Esteves F. Emotion drives attention: Detecting the snake in the grass. J Exp Psychol Gen. 2001;130:466–478. doi: 10.1037//0096-3445.130.3.466. [DOI] [PubMed] [Google Scholar]
- Ongur D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol. 2003;460:425–449. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- Ouimet AJ, Gawronski B, Dozois DJA. Cognitive vulnerability to anxiety: A review and an integrative model. Clin Psychol Rev. 2009;29:459–470. doi: 10.1016/j.cpr.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Pandya DN. Anatomy of the auditory cortex. Rev Neurol(Paris) 1995;151:486–494. [PubMed] [Google Scholar]
- Parrott WG, Sabini J. Mood and memory under natural conditions: Evidence for mood incongruent recall. J Pers Soc Psychol. 1990;59:321–336. [Google Scholar]
- Penny, et al. Comparing dynamic causal models. NeuroImage. 2004;22:1157–1172. doi: 10.1016/j.neuroimage.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Penny WD, et al. Comparing families of dynamic causal models: PLoS Comput. Biol. 2010;6:e1000709. doi: 10.1371/journal.pcbi.1000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlstein WM, Elbert T, Stenger VA. Dissociation in human prefrontal cortex of affective influences on working memory-related activity. Proc Natl Acad Sci U S A. 2002;99:1736–1741. doi: 10.1073/pnas.241650598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. On the relationship between emotion and cognition. Nat Rev Neurosci. 2008;9:148–158. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- Petrides M, Mackey S. Topography of the human OFC. In: Zald DH, Rauch SL, editors. Orbitofrontal Cortex. Oxford University Press; 2006. [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polmorphism impacts human cingulate-amygdala interactions: A genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde T, Tancer ME. Neural Substrates for Voluntary Suppression of Negative Affect: A Functional Magnetic Resonance Imaging Study. Biol Psychiatry. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction Learning in Humans: Role of the Amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: Implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13:833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piech RM, Lewis J, Parkinson CH, Owen AM, Roberts AC, Downing PE, Parkinson JA. Neural correlates of affective influence on choice. Brain Cogn. 2010;72:282–288. doi: 10.1016/j.bandc.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Postle BR. Working memory as an emergent property of the mind and brain. Neuroscience. 2006;139:23–38. doi: 10.1016/j.neuroscience.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL. Architectonic Structure of the Orbital and Medial Prefrontal Cortex. In: Zald DH, Rauch SL, editors. Orbitofrontal Cortex. Oxford University Press; Oxford, UK: 2006a. pp. 3–18. [Google Scholar]
- Price JL. Connections of the Orbital Cortex. In: Zald DH, Rauch SL, editors. Orbitofrontal Cortex. Oxford University Press; Oxford, UK: 2006b. pp. 39–56. [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: Convergence of rat and human studies. Curr Opin Neurobiol. 2006;16:723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizada RD, Grossberg S. Towards a theory of the laminar architecture of cerebral cortex: Computational clues from the visual system. Cereb Cortex. 2003;13:100–113. doi: 10.1093/cercor/13.1.100. [DOI] [PubMed] [Google Scholar]
- Ranganath C. Working memory for visual objects: Complementary roles of inferior temporal, medial temporal, and prefrontal cortex. Neuroscience. 2006;139:277–289. doi: 10.1016/j.neuroscience.2005.06.092. [DOI] [PubMed] [Google Scholar]
- Ray R, Wilhelm FH, Gross JJ. All in the mind’s eye: Anger rumination and reappraisal. J Pers Soc Psychol. 2008;94:133–145. doi: 10.1037/0022-3514.94.1.133. [DOI] [PubMed] [Google Scholar]
- Rempel-Clower NL, Barbas H. Topographic organization of connections between the hypothalamus and prefrontal cortex in the rhesus monkey. J Comp Neurol. 1998;398:393–419. doi: 10.1002/(sici)1096-9861(19980831)398:3<393::aid-cne7>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Rockland KS, Pandya DN. Laminar origins and terminations of cortical connections of the occipital lobe in the rhesus monkey. Brain Res. 1979;179:3–20. doi: 10.1016/0006-8993(79)90485-2. [DOI] [PubMed] [Google Scholar]
- Roland PE, Hanazawa A, Undeman C, Eriksson D, Tompa T, Nakamura H, et al. Cortical feedback depolarization waves: A mechanism of top-down influence on early visual areas. Proc Natl Acad Sci U S A. 2006;103:12586–12591. doi: 10.1073/pnas.0604925103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg J, Gross JJ. When emotion goes wrong: Realizing the promise of affective science. Clin Psychol Sci Pract. 2003;10:227–232. [Google Scholar]
- Rottenberg J, Johnson SL, editors. Emotion and psychopathology: Bridging affective and clinical science. APA Books; Washington, D.C: 2007. [Google Scholar]
- Rowe J, et al. Rule-selection and action-selection have a shared neuroanatomical basis in the human prefrontal and parietal cortex. Cerebral Cortex. 2008;18:2275–2285. doi: 10.1093/cercor/bhm249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusting CL, DeHart T. Retrieving positive memories to regulate negative mood: Consequences for mood congruent memory. J Pers Soc Psychol. 2000;78:737–752. doi: 10.1037//0022-3514.78.4.737. [DOI] [PubMed] [Google Scholar]
- Rypma B, Berger JS, D’Esposito M. The influence of working-memory demand and subject performance on prefrontal cortical activity. J Cogn Neurosci. 2002;14:721–731. doi: 10.1162/08989290260138627. [DOI] [PubMed] [Google Scholar]
- Saalmann YB, Pigarev IN, Vidyasagar TR. Neural mechanisms of visual attention: How top-down feedback highlights relevant locations. Science. 2007;316:1612–1615. doi: 10.1126/science.1139140. [DOI] [PubMed] [Google Scholar]
- Saddoris MP, Gallagher M, Schoenbaum G. Rapid associative encoding in basolateral amygdala depends on connections with orbitofrontal cortex. Neuron. 2005;46:321–331. doi: 10.1016/j.neuron.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Sanides F. Comparative architects of the neocortex of mammals and their evolutionary interpretation. Ann N Y Acad Sci. 1969;167:404–423. [Google Scholar]
- Savine AC, Braver TS. Motivated cognitive control: Reward incentives modulate preparatory neural activity during task-switching. J Neurosci. 2010;30:10294–10305. doi: 10.1523/JNEUROSCI.2052-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Seminowicz DA, Mayberg HS, McIntosh AR, Goldapple K, Kennedy S, Segal Z, et al. Limbic-frontal circuitry in major depression: a path modeling metanalysis. Neuroimage. 2004;22:409–418. doi: 10.1016/j.neuroimage.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Shulman GI, Fiez J, Corbetta M, Buckner RL, Miezin FM, Raichle M, et al. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cogn Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Siemer M. Mood-congruent cognitions constitute mood experience. Emotion. 2005;5:296–308. doi: 10.1037/1528-3542.5.3.296. [DOI] [PubMed] [Google Scholar]
- Smith APR, et al. Task and content modulate amygdala-hippocampal connectivity in emotional retrieval. Neuron. 2006;49:631–638. doi: 10.1016/j.neuron.2005.12.025. [DOI] [PubMed] [Google Scholar]
- Stefanacci L, Amaral DG. Topographic organization of cortical inputs to the lateral nucleus of the macaque monkey amygdala: A retrograde tracing study. J Comp Neurol. 2000;421:52–79. doi: 10.1002/(sici)1096-9861(20000522)421:1<52::aid-cne4>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Stefanacci L, Amaral DG. Some observations on cortical inputs to the macaque monkey amygdala: An anterograde tracing study. J Comp Neurol. 2002;451:301–323. doi: 10.1002/cne.10339. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Benson DF. The Frontal Lobes. Raven; New York: 1986. [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. Thieme; New York: 1988. [Google Scholar]
- Taylor Tavares JV, Clark L, Furey ML, Williams GB, Sahakian BJ, Drevets WC. Neural basis of abnormal response to negative feedback in unmedicated mood disorders. Neuroimage. 2008;42:1118–1126. doi: 10.1016/j.neuroimage.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale &, Rezin V. The effects of reducing frequency of negative thoughts on the mood of depressed patients: Tests of a cognitive model of depression. Brit J Soc Clin Psychol. 1978;17:65–74. doi: 10.1111/j.2044-8260.1978.tb00898.x. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Jonides J, Marshuetz C, Smith EE, D’Esposito M, Kan IP, Knight RT, Swick D. Effects of frontal lobe damage on interference effects in working memory, Cogn. Affect Behav Neurosci. 2002;2:109–120. doi: 10.3758/cabn.2.2.109. [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Reekum CM, Johnstone T, Urry HL, Thurow ME, Schaefer HS, Alexander AL, Davidson RJ. Gaze fixations predict brain activation during the voluntary regulation of picture-induced negative affect. NeuroImage. 2007;36:1041–1055. doi: 10.1016/j.neuroimage.2007.03.052. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN. Cingulate cortex of the rhesus monkey: II. Cortical afferents. J Comp Neurol. 1987;262:271–289. doi: 10.1002/cne.902620208. [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AE. A cytoarchitectural study of the prefrontal area of the macaque monkey. J Comp Neurol. 1940;73:59–86. [Google Scholar]
- Wallis JD, Miller EK. Neuronal activity in primate dorsolateral and orbital prefrontal cortex during performance of a reward preference task. Eur J Neurosci. 2003;18:2069–2081. doi: 10.1046/j.1460-9568.2003.02922.x. [DOI] [PubMed] [Google Scholar]
- Walther D, Koch C. Modeling attention to salient proto-objects. Neural Networks. 2006:1395–1407. doi: 10.1016/j.neunet.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Tegner J, Constantinidis C, Goldman-Rakic PS. Division of labor among distinct subtypes of inhibitory neurons in a cortical microcircuit of working memory. Proc Natl Acad Sci U S A. 2004;101:1368–1373. doi: 10.1073/pnas.0305337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ, Bush G, McNally RJ, Wilhelm S, McInerney SC, Jenike MA, Rauch SL. The emotional counting Stroop paradigm: a functional magnetic resonance imaging probe of the anterior cingulate affective division. Biol Psychiatry. 1998;44:1219–1228. doi: 10.1016/s0006-3223(98)00251-0. [DOI] [PubMed] [Google Scholar]
- Williams JMG, Mathews A, MacLeod C. The emotional stroop task and psychopathology. Psychol Bull. 1996;120:3–24. doi: 10.1037/0033-2909.120.1.3. [DOI] [PubMed] [Google Scholar]
- Woodward TS, Cairo TA, Ruff CC, Takane Y, Hunter MA, Ngan ET. Functional connectivity reveals load dependent neural systems underlying encoding and maintenance in verbal working memory. Neuroscience. 2006;139:317–325. doi: 10.1016/j.neuroscience.2005.05.043. [DOI] [PubMed] [Google Scholar]
- Yeterian EH, Pandya DN. Prefrontostriatal connections in relation to cortical architectonic organization in rhesus monkeys. J Comp Neurol. 1991;312:43–67. doi: 10.1002/cne.903120105. [DOI] [PubMed] [Google Scholar]
- Zald DH. Orbital versus dorsolateral prefrontal cortex: Anatomical insights into content versus process differentiation models of the prefrontal cortex. Ann N Y Acad Sci. 2007;1121:395–406. doi: 10.1196/annals.1401.012. [DOI] [PubMed] [Google Scholar]
- Zald DH, Donndelinger MJ, Pardo JV. Elucidating dynamic brain interactions with across-subjects correlational analyses of positron emission tomographic data - The functional connectivity of the amygdala and orbitofrontal cortex during olfactory tasks. J Cereb Blood Flow Metab. 1998;18:896–905. doi: 10.1097/00004647-199808000-00010. [DOI] [PubMed] [Google Scholar]
- Zald DH, Kim SW. Anatomy and function of the orbital frontal cortex, II: Function and relevance to obsessive-compulsive disorder. J Neuropsychiatry Clin Neurosci. 1996;8:249–261. doi: 10.1176/jnp.8.3.249. [DOI] [PubMed] [Google Scholar]
- Zald DH, Mattson DL, Pardo JV. Brain activity in ventromedial prefrontal cortex correlates with individual differences in negative affect. Proc Natl Acad Sci U S A. 2002;99:2450–2454. doi: 10.1073/pnas.042457199. [DOI] [PMC free article] [PubMed] [Google Scholar]