Abstract
Aim
Micropapillary/borderline (LMP) ovarian tumors are rarely included in clinical trials and are intrinsically resistant to radiation and chemotherapy. Platinum resistant epithelial ovarian cancer (EOC) has a poor prognosis. The histone deacetylase inhibitor belinostat demonstrated antitumor activity in preclinical ovarian cancer models.
Methods
A phase II study was performed to evaluate the activity of belinostat in two patient populations: women with metastatic or recurrent platinum resistant (progression within 6 months) EOC and LMP ovarian tumors, both groups had received no more than 3 prior lines of chemotherapy. Belinostat 1000mg/m2/day was administered iv days 1-5 of a 21 day cycle. Peripheral blood mononuclear cells (PBMCs) and tumor biopsies, where possible, for correlative studies were obtained prior to and following treatment.
Results
18 patients with EOC and 14 patients with LMP tumors were enrolled on study. Belinostat was well tolerated with no grade 4 toxicity (179 cycles). Grade 3 toxicity consisted of thrombosis (3 patients), hypersensitivity (1) and elevated ALP (1). One patient with LMP tumor had a partial response (unconfirmed) and 10 had stable disease (SD), 3 were non-evaluable. Median progression-free survival (PFS) was 13.4 months (95% CI, 5.6 - not reached). Best response in patients with EOC was SD (9 patients) and median PFS was 2.3 months (95% CI, 1.2-5.7 months). An accumulation of acetylated histones H3 and H4 was noted in PBMCs and in tumor tissue.
Conclusions
Belinostat is well tolerated in both patient groups and shows some activity in patients with micropapillary (LMP) disease.
Introduction
Epithelial ovarian cancer (EOC) is the fifth leading cause of death in women in North America (1). Patients whose disease does not respond or in whom, the duration of response is short to initial platinum based chemotherapy have a median survival of only 6-9 months (2). Borderline ovarian tumors or tumors of low malignant potential (LMP) account for up to 20% of all ovarian tumors (3). Although survival is excellent in early stage disease between 10-20% of women will die of their disease (3,4,5) If surgery is not feasible there is no established standard of care. Chemotherapy and radiation are ineffective and these patients are rarely included in clinical trials (6,7,8). New therapeutic approaches are urgently required for both of these diseases.
Histone deacetylases (HDAC) are involved in post-translational acetylation of core nucleosomal histones thus playing a key role in the epigenetic regulation of gene expression (9). Inhibition of HDACs has emerged as a promising therapeutic strategy, restoring expression of silenced genes leading to cell differentiation and subsequent cell cycle arrest or apoptosis in transformed cells (10), including in ovarian cancer cell lines (11,12,13).
Belinostat (formally known as PXD101) is a low molecular weight, hydroxamic acid inhibitor, of class I and II HDACs (14,15). Pre clinical studies suggest belinostat may have activity in women with EOC (16).
The aim of this 2-stage, multicenter, phase II study was to evaluate the efficacy and safety of belinostat in two patient populations: metastatic or recurrent platinum resistant (progression within 6 months) EOC and micropapllilary/LMP ovarian tumors. Secondary aims were survival and pharmacodynamic effects of belinostat in peripheral blood mononuclear cells (PBMCs) and tumor tissue. The dose and scheduling for this study were established by a phase I study with belinostat in patients with solid tumors (17).
Materials and Methods
This study was conducted by the Princess Margaret Phase II Consortium according to Good Clinical Practice guidelines and with full research ethics board approval. All patients signed written informed consent before study entry.
Eligibility
Patients with histologically or cytologically confirmed platinum resistant (defined as progression within 6 months of platinum therapy) recurrent EOC or micropapillary/borderline(LMP) ovarian tumors who had received ≤ 3 prior lines of chemotherapy for advanced disease were eligible for this trial. Eligibility criteria included: life expectancy ≥12 weeks; Eastern Cooperative Group (ECOG) performance status ≤2, adequate hematologic, hepatic and renal function and Response Evaluation Criteria In Solid Tumors (RECIST) measurable disease (18); prior treatment had to be completed >4 weeks before study entry. Exclusion criteria included: bowel obstruction, significant cardiovascular disease, prolongation of QT/QTc interval, uncontrolled hypertension or a history of/or known brain metastases.
Study design
Belinostat 1000mg/m2 was administered intravenously on days 1 to 5 of a 21 day cycle. No pre-medication was required. Treatment was discontinued for disease progression, patient choice or toxicity.
Patient evaluation
Baseline evaluations included medical history, physical examination, laboratory tests, CA125 and ECG and were performed within 7 days of starting protocol therapy and repeated day 1 of each cycle. Tumor response was evaluated every 2 cycles (6 weeks) by RECIST (18). CA125 was measured every 3 weeks according to Gynecologic Cancer InterGroup (GCIG) criteria for response and progression (19). Toxicity was evaluated according to NCI Common Terminology Criteria for Adverse Events (CTCAE) Version 3.0 resolution to baseline or grade 1 was required prior to re treatment.
Correlative studies
Representative HE sections of the tumors were reviewed by an expert gynecopathologist (TC).
Blood samples, for peripheral blood mononuclear cells (PBMCs), and tumor biopsies were collected ≤ 7 days prior to and post belinostat on day 4 or 5 of cycle 1. Histone acetylation was evaluated using Western Blotting for histone H3 and H4 isolated from PBMCs. Acetylated histones were detected using anti-Acetyl-Histone H3 or H4 (Lys9,Lys8) antibodies (Cell Signaling Technology,Inc)(20).
Paraffin sections of pre- and post-treatment biopsies from two patients were processed using standard operating procedures at the Applied Molecular Profiling Laboratory (University Health Network, Toronto, ON). Microwave antigen retrieval was followed by detection of acetylated histone H3 using anti-Acetyl–histone H3 antibody (1:400 dilution) labeled on the Ventana Benchmark XT using the uVIEW DAB (Tucson, AZ). Positive staining was visually evaluated (M-S T) and image analysis performed to obtain positive average staining intensity in regions of interest as previously described (21).
Statistical Methods and Endpoints
The primary end point of this study was objective response according to RECIST and Rustin criteria (18,19). A two-stage design was used within each cohort (22). Using response hypotheses of H0 ≤ 5% and Ha ≥ 20%, 15 evaluable patients with LMP/borderline tumors were required for the first stage, with ≥1 response required to continue into second stage accrual (significance level, α= .05 and β =0.20).
Using response hypotheses of Ho ≤ 10% and Ha ≥30%, 12 evaluable patients with platinum resistant EOC were required for the first stage requiring ≥ 1 response to proceed to the second stage (significance level, α= .10 and β =0.10) PFS and OS were summarized using the Kaplan Meier method.
Results
Eighteen patients with platinum resistant EOC and 14 with micropapillary/LMP tumors were enrolled at 3 centers across Canada.
At the time of this analysis all 18 patients with EOC were off study with: progression (14 patients), bowel obstruction (1), patient choice (2) and other (1). Thirteen patients with micropapillary/LMP tumors were off study: progression (5), bowel obstruction (1), toxicity (2), patient choice (5). One patient remains on study.
Patient characteristics
Table 1 summarizes the pretreatment patient and disease characteristics in both groups of eligible patients. All patients had a diagnosis of micropapillary/ LMP tumor confirmed on expert pathology review (TC) and were actively progressing on the basis of CT scan prior to entering the study (confirmed by expert radiological review).
Table 1.
Patient Characteristics
| Characteristic | Micropapillary/ Borderline ovarian tumors N=14 |
Platinum resistant EOC N=18 |
|---|---|---|
| Age (years) Median Range |
50 28-82 |
59.5 31-78 |
| Performance status 0 1 2 |
11 2 1 |
11 6 1 |
| Primary Site Ovarian Peritoneal Unknown |
14 |
16 1 1 |
| Target: Non-target sites Median (range) |
4.5 1-9 |
5 1-10 |
| Prior radiotherapy Yes No |
1 13 |
0 18 |
| No. of prior chemotherapy regimens 0 1 2 3+ |
3 6 5 0 |
0 4 5 9 |
Treatment administration
32 patients received 179 treatment cycles (115 cycles, LMP: 64 cycles EOC). Median 2 cycles/ patient EOC (range 1-14 cycles) and 8 cycles /patient micropapillary/LMP (range 1-20 cycles). 16 patients had missed or delayed doses due to: patient request (4), adverse events (3) and other (3). Three patients had dose delays. There were no dose reductions. 2 patients with LMP tumors were taken off study for possibly drug related adverse events hypersensitivity (1 patient) and grade 3 elevation of ALP (1 ).
Toxicity
All patients were evaluable for toxicity. Table 2 displays the adverse events considered to be at least possibly drug related for both patient groups The most common events (all grade 1 or 2) were fatigue (69% of patients) and nausea (59%). No patients died on study. Four patients developed disease related bowel obstruction.
Table 2. Adverse Events on Treatment (n = 32 evaluable patients).
| Belinostat related toxicity CTCAE version 3. |
Grade 1/2 |
Grade 3/4 |
Patients Total no. % |
|---|---|---|---|
| Fatigue | 22 | - | 22 (69%) |
| Nausea | 22 | - | 19 (59%) |
| Vomiting | 11 | - | 11(34%) |
| Diarrhea | 9 | - | 9(28%) |
| Pneumonitis | 2 | - | 2(6%) |
| Sinus bradycardia | 6 | - | 6(19%) |
| Sensory peripheral neuropathy |
5 | - | 5(16%) |
| Headache | 5 | - | 5(16%) |
| Hypersensitivity | 2 | 1 | 3(9%) |
| Thrombosis | 0 | 3 | 3(9%) |
| Leukopenia | 2 | - | 2(6%) |
| Anemia | 4 | - | 4(13%) |
| Elevated alkaline phosphatase |
1 | 1 | 2(6%) |
Efficacy
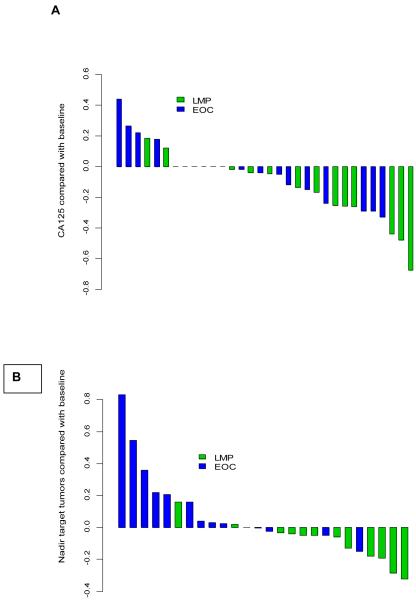
Fifteen patients with platinum resistant EOC were evaluable for response: 9 had SD and 6 progressive disease (PD) (figure 1a). Median progression-free survival (PFS) was 2.3 months (95% CI, 1.2-5.7 months), 6-month progression free proportion (PFP) was estimated at 13% (95% CI, 4-49%).
Figure 1.
Waterfall plots for response to treatment A) CA125 and B) by RECIST criteria .
Twelve patients with micropapillary/LMP tumors were evaluable for response: 1 had an unconfirmed partial response (uPR), reduction in target lesions of 32% post cycle 8 increasing by 4% post cycle 10, ten had SD and 1 PD (figure 1a). The patient with uPR had received prior chemotherapy (carboplatin/paclitaxel). An additional patient had a CA125 response (figure 1b). Median PFS was 13.4 months (95% CI, 5.6 - not reached), 6 month PFP was estimated at 56% (95% CI, 32-96%). Median duration of SD was 5.8 months (range 2.8 to 13.6 months) Twelve patients have died (10 in the EOC group and 2 LMP patients) Median overall survival (OS) for patients with platinum resistant EOC was 7.5 months (95% CI: 3.7-not reached) and median OS for the LMP patients has not been reached.
Pharmacodynamic analyses
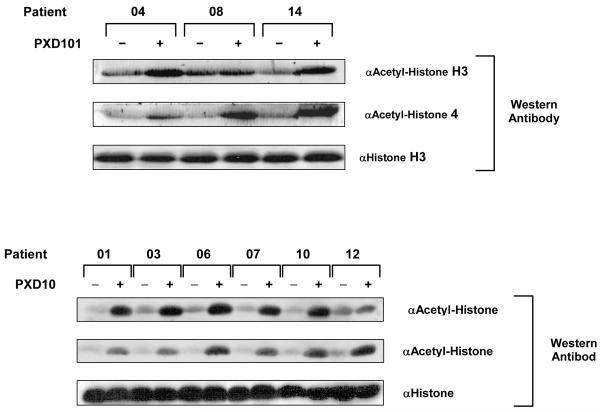
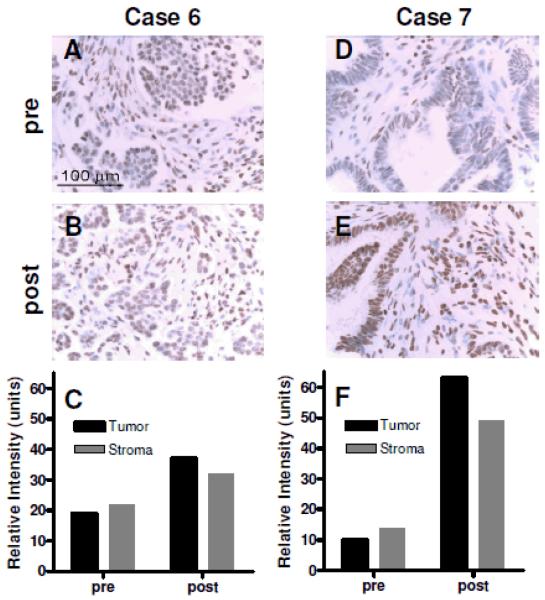
Paired samples of PBMCS prior to and following belinostat were available for 9 patients with micropapillary/LMP tumors. A marked increase in acetylated histones H3 and H4 was demonstrated for all patients, Figure 2. Paired tumor samples were available for two patients, patient no 6 and 7. A marked increase in acetylation was observed following treatment with belinostat in both patients, Figure 3.
Figure 2.
Histone acetylation evaluated using Western Blotting for histone H3 and H4 isolated from PBMCs Acetylated histones detected using anti-Acetyl-Histone H3 or H4 (Lys9,Lys8) antibodies (Cell Signaling Technology,Inc) in PBMCs taken prior to and following Belinostat in patients with Micropapillary/LMP tumors.
Figure 3.
Acetylated histone H3 measured prior to and following Belinostat (cycle 1). For patient 6 the average mean intensity in tumor was pre 19 units and post 37 units, for stroma pre 22 units and post 32 units. For patient 7 in tumor pre 10 units and post 63 units and stroma pre 14 units and post 49 units
Discussion
When surgery is no longer an option, there is no standard of care for women with micropapillary (LMP) tumors (7). To the best of the authors knowledge, this represents the first prospective study conducted in this group of women. Belinostat shows modest activity (Figure 1A/B) in this patient population. Although the study did not meet criteria to proceed to the second stage we feel the PFS and waterfall plots of both tumor size and CA125 suggest activity that may warrant further investigation. Defining activity by objective response, however, may not be the best approach for patients with this, indolent, disease. Future trials incorporating a randomized discontinuation design to allow assessment of prolonged SD may be preferable.
Interpreting and designing studies for women with micropapillary/borderline LMP tumors is challenging. The histological diagnosis and correct classification of these tumors requires expert pathological review (TC in this study) (6,23). Prognosis for these women differs depending on the presence of non-invasive (mortality rate 6%) vs. invasive peritoneal (25%) implants (24). Eight patients had invasive implants in this study. A further confounding factor is the histology of the recurrence one series suggesting 73% may recur as low grade serous EOC (7). Data on outcome for non surgical therapies are scarce and retrospective (7). One study suggesting chemotherapy and radiation may even be detrimental for those with non invasive implants (6). The highest reported response rate to first line chemotherapy (retrospect review) is 26 %. No response was observed >1 non-surgical therapy (7). In our study 11 patients had received prior chemotherapy. Although only one uPR and one CA125 response were seen 10 patients had a fall in CA125 and 10 a reduction in tumor size, having previously demonstrated actively progressing disease. Changes in the patterns of calcification observed on CT scanning also suggest a biological effect. Again the lack of comparative data makes interpreting survival problematic. In a retrospective series of 21 patients receiving first line chemotherapy median duration of SD was 12 months (range 3 to 77.5 months) (7). Based on this a median PFS of 13.4 months in a ,largely, pre-treated population appears promising.
An increase in H3 and H4 histone acetylation was observed in all nine LMP patients who had paired PBMC samples Figure 2. In pre clinical studies with belinostat acetylated histones measured in mice showed a similar dose response (15). In two patients (patient 6 and 7) additional paired tumor samples showed an increase in acetylated histone H3 in tumor cells and stroma (figure 3). Both patients had SD, patient 6 receiving 8 and patient 7 four treatment cycles. These data would suggest in these patients belinostat was having a pharmacodynamic effect in tumor tissue.
The outlook for women with platinum resistant EOC is very poor. Despite promising activity in pre-clinical ovarian cancer models single agent belinostat did not display sufficient efficacy to warrant further investigation results similar to those seen with Vorinostat, another HDAC inhibitor (25). Belinostat in combination with conventional cytotoxics may be a more promising approach in women with EOC (15,16,26).
Belinostat was very well tolerated in both study populations with fatigue the most common adverse event. Three patients developed thombosis on study although not reported previously for belinostat this is a recognized adverse event in other HDAC inhibitor studies (27,28).
Epigenetic changes at an intermediate level between normal ovarian tissue and EOC are well recognized for micropapillary (LMP) tumors (29). It is possible that Belinostat in this low grade, indolent tumor is inducing differentiation and disease stabilization similar to that which is seen in another indolent condition, myelodysplastic syndrome following administration of demethylating agents (30). Whilst the lack of single agent activity in platinum resistant EOC is disappointing the future for epigenetic therapy in this disease may lie in combination with other agents as a response modifier.
Further investigation into the biology and treatment of micropapillary/borderline (LMP) tumors is urgently needed. Belinostat is the first agent biological agent to show promising activity in this group of women and this warrants further investigation.
Acknowledgments
Support: This trial was supported by funding from N01-CM-62203 and drug was supplied by the Cancer Therapeutics Evaluation Program, US National Cancer Institute and the Campbell Family Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No conflict of interest to declare
Presented: Poster discussion ASCO 2008
References
- 1.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–29. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 2.Cannistra SA. Is There a “Best” Choice of Second-Line Agent in the Treatment of Recurrent, Potentially Platinum-Sensitive Ovarian Cancer? J Clin Oncol. 2002;20:1158–60. doi: 10.1200/JCO.2002.20.5.1158. [DOI] [PubMed] [Google Scholar]
- 3.Tinelli R, Tinelli A, Tinelli FG, et al. Conservative surgery for borderline ovarian tumors: a review. Gynecol Oncol. 2006;100:185–191. doi: 10.1016/j.ygyno.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 4.Leake JF, Currie JL, Rosenshein NB, et al. Longterm follow up of serous ovarian tumors of low malignant potential. Gynaecol. Oncol. 1992;47:150–158. doi: 10.1016/0090-8258(92)90099-5. [DOI] [PubMed] [Google Scholar]
- 5.Longacre TA, Mckenney JK, Tazelaar HD, et al. Ovarian serous tumors of low malignant potential (borderline tumors): Outcome based study of 276 patients with longterm (>5 year) follow up. Am. J. of Surg. Path. 2005;29:707–723. doi: 10.1097/01.pas.0000164030.82810.db. [DOI] [PubMed] [Google Scholar]
- 6.Seidman J, Kurman RJ. Subclassification of Serous Borderline Tumors of the Ovary into Benign Malignant Types: A Clinicopathologic Study of 65 Advanced Stage Cases. The American Journal of Surgical Pathology. 1996;20:1331–1345. doi: 10.1097/00000478-199611000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Crispens MA, Bodurka D, Deavers M, et al. Response and Survival in Patients With Progressive or Recurrent Serous Ovarian Tumors of Low Malignant Potential. Obstetrics and Gynecology. 2002;99:3–10. doi: 10.1016/s0029-7844(01)01649-0. [DOI] [PubMed] [Google Scholar]
- 8.Trope C, Kaern J, Vergote IB, et al. Are borderline tumors of the ovary overtreated both surgically and systemically? A review of four prospective randomized trials including 253 patients with borderline tumors. Gynecol Oncol. 1993;51:236–243. doi: 10.1006/gyno.1993.1279. [DOI] [PubMed] [Google Scholar]
- 9.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nature Reviews Drug Discovery. 2006;5:769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 10.Caslini C, Capo-Chici CD, Roland IH, Nicholas E, Yeung AT, Xu X. Histone modification silences the GATA transcription factor genes in ovarian cancer. Oncogene. 2006;25:5446–5461. doi: 10.1038/sj.onc.1209533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terasawa K, Sagae S, Toyota T, Tsukada K, Ogi K, Satoh A, Mita H, Imai K, Tokino T, Kudo R. Epigenetic Inactivation of TMS1/ASC in Ovarian Cancer. Clinical Cancer Research. 2004;10:2000–2006. doi: 10.1158/1078-0432.ccr-0932-03. [DOI] [PubMed] [Google Scholar]
- 12.Yarden I, Brody LC. BRCA1 interacts with components of the histone deacetylase complex. Proceedings of the National Academy of Science USA. 1996;96:4983–4988. doi: 10.1073/pnas.96.9.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finn P, Watkins C, Romero R. Discovery and structure-activity relationships of novel classes of histone deacetylase inhibitors. Proceedings of the American Association of Cancer Research. 43:A3672. [Google Scholar]
- 14.Plumb JA, Finn PW, Williams RJ, Bandara MJ, Romero MR, Watkins CJ, La Thangue NB, Brown R. Pharmacodynamic Response and Inhibition of Growth of Human Tumor Xenografts by the Novel Histone Deacetylase Inhibitor PXD101. Molecular Cancer Therapeutics. 2003;2:721–728. [PubMed] [Google Scholar]
- 15.Gian X, LaRochelle WJ, Guishan A, Wu F, Petersen KD, Thougaard A, Sehested M, Lichenstein HS, Jeffers M. Activity of PXD101, a histone deacetylase inhibitor, in preclinical ovarian cancer studies. Molecular Cancer Therapeutics. 2006;5:2086–2095. doi: 10.1158/1535-7163.MCT-06-0111. [DOI] [PubMed] [Google Scholar]
- 16.Steele N, Plumb J, Vidal L, Plumb J, et al. A phase 1 pharmacokinetic and pharmacodynamic study of the histone deacetylase inhibitor Belinostat in patients with advanced solid tumour. Clin. Cancer Res. 2008;14:804–810. doi: 10.1158/1078-0432.CCR-07-1786. [DOI] [PubMed] [Google Scholar]
- 17.Therasse P, Arbuck S, Eisenhauer EA, et al. European Organization for Research and Treatment of Cancer. National Cancer Institute of the United States. National Cancer Institute of Canada New guidelines to evaluate the response to treatment in solid tumors. J. Natl. Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 18.Rustin GJ, Quinn M, Thigpen T, et al. Re: New guidelines to evaluate the response to treatment in solid tumors (ovarian cancer) J Natl Cancer Inst. 2004;96:487–488. doi: 10.1093/jnci/djh081. [DOI] [PubMed] [Google Scholar]
- 19.Shechter D, Dormann HL, Allis CD, et al. Extraction, purification and analysis of histones. Nature Protocols. 2007;2:1445–1457. doi: 10.1038/nprot.2007.202. [DOI] [PubMed] [Google Scholar]
- 20.Pham NA, Morrison A, Schwock J, et al. Quantitative image analysis of immunohistochemical stains using a CMYK color model. Diagnostic Pathology. 2007;2:8. doi: 10.1186/1746-1596-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simon R. Optimal Two-Stage Designs For Phase II Clinical Trials. Controlled Clinical Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 22.Bell K, Smith Sehdev AE, Kurman R. Refined diagnostic criteria for implants associated with ovarian atypical proliferative serous tumors (borderline) and micropapillary serous carcinomas. Am .J .of Surg. Pathol. 2001;25:419–432. doi: 10.1097/00000478-200104000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Gershenson DM. Clinical management potential tumours of low malignancy. Best Pract Res Clin Obstet Gynaecol. 2002;16:513–527. doi: 10.1053/beog.2002.0308. [DOI] [PubMed] [Google Scholar]
- 24.Cusido M, Balaguero L, Hernandez G, et al. Results of the national survey of borderline ovarian tumors in Spain. Gynecol. Oncol. 2007;104:617–622. doi: 10.1016/j.ygyno.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Modesitt S, Sill MB, Hoffman JS, et al. A phase II study of vorinostat in the treatment of persistent or recurrent epithelial ovarian or primary peritoneal carcinoma:A Gynecologic Oncology Group study. Gynecol. Oncol. 2008;109:182–186. doi: 10.1016/j.ygyno.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Finkler N, Dizon DS, Braly P, et al. Phase II multicentre trial of the histone deacetylase inhibitor (HDACi) Belinostat, carboplatin and paclitaxel (BelCAP) in patients with relapsed epithelial ovarian cancer. J Clin Oncol. 2008 May 20;26(suppl) 2008. abstr 5519. [Google Scholar]
- 27.Woyach JA, Kloos RT, Ringel MD, et al. Lack of therapeutic effect of the histone deacetylase inhibitor Vorinostat in patients with metastatic radioiodine-refractory thyroid carcinoma. J Clin Endocrinol Metab. 2009;94:164–170. doi: 10.1210/jc.2008-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramalingam SS, Belani CP, Reul C, et al. Phase II study of belinostat (PXD101), a histone deacetylase inhibitor, for second line therapy of advanced malignant pleural mesothelioma. J. Thoracic Oncology. 2009;4:97–101. doi: 10.1097/JTO.0b013e318191520c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wiley A, Katsaros D, Chen H, Rigault de la Longrais IA, Beeghly A, Puopolo M, Singal R, Zhang Y, Amoaka A, Zelterman D, Yu H. Aberrant promoter methylation of multiple genes in malignant ovarian tumors and ovarian tumors with low malignant potential. Cancer. 2006;107:299–308. doi: 10.1002/cncr.21992. [DOI] [PubMed] [Google Scholar]
- 30.Jabbour E, Issa J-P, Garcia-Manero G, et al. Evolution of decitabine development: Accomplishments, ongoing investigations, and future strategies. Cancer. 2008;112:2341–2351. doi: 10.1002/cncr.23463. [DOI] [PMC free article] [PubMed] [Google Scholar]