Abstract
The objective of this study was to compare bone marrow (BM) aspirates from the sternum and the tuber coxae of middle-aged horses. Bone marrow was obtained from the sternum and both tubera coxae of 12 healthy, 13-year-old geldings. Two different puncture techniques were used for the tuber coxae. The 2 syringes used for sternal sampling were evaluated separately. The mononuclear cell (MNC) fraction of the BM was isolated and the mesenchymal stem cells (MSCs) were culture-expanded. At the sternum, BM aspiration was always possible. Bone marrow aspiration at the tuber coxae required straight and deep needle penetration combined with high negative pressure. With this technique a median sample amount of 11.0 mL with large individual variation was obtained. A median of 3.06 × 106 MNC/mL BM (1st syringe) and 2.46 × 106 MNC/mL BM (2nd syringe) was isolated from sternal samples. In contrast, the tuber coxae yielded a median of 0.27 × 106 MNC/mL BM. The first passage yielded a median of 2.19 × 106 MSC (1st syringe) and 1.13 × 106 MSC (2nd syringe) from sternal samples, compared to a significantly lower median number of MSC from tuber coxae BM (0.06 × 106 MSC). The number of MNC and MSC obtainable from the BM aspirates taken from the tuber coxae is significantly lower than that obtained from the sternal BM aspirates. Autologous BM for the equine athlete is particularly clinically relevant at an advanced age. Based on our findings, the tuber coxae cannot be recommended for BM aspiration in middle-aged horses.
Résumé
Cette étude visait à comparer les aspirations de moelle osseuse (MO) provenant du sternum et de la tubérosité de la hanche de chevaux d’âge moyen. La MO fut obtenue du sternum et des deux tubérosités de la hanche de 12 hongres âgés de 13 ans. Deux techniques de ponctions différentes ont été utilisées pour la tubérosité de la hanche. Les 2 seringues utilisées pour l’échantillonnage du sternum furent évaluées séparément. La fraction des cellules mononucléaires (MNC) de la MO a été isolée et les cellules souches mésensychamenteuses (CSM) ont été multipliées par culture. À partir du sternum, les aspirations de MO étaient toujours possibles. L’aspiration de moelle osseuse à partir de la tubérosité de la hanche nécessitait de combiner une pénétration droite et profonde de l’aiguille ainsi qu’une pression négative élevée. Au moyen de cette technique une quantité médiane de 11,0 mL d’échantillon était obtenue, avec de grandes variations individuelles. Une quantité médiane de 3,06 × 106 CSM/mL de MO (1ère seringue) et de 2,46 × 106 CSM/mL de MO (2e seringue) était obtenue à partir des échantillons du sternum. Ceci contrastait avec la médiane de 0,27 × 106 CSM/mL de MO obtenue de la tubérosité de la hanche. Une médiane de 2,19 × 106 CSM (1ère seringue) et de 1,13 × 106 CSM (2e seringue) a été obtenu des échantillons provenant du sternum, comparativement à une quantité médiane significativement moindre de MSC provenant de la MO de la tubérosité de la hanche (0,06 × 106 CSM). Le nombre de cellules mononucléaires et de CSM pouvant être obtenues à partir d’aspiration de MO provenant de la tubérosité de la hanche est significativement plus bas que celui obtenu à partir d’une aspiration de MO provenant du sternum. Pour un athlète équin, de la MO homologue a une signification clinique particulière chez un animal plus âgé. Selon nos trouvailles, la tubérosité de la hanche ne peut être recommandée pour une aspiration de MO chez des chevaux d’âge moyen.
(Traduit par Docteur Serge Messier)
Introduction
The aspiration of BM and its use in various forms for regenerative treatment have gained increased attention in recent years. The two sites described for obtaining BM in horses are the sternum (1) and the tuber coxae (2,3). While most equine veterinarians probably prefer the sternum, this site forces them to assume an unsafe position when performing this procedure. Furthermore, severe complications have been reported following sternal BM aspiration (4). In principle, all bones containing BM can be used if they are accessible for transcutaneous puncture. The ilium (tuber coxae or iliac crest) is used in humans (5,6), in dogs and cats (7), and in small ruminants and pigs (8) used for experimental purposes.
If aspiration at the tuber coxae is as efficient as sternal aspiration, it would be a safe alternative for both the equine veterinarian and the horse. Therefore, the goal of this study was to compare BM aspirates from the sternum with those taken from the tuber coxae in terms of yield of marrow and obtainable MSC in a group of healthy, middle-aged horses.
Materials and methods
In-vivo sampling
Twelve geldings (13 y of age, mean body weight of 580 kg) were included in this study (2 Thoroughbreds, 2 Standardbreds, and 8 Warmbloods). The horses belonged to a research herd and were kept on pasture at the time of sampling. On physical examination of the horses before BM aspiration, all parameters were within normal limits. None of the horses had undergone BM aspiration before. Aspirations were performed at the right and left tuber coxae at 2 separate times.
Before the procedure, each horse was sedated with 0.06 mg/kg romifidine (Sedivet; Boehringer Ingelheim, Ingelheim, Germany) and 0.02 mg/kg body weight (BW) butorphanol (Torbugesic; Fort Dodge Veterinär, Würselen, Germany). The preparation for puncture was the same at all sites: the ventral chest wall and the point of the hips were clipped with a No. 40 blade and the skin was aseptically prepared using iodine soap and alcohol. The soft tissue overlying the targeted bones was anesthetized with 6 mL 2% lidocaine (Lidocain HCl 2%; Bela-Pharm, Vechta, Germany) and incised using a No. 15 scalpel blade. The sternum was punctured before the tuber coxae. The puncture site for BM aspiration at the sternum was located in the sternal midline, about 1 hand caudal to the olecranon. The trocar with the sharp obturator of an 11-ga and 10-cm long BM biopsy needle (Bone Marrow Harvest Needle; Angiotech, Gainsville, Florida, USA) was inserted perpendicularly into the skin incision and advanced with gentle force and rotary to-and-fro motion approximately 2 cm into the bone. After the obturator was removed, a heparinized 12-mL syringe [5000 IU Heparin-Sodium/10 mL BM aspirate (Heparin-Natrium Braun; B. Braun, Melsungen, Germany)] was attached and BM was aspirated. If possible, a 2nd heparinized syringe was filled.
For BM aspiration at the right tuber coxae, the puncture site was located in the center of the tuber coxae. The biopsy needle was pointed in a medial and slightly ventrocaudal direction. After the needle was advanced approximately 5 cm into the bone, the sharp obturator was removed. Again, a heparinized 12-mL syringe was attached and BM was aspirated. If no BM was obtainable, the needle was redirected at least twice within the bone. Negative pressure was applied for at least 1 min before the procedure was terminated.
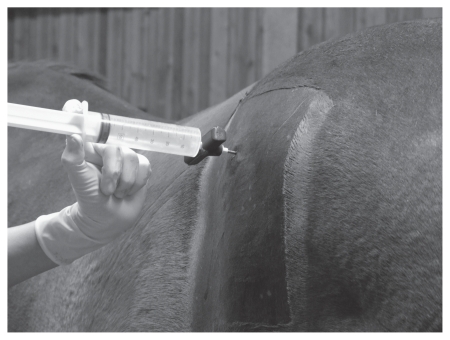
A modified technique was used for BM aspiration at the left tuber coxae. The puncture site was identical to that on the right side, with the biopsy needle pointing straight in a medial direction, perpendicular to the horse’s midline. The needle was advanced up to 8 cm into the bone. For aspiration, a 30-mL or 50-mL heparinized syringe was used (Figure 1). If necessary, the needle was redirected and negative pressure was applied. The maximum amount of BM obtainable was sampled. If no BM could be extracted, the procedure was terminated after 2 min. All amounts of BM aspirated were recorded.
Figure 1.
Aspiration at the left tuber coxae using a 50-mL syringe, placing the bone marrow aspiration needle straight and advancing it up to 8 cm into the bone.
The horses and the site of aspiration were closely monitored for at least 3 d after the procedure. All procedures used in this study were approved by the State Animal Care Committee.
In-vitro processing of the bone marrow aspirates
Bone marrow aspirates were processed within 8 h after sampling. Two methods were used to isolate the MNC fraction. Red blood cell (RBC) lysis was used to isolate MNCs from sternal samples and from the samples from the right tuber coxae. Density gradient centrifugation was used to isolate MNCs from the left tuber coxae samples. The 2 syringes with sternal BM were processed separately. Bone marrow from the left tuber coxae was pooled.
Red blood cell lysis was performed by resuspending the BM aspirate in phosphate buffered saline (PBS; Biochrom, Berlin, Germany) at a ratio of 1:2 and pouring it through a 100-μm cell strainer (BD Biosciences, Erembodegem, Belgium). The suspension was centrifuged [2000 × g, 10 min, room temperature (RT)] and the plasma supernatant was removed. The remnant obtained underwent RBC lysis adding buffered erylysis solution (ammonium chloride; Riedel-de Haën, Seelze, Germany), potassium hydrogencarbonate (Kaliumhydrogencarbonat; Sigma-Aldrich Chemie, Taufkirchen, Germany), ehtylenediamine tetra-acetic acid (EDTA) (Fluka; Feinchemiekalien, Sigma-Aldrich Chemie), aqua bidest (MembraPure, Bodenheim, Germany) and careful resuspension of the cell pellet. After 5 min, the material was centrifuged again (2000 × g, 10 min, RT) and the supernatant was removed. The cells obtained were washed twice in Dulbecco’s Modified Eagle Medium (DMEM +/− Glutamax; Gibco Invitrogen, Paisley, United Kingdom). The MNCs obtained were counted manually and plated in T175 cm2 tissue culture flasks in culture medium, DMEM Glutamax, supplemented with 10% fetal calf serum (Sigma-Aldrich Chemie) and 1% Penicillin/Streptomicin (PAA, Pasching, Austria), and incubated at 37°C in 5% CO2. Media were changed first after 1 to 2 d and then every 3 to 4 d. All samples were passaged first after 15 d and a second passage was performed when cells reached 80% confluency.
For density gradient centrifugation, the BM was resuspended in PBS (ratio of BM to PBS 1:2), loaded onto 70% Ficoll gradient solution (Ficoll-Paque Premium; GE Healthcare, Uppsala, Sweden), and centrifuged (328 × g, 30 min, RT). The MNCs were collected from the interface and washed twice in PBS and centrifuged (437 × g, 5 min, 10°C). The MNCs obtained were counted manually and placed in cell culture flasks at a density of 4 × 105/cm2 and cultured as previously described. First and second passage were performed when 80% confluency was reached.
Generation time between first and second passage was calculated for all MSC lines to allow comparison of growth characteristics between the 2 aspiration sites (9). Briefly, generation time was calculated using the following equation:
(tg = generation time; t = duration of culture time at the time of evaluation; N = number of cells at the time of evaluation; N0 = number of cells at the time point 0).
Statistical analysis
Statistical analysis was performed with SPSS for Windows (Version 18.0; SPSS, Chicago, Illinois, USA) using a Wilcoxon signed rank test and a Mann-Whitney rank sum test to compare the median obtained MNC and MSC count and the median generation time, respectively. Significance was set at P < 0.05 for all tests.
Results
In-vivo sampling
All horses tolerated the procedure well. None of the horses showed any signs of discomfort or wound morbidity after the procedure and throughout the following days. Bone marrow aspiration was always possible at the sternum with median samples of 11.0 mL (1st syringe) and 11.3 mL (2nd syringe) (Table I). Two syringe samples were lost due to a technical error. Bone marrow (4.5 mL) could be obtained at the right tuber coxae from only 1 horse. At the left tuber coxae, using a modified sampling technique, BM was obtained from all horses, with a median sample amount of 11.0 mL. However, a large variation was noticed (a minimum of 0.5 mL to a maximum of 33 mL BM). Macroscopically, several of the samples from the tuber coxae contained small fat droplets and were lighter red in color than the BM obtained from the sternum.
Table I.
Amount of bone marrow (BM), total number of mononuclear cells (MNCs) number of MNCs per milliliter of BM, and number of mesenchymal stem cells (MSCs) at the first passage from the sternum and the left tuber coxae in 12 middle-aged horses
| Sternal sample 1st syringe | Sternal sample 2nd syringe | Pooled left tuber coxae sample | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Horse number | BM (mL) | MNC × 106 | MNC × 106/mL BM | 1st pass. MSC × 106 | BM (mL) | MNC × 106 | MNC × 106/mL BM | 1st pass. MSC × 106 | BM (mL) | MNC × 106 | MNC × 106/mL BM | 1st pass. MSC × 106 |
| 1 | 11.0 | 30.25 | 2.75 | 1.13 | 11.0 | 29.50 | 2.68 | Cont | 33.0 | 7.38 | 0.22 | 1.13 |
| 2 | 10.5 | 53.00 | 5.05 | 1.38 | lost | 15.0 | 15.13 | 1.01 | 1.81 | |||
| 3 | 10.5 | 18.38 | 1.75 | 4.88 | lost | 10.0 | 10.50 | 1.05 | 0.19 | |||
| 4 | 11.5 | 70.00 | 6.09 | 3.38 | 11.0 | 11.00 | 1.00 | 0.13 | 9.0 | 0.06 | 0.01 | 0.31 |
| 5 | 11.0 | 108.00 | 9.82 | 2.00 | 13.0 | 67.00 | 5.15 | 1.00 | 5.0 | 0.31 | 0.06 | Ng |
| 6 | 10.5 | 40.00 | 3.81 | 5.37 | 12.0 | 41.00 | 3.42 | 2.88 | 12.0 | 8.38 | 0.70 | 0.38 |
| 7 | 12.0 | 26.00 | 2.17 | 3.88 | 12.0 | 121.00 | 10.08 | 2.75 | 18.0 | 25.70 | 1.43 | 2.00 |
| 8 | 11.5 | 13.00 | 1.13 | 4.75 | 11.0 | 5.25 | 0.48 | 0.13 | 17.0 | 3.84 | 0.23 | 0.78 |
| 9 | 11.5 | 38.70 | 3.37 | 0.75 | 11.0 | 23.75 | 2.16 | 0.63 | 5.0 | 0.13 | 0.03 | 0.02 |
| 10 | 11.0 | 23.25 | 2.11 | 1.88 | 11.0 | 20.50 | 1.86 | Cont | 30.0 | 9.50 | 0.32 | 0.13 |
| 11 | 11.0 | 59.75 | 5.43 | 2.38 | 11.5 | 61.25 | 5.33 | 2.50 | 10.0 | 4.00 | 0.40 | 0.44 |
| 12 | 12.0 | 16.00 | 1.33 | 0.50 | 12.0 | 26.75 | 2.23 | 1.25 | 0.5 | Not platable | ||
| Q1 | 10.9 | 22.03 | 2.02 | 1.31 | 11.0 | 21.31 | 1.94 | 0.50 | 8.0 | 2.08 | 0.05 | 0.04 |
| Median | 11.0 | 34.48 | 3.06 | 2.19 | 11.3 | 28.13 | 2.46 | 1.13 | 11.0 | 7.38 | 0.27 | 0.06 |
| Q3 | 11.5 | 54.69 | 5.14 | 4.09 | 12.0 | 56.19 | 4.72 | 2.56 | 17.3 | 10.00 | 0.78 | 0.17 |
BM — bone marrow; MNC — mononuclear cells; MSC — mesenchymal stem cells; pass. — passage; Cont — contaminated culture; Ng — No growth; Q1 — Lower quartile; Q3 — Upper quartile.
In-vitro processing of the bone marrow aspirates
A median of 3.06 × 106 MNC/mL BM (1st syringe) and 2.46 × 106 MNC/mL BM (2nd syringe) could be isolated from sternal samples. The single sample obtained from the right tuber coxae yielded 0.5 × 106 MNC and a total of 0.025 × 106 MSC at the time of first passage. This sample was not pooled with the sample from the left side. A median of 0.27 × 106 MNC/mL of BM could be isolated from the left tuber coxae samples, with a wide range between the minimum and maximum values (0.01 to 1.43 × 106 MNC/mL BM, respectively).
First passage for the sternal samples was conducted after 15 d in culture, while tuber coxae samples were first passaged when 80% confluency was reached (on average after 16.9 d). A median of 2.19 × 106 MSC (1st syringe) and 1.13 × 106 MSC (2nd syringe) was yielded from sternal samples, compared to a significantly lower median number of MSC from tuber coxae samples, with 0.06 × 106 MSC. It is important to note that cell growth was feasible in only 10 out of 12 tuber coxae samples. Among those, 7 samples yielded 0.78 × 106 MSC or less.
Differences were also noticed between the cell numbers yielded from the 1st and 2nd sternal syringe. The 1st syringe contained a higher MNC count per milliliter of BM, which further generated a higher number of MSC than the 2nd syringe. The generation time of the MSC from the tuber coxae and from the sternum from first to second passage was not significantly different (P = 0.106).
Discussion
In the present study we compared BM aspiration at the sternum and at the tuber coxae in a group of middle-aged horses.
Evaluating the 2 different puncture techniques at the tuber coxae, it becomes apparent that a straight needle direction and deep penetration combined with a considerable high negative pressure, for example, using a 30-mL or 50-mL syringe, is important in order to obtain BM from this location. We developed the technique used at the tuber coxae as well as its modification by studying the pelvic bones of equine cadavers. Ilial bone marrow aspiration techniques are described on various commercial Web sites (10,11). Both sources emphasize the importance of sufficient high negative pressure, which is applied long enough, for successful aspiration of BM at this location. However, we were not able to aspirate clinically relevant amounts of BM in all our horses. In 6 out of 12 animals, only 10 mL of BM or less could be obtained. Contrary to that, aspiration was possible at the sternum in all cases of the present study even though less negative pressure was applied through use of a 12-mL syringe.
The cell number that is yielded per milliliter of BM is more important than the total amount of BM. The total numbers of MNCs and MSCs, as well as the cell count per milliliter of BM were significantly higher in aspirates from the sternum than in aspirates from the tuber coxae.
For therapeutic application in horses, a minimum of 10 × 106 MSC per lesion site is reported (12–14). At the first passage, the median MSC number from the tuber coxae samples was 60 000 cells. As further passages become necessary to acquire a sufficient amount of MSCs, cell senescence and decreased growth as well as differentiation capacity may diminish the quality and therapeutic applicability of cultured MSC.
The age of the animals is a possible explanation for the discrepancy between our results and those of previous reports that describe the tuber coxae as a valid source for BM in horses. In other studies, 2- to 5-year-old horses (2) or even immature animals less than 2 y of age were used (3,15). The specific goal of the present study was to assess the efficiency of the tuber coxae as a BM aspiration site in horses at an advanced age. These horses represent a potential patient population for orthopedic treatment, particularly in warm-blooded sport horses.
An interesting finding is the large individual variation of obtainable BM from the tuber coxae (a minimum of 0.5 mL to a maximum of 33 mL BM), although all punctured horses were the same age and had been in the research herd for several years. This was also observed by Stewart et al (3). Possible explanations are remaining variations of the sampling technique from horse to horse or true individual differences.
Another important finding is the variation in the number of MSCs obtained in the 1st and 2nd syringe in the sternal BM samples. This is in agreement with a previous report on BM aspiration at the same location (16). Variation between samples was also described by Stewart et al (3) when tuber coxae samples were taken, although they did not specify whether the 1st or the following syringes yielded a higher amount of MSC. Kasashima et al (16) therefore recommend multiple punctures of the same sternebra with an aspiration of smaller amounts, such as 5 mL. Alternatively, multiple sternebrae can be used for aspiration. The sternum contains up to 7 sternebrae, of which the 4th and 5th sternebra are commonly used for aspiration, preferably with sonographic guidance.
The main criticism of this study is that 2 different methods were used to isolate MNCs. Density gradient centrifugation and RBC lysis are both valid and established isolation procedures. Density gradient centrifugation is a frequently described isolation technique in most of the articles pertaining to equine MSCs (17–20). Two density gradient centrifugation methods using either Percoll or Ficoll and the classic isolation technique of adherence to a plastic culture dish to isolate equine BM-derived MSC were recently compared (21). It was found that the Percoll protocol was best in terms of MSC yield and self-renewal potential. Horn et al (22) and Peterbauer-Scherb et al (23), however, used RBC lysis to isolate human and pig MNC, respectively. They found the RBC lysis technique to be more efficient than density gradient centrifugation.
Based on these findings, we can speculate that the difference between cell numbers obtained at the sternum and tuber coxae in our study may in part be due to the different isolation techniques used. By calculating the generation time of all MSC lines from the first to the second passage, it was attempted to indirectly measure the growth performance of the isolated MSCs. Generation time is the time between 2 cell divisions and is therefore a measure of the growth potential of cells. As no significant difference in generation time was observed, we assume that the isolation protocols are comparable. A dedicated study comparing the efficiency of density gradient centrifugation and RBC lysis for isolating equine MSC from BM is currently underway at the Large Animal Clinic for Surgery at the University of Leipzig. Preliminary data suggest that there is no significant difference in isolated MNCs or growth characteristics of the cultured MSCs.
In this study we compared BM aspiration at the sternum and the tuber coxae in a group of middle-aged horses. In terms of the quantity of BM, the tuber coxae is an unreliable source of BM. More importantly, the quality of BM, measured by MSC yield, is inferior to samples taken from the sternum. The sternum therefore remains the preferred site for BM aspiration in middle-aged horses. To achieve an even higher quality of BM from the sternum, we recommend aspirating smaller volumes of BM from multiple punctures of a single sternebra or aspirating BM from more than 1 sternebra.
References
- 1.Fortier LA, Nixon AJ, Williams J, Cable CS. Isolation and chondrocytic differentiation of equine bone marrow-derived mesenchymal stem cells. Am J Vet Res. 1998;59:1182–1187. [PubMed] [Google Scholar]
- 2.Kisiday JD, Kopesky PW, Evans CH, Grodzinsky AJ, McIlwraith CW, Frisbie DD. Evaluation of adult equine bone marrow- and adipose-derived progenitor cell chondrogenesis in hydrogel cultures. J Orth Res. 2008;26:322–331. doi: 10.1002/jor.20508. [DOI] [PubMed] [Google Scholar]
- 3.Stewart AA, Byron CR, Pondenis HC, Stewart MC. Effect of dexamethasone supplementation on chondrogenesis of equine mesenchymal stem cells. Am J Vet Res. 2008;69:1013–1021. doi: 10.2460/ajvr.69.8.1013. [DOI] [PubMed] [Google Scholar]
- 4.Durando MM, Zarucco L, Schaer TP, Ross M, Reef VB. Pneumopericardium in a horse secondary to sternal bone marrow aspiration. Equine Vet Educ. 2006;18:75–79. [Google Scholar]
- 5.Bain BJ. Bone marrow trephine biopsy. J Clin Pathol. 2001;54:737–742. doi: 10.1136/jcp.54.10.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abla O, Friedman J, Doyle J. Performing bone marrow aspiration and biopsy in children: Recommended guidelines. Paediatr Child Health. 2008;6:499–501. doi: 10.1093/pch/13.6.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Townsend FI. Bone marrow aspiration in dogs and cats. Lab Anim. 2008;37:497–498. doi: 10.1038/laban1108-497. [DOI] [PubMed] [Google Scholar]
- 8.Abukawa H, Phelps M, Jackson P, et al. Effect of ibuprofen on osteoblast differentiation of porcine bone marrow-derived progenitor cells. J Oral Maxillofac Surg. 2009;67:2412–2417. doi: 10.1016/j.joms.2009.05.434. [DOI] [PubMed] [Google Scholar]
- 9.Lindl T, Gstraunthaler G. Zell- und Gewebekultur [Cell- and tissue culture] 6th ed. Heidelberg: Spektrum Akademischer Verlag; 2008. pp. 150–151.pp. 380–381. [Google Scholar]
- 10.Ilial Bone Marrow Aspiration Protocol. [Last accessed October 19, 2011]. Available from www.art4dvm.com/PDF/Getting-Started/Equine-Ilial-Aspiration-protocol.pdf.
- 11.StemRegen Procedurec. [Last accessed October 19, 2011]. Available from www.vetcell.com/stemregen-procedures; and www.vetcell.com/assets/Uploads/StemRegen-BMA-Procedures-Tuber-Coxa2.pdf.
- 12.Smith RKW. Mesenchymal stem cell therapy for equine tendinopathy. Disabil Rehabil. 2008;30:1752–1758. doi: 10.1080/09638280701788241. [DOI] [PubMed] [Google Scholar]
- 13.Frisbie DD, Kisiday JD, Kawcak CE, Werpy NM, McIlwraith CW. Evaluation of adipose-derived stromal vascular fraction or bone marrow-derived mesenchymal stem cells for treatment of osteoarthritis. J Orthop Res. 2009;27:1675–1680. doi: 10.1002/jor.20933. [DOI] [PubMed] [Google Scholar]
- 14.Ferris DJ, Frisbie DD, Kisiday JD, et al. Clinical follow-up of horses treated with bone-marrow derived mesenchymal stem cells for muskuloskeletal lesions. Proc AAEP. 2009;59 [Google Scholar]
- 15.Toupadakis CA, Wong A, Genetos DC, et al. Comparison of the osteogenic potential of equine mesenchymal stem cells from bone marrow, adipose tissue, umbilical cord blood, and umbilical cord tissue. Am J Vet Res. 2010;71:1237–1245. doi: 10.2460/ajvr.71.10.1237. [DOI] [PubMed] [Google Scholar]
- 16.Kasashima Y, Smith RKW, Goodship A. Optimizing the recovery of mesenchymal progenitor cells from bone marrow. Proc World Conf on Regenerative Medicine. 2009:S19–20. [Google Scholar]
- 17.Smith RKW, Korda M, Blunn GW, Goodship AE. Isolation and implantation of autologous equine mesenchymal stem cells from bone marrow into the superficial digital flexor tendon as a potential novel treatment. Equine Vet J. 2003;35:99–102. doi: 10.2746/042516403775467388. [DOI] [PubMed] [Google Scholar]
- 18.Pacini S, Spinabella S, Trombi L, et al. Suspension of bone marrow-derived undifferentiated mesenchymal stromal cells for repair of superficial digital flexor tendon in race horses. Tissue Eng. 2007;13:2949–2955. doi: 10.1089/ten.2007.0108. [DOI] [PubMed] [Google Scholar]
- 19.Vidal MA, Robinson SO, Lopez MJ, et al. Comparison of chondrogenic potential in equine mesenchymal stromal cells derived from adipose tissue and bone marrow. Vet Surg. 2008;37:713–724. doi: 10.1111/j.1532-950X.2008.00462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hackett CH, Flaminio MJ, Fortier LA. Analysis of CD14 expression level in putative mesenchymal progenitor cells isolated from equine bone marrow. Stem Cells Dev. 2011;20:721–735. doi: 10.1089/scd.2010.0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bourzac C, Smith LC, Vincent P, Beauchamp G, Lavoie J-P, Laverty S. Isolation of equine bone marrow-derived mesenchymal stem cells: A comparison between three protocols. Equine Vet J. 2010;42:519–527. doi: 10.1111/j.2042-3306.2010.00098.x. [DOI] [PubMed] [Google Scholar]
- 22.Horn P, Bork S, Diehlmann A, et al. Isolation of human mesenchymal stromal cells is more efficient by red blood cell lysis. Cytotherapy. 2008;10:676–685. doi: 10.1080/14653240802398845. [DOI] [PubMed] [Google Scholar]
- 23.Peterbauer-Scherb A, van Griensven M, Meinl A, Gabriel C, Redl H, Wolbank S. Isolation of pig bone marrow mesenchymal stem cells suitable for one-step procedures in chondrogenic regeneration. J Tissue Eng Regen Med. 2010;4:485–490. doi: 10.1002/term.262. [DOI] [PubMed] [Google Scholar]