Abstract
The purpose of this study was to analyze cases undergoing imaging guided localization prior to surgical excisional biopsy of abnormalities in the breast and to describe the methodology utilized to perform such presurgical localization procedures. Presurgical localization of non palpable breast abnormalities is a simple, safe and effective procedure; it is now used more selectively for this indication due to availability of minimally invasive percutaneous biopsy procedures that can be performed under ultrasound or stereotactic guidance.
Keywords: Mammography, Presurgical localization, Breast, Sonography
Introduction
Presurgical localization is performed to obtain histological diagnosis of mammographic screen detected abnormalities of the breast. It is an integral part of a breast cancer screening program and helps in the early diagnosis of non palpable breast cancer. Over the last two decades there has been a dramatic drop in the number of these open biopsy procedures due to widespread use of minimally invasive percutaneous biopsy procedures performed under local anesthesia with ultrasound or stereotactic mammographic guidance. Apart from the morbidity factor the cost benefit of performing imaging guided percutaneous outpatient procedures has been shown by multiple studies [1–4]. Preoperative diagnosis of cancer decreases or eliminates positive operative margins and need to reexcise tissue. Stereotactic percutaneous biopsy has been recommended as the procedure of choice for mammographically detected abnormalities [1]
Presurgical localization is now performed for selected indications, such as in those patients with a biopsy proven cancer, in those who have imaging pathological discordance at core needle biopsy, in those with high risk lesions such as atypical ductal hyperplasia, radial scar, papillary lesions diagnosed at percutaneous biopsy or where core needle biopsy is not an option or fails to provide a definitive histological diagnosis. It has been reported that with selective use of excisional biopsy for indications noted above, missed diagnosis of breast cancer is rare [1]. Compared with surgical excisional biopsy preoperative diagnosis by core needle biopsy allows for wider margins of excision, fewer positive margins and fewer surgical procedures to achieve adequate treatment than diagnosis by surgical excisional biopsy alone would permit [1]. A study testing the cost effectiveness of stereotactic biopsy versus needle localized open surgical biopsy reported that there was no difference in cost benefit in cases where there are lesions highly suggestive of breast cancer (BIRADS 5) or those cases suspicious for ductal carcinoma in situ [4]. However in cases of intermediate risk lesions classified as BIRADS 4 these investigators noted significant cost savings when stereotactic percutaneous biopsy was performed instead of needle localized breast biopsy [4].
Materials and Methods
During a one-year period from November 2008 to October 2009 we performed a total of 202 presurgical image guided localization procedures at our Breast center. During this period 15,875 screening mammograms were performed. Patients ranged in age from 18 to 81 years with a mean age of 52 years. 155 localizations were performed under mammographic guidance and 47 were ultrasound guided.
Mammographic Guided Needle Wire Localization
In this method there are three variables to be considered, the type of needle wire, the length of the needle and the type of approach. A modified hook wire system with a reinforced 2 cm segment 1.2 cm from its hook was used for all procedures regardless of whether localization was performed under mammographic or sonographic guidance. We used 5, 7 or 9 cm needle length depending on the depth of the abnormality being localized in the breast. All procedures were performed using the parallel to the chest wall approach; none were performed using free hand or anterior approach [5].
Two kinds compression paddles were utilized one with an alphanumeric grid was the most commonly used. A second one called the Swiss cheese paddle is a smaller paddle with multiple holes and was used in women with small breasts, for lesions in the subareoalar region, high up in the axilla or close to the chest wall, where the small size of the paddle allows for easier access and optimal immobilization. Prior to the procedure, informed consent was obtained in all patients routinely. This was done after explanation of the procedure, and a description of the potential complications including informing of the patient of the possibility of failure to adequately excise the abnormality localized. Local anesthesia is not administered at our institution for mammographic-guided localization procedures but used in most instances of ultrasound-guided localization. The decision to use local anesthesia for sonographic-guided localization procedure is dependent on both physician and patient preference. The author for example used local anesthetic for sonographic-guided procedures only if patient preferred to have it.
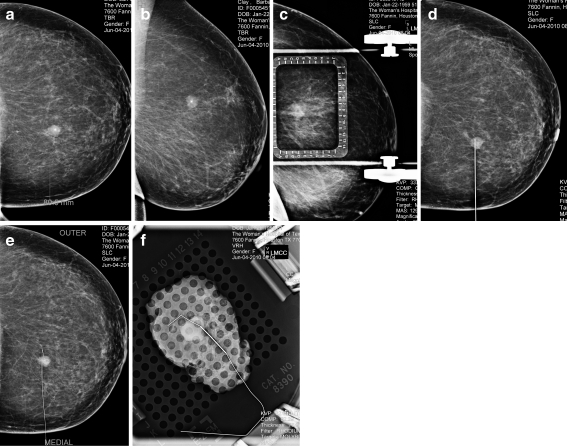
For the parallel to the chest wall approach the breast is positioned such that the lesion to be localized is closest to the skin surface through which the needle wire combination was to be introduced. A lesion at the 12’o clock position in the upper breast for instance the approach is superior with breast placed in the craniocaudal position, a lesion at the 3’o clock position of the right breast is best approached medially with the breast in compression in the mediolateral position. The length of the needle selected depended on the depth of the lesion, keeping in mind that the final wire placement should be such that the tip of the wire extends beyond the lesion and is ideally with 0.5 cm from the abnormality. Once the approach and length of the needle is decided the breast is placed under compression .The lesion coordinates are obtained from this initial mammogram based on its location within the alphanumeric grid (Fig. 1a). Using the collimator cross hairs the point of entry is determined and the needle wire is advanced to the predetermined depth, satisfactory placement of the needle wire is determined by obtaining two views in the orthogonal plane and needle position is adjusted based on these two views as needed (Fig. 1b,c). Once this is satisfactory, the wire is advanced so that the hook wire anchors to the tissue and the needle is gently withdrawn. A final two-view mammogram is obtained to confirm that the wire tip is located within 5 mm of the lesion (Fig. 1d, e). The patient is then transported to the operating suite with the films showing the position of the localizing wire for the surgeon to review prior to performing the excisional biopsy.(Fig. 1f)
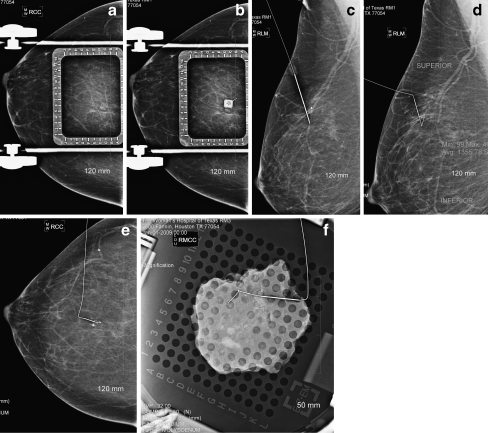
Fig 2.
Mammographic presurgical localization for a mass that was histologically proven to be a Fibroadenoma a Craniocaudal view showing the mass b Mediolateral view showing the mass c Mediolateral view with breast under compression in a fenestrated paddle with an alphanumeric grid d Craniocaudal view with satisfactory placement of the needle wire e Craniocaudal view showing satisfactory position of the wire f Specimen radiograph showing the wire adjacent to the localized mass
Fig 1.
Mammographic presurgical localization for clustered micorcalcifications that were histologically proven to be DCIS. a Craniocaudal view with breast under compression with an alphanumeric grid showing micorcalcifications at 6D coordinates b Craniocaudal view with breast under compression with an alphanumeric grid and needle wire in satisfactory position c Mediolateral view confirming satisfactory placement of the needle wire d Mediolateral view following showing satisfactory deployment of the wire e Craniocaudal view showing satisfactory position of the wire f Specimen radiograph showing the wire adjacent to the localized microcalcifications
Ultrasound Guided Localization
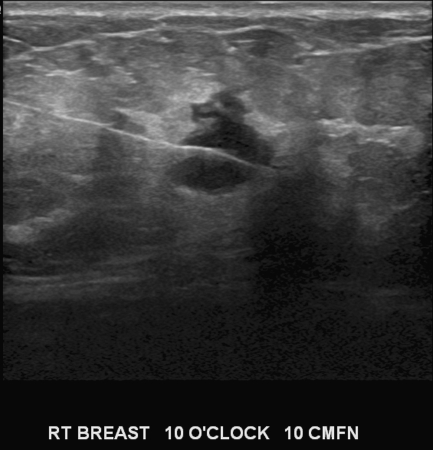
When an abnormality is seen well on ultrasound and concordance with mammographic finding has been proven for those abnormalities that are seen on mammograms, sonographic localization is the preferred modality for localization. These lesions are usually solid masses. The same needle wire utilized for mammographic localization is used when localizing abnormalities under ultrasound guidance. The needle wire is introduced through a point on the skin determined to be the shortest to the lesion. Under real time guidance the needle wire is advanced 1 cm beyond the lesion and once position is determined to be satisfactory the wire is advanced over the wire and the needle is withdrawn gently taking care not to withdraw the wire with the needle. An image with wire in satisfactory position is obtained and sent with the patient for the surgeon (Fig. 3).
Fig. 3.
Ultrasound guided presurgical needle localization. Image showing the hookwire passing through the solid mass that was preoperatively localized and was histologically proven to be a Fibroadenoma
In both types of presurgical localization procedures the wire is taped firmly in position and patient is advised not to move the arm in question to avoid movement of the wire during transportation to the operating room. All surgical excisional procedures were performed under general anesthesia.
Results
Microcalcifications were the most frequent abnormality for which surgical excisional biopsy was performed with 94 patients undergoing localizations for this mammographic finding. There were 76 patients who underwent this procedure for solid masses, of which 5 were palpable. Three patients had localized architectural distortion and ten patients had focal asymmetry. In 19 patients there were a combination of the above findings. In 200 patients the abnormality was successfully excised. In two patients both of whom had microcalcifications the surgical specimen did not demonstrate the calcifications accounting for a procedure failure rate of 1%.
There were 59 cancers in the group studied (29.5%) of which 35 were invasive ductal cancers one was invasive lobular cancer, one was malignant Phylloides tumor and 22 were ductal carcinomas in situ [DCIS]. All cases of ductal carcinoma in situ were clustered microcalcifications. Microcalcifications with or without a mass was the most frequent mammographic finding in non palpable abnormalities proven to be invasive ductal cancer and seen in 30 of 35 cases (Table 1).
Table 1.
BIRADS Assessment correlation with histological outcome
| Benign | Malignant | |
|---|---|---|
| BIRADS 5 (N = 26) | 3 | 23 |
| BIRADS 4 (N = 152) | 117 | 35 |
| BIRADS 3 (N = 21) | 20 | 1 |
| BIRADS 2 (1) | 1 | 0 |
Amongst the 155 patients undergoing excisional biopsy, in nine patients a specimen radiograph was not done. In this group in two patients frozen section revealed cancer, in three at 12 month lesion was not seen, in one a mass was seen in the gross specimen, one had distortion with radial scar, and in two of the remaining patients the histology findings were concordant with abnormality localized.
Amongst abnormalities categorized as BIRADS 4 AND 5, the positive predictive value for breast cancer was 31%. In 21 patients surgical excision was performed despite a recommendation for follow up (BI-RADS 3), all but one had a benign diagnosis (Table 2). One patient with microcalcifications considered probably benign had DCIS.
Table 2.
Mammographic abnormalities in patients with breast cancer
| Ductal carcinoma in situ (N = 22) | Invasive ductal cancer (N = 35) | Malignant phylloides tumor (N = 1) | Invasive lobular cancer (N = 1) | |
|---|---|---|---|---|
| Microcalcifications | 22 | 7 | 0 | 1 |
| Mass | 0 | 9 | 1 | 0 |
| Focal asymmetry | 0 | 3 | 0 | 0 |
| Architectural distortion | 0 | 2 | 0 | 0 |
| Mass associated with calcifications | 0 | 14 | 0 | 0 |
Discussion
Presurgical localization is performed generally for non palpable abnormalities considered suspicious for breast cancer based on mammographic and or sonographic work up of screen detected breast abnormalities. The most common of these mammographic abnormalities include micorcalcifications, solid or complex cystic masses, areas of architectural distortions and focal asymmetry. In some instances a surgeon may request imaging guidance prior to surgical excision of palpable abnormalities to ensure optimal correlation between clinical and mammographic or sonographic findings [5]
The hook wire technique using the parallel to the chest wall approach that we use at our institution for needle localization is the most commonly used method and is described in the materials and methods section of this article. An alternate approach using the hook wire is the free hand technique or an anterior approach. In this method the radiologist extrapolates the location of the abnormality from the mammograms to a decompressed breast and advances the needle blindly towards the chest wall. Two view mammograms are obtained and repeated as needed after repositioning until the wire is seen to be within 1 cm of the lesion being localized. Due to the inherent nature of the procedure this technique is time consuming and has a higher complication rate [5]
There are other techniques that have been used to localize breast abnormalities under mammographic guidance. When an abnormality is located within 1 cm of the skin surface, placing a BB on the skin overlying the abnormality may be adequate to perform localization. The position of the BB is then be confirmed by a two-view mammogram. Skin localization is however not recommended for lesions at a depth greater than 1 cm and may lead to excision of unacceptably large volume of tissue. There is a dye method of localization and this involves injection of 0.2 ml of dye through a needle positioned under mammographic guidance near the abnormality localized. As the needle is withdrawn a dye outlined track is left behind which the surgeon uses as a guide to find the abnormality. Mehtylene blue dye or Alician blue dye can be used, care should be taken to inject not more than 0.2 ml to avoid dye diffusion. The advantage of this method is that there is no need to leave a wire in the breast and hence avoids the problem of potential wire displacement or migration during transfer to the operating suite [5]. The practice of using local anesthetic prior to introduction of the needle wire is variable. At our institution we do not use local anesthetic during mammographic localizations and often do for sonographic guided procedures. The value of local anesthetic has been questioned. In a study of 89 patients undergoing mammographic localized excisional biopsy, 46 patients received local anesthetic and 43 did not. Patients who did not receive the local anesthetic reported a lower mean pain score than those who did [6]
Complications of the presurgical imaging guided localization procedures may involve failure to excise the localized abnormality or procedure related complications. A failure to excise the localized mammographic abnormality has been reported in 2.5% to 6.7% of cases. Jackman and Marzoni looked at a series of 280 lesions undergoing presurgical localization [7]. Failure to localize occurred in 7 (2.5%), 21 lesions that were not initially excised were done so on repeat excision of more than one tissue specimen in 14 of 21 cases where specimen radiography did not demonstrate the abnormality [7]. These authors concluded that failure was more likely with two lesions, small breast, for microcalcifications, small specimen and small lesions [7]. In another study Abrahamson and others reported a success rate of 93.3% (254 of 272 lesions), and concluded that placement of the localization wire within 5 mm was a significant predictor of successful removal of the localized lesion and that failure rate was higher when wire was greater than 5 mm from the lesion, in small breasts and in small specimen [8]. In our series the failure rate was 1%. Potential reasons for failure to excise include placement of the wire greater than 1 cm from the lesion, placing the wire short of the lesion or advancing the wire significantly beyond the lesion, wire movement during patient transfer to the operating suite, bleeding leading to hematoma formation. In both the cases in our series post wire placement mammograms demonstrated satisfactory placement, and the presumed reason for failure to excise was movement of the wire during patient transfer from the Breast center.
Procedure related complications are relatively rare. Of the known complications the most common ones are vasovagal reactions and bleeding both of which are self limited and easy to manage and almost never lead to cancellation or failure of the procedure. Migration of the wire, fragmentation of the wire, and pneumothorax, are very rare complications [9, 10]. There has been a report of a hook wire causing delayed cardiac injury by penetrating the pericardium and myocardium and lodging in the aorta with patient presenting with chest pain. Following an echocardiogram and CT scan the wire was surgically removed. [9] We did not encounter any procedure related complications during the one year study period,
Specimen radiography following imaging guided localization is performed for several reasons. Primarily it verifies that the entire lesion has been removed. It provides a guide for the pathologist to the location of the lesion in the specimen. It verifies that the wire has been removed from the breast. Other advantages include detecting additional abnormalities that may not have been suspected and is a good learning tool to correlate lesion morphology with histology [5]. At our institution we routinely perform specimen radiography on almost all cases undergoing mammographic localizations and inconsistently so when ultrasound is used to localize masses. The benefit of specimen radiography has been questioned, in one study only three of 165 patients (1.8%) benefited from performance of specimen radiography. [11]. A technique of immersion ultrasonography of excised specimens has been reported for lesions localized under ultrasound guidance and those that are not mammographically visible [12]. In our series seven of 47 patients undergoing ultrasound guided localizations had specimen radiography and among these in five specimen radiography confirmed excision of the abnormality. The diagnostic accuracy of the procedure itself was 100% in our series, when the localized lesion was seen on the specimen radiograph. A study looking at the diagnostic accuracy of needle-localized open breast biopsy reported 96% accuracy at 5 year follow up. However on review of the cases of missed breast cancer revealed that 6 of the seven were in fact failure to excise the localized abnormalities and in one instance cancer developed after the surgical excision [13].
In our series, breast carcinoma was diagnosed in 59 of the 200 patients in whom successful excision of the abnormality localized was achieved [29.5%], 22 of these 59 cancers [37.3%] were ductal carcinoma in situ. This compares with a cancer rate of 20% in a series of 167 excisional biopsies, a 21.8% rate in a series of 156 excisional biopsies and 27% of 809 needle localized biopsies [14–16]. Microcalcifications without an associated mass were seen in all cases proven to be ductal carcinoma in situ and in seven cases of invasive ductal cancer. 26 patients were 40 years or younger and this age group four cancers were diagnosed, three DCIS and one invasive ductal cancer. Lein and others in a series of 809 presurgical needle localization reported that three of 60 patients under 40 years had breast cancer [16].
Imaging guided presurgical localization of non-palpable mammographic screen detected abnormalities of the breast is a simple, safe and accurate way of diagnosing early stage breast cancers. Over the last two decades a rapid decrease in the number of these procedures has been noted due to advent of minimally invasive percutaneous biopsy procedures performed under mammographic or sonographic guidance. Nevertheless it still remains a useful method and is now indicated in selected cases where a cancer diagnosis has been made based on a needle biopsy, when there is imaging pathological discordance following needle biopsy or in those patients where imaging guided biopsy is not an option due to patient related factors.
Acknowledgement
Ms Samantha Christians RT, Research Assistant
References
- 1.Yim JH, Barton PM, Weber B, Radford D, et al. Mammographically detected breast cancer. Benefits of stereotactic core vs. wire localization biopsy. Ann Surg. 1996;223(6):688–700. doi: 10.1097/00000658-199606000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White RR, Halperin TJ, Olson AJ et al Impact of Core-needle biopsy on the surgical management of mammographic abnormalities annals of surgery 233 (6): 769–777 [DOI] [PMC free article] [PubMed]
- 3.Whitten TM, Wallace TW. Image guided core biopsy has advantages over needle localization biopsy for the diagnosis of Nonpalpable breast cancer. Am Surg. 1997;63:1072. [PubMed] [Google Scholar]
- 4.Fahy BN, Bold RJ, Schneider PD, Khatri V, Goodnight JE. Cost-benefit analysis of biopsy methods for suspicious mammographic lesions. Arch Surg. 2001;136:990–994. doi: 10.1001/archsurg.136.9.990. [DOI] [PubMed] [Google Scholar]
- 5.Cardenosa G. Breast imaging companion. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 414–433. [Google Scholar]
- 6.Reynolds HE, Jackson VP, Musick BS. Preoperative needle localization in the breast: utility of local anesthesia. Radiology. 1993;187:503–505. doi: 10.1148/radiology.187.2.8475298. [DOI] [PubMed] [Google Scholar]
- 7.Jackman RJ, Marzoni FJ (1997) Needle localized Breast Biopsy. Why do we fail? Radiology 204 [DOI] [PubMed]
- 8.Abrahamson PE, Dunlap LA, Amamoo MA, Schell MJ, Braeuning MP, Pisano ED. Factors predicting successful needle-localized breast biopsy. Acad Radiol. 2003;10:601–606. doi: 10.1016/S1076-6332(03)80077-6. [DOI] [PubMed] [Google Scholar]
- 9.Martinez SR, Gelfand M, Hourani HS, Sorrento JJ, Mohan EP. Cardiac injury during needle localized surgical breast biopsy. J Surg Oncol. 2003;82(4):261–265. doi: 10.1002/jso.10218. [DOI] [PubMed] [Google Scholar]
- 10.Helvie MA, Ikeda DM, Adler DD Localization and Needle aspiration of Breast Lesions: complications in 370 cases. AJR Am J Roentgenol 157:711–714 [DOI] [PubMed]
- 11.Bimston DN, Bebb GG, GG WLD. Is specimen mammography beneficial? Arch Surg. 2000;135:1083–1088. doi: 10.1001/archsurg.135.9.1083. [DOI] [PubMed] [Google Scholar]
- 12.Lee KY, Seo BK, Ann Y, et al. Immersion ultrasonography of excised non palpable breast lesion specimens after ultrasound-guided needle localization. Korean J Radiol. 2008;9:312–319. doi: 10.3348/kjr.2008.9.4.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verkooijen HM, Peeters PHM, Pijnappel RM, Koot VCM, Schipper MEI. Borel rinkes IHM. Diagnostic accuracy of needle-localized open breast biopsy for impalpable breast disease. Br J Surg. 2000;87:344–347. doi: 10.1046/j.1365-2168.2000.01380.x. [DOI] [PubMed] [Google Scholar]
- 14.Markopoulos C, Phil M, Kakisis J, et al. Management of nonpalpable, mammographically detectable breast lesions. World J Surg. 1999;23:434–489. doi: 10.1007/PL00012323. [DOI] [PubMed] [Google Scholar]
- 15.Tran DQ, Wilkerson DK, Namm J, Zeis MA, Cottone FJ (1999) Needle-localized breast biopsy for mammographic abnormalities: a community hospital experience. The American Surgeon 281–287 [PubMed]
- 16.Lein BC, Alex WR, Zebley DM, Pezzi CM. Results of needle localized breast biopsy in women under age 50. Am J Surg. 1996;171:356–359. doi: 10.1016/S0002-9610(97)89641-9. [DOI] [PubMed] [Google Scholar]