Abstract
In this article, we describe detailed protocols for the isolation and transfer of spindle–chromosomal complexes between mature, metaphase II-arrested oocytes. In brief, the spindle–chromosomal complex is visualized using a polarized microscope and extracted into a membrane-enclosed karyoplast. Chromosomes are then reintroduced into an enucleated recipient egg (cytoplast), derived from another female, by karyoplast–cytoplast membrane fusion. Newly reconstructed oocytes consist of nuclear genetic material from one female and cytoplasmic components, including mitochondria and mitochondrial DNA (mtDNA), from another female. This approach yields developmentally competent oocytes suitable for fertilization and producing embryonic stem cells or healthy offspring. The protocol was initially developed for monkey oocytes but can also be used in other species, including mouse and human oocytes. Potential clinical applications include mitochondrial gene replacement therapy to prevent transmission of mtDNA mutations and treatment of infertility caused by cytoplasmic defects in oocytes. Chromosome transfer between the cohorts of oocytes isolated from two females can be completed within 2 h.
INTRODUCTION
Transplantation of genetic material in mammalian oocytes and embryos
During oogenesis, mammalian oocytes undergo two subsequent meiotic divisions that result in a single, haploid egg. The first meiotic division begins in the fetal ovary, but oocytes arrest at prophase I of the first meiotic cell cycle. Primary oocytes at this stage have a distinct large nucleus known as a ‘germinal vesicle’ (GV). At puberty, oocytes resume meiosis and undergo germinal vesicle breakdown, followed by condensation of chromosomes and segregation of the first polar body. Mature oocytes arrest again at the metaphase II (MII) stage. Completion of meiosis and separation of chromosomes into the second polar body are incited by sperm entry at fertilization. Transplantation of genetic material between mammalian oocytes offers many opportunities to study various aspects of nuclear-cytoplasmic interactions during oogenesis, fertilization and embryo development1,2. Such technologies may also have far-reaching clinical applications for overcoming cytoplasmic defects in human oocytes. Particularly, new assisted reproductive options have been sought that would prevent the transmission of mitochondrial diseases, caused by mutations in mitochondrial DNA (mtDNA), from affected women to their children3. Furthermore, whereas the mechanisms responsible for reproductive aging in older women are unclear, assisted reproductive technology (ART) results show that women even in their sixties can have healthy children as long as they use oocytes donated by younger women4. If the factors responsible for oocyte aging are confined to the cytoplasm and not to the nucleus itself, the nuclear transfer strategy may well prove valuable for overcoming this form of reproductive aging and allow older women to have their own biological children.
In model animals, successful nuclear transfer has been accomplished between GV oocytes (GVT)5. The choice of this particular stage of oocytes has been mainly dictated by the visibility of the nucleus, and by the possibility of isolating and transplanting intact nuclear material surrounded by a nuclear membrane. Similarly, nuclear transfer techniques have also been expanded to pronuclear-stage zygotes6. Until recently, transfer of genetic material in mature oocytes was thought to be unattainable because of the unique biological characteristics of MII-arrested oocytes. However, transplantation of MII chromosomes has several clear advantages over GV oocytes or pronuclear-stage zygotes. (i) In contrast to GV oocytes, mature eggs do not require in vitro maturation before fertilization. In humans, in vitro maturation of GV-intact oocytes is inefficient and associated with poor developmental competence following fertilization. Mature MII eggs, however, are routinely retrieved and used in clinical in vitro fertilization (IVF) programs. (ii) Pronuclear transfer in fertilized human zygotes is associated with serious ethical and moral issues involving the destruction of human embryos. (iii) Nuclear transplantation in GV oocytes and pronuclear-stage zygotes inevitably results in significant mtDNA carryover because of an uneven concentration of mitochondria in the perinuclear space6–9. Thus, transmission of mtDNA from nuclear donor oocytes generates a notable heteroplasmy in embryos and offspring, rendering these approaches inappropriate for patients with mtDNA mutations. Our recent findings indicate that mitochondria are evenly distributed in MII oocytes, and that chromosome transfer does not cause any detectable mtDNA heteroplasmy in resulting embryos and offspring10.
Despite these advantages, one of the main difficulties in nuclear transplantation in mature oocytes is related to the detection of nuclear material in mature eggs, using conventional microscopes. This is because of the fact that a nuclear membrane in MII oocytes is absent and chromatin is condensed into chromosomes. Furthermore, metaphase chromosomes and the spindle apparatus in MII oocytes are prone to damage or premature resumption of meiosis and abnormal segregation of chromosomes during manipulations. Early attempts to transfer MII chromosomes in human MII oocytes resulted in limited success because of low fertilization rates, pronuclear anomalies and poor embryo development11,12. The developmental potential of such embryos was only monitored in vitro by blastocyst formation rates.
Chromosome transfer in metaphase II oocytes
We recently implemented several methodological advances during manipulation of rhesus macaque MII oocytes to surmount these biological barriers10. We incorporated and adopted the spindle imaging system (Oosight from CRi) for detection and isolation of MII spindle–chromosomal complexes. This innovative approach allowed efficient, noninvasive visualization and removal of intact MII spindles into small karyoplasts with nearly 100% efficiency. Furthermore, this technique also enables isolation of karyoplasts containing very small amounts of cytoplasm, thus minimizing the amount of cotransferred mtDNA. Another modification was implemented to avoid spontaneous activation and premature segregation of MII chromosomes during introduction of karyoplasts into recipient cytoplasts by electrofusion. We developed the karyoplast–cytoplast fusion method that uses the natural cell membrane fusion property of the viral envelope isolated from the Hemagglutinating virus of Japan (HVJ, also referred to as inactivated SeV).
Using these modifications, we recently showed that reconstructed MII oocytes are capable of supporting normal fertilization, embryo development and production of healthy offspring or embryonic stem cells10. Moreover, genetic analysis confirmed that mtDNA in all analyzed offspring and stem cells originated exclusively from cytoplasts with no contribution of spindle donor mtDNA. In this study, we describe detailed methods for chromosome transfer in MII-arrested oocytes in a clinically relevant nonhuman primate model. Protocols include specific reagents, supplies and equipment required to conduct these procedures. We also illustrate step-by-step micromanipulation techniques that involve isolation of chromosomes into karyoplasts, followed by transfer to and fusion with recipient cytoplasts. Descriptions of these procedures are supported by illustrations in figures and a video. Because of close similarities in oocyte biology and embryo development between rhesus macaques and humans, described protocols are directly applicable to human oocytes in standard clinical IVF settings. Most supporting ART techniques required for chromosome transfer are routine in clinical IVF laboratories. These include collection of MII oocytes, fertilization by intracytoplasmic sperm injection (ICSI) and in vitro embryo culture. Therefore, we focused here on the detailed description of manipulation procedures specifically required for chromosome transfer. In addition to standard IVF equipment, our procedures require a spindle imaging system and a laser objective.
We also believe that the described manipulation procedures are universal, meaning that critical equipment, reagents and micro-manipulation steps described here are optimal to successfully carry out chromosome transfer in other species. Therefore, our protocols can be applied without further adaptations to other species, including mice and farm animals. During somatic cell nuclear transfer procedures, MII spindles in selected mouse strains can be located and removed without a special imaging system. However, unlike cloning, in which nuclear material is normally discarded, spindle-chromosomal integrity during chromosome transfer must be maintained. Therefore, we find that spindle imaging is extremely helpful to maintain intact mouse MII spindles during the enucleation step.
In addition to the specific chromosome transfer steps, we also included a detailed description of monkey oocyte and embryo culture media preparations and fertilization by ISCI. These media and protocols are specific to the rhesus monkey (particularly in our laboratory) and should be replaced with appropriate protocols for other species.
MATERIALS
REAGENTS
Rhesus macaque MII-stage oocytes and semen were used (detailed protocols for controlled ovarian stimulations, oocyte and sperm collections are available in ref. 13). All our animal procedures were approved by the institutional animal care and use committee at the Oregon National Primate Research Center. ! CAUTION Experimenters must comply with national regulations about animals and their use.
Talp HEPES medium (TH; see REAGENT SETUP and Table 1)
Talp HEPES medium + 0.3% BSA (TH3; see REAGENT SETUP)
Hamster embryo culture medium (HECM-9; see REAGENT SETUP and Table 1)
Cytochalasin B (Sigma, cat. no. C6762)
Dimethylsulfoxide (DMSO; Sigma, cat. no. D2650)
HVJ Envelope Cell Fusion Kit (Ishihara Sangyo Kaisha Ltd, cat. no. GenomONE-CF EX)
Mineral oil (SAGE Assisted Reproduction Products, cat. no. ART-4008)
High-viscosity silicon oil (Fluka, cat. no. 85416)
TABLE 1.
Reagents and quantities for media preparations
| Reagents | TH medium (1,000 ml) | HECM-9 (1,000 ml) | AA 100× stock (100 ml) |
|---|---|---|---|
| NaCl (Sigma, cat. no. S5886) | 6.660 g | 6.639 g | |
| KCl (Sigma, cat. no. P5405) | 0.239 g | 0.2240 g | |
| CaCl2-2H2O (Sigma, cat. no. C7902) | 0.294 g | 0.2790 g | |
| MgCl2-6H2O (Sigma, cat. no. M0250) | 0.102 g | 0.1020 g | |
| Na2HPO4 (Sigma, cat. no. S5136) | 0.048 g | ||
| Glucose (Sigma, cat. no. G6152) | 0.900 g | ||
| Na lactate (Sigma, cat. no. L7900) | 1.870 ml | 0.6320 ml | |
| Phenol Red (Sigma, cat. no. P3532) | 0.010 g | ||
| NaHCO3 (Sigma, cat. no. S5761) | 0.168 g | 2.1000 g | |
| Gentamycin Sulfate (Sigma, cat. no. G1264) | 0.050 g | 0.0100 g | |
| HEPES (Sigma, cat. no. H6147) | 2.603 g | ||
| Na pyruvate (Sigma, cat. no. P4562) | 0.060 g | ||
| PVA (Sigma, cat. no. P8136) | 0.1000 g | ||
| HCl, 1 M (Sigma, cat. no. H7020) | 1.4000 ml | ||
| Taurine (Sigma, cat. no. T8691) | 0.626 g | ||
| Asparagine (Sigma, cat. no. A4159) | 0.013 g | ||
| Cytseine (Sigma, cat. no. C6852) | 0.018 g | ||
| Histidine (Sigma, cat. no. H5659) | 0.021 g | ||
| Lysine (Sigma, cat. no. L8662) | 0.018 g | ||
| Proline (Sigma, cat. no. P5607) | 0.012 g | ||
| Serine (Sigma, cat. no. S4311) | 0.011 g | ||
| Aspartic acid (Sigma, cat. no. A4534) | 0.013 g | ||
| Glycine (Sigma, cat. no. G8790) | 0.008 g | ||
| Glutamic acid (Sigma, cat. no. G5889) | 0.017 g | ||
| Glutamine (Sigma, cat. no. G8540) | 0.292 g | ||
| Pantothenic acid (Sigma, cat. no. P5155) | 0.007 g |
EQUIPMENT
Inverted microscope with Hoffman or Relief Contrast optics (Olympus IX70 or IX71)
Dissecting stereomicroscope (Olympus SZX-7)
Heated microscope stages (Tokai Hit, MATS-U55R30 and MATS-U4020WF)
Micromanipulators (Narishige, cat. no. MMO-202ND)
Micropipette holders (Narishige, cat. no. IM-7)
Microinjection system and microsyringe (Narishige, cat. no. IM-5B; Hamilton, cat. no. 81101, 1725LT 250 μl SYR)
Teflon tubing (Narishige, cat. no. CT-2)
Holding pipettes, inner diameter (i.d.) 25–30 μm, outer diameter (o.d.) 120–140 μm, 20° angle (Humagen Fertility Diagnostics, cat. no. MPH-LG-20)
Enucleation pipettes (Biomedical Instruments, ES blastocyst injection pipette, with spike, i.d. = 13.4–14.1 μm (size 38 or 39), L = 5.5 cm, BA = 20°, bending orientation C, BL 1 mm) ▴ CRITICAL The size of holding and enucleation pipettes may vary between species. For spindle–chromosomal complex isolations in the mouse, we recommend to use enucleation pipette i.d. = 12–13 μm. It is larger than the enucleation pipette routinely used during mouse somatic cell nuclear transfer. The holding pipette size for mouse oocytes is o.d. = 90–100 μm and i.d. = 15–20 μm.
Intracytoplasmic sperm injection (ICSI) pipette (Humagen Fertility Diagnostics, cat. no. MIC-NSP-0)
Oosight spindle imaging system (CRi)
XYClone RED-i laser objective (Hamilton Thorne)
CO2/O2 incubator to maintain 6% CO2, 5% O2 and 89% N2 embryo culture conditions
Alternatively, premixed gas can be used to fill up the modular incubator chamber (Billups-Rothenberg, cat. no. MIC-101)
FluoroDish Cell Culture Dish, Oosight-compatible glass-bottom micromanipulation dishes (World Precision Instruments, cat. no. FD5040-100).
Nunclon four-well dishes (Thermo Sci Nunc, cat. no. 176740)
50 × 9-mm Petri dishes (Becton-Dickinson Falcon, cat. no. 35-1006)
REAGENT SETUP
Preparation of base Talp HEPES medium
To prepare 1,000 ml of TH medium, you should add exact amounts of chemicals listed in Table 1 to 1,000 ml of Milli-Q water. Adjust the pH of the TH medium to 7.4 and osmolarity to 275–290. Filter the TH medium through a 0.22-μm filter and store up to 1 month at 4 °C. TH is used as a base medium for TH3 or for diluting reagents. ▴ CRITICAL TH and HECM manipulation and embryo culture media are specific to the rhesus monkey. These media should be replaced with appropriate 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES)-buffered manipulation and embryo culture media for different species. For example, we routinely use M2 medium for mouse embryo manipulations, and potassium simplex optimization medium for mouse embryo culture. ▴ CRITICAL It is important to use highly purified embryo-tested water during all medium and solution preparations.
Preparation of TH3 manipulation medium
Add 3 mg ml−1 BSA (Sigma, cat. no. A3311) to the TH medium, filter through a 0.22-μm filter and store up to 1 week at 4 °C. However, once warmed, use within 24 h. We usually prepare and warm up this medium the evening before an experiment. This medium is used for oocyte and sperm handling during collection and manipulations. ▴ CRITICAL Except TH and HECM media, it is crucial that a recommended supplier, manufacturer, reagent or catalog number is used for all reagents rather than than alternative. These reagents were meticulously tested in our laboratory over the years and alternative sources may not produce desirable results.
Preparation of hamster embryo culture medium
To prepare 1,000 ml HECM-9 base medium, you should add exact amounts of chemicals listed in Table 1 to 1,000 ml Milli-Q water. Adjust the pH to 7.4 and osmolarity to 277 ± 5. Sterilize by filtering through a 0.22-μm filter and store up to 1 week at 4 °C.
Preparation of 100× amino acid stock solution
To prepare 100 ml of stock solution, add amounts of amino acids listed in Table 1 to 100 ml of Milli-Q water. Filter through a 0.22-μm filter and aliquot into 500 μl. This stock solution can be stored for up to 3 months at − 20 °C.
Preparation of HECM-9 + AA medium
To prepare 10 ml HECM-9 + amino acid (AA) medium, you should add 0.1 ml AA 100× stock solution to 9.9 ml base HECM-9 medium. Preequilibrate in an incubator at 37 °C in 5% for a minimum of 4 h before use. This medium is used for culture of CO2 monkey oocytes and embryos up to the eight-cell stage.
Preparation of HECM-9 + AA + 5% FBS
Add 0.1 ml of AA 100× stock and 0.5 ml fetal bovine serum (FBS; Hyclone, cat. no. SH30070.03) to 9.4 ml of base HECM-9 medium. Preequilibrate in an incubator at 37 °C in 5% CO2 for a minimum of 4 h before use. This medium is used for culture of monkey embryos from the eight-cell stage to blastocysts.
Preparation of TH + Polyvinylpyrrolidone
Reconstitute a vial of lyophilized polyvinylpyrrolidone (PVP; Irvine Scientific, cat. no. 99219) with 1 ml of base TH medium and divide into 20-μl aliquots. TH + PVP can be stored for up to 3 months at 4 °C. This medium is used for sperm immobilization during ICSI.
Preparation of cytochalasin B
To prepare 1,000× stock solution of cytochalasin B (CB), it is recommended to reconstitute a vial containing 1 mg CB (Sigma) with 200 μl DMSO (Sigma). Divide this master stock of CB (5 mg ml−1) into 5-μl aliquots and store for up to 6 months at − 20 °C. For preparation of micromanipulation medium, it is recommended to add 1 μl CB master stock solution to 1 ml TH3 medium (final concentration 5 μg ml−1). All described chromosome transfer manipulations are carried out in this medium. ▴ CRITICAL Prepare this micromanipulation medium fresh just before use. ▴ CRITICAL Avoid multiple freezing and thawing of CB stock solution to maintain its activity. The CB working concentration may vary from 2.5 to 7.5 μg ml−1 depending on species and requirement of different manipulation procedures.
Preparation of HVJ-E solution
To prepare HVJ-E solution, you should reconstitute a vial of freeze-dried inactivated Sendai virus envelope (Ishihara Sangyo Kaisha Ltd) with 260 μl of suspension buffer (comes with the kit). Keep the solution on ice during preparation. Aliquot into 5-μl vials and store at − 80 °C for up to 3 months. For fusion of karyoplast/cytoplast couples, thaw a vial of HVJ-E solution just before manipulations and use undiluted. ▴ CRITICAL Avoid multiple freezing and thawing of HVJ-E stock solution to maintain its activity.
EQUIPMENT SETUP
Setting up micromanipulators and micropipettes
The micromanipulation station consisting of an Olympus inverted microscope, Narishige micromanipulators, a microinjector, a spindle imaging system and a laser objective is depicted in Figure 1a. Attach a metal micropipette holder (Narishige) to Teflon tubing connected to a 20-ml plastic syringe mounted on a stand (Fig. 1b). Insert and tighten a holding glass micropipette into a metal micropipette holder. Fill in approximately half of the holding micropipette with TH3 + CB manipulation medium. Air-fill the rest of the holding line.
Figure 1.
Equipment and pipette setting for chromosome transfer. (a) Micromanipulation station consisting of an inverted microscope, two micromanipulators, microinjectors, spindle imaging and laser systems controlled by a PC. (b) Setting up the holding pipette. The entire line consists of a metal Narishige pipette holder, Teflon tubing and a 20-ml plastic syringe filled with air. Syringe is mounted on a stand. (c) Setting up the enucleation pipette. The entire line consists of a metal Narishige pipette holder, Teflon tubing and a 250-μl Hamilton glass syringe filled with water. The syringe is mounted on the Narishige microinjection system.
Attach a second metal micropipette holder to a Teflon tubing connected to a 200–250-μl volume microsyringe controlled by a microinjector (Narishige; Fig. 1c). Fill the entire system with water. Load the enucleation micropipette completely with high-viscosity silicon oil to improve control over aspirations and injections. Insert and tighten an enucleation micropipette into a metal micropipette holder. ▴ CRITICAL The entire line including microsyringe, tubing and enucleation micropipette must be completely free of air bubbles.
Setting up the micromanipulation chamber
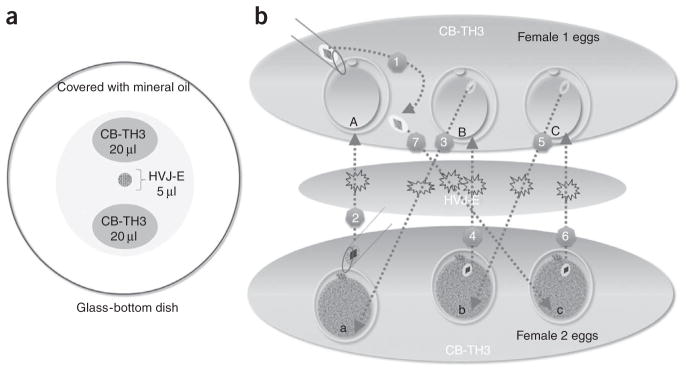
Place two 20-μl micromanipulation drops of TH3 medium containing CB (5 μg ml−1) and one 5-μl drop of HVJ-E solution in the center of glass-bottom dish as shown in Figure 2a. Cover the dish with approximately 3.2 ml SAGE oil. ▴ CRITICAL Separate micromanipulation drops are necessary to keep apart oocytes derived from different females. Inactivated SeV extract (HVJ-E) must be thawed immediately before use as its activity quickly declines, resulting in low fusion rates. ! CAUTION The micromanipulation equipment, settings and techniques may vary in each laboratory. We recommend the described settings because these techniques have been tested in our laboratory over the years on several species and for many types of micromanipulation needs.
Figure 2.
Experimental design for serial spindle–chromosomal complex transfer between two cohorts of oocytes. (a) Suggested layout of micromanipulation drops on the manipulation chamber. (b) Recommended experimental plan for serial chromosome transfer between oocytes from two females. Step 1: Isolate a karyoplast from the first (A) oocyte of female 1 and place it next to the cytoplast until the last transfer. Step 2: Isolate a karyoplast from the first (a) oocyte of female 2, briefly soak in HVJ-E and transfer into a perivitelline space of cytoplast A from female 1. Step 3: Isolate and transfer a karyoplast from the second (B) oocyte of female 1 and transfer it into the cytoplast (a) of female 2. Proceed in a similar manner for Steps 4, 5 and 6. During the last step (Step 7), pick up the karyoplast isolated from the oocyte (A) of female 1 and transfer into the last cytoplast (c) of female 2.
PROCEDURE
Karyoplast isolation ● TIMING 0–1 h
-
1|
Place three to five oocytes from each of the two females into separate manipulation drops and mount the chamber on the microscope stage. Incubate oocytes in CB containing manipulation medium for at least 5 min before manipulations. Set up the Oosight and laser system (next step) while waiting. We suggest an experimental plan for serial chromosome transfer between several oocytes from two different females as shown in Figure 2b. Step-by-step manipulations of an entire chromosome transfer procedure can also be found in Supplementary Video.
▴ CRITICAL STEP Oocytes and embryos are temperature sensitive and must be maintained at 37 °C at all times during retrieval, scoring, manipulation and culture. All oocyte manipulation procedures should be conducted in a dimly lit and warm room (30 °C).
-
2|
Switch the condenser turret to a slot with a circular polarizer and interference filter and insert a crystal universal compensator into the analyzer slot of the microscope. Open the side camera port in the microscope and start Oosight control software. Run a background acquisition using a 20× laser objective and initiate the Oosight mode according to the manual. Start XYClone laser control program to operate the laser objective. Adjust the laser power output to 100 and adjust pulse to a range of 100–300 μs.
▴ CRITICAL STEP Do not use full XYClone software to operate the laser during enucleation; instead use the laser control panel mode that is integrated with Oosight software.
-
3|
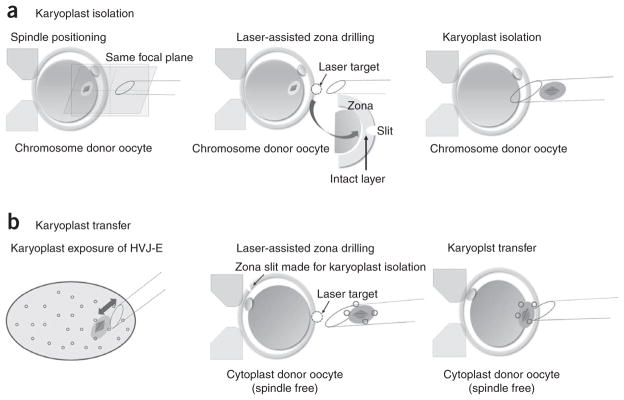
Lower both the holding and enucleation pipettes into the first manipulation drop and immobilize an individual oocyte on the holding pipette, with the sharpest spindle image situated in the equatorial plane close to the 3 o’clock position. Rotating an oocyte is essential to find the spindle (Fig. 3a; spindle positioning; see also Supplementary Video).
? TROUBLESHOOTING
-
4|
Navigate the holding pipette with an attached oocyte to the visible REDi laser target and position the target to the zona pellucida adjacent to the spindle.
-
5|
Slightly lower the holding pipette with oocyte attached and gently press an oocyte to the bottom of the plate to stabilize it during chromosome isolation.
-
6|
Focus the objective on the spindle and bring the tip of the enucleation pipette to the same focal plane.
▴ CRITICAL STEP Ensure that the enucleation pipette and spindle are in the same equatorial focal plane by gently poking an oocyte with the pipette without piercing the zona pellucida, as a result of which the spindle should move away (see Supplementary Video).
-
7|
Drill a hole in the zona next to the spindle with the laser, starting from the outer layer of the zona, using the lowest laser settings (Fig. 3a; laser-assisted zona drilling).
▴ CRITICAL STEP Avoid making a large gap in the zona and make sure to leave a thin intact inner layer in the zona pellucida. This will prevent leakage of the cytoplast from the zona.
? TROUBLESHOOTING
-
8|
Slowly insert the pipette through the slit in the zona pellucida without piercing the plasma membrane and navigate its opening close to the spindle.
-
9|
Slowly aspirate the spindle with a minimum amount of the underlying cytoplasm into the enucleation pipette. Confirm the presence of the spindle in the pipette, which can be easily observed within the pipette (see Supplementary Video; karyoplast isolation). There is no need for any further confirmation of enucleation by DNA staining.
? TROUBLESHOOTING
-
10|
Slowly withdraw the pipette from the slit in the zona pellucida. As the pipette is pulled away from the egg, the plasma membrane will stretch and form a thin bridge between the egg and the karyoplast. A rapid movement of the enucleation pipette toward the 6 o’clock position will break this membrane bridge (Fig. 3a; karyoplast isolation).
? TROUBLESHOOTING
-
11|
Expel the karyoplast into the manipulation drop next to the enucleated egg (cytoplast). Move both pipettes to the second manipulation drop containing eggs from the second female. Repeat the enucleation steps described above for the first oocyte in this group. Keep the karyoplast inside the pipette.
▴ CRITICAL STEP To avoid accidental mixing, store oocytes, cytoplasts and karyoplasts from each female in separate drops.
▴ CRITICAL STEP Avoid contact between the karyoplast and oil in the pipette as this may cause lysis.
▴ CRITICAL STEP We highly recommend that our experimental plan be followed (see Fig. 2b). To minimize manipulation steps (expelling karyoplasts from the pipette during an intermediate step) and avoid karyoplast lysis, we recommend the immediate transfer of a karyoplast from one female into an enucleated recipient oocyte from another female. Leaving isolated karyoplasts in the manipulation drop for an extended time may cause sticking of the membrane to the bottom of the dish and eventual lysis. However, the first karyoplast from the first female must be expelled from the pipette and placed into the drop until the last oocyte from the second female is enucleated.
? TROUBLESHOOTING
Figure 3.
Schematic diagram of the chromosome transfer procedure. Chromosome transfer in MII oocytes consists of two chief procedural steps: (a) isolation of the spindle–chromosomal complex into a karyoplast and (b) introduction of the karyoplast into a recipient cytoplast. Step-by-step manipulations of rhesus monkey oocytes can also be found in Supplementary Video.
Karyoplast transfer and oocyte assessment ● TIMING 2–3 h
-
12|
Release an enucleated egg from the second female and lift up the holding pipette from the manipulation chamber. Move the enucleation pipette containing the karyoplast into the middle HVJ-E drop.
-
13|
Gently expel the karyoplast from the pipette into the drop to soak in the HVJ-E for approximately 10 s (Fig. 3b). Aspirate the karyoplast again into the pipette and return the pipette to the first manipulation drop containing oocytes from the first female.
-
14|
Lower the holding pipette into the drop and immobilize previously prepared cytoplasts with the first polar body positioned at 9 o’clock, but avoid holding over the hole in the zona made previously during spindle isolation.
-
15|
Drill the hole in the zona in the 3 o’clock position, using the laser as described above (Fig. 3b; laser-assisted zona drilling).
-
16|
Insert the transfer pipette through the zona slit and gently eject the karyoplast into the perivitelline space (Fig. 3b; karyoplast transfer).
▴ CRITICAL STEP It is important to place a karyoplast as close as possible to the cytoplast, ensuring good contact between the membranes. Avoid injecting an excessive amount of medium that would create a large pocket in the perivitelline space during karyoplast transfer (see Supplementary Video; karyoplast transfer).
? TROUBLESHOOTING
-
17|
Isolate the spindle from the second oocyte of the first female. Soak the karyoplast in HVJ-E and transfer into the first cytoplast from the second female.
-
18|
Repeat swapping of chromosomes between the two female oocytes. During the last step, transfer the karyoplast isolated from the first oocyte into the last cytoplast from the second female.
-
19|
Rinse reconstructed oocytes at least three times in TH3 to remove the CB residue and place into embryo culture medium (HECM-9) at 37 °C in 5% CO2 for 20–30 min.
▴ CRITICAL STEP Manipulated oocytes must be thoroughly rinsed to remove CB residues as it may cause difficulties during the ICSI procedure.
-
20|
Evaluate fusion in 20–30 min after karyoplast transfer by noting the presence or absence of karyoplasts in the perivitelline space. Separate fused and unfused couples into different wells.
? TROUBLESHOOTING
-
21|
Return unfused oocytes into culture for an additional 30 min and check fusion again.
▴ CRITICAL STEP Fusion rates in our laboratory are nearly 100%. However, if fusion does not occur within 60 min, unfused oocytes can be directly exposed to HVJ-E solution for a brief time (5–10 s). Oocytes should be placed back into culture and evaluated for fusion after 20–30 min.
Intracytoplasmic sperm injection and ICSI preparation ● TIMING 4.5–5.5 h
-
22|
Wash collected spermatozoa twice by resuspension in TH3 medium, followed by centrifugation of the liquid portion of the ejaculate for 7 min, at 200g.
-
23|
Take an aliquot and determine motility and concentration and adjust sperm concentration to 1 × 106 motile spermatozoa per ml in TH3 medium, then store for approximately 3 h at room temperature before ICSI.
-
24|
Set up an inverted Olympus IX70 or IX71 microscope equipped with Hoffman or Relief Contrast optics, heating stage (set at 37 °C) and micromanipulators ready for the ICSI procedure.
-
25|
Set up micromanipulators and micropipettes in the same manner as for chromosome transfer, except use a smaller-diameter ICSI pipette instead of an enucleation pipette.
▴ CRITICAL STEP Use CB-free TH3 medium for manipulations during ISCI to avoid difficulties in penetrating the oocyte membrane.
-
26|
Fill approximately half the holding micropipette with TH3 medium before the micromanipulation procedure.
-
27|
Dilute a small aliquot of sperm with 10% PVP (1:4) and place a 5-μl drop in the center of the glass-bottom dish. It is not critical to use a glass-bottom manipulation dish for ISCI; you can use the plastic lid of 50 × 9-mm Falcon dish. Next, place a 30-μl drop of TH3 adjacent to the sperm drop. Slowly cover the manipulation dish with 3.2 ml of prewarmed (37 °C) SAGE oil.
-
28|
Transfer manipulated oocytes into the 30-μl drop of TH3 manipulation medium and place the dish on the heated stage (37 °C) of the microscope.
-
29|
Lower the ICSI pipette into the sperm drop. Select a motile sperm and quickly immobilize it by striking the midpiece with the tip of the pipette. Slowly aspirate the immobilized sperm into the ISCI pipette tail first.
-
30|
Move the ISCI pipette to the manipulation drop containing oocytes and lower the holding pipette.
-
31|
Immobilize an individual oocyte on the holding pipette with the polar body positioned at either the 12 o’clock or 6 o’clock position, but avoid holding over the hole in the zona made previously during chromosome transfer.
-
32|
Slightly press down the holding pipette with oocyte attached until it touches the bottom of the plate to stabilize the egg during injection.
-
33|
Focus the objective on the oocyte membrane and then bring the tip of the ICSI pipette into sharp focus next to the 3 o’clock position on the oocyte.
-
34|
Slowly push the sperm to the pipette tip using the microsyringe and insert the ICSI pipette through the zona pellucida and ‘into’ the oocyte across approximately one-third of the egg’s diameter. Piercing the plasma membrane during the ICSI procedure is critical but often difficult to achieve, as the needle can grossly invaginate the membrane without breaking through.
? TROUBLESHOOTING
-
35|
Pierce the plasma membrane by slowly aspirating egg cytoplasm into the ICSI pipette (as far back as the needle junction with the zona pellucida) until the plasma membrane breaks. A ‘pop’ or sudden movement of cytoplasm into the pipette will indicate the release of membrane tension. Once the membrane is penetrated, expel the sperm into the cytoplasm with a minimal amount of medium.
? TROUBLESHOOTING
Embryo culture to the blastocyst stage ● TIMING 7–9 d
-
36|
After ICSI, transfer embryos into HECM-9 + AA culture medium (containing amino acids) covered with SAGE oil and culture at 37 °C, in 6% CO2, 5% O2 and 89% N2.
-
37|
Check for fertilization by noting the presence of pronuclei the next morning. This should be carried out no later than 14–16 h after ICSI. The presence of two pronuclei indicates normal fertilization. You will find the second polar body on the opposite side of the first polar body.
? TROUBLESHOOTING
-
38|
Monitor embryos daily for cleavage and embryonic development. Eight-cell–stage embryos should be transferred into fresh HECM-9 medium containing amino acids and 5% FBS and cultured for a maximum of 9 d with medium changes every other day. On reaching the blastocyst stage, embryos can be transferred into surrogate females or used for the derivation of embryonic stem cells.
? TROUBLESHOOTING
● TIMING
Steps 1–11, karyoplast isolation: 2–4 min per oocyte
Steps 12–16, karyoplast transfer: 1–2 min per oocyte
Steps 17 and 18, repeat serial chromosome transfer for remaining oocytes: 3–6 min × number of oocytes, 30–60 min (for a total of 10 oocytes)
Step 19, rinse oocytes: 3 min
Step 20, evaluation of fusion: 30 min after reconstruction
Step 21, preculture before ICSI: further 30 min
Steps 22 and 23, preparation of sperm for ICSI: 3–4 h before ICSI
Steps 24–35, fertilization by ICSI: 2–3 min per oocyte
Step 36, transfer embryos back into culture media: immediately after ICSI
Step 37, fertilization check: no later than 14–16 h after ICSI
Step 38, embryo culture: up to 9 d
? TROUBLESHOOTING
Troubleshooting advice can be found in Table 2.
TABLE 2.
Troubleshooting table
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 3 | Complete absence of or poor spindle image | Incorrect setting of the imaging system Improper position of an oocyte Immature oocytes or premature activation Suboptimal temperature |
Make sure that all Oosight parts are properly installed (polarizer, CRi lens, camera port, etc.) and the camera port is open. Run background acquisition and make sure that all Oosight modes are working properly. Refer to the manual The spindle of primate oocytes is much smaller than in rodents and can only be seen at a certain position within an oocyte. Slow rotation of oocytes in different directions is always required to visualize spindles If a meiotic mid-body is present adjacent to the first polar body, the oocyte is still completing meiosis I. Place oocytes back into culture medium for 30–60 min and attempt imaging again. Wash oocytes before culture to remove cytochalasin B residue. If a second PB is observed, meiosis has been completed, possibly due to premature activation The microtubules of the spindle apparatus are temperature sensitive. Always maintain and operate oocytes at 37 °C |
| 7 | Difficulties in the laser-assisted zona pellucida drilling | Incorrect setting(s) on the laser system Laser power level set too low |
Check all connections of the laser system. Contact Hamilton Thorne representatives for troubleshooting Increase laser output gradually |
| 9 | Difficulties in isolation of spindles | Enucleation pipette is out of focus The pipette is too small |
Make sure to bring the spindle into an equatorial plane close to the 3 o’clock position and move the pipette tip to the same focal plane. Proper alignment can be confirmed by gently poking the spindles through the zona with the pipette tip. You should be able to push the spindle away The optimal size of the pipette is important. The spindle may not fit easily if the pipette is too small. However, too-large pipettes may remove excess volume of cytoplasm during karyoplast isolation |
| 10 | Lysis of cytoplasts during spindle isolation | Incorrect concentration of CB Sharp edges of the enucleation pipette | Check the CB concentrations and make sure to use fresh CB stock The tip of enucleation pipettes must be polished to avoid cutting the membrane. This is normally done by the pipette manufacturer |
| 11 | Karyoplast lysis | Incorrect concentration of CB Sticking of the karyoplast membrane to the bottom of the dish, pipette and pipette oil |
Check the CB concentrations and make sure to use fresh CB stock Avoid keeping isolated karyoplasts too long within the pipette or in the manipulation drop. Transfer karyoplasts immediately into recipient cytoplasts. Avoid contact between a karyoplast and oil in the pipette |
| 16 | ‘Leaking’ of the cytoplasm out of the opposite hole in the zona pellucida during karyoplast transfer | Excessive pressure on the zona pellucida during insertion of the pipette Too large a hole in the zona |
Avoid squishing the zona during karyoplast transfer. See Figure 4d Minimize the size of the zona slit and always leave a thin intact layer in the zona wall. Review Step 7 and see Figure 4a and b |
| 20 | Poor fusion rates | Insufficient contact between karyoplasts and cytoplasts Inactive HVJ-E |
Make sure to remove extra fluid surrounding karyoplasts from the perivitelline space to ensure sufficient membrane contact. Review Step 16 and see Figure 4e and f Thaw HVJ-E just before use |
| 34 | ‘Leaking’ of cytoplasm out of the hole in the zona pellucida during ICSI | Excessive pressure on the zona pellucida during insertion of the pipette Too large a hole in the zona |
Poking with injection pipette away from the center of the oocyte may cause cytoplasmic leakage during ICSI. To prevent this, you may insert the pipette through the gap made earlier during chromosome transfer Once again, minimize the size of the zona slit and always leave a thin intact layer in the zona wall as we suggest during Steps 7 and 15. See Figure 4a and b |
| 35 | Difficulties of breaking oocyte membrane during ICSI | Excessive softening of oocytes due to CB residue | ICSI should be performed in CB-free media and oocytes must be thoroughly rinsed after chromosome transfer manipulations |
| 37, 38 | Poor fertilization and embryo development | Premature activation Inadequate culture condition |
Check your reconstructed oocytes carefully before ICSI. If you observe the second PB at the time of ICSI, oocytes are prematurely activated and have completed meiosis Check the quality of all reagents, media and culture conditions |
ANTICIPATED RESULTS
All techniques described above will require certain micromanipulation skills, necessitating diligent practice before attempting this procedure. Chromosome transfer in MII oocytes itself does not have any adverse effect on fertilization or embryo development. If a highly skilled person performs manipulations, spindle visualization and karyoplast isolation can be successfully achieved at a rate of 90% or higher. If one is having difficulties with spindle/karyoplast isolation, it is possible that the positioning of an oocyte on the holding pipette is not appropriate. Spend more time rotating an oocyte with your holding pipette so that you can position the metaphase spindle exactly where it should be; this will save you a lot of time when proceeding to remove the spindle with the enucleation pipette. Importantly, this will allow you to isolate chromosomes into a karyoplast with a minimum amount of cytoplasm. To avoid mtDNA carryover during chromosome transfer, we recommend that the size of the karyoplast should be minimized. Moreover, large karyoplasts (more that 1% of oocyte size) are difficult to transfer, often resulting in lysis during manipulation. It is often difficult to see spindles in freshly matured MII oocytes (within 30 min of polar body extrusion). We recommend waiting for an additional 1–2 h before attempting chromosome transfer with these oocytes. Transferring spindles back into enucleated oocytes should be relatively smooth and quick once you have practiced for a while. Experimental examples of good and poor outcomes during chromosome transfer are shown in Figure 4. To increase fusion rates, it is suggested to literally squeeze out all extra media that were transferred to the perivitelline space with the karyoplast. If the karyoplast and plasma membrane are in tight contact, fusion usually occurs within 20–30 min at a 90–100% rate. We do not recommend electrofusion because of accidental activation of MII spindle–chromosomal complexes induced by electric pulses10. We consider fertilization and cleavage rates of 80–90% acceptable, although we always strive for 100%. As a control for chromosome transfer procedures, we recommend monitoring the development of nonmanipulated ICSI embryos from the same cohort of oocytes. Blastocyst development may vary between experiments because of variation in oocyte quality following controlled ovarian stimulation protocols in nonhuman primates, but you should consistently be producing blastocysts from each experiment.
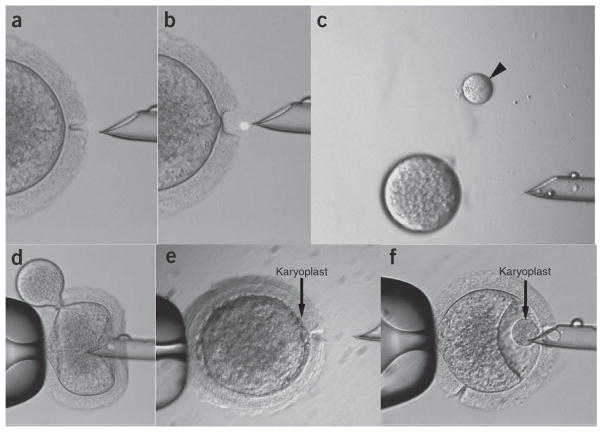
Figure 4.
Experimental examples of good and poor outcomes during chromosome transfer. (a) Optimal (partial) zonal drilling with laser, leaving a thin intact layer. This will prevent cytoplasm leakage during further manipulations. (b) An example of too large a gap in the zona pellucida. (c) Examples of small (desirable, depicted by arrow) and oversized karyoplasts. (d) Cytoplasmic leakage during karyoplast transfer (or ICSI) as a result of a large zona gap. (e) Good contact between karyoplast and cytoplast that is critical for efficient fusion. (f) An example of poor karyoplast–cytoplast contact due to a large pocket in the perivitelline space.
Supplementary Material
Acknowledgments
This work was supported by start-up funds from the Oregon National Primate Research Center, the Oregon Stem Cell Center and grants from the National Institutes of Health HD057121, HD059946, HD063276, HD047721, HD047675, RR0000163 and U54 HD18185.
Footnotes
Note: Supplementary information is available via the HTML version of this article.
AUTHOR CONTRIBUTIONS S.M., M.S. and M.T. developed and wrote this protocol.
COMPETING FINANCIAL INTERESTS The authors declare no competing financial interests.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/.
References
- 1.Cui LB, Huang XY, Sun FZ. Transfer of germinal vesicle to ooplasm of young mice could not rescue ageing-associated chromosome misalignment in meiosis of oocytes from aged mice. Hum Reprod. 2005;20:1624–1631. doi: 10.1093/humrep/deh826. [DOI] [PubMed] [Google Scholar]
- 2.Liu H, Wang CW, Grifo JA, Krey LC, Zhang J. Reconstruction of mouse oocytes by germinal vesicle transfer: maturity of host oocyte cytoplasm determines meiosis. Hum Reprod. 1999;14:2357–2361. doi: 10.1093/humrep/14.9.2357. [DOI] [PubMed] [Google Scholar]
- 3.Roberts RM. Prevention of human mitochondrial (mtDNA) disease by nucleus transplantation into an enucleated donor oocyte. Am J Med Genet. 1999;87:265–266. doi: 10.1002/(sici)1096-8628(19991126)87:3<265::aid-ajmg14>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 4.Paulson RJ, et al. Pregnancy in the sixth decade of life: obstetric outcomes in women of advanced reproductive age. JAMA. 2002;288:2320–2323. doi: 10.1001/jama.288.18.2320. [DOI] [PubMed] [Google Scholar]
- 5.Takeuchi T, Ergun B, Huang TH, Rosenwaks Z, Palermo GD. A reliable technique of nuclear transplantation for immature mammalian oocytes. Hum Reprod. 1999;14:1312–1317. doi: 10.1093/humrep/14.5.1312. [DOI] [PubMed] [Google Scholar]
- 6.Sato A, et al. Gene therapy for progeny of mito-mice carrying pathogenic mtDNA by nuclear transplantation. Proc Natl Acad Sci USA. 2005;102:16765–16770. doi: 10.1073/pnas.0506197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meirelles FV, Smith LC. Mitochondrial genotype segregation in a mouse heteroplasmic lineage produced by embryonic karyoplast transplantation. Genetics. 1997;145:445–451. doi: 10.1093/genetics/145.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meirelles FV, Smith LC. Mitochondrial genotype segregation during preimplantation development in mouse heteroplasmic embryos. Genetics. 1998;148:877–883. doi: 10.1093/genetics/148.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilding M, et al. Mitochondrial aggregation patterns and activity in human oocytes and preimplantation embryos. Hum Reprod. 2001;16:909–917. doi: 10.1093/humrep/16.5.909. [DOI] [PubMed] [Google Scholar]
- 10.Tachibana M, et al. Mitochondrial gene replacement in primate offspring and embryonic stem cells. Nature. 2009;461:367–372. doi: 10.1038/nature08368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen J, et al. Ooplasmic transfer in mature human oocytes. Mol Hum Reprod. 1998;4:269–280. doi: 10.1093/molehr/4.3.269. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka A, et al. Metaphase II karyoplast transfer from human in-vitro matured oocytes to enucleated mature oocytes. Reprod Biomed Online. 2009;19:514–520. doi: 10.1016/j.rbmo.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Mitalipov SM, Wolf DP. Nuclear transfer in nonhuman primates. Methods Mol Biol. 2006;348:151–168. doi: 10.1007/978-1-59745-154-3_10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.