Abstract
Individuals can now obtain their personal genomic information via direct-to-consumer genetic testing, but what, if any, impact will this have on their lifestyle and health? A recent longitudinal cohort study of individuals who underwent consumer genome scanning found minimal impacts of testing on risk-reducing lifestyle behaviors, such as diet and exercise. These results raise an important question: is personal genomic information likely to beneficially impact public health through motivation of lifestyle behavioral change? In this article, we review the literature on lifestyle behavioral change in response to genetic testing for common disease susceptibility variants. We find that only a few studies have been carried out, and that those that have been done have yielded little evidence to suggest that the mere provision of genetic information alone results in widespread changes in lifestyle health behaviors. We suggest that further study of this issue is needed, in particular studies that examine response to multiplex testing for multiple genetic markers and conditions. This will be critical as we anticipate the wide availability of whole-genome sequencing and more comprehensive phenotyping of individuals. We also note that while simple communication of genomic information and disease susceptibility may be sufficient to catalyze lifestyle changes in some highly motivated groups of individuals, for others, additional strategies may be required to prompt changes, including more sophisticated means of risk communication (e.g., in the context of social norm feedback) either alone or in combination with other promising interventions (e.g., real-time wireless health monitoring devices).
Keywords: behavioral intervention, consumer genomics, direct-to-consumer, genetic risk, genetic testing, nudging, personalized medicine, social norm feedback, wireless monitoring
More than a decade following completion of the Human Genome Project there are high expectations that the provision of personalized genomic disease risk information to individuals will motivate risk-reducing lifestyle heath behavior changes [1,2]. In this regard, it has been suggested that genomic information may be inherently more powerful relative to other types of non-genetic information, a notion commonly referred to as ‘genetic exceptionalism’ [3]. In part, recent evidence of these high expectations for genomics can be seen in the current availability of direct-to-consumer (DTC) genomic testing for multiple markers conferring genetic risk for multiple common conditions. In this context, testing is initiated by and results are provided directly to individuals without the involvement of a health-care provider. It has been argued that this potentially empowers the individual consumer to make appropriate changes in risk-reducing lifestyle and other health behaviors.
In this article, we provide a brief overview of studies that have examined lifestyle behavioral responses to genetic testing. We define this to mean dietary behavior, physical activity and smoking, given that these represent the three main factors that increase risk for chronic diseases with the highest worldwide mortality rates (cardiovascular disease, cancer, chronic respiratory disease and Type 2 diabetes). As will be shown, we find only a few studies that have examined this issue, and that many of the studies that have been carried out have been limited in terms of design and sample size, and also tend to focus on monogenic conditions. Indeed, we know of only one study that has evaluated this issue in the context of multiplex testing for multiple genetic variations and risk for multiple common diseases [4]. Also, of the studies that have been carried out, results generally show no positive (disappointingly) or negative effects of testing (e.g., increases in unhealthy behaviors in response to estimates of low genetic risk). These findings raise an important question: can direct access to personal genomic information be used in ways that will motivate positive lifestyle behavior change and thus benefit public health and lead to chronic disease prevention?
In anticipation of the wide availability of whole-genome sequencing and more comprehensive phenotyping of individuals [5], careful consideration of this question is now more critical than ever. In addition, lifestyle behaviors deserve separate consideration from more ‘medical’ health behaviors for two reasons: first, there is existing evidence that medical health behaviors that require involvement of a healthcare provider (e.g., altering medications or clinical screening) are impacted quite differently than lifestyle behaviors in terms of response to genetic testing [4,6]. Second, while best practice medical guidelines currently do not include recommendations for individuals found to be at increased or decreased risk based on genomic testing, there are clear public health goals for diet pattern, physical activity and smoking (i.e., for all individuals, see [101]). As such, for providers and consumers alike, DTC genomic testing presents a clear opportunity for providing personalized health information that may motivate improvements in lifestyle behaviors, potentially leading to a reduction of disease morbidity and premature mortality [7].
In the latter half of this article we propose several constructs that may be useful in guiding studies of, and the development of interventions for, positive lifestyle behavioral change in the context of personalized genomic risk testing. Furthermore, while simple communication of genomic information and disease susceptibility may be sufficient to catalyze lifestyle changes in some highly motivated groups of individuals, for others, additional strategies may be required. Thus, we conclude by suggesting some possible strategies for more novel means of communication of genomic information (e.g., in the context of social norm feedback) either alone or in combination with other promising interventions (e.g., real-time wireless health monitoring devices) in order to promote more widespread adoption of healthy behaviors.
Studies of lifestyle behavioral response to genetic testing
Studies of the lifestyle behavioral response to genetic testing have included examination of response to testing for single-gene conditions (e.g., hereditary breast ovarian cancer syndrome caused by highly penetrant mutations in BRCA1 or BRCA2), as well as common conditions (e.g., Alzheimer’s disease) in which known genetic markers typically account for only a small percentage of risk. Furthermore, as previously mentioned, there is also the relatively recent introduction of SNP-based whole-genome testing for multiple markers conferring risk for multiple common conditions with results provided directly to consumers [102,103]. Arguably, expectations are highest in terms of anticipated health benefit with respect to this latter category of testing. Indeed, many have espoused the expected power of this type of testing to motivate adoption of risk-reducing lifestyle behaviors among individuals [2,102].
A small number of informative reviews have been written on this topic [6,8–11], selected findings of which are summarized in Table 1. As shown, few studies have been carried out, and the literature that is available has often been criticized for the use of small samples and suboptimal study designs [8]. Furthermore, if one limits the studies of interest to those that have tested for common genetic susceptibility markers and diseases, there are even fewer reports (see Table 2 for selected studies). To the best of our knowledge, only one study has systematically evaluated lifestyle behavioral response to DTC testing for multiple susceptibility markers and multiple conditions [4], though smaller scale qualitative studies have been carried out [12]. Finally, from the aggregate of the few studies that have been conducted, there is little evidence that the simple provision of risk information leads to sustained beneficial changes in lifestyle behaviors [8]. We stress that these conclusions are with respect to changes in lifestyle behaviors, and that other behavioral means of risk reduction, for instance increased clinical screening test adherence or intent to screen in individuals found to be at high genetic risk, have been observed [4,6].
Table 1.
Selected recent review articles on the behavioral impact of genetic risk disclosure for different categories of genetic testing.
| Source (year) | Population | Studies pertaining to lifestyle | Findings | Ref. |
|---|---|---|---|---|
| Marteau et al. (2010) | Adults receiving actual or imagined genetic risk estimates for lung or esophageal cancer, Crohn’s disease, obesity, heart disease or AD | All 13 studies reviewed assessed lifestyle behaviors | All but one study failed to find a significant effect of genetic testing on smoking cessation behaviors; other behaviors assessed included physical activity, medication or vitamin use and dietary behavior, and significant effects of testing were small and found only for diet | [8] |
| Heshka et al. (2008) | Adults tested for HBOC, hereditary colon cancer and AD | Only two studies included lifestyle behaviors | Carriers and noncarriers tested for HBOC reported risk-reducing lifestyle changes (e.g., changing diet, exercise or smoking); APOE-ε4 carriers reported risk-reducing lifestyle changes (e.g., adding vitamin E, changing diet or exercise) | [6] |
| Scheuner et al. (2008) | Adults tested for HBOC and attendees at a weight management clinic | Only three studies included lifestyle or clinical behaviors | Weight loss did not differ between patients who received nutrigenomic testing versus patients who did not | [9] |
| Beery et al. (2007) | Adults tested for HBOC, hereditary colon cancer and HD | Only two studies included ‘complementary alternative medicine’ behaviors | One study reported correlation between cancer risk perception and use of complementary alternative medicine | [10] |
AD: Alzheimer’s disease; HBOC: Hereditary breast and ovarian cancer; HD: Huntington’s disease.
Table 2.
Selected studies of the behavioral impact of genetic susceptibility testing and risk disclosure.
| Source (year) | Population | Findings | Ref. |
|---|---|---|---|
| Bloss et al. (2011)† | Adults receiving DTC testing for multiple markers conferring genetic risk for multiple chronic conditions | No effect of testing or specific disease risk (e.g., increased risk) on brief measures of dietary fat intake or physical activity were observed across the sample as a whole | [4] |
| Chao et al. (2008) | Adults tested for APOE-ε4 conferring genetic risk for AD | Increased disease risk associated with reporting of at least one of three health behavior changes (i.e., diet, exercise or medication/vitamin use), but most common change was to medication/vitamin use | [31] |
| McBride et al. (2002) | Adults who were smokers tested for GSTM1 conferring genetic risk for lung cancer | Compared standard intervention to standard intervention plus genetic feedback and found that the latter group had significantly greater rates of cessation at 6-month follow-up. Did not, however, have a genetics-only intervention comparison group | [27] |
Only known systematic study to date on response to multiplex DTC genomic testing.
AD: Alzheimer’s disease; DTC: Direct-to-consumer.
Proposed framework for understanding the relationship between genomic information & behavior change
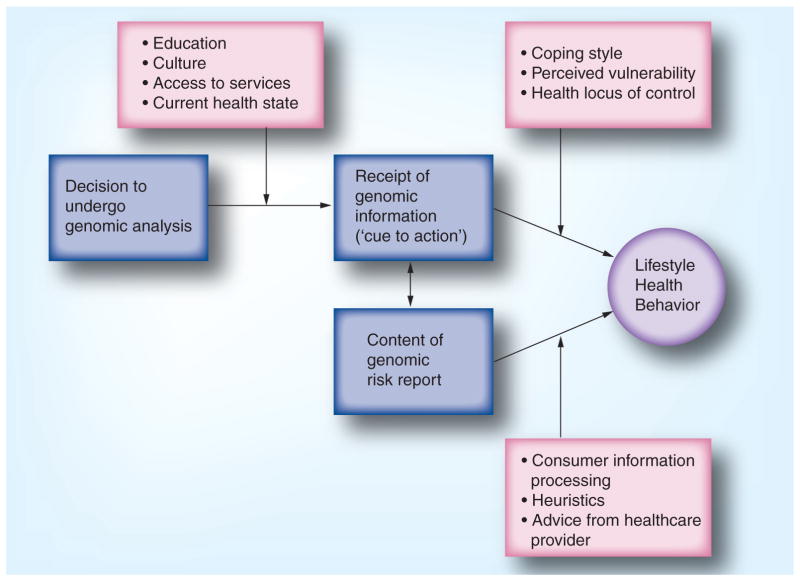
Hypothesizing that genomic information might motivate healthful behavior change is reasonable given that several established health behavior theories incorporate concepts that are consistent with this and could guide studies of genomic health. The framework depicted in Figure 1 summarizes some concepts that may predict health behavior change in the genomic setting; this is not intended to be a complete theory but rather a starting point for discussion. First, an understanding of the baseline behaviors and the characteristics of the population undergoing genomic testing are needed. Next, we propose that the receipt of genomic information itself can be conceptualized in two ways: as content, and as a process. Finally, the framework includes some constructs from various health behavior theories that may be informative for understanding how receipt of genomic information might motivate behavior change.
Figure 1.
Framework of possible constructs that may predict health behavior change in the genomic setting.
Who is undergoing genetic testing?
The first step in designing studies of behavior change prompted by genomic testing is to understand who comprises the population of individuals undergoing testing. Notably, the first published study of response to DTC genomic services included individuals who were representative of the current population of consumers of DTC genomic tests [4] and not of the general population. As such, not surprisingly, the sample as a whole was found to already be quite healthy prior to undergoing testing. Specifically, at the baseline assessment, 69% of the sample reported almost daily consumption of fruits and vegetables, and 79% reported a low fat intake. Only 6% reported using tobacco, and 57% were already engaging in a level of physical activity (at least 21 metabolic equivalent hours per week) consistent with recommendations from the Institute of Medicine. This suggests that the majority of participants were already meeting or exceeding healthy lifestyle guidelines [101]. Furthermore, the vast majority of individuals surveyed (88%) self-reported his or her health to be either ‘good’ or ‘very good’. This suggests that these early adopters of DTC genomic testing appear to represent a segment of the population already engaging in healthy behaviors and may have ‘little room for improvement’ so-to-speak. It may be that other segments of the more mainstream population will likely benefit more from DTC testing in terms of lifestyle behavioral health.
Process of receiving genomic information: a cue to action?
The receipt of genomic information can be considered a ‘cue to action’, as conceptualized within the health belief model [13]. This concept suggests that an information-giving event is needed to drive individuals to consider undertaking a change in a health behavior. The volume and complexity of genomic information that is becoming available to consumers has led to new decision points that need to be made on an ongoing basis by consumers. For example, the first versions of the 23andMe test [103] reports did not include risk assessments for Alzheimer’s disease based on APOE genotype, but later versions offered customers the opportunity to ‘opt-in’ to be able to view their results [14]. From a behavioral perspective, the opportunity to opt-in or -out of specific genomic test results is an important part of the process, as opting in may indicate a willingness or motivation to take action, while opting out could indicate that the individual would not make any lifestyle changes based on the information. Finally, since genomic testing is an evolving field, the interpretation of genomic information is subject to change, and individuals may receive updates to their genomic test reports that increase or decrease their risk of disease. These ongoing update events could potentially serve as ongoing cues to action if the updated risk estimate is perceived to have greater accuracy than the original estimate. Conversely, ongoing updates could undermine the potential for genomic information to motivate health behavior change if they are too frequent or if they lead to the perception that genomic information is a less credible source of disease risks. The latter may be particularly relevant if the direction of an individual’s risk estimate changes (i.e., an increased risk of disease is later updated to a decreased risk of disease).
Genomic information as ‘content’
The content of the genomic information itself might be predicted to suggest certain types of health behavior changes depending on the size and direction of the genomic risks provided to the consumer, as well as the consumer’s interpretation of the results. Current DTC genomic testing companies emphasize relative risks (compared with actual risks) in their reports, typically highlighting those conditions for which the consumer has a calculated increase in risk compared with the population. Here, going back to the notion of genetic exceptionalism, it is also important to note that there are currently no studies of which we are aware that have explicitly evaluated the ‘motivational capacity’ of genomic information relative to other types of health risk information (e.g., cholesterol screening).
We anticipate that novel ways of conceptualizing the potential relationship between genomic information and behavior change will emerge, given that genomic information is unique in many respects. For example, consideration should be given to the volume of information and competing risks. Mason et al. described how the simultaneous receipt of multiple health messages can be confusing and sometimes overwhelming [15]. Genomic information offers the individual consumer, for the first time, information regarding multiple (and potentially tens to hundreds of) disease risks simultaneously. The scope and volume of information may only serve as a cue to action if the consumer is able to ‘drill down’ to the pieces of information most relevant and actionable in their lives at that time. Accordingly, adopting concepts from theories used in the disciplines of marketing or economics may adapt well to large volumes of information. For example, consumer information processing theory [16] and heuristic reasoning [17] are both models of how individuals process and integrate large amounts of complex information into usable pieces that make sense in the context of their current setting and priorities. Finally, since the information often adjusts an individual’s risk for multiple diseases and health conditions, consumers may elect to seek advice from a physician, genetic counselor or other healthcare provider. These professionals may be able to assist with sifting through the information and help identify specific areas where health behavior change may be recommended based on genomic information combined with family history and current lifestyle health behavior practices.
Health behavior constructs that may be informative in the genomic setting
Coping style
The cognitive-social health information processing model [18] conceptualizes two basic ways of coping with threatening information: monitoring or ‘information-seeking’, and blunting or ‘information avoidance’. This model further suggests how individuals with these coping styles would be predicted to react to complex health information. Clearly the early adopters of genomic testing are likely to be information seekers, such that this construct may not be predictive of behavior change itself, but might be predictive of uptake of genomic testing.
Perceived vulnerability
Protection motivation theory [19] and the health belief model [13] suggest that healthy behaviors are more likely to be pursued if the individual perceives a personal, specific health risk. It may be that adapting ‘perceived vulnerability’ to the context of genomic risk information (particularly since current consumer genomic reports emphasize relative risks) would shed light on how individuals integrate novel, personalized genomic information into their individual constructions of health and disease risk.
Locus of control
Health locus of control measures the extent to which patients perceive their health to be influenced by their own behavior and choices (an ‘internal’ locus of control) versus by others, such as their healthcare providers (a ‘powerful others’ external locus of control), versus by chance or random events (a ‘chance-external’ locus of control) [20]. Health locus of control may be associated with perceptions of ‘genetic determinism’ such that individuals with a ‘chance’ or fatalistic locus of control may not be at all motivated to change their behaviors, even in the face of modifiable disease risk.
Social norms/accountability
There is a culture shift occurring, catalyzed by the public space of the internet, where personal health information and health behaviors are more likely to be shared by and among social groups than ever before [104]. Paradoxically, this increased sharing of highly personalized information sets the stage for social theories of behavior change. Even though the information itself is highly individualized (the very definition of genomic profiling), finding others with shared traits or risks of interest may lead to the development of social groups with common genetic predispositions [21] and facilitation of shared goal-setting and accountability that in turn can predict successful behavior change [22]. Others have also suggested that the motivational potency of genetic information might be enhanced by engaging ‘kinship networks’ [11].
Combining genomics & other technologies for health promotion
As we have previously stated, while simple communication of genomic information and disease susceptibility may be sufficient to catalyze lifestyle health behavior changes in some highly motivated groups of individuals, for others, different or additional strategies may be required. Below, we propose some possible ways of creatively delivering genomic information (e.g., in the context of social norm feedback), as well as ways of combining it with other interventions or technologies (e.g., real-time wireless health monitoring devices) and novel health education strategies.
Social norm feedback
Social norms interventions have gained attention through their use in reducing risky behaviors, a prominent example being use among college students to reduce alcohol consumption and alcohol-related problems [23]. Although they have been used in different ways (e.g., to provide standard information about normative behaviors to individuals versus correcting misperceptions in norms), the notion is that humans are easily ‘nudged’ by other humans and that individuals can be encouraged to engage in healthy behaviors if they believe other people are performing those same behaviors [24]. Thus, in the context of genomic information, one could imagine a scenario in which genetic risk results are delivered in such a way that draws on these principles.
For instance, say an individual’s genomic test result indicates they are a carrier of the high-risk variant for coronary artery disease at position 9p21 in the genome. If the goal is then to get them to engage in lifestyle risk-reducing behaviors (e.g., increase physical activity), a social norm feedback approach would suggest framing this recommendation in terms of what others who have also tested positive are doing in terms of prevention (e.g., ‘90% of individuals who tested positive for this variant have initiated an increase in their physical activity’). Although this is an overly simplistic example, the general notion is that delivery of information in this context may be more likely to promote sustained behavioral change. ‘Nudging’ strategies for improving population health have previously been considered [25], including online communities for the sharing of health information with other patients with similar conditions (see [104]). Furthermore, the approach, broadly, of combining social norm feedback with provision of genomic information is also generally consistent with the development of online support groups based on genetic disease status [21].
Continuous health feedback & monitoring
It has also been noted that genomic information may be more likely to motivate risk-reducing lifestyle behaviors when combined with other interventions [11], including interventions that provide real-time continuous feedback [26]. In the context of models of behavioral change, this makes sense insofar as for a given behavioral change to occur, multiple needs may have to be addressed and multiple variables considered (see Figure 1). Furthermore, in one of the only studies in the literature in which a lifestyle behavioral change occurred in an intervention arm that included provision of genetic risk estimates to participants [27], the genetic risk estimates were provided as part of a multicomponent intervention that also included telephone counseling to assist in smoking cessation. Thus, bolstering self-efficacy by incorporating other interventions, technologies, or skill building, may have a synergistic effect on health behaviors when combined with the delivery of genomic risk information.
One technology that has recently been given attention for its potential to impact medicine and health are noninvasive wearable wireless sensors that can continuously track a variety of physiological metrics relevant for lifestyle behaviors (e.g., blood pressure, heart rhythm and respiratory rate) [5,26]. Data are collected and can be sent anywhere, including back to the wearer’s cell phone or computer, or to the wearer’s physician. One specific example is the Nike+iPod® Sport Kit (Apple, CA, USA), which contains a wireless sensor placed in the sole of one’s Nike shoe and tracks exercise metrics such as distance traveled, velocity and oxygen consumption. Other devices are available that can help track calories consumed and other aspects of diet, such as the Fitbit Wireless Tracker [104]. The combined effects of providing genomic risk information together with continuous feedback regarding health behaviors from wireless monitoring technologies (or even more traditional face-to-face interventions), may ultimately have more potential with respect to motivating and facilitating lifestyle behavioral changes for some groups of individuals. This may be particularly true for the subset of the population who are both ‘information seeking’ and technologically savvy enough to have pursued genomic information in the earliest phase of availability.
Health education strategies
Many health education strategies have been tried and tested and could be useful in combining with genomic risk information for motivating lifestyle behavioral changes. Traditional approaches, as well as approaches that leverage social media and networks (e.g., the Center for Disease Control and Prevention on YouTube [105]; Alabama Department of Public Health on Facebook [106]) could be considered, as could more novel strategies such as serious video games designed for health-related behavior change [28].
We stress that these are just some suggestions for use in refining ‘genomic health interventions’. We recognize that there are most certainly several ‘roads to Rome’ in terms of engendering health behavior change and that the ‘right’ intervention will most certainly vary across individuals, populations, time and circumstances, and in other words, require personalization.
Conclusion
In our overview of the literature on lifestyle behavioral change in response to a range of types of genetic testing, we find that only a few studies have addressed this issue, and only one study to date has systematically evaluated this in the context of multiplex testing for multiple genetic variations and risk for multiple common diseases [4]. Accordingly, we suggest that additional research in this area is needed and propose several constructs that may be useful in guiding relevant studies. We also note that while simple communication of genomic information and disease susceptibility may be sufficient to catalyze lifestyle changes in some highly motivated groups of individuals, for others, additional strategies may be required. For some groups of individuals, creatively delivering genomic risk information, as well as combining it with other technologies and interventions may increase its impact on lifestyle behaviors.
There are a few other noteworthy points. First, the genomic data delivered and emphasized to the consumer has to be so-called ‘actionable’ in that the lifestyle behavior that is being encouraged would conceivably reduce risk. Without this sense of actionability, genomic information may do little to promote healthy lifestyle behaviors. Second, there is a need to target individuals for whom a change in lifestyle would be beneficial. As described above, in the first study of lifestyle behavioral response to DTC genomic testing, few changes in lifestyle health behaviors were observed, however this observation is confounded by the fact that most study participants were already practicing healthy behaviors at baseline. Indeed, to take an extreme example, it would make little sense to encourage a frequent marathon runner who happens to carry a genetic variant placing him or her at risk for obesity to get more aerobic exercise. Third, the majority of common conditions for which genetic risk estimates are provided to consumers (in the context of DTC genomic testing) are characterized by a large fraction of risk known to be owing to environmental factors. Thus, assessment of these factors in individuals may also point to possible interventions and/or could serve as additional ‘cues to action.’
In addition, another issue that has been raised concerning DTC genomic testing is the extent to which genomic test results are consistent with a person’s known characteristics or health risks based on other factors (e.g., family history). For instance, if trait predictions (e.g., eye color, hair curliness or even ancestry) reported on by some DTC companies are discordant with the consumer’s actual phenotype, that discordance may in turn undermine the consumer’s perception of the utility of their health-related information, making it less likely that any lifestyle health behavior change might be undertaken as a result. Along these lines, with regards to current DTC genomic testing for disease susceptibility, our knowledge of what the test actually means in terms of clinical validity and utility is scant, thus some may argue that personalized behavioral health recommendations based on genetic test results alone may be premature. This also brings up the often raised issue that all people, irrespective of very small increases in risk for common disease, could likely benefit from more healthful diets, increases in physical activity, and smoking abstinence. Finally, there is also the issue of regulation [107] and whether or not DTC personalized genomic testing will continue to be delivered to consumers without the involvement of a healthcare provider. Indeed, mandating physicians as gatekeepers would have important implications with respect to who would be the target of relevant interventions (consumers or physicians?) and the extent to which preventative strategies will gain traction.
Future perspective
Whole-genome sequencing and comprehensive phenotyping technologies [5] are on the cusp of being widely available to individuals, possibly without the involvement of a healthcare provider. Furthermore, once whole-genome sequencing is commonplace and an individual has had his or her genome sequenced, that individual will continue to learn about disease susceptibilities and other genetic predispositions over time as genomic findings accumulate. Current DTC genomic testing for multiple markers conferring genetic risk for multiple common chronic conditions is a precursor to these events. Further studies are needed to understand how the strong potential of these technologies (already beginning to be realized in some areas such as pharmacogenomics and clinical screening adherence) can be leveraged with respect to improved lifestyle behaviors, public health and chronic disease prevention. In realizing this goal, consideration should be given to novel, multicomponent strategies that include delivery of genomic information to consumers, together with complementary individualized physiological data of high relevance and consequence in order to motivate healthful lifestyle behavioral change [29,30].
Executive summary.
Individuals can now obtain personal genomic information via direct-to-consumer testing, but few studies have evaluated the impact of this type of testing on consumer behavior.
Although many have suggested the potential for this type of information to motivate consumers to engage in risk-reducing lifestyle behavioral changes, there is little evidence to support such an effect.
Studies are needed that consider the development of interventions that leverage genomic information in the promotion of positive lifestyle behavioral change.
Suggested strategies for such interventions might include more novel means of communication of genomic information, either alone or in combination with other promising tools, such as real-time wireless health monitoring devices.
Acknowledgments
The authors thank Burcu Darst for her assistance with the manuscript preparation.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
This work was supported in part by a NIH/NHGRI R21 grant (1R21HG005747–01; PI: Cinnamon S Bloss, PhD), a NIH/NCRR flagship Clinical and Translational Science Award grant (1UL1RR025774-01; primary investigator: Eric J Topol, MD), and Scripps Genomic Medicine Division of Scripps Health. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
- 1.Collins FS, Green ED, Guttmacher AE, Guyer MS. A vision for the future of genomics research. Nature. 2003;422(6934):835–847. doi: 10.1038/nature01626. [DOI] [PubMed] [Google Scholar]
- 2.Evans JP, Meslin EM, Marteau TM, Caulfield T. Genomics. Deflating the genomic bubble. Science. 2011;331(6019):861–862. doi: 10.1126/science.1198039. [DOI] [PubMed] [Google Scholar]
- 3.Green MJ, Botkin JR. “Genetic exceptionalism” in medicine: clarifying the differences between genetic and nongenetic tests. Ann Intern Med. 2003;138(7):571–575. doi: 10.7326/0003-4819-138-7-200304010-00013. [DOI] [PubMed] [Google Scholar]
- 4▪.Bloss CS, Schork NJ, Topol EJ. Effect of direct-to-consumer genomewide profiling to assess disease risk. N Engl J Med. 2011;364(6):524–534. doi: 10.1056/NEJMoa1011893. First study of the behavioral response to multiplex direct-to-consumer genomic testing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Topol EJ. Transforming medicine via digital innovation. Sci Transl Med. 2010;2(16):16cm4. doi: 10.1126/scitranslmed.3000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6▪.Heshka JT, Palleschi C, Howley H, Wilson B, Wells PS. A systematic review of perceived risks, psychological and behavioral impacts of genetic testing. Genet Med. 2008;10(1):19–32. doi: 10.1097/GIM.0b013e31815f524f. Comprehensive review of clinical and behavioral impacts of genetic testing and risk disclosure for single-gene conditions. [DOI] [PubMed] [Google Scholar]
- 7.Willett WC. Balancing life-style and genomics research for disease prevention. Science. 2002;296(5568):695–698. doi: 10.1126/science.1071055. [DOI] [PubMed] [Google Scholar]
- 8▪.Marteau TM, French DP, Griffin SJ, et al. Effects of communicating DNA-based disease risk estimates on risk-reducing behaviours. Cochrane Database Syst Rev. 2010;10:CD007275. doi: 10.1002/14651858.CD007275.pub2. 2010. Review of behavioral impacts of genetic susceptibilty testing. [DOI] [PubMed] [Google Scholar]
- 9.Scheuner MT, Sieverding P, Shekelle PG. Delivery of genomic medicine for common chronic adult diseases: a systematic review. JAMA. 2008;299(11):1320–1334. doi: 10.1001/jama.299.11.1320. [DOI] [PubMed] [Google Scholar]
- 10.Beery TA, Williams JK. Risk reduction and health promotion behaviors following genetic testing for adult-onset disorders. Genet Test. 2007;11(2):111–123. doi: 10.1089/gte.2006.0527. [DOI] [PubMed] [Google Scholar]
- 11.McBride CM, Koehly LM, Sanderson SC, Kaphingst KA. The behavioral response to personalized genetic information: will genetic risk profiles motivate individuals and families to choose more healthful behaviors? Annu Rev Public Health. 2010;31:89–103. doi: 10.1146/annurev.publhealth.012809.103532. [DOI] [PubMed] [Google Scholar]
- 12.O’Daniel JM, Haga SB, Willard HF. Considerations for the impact of personal genome information: a study of genomic profiling among genetics and genomics professionals. J Genet Couns. 2010;19(4):387–401. doi: 10.1007/s10897-010-9297-x. [DOI] [PubMed] [Google Scholar]
- 13.Maiman LA, Becker MH. The health belief model: origins and correlates in psychological theory. Health Education Monographs. 1974;2:336–353. [Google Scholar]
- 14.MacArthur D. 23andMe releases results for major Alzheimer’s risk marker. In: Anderson C, editor. Wired. NY, USA: 2011. [Google Scholar]
- 15.Mason JO, Ogden HG, Berreth DA, Martin LY. Interpreting risks to the public. Am J Prev Med. 1986;2(3):133–139. [PubMed] [Google Scholar]
- 16.Rudd J, Glanz K. How individuals use information for health action: consumer information processing. In: Glanz K, Lewis FM, Rimer BK, editors. Health Behavior and Health Education: Theory, Research, and Practice. Jossey-Bass; CA, USA: 1990. [Google Scholar]
- 17.Tversky A, Kahneman D. Judgment under uncertainty: heuristics and biases. Science. 1974;185(4157):1124–1131. doi: 10.1126/science.185.4157.1124. [DOI] [PubMed] [Google Scholar]
- 18.Miller SM, Diefenbach MA. C-SHIP: a cognitive-social health information processing approach to cancer. In: Krantz D, editor. Perspectives in Behavioral Medicine. Lawrence Erlbaum; NJ, USA: 1998. pp. 219–244. [Google Scholar]
- 19.Rogers RW. Cognitive and physiological processes in fear appeals and attitude change: a revised theory of protection motivation. In: Cacioppo JT, Petty RE, editors. Social Psychophysiology. Guilford Press; NY, USA: 1983. pp. 153–176. [Google Scholar]
- 20.Wallston KA, Wallston BS, Smith S, Dobbins CJ. Perceived control and health. Curr Psychol Res Rev. 1987;6(1):5–25. [Google Scholar]
- 21.Coulson NS, Buchanan H, Aubeeluck A. Social support in cyberspace: a content analysis of communication within a Huntington’s disease online support group. Patient Educ Couns. 2007;68(2):173–178. doi: 10.1016/j.pec.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Bandura A. Social learning theory. Englewood Cliffs; Prentice-Hall, NJ, USA: 1977. [Google Scholar]
- 23.Lewis MA, Neighbors C. Social norms approaches using descriptive drinking norms education: a review of the research on personalized normative feedback. J Am Coll Health. 2006;54(4):213–218. doi: 10.3200/JACH.54.4.213-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thaler RH, Sunstein CR. Nudge: Improving Decisions About Health, Weath, and Happiness. Yale University Press; New Haven, CT, USA: 2008. [Google Scholar]
- 25▪.Marteau TM, Ogilvie D, Roland M, Suhrcke M, Kelly MP. Judging nudging: can nudging improve population health? BMJ. 2011;342:d228. doi: 10.1136/bmj.d228. Commentary on the use of ‘nudging’ as a population health intervention. [DOI] [PubMed] [Google Scholar]
- 26.Lillie EO, Patay B, Diamant J, Issell B, Topol EJ, Schork NJ. The n-of-1 clinical trial: the ultimate strategy for individualizing medicine? Per Med. 2011;8(2):161–173. doi: 10.2217/pme.11.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McBride CM, Bepler G, Lipkus IM, et al. Incorporating genetic susceptibility feedback into a smoking cessation program for African–American smokers with low income. Cancer Epidemiol Biomarkers Prev. 2002;11(6):521–528. [PubMed] [Google Scholar]
- 28.Baranowski T, Buday R, Thompson DI, Baranowski J. Playing for real: video games and stories for health-related behavior change. Am J Prev Med. 2008;34(1):74–82. doi: 10.1016/j.amepre.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goetz T. Harnessing the power of feedback loops. In: Goetz T, editor. Wired. 125–133. NY, USA: 2011. pp. 162–164. [Google Scholar]
- 30.Bandura A. Health promotion by social cognitive means. Health Educ Behav. 2004;31(2):143–164. doi: 10.1177/1090198104263660. [DOI] [PubMed] [Google Scholar]
- 31.Chao S, Roberts JS, Marteau TM, Silliman R, Cupples LA, Green RC. Health behavior changes after genetic risk assessment for Alzheimer disease: The REVEAL Study. Alzheimer Dis Assoc Disord. 2008;22(1):94–97. doi: 10.1097/WAD.0b013e31815a9dcc. [DOI] [PMC free article] [PubMed] [Google Scholar]
Websites
- 101.Healthy People. www.healthypeople.gov.
- 102.Navigenics. www.navigenics.com.
- 103.23andMe. www.23andme.com.
- 104.Fitbit. www.fitbit.com.
- 105.Patients Like Me. www.patientslikeme.com.
- 106.Centers for Disease Control and Prevention on Youtube. www.youtube.com/user/CDCStreamingHealth.
- 107.Alabama Department of Public Health on Facebook. www.facebook.com/alabamapublichealth.
- 108.United States Government Accountability Office. Direct-to-consumer genetic tests: misleading test results are further complicated by deceptive marketing and other questionable practices. www.gao.gov/new.items/d10847t.pdf.