Abstract
Lymphedema of the upper extremity, in addition to being unsightly, can be painful, can limit the arm movements, increases the risk of infection and is psychologically distressing, serving as a constant reminder of cancer. 1. To ascertain the incidence of lymphedema in a hospital based population (in patients undergoing axillary dissection for breast cancer. 2. To determine the clinico-epidemilogical factors associated with the occurrence of lymphedema in these patients. For all patients undergoing axillary dissection, arm measurements were taken in the pre-& post-operative period from at least 3 sites; one in the arm, forearm and wrist (points fixed in reference to fixed bony landmarks). Patients included in this study were followed up for at least 12 months. Circumference difference of more than 5% was taken as mild lymphedema; more than 10% as moderate lymphedema and more than 15% as severe lymphedema. Data was analyzed using SPSS 11.0 statistical software. Of the 231 patients in this study mean age was 51.2 years, majority were housewives (71.9%) and postmenopausal (58.5%). Modified radical mastectomy (MRM), was performed on 203 (87.9%) patients. 57.2% patients had positive lymph nodes. The mean number of positive nodes was 6.52. Majority of the patients received chemo and radio therapy. Overall incidence of lymphedema was 41.1%. The definition of 5–10% increase as mild lymphedema may be a bit severe as in most patients with this increase, lymphedema is not clinically apparent. The incidence of moderate and severe lymphedema in our series is only 7.4%. The incidence of clinically significant lymphedema (moderate to severe lymphedema & symptomatic mild lymphedema) was 16.8%. Only axillary irradiation and pathological nodal status (pN3) emerged as significant risk factors for lymphedema development on multivariate analysis. Lymphedema once established is difficult to treat. Combination of axillary dissection with radiation and more nodal positivity seems to predispose to lymphedema. Prevention by means of sentinel node biopsy in early cases, good surgical technique, arm care post surgery, exercises and massage therapy may help reducing the incidence and/or severity.
Keywords: Lymphedema, Breast cancer, Irradiation
Introduction
Breast cancer is by far the most commonly diagnosed cancer in women worldwide, accounting for 21% of all cancers diagnosed in women [1]. Breast cancer incidence, in India, is on the rise and has now become the most common cancer among women, having overtaken cervix in all the cancer registries, rural or urban [2].
With increasing incidence and longer survival because of improved treatment, quality of life issues are becoming an integral part of the treatment. Lymphedema of the upper extremity, in addition to being unsightly, can be painful, can limit the arm movements, increases the risk of infection and is psychologically distressing, serving as a constant reminder of cancer [3–7]. The incidence of lymphedema has been reported with a very wide range from 2%–40% in women treated with modified radical mastectomy or breast conserving surgery with no apparent difference between the two surgeries [4, 5, 8–11]. The factors quoted to influence incidence of lymphedema include the level of nodal dissection, the number of nodes removed, the number of involved nodes, presence of extra capsular spread, size and grade of the primary tumor, co-morbid conditions, anthracycline based chemotherapy, axillary irradiation, experience of the surgeon, dominant limb and body mass index [12–16].
There is considerable lack of clinico-epidemiological data on this condition from Indian patient population, which is in epidemiological transition. It is necessary to report the incidence and epidemiology of this complication in Indian population, as it may be considerably different compared to disease in western world.
Aims & Objectives
To ascertain the incidence of lymphedema in a hospital based population (in patients undergoing axillary dissection for breast cancer) in the Indian scenario.
To determine the clinico-epidemilogical factors associated with the occurrence of lymphedema in these patients.
Methodology
We did an analysis in the Department of Surgical Oncology at Cancer Institute, Amrita Institute of Medical Sciences, Cochin of patients of breast cancer who were operated over a 4-year period from 1st January 2004 to 31st December 2007. All patients of breast cancer who underwent breast and axillary surgery in our department were included in the study if they had a minimum follow up of at least 12 months. Patients who had undergone surgery elsewhere, had a history of previous surgery in the axilla or history of filariasis and patients with a follow up of less than 1 year after surgery were excluded,
Patients were staged according to AJCC staging guidelines (6th edition) [17]. Hormone receptor status was assessed by Immunohistochemistry for estrogen & progesterone receptors (ER/PR) and for Her-2/neu receptors. Patients received either surgery or neoadjuvant chemotherapy according to departmental protocol taking into account the disease stage and patient characteristics.
Surgery Patients were offered either modified radical mastectomy or wide local excision with axillary dissection or toilet mastectomy as dictated by the disease extent & stage. All patients who had positive surgical margins underwent re-excision to negative margins.All patients undergoing axillary dissection at our institute are subjected to the rehabilitation program for prevention of lymphedema. As a part of this and departmental policy, arm measurements are taken for each patient on regular basis in the pre-operative & post-operative period at fixed distances from bony references.Data was gathered from a prospectively maintained database at the hospital to retrieve the measurements of arm, forearm and wrist circumference at fixed intervals of 5 cm from wrist and olecranon taken pre-operatively and two post-op measurements—first between 4–6 months and second 12 months after surgery.For purpose of this study lymphedema was defined as increase in circumferential measurement of the operated side upper limb of at least 5% or more from baseline, recorded at three different pre-defined levels, and persisting for two follow ups at least 6 months apart. Grading was done as follows: 5%–10% increase was considered as mild lymphedema, 10%–15% increase as moderate lymphedema and more than 15% increase as severe lymphedema. Clinically significant lymphedema was defined as either lymphedema (of any extent) in a symptomatic patient or moderate to severe lymphedema irrespective of symptoms. If there was a difference in the percent change in measurement at different points in the arm, then the maximum change noted at any point in that arm was taken to grade severity of lymphedema.
Chemotherapy Indications for adjuvant chemotherapy included all tumors larger than 1 cm, node positive disease, ER negative disease, or Her-2/neu receptor positive disease. Indications for neoadjuvant chemotherapy included locally advanced breast cancer (LABC), metastatic breast cancer (MBC) or primaries in small breast where conservation was strongly desired. Chemotherapy protocols included anthracycline based (Epirubicin or Adriamycin) or taxol based (paclitaxel or docetaxel). However, some patients were also offered other protocols like CMF (cyclophosphamide, methotrexate and 5-FU) for reasons of cost or general condition of the patient at the discretion of treating medical oncologist. Hormonal therapy was advised for all patients who were positive for either ER or PR or both, after completion of treatment for a continuous period of 5 years. Agents included anti-estrogens (Tamoxifen; 20 mg per day) or aromatase inhibitors (Letrozole; 2.5 mg per day).
Radiotherapy All patients who received breast conserving surgery, had T3 or more disease at presentation (>5 cm or with skin or chest involvement locally), close surgical margins or those who had more than three lymph nodes positive on histopathology were treated with adjuvant radiation.
Statistics Data was analyzed using SPSS 11.0 statistical software (SPSS Inc, USA). Descriptive analysis was used for demographics, patient and disease characteristics. Paired t-test and chi-square test were used to evaluate statistically significant association between groups as appropriate.Sample size was calculated in consultation with a biostatistician, taking into account the only published incidence 33.5% of lymphedema in Indian population [16]. The minimum sample size to establish any significant difference from this was calculated to be 200.
(Calculation: 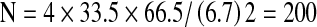 ) [p = 33.5; alpha = 0.05; confidence = 95%; allowable error on the estimate = 20%]
) [p = 33.5; alpha = 0.05; confidence = 95%; allowable error on the estimate = 20%]
Results
Of a cohort of 526 patients of malignancy treated in the department over the same period, 313 (59.5%) were cases of breast cancer. Of these, 231 (73.8%) were found eligible for inclusion in the study.
Mean age of patients was 51.21 (range 26–85; SD 12.21), majority being housewives (143/231; 71.9%) and postmenopausal (135/231; 58.5%) (Table 1).
Table 1.
Clinico-demographic profile of patients
| Parameter | Mean | Range | SD | |
|---|---|---|---|---|
| Age (years) | 51.21 | 26–85 | 12.21 | Median |
| Duration of illness (months) | 6.10 | 0.25–108 | 11.29 | Median |
| Occupation (n = 199) | ||||
| Housewife | 143 | 71.9% | ||
| Unskilled | 4 | 2.0% | ||
| Skilled | 41 | 20.6% | ||
| Professional | 11 | 5.5% | ||
| Menstrual status | ||||
| Pre-menopausal | 96 | 41.5% | ||
| Postmenopausal | 135 | 58.5% | ||
Nearly 60% of patients (137/231; 59.3%) presented within 3 months of noticing symptoms although some waited longer before seeking medical advice. Hormone receptor status was available for 219 (94.8%) patients. Of these 29.2% (64/219) were positive for ER, 38.8% (85/219) were positive for PR and 31.9% (70/219) were triple negative (Table 2).
Table 2.
Hormone receptor status
| Her-2/neu | PR | |||
|---|---|---|---|---|
| Positive | Negative | |||
| Positive | ER | Positive | 15 | 10 |
| Negative | 10 | 44 | ||
| Negative | ER | Positive | 29 | 10 |
| Negative | 31 | 70 | ||
Histologically, majority of the patients had invasive ductal carcinoma (IDC) (207/231; 89.6%) (Table 3).
Table 3.
Histopathology of breast cancer in our patients (n = 307)
| Histology | N | Percent |
|---|---|---|
| Invasive Ductal Carcinoma (IDC) | 207 | 89.6 |
| Invasive Lobular Carcinoma (ILC) | 9 | 3.8 |
| Ductal Carcinoma In-situ | 4 | 1.7 |
| Others | 11 | 4.7 |
In 104 (42.4%) patients, at presentation, the disease was locally advanced (T3N0 or more) (Table 4), while 37 (16.0%) patients were metastatic.
Table 4.
Disease staging at presentation
| Stage | N | Percent |
|---|---|---|
| I | 8 | 3.5 |
| IIA | 44 | 19.0 |
| IIB | 81 | 35.0 |
| IIIA | 38 | 16.5 |
| IIIB | 15 | 6.5 |
| IIIC | 8 | 3.5 |
| IV | 37 | 16.0 |
The most common procedure done was modified radical mastectomy (MRM), performed on 203 (87.9%) patients (Table 5).
Table 5.
Extent of surgery
| Nature of surgery | N | Percent |
|---|---|---|
| Modified radical mastectomy | 203 | 87.9 |
| Breast conserving surgery | 28 | 12.1 |
Five pre-menopausal patients (2.1%) underwent bilateral oopherectomy along with the surgery for breast cancer. All these patients were estrogen receptor positive on core biopsy specimens.
In 231 patients, the mean yield of lymph nodes in the axillary dissection specimen was 17.71 (range 3–45; SD 6.67). In 198 (85.7%) patients we were able to dissect more than ten nodes (Table 6). On hematoxylin & eosin staining, 99 (42.8%) patients were free of disease in the lymph nodes (Table 6).
Table 6.
Nodal yield and disease burden in axillary dissection specimen
| Nodal yield | N | Percent | |
|---|---|---|---|
| No nodes dissected/Inoperable axilla | 3 | 1.3 | |
| Less than 10 nodes dissected | 30 | 12.9 | |
| 11–20 nodes dissected | 131 | 56.8 | |
| More than 20 nodes dissected | 67 | 29.0 | |
| Pathological N staging | N = 228 | ||
| pN0 (no nodes positives) | 99 | 42.8 | |
| pN1 (1–3 nodes positive) | 61 | 26.5 | |
| pN2 (4–10 nodes positive) | 42 | 18.2 | |
| pN3 (>10 nodes positive) | 29 | 12.5 | |
| Percent positive nodes | N = 228 | ||
| No positive nodes | 99 | 42.8 | |
| Less than 25% positive | 69 | 29.9 | |
| 26%–50% positive | 20 | 8.7 | |
| 51%–75% positive | 23 | 9.9 | |
| More than 75% positive | 20 | 8.7 |
Of the 129 (56.6%) patients who had nodal disease in their axilla, the mean number of positive nodes was 6.52 (range1–37; SD 6.18; median 4).
Of the 231 patients, 37 (16.0%) did not receive any chemotherapy (not indicated or refused) and details are not available for 2 (0.8%) patients. Chemotherapy details of remaining 192 patients are given in Table 7.
Table 7.
Chemotherapy and radiotherapy details
| Chemotherapy | (N = 192) | N | Percent |
|---|---|---|---|
| Adjuvant chemotherapy alone | 94 | 48.9 | |
| Neoadjuvant chemotherapy | 98 | 51.1 | |
| Nature of Chemotherapy | |||
| Taxol based | 63 | 32.8 | |
| Non Taxol based | 129 | 67.2 | |
| Radiotherapy | (N = 126) | ||
| EBRT | 121 | 96.2 | |
| APBIa | 3 | 2.3 | |
| EBRT+Brach therapy | 2 | 1.5 |
EBRT External beam radiotherapy, APBI Accelerated partial breast irradiation
aPart of research protocol
Ninety-nine (42.8%) patients did not receive any form of radiotherapy and details were not available for 6 (2.5%) patients. Details of radiotherapy for the remaining 126 patients are given in Table 7.
Median number of chemotherapy cycles received in neoadjuvant setting was 3.0 (mean 3.57; range 2–16; SD 1.67) while that in adjuvant setting was 4.0 (mean 4.54; range 1–13; SD 1.73). Median total number of chemotherapy cycles was 6.0 (mean 6.10; range 2–19; SD 1.87).
One hundred and five (45.5%) patients received hormonal therapy either with Tamoxifen or Aromatase inhibitors (Letrozole).
At a mean follow up of 2.2 years (range 0–4.4 years; SD 0.98) we had a recurrence in 34 (14.7%) patients either locally or at distant sites.
Overall incidence of lymphedema (all grades) of the operated side upper extremity was noted in 95 (41.1%) patients (Table 8). This incidence is not significantly different from that reported in the only Indian study (p = 0.07). Of these 95 patients, 25 had clinically significant lymphedema (17 patients had moderate to severe lymphedema and eight patients with mild lymphedema were symptomatic for it) of the upper limb on the operated side. Thus our overall incidence of clinically significant lymphedema is 16.8%. This difference is highly significant (p < 0.0001). The incidence of moderate and severe lymphedema is 7.4%
Table 8.
Incidence of lymphedema
| Lymphedema | N | Percent |
|---|---|---|
| No edema (<5% change from baseline) | 136 | 58.9 |
| Mild edema (5%–10% change from baseline) | 78 | 33.8 |
| Moderate edema (10%–15% change from baseline) | 15 | 6.5 |
| Severe edema (>15% change from baseline) | 2 | 0.9 |
Univariate analysis of risk factors in the current study showed a correlation between increased lymphedema rates and advanced stage of disease, presence of co-morbid conditions, and postoperative loco-regional radiotherapy. However only axillary irradiation and pathological nodal status of 3 (pN3) have emerged as significant risk factors (p < 0.01) for lymphedema development in multivariate analysis.
Discussion
Majority of the breast cancer patients in developing countries, including India, still present with locally advanced stage that necessitates a comprehensive axillary dissection. Our own audit (unpublished data) shows that nearly 40% of patients present in locally advanced stage. Better understanding of the disease process, advances in drug development and consequent improvement in treatment have increased the cure rates and prolonged survival. With this, there is an even greater emphasis on quality of life and long-term post treatment sequelae.
Lymphedema, following breast cancer treatment, occurs due to lymphatic interruption following surgical trauma or radiotherapy induced fibrosis that leads to chronic inflammation and consequent fibrosis of the hypodermal and dermal connective tissue [18].
Once established, lymphedema is difficult to treat. Prevention by means of good surgical technique, arm care post surgery, exercises and massage therapy may help reducing the incidence and/or severity, although there is no definite known or established method of preventing the occurrence of this dreaded complication.
This study should help us generate some more information on the incidence of lymphedema in the patients operated here, so that we can base our future strategies for management of lymphedema more effectively.
The risk of lymphedema persists throughout the lifetime of patients and except for cancer recurrence, no other event is more dreaded than the development of lymphedema.
Impact of lymphedema to patients’ physical and psychological health can be enormous, with long lasting and often permanent sequelae to their quality of life [19, 20].
Tobin and colleagues reported that patients with edema due to ALND had more psychosocial and adaptational problems than a comparable group of patients without edema [21]. Other authors have also reported similar problems [6]. These include (but not limited to) less interest in maintaining family relationships, less active socially, less interest in their personal appearance and were less active sexually. Of the most troublesome issues in patients with severe lymphedema of upper extremity were problems related to the weight of the arm and its impact on arm utility along with issues with dress fitting.
It is unfortunate in this scenario that most often clinical attention is directed toward cancer recurrence rather than concerns about arm disability and its impact on quality of life issues. This is evident by scant research interest and available support for lymphedema.
A wide variation in prevalence (2%–40%) is due to lack of consensus regarding the clinical criteria, method of assessment and timing of assessment for lymphedema [4, 5, 8, 10, 11]. The incidence of severe lymphedema is less variable with most studies reporting it in less than 10% of the patients with lymphedema [22, 23].
There is very little literature available on the incidence of lymphedema in the Indian scenario with the only study by Deo et al. in a group of 299 patients reporting an incidence of 33.5% [16].
Petrek et al. analyzed seven reports over an 8-year period and found the incidence of lymphedema ranging from 6%–30% in breast cancer patients in these studies. They also noted that comparisons were difficult due to heterogeneity of definition of lymphedema, measurement techniques, etc. [24].
It is usually presumed that the more radical the procedure in the nodal basin, the more likely it is for the extremity to develop a postoperative lymphedema [25]. With a high rate of 49%–63% reported during the era of radical mastectomy [26, 27], the incidence of lymphedema has shown a decreasing trend following introduction and wide spread adaptation of technique of modified radical mastectomy[28, 29]. Simple mastectomy without axillary dissection carried an incidence of 9.1% of lymphedema compared to 31.5% in patients following modified radical mastectomy in a report by Say et al. [30]. However some authors have noted that that the extent or level of axillary node dissection does not show statistically significant association with the risk of development of lymphedema [18, 31].
Postoperative radiotherapy (and its sequelae) increase the risk of and aggravate lymphedema [13, 28, 29] although not all will agree [12]. Edwards et al. studied the incidence of lymphedema after breast cancer treatment by volumetric method and subjective assessment of swelling and found no significant relationship between axillary irradiation and lymphedema [12]. Apart from surgery and radiotherapy various other risk factors for lymphedema development are described in literature including age, presence of co-morbid conditions, wound infection, obesity, stage of disease and systemic therapy [13–15].
Various different methods have been used for the assessment of lymphedema and include volumetric methods, serial circumferential measurement method, MRI, bioelectrical impedance and tonometry [3, 32, 33]. Usually, lymphedema has been defined based on the difference in the arm measurements (volumetric or circumferential) between the treated and untreated sides. However, clinically significant lymphedema varies due to individual differences in subjective perception of symptoms and the severity of lymphedema, with self-rating showing a lower incidence compared to objective assessment [7].
Comparison across the studies is hampered not only by the different methods of assessment but also by different definitions. While some authors have used an absolute difference in circumference to define lymphedema, others have reported significant lymph edema as volumetric difference of >200 ml between both arms. Here, again, heterogeneities are reported between volumes of the non-operated & operated arms and therefore hamper the comparability.
The most traditional method is the measurement of arm circumferences at predefined positions relative to the olecranon. It has the advantages of being the simplest, cheapest and most reliably reproducible method of objective assessment of lymphedema. However, in the reported studies using this method the point of measurement as well as the differences between both arms considered as significant lymph edema vary significantly [10, 34].
Chau et al. defined a volume difference of 10%–15% as moderate, and a difference of >15% as severe lymph edema after reporting that variance in the difference in two arms in the same individual can be as high as 3.1%–4.1% [35]. We decided to use serial circumferential measurements at fixed distances to bony prominences in the affected arm alone and compared the serial change in these measurements to avoid such inter-individual volume differences and used the criteria set by Chau et al. to define significant moderate and severe lymphedema.
The definition of 5%–10% increase as mild lymphedema may be a bit severe as in most patients with this increase, lymphedema is not clinically apparent. The incidence of moderate and severe lymphedema in our series is only 7.4%. Precautions during surgery routinely include keeping the axillary skin flaps as thick as possible, preserving the entire fascia over the axillary vein, preserving the intercostobrachial nerves in majority and preserving as many venous tributaries as possible, all without affecting the nodal yield.
In our study, only axillary irradiation and pathological nodal status of three (pN3) have emerged as significant risk factors for lymphedema development in multivariate analysis. No definite conclusion could be drawn in relation to the prevalence of lymphedema in radical mastectomy and breast conservation groups because of the small sample size of patients undergoing breast conservation. We perform a complete axillary dissection including level I–III clearance as a routine and take intra operative precautions as described above. This is, in our opinion, along with thick axillary flaps, could be an important reason for our low incidence of lymphedema.
Conclusions
Lymphedema following breast cancer treatment continues to be a significant long-term morbidity in the current era. Approximately 10% of breast cancer patients develop clinically significant lymphedema. Postoperative radiotherapy to axilla and large nodal burden are significant risk factors for lymphedema development. Since there is no ideal treatment available for established lymphedema, future efforts should be focused on optimizing treatment combinations and evolving minimally invasive methods like sentinel node biopsy [25] for staging axilla.
Contributor Information
Pramod R. Pillai, Email: 58drpramod@gmail.com
Shekhar Sharma, Email: drshekharsharma@gmail.com.
Sheikh Zahoor Ahmed, Email: sheikhzahoorahmed@aims.amrita.edu.
D. K. Vijaykumar, Phone: +91-944-6507751, Email: dkvijaykumar@aims.amrita.edu
References
- 1.Parkin DM. The global burden of cancer. Semin Cancer Biol. 1998;8:219–235. doi: 10.1006/scbi.1998.0080. [DOI] [PubMed] [Google Scholar]
- 2.Development of cancer atlas of India—A project of National Cancer Registry Program (Indian Council of Medical Research), First All India Report 2002;1:11–18.
- 3.Margaret LF. Lymphedema. In: Berger AM, Portenoy RK, Weiss man DE, editors. Principles and practice of supportive oncology. New york: Lippinctt-Raven; 1998. pp. 275–289. [Google Scholar]
- 4.Brennan MJ, DePompolo RW, Garden FH. Focused review: post mastectomy lymphedema. Arch Phys Med Rehabil. 1996;77(Suppl):S74–S80. doi: 10.1016/S0003-9993(96)90248-8. [DOI] [PubMed] [Google Scholar]
- 5.Tasmuth T, Smitten K, Kalso E. Pain and other symptoms during the first year after radical and conservative surgery for breast cancer. Br J Cancer. 1996;74:2024–2031. doi: 10.1038/bjc.1996.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maunsell E, Brisson J, Deschênes L. Arm problems and psychological distress after surgery for breast cancer. Can J Surg. 1993;36:315–320. [PubMed] [Google Scholar]
- 7.Woods M, Tobin M, Mortimer P. The psychosocial morbidity of breast cancer patients with lymphoedema. Cancer Nurs. 1995;18:467–471. doi: 10.1097/00002820-199512000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Hoe AL, Iven D, Royle GT, Taylor I. Incidence of arm swelling following axillary clearance for breast cancer. Br J Surg. 1992;79:261–262. doi: 10.1002/bjs.1800790326. [DOI] [PubMed] [Google Scholar]
- 9.Querci della Rovere G, Ahmad I, Singh P, Ashley S, Daniels IR, Mortimer P. An audit of the incidence of arm edema after prophylactic level I/II axillary dissection without division of pectoralis minor muscle. Ann R Coll Surg Engl. 2003;85(3):158–161. doi: 10.1308/003588403321661299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ozalslan C, Kuru B. Lymphedema after treatment of breast cancer. Am J Surg. 2004;187:69–72. doi: 10.1016/j.amjsurg.2002.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Logan V. Incidence and prevalence of lymphedema: a literature review. J Clin Nurs. 1995;4:213–219. doi: 10.1111/j.1365-2702.1995.tb00209.x. [DOI] [PubMed] [Google Scholar]
- 12.Edwards TL. Prevalence and aetiology of lymphoedema after breast cancer treatment in southern Tasmania. Aust N Z J Surg. 2000;70(6):412–418. doi: 10.1046/j.1440-1622.2000.01839.x. [DOI] [PubMed] [Google Scholar]
- 13.Kissin MW, Querrci DR, Easton D, Westbury G. Risk of lymphedema following the treatment of breast cancer. Br J Surg. 1986;73:580–584. doi: 10.1002/bjs.1800730723. [DOI] [PubMed] [Google Scholar]
- 14.Ryttov N, Holm NV, Quist N, Blichert Influence of adjuvant radiotherapy on the development of late arm lymphedema and impaired shoulder mobility after mastectomy for carcinoma of breast. Acta Oncol. 1988;27:667–668. doi: 10.3109/02841868809091766. [DOI] [PubMed] [Google Scholar]
- 15.Segerstorm K, Bjerle P, Graffman S, Nystrom A. Factors that influence the incidence of brachial oedema after treatment of breast cancer. Scand J Plast Reconstr Surg Hand Surg. 1992;26:223–237. doi: 10.3109/02844319209016016. [DOI] [PubMed] [Google Scholar]
- 16.Deo SV, Ray S, Rath GK, Shukla NK, Kar M, Asthana S, Raina V. Prevalence and risk factors for development of lymphedema following breast cancer treatment. Indian J Cancer. 2004;41(1):8–12. [PubMed] [Google Scholar]
- 17.(2002) AJCC cancer staging manual. Greene FL, Compton CC, Fritz AG, Shah J, Winchester DP (eds) 6th ed
- 18.Karakousis CP. Surgical procedures and lymphedema of the upper and lower extremity. J Surg Oncol. 2006;93:87–91. doi: 10.1002/jso.20349. [DOI] [PubMed] [Google Scholar]
- 19.Beaulac SM, McNair LA, Scott TE, LaMorte WW, Kavanah MT. Lymphedema and quality of life in survivors of early—stage breast cancer. Arch Surg. 2002;137:1253–1257. doi: 10.1001/archsurg.137.11.1253. [DOI] [PubMed] [Google Scholar]
- 20.Johansson K, Holmostrom H, Nilsson I, Ingvar C, Albertsson M, Ekdahl C. Breast cancer patients’ experiences of lymphedema. Scan J Caring Sci. 2003;17:35–42. doi: 10.1046/j.1471-6712.2003.00119.x. [DOI] [PubMed] [Google Scholar]
- 21.Tobin MB, Lacey HJ, Meyer L, Mortimer PS. The psychological morbidity of breast cancer-related arm swelling. Psychological morbidity of lymph oedema. Cancer. 1993;72:3248–3252. doi: 10.1002/1097-0142(19931201)72:11<3248::AID-CNCR2820721119>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 22.Ivens D, Hoe AL, Podd TJ, Hamilton CR, Taylor I, Royle GT. Assessment of morbidity from complete axillary dissection. Br J Cancer. 1992;66:136–138. doi: 10.1038/bjc.1992.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dam MSW, Hennipman A, Kruif JT, Tweel I, Graaf PW. Complications following axillary dissection for breast carcinoma. Ned Tijdschr Geneeskd. 1993;137:2395–2398. [PubMed] [Google Scholar]
- 24.Petrek JA, Heelan MC. Incidence of breast carcinoma-related lymphedema. Cancer. 1998;83:2776–2781. doi: 10.1002/(SICI)1097-0142(19981215)83:12B+<2776::AID-CNCR25>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 25.Golshan M, Martin WJ, Dowlatshahi K. Sentinel lymph node biopsy lowers the rate of lymphedema when compared with standard axillary lymph node dissection. Am Surg. 2003;69:209–211. [PubMed] [Google Scholar]
- 26.Britton RE, Nelson PA. Causes and treatment of post mastectomy lymphedema of arm—report of 114 cases. JAMA. 1962;180:95–102. doi: 10.1001/jama.1962.03050150001001. [DOI] [PubMed] [Google Scholar]
- 27.Huges JH, Patel AR. Swelling of the arm following radical mastectomy. Br J Surg. 1966;53:4–14. doi: 10.1002/bjs.1800530150. [DOI] [PubMed] [Google Scholar]
- 28.Schunemann H, Willich N. Lymphedema of the arm after primary treatment of breast cancer. Anti Cancer Res. 1998;18:2235–2236. [PubMed] [Google Scholar]
- 29.Mortiner PS, Bates DO, Brassington HD, Stanton AWB. The prevalence of arm oedema following treatment for breast cancer. QJ Med. 1996;89:377–380. [Google Scholar]
- 30.Say CC, Donegen W. A biostatistical evaluation of complications from mastectomy. Surg Gynaecol Obstet. 1974;138:370–376. [PubMed] [Google Scholar]
- 31.Werner RS, McCormick B, Petrek J, Cox L, Cirrincione C, Gray JR, et al. Arm oedema in conservatively managed breast cancer: obesity is a more predictive factor. Radiology. 1991;180:177–184. doi: 10.1148/radiology.180.1.2052688. [DOI] [PubMed] [Google Scholar]
- 32.Pain SJ, Purushotham AD. Lymphedema following surgery for breast cancer. Br J Surg. 2000;87:1128–1141. doi: 10.1046/j.1365-2168.2000.01569.x. [DOI] [PubMed] [Google Scholar]
- 33.Markowski J, Wilcox JP, Helm PA. Lymphedema incidence after specific post-mastectomy therapy. Arch phys Med Rehabil. 1981;62:449–452. [PubMed] [Google Scholar]
- 34.Schulze T, Mucke J, Markwardt J, Schlag PM, Bembener A. Long-term morbidity of patients with early breast cancer after sentinel lymph node biopsy compared to axillary lymph node dissection. J Surg Oncol. 2006;93:109–119. doi: 10.1002/jso.20406. [DOI] [PubMed] [Google Scholar]
- 35.Chau N, Remy E, Petry D. Asymmetry correction equations for hand volume, grip and pinch strengths in healthy working people. Eur J Epidemiol. 1998;14:71–77. doi: 10.1023/A:1007436000228. [DOI] [PubMed] [Google Scholar]


