Abstract
This project investigated the use of ultrasonography at first diagnosis of presumptive early bovine respiratory disease (BRD) in feedlot cattle from western Canada. One hundred seventy-four cattle (116 cases and 58 controls) at high risk of developing BRD were enrolled in a prospective longitudinal study over 2 y (2006–2007). Cattle with clinical signs relating to the respiratory system and assessed as sick at the time of feedlot arrival (arrival fever cases) or assessed as sick in the pen 3 to 30 d post-arrival (post-arrival fever cases, post-arrival no fevers cases) were eligible for enrollment. Control animals were identified at the time of case enrollments. Ultrasonography was done using a 3.5 sector transducer at enrollment and at 2, 4, and 6 wk post-enrollment. Lung lesions were identified at least 1 time over the course of the trial in 32/116 (28%) cases and 9/58 (16%) controls. At enrollment, lung lesions were identified in 20/115 (17%) cases and 2/55 (4%) controls (data unreadable n = 4). Post-arrival fever cases (14/48) were the most likely to have a lesion identified using ultrasound. In arrival fever cases, average daily gain (enrollment to last ultrasound, average 34 d) was improved (P = 0.007) in cattle identified with a lesion at enrollment using ultrasound compared with those not identified with a lesion at that time, potentially demonstrating the effects of gut fill at arrival weighing, as these sicker animals may have eaten less prior to arrival and, therefore, had more room for improvement in weight over time due to restoration of normal gut fill. None of the ultrasound time points explored (enrollment, 2, 4, or 6 wk post-enrollment) were associated with the animal health outcomes of interest (subsequent treatment, chronicity, wastage, or mortality) for cattle enrolled at arrival or post-arrival.
Ultrasonography using a 3.5 sector transducer was not particularly effective as a prognostic/diagnostic tool for early detection of BRD, but may be useful in targeted populations of animals with respiratory disease of longer duration (such as chronic pens).
Résumé
Ce projet visait à étudier l’utilisation de l’échographie au moment du premier diagnostic de cas présumés de maladie du complexe respiratoire bovin (CRB) chez des bovins de parcs d’engraissement de l’ouest canadien. Cent soixante-quatorze bovins (116 cas et 58 témoins) à risque élevé de développer CRB ont été inclus dans une étude prospective longitudinale d’une durée de 2 ans (2006–2007). Les bovins avec des signes cliniques associés au système respiratoire et évalués comme malade au moment de leur arrivée au parc d’engraissement (cas fiévreux à l’arrivée) ou évalués comme malade dans les enclos 3 à 30 jours post-arrivée (cas fiévreux post-arrivée, cas non-fiévreux post-arrivée) étaient éligibles à être inclus dans l’étude. Des animaux témoins ont été identifiés au moment du recrutement des cas. L’échographie a été réalisée à l’aide d’une sonde sectorielle 3,5 au moment du recrutement et à 2, 4 et 6 sem post-recrutement. Les lésions pulmonaires ont été identifiées au moins 1 fois durant la durée de l’essai chez 32/116 (28 %) des cas et 9/58 (16 %) des témoins. Au moment du recrutement, les lésions pulmonaires ont été identifiées chez 20/115 (17 %) des cas et 2/55 (4 %) des témoins (données non-traitables n = 4). Les cas fiévreux post-arrivée (14/48) étaient les plus susceptibles à avoir des lésions identifiées par échographie. Chez les cas fiévreux à l’arrivée, le gain moyen quotidien (recrutement à la dernière échographie, moyenne de 34 j) était amélioré (P = 0,007) chez les bovins avec des lésions identifiées par échographie au moment du recrutement comparativement à ceux non-identifiés avec des lésions au même moment, démontrant potentiellement les effets du remplissage intestinal sur la pesée au moment de l’arrivée étant donné que ces animaux plus malades pourraient avoir mangé moins avant leur arrivée et, ainsi, avaient une plus grande marge pour améliorer leur poids en fonction du temps compte tenu du rétablissement du remplissage normal de l’intestin. Aucun des moments où une échographie a été réalisée (recrutement, 2, 4, ou 6 sem post-recrutement) n’était associé à un résultat d’intérêt en rapport avec la santé des animaux (traitement subséquent, chronicité, dépérissement, ou mortalité) pour ceux recrutés à l’arrivée ou post-arrivée.
L’échographie utilisant une sonde sectorielle 3,5 ne s’est pas montrée particulièrement efficace comme outil de pronostic/diagnostic pour la détection hâtive de CRB, mais pourrait être utile dans des populations ciblées d’animaux avec des maladies respiratoires de plus longue durée (enclos d’animaux avec maladie chronique).
(Traduit par Docteur Serge Messier)
Introduction
The clinical syndrome commonly known as bovine respiratory disease (BRD) continues to be one of the most common animal health concerns in commercial feedlot production (1–3), and can result in significant economic losses. It is generally accepted that early recognition and treatment of BRD improves both prognosis and outcome, while delayed diagnosis and treatment may result in treatment failure (4).
The current methods used to diagnose BRD in feedlot cattle can be divided into 2 main categories: subjective and objective methods. The subjective method includes the assessment of respiratory signs (such as cough, nasal discharge, increased respiratory rate or effort) and animal demeanor and behavior by trained feedlot workers (pen checkers). The main objective method is transrectal temperature. The serial use of these 2 types of evaluations is currently the most practical, economically feasible, and common means of recognizing BRD in feedlot cattle. These subjective and objective assessments have limited sensitivity and specificity: demeanor and behavior changes associated with BRD are possible with a variety of diseases; animals (especially those in chronic stages of the disease) may not exhibit clinical signs indicative of BRD; and rectal temperatures can be elevated in healthy individuals or within acceptable limits in animals with BRD. These limitations may lead to unnecessary treatment of clinically normal animals, or delayed or missed detection of BRD in sick animals; both of which have adverse animal well-being and economic implications.
Current field research often focuses on evaluating biological methods of prevention and treatment of BRD in feedlot cattle (5–7). However, few studies focus on improving methods for early recognition and detection of BRD. Ultrasonography is a non-invasive diagnostic tool that has been used to describe and assess respiratory diseases in cattle (8–10). Compared with other diagnostic methods, such as radiography, ultrasonography has been found to be more sensitive in detecting lung consolidation and pleural effusion in cattle and horses (11). Also, when ultrasonographic and lung function indicators were compared with clinical signs and lung pathology in cattle experimentally inoculated with Pasteurella multocida, significant correlations between the ultrasound scores and pathological findings, tidal volumes, and respiratory rates were identified (10). Ultrasound changes associated with respiratory diseases in cattle have been described in the literature (8,9,12); however, the technique has not been adopted as a diagnostic or prognostic tool in commercial feedlot production related to the management of respiratory disease.
In this study, thoracic ultrasonography using a 3.5 sector transducer was evaluated as a diagnostic and prognostic tool at first diagnosis of presumptive early respiratory disease in commercial feedlot cattle. The objective of this study was to determine if live-animal thoracic ultrasound at arrival and at 2-week intervals could be associated with animal health and production outcomes, such as subsequent treatment, chronicity, wastage, mortality, and rate of gain, in groups of cattle.
Materials and methods
Study facilities
Initially, thoracic ultrasonography was conducted at commercial feedlots in southern Alberta to validate the study technique. A prospective longitudinal ultrasound study was then conducted at a commercial feedlot near High River, Alberta with a one-time capacity of 35 000 head. For the longitudinal study, cattle were maintained in large, open-air, dirt-floor pens arranged side by side with central feed alleys and 20% porosity wood-fence windbreaks. Ultrasonography was conducted in feedlot facilities containing a hydraulic chute, an individual animal scale [Animal Weighing and Controlled Sampling (AWACS), Mix-Weigh, Calgary, Alberta], and a chute-side computer for recording animal health data [Feedlot Health Animal Record Management (FHARM), Feedlot Health Management Services Ltd (FHMS), Okotoks, Alberta]. In addition, a percutaneous lung biopsy was collected from each animal in the 2nd y of the study (2007) after every ultrasonography assessment (13). Animals were returned to their home pens once ultrasonography and biopsy were done. This study was approved by the Feedlot Health Management Services Ltd. Animal Care Committee and the University of Saskatchewan Committee on Animal Care and Supply.
Study animals
Candidate animals for the prospective longitudinal study were auction-market derived, exotic crossbred steer or heifer calves at high risk of developing BRD. The same day as arrival at the feedlot, animals were subject to standardized animal health management and feedlot production procedures as per the protocols developed by the feedlot animal health/production consultants (FHMS). In brief, each animal received a unique individual animal identification ear tag, a trial-specific ear tag to help identify individuals for follow-up ultrasonography, a subcutaneous hormonal growth implant in the middle third of the ear, vaccine(s) to immunize against selected bacteria and viruses and a metaphylactic antimicrobial as part of prevention and control strategies for BRD, and application of topical avermectin for internal and external parasite control. There was no difference in the way animals were processed between any of the experimental and control groups except related to antimicrobial treatment, as described in the enrollment section below. Water and standard mixed complete feedlot diets formulated to meet or exceed the National Research Council nutritional requirements for beef cattle were offered ad libitum throughout the feeding period.
Subsequent to arrival processing, animals were housed in designated feedlot pens and managed as per standard feedlot operating procedures. Animals were observed once or twice daily by experienced animal health personnel for evidence of disease. Animals deemed sick by the animal health personnel were moved to a hospital facility, diagnosed, and treated as per the written treatment protocols provided by FHMS. All animal health events including treatment date, presumptive diagnosis, drugs used, and doses used were recorded on the chute-side computer system (FHARM). All animals that died during the study were necropsied by the attending feedlot veterinarian and the cause of death was determined for each animal based on the findings of the gross postmortem examination.
Enrollment
In 2006, 74 animals, weighing between 238 kg (524 lb) and 367 kg (846 lb), were enrolled in the longitudinal study from December 14 to 20. In 2007, 100 animals, weighing between 211 kg (465 lb) and 377 kg (830 lb), were enrolled in the longitudinal study from November 8 to 24. In total, 174 cattle were enrolled in the study. Study animals were followed from enrollment to harvest. Two populations were targeted for enrollment: 1) cattle assessed as sick with BRD at the time of feedlot arrival (arrival fever cases) and 2) cattle identified with clinical illness by animal health personnel during the first 3 to 30 d of the feeding period (post-arrival fever cases, post-arrival no fever cases). In addition, control animals were enrolled at the same time as case subjects. See Appendix I for inclusion criteria for all enrollment groups. All animals maintained their enrollment group throughout the study, regardless of subsequent disease or treatment.
Appendix I.
Inclusion criteria for all enrollment groups
|
Arrival (n= 45): Each arrival fever case (n = 30)
|
|
Post-arrival (n= 129): Each post-arrival fever case (n = 48):
|
Thoracic ultrasonography field validation
Prior to the start of the study, ultrasonography was done on 62 commercial feedlot cattle. Ultrasonography was conducted to determine the most suitable ultrasound machine for field conditions based on ease of use and image quality; 3 different ultrasound machines were evaluated (data not shown). As well, landmarks for the procedure were established in different sized feedlot cattle, the time required to do the procedure under field conditions was estimated, and a rough evaluation of the ability of the ultrasound to detect the presence or absence of gross pulmonary pathology was made. The thoracic ultrasonography images were compared with the gross postmortem findings, histological tissue assessment from live-animal percutanous lung biopsy, or both. Ultrasound results were observed to be consistent with gross postmortem findings based on visual assessment by the attending veterinarian that did both the necropsy and ultrasound.
Based on the image quality, ease of use and features for recording images, the 3.5 MHz sector scanner (InterSonoScan 3.56; Direct Medical Systems, Pleasanton, California, USA) was selected as the most appropriate ultrasound machine for the longitudinal study. It was determined that the 3rd, 5th, and 7th intercostal spaces on the right hemithorax were the most representative and accessible scanning areas for assessing the lungs and thoracic cavity of feedlot cattle. Each intercostal space was scanned from dorsal to ventral. The time required to do thoracic ultrasounds on feedlot cattle under commercial conditions was determined to be 10 min/animal when images and recordings were obtained for all 3 intercostal spaces.
Methodology
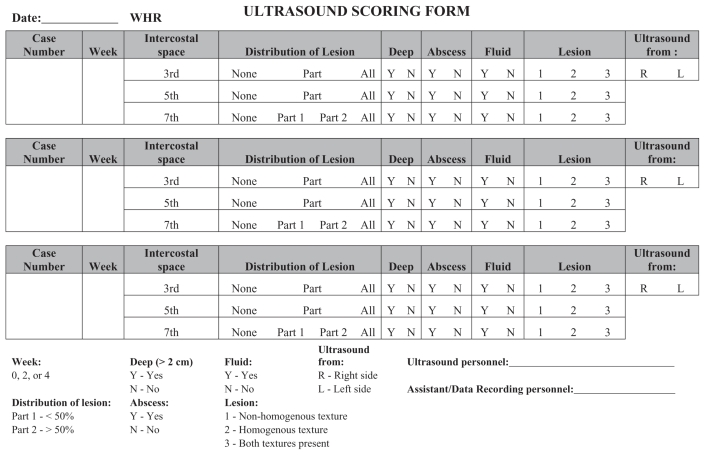
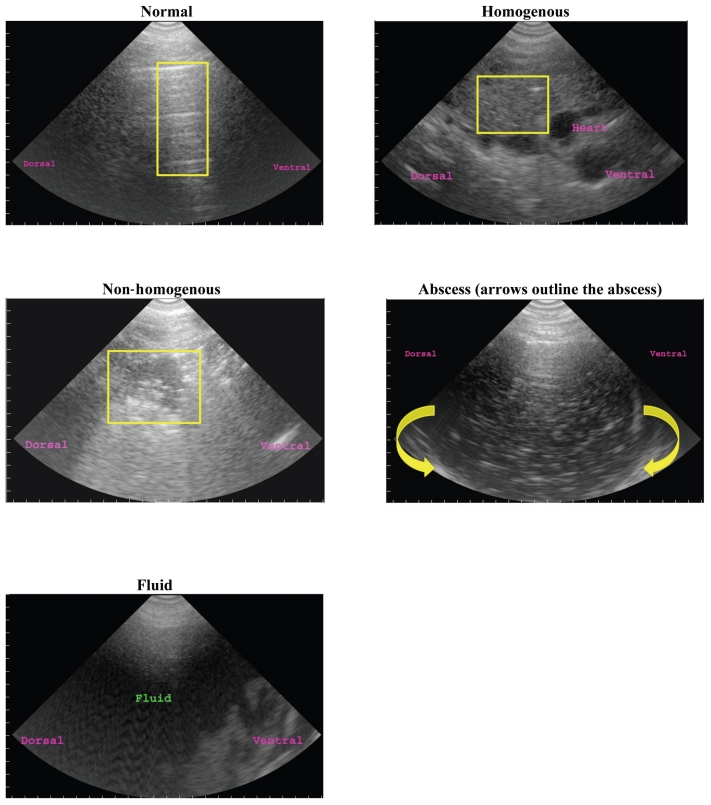
For thoracic ultrasonography, each animal was restrained in a hydraulic chute and an area of hair on the right thorax (approximately 25 cm wide and caudal to the right front leg) was removed using electronic clippers. The area was scrubbed with alcohol, ultrasonographic gel was applied, and the ultrasound was done using the 3.5 sector transducer in a transverse orientation through the 3rd, 5th, and 7th intercostal spaces. Images were recorded for each intercostal space and every set of images was evaluated using a standardized scoring system developed from previously published criteria (8,12,14, Figure 1). The lung was considered normal if the characteristic pattern of well-ventilated lung tissue with a smooth visceral surface was observed and if a pleural reflective band and reverberation artifacts were seen (8). Lung lesions were characterized based on distribution of the lesion over the length of the intercostals space (partial versus complete), the depth of the lesion (≤ 2 cm versus > 2 cm), the textural appearance of the lesion (homogenous versus non-homogenous), the presence of an abscess, and the presence of fluid. A lesion was considered homogenous when fine-grained, homogenous, echogenic zones were seen. The lesion was considered non-homogenous when anechoic and hyper-echoic coarse-grained zones were seen. An abscess was considered to be present when anechoic or hypoechoic zones, sometimes mixed with hyperechoic dots, were surrounded by a well circumscribed hyperechoic band. Fluid was considered to be present when an anechoic zone was seen. See Figure 2 for labeled images of thoracic ultrasonographic images.
Figure 1.
Ultrasound scoring form.
Figure 2.
Ultrasonographic image examples from study.
Data collection and management
All numerical study data were subsequently compiled, collated in a computer spreadsheet program (Microsoft Office Excel; Microsoft Corporation, Redmond, Washington, USA), and verified. The ultrasound findings from each animal were recorded on a standard data collection template at each time point (Figure 1). The veterinarian evaluating the images was blinded to the enrollment groups of study animals.
Statistical analysis
All analyses were done using a commercial software program (SAS for Windows, release 9.1; SAS Institute, Cary, North Carolina, USA). Frequency distributions and descriptive statistics were calculated for all design variables and animal health outcomes. In unconditional analyses (1 explanatory variable and the outcome in the model), each lung lesion explanatory variable (lesion at enrollment, lesion at 2 wk, at 4 wk, and at 6 wk post-enrollment) was tested for potential associations with animal health [subsequent treatment for any disease including BRD, subsequent treatment for BRD (a subset of the above that describes only BRD treatments), chronicity, wastage, and mortality from all causes] and production outcomes [average daily gain (ADG) from enrollment to last ultrasound — average 34 d, and ADG from enrollment to final rehandling — average of 128 d]. See Appendix II for description of explanatory and outcome variables. In addition, enrollment group comparisons were made with lesion at enrollment as the outcome variable. Parameters were considered associated with an outcome if the P-value obtained was < 0.050.
Appendix II.
Definitions of groups, explanatory variables and outcome variables explored in analysis
| Enrollment parameters | |
| Enrollment groups | Arrival: Cattle designated as arrival fever cases or arrival controls. |
| Post-arrival: Cattle designated as post-arrival fever cases, post-arrival no fever cases, or post-arrival controls. | |
| Animal health and production outcomes | |
| Subsequent treatment-ANY | Animals treated for any disease (including BRD) post-enrollment. |
| Subsequent treatment-BRD | Animals treated for bovine respiratory disease (BRD) post-enrollment. |
| Chronicity | Animals that were treated 3 times after arrival for the same disease, or animals designated as unable to return to their pens due to sickness by animal health personnel. |
| Wastage | Animals that developed chronic disease post-enrollment but did not die during the study. |
| Mortality | Animals that died during the study but were not sacrificed as part of the study design. |
| ADG to last ultrasound | Average daily gain: weight difference (kg) from enrollment to last ultrasound date divided by number of days for the same time frame (average 26 d, range 23–49 d post-enrollment). |
| ADG to final rehandling | Average daily gain: weight difference (kg) from enrollment to last feedlot management date (last date weighed) divided by number of days for the same time frame (average 28 d, range 112–140 d post-enrollment). |
| Ultrasonography lung lesion explanatory variables | |
| Lesion at enrollment | Whether or not a lung lesion was identified using thoracic ultrasonography at the time of enrollment. |
| Lesion at 2 wk | Whether or not a lesion was identified using thoracic ultrasonography 2 wk post-enrollment. |
| Lesion at 4 wk | Whether or not a lesion was identified using thoracic ultrasonography 4 wk post-enrollment. |
| Lesion at 6 wk | Whether or not a lesion was identified using thoracic ultrasonography 6 wk post-enrollment. |
| Lesion at enrollment was an outcome in some models and an explanatory variable in other models. | |
For animal health outcomes, Poisson regression in a log-linear model using generalized estimating equations and controlling for clustering of observation within pens was conducted (15). Associations with production outcomes were assessed using least squares analysis of variance and accounting for pen-level clustering (16). Year was tested as a potential covariate in all models and days on feed (DOF) was explored as a potential covariate in the ADG models; a covariate was retained in a model if it was statistically significant at a P < 0.050 level in unconditional analysis. For the ADG outcomes, animals that died of natural causes or were sacrificed for study purposes were excluded, as the question of interest was whether identifying a lesion using ultrasonography was associated with ADG in animals that lived.
Results
One hundred seventy-four animals were examined for lung lesions (45 arrival, 129 post-arrival) using ultrasounds. The categorical distributions of animals by enrollment group for animal health and production outcomes are summarized in Table I. In 2006, data were available for all 74 cattle enrolled in the study at all 4 time points (enrollment, 2, 4, and 6 wk post-enrollment); 22 animals had lesions identified at least once over the 4 assessments, see Table II. Four animals had lesions identified at every time point, and 3 of these 4 were classified as cases (had an increased rectal temperature, clinical signs of BRD, or both) at the time of enrollment to the study. In 2007, data were unavailable for 4 animals at enrollment, 91 animals at 2 wk, 4 animals at 4 wk, and 100 animals at 6 wk post-enrollment due to readability of the ultrasonographic recordings and malfunctioning of the equipment at the 2 and 6 wk time points.
Table I.
Animal numbers by outcomes of interesta
| Enrollment | Animal health outcomeb | Production outcomesc | ||||||
|---|---|---|---|---|---|---|---|---|
| Group | N | ST-ANYd | ST-BRDe | Chronicity | Wastage | PMf | ADG-USg | ADG-Finalh |
| Arrival | ||||||||
| Arrival fever cases | 30 | 3/30 | 2/30 | 0/30 | 0/30 | 2/30 | 28/30 | 28/30 |
| Arrival controls | 15 | 5/15 | 5/15 | 2/15 | 2/15 | 0/15 | 14/15 | 14/15 |
| Post-arrival | ||||||||
| Post-arrival fever cases | 48 | 5/48 | 3/48 | 2/48 | 1/48 | 3/48 | 45/48 | 43/48 |
| Post-arrival no fever cases | 38 | 5/38 | 2/38 | 2/38 | 2/38 | 0/38 | 38/38 | 37/38 |
| Post-arrival controls | 43 | 4/43 | 1/43 | 2/43 | 2/43 | 1/43 | 40/43 | 38/43 |
See Appendix 2 for definitions.
Animal health outcomes: number of animals with the animal health outcome divided by the number of animals enrolled.
Production outcomes: number of animals with available data divided by the number of animals enrolled.
ST-ANY is subsequent treatment for any disease (including bovine respiratory disease, BRD).
ST-BRD is subsequent treatment for BRD.
PM is mortality from any cause.
ADG-US is average daily gain (lb/animal/day) from enrollment to last ultrasound (average 34 d).
ADG-Final is average daily gain (lb/animal/day) from enrollment to final rehandling (average 128 d).
Table II.
Lesions identified in animals using ultrasound at the 4 time points in 2006a
| Animal | Enrollment subgroup | LAE | 2 wk | 4 wk | 6 wk |
|---|---|---|---|---|---|
| G8267 | Arrival fever case | Yes | Yes | Yes | Yes |
| G7992 | Arrival fever case | Yes | No | No | No |
| G8172 | Arrival fever case | Yes | No | No | No |
| G7955 | Arrival fever case | No | Yes | No | No |
| B3655 | Post-arrival fever case | Yes | Yes | Yes | Yes |
| B3874 | Post-arrival fever case | Yes | Yes | Yes | No |
| B3759 | Post-arrival fever case | Yes | No | Yes | No |
| B3502 | Post-arrival fever case | Yes | No | No | No |
| B3979 | Post-arrival fever case | Yes | No | No | Yes |
| B4023 | Post-arrival fever case | Yes | No | No | Yes |
| B3853 | Post-arrival fever case | Yes | No | No | No |
| B4166 | Post-arrival fever case | Yes | No | No | No |
| G7933 | Post-arrival fever case | No | No | No | Yes |
| B3698 | Post-arrival no fever case | Yes | Yes | Yes | Yes |
| B3847 | Post-arrival no fever case | Yes | No | No | No |
| G7585 | Post-arrival no fever case | Yes | No | No | No |
| B3739 | Post-arrival no fever case | No | Yes | No | No |
| G8029 | Post-arrival no fever case | No | Yes | No | No |
| G8062 | Post-arrival no fever case | No | No | Yes | Yes |
| B3555 | Post-arrival control | No | Yes | No | No |
| B3557 | Post-arrival control | No | Yes | No | No |
| B4277 | Post-arrival control | Yes | Yes | Yes | Yes |
Includes animals identified with a lesion and examined using ultrasonography at each of the 4 time points in the study in 2006; 52 animals enrolled in that same year were examined at all 4 time points but were not identified with a lesion at any time.
LAE — lesion at enrollment.
For arrival and post-arrival groups, 1 control animal was humanely euthanized for every 2 cases that died so that postmortem changes in cases could be objectively assessed in relation to tissue changes in non-case animals (Table III). Only the 6 animals that died naturally were included in the assessment of mortality. The 9 animals that died or were euthanized were excluded from the ADG data, and another 4 animals in 2007 were missing body weights for the calculation of ADG to final rehandling.
Table III.
Description of animal mortality over the course of the triala
| Tag # | Enrollment | LAE | ST-ANY | ST-BRD | Wastage | Mortality | Postmortem findings |
|---|---|---|---|---|---|---|---|
| 2006 | |||||||
| G8026 | Post-arrival control | Absent | No | No | No | Died | congestive heart failure |
| 2007 | |||||||
| W6624 | Arrival fever case | Absent | Yes | No | No | Died | pneumonia and arthritis |
| W6174 | Arrival fever case | Absent | No | No | No | Died | no visible lesions |
| W6625 | Arrival control | Absent | Yes | Yes | No | Sacb | no visible lesions |
| R4794 | Post-arrival fever case | Absent | Yes | Yes | No | Died | myocarditis |
| O6790 | Post-arrival fever case | Absent | Yes | No | Yes | Died | chronic fibrotic pleuritis |
| W5838 | Post-arrival fever case | Present | Yes | No | No | Died | chronic fibrotic pleuritis |
| W5309 | Post-arrival control | Absent | No | No | No | Sacb | no visible lesions |
| W6695 | Post-arrival control | Absent | No | No | No | Sacb | no visible lesions |
See Appendix 2 for definitions of variables.
Sac: For every 2 case deaths, 2 control animal was sacrificed to compare gross postmortem examination findings between the case and non-case animals.
LAE — lesion at enrollment; ST-ANY — subsequent treatment for any disease (including bovine respiratory disease, BRD); ST-BRD — subsequent treatment for BRD.
A summary of the number of animals identified with lung lesions using ultrasonography over the course of the study is found in Table IV; lung lesions were identified at least once in 32/116 (28%) cases and 9/58 (16%) controls. At enrollment, lung lesions were identified in 17% (20/115) of cases and 4% (2/55) of controls; data were unreadable for 4 cattle. In approximately half (20/41) of cattle identified with lung lesions, those same lesions were not identified in subsequent ultrasound time points based on 2 or more ultrasound assessments. Abscesses were found using ultrasonography in 13 animals (32% of those with lesions) and lung lesions were characterized as deep in 23 animals. Fluid was identified in the thoracic cavity of 16 cattle over the course of the study; 14 animals had accompanying lesions while 2 animals were identified with fluid only. The presence of fluid alone was not considered a lesion as it can be a normal ultra-sonographic finding in the thorax of some healthy cattle and other indications of thoracic disease (fibrin, pleuritis) were not observed using ultrasonography in these cases.
Table IV.
| Enrollment group | LAE | 2 wk | 4 wk | 6 wk |
|---|---|---|---|---|
| Arrival | ||||
| Arrival fever cases | 3/29 | 3/7 | 2/30 | 1/3 |
| Arrival controls | 1/14 | 1/4 | 3/15 | 0/2 |
| Post-arrival group | ||||
| Post-arrival fever cases | 14/48 | 2/22 | 5/46 | 4/22 |
| Post-arrival no fever cases | 3/38 | 4/26 | 5/38 | 2/24 |
| Post-arrival controls | 1/41 | 3/24 | 2/41 | 1/23 |
Lesions are not necessarily in the same animals over time.
Number of animals identified with lesions using ultrasound divided by the number of animals tested with ultrasound at the different time points.
LAE — lesion at enrollment.
In exploration of both the arrival and post-arrival groups, associations between the lung lesion explanatory variables and the animal health outcomes were either not statistically significant (P ≥ 0.050) or would not converge due to the small number of events (Table V). However, for arrival fever cases, ADG (enrollment to last ultrasound, average 34 d) was improved (P = 0.007) if animals had lesions identified at enrollment using ultrasound compared with those not identified with lesions at that time. As well, post-arrival fever cases were approximately 12 times more likely to have lesions at enrollment than post-arrival controls [95% confidence interval (CI) 2.34 to 61.07, P = 0.003], and approximately 4 times more likely to have lesions at enrollment than post-arrival no fever cases (95% CI 1.38 to 9.97, P = 0.010, Table VI).
Table V.
Associations between the identification of a lesion at enrollment using ultrasonography and average daily gaina
| Outcomeb | LAE present mean ADG | LAE absent mean ADG | Average difference | 95% CI of the difference | P-value |
|---|---|---|---|---|---|
| Arrival fever cases | |||||
| ADG to last ultrasound | 2.35 | 1.32 | 1.03 | 0.31 to 1.74 | 0.007 |
| ADG to final rehandling | 2.00 | 1.78 | 0.22 | −0.17 to 0.61 | 0.257 |
| Arrival controls | |||||
| ADG to last ultrasound | 2.49 | 1.48 | 1.01 | −0.73 to 2.75 | 0.226 |
| ADG to final rehandling | 2.15 | 1.83 | 0.32 | −0.27 to 0.90 | 0.256 |
| Post-arrival fever cases | |||||
| ADG to last ultrasoundc | 2.18 | 2.06 | 0.12 | −0.21 to 0.44 | 0.477 |
| ADG to final rehandling | 2.03 | 1.86 | 0.17 | −0.02 to 0.35 | 0.084 |
| Post-arrival no fever cases | |||||
| ADG to last ultrasound | 2.01 | 1.88 | 0.13 | −0.57 to 0.83 | 0.713 |
| ADG to final rehandlingd | 2.05 | 1.81 | 0.24 | −0.12 to 0.59 | 0.185 |
| Post-arrival controls | |||||
| ADG to last ultrasoundc | 2.02 | 2.12 | −0.10 | −1.07 to 0.87 | 0.833 |
| ADG to final rehandlingd | 1.47 | 1.84 | −0.37 | −0.86 to 0.11 | 0.128 |
Data were analyzed using mixed models for lesion at enrollment group effects and corrected for intra-pen clustering of observations.
ADG is average daily gain (kg/animal/day) from enrollment to last ultrasound (average 34 d), or from enrollment to final rehandling (average 128 d).
Models include days on feed as a covariate.
Models include year as a covariate.
LAE — lesion at enrollment; CI — confidence interval.
Table VI.
Associations between enrollment groups and identification of lesions at enrollment using ultrasonography
| Comparison groups (LAE present) | Relative riska | 95% CI | P-value |
|---|---|---|---|
| Case versus controls | |||
| Arrival fever cases versus arrival controls | 1.45 | 0.27 to 7.67 | 0.663 |
| Post-arrival fever cases versus post-arrival controls | 11.96 | 2.34 to 61.07 | 0.003 |
| Post-arrival no fever cases versus post-arrival controls | 3.24 | 0.33 to 31.42 | 0.311 |
| Post-arrival fever cases versus post-arrival no fever cases | 3.69 | 1.37 to 9.97 | 0.010 |
| Case versus case | |||
| Arrival fever cases versus post-arrival cases (fever and no fever cases combined) | 0.52 | 0.13 to 2.04 | 0.352 |
Relative risk is the ratio of the rate of disease in the first group divided by the rate of disease in the second group in that comparison.
LAE — lesion at enrollment; 95% CI — 95% confidence interval calculated for each relative risk using Poisson regression in a log linear model for comparison group effects and correcting for intra-pen clustering of disease with generalized estimating equations.
Discussion
Despite arriving into the feedlots during the fall of the year, few morbidity or mortality events occurred in the cattle in this study. While the identification of lung lesions at enrollment using 3.5 sector transducer was not associated with subsequent animal health outcomes (treatment, wastage, or mortality), several interesting findings were identified during the study. In 49% (20/41) of cattle identified with lung lesions, those same lesions were not identified in subsequent ultrasound sessions. This may reflect success of treatment protocols (lesion resolved), lack of sensitivity of the ultrasound methodology to identify lesions that may be in the process of resolving, or lack of sensitivity due to the requirement of limiting the procedure time (10 min per animal) in the commercial production setting. Of the 4 time points evaluated, the most relevant to commercial feedlot production for ease of implementation and significance of findings would likely be at enrollment. That said, the length of time required to evaluate each animal (10 min per animal) would significantly reduce arrival processing efficiency, and would not be generally adopted without the tool providing robust, repeatable, and economically significant information about animal health.
In arrival fever cases, cattle diagnosed with lung lesions at enrollment had significantly better short-term rates of gain (ADG to last ultrasound) compared with animals without lung lesions. A possible explanation for this finding may be that cattle with lung lesions at enrollment have relatively less gut fill due to sickness and inappetance compared with cattle without lung lesions at enrollment, which could artificially reduce enrollment weight and increase apparent post-enrollment weight gain. However, for the long-term production parameter evaluated (ADG to final rehandling, average 128 d) the presence of a lung lesion at enrollment did not significantly affect rate of gain in any of the groups in the study.
Interestingly, ultrasonography identified lung lesions at enrollment in 29% of post-arrival fever cases compared with 10% of arrival fever cases. Arrival fever cases were enrolled in the study based primarily on elevated rectal temperature at processing; a vague clinical finding that may result from excitement or stress in addition to clinical disease. Post-arrival fever cases were enrolled based on primary BRD identification by pen checkers and subsequent confirmation of elevated rectal temperature. In order for sick cattle to have been identified by pen checkers amidst the healthy cattle in the pen, the post-arrival fever cases may have been sick for a time prior to identification, and, as such, may have had more severe pathology than those animals identified as sick at feedlot arrival. Therefore, it is perhaps not surprising that more lung lesions were identified in the post-arrival fever cases, a population of potentially sicker animals compared with the arrival fever cases.
Ultrasonography identified lung lesions at enrollment in only 8% of post-arrival no fever cases. However, these cattle exhibited clinical signs of BRD that were similar, if not identical, to the cattle enrolled as post-arrival fever cases. In spite of this, the rectal temperatures of animals in the post-arrival no fever cases group were lower than those post-arrival fever cases, which may be indicative of less severe disease. These results suggest that clinical assessment combined with measurement of rectal temperature may be used successfully to classify animals into groups with higher and lower probabilities of lung lesions based on thoracic ultrasonography.
This study successfully assessed the utility of thoracic ultrasonography in commercial feedlot cattle related to the first diagnosis of presumptive BRD. Parenchymal lung lesions, abscesses, and thoracic fluid were visualized using a 3.5 sector ultrasound transducer. Based on thoracic ultrasonography at enrollment, animals diagnosed with severe clinical BRD (post-arrival fever cases) were more likely to have lung lesions than animals diagnosed with less severe clinical disease (post-arrival no fever cases or post-arrival controls). However, the identification of lung lesions at enrollment was not associated with subsequent animal health outcomes (treatment, wastage, or mortality) in any of the explorations, and ultrasonography, therefore, would not be considered useful in general commercial production settings. Nonetheless, the technique developed in this study may be useful as diagnostic and prognostic aid in targeted populations of animals with respiratory disease of longer duration, such as cattle within feedlot chronic pens, where the number of animals to be assessed would be smaller and the time required to do the ultrasound would be more acceptable as part of commercial production practices.
Acknowledgments
This project was wholly supported by a research grant from Alberta Beef Producers, Calgary, Alberta. The authors thank the management and staff of Western Feedlots Ltd., High River, Alberta, for their assistance and cooperation in conducting this project, and Ms. Lindsay Palmer (FHMS) for her work associated with the collection of ultrasound images over the course of this project.
References
- 1.Edwards TA. Control methods for bovine respiratory disease for feedlot cattle. Vet Clin Food Anim. 2010;26:273–284. doi: 10.1016/j.cvfa.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Gagea MI, Bateman KG, van Dreumel T, et al. Diseases and pathogens associated with mortality in Ontario beef feedlots. J Vet Diagn Invest. 2006;18:18–28. doi: 10.1177/104063870601800104. [DOI] [PubMed] [Google Scholar]
- 3.Woolums AR, Loneragan GH, Hawkins LL, Williams SM. Baseline management practices and animal health data reported by US feedlots responding to a survey regarding acute interstitial pneumonia. Bov Prac. 2005;39:116–124. [Google Scholar]
- 4.Smith RA, Stokka GL, Radostits OM, Griffin DD. Health and production management in beef feedlots. In: Radostits O, editor. Herd Health: Food Animal Production. Vol. 14. Philadelphia, Pennsylvania: WB Saunders; 2001. pp. 581–633. [Google Scholar]
- 5.Apley M. Bovine respiratory disease: Pathogenesis, clinical signs and treatment in lightweight calves. In: Spire MF, Smith RA, editors. Veterinary Clinics Food Animal Practice. Vol. 22. Philadelphia, Pennsylvania: 2006. pp. 399–411. [DOI] [PubMed] [Google Scholar]
- 6.Wildman BK, Jim GK, Perrett T, et al. A comparison of two multivalent viral vaccine programs in feedlot calves at high risk of developing undifferentiated fever/bovine respiratory disease. Bov Pract. 2009;43:130–139. [Google Scholar]
- 7.Nickell JS, White BJ. Metaphylactic antimicrobial therapy for bovine respiratory disease in stocker and feedlot cattle. Vet Clin Food Anim. 2010;26:285–310. doi: 10.1016/j.cvfa.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Rabeling B, Rehage J, Döpfer D, Scholz H. Ultrasonographic findings in cattle with respiratory disease. Vet Rec. 1998;143:468–471. doi: 10.1136/vr.143.17.468. [DOI] [PubMed] [Google Scholar]
- 9.Flöck M. Diagnostic ultrasonography in cattle with thoracic disease. Vet J. 2004;167:272–280. doi: 10.1016/S1090-0233(03)00110-2. [DOI] [PubMed] [Google Scholar]
- 10.Reinhold P, Rabeling B, Günther H, Schimmel D. Comparative evaluation of ultrasonography and lung function testing with the clinical signs and pathology of cattle inoculated experimentally with Pasteurella multocida. Vet Rec. 2002;150:109–114. doi: 10.1136/vr.150.4.109. [DOI] [PubMed] [Google Scholar]
- 11.Reef VB, Boy MG, Reid CF, Elser A. Comparison between diagnostic ultrasonography and radiography in the evaluation of horses and cattle with thoracic disease: 56 cases (1984–1985) J Am Vet Med Assoc. 1991;198:2112–2118. [PubMed] [Google Scholar]
- 12.Braun U, Sicher D, Pusterla N. Ultrasonography of the lungs, pleura, and mediastinum in healthy cows. Am J Vet Res. 1996;57:432–438. [PubMed] [Google Scholar]
- 13.Burgess BA, Hendrick SH, Pollock CM, Abutarbush SM, Vogstad A, Jim GK, Booker CW. The development of a percutaneous lung biopsy procedure for use on feedlot steers. Can J Vet Res. 2011;75:254–260. [PMC free article] [PubMed] [Google Scholar]
- 14.Anzböck W, Stellamor K, Braun U, Hruby W. Sonography of the lungs and pleura. Rofo. 1990;153:278–282. doi: 10.1055/s-2008-1033379. [DOI] [PubMed] [Google Scholar]
- 15.Dohoo I, Martin W, Stryhn H. Alternative Approaches to dealing with clustered data. In: McPike SM, editor. Veterinary Epidemiologic Research. Charlottetown, Prince Edward Island: AVC Inc; 2003a. pp. 521–540. [Google Scholar]
- 16.Dohoo I, Martin W, Stryhn H. Mixed Models for Continuous Data. In: McPike SM, editor. Veterinary Epidemiologic Research. Charlottetown, Prince Edward Island: AVC Inc; 2003b. pp. 473–496. [Google Scholar]