SUMMARY
The speed of muscle contraction is largely controlled at the sarcomere level by the ATPase activity of the motor protein myosin. Differences in amino acid sequence in catalytically important regions of myosin yield different myosin isoforms with varying ATPase activities and resulting differences in cross-bridge cycling rates and interfilamentary sliding velocities. Modulation of whole-muscle performance by changes in myosin isoform ATPase activity is regarded as a universal mechanism to tune contractile properties, especially in vertebrate muscles. Invertebrates such as squid, however, may exhibit an alternative mechanism to tune contractile properties that is based on differences in muscle ultrastructure, including variable myofilament and sarcomere lengths. To determine definitively whether contractile properties of squid muscles are regulated via different myosin isoforms (i.e. different ATPase activities), the nucleotide and amino acid sequences of the myosin heavy chain from the squid Doryteuthis pealeii were determined from the mantle, arm, tentacle, fin and funnel retractor musculature. We identified three myosin heavy chain isoforms in squid muscular tissues, with differences arising at surface loop 1 and the carboxy terminus. All three isoforms were detected in all five tissues studied. These results suggest that the muscular tissues of D. pealeii express identical myosin isoforms, and it is likely that differences in muscle ultrastructure, not myosin ATPase activity, represent the most important mechanism for tuning contractile speeds.
KEY WORDS: myosin, squid, muscle, contraction, myofilament
INTRODUCTION
Muscular performance, including contractile speed and power output, is largely dictated at the molecular level by the motor protein myosin. Composed of two heavy chains and four light chains, muscle myosin-II is a hexameric protein that composes the thick filaments in muscle sarcomeres. The myosin heavy chain is composed of three distinct regions: the globular subfragment-1 (S1) head at the N terminus, the flexible subfragment-2 (S2) neck or linker region, which gives flexibility to the protein, and the light meromyosin (LMM) rod at the C terminus. The myosin S1 heads (two per myosin protein) include binding sites for ATP and for the thin filament protein actin. Myosin is an enzyme that uses the energy from ATP hydrolysis to bind actin, undergo a conformational change (the power stroke) and induce filament sliding, as described in the sliding filament model of muscle contraction (Huxley, 1969). Variations in the arrangement of myosin proteins in the sarcomere or in the myosin protein sequence can lead to changes in myosin activity, thus affecting interfilament sliding velocity and muscle performance.
The contractile speed of striated muscles is correlated at the molecular level with the myosin isoform. Myosin isoforms with higher ATPase activities (i.e. higher ATP hydrolysis rates) are usually found in muscles that display fast shortening velocities. For example, mammalian fast-twitch muscle fibres IIa, IIb and IIx contain the fast myosin isoforms IIA, IIB and IIX, respectively (Bottinelli et al., 1991). These fast myosin isoforms possess higher ATPase activities compared with the slow myosin isoform found in slow-twitch muscle fibres (also found in cardiac muscle). The relationship between muscle shortening velocity and ATPase activity was elegantly demonstrated by Barany (Barany, 1967). Using muscles from invertebrates, lower vertebrates and higher vertebrates, Barany showed that actin- and Ca2+-activated myosin ATPase activities were proportional to shortening velocities, from 0.1 to 24 muscle lengths s–1. The myosin ATPase activities from these muscles were also inversely proportional to the muscle isometric twitch contraction time. Taken together, these results demonstrated that myosin ATPase activity plays a crucial role in determining the speed of muscle contraction.
The ATPase activity of myosin is dependent on the primary amino acid sequence of the myosin heavy chain (Spudich, 1994; Gauvry et al., 1997). Two regions of amino acids in the myosin S1 heads are thought to play an especially important role. Referred to as surface loops because they are thought to be flexible and did not crystallize in the S1 crystal structure shown by Rayment et al. (Rayment et al., 1993), these regions may contribute to regulating myosin ATPase activity by affecting ADP dissociation rates [surface loop 1 (Murphy and Spudich, 1998)] and/or by modulating interactions between myosin and actin [surface loop 2 (Uyeda et al., 1994)]. Perreault-Micale et al. (Perreault-Micale et al., 1996b) demonstrated that amino acid differences in surface loop 1 in scallop striated and catch muscle fibres contributed to differences in myosin ATPase activity.
The squid Doryteuthis pealeii possesses muscle fibres with unique contractile properties. The squid has eight arms and two tentacles, the latter of which can double their length in only 15–35 ms to capture prey (Kier, 1982; Van Leeuwen and Kier, 1997). This rapid strike is caused by contraction of transverse muscle in the core of the tentacular stalk. The tentacle transverse muscle is unusual amongst cephalopods, as it includes fibres that are cross-striated with short thick filaments and sarcomeres (∼0.8 μm thick filament length), whereas most cephalopod muscle is obliquely striated with long thick filaments [e.g. the thick filaments of arm transverse muscle fibres are ∼7.4 μm (Kier and Curtin, 2002)]. Measurements of shortening velocities in transverse muscle tissue preparations revealed that the transverse muscle of the tentacle contracts nearly 10 times faster than the transverse muscle of the arm [15 vs 1.5 muscle lengths s–1, respectively (Kier and Curtin, 2002)]. This 10-fold greater contractile speed in the tentacles is expected given the observed differences between the transverse muscle ultrastructure in the arms and tentacles. Because the tentacle fibres contain 10-fold more sarcomeres per unit length than the arm muscle and the shortening velocity is proportional to the number of elements in series (Huxley and Simmons, 1973; Josephson, 1975), the contractile speed of the transverse muscle of the tentacle is predicted to be 10-fold faster than that of the arm, which indeed it is (Kier and Curtin, 2002).
As demonstrated by Barany (Barany, 1967), myosin isoforms and ATPase activity play an important role in regulating shortening velocities in striated muscle fibres, but there has been relatively little research on the biochemistry of myosin isoforms in squid muscles. Kodama and Konno (Kodama and Konno, 1983) isolated myosin from mantle and brachial muscles of the squid Todarodes pacificus and found that myosin from both tissues exhibited similar Ca2+, Mg2+ and EDTA-ATPase activity. Several groups have measured the myosin ATPase activities of myosin isolated from the mantle musculature, but no comparisons were made with other squid tissues (Konno, 1978; Tsuchiya et al., 1978; Konno et al., 1981). Lehman and Szent-Gyorgyi (Lehman and Szent-Gyorgyi, 1975) measured the ATPase activity of myosin isolated from the ventral pharynx retractor muscle, but again no comparisons were made with other squid musculature. Kier and Schachat (Kier and Schachat, 1992) found that myosin extracted from arm and tentacle muscles was identical based on comparisons of V8 protease and cyanogen bromide digested SDS-PAGE bands and low-percentage polyacrylamide and Neville gels (Neville, 1971). Matulef et al. (Matulef et al., 1998) sequenced the myosin heavy chain transcript from the funnel retractor muscle of D. pealeii and found two isoforms that varied in sequence in surface loop 1, suggesting that these myosin isoforms may display different ATPase activities (Matulef et al., 1998). Even if the two isoforms do possess different ATPase activities, Kier and Schachat (Kier and Schachat, 2008), using semi-quantitative RT-PCR, found that the expression of these isoforms was approximately the same in the arm and tentacle muscles, again providing evidence that squid arm and tentacle muscles possess similar myosin heavy chain proteins.
Despite the evidence that the arm and tentacles do not differ with regards to myosin heavy chain composition, a full analysis of the myosin heavy chain sequence from arm and tentacle tranverse muscle fibres has not been completed. In addition to the previously described A and B isoforms that differ in the actin binding site of surface loop 1 (Matulef et al., 1998), other differences in the sequence may exist that could influence myosin ATPase activity. To resolve this issue, we sequenced transcripts for the myosin heavy chain from cross-striated muscle fibres (tentacle) and obliquely striated muscle fibres (arm, mantle, fin and funnel retractor) of the squid D. pealeii. We found that the myosin heavy chain was identical in all muscles studied. Both previously identified myosin heavy chain isoforms (A and B) were found in all muscles studied, in addition to a third isoform (referred to as isoform C). These results suggest that the contractile properties of squid muscles are not regulated by myosin heavy chain isoforms (and therefore variable ATPase activities); rather, they are likely regulated by differences in muscle-fibre ultrastructure, in particular the arrangement and dimensions of the myofilaments. This study provides evidence for an alternative method of the regulation of muscle contractile properties.
MATERIALS AND METHODS
Animal preparation
Specimens of Doryteuthis pealeii (Lesueur 1821) were collected at night in shallow water with cast nets near the Darling Marine Center, University of Maine (Walpole, ME, USA). They were maintained in a flow-through seawater system and fed daily until used. Animals were anesthetized in cold seawater and then killed. Tissue samples from the tentacular stalk, arm, fin, mantle and funnel retractor musculature were removed, flash frozen in liquid nitrogen and stored at –80°C until use.
RNA extraction and cDNA synthesis
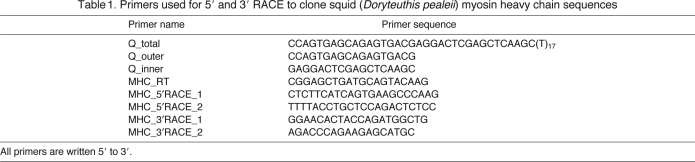
Total RNA was extracted from 30–50 mg of each tissue using the GeneJET RNA Purification Kit (Fermentas, Glen Burnie, MD, USA) and treated with DNase I (Fermentas). First strand cDNA synthesis was performed using 125–250 ng total RNA with the Moloney murine leukemia virus reverse transcriptase (M-MuLV RT) from the Phusion RT-PCR Kit according to the manufacturer's instructions (Finnzymes, Espoo, Finland). An oligo(dT)15 primer was used to synthesize cDNA for conventional PCR, an anchor primer (Q_total) was used for 3′ rapid amplification of cDNA ends (RACE) cDNA synthesis and a myosin-specific primer (MHC_RT) was used for 5′ RACE cDNA synthesis (Table 1). The MHC_RT primer was based on the previously published myosin heavy chain sequence from the funnel retractor muscle of D. pealeii (Matulef et al., 1998).
Table 1.
Primers used for 5′ and 3′ RACE to clone squid (Doryteuthis pealeii) myosin heavy chain sequences
Cloning of squid myosin heavy chain
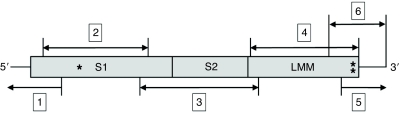
Because the myosin heavy chain transcript is large (∼6600 bp in length), we used a cloning strategy that divided the myosin heavy chain transcript into six regions (Fig. 1). Conventional PCR was used to clone regions 2, 3 and 4 (internal coding sequences) and region 6, which spanned the internal coding region and the 3′ untranslated region (UTR). 5′ and 3′ RACE methods were used to clone regions 1 and 5. For conventional PCR, 1 μl of oligo(dT)15-synthesized cDNA was used as a template with Phusion Hot Start II High-Fidelity DNA Polymerase (Finnzymes). PCR was carried out according to the manufacturer's instructions using primers specific for myosin heavy chain regions 2, 3, 4 and 6 (Table 2) [based on the previously published myosin heavy chain sequence for the funnel retractor muscle of D. pealeii (Matulef et al., 1998)]. The cycling conditions were as follows: an initial denaturation of 98°C for 30 s; 40 cycles of 98°C for 10 s, 59–67°C for 10 s (depending on the primer pair), 72°C for 2.5 min; and a final extension of 72°C for 5 min. PCR products were separated on 1% agarose gels, extracted and purified using the GeneJET Gel Extraction Kit (Fermentas). The purified PCR products were mixed in a 3:1 molar ratio with the pJET1.2/blunt vector (Fermentas) and ligated with T4 DNA ligase according to the manufacturer's instructions (CloneJET PCR Cloning Kit, Fermentas). The ligation mixture was transformed into competent JM107 Escherichia coli cells prepared using the TransformAid Bacterial Transformation Kit (Fermentas). Colonies that contained the proper insert were selected using colony PCR and grown overnight. Plasmid DNA was isolated using miniprep kits (Fermentas and Qiagen, Valencia, CA, USA).
Fig. 1.
Schematic of the strategy used to clone and sequence the myosin heavy chain from Doryteuthis pealeii. The numbers in boxes indicate different cloning regions that were amplified by PCR or RACE and sequenced. The single asterisk indicates where isoforms A and B differ, and the double asterisk indicates where isoforms A and C differ. S1 and S2, subfragments 1 and 2 of the myosin heavy chain; LMM, light meromyosin.
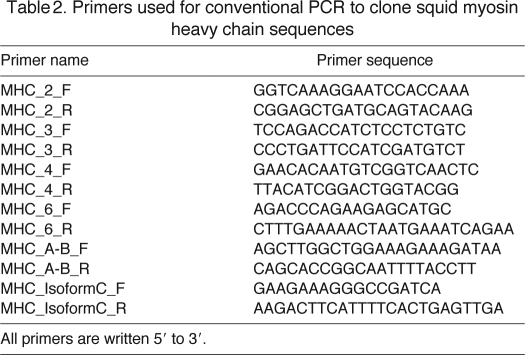
Table 2.
Primers used for conventional PCR to clone squid myosin heavy chain sequences
5′ and 3′ RACE methods based on those described previously (Scotto-Lavino et al., 2006a; Scotto-Lavino et al., 2006b) were used to clone myosin heavy chain regions 1 and 5, respectively (Fig. 1). For 5′ RACE, the MHC_RT-synthesized cDNA was purified using the GeneJET PCR Purification Kit (Fermentas) to remove dNTPs. A synthetic polyA tail was added to the 3′ end of 15 μl of cDNA by adding 0.5 μl terminal deoxynucleotidyl transferase (TdT; Fermentas), 1 μl of 1 mmol l–1 dATP, 4 μl of 5× buffer, and heating at 37°C for 15 min, followed by 70°C for 10 min to deactivate the TdT. The first complementary strand to the polyA-tailed cDNA was generated using the anchor primer (Q_total, Table 1) and the Phusion polymerase (Finnzymes) by heating at 98°C for 1 min, 48°C for 2 min and 72°C for 25 min. The first PCR reaction was then carried out using the Phusion polymerase (Finnzymes), the outer anchor primer (Q_outer) and the MHC_5′RACE_1 primer (Table 1), with 30 cycles of 98°C for 10 s, 57°C for 10 s and 72°C for 90 s, and a final extension of 72°C for 10 min. A second, nested PCR was performed to increase specificity of the final PCR product. An aliquot of the first PCR reaction was used as the template with the Phusion polymerase (Finnzymes), the inner anchor primer (Q_inner) and the MHC_5′RACE_2 primer (Table 1). The following cycling conditions were used: 98°C for 1 min; 30 cycles of 98°C for 10 s, 57°C for 10 s and 72°C for 90 s; and a final extension of 72°C for 10 min. The PCR products were then purified, cloned and analyzed as described above.
The 3′ RACE methods used were based on previously published protocols (Scotto-Lavino et al., 2006a). The first complementary strand to the anchor primer (Q_total) synthesized cDNA was generated using the outer anchor primer (Q_outer, Table 1) and the Phusion polymerase (Finnzymes) by heating at 98°C for 1 min, 61°C for 2 min and 72°C for 25 min. The first PCR reaction was then carried out using the Phusion polymerase (Finnzymes), the outer anchor primer (Q_outer) and the MHC_3′RACE_1 primer (Table 1) with 30 cycles of 98°C for 10 s, 61°C for 10 s and 72°C for 90 s, and a final extension of 72°C for 10 min. A second, nested PCR was performed using an aliquot of the first PCR reaction, the Phusion polymerase (Finnzymes), the inner anchor primer (Q_inner) and the MHC_3′RACE_2 primer (Table 1). The following cycling conditions were used: 98°C for 1 min; 30 cycles of 98°C for 10 s, 61°C for 10 s and 72°C for 90 s; and a final extension of 72°C for 10 min. The PCR products were then purified, cloned and analyzed as described above.
Detection of myosin heavy chain isoforms
Matulef et al. (Matulef et al., 1998) identified two myosin heavy chain isoforms (A and B) that differ within a 90 bp region. Conventional PCR was used to amplify DNA surrounding this region (total 202 bp for isoform A and 187 bp for isoform B) using primers described previously (Table 2) (Kier and Schachat, 2008). In addition, a third isoform (isoform C) was detected that consisted of 36 additional base pairs at the 3′ end of the coding region as well as a shorter 3′ UTR (see Results). Conventional PCR was used to amplify DNA specific for isoform C (total 141 bp) using primers listed in Table 2. The PCR products were cloned and analyzed as described above.
DNA sequencing and analysis
Plasmid DNA was sequenced using pJET forward and reverse primers (Fermentas) as well as primers specific to internal myosin heavy chain sequences. DNA sequencing was carried out by Eton Biosciences (Durham, NC, USA). Sequence data were analyzed with Sequence Scanner software (v1.0, Applied Biosystems, Carlsbad, CA, USA) as well as tools on the ExPASy web server (Gasteiger et al., 2003). Sequences were compared with the previously published D. pealeii myosin heavy chain sequence (Matulef et al., 1998) and other myosin heavy chain sequences and aligned using the webtools LALIGN (Pearson, 2006) and ClustalW2 (Chenna et al., 2003).
RESULTS
Determination of myosin heavy chain sequence
The transcripts encoding the myosin heavy chain protein from five muscular tissues of the squid D. pealeii were sequenced using conventional PCR, 5′ and 3′ RACE, and standard molecular cloning methods. Strikingly, the nucleotide and translated amino acid sequences for the coding region were 100% identical amongst all five myosin heavy chain transcripts studied, suggesting that the squid D. pealeii expresses the same myosin heavy chain isoforms in the mantle, arm, tentacle, fin and funnel retractor muscular tissues.
The complete amino acid sequence of the D. pealeii myosin heavy chain protein is shown in supplementary material Fig. S1, aligned with closely related cephalopod and molluscan sequences (because the myosin heavy chain sequence was identical for all five tissues studied, only one sequence is shown and labeled D. pealeii). The coding region for the D. pealeii myosin heavy chain was found to comprise 5808 nucleotides encoding 1936 amino acids and yielding a protein with expected molecular mass of 221,891 Da. The sequence is highly similar to that previously described as myosin heavy chain isoform A for D. pealeii funnel retractor muscle by Matulef et al. (Matulef et al., 1998), and thus is referred to as isoform A here. The sequence obtained in the present study was one amino acid longer than that described previously, and also contained 16 amino acid substitutions. Differences in experimental techniques (e.g. the use of the proofreading Phusion polymerase in this study rather than the Taq polymerase used previously) and advances in sequencing technologies are likely responsible for these differences.
The 5′ UTR sequences of the myosin heavy chain transcripts were sequenced using 5′ RACE (region 1, Fig. 1). The 5′ UTR sequence was 152–157 nucleotides in length, depending on the tissue-specific transcript (supplementary material Fig. S2A). The differences in the 5′ UTR were found at the most extreme 5′ end of the transcript, likely because of experimental or technical limitations [i.e. premature termination of the reverse transcription reaction (Scotto-Lavino et al., 2006b)]. The 5′ UTRs of the different tissue myosin heavy chain transcripts were identical except for the first six nucleotides, with one exception: one sequencing reaction of the 5′ UTR from the tentacle myosin heavy chain transcript yielded a four-nucleotide insert 26 nucleotides downstream from the 5′ end of the transcript (Tentacle2, supplementary material Fig. S2A). This insert was only found once and may represent a different transcript, but further investigation is needed. The 5′ UTR has been reported for the Japanese flying squid, T. pacificus (Genbank GU338005.1), and has a length of 153 bp. The 5′ UTR for D. pealeii was 92.2% identical to that of T. pacificus.
The 3′ UTR sequences of the myosin heavy chain transcripts were sequenced using 3′ RACE and conventional PCR (regions 5 and 6, Fig. 1). Initial results with 3′ RACE yielded a 3′ UTR of 408 nucleotides (not counting the polyA tail), which was identical for the myosin heavy chain transcript for all five tissues studied. This 3′ UTR sequence was 273 nucleotides shorter than that reported by Matulef et al. (Matulef et al., 1998) for the D. pealeii funnel retractor myosin heavy chain transcript (681 nucleotides, GenBank AF042349). In order to attempt to detect the remainder of the 3′ UTR, a reverse primer specific for the 3′ UTR detected by Matulef et al. (Matulef et al., 1998) was used for conventional PCR along with a forward primer in the coding sequence (Table 2). Using this primer set, a longer 3′ UTR was identified in all five myosin heavy chain transcripts (675 nucleotides in length, not counting the polyA tail, supplementary material Fig. S2B). The 3′ UTRs differed by only two nucleotides between all five myosin heavy chain transcripts studied.
Identification of multiple myosin isoforms
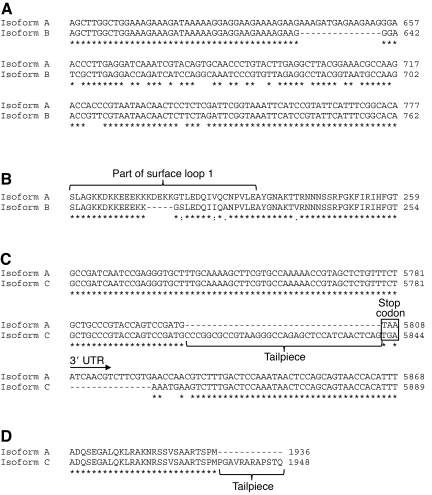
Matulef et al. (Matulef et al., 1998) determined the nucleotide and amino acid sequence (isoform A) of the myosin heavy chain from the funnel retractor muscle of D. pealeii, and also found a second isoform (isoform B) that differed in surface loop 1 of the S1 head region of myosin. Isoform B contained a five-amino-acid deletion and seven amino acid substitutions compared with isoform A. In this study, we wished to determine whether isoform B was found in muscular tissues of D. pealeii in addition to isoform A. A short region of the myosin heavy chain transcript was cloned via PCR, which would yield a 202 bp product for isoform A and a 187 bp product for isoform B [because of the five-amino-acid (15-nucleotide) deletion]. Plasmid DNA from multiple clones for each tissue type (mantle, arm, tentacle, fin and funnel retractor) was prepared and sequenced, and isoform B was found in all five muscle types studied; the sequence was identical for all tissues. Isoform B contained a five-amino-acid deletion as well as four amino acid substitutions compared with isoform A. Sequence comparisons for isoforms A and B identified in this study are shown in Fig. 2A,B (only one sequence is shown for each because the sequences were identical for all five muscle tissues studied). These results do not provide any information regarding the relative abundances of isoforms A and B in the tissues studied. However, they do suggest that all five tissues studied express both myosin isoforms A and B.
Fig. 2.
Comparisons of myosin isoforms A, B and C. Nucleotide (A) and translated amino acid sequences (B) of Doryteuthis pealeii myosin heavy chain isoforms A and B. Isoform B contains a 15-nucleotide (five-amino-acid) deletion and four amino acid substitutions compared with isoform A. Nucleotide (C) and translated amino acid sequences (D) of D. pealeii myosin heavy chain isoforms A and C. Isoform C contains a 36-nucleotide (12-amino-acid) extension compared with isoform A, as well as differences in the stop codon and 3′ UTR. The remainder of the 3′ UTR is not shown because it is the same as that for isoforms A and C. Asterisks (*) denote identical amino acids (or nucleotides), colons (:) denote well-conserved amino acids, periods (.) denote somewhat conserved amino acids, and a blank space denotes no conservation. Nucleotide numbering starts from the start codon.
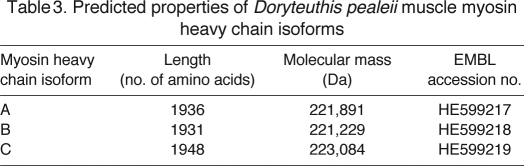
While cloning and sequencing the 3′ end of the myosin heavy chain transcript from RNA isolated from arm tissue, we found a third myosin heavy chain isoform (referred to as isoform C) that has not been previously described. Using primers specific for isoform C that flanked the 3′ end of the coding region and the beginning of the 3′ UTR, we amplified a short region of the myosin heavy chain transcript using PCR and analyzed multiple clones from each tissue to determine whether isoform C was expressed in the other four tissues. Myosin isoform C was detected in all five tissues studied, and all had 100% identical sequences. Isoform C contained a 36 bp extension to the 3′ end of the coding region, equivalent to a 12-amino-acid extension, or tailpiece, to the C terminus of the translated myosin heavy chain protein. Isoform C also contained a different stop codon than observed in isoform A, and a unique 7 bp region to begin the 3′ UTR, followed by 100% homology to the 3′ UTR described above (supplementary material Fig. S2B). Together, isoform C is 21 bp longer than isoform A (36 bp longer in the coding region, and 15 bp shorter in the 3′ UTR). The nucleotide and amino acid sequences for isoform C compared with isoform A are shown in Fig. 2C,D, respectively. As with the detection of isoforms A and B described above, we could not determine the relative quantities of these isoforms from these data. Table 3 summarizes the predicted properties of the three squid muscle myosin isoforms. Sequence data for all three isoforms were uploaded to the EMBL Nucleotide Sequence Database under the following accession numbers: isoform A, HE599217; isoform B, HE599218; and isoform C, HE599219.
Table 3.
Predicted properties of Doryteuthis pealeii muscle myosin heavy chain isoforms
Comparison with other molluscs
The relationship of the D. pealeii myosin heavy chain protein sequence to that of other molluscs was examined by aligning the sequences one pair at a time using LALIGN (Pearson, 2006). The D. pealeii myosin heavy chain sequence was highly similar to that of other squids, e.g. Doryteuthis bleekeri (unknown tissue, 96.9% identity) and T. pacificus (unknown tissue, 93.6% identity). It was also highly similar (94.2% identity) to that of the golden cuttlefish, Sepia esculenta (unknown tissue), another coleoid cephalopod. No other cephalopod sequences were available in databases, so comparisons were made with a scallop, Aequipecten irradians (striated adductor muscle, 73.6% identity). The complete amino acid sequence alignment for these species is shown in supplementary material Fig. S1.
DISCUSSION
The major result from this study is that identical myosin heavy chain isoforms are expressed in five different muscular tissues of the squid D. pealeii. This is the first time that myosin sequence information has been obtained for squid muscular tissues other than the funnel retractor muscle, and this is also the first description of a third myosin heavy chain isoform in squid muscular tissues. These results indicate that squid muscle fibres do not express different myosin isoforms with expected different ATPase activities and contractile speeds; rather, squid appear to use ultrastructural differences to achieve a range of shortening velocities (Kier and Curtin, 2002).
Transverse muscle fibres of squid tentacles shorten 10-fold faster than transverse muscle fibres of the arms (Kier and Curtin, 2002). This could be the result of two possible mechanisms. First, tentacle muscle fibres might contain a myosin isoform with a higher ATPase activity than the myosin isoform found in the slower-contracting arm muscles, as described for other muscles by Barany (Barany, 1967). Alternatively, the ultrastructural arrangement of the sarcomeres of the arm and tentacle transverse muscle fibres could differ, with shorter sarcomeres and, consequently, higher shortening velocity in the tentacle fibres. This type of ultrastructural arrangement has been described previously for squid musculature (Kier, 1985; Kier, 1991; Kier and Curtin, 2002). In the present study, our goal was to determine definitively whether the mechanism of multiple myosin isoform expression applies to differences in squid muscle shortening velocities. To do so we determined the primary amino acid sequence of the myosin heavy chain from five different muscles of the squid D. pealeii and found that the myosin heavy chain sequence was 100% identical in all tissues studied (mantle, arm, tentacle, fin and funnel retractor), suggesting that the ATPase activities are identical and thus differences in ultrastructure, not differences in myosin isoforms, are likely responsible for the observed difference in contractile properties. Consistent with our results, Kodama and Konno (Kodama and Konno, 1983) measured ATPase activities from squid mantle and arm musculature and found that they were not significantly different. To our knowledge, this is the first report of an animal that uses differences in the ultrastructural arrangement of contractile proteins and sarcomeres to modulate muscle-fibre contractile properties in the absence of expression of tissue-specific myosin isoforms. This may represent a new mechanism for controlling the contractile properties of muscle.
Other molluscs employ variations in sarcomere length to modulate shortening velocity and force generation, but they also use different myosin isoforms. The adductor muscles of scallops are composed of fast fibres used for swimming and slow catch fibres used for prolonged shell closure with minimal metabolic expenditure. The fast fibres are cross-striated with relatively short sarcomeres, whereas the catch fibres are smooth and contain long myofilaments (Nunzi and Franzini Armstrong, 1981). The striated and catch fibres also shorten at dramatically different speeds; the striated fibres shorten ∼20 times faster than the catch fibres (Millman, 1967). In addition to longer myofilaments and slower shortening velocities, catch muscle fibres possess a myosin isoform that has a twofold to fivefold lower ATPase activity than that of a myosin isoform from striated adductor fibres (Ruegg, 1971; Perreault-Micale et al., 1996a; Perreault-Micale et al., 1996b). The difference in ATPase activity between molluscan striated and catch fibres appears to be due to the presence of distinct striated and catch myosin isoforms within these tissues. Perreault-Micale et al. (Perreault-Micale et al., 1996a) found that the amino acid sequence of surface loop 1 of the myosin heads differed between striated and catch adductor fibres of the scallop Placopecten magellanicus (Perreault-Micale et al., 1996b). These isoforms are produced via alternative splicing of a single gene (Nyitray et al., 1994). Although molluscs such as bivalves vary myofilament and sarcomere lengths, they also express different myosin isoforms with varying myosin ATPase activities, and thus the differences in contractile properties observed are the result of both biochemical and ultrastructural differences.
Other invertebrates also employ both sarcomere length and myosin isoform variation to tune muscle contractile properties. Crustaceans have both long-sarcomere slow and short-sarcomere fast muscles (Fahrenbach, 1967). In addition to differences in sarcomere length, the ATPase activity of myosin from fast crustacean muscles is at least fourfold higher than that of myosin from slow muscles (Lehman and Szent-Gyorgyi, 1975; Mykles, 1985). This difference is likely a result of differences in myosin heavy chain amino acid sequence (Li and Mykles, 1990). Medler and Mykles (Medler and Mykles, 2003) demonstrated using RT-PCR that fast and slow muscles of the American lobster (Homarus americanus) express distinct fast and slow myosin heavy chain isoforms (Medler and Mykles, 2003). Distinct myosin heavy chain isoforms with differences in the surface loop 1 sequence have also been described in fast and slow muscles of gammarid amphipods (Whiteley et al., 2010), which display corresponding fast and slow ATPase activities (Ogonowski and Lang, 1979).
Sarcomeric proteins other than myosin may play a role in modulating shortening velocity. Each myosin molecule is composed of two heavy chains, two essential light chains and two regulatory light chains. Both types of light chains are required for physiological shortening velocities in vertebrate skeletal muscles (Lowey et al., 1993). Two isoforms of the essential light chain are found in vertebrate skeletal muscles, and variations in the ratio of these essential light chain isoforms have been shown to affect shortening velocities in skeletal muscle fibres (Sweeney et al., 1988). Variations in troponin isoforms may also influence contractile dynamics. Reconstitution of mouse and rat cardiac fibres with the other species' recombinant troponin complexes resulted in differences in contractile kinetics (Chandra et al., 2007). It is unknown whether differences in light chain and troponin isoforms exist in cephalopod muscle. Kier and Schachat (Kier and Schachat, 1992) showed with SDS-PAGE that the myofibrillar proteins from arm and tentacle musculature were remarkably similar, suggesting that there is little variation in troponin or light chain isoforms between these tissues. It is possible, however, that these techniques might not detect minor sequence variation that could nevertheless contribute to the observed differences in shortening velocities between arm and tentacle transverse musculatures.
External biochemical factors also have been found to affect shortening velocity in vertebrate muscles. Nitric oxide (NO) is known to decrease shortening velocities in both vertebrate skeletal and cardiac muscle fibres (Galler et al., 1997; Perkins et al., 1997). Recently, Evangelista et al. demonstrated that NO acts directly on skeletal and alpha-cardiac myosin heavy chains by S-nitrosylating cysteine residues in the myosin heavy chain, resulting in slowed actin velocity in an in vitro motility assay and increased myosin force production in a laser trap (Evangelista et al., 2010). NO has been well studied in cephalopods, most notably the cuttlefish Sepia officinalis, and has been linked to variations in manipulative behavior, regulation of blood flow and pressure, statocyst activity and the ink defense response (reviewed in Palumbo, 2005). Despite the evidence of physiological effects of NO in Sepia and other cephalopods, no studies have directly examined the effects of NO on muscular contractile properties in these animals. It is thus an interesting possibility that NO may differentially affect the myosin heavy chain of the arm and tentacle musculature of squid, thereby resulting in differences in shortening velocity.
We have identified three myosin isoforms (A, B and C) in squid muscles. Although it is clear that all myosin isoforms are found in all five tissues studied, a limitation of this study is that we have not measured the relative abundance of these isoforms in the different tissues. Kier and Schachat (Kier and Schachat, 2008) demonstrated using semi-quantitative RT-PCR that myosin B comprises less than 5 and 10% of the total myosin heavy chain transcripts in the arm and tentacle musculature of D. pealeii, respectively, suggesting that similar quantities of isoform A and B transcripts are found in each of these tissues. An interesting possibility, however, is that myosin isoforms A, B and C may be differentially expressed in the various muscle groups within each tissue type. For instance, the arms and tentacles include, in addition to the transverse muscle fibres, longitudinal, helical and oblique muscle fibres (Kier, 1982). Because of the small size of these appendages and the interdigitation of many of the fibres, it is difficult to dissect pure samples from each muscle mass. We thus used entire cross-sections, which prevented us from assessing potential differences in expression between the muscle groups. Additional studies that measure transcript levels, detect transcript expression patterns, and investigate protein levels and expression patterns using isoform-specific antibodies are warranted to determine the distribution and possible functional importance of myosin isoforms A, B and C in squid muscles.
Myosin heavy chain isoform C contains a 12-amino-acid C-terminal extension, or tailpiece. A myosin heavy chain isoform with a tailpiece has been described previously for scallop catch muscle myosin (Perreault-Micale et al., 1996b). The myosin heavy chains of Argopecten and Placopecten sequenced from catch muscle both contained a 10-amino-acid extension at the C terminus. The scallop tailpiece is not homologous in sequence to that of the squid tailpiece described in this study. However, the existence of a myosin heavy chain isoform with an extended C terminus in squid muscles may indicate that this isoform (isoform C) is fibre specific, similar to fibre expression in the scallop. In addition, vertebrate smooth muscle myosin is expressed as two isoforms with different tailpiece lengths (SM1, 43 amino acids; SM2, nine amino acids), which affects thick filament assembly (Rovner et al., 2002). Although the functional implications of the myosin heavy chain isoform C tailpiece in squid musculature are unknown, its existence opens the interesting possibility of additional muscular specialization in cephalopods.
One of the striking differences between the ultrastructure of the squid arm and tentacle transverse muscle fibres is the length of the thick filaments: thick filaments are nearly 10 times longer in the arm than the tentacle fibres (Kier and Curtin, 2002). In addition, the tentacle transverse muscle fibres are cross-striated, whereas the arm transverse muscle fibres are obliquely striated. There are thus significant differences in thick filament organization and arrangement, and it is possible that differences in the rod portion of myosin (where thick filament assembly occurs) are necessary or even dictate these differences, as has been suggested by a previous study (Rovner et al., 2002). In the present study, however, the myosin isoforms found in the arm and tentacle were 100% identical, suggesting that differences in myosin sequence do not contribute to differences in filament assembly. It is possible that other proteins, such as paramyosin, which has been found in different levels in arm and tentacle muscle fibres (Kier and Schachat, 1992), contribute to thick filament length and organization.
Although the present study has revealed that squid muscle fibres likely express identical myosin isoforms, further investigation is needed to determine whether other squids and cephalopods also possess identical myosin isoforms. Of particular interest is the cuttlefish, a closely related coleoid cephalopod that also possesses eight arms and two tentacles. The transverse muscles of the tentacles are cross-striated, whereas the transverse muscle of the arms are obliquely striated, an arrangement that is homologous to that found in squid tentacle and arm muscles (W.M.K., unpublished).
In conclusion, we have determined the sequences of the myosin heavy chain protein for five muscular tissues of the squid D. pealeii. All five tissues express 100% identical myosin heavy chain proteins, suggesting that squid, and possibly cephalopods in general, do not tune muscle contractile performance through different myosin isoforms. Rather, these animals likely use changes in muscle ultrastructure to modulate whole-muscle performance.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Joe Thompson and the staff of the Darling Marine Center (Walpole, ME, USA) for assistance in animal collection and handling, and Kathleen Smith for the loan of equipment. We also thank Fred Schachat for helpful discussions regarding our results.
LIST OF ABBREVIATIONS
- LMM
light meromyosin
- NO
nitric oxide
- RACE
rapid amplification of cDNA ends
- S1
myosin subfragment 1
- S2
myosin subfragment 2
- TdT
terminal deoxynucleotidyl transferase
- UTR
untranslated region
FOOTNOTES
Supplementary material available online at http://jeb.biologists.org/cgi/content/full/215/2/239/DC1
FUNDING
This work was supported by the Seeding Postdoctoral Innovators in Research and Education (SPIRE) program funded by the National Institutes of Health, Minority Opportunities in Research division of the National Institute of General Medical Sciences [K12GM000678 to J.F.S.], and the National Science Foundation [IOS-0951067 to W.M.K.]. Deposited in PMC for release after 12 months.
REFERENCES
- Barany M. (1967). ATPase activity of myosin correlated with speed of muscle shortening. J. Gen. Physiol. 50, 197-218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottinelli R., Schiaffino S., Reggiani C. (1991). Force–velocity relations and myosin heavy-chain isoform compositions of skinned fibres from rat skeletal muscle. J. Physiol. 437, 655-672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra M., Tschirgi M. L., Ford S. J., Slinker B. K., Campbell K. B. (2007). Interaction between myosin heavy chain and troponin isoforms modulate cardiac myofiber contractile dynamics. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R1595-R1607 [DOI] [PubMed] [Google Scholar]
- Chenna R., Sugawara H., Koike T., Lopez R., Gibson T. J., Higgins D. G., Thompson J. D. (2003). Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 31, 3497-3500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelista A. M., Rao V. S., Filo A. R., Marozkina N. V., Doctor A., Jones D. R., Gaston B., Guilford W. H. (2010). Direct regulation of striated muscle myosins by nitric oxide and endogenous nitrosothiols. PLoS ONE 5, e11209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrenbach W. H. (1967). Fine structure of fast and slow crustacean muscles. J. Cell Biol. 35, 69-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galler S., Hilber K., Gobesberger A. (1997). Effects of nitric oxide on force-generating proteins of skeletal muscle. Pflugers Arch. 434, 242-245 [DOI] [PubMed] [Google Scholar]
- Gasteiger E., Gattiker A., Hoogland C., Ivanyi I., Appel R. D., Bairoch A. (2003). ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 31, 3784-3788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauvry L., Mohan-Ram V., Ettelaie C., Ennion S., Goldspink G. (1997). Molecular motors designed for different tasks and to operate at different temperatures. J. Therm. Biol. 22, 367-373 [Google Scholar]
- Huxley A. F., Simmons R. M. (1973). Mechanical transients and origin of muscular force. Cold Spring Harb. Symp. Quant. Biol. 37, 669-680 [Google Scholar]
- Huxley H. E. (1969). Mechanism of muscular contraction. Science 164, 1356-1366 [PubMed] [Google Scholar]
- Josephson R. K. (1975). Extensive and intensive factors determining performance of striated muscle. J. Exp. Zool. 194, 135-154 [DOI] [PubMed] [Google Scholar]
- Kier W. M. (1982). The functional morphology of the musculature of squid (Loliginidae) arms and tentacles. J. Morphol. 172, 179-192 [DOI] [PubMed] [Google Scholar]
- Kier W. M. (1985). The musculature of squid arms and tentacles: ultrastructural evidence for functional differences. J. Morphol. 185, 223-239 [DOI] [PubMed] [Google Scholar]
- Kier W. M. (1991). Squid cross-striated muscle: the evolution of a specialized muscle-fiber type. Bull. Mar. Sci. 49, 389-403 [Google Scholar]
- Kier W. M., Curtin N. A. (2002). Fast muscle in squid (Loligo pealei): contractile properties of a specialized muscle fibre type. J. Exp. Biol. 205, 1907-1916 [DOI] [PubMed] [Google Scholar]
- Kier W. M., Schachat F. H. (1992). Biochemical comparison of fast-contracting and slow-contracting squid muscle. J. Exp. Biol. 168, 41-56 [DOI] [PubMed] [Google Scholar]
- Kier W. M., Schachat F. H. (2008). Muscle specialization in the squid motor system. J. Exp. Biol. 211, 164-169 [DOI] [PubMed] [Google Scholar]
- Kodama S., Konno K. (1983). Isolation and biochemical properties of myosin from squid brachial muscle. Bull. Jpn. Soc. Sci. Fish. 49, 437-442 [Google Scholar]
- Konno K. (1978). Two calcium regulation systems in squid (Ommastrephes sloani pacificus) muscle preparation of calcium-sensitive myosin and troponin-tropomyosin. J. Biochem. 84, 1431-1440 [DOI] [PubMed] [Google Scholar]
- Konno K., Arai K., Yoshida M., Watanabe S. (1981). Calcium regulation in squid mantle and scallop adductor muscles. J. Biochem. 89, 581-589 [DOI] [PubMed] [Google Scholar]
- Lehman W., Szent-Gyorgyi A. G. (1975). Regulation of muscular-contraction. Distribution of actin control and myosin control in the animal kingdom. J. Gen. Physiol. 66, 1-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. L., Mykles D. L. (1990). Analysis of myosins from lobster muscles – fast and slow isozymes differ in heavy-chain composition. J. Exp. Zool. 255, 163-170 [Google Scholar]
- Lowey S., Waller G. S., Trybus K. M. (1993). Skeletal muscle myosin light chains are essential for physiological speeds of shortening. Nature 365, 454-456 [DOI] [PubMed] [Google Scholar]
- Matulef K., Sirokman K., Perreault-Micale C. L., Szent-Gyorgyi A. G. (1998). Amino acid sequence of squid myosin heavy chain. J. Muscle. Res. Cell Motil. 19, 705-712 [DOI] [PubMed] [Google Scholar]
- Medler S., Mykles D. L. (2003). Analysis of myofibrillar proteins and transcripts in adult skeletal muscles of the American lobster Homarus americanus: variable expression of myosins, actin and troponins in fast, slow-twitch and slow-tonic fibres. J. Exp. Biol. 206, 3557-3567 [DOI] [PubMed] [Google Scholar]
- Millman B. M. (1967). Mechanism of contraction in molluscan muscle. Am. Zool. 7, 583-591 [Google Scholar]
- Murphy C. T., Spudich J. A. (1998). Dictyostelium myosin 25-50K loop substitutions specifically affect ADP release rates. Biochemistry 37, 6738-6744 [DOI] [PubMed] [Google Scholar]
- Mykles D. L. (1985). Heterogeneity of myofibrillar proteins in lobster fast and slow muscles: variants of troponin, paramyosin, and myosin light-chains comprise four distinct protein assemblages. J. Exp. Zool. 234, 23-32 [DOI] [PubMed] [Google Scholar]
- Neville D. M. (1971). Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J. Biol. Chem. 246, 6328-6334 [PubMed] [Google Scholar]
- Nunzi M. G., Franzini Armstrong C. (1981). The structure of smooth and striated portions of the adductor muscle of the valves in a scallop. J. Ultrastruct. Res. 76, 134-148 [DOI] [PubMed] [Google Scholar]
- Nyitray L., Jancso A., Ochiai Y., Graf L., Szent-Gyorgyi A. G. (1994). Scallop striated and smooth muscle myosin heavy-chain isoforms are produced by alternative RNA splicing from a single gene. Proc. Natl. Acad. Sci. USA 91, 12686-12690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogonowski M. M., Lang F. (1979). Histochemical evidence for enzyme differences in crustacean fast and slow muscle. J. Exp. Zool. 207, 143-151 [Google Scholar]
- Palumbo A. (2005). Nitric oxide in marine invertebrates: a comparative perspective. Comp. Biochem. Physiol. 142A, 241-248 [DOI] [PubMed] [Google Scholar]
- Pearson W. R. (2006). Local Protein Alignments (LALIGN). Available at http://fasta.bioch.virginia.edu/fasta_www2/fasta_www.cgi?rm=lalign&pgm=lal
- Perkins W. J., Han Y. S., Sieck G. C. (1997). Skeletal muscle force and actomyosin ATPase activity reduced by nitric oxide donor. J. Appl. Physiol. 83, 1326-1332 [DOI] [PubMed] [Google Scholar]
- Perreault-Micale C. L., Jancso A., Szent-Gyorgyi A. G. (1996a). Essential and regulatory light chains of Placopecten striated and catch muscle myosins. J. Muscle Res. Cell Motil. 17, 533-542 [DOI] [PubMed] [Google Scholar]
- Perreault-Micale C. L., Kalabokis V. N., Nyitray L., Szent-Gyorgyi A. G. (1996b). Sequence variations in the surface loop near the nucleotide binding site modulate the ATP turnover rates of molluscan myosins. J. Muscle Res. Cell Motil. 17, 543-553 [DOI] [PubMed] [Google Scholar]
- Rayment I., Rypniewski W. R., Schmidtbase K., Smith R., Tomchick D. R., Benning M. M., Winkelmann D. A., Wesenberg G., Holden H. M. (1993). Three-dimensional structure of myosin subfragment-1-a molecular motor. Science 261, 50-58 [DOI] [PubMed] [Google Scholar]
- Rovner A. S., Fagnant P. M., Lowey S., Trybus K. M. (2002). The carboxyl-terminal isoforms of smooth muscle myosin heavy chain determine thick filament assembly properties. J. Cell Biol. 156, 113-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruegg J. C. (1971). Smooth muscle tone. Physiol. Rev. 51, 201-248 [DOI] [PubMed] [Google Scholar]
- Scotto-Lavino E., Du G., Frohman M. A. (2006a). 3′ end cDNA amplification using classic RACE. Nat. Protoc. 1, 2742-2745 [DOI] [PubMed] [Google Scholar]
- Scotto-Lavino E., Du G., Frohman M. A. (2006b). 5′ end cDNA amplification using classic RACE. Nat. Protoc. 1, 2555-2562 [DOI] [PubMed] [Google Scholar]
- Spudich J. A. (1994). How molecular motors work. Nature 372, 515-518 [DOI] [PubMed] [Google Scholar]
- Sweeney H. L., Kushmerick M. J., Mabuchi K., Sreter F. A., Gergely J. (1988). Myosin alkali light chain and heavy chain variations correlate with altered shortening velocity of isolated skeletal muscle fibers. J. Biol. Chem. 263, 9034-9039 [PubMed] [Google Scholar]
- Tsuchiya T., Kaneko T., Matsumoto J. J. (1978). Calcium sensitivity of mantle muscle of squid. J. Biochem. 83, 1191-1193 [DOI] [PubMed] [Google Scholar]
- Uyeda T. Q. P., Ruppel K. M., Spudich J. A. (1994). Enzymatic activities correlate with chimeric substitutions at the actin-binding face of myosin. Nature 368, 567-569 [DOI] [PubMed] [Google Scholar]
- Van Leeuwen J. L., Kier W. M. (1997). Functional design of tentacles in squid: linking sarcomere ultrastructure to gross morphological dynamics. Philos. Trans. R. Soc. Lond. B 352, 551-571 [Google Scholar]
- Whiteley N. M., Magnay J. L., McCleary S. J., Nia S. K., El Haj A. J., Rock J. (2010). Characterisation of myosin heavy chain gene variants in the fast and slow muscle fibres of gammarid amphipods. Comp. Biochem. Physiol. 157A, 116-122 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.