Abstract
Importance of the field
The evolution of targeted therapies is dependent upon identification of cellular moieties that can be pharmacologically modulated. As one such example, the serine-threonine kinase Akt was identified nearly two decades ago. Since then, its role in mediating multiple signaling cascades (ultimately leading to cell growth and proliferation) has since been identified. More recently, several agents have been developed that antagonize Akt – these agents are in various stages of clinical testing.
Areas covered in this review
Herein, we outline development of several promising Akt inhibitors, including perifosine, MK-2206, RX-0201, PBI-05204, GSK2141795, and others.
What the reader will gain
The reader will gain insight into the current pipeline of Akt inhibitors, and the degree to which these agents have been examined both clinically and preclinically.
Take home message
With an emerging pipeline of agents targeting Akt, it will be critical to decipher which amongst them holds the greatest promise. Herein, we explore this drug pipeline and provide strategies for determining the future clinical application of these agents.
Keywords: Akt, perifosine, MK-2206, RX-0201, PBI-05204, GSK2141795, biomarker
1.0 INTRODUCTION
In the field of oncology, the advent of targeted agents has allowed clinical outcomes to surpass the plateau achieved by traditional chemotherapeutic and immunotherapeutic agents. This applies to a wide spectrum of malignancies – several prominent examples include the use of imatinib in chronic myelogenous leukemia, the use of trastuzumab for HER2-overexpressing breast cancer, and the use of sunitinib, sorafenib and other angiogenesis inhibitors for metastatic renal cell carcinoma (mRCC).1–4 mRCC serves as an excellent paradigm for the impact of targeted therapies. A largely chemotherapy-resistant disease, the only FDA approved agent for use in mRCC prior to this decade was interleukin-2 (IL-2), with approval granted in 1992.5 Since 2005, a total of six targeted agents have been approved for the treatment of mRCC (sunitinib, sorafenib, bevacizumab, pazopanib, temsirolimus and everolimus).6 Though these drugs have dramatically improved clinical outcomes for mRCC, a new therapeutic plateau is being realized. For the investigator, development of novel targeted therapies to complement or replace existing agents remains an imperative. Looking upstream or downstream of existing therapeutic targets is one method of identifying new targets for drug therapy. One such target is the serine-threonine kinase protein kinase B (PKB), or Akt.7 Herein, efforts to exploit Akt-mediated signaling are described.
2.0 ROLE OF Akt IN CARCINOGENESIS
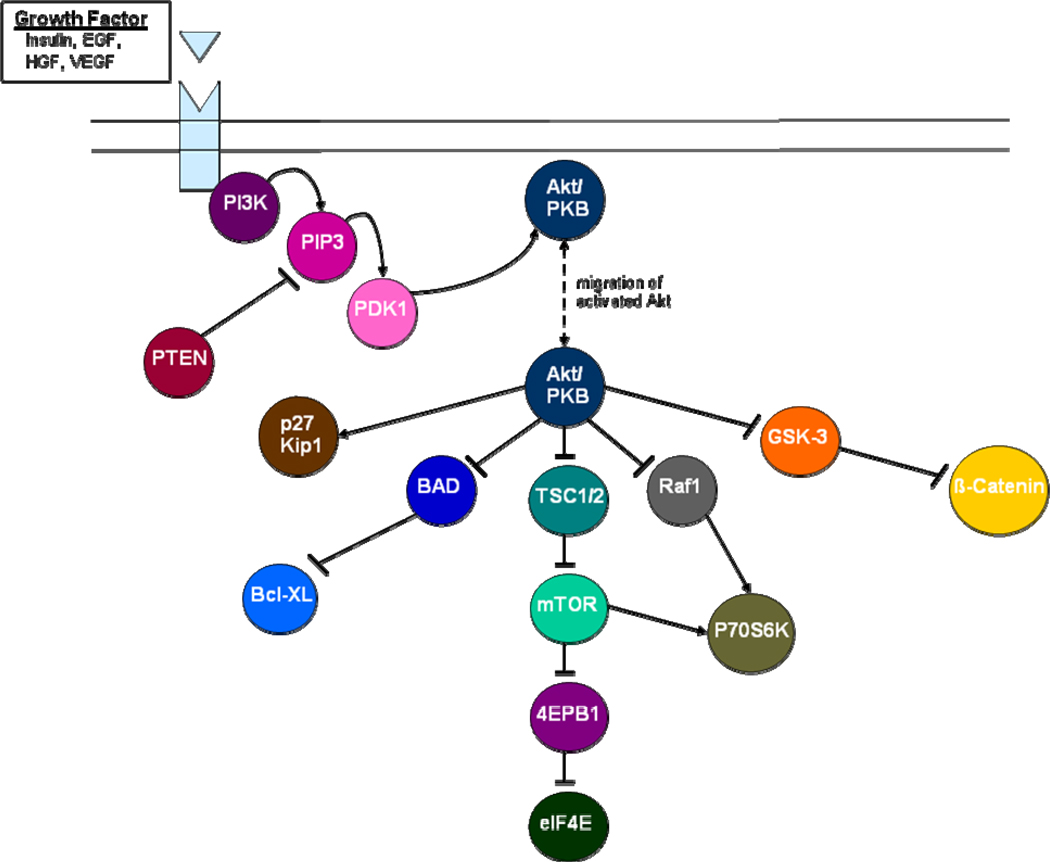
Akt was first identified in three separate efforts on the basis of its homology to protein kinase A (PKA), protein kinase C (PKC) and the retroviral oncogene, viral Akt (v-Akt).7–10 There are three distinct genes encoding unique isoforms of Akt located at 14q32, 19q13, and 1q44, encoding Akt1, Akt2 and Akt3, respectively.11–13 All three isoforms of Akt share structural features, including an N-terminal pleckstrin homology (PH) domain, a regulatory hydrophobic C-terminal domain, and a central kinase domain.7 The PH domain interacts with products of phosphatidylinositol 3-kinase (PI3K), including phosphatidylinositol-3,4,5-trisphosphate (PIP3) and phosphatidylinositol-4,5-diphosphate (PIP2).14, 15 This interaction appears to direct Akt to the plasma membrane, where phosphorylation of residues in the catalytic and C-terminal domains occurs via phosphoinositide-dependent kinase 1 (PDK1).7
The three isoforms of Akt may each have distinct biological relevance. For instance, in 3 glioma cell lines (U87MG, T98G and TG), dowregulation of Akt2 or Akt3 by RNA interference led to induction of caspase-dependent apoptosis.16 In contrast, Akt1 knock-down did not lead to growth inhibition. In the setting of non-small cell lung cancer (NSCLC), a series of 335 tumors from patients with stage I to IIIA disease was assessed for Akt isoform expression.17 In this study, it was suggested that non-phosphorylated Akt2 levels were an independent predictor of survival. As a third example, activating mutations in Akt1 have been described in the setting of prostate cancer.18 Thus, across a spectrum of three malignancies, three relevant Akt isoform profiles have been elicited. These data point to the need to selectively target Akt isoforms in clinical drug development.
Phosphorylation activates Akt, which then triggers a number of downstream signals (Figure 1). Particularly relevant in cancer is stimulation of tuberous sclerosis complex 2 (TSC2) protein, which associates with tuberous sclerosis 1 (TSC1) and is a key mediator of the mammalian target of rapamycin (mTOR).19 Akt further regulates transcriptional control of apoptosis through regulation of IKK-, MDM2-, and CREB-mediated signaling.7 More direct regulation of apoptosis occurs via the mediators BAD and caspase-9. Given its role in guiding apoptosis, Akt is an attractive target for drug development in oncology.
Figure 1.
Signal transduction cascades relevant to Akt-mediated cell growth and proliferation.
3.0 CLINICALLY RELEVANT Akt INHIBITORS
3.1 Perifosine
3.1.1 Preclinical Studies
Perifosine is a phospholipid derivative of alkylphosphocholine, and appears to inhibit not only Akt-mediated signaling but MAPK and JNK pathways as well.20–22 In several preclinical models, the agent demonstrated substantial activity. For instance, in murine models of neuroblastoma, treatment with perifosine resulted in substantial antitumor activity.23 Combination of perifosine with irradiation in human squamous cell carcinoma xenografts resulted in complete tumor regression.24 In rat mammary carcinoma models, miltefosine (a related compound) was compared to perifosine.25 In these experiments, the activity of perifosine appeared to be superior to miltefosine with respect to both efficacy and toxicity when using a loading dose (68.1 mg/kg) and maintenance dose (2.15 mg/kg/day).25 These encouraging data led to subsequent clinical examination of perifosine.
3.1.2 Phase I Studies
Several phase I trials have been conducted to assess the safety and tolerability of perifosine. Van Ummersen et al have reported an experience with 42 patients with incurable solid malignancies.26 The maximum tolerated dose (MTD) was determined to be a loading dose of 600 mg (150 mg oral every 6 hours × 4) followed by 100 mg oral daily. Dose limiting toxicities (DLTs) encountered with the loading dose included nausea, vomiting, dehydration, diarrhea and fatigue. These toxicities were easily managed with standard supportive care modalities. In contrast, toxicities were more challenging to treat during the maintenance phase. These toxicities resembled those encountered with the loading phase, but also included leg/foot pain, gout, arthralgias and gastrointestinal bleeding. A confirmed partial response (PR) was observed in a patient with leiomyosarcoma, and two patients with mRCC had stable disease through 6 and 14 courses of perifosine therapy, respectively. These malignancies were therefore considered attractive for further drug development.
Phase I studies have also been performed exploring the combination of perifosine with radiotherapy.27 In a study including 21 patients (17 with NSCLC), an MTD of 150 mg/day maintenance was identified. The regimen appeared to be well tolerated, and further study of perifosine and radiation was recommended in both NSCLC and bladder cancer given observed responses. Perifosine is also being studied in combination with other targeted therapies. In patients with advanced cancer, these phase I studies have preliminarily identified that perifosine can be safely combined with temsirolimus and sorafenib.28, 29 Combining the mTOR inhibitor temsirolimus with perifosine comes with strong scientific rationale; the mTORc2 (mTOR/rictor) complex phosphorylates Akt at S473 in a positive feedback loop.30 In preclinical studies employing a wide array of cell lines, use of everolimus alone did not lead to abrogate Akt activation, since this class of agents primarily exerts an effect on mTORc1. However, use of a dual PI3K/Akt inhibitor (NVP-BEZ235) did inhibit both mTORc1 and Akt activation. Building on this observation, the combination of an mTOR inhibitor with an Akt inhibitor may similar promote dual blockade of these moieties.
3.1.3 Phase II Studies
With the MTD identified from phase I studies, several phase II experiences have classified the activity of perifosine in a spectrum of malignancies. Regarding hematologic malignancies, in a phase II study including 37 patients with Waldenstrom’s macroglobulinemia, 1 PR (3%) and 10 minimal responses (32%) were observed amongst 31 evaluable patients.31 Encouraging activity has also been observed in a phase II trial in multiple myeloma.32 Patients in this trial had either symptomatic relapsed or relapsed/refractory disease and received perifosine with or without dexamethasone. A total of 64 patients were treated. Amongst 48 evaluable patients, the combination of dexamethasone with perifosine achieved either a partial response or minimal response in 12 patients (38%) and resulted in stable disease (SD) in 15 patients (47%). More impressive data were seen with the combination of perifosine, bortezomib and dexamethasone.33 In this phase I/II effort, a total of 84 patients with relapsed or refractory multiple myeloma were enrolled. At the time of most recent assessment, median OS had not been reached. Fifteen patients (20%) had either a complete response (CR) or PR, and median TTP was 6.4 months. On the basis of these encouraging data, a phase III effort has been launched examining bortezomib and dexamethasone with or without perifosine.34 Perifosine is also being evaluated in patients with acute myelogenous leukemia and other hematologic malignancies.35
Amongst solid tumors, two phase II studies have assessed the activity of perifosine in mRCC. First, Vogelzang et al performed a study including 46 patients with mRCC who had been previously treated with either a vascular endothelial growth factor-tyrosine kinase inhibitor (VEGF-TKI) alone (Group A) or both a VEGF-TKI and an mTOR inhibitor (Group B).36 Amongst 44 patients evaluable for response, 2 PRs (5%) were recorded, and 19 patients (43%) had SD lasting greater than 12 weeks. Median progression-free survival (PFS) was 13 weeks in Group A and had not been reached at the time of report in Group B. In a separate study evaluating perifosine, Cho et al enrolled 24 patients with mRCC; all patients had received prior therapy with a VEGF-TKI (12 with sunitinib, 12 with sorafenib).37 Mirroring results from the previous study, 2 PRs (8%) were recorded, and 10 patients (42%) had SD lasting greater than 12 weeks. Median PFS was 19 weeks. Amongst the genitourinary malignancies, perifosine has also been assessed in a phase II study in patients with biochemically recurrent, hormone sensitive prostate cancer.38 A total of 25 patients were enrolled, and at the time of most recent report, 24 were evaluable for response. With a median of 8 months of follow-up, 5 patients (20%) had a decrease in serum PSA levels, although none met stringent criteria set forth for PSA response (a 50% or greater reduction in baseline PSA value).
Encouraging activity with perifosine has also been documented in NSCLC. In a European multi-center phase II trial, 177 patients with non-metastatic but inoperable NSCLC were randomized to receive radiation with either perifosine or placebo.39 A total of 26 patients survived for 1 year without relapse or disease progression, with 14 patients (14.7%) on the perifosine arm and 12 patients (14.2%) on the placebo arm. Though little difference was seen in this endpoint, there was a trend towards an improvement in overall survival (OS) favoring perifosine. Further report of these data is eagerly anticipated. A randomized phase II trial has also been conducted in the setting of colorectal cancer. In this study, patients with metastatic disease who had failed first-line therapy were treated with either capecitabine alone or capecitabine in combination with perifosine.40 With 37 patients randomized to date, the addition of perifosine to capecitabine more than doubled median time to progression (TTP) from 11 to 29 weeks (P=0.01). These encouraging data have led to the initiation of a phase III trial employing a similar randomization.41
A separate phase II trial assessed perifosine in the setting of hormone-sensitive prostate cancer (HSPC).42 In a total of 25 patients (with 22 evaluable for response), none had a PSA decline ≥ 50%, leading to early termination of accrual. However, a total of 5 patients (23%) did have a PSA decline of < 50%, and median PFS with perifosine was an appreciable 9.5 months. Furthermore, the agent appeared to be well tolerated, with moderative to severe toxicities limited to grade 3 hyponatremia, arthritis, hyperuricemia and vision changes. These studies were suggested to serve as further impetus to study the agent in combination with hormonal therapy or chemotherapy in the setting of HSPC.
While the outlined data from trials of perifosine is largely encouraging, several phase II studies have produced sobering results. For instance, a trial conducted in 10 patients with locally advanced, unresectable or metastatic pancreatic adenocarcinoma showed no responses and three deaths during therapy.43 Median OS was only 1.85 months in this experience. Similarly, a study of perifosine in metastatic melanoma showed no objective responses; of 14 evaluable patients, only 3 patients (21%) achieved SD after two cycles of therapy.44 Finally, a study of perifosine in 19 patients with head and neck cancer showed no responses and a median PFS and OS of 1.7 and 5.5 months, respectively.45
3.2 MK-2206
3.2.1 Preclinical Studies
MK-2206 is an allosteric inhibitor of Akt.46 Synergy has been observed with combined use of MK-2206 and other targeted therapies in preclinical models. For instance, combination of MK-2206 with erlotinib in NSCLC cell lines and with lapatinib in breast cancer cell lines led to synergistic growth inhibition.46 In xenograft studies utilizing mice bearing the A2780 ovarian cancer cell line, treatment with MK-2206 led to roughly 60% growth inhibition and generated sustained inhibition of all three Akt isoforms.47 Subsequent in vitro data suggested that inhibition of each isoform occurred at nanomolar concentrations.
3.2.2 Phase I Studies
In a study including 24 healthy male volunteers, doses of MK-2206 ranging between 0.25 to 100 mg oral were well tolerated.48 Maximal inhibition of Akt occurred roughly 6 hours after an oral dose was administered and led to Akt inhibition (measured in whole blood) for up to 24 hours. A subsequent phase I study in 19 patients with advanced solid tumors assessed dosing of MK-2206 on an every other day (QOD) schedule.49 With QOD dosing, the MTD was determined to be 60 mg (grade 3/4 mucositis and skin rash were DLTs noted at the next dose level, 90 mg). In this dose-finding study, treatment with MK-2206 was noted to cause central tumor necrosis, a reduction in index lesions and improvement in ascites and peripheral edema. As in the healthy volunteers study, it appeared that the dose of 60 mg led to sustained inhibition of Akt in whole blood. A larger phase I exploration is underway, examining two formulations of MK-2206 – one administered on a weekly schedule (QW) and another on an every other day schedule (QOD).50 Preliminary data from this study (with a total of 70 patients accrued thus far) showed DLTs of rash and mucositis at doses of 75 mg and 90 mg QOD, and a DLT of rash at 300 mg QW.51 Correlative studies paired with this analysis showed sustained declines in pAkt with MK-2206 therapy. Furthermore, of 23 evaluable patients, 18 (78.3%) had a decline in circulating endothelial cells (CECs).
Several combinations of both cytotoxic agents and targeted therapies with MK-2206 have been proposed. In an ongoing study, patients with advanced solid tumors will be randomized to MK-2206 with either carboplatin and paclitaxel, docetaxel or erlotinib.52 The study will enroll a total of 148 patients and is estimated to complete data collection in September of 2011. A second phase I trial in breast cancer will enroll 58 patients with HER2-overexpressing metastatic breast cancer and treat with MK-2206 and trastuzumab or MK-2206, trastuzumab and lapatinib.53 This study builds on two elements: (1) data supporting the combination of trastuzumab and lapatinib in HER2-overexpressing disease, and (2) preclinical data showing synergy between MK-2206 and HER2-directed therapies.54, 55 Specifically, MK-2206 with lapatinib exhibited combination indices (CIs) in the range of 0.07 to 0.69 in HCC70 breast cancer cell lines.55
3.3 RX-0201
3.3.1 Preclinical Studies
RX-0201 represents an antisense oligonucleotide (AO) to mRNA encoding Akt1. In in vitro models, culture with Akt1 AO at nanomolar concentrations resulted in growth inhibition of various human cancer cell lines.56 When tested in two in vivo models (nude mice implanted with U251 human glioblastoma cells or MIA human pancreatic cells), substantial growth inhibition was also observed. In the radiation resistant H1299 NSCLC cell line, treatment with Akt1 AO resulted in cytotoxicity.57 These encouraging studies led to further clinical development of the agent.
3.3.2 Phase I Studies
A preliminary report of a phase I experience with RX-0201 included 17 patients with advanced solid tumors.58 RX-0201 was administered as a continuous infusion for 14 days on a 21 day cycle. Grade 3 fatigue was observed in 2 patients treated at a dose of 315 mg/m2/d, leading to a recommended phase II dose of 250 mg /m2/d. Phase II studies of RX-0201 are planned in mRCC. Furthermore, an ongoing trial in pancreatic cancer is assessing the combination of RX-0201 and gemcitabine.57, 59 A liposomal formulation of RX-0201 (RX-0201-nano) is currently under development to enhance nuclear delivery of the Akt1 AO.
3.4 Erucylphosphocholine (ErPC)
ErPC is structurally related to perifosine and is currently in preclinical development. Like perifosine, the agent appears to inhibit Akt, but also impacts other signaling pathways (most prominently, Raf-MEK-ERK).60–62 Ovarian and endometrial cancer cell lines appear to be sensitive to growth inhibition with ErPC treatment.63 In contrast, growth of normal ovarian and endometrial epithelial cells is not retarded.
3.5 PBI-05204
A derivative of Nerium oleander, PBI-05204 is an inhibitor of several moieties, including Akt, fibroblast growth factor 2 (FGF2), NF-kappa B and p70S6K.64, 65 The agent is currently being explored in a phase I clinical trial in patients with advanced solid tumors. At the time of most recent report, 15 patients had received doses ranging from 0.6 mg to 10.2 mg oral daily.64 Three patients (with bladder, colorectal and fallopian tube carcinoma, respectively) had stable disease lasting greater than 4 months. Assessment of Akt phosphoyrlation in peripheral blood mononuclear cells (PBMCs) suggested a time dependent decline in the level of Akt phosphorylation. Further data for this compound are eagerly anticipated.
3.6 GSK690693
In preclinical experiments, GSK690693 was noted to inhibit all isoforms of Akt at nanomolar concentrations (2 nM for Akt1, 13 nM for Akt 2 and 9 nM for Akt3).65, 66 In xenograft models, administration of the agent led to significant growth inhibition in mice bearing SKOV-3 ovarian, LNCaP prostate, and BT474 breast tumors.67 Although GSK690693 appeared to delay tumor growth irrespective of Akt activation status, it was noted to be particularly effective in MyrAkt2 mice expressing a constitutively activated, membrane bound form of Akt.68 Despite these encouraging preclinical data, clinical development of the agent was halted due to interim results from a phase I trial testing an IV formulation of the agent.69 No published reports describe the rationale for terminating the study; however, an interim report does cite transient drug-related hyperglycemia as an associated side effect.70 Murine studies indicate that GSK690693 may inhibit glycogen synthesis and activate glycogenolysis.71 Furthermore, fasting appeared to inhibit hyperglycemia in these models. The amalgam of data suggests that GSK690693 may induce peripheral insulin resistance.
Lesser preclinical data are available for an oral Akt inhibitor currently under development by GlaxoSmithKline. The agent, GSK2141795, is being assessed in a phase I trial expected to enroll 70 patients with advanced solid tumors or lymphoma by January of 2011.72
3.7 XL-418
XL-418, a dual inhibitor of Akt and p70S6K, has been assessed in a phase I trial in advanced solid tumors.63 In a study including 63 patients, low drug exposure was achieved and the trial was therefore halted.
4.0 CONCLUSIONS
With a spectrum of new agents directed at inhibiting Akt, it will be critical to determine where these agents fit into existing management paradigms. Studies assessing the safety and efficacy of Akt inhibitors combined with either traditional cytotoxic agents or other targeted therapies are therefore of prime importance -- these may allow novel Akt inhibitors to complement existing treatments. These studies should proceed only with appropriate preclinical justification. For instance, a recently reported preclinical study assessing four RCC cell lines suggested subadditive growth inhibition with the combination of perifosine and rapamycin (an mTOR inhibitor).82 Such data makes clinical forays exploring the same combination less promising.
The research community is also advised to focus efforts with Akt inhibitors in those disease subtypes where other small molecules have had the largest impact. As noted in Figure 1, Akt sits along a vertical axis and serves as an intermediary between VEGFR and mTOR. Diseases such as mRCC, where agents targeting VEGFR and mTOR have had the most tangible impact, therefore represent the most promising targets. Three VEGF-TKIs (sunitinib, sorafenib and pazopanib) have been approved for the treatment of advanced disease, as well as two mTOR inhibitors (temsirolimus and everolimus).6 Presumably, in diseases such as breast cancer where agents such as sunitinib and sorafenib have had a modest impact at best, the impact of Akt-directed therapies is destined to be less profound.83–85 There is a burgeoning pipeline of agents that target elements of the axis between VEGFR and mTOR, and perhaps this paradigm can be applied to each of these drugs. For instance, preclinical studies suggest that the dual PI3K/mTOR inhibitor NVP-BEZ235 more efficiently induces growth arrest than TORC1 inhibition alone in RCC cell lines 786O and A498.86 In contrast, activity in breast cancer models was only observed amongst cell lines with either HER2 amplification and/or PIK3CA mutation.87 With phase I data for NVP-BEZ235 in advanced solid tumors recently reported, these observations may be used to guide further clinical development of the agent.88
With the signing of the American Recovery and Reinvestment Act (ARRA) in 2009, there is now substantial impetus to conduct comparative effectiveness (CE) research.89 The oncology community should therefore be focused on selecting the most promising of the Akt inhibitors to fare in comparative trials against existing standards. Because such comparative trials are only appropriately organized as large, phase III efforts, there will have to be discretion in to what extent Akt inhibitors are carried forward. Utilizing the biomarker strategies presented herein may allow for identification of unique populations that benefit from this class of agents. Validation of a biomarker to predict response would allow future studies to be conducted with a narrower population, sparing clinical trial resources allocated to marker-negative patients that may have a suboptimal response. Although Akt inhibitors hold great promise, the challenge that lies ahead is their incorporation into available treatment algorithms across malignancies.
5.0 EXPERT OPINION
5.1 Deciphering the Data: Predicting the Activity of Akt Inhibitors
Given an enlarging portfolio of targeted agents, it will be critical to implement these therapies in selected populations with the greatest likelihood for response. Biomarker studies to accompany assessments of Akt inhibitors are therefore of prime importance. To date, early clinical studies have attempted to establish a proof of principle – for instance, it was demonstrated that MK-2206 decreased Akt phosphorylation in whole blood and that RX-0201 decreased Akt mRNA levels.49, 58 Moving beyond these experiences, it will be important to determine if Akt phosphorylation or activation/mutation of other related mediators can predict the efficacy of this class of agents.
5.1.1 Akt Phosphorylation
As noted previously, phosphorylation of critical residues in Akt results in migration of the moiety to the cellular membrane and subsequent activation via PDK1-dependent processes.7 Several studies have suggested that the extent of Akt phosphorylation may be related to clinical outcome. A study in acute myelogenous leukemia suggested that Akt phosphorylation at Thr308 portends a poorer prognosis.53 Outside of its prognostic role, Akt phosphorylation may predict the efficacy of certain targeted agents. As one example, a phase II trial of gefitinib (an epidermal growth factor receptor, EGFR, inhibitor) assessed 106 patients with advanced NSCLC that had progressed on standard therapy.50 Correlative studies suggested that increased baseline phosphorylated Akt (pAkt) predicted a superior response rate (26.1% v 3.9%, P=0.003), disease control rate (60.9% v 23.5%, P<0.001), and time to progression (5.5 v 2.8 months, P=0.004). In contrast to pAkt, levels of phosphorylated MAPK had no bearing on clinical outcome. Using these studies as a paradigm, it will be critical to see if the activity of Akt inhibitors can be predicted by baseline levels of pAkt.
5.1.2 Related Mediators
Modulation of signal transducers relevant to Akt signaling may affect the activity of Akt inhibitors. PI3K sits directly upstream from Akt, and gain-of-function mutations have been described in the PIK3CA gene (which encodes the p110α catalytic subunit of PI3K) in breast, ovarian and colorectal cancer.73 Although agents are being crafted to selectively target PIK3CA mutated cells, the mutations could serve as a biomarker for response to Akt-directed therapies.74, 75 This is supported by in vitro experiments using thyroid cancer cell lines – in cells bearing PIK3CA mutations (as compared to cell lines without), sensitivity to perifosine was blunted.76 Status of the tumor suppressor PTEN may also predict response to Akt inhibitors. The function of PTEN may be altered by gene deletion, epigenetic silencing or mutation. In multiple series, these mutations have been associated with poorer clinical outcome.77–79 Deregulation of PTEN may lead to activation of activating pathways upstream of Akt. Outside of PTEN and PI3K, a number of other moieties may impact the activity of Akt inhibitors. At the level of the cell membrane, aberrant activation of transmembrane proteins (i.e., EGFR or IGF-1R) could drive downstream pathways. Recently, the tumor suppressor FBXW7 has been shown to predict sensitivity of tumor cells to PI3K inhibitors; assessing the role of this moiety in predicting Akt inhibitor response may therefore be of utility.80
5.2 Relevant Trial Designs
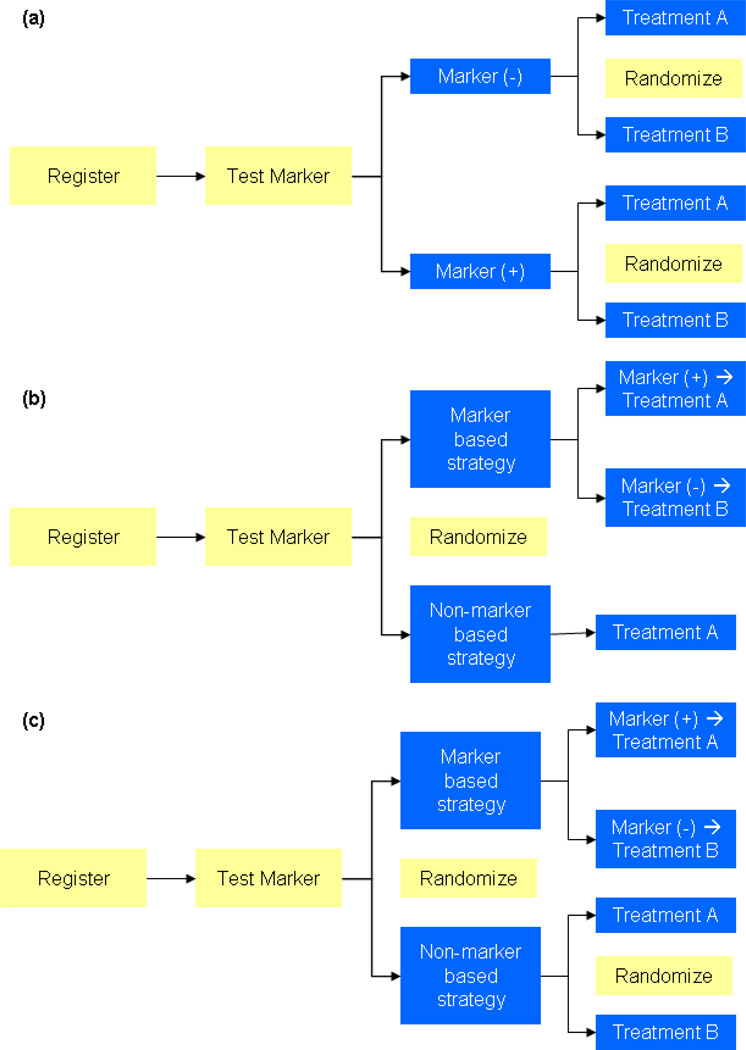
At this time, further retrospective validation is needed to elicit the role of pAkt and other mediators as a biomarker of response. Assuming that these studies carry forward, a number of prospective trial designs can be considered, as outlined in Figure 2.81 As in Figure 2a, registration of patients can be followed by stratification of patients by biomarker status. Patients in subgroups based on biomarker status can then be independently randomized to either an Akt inhibitor or a mechanistically distinct agent (i.e., cytotoxic therapy). In contrast to this approach, the design proposed in Figure 2b essentially conducts two separate clinical studies – one assessing a marker based strategy and the other assessing a non-marker based approach. The design posited in Figure 2c has the added advantage of determining the effect of randomization to Akt inhibitor or not, using a non-marker based strategy. Admittedly, such a design would require an even greater number of patients.
Figure 2.
Suggested designs for clinical trials evaluating biomarkers. (Adapted from Pal SK, Figlin RA: Renal Cell Carcinoma in 2010: Many Options with Little Comparative Data Clin Adv Hem Onc 8(3):1–7.)
Before embarking on one of the schema proposed herein, the investigator should be somewhat confident regarding the role of the putative biomarker – each of the trial designs require an immense number of patients and resources.81 Consider the example of evaluating pAkt as a biomarker for response to an Akt inhibitor for second-line treatment of NSCLC, using a standard cytotoxic agent (i.e., docetaxel) as a comparator. Using the design proposed in Figure 2a, let us assume that the prevalence of pAkt is 50%, and that we wish to have 90% power with a two-sided α of 0.05. Assuming that the hazard ratio for survival in the high baseline pAkt group is 1.25 (favoring the Akt inhibitor) and the hazard ratio for survival in the low baseline pAkt group is 0.86 (favoring docetaxel), over 2200 patients per treatment arm would be required. Given the immensity of such a trial, it is imperative that efforts to characterize biomarkers undergo careful forethought. While each of the designs in Figure 2 is reasonable, a thorough consultation with a biostatistician may allow the translational researcher to select the trial design that minimizes expenditure of valuable patient resources while maintaining sufficient statistical power.
Article Highlights.
Akt is a serine-threonine kinase that plays a critical role in a number of apoptotic pathways
Encouraging phase II data has emerged for perifosine in renal cell carcinoma, colorectal cancer and multiple myeloma, with phase III studies of the agent ongoing in the latter two malignancies
The MTD of MK-2206 has been established in phase I studies, and ongoing efforts are characterizing the MTD of the agent in combination with other cytotoxic and targeted therapies
RX-0201 uses a novel antisense oligonucleotide technology to antagonize Akt signaling; the agent is being assessed in phase II studies in renal cell carcinoma and pancreatic cancer
PBI-05204 and GSK2141795 are currently being examined in phase I studies
Erucylphosphocholine is in preclinical development
To optimize the activity of Akt inhibitors, identification of relevant biomarkers that predict activity is critical
Potential biomarkers that should be explored include pAkt, PIK3CA mutation, loss of PTEN, and FBXW7 expression
Biomarker validation is a laborious albeit necessary task to move the field forward
Table 1.
Signal transduction cascades relevant to Akt-mediated cell growth and proliferation.
| Drug | Manufacturer | Description |
|---|---|---|
| Perifosine | AEterna Zentaris | Phase II trials show activity in hematologic malignancies (Waldenstrom’s macroglobulinemia and multiple myeloma) and solid tumors (RCC, NSCLC, colorectal cancer). Phase III trials underway in metastatic colorectal cancer and multiple myeloma. |
| MK-2206 | Merck & Co | Single agent MTD defined in a phase I study in advanced solid tumors. Ongoing phase I studies are assessing the combination of MK-2206 with cytotoxic therapy, and disease specific studies in HER2-overexpressing breast cancer are assessing MK-2206 with trastuzumab and trastuzumab/lapatinib. |
| RX-0201 | Rexahn | Phase II studies ongoing in RCC and pancreatic cancer. |
| PBI-05204 | Phoenix Biotechnology | Phase I trial in advanced solid tumors ongoing. |
| GSK2141795 | GlaxoSmithKline | Phase I trial in advanced solid tumors ongoing. |
| Erucylphosphocholine (ErPC) | AEterna Zentaris | In preclinical development. |
| GSK690693 | GlaxoSmithKline | Clinical development suspended. |
| XL-418 | Exelixis | Clinical development suspended. |
Acknowledgments
SK Pal’s efforts are supported by the NIH Loan Repayment Plan (LRP), the CBCRP 15IB-0140 (California Breast Cancer Research Program Junior IDEA Award) and NIH K12 2K12CA001727-16A1. He has also received research funding from Amgen and Honoraria from Pfizer and Novartis.
R Figlin has received research funding from Novartis, GSK, Pfizer, Argos and Antisoma, and has served as a consultant for Aveo, Enzon, GSK, Clovis and Pfizer.
K Reckamp’s efforts are supported by NIH K12 2K12CA001727-16A1, the Phase One Foundation, and the Baumel Family Research Fund. She has served on the Speaker’s Bureau for Genetech and has received research funding from OSI Pharmaceuticals.
Footnotes
Declaration of interest
Contributor Information
Sumanta Kumar Pal, Department of Medical Oncology & Experimental Therapeutics, City of Hope Comprehensive Cancer Center, Phone: (626) 256-4673, Fax: (626) 301-8233, spal@coh.org.
Karen Reckamp, Department of Medical Oncology & Experimental Therapeutics, City of Hope Comprehensive Cancer Center, Phone: (626) 256-4673, kreckamp@coh.org.
Hua Yu, Department of Immunology, City of Hope Comprehensive Cancer Center, Phone: (626) 256-4673, hyu@coh.org.
Robert A. Figlin, Department of Medical Oncology & Experimental Therapeutics, City of Hope Comprehensive Cancer Center, Phone: (626) 256-4673, Fax: (626) 301-8233, rfiglin@coh.org.
References
- 1.Druker BJ, Guilhot F, O'Brien SG, Gathmann I, Kantarjian H, Gattermann N, et al. Five-Year Follow-up of Patients Receiving Imatinib for Chronic Myeloid Leukemia. N Engl J Med. 2006 December 7;355(23):2408–2417. doi: 10.1056/NEJMoa062867. 2006. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of Chemotherapy plus a Monoclonal Antibody against HER2 for Metastatic Breast Cancer That Overexpresses HER2. N Engl J Med. 2001 March 15;344(11):783–792. doi: 10.1056/NEJM200103153441101. 2001. [DOI] [PubMed] [Google Scholar]
- 3.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in Advanced Clear-Cell Renal-Cell Carcinoma. N Engl J Med. 2007 January 11;356(2):125–134. doi: 10.1056/NEJMoa060655. 2007. [DOI] [PubMed] [Google Scholar]
- 4.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007 Jan 11;356(2):115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 5.Clark JI, Atkins MB, Urba WJ, Creech S, Figlin RA, Dutcher JP, et al. Adjuvant High-Dose Bolus Interleukin-2 for Patients With High-Risk Renal Cell Carcinoma: A Cytokine Working Group Randomized Trial. J Clin Oncol. 2003 August 15;21(16):3133–3140. doi: 10.1200/JCO.2003.02.014. 2003. [DOI] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network Clinical Practice Guidelines: Renal Cell Carcinoma. [last accessed March 29, 2010]; Available at http://www.nccn.org.
- 7.Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. Journal of Cellular and Molecular Medicine. 2005;9(1):59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coffer PJ, Woodgett JR. Molecular cloning and characterisation of a novel putative protein-serine kinase related to the cAMP-dependent and protein kinase C families. Eur J Biochem. 1991 Oct 15;201(2):475–481. doi: 10.1111/j.1432-1033.1991.tb16305.x. [DOI] [PubMed] [Google Scholar]
- 9.Jones PF, Jakubowicz T, Pitossi FJ, Maurer F, Hemmings BA. Molecular cloning and identification of a serine/threonine protein kinase of the second-messenger subfamily. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4171–4175. doi: 10.1073/pnas.88.10.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellacosa A, Testa JR, Staal SP, Tsichlis PN. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science. 1991 Oct 11;254(5029):274–277. doi: 10.1126/science.254.5029.274. [DOI] [PubMed] [Google Scholar]
- 11.Jones PF, Jakubowicz T, Hemmings BA. Molecular cloning of a second form of rac protein kinase. Cell Regul. 1991 Dec;2(12):1001–1009. doi: 10.1091/mbc.2.12.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng JQ, Godwin AK, Bellacosa A, Taguchi T, Franke TF, Hamilton TC, et al. AKT2, a putative oncogene encoding a member of a subfamily of protein-serine/threonine kinases, is amplified in human ovarian carcinomas. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9267–9571. doi: 10.1073/pnas.89.19.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brodbeck D, Cron P, Hemmings BA. A human protein kinase Bgamma with regulatory phosphorylation sites in the activation loop and in the C-terminal hydrophobic domain. J Biol Chem. 1999 Apr 2;274(14):9133–9136. doi: 10.1074/jbc.274.14.9133. [DOI] [PubMed] [Google Scholar]
- 14.James SR, Downes CP, Gigg R, Grove SJ, Holmes AB, Alessi DR. Specific binding of the Akt-1 protein kinase to phosphatidylinositol 3,4,5-trisphosphate without subsequent activation. Biochem J. 1996 May 1;315(Pt 3):709–713. doi: 10.1042/bj3150709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frech M, Andjelkovic M, Ingley E, Reddy KK, Falck JR, Hemmings BA. High affinity binding of inositol phosphates and phosphoinositides to the pleckstrin homology domain of RAC/protein kinase B and their influence on kinase activity. J Biol Chem. 1997 Mar 28;272(13):8474–8481. doi: 10.1074/jbc.272.13.8474. [DOI] [PubMed] [Google Scholar]
- 16.Mure H, Matsuzaki K, Kitazato KT, Mizobuchi Y, Kuwayama K, Kageji T, et al. Akt2 and Akt3 play a pivotal role in malignant gliomas. NEURO ONCOL. 2010 March 1;12(3):221–232. doi: 10.1093/neuonc/nop026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Saad S, Donnem T, Al-Shibli K, Persson M, Bremnes RM, Busund L-T. Diverse Prognostic Roles of Akt Isoforms, PTEN and PI3K in Tumor Epithelial Cells and Stromal Compartment in Non-small Cell Lung Cancer. Anticancer Research. 2009 October 29;(10):4175–4183. 2009. [PubMed] [Google Scholar]
- 18.Boormans JL, Hermans KG, Leenders GJLHv, Trapman J, Verhagen PCMS. An activating mutation in <I>AKT1</I> in human prostate cancer. International Journal of Cancer. 2008;123(11):2725–2726. doi: 10.1002/ijc.23787. [DOI] [PubMed] [Google Scholar]
- 19.Zhang HH, Huang J, Duvel K, Boback B, Wu S, Squillace RM, et al. Insulin stimulates adipogenesis through the Akt-TSC2-mTORC1 pathway. PLoS One. 2009;4(7):e6189. doi: 10.1371/journal.pone.0006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gills JJ, Dennis PA. Perifosine: update on a novel Akt inhibitor. Curr Oncol Rep. 2009;11(2):102–110. doi: 10.1007/s11912-009-0016-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hideshima T, Catley L, Yasui H, Ishitsuka K, Raje N, Mitsiades C, et al. Perifosine, an oral bioactive novel alkylphospholipid, inhibits Akt and induces in vitro and in vivo cytotoxicity in human multiple myeloma cells. Blood. 2006 May 15;107(10):4053–4062. doi: 10.1182/blood-2005-08-3434. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruiter GAab, Zerp SFab, Bartelink Ha, van Blitterswijk WJb, Verheij Mab. Anti-cancer alkyl-lysophospholipids inhibit the phosphatidylinositol 3-kinase-Akt/PKB survival pathway. Anti-Cancer Drugs. 2003;14(2):167–173. doi: 10.1097/00001813-200302000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Li Z, Tan F, Tong J, McKee A, Thiele C. Perifosine, as a single agent, inhibits neuroblastoma tumor cell growth in in vitro and in vivo preclinical models; AACR Meeting Abstracts; 2009. p. 3205. [Google Scholar]
- 24.Vink SR, Schellens JHM, Beijnen JH, Sindermann H, Engel J, Dubbelman R, et al. Phase I and pharmacokinetic study of combined treatment with perifosine and radiation in patients with advanced solid tumours. Radiotherapy and Oncology. 2006;80(2):207–213. doi: 10.1016/j.radonc.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 25.Hilgard P, Klenner T, Stekar J, Nössner G, Kutscher B, Engel J. D-21266, a new heterocyclic alkylphospholipid with antitumour activity. European Journal of Cancer. 1997;33(3):442–446. doi: 10.1016/s0959-8049(97)89020-x. [DOI] [PubMed] [Google Scholar]
- 26.Van Ummersen L, Binger K, Volkman J, Marnocha R, Tutsch K, Kolesar J, et al. A Phase I Trial of Perifosine (NSC 639966) on a Loading Dose/Maintenance Dose Schedule in Patients with Advanced Cancer. Clinical Cancer Research. 2004;10(22):7450–7456. doi: 10.1158/1078-0432.CCR-03-0406. [DOI] [PubMed] [Google Scholar]
- 27.Verheij M, Vink SR, Schellens JHM, Beijnen JH, Sindermann H, Engel J, et al. Phase I study of combined treatment with the oral alkyl-lysophospholipid (ALP) Perifosine and radiation in patients with advanced solid tumors. J Clin Oncol (Meeting Abstracts) 2004 July 15;22(14_suppl):3064. [Google Scholar]
- 28. NCT00398814: Phase I Study of Perifosine + Sorafenib for Patients With Advanced Cancers. [last accessed March 4, 2010]; Available at http://clinicaltrials.gov/ct2/show/NCT00398814.
- 29. NCT01049841: Perifosine With Temsirolimus for Recurrent Pediatric Solid Tumors. [last accessed March 4, 2010]; Available at http://clinicaltrials.gov/ct2/show/NCT01049841.
- 30.Breuleux M, Klopfenstein M, Stephan C, Doughty CA, Barys L, Maira S-M, et al. Increased AKT S473 phosphorylation after mTORC1 inhibition is rictor dependent and does not predict tumor cell response to PI3K/mTOR inhibition. Molecular Cancer Therapeutics. 2009 April;8(4):742–753. doi: 10.1158/1535-7163.MCT-08-0668. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghobrial IM, Leleu X, Rubin N, Leduc R, Chuma S, Nelson M, et al. Phase II trial of the novel oral Akt inhibitor perifosine in relapsed and/or refractory Waldenstrom macroglobulinemia (WM) J Clin Oncol (Meeting Abstracts) 2008 May 20;26(15_suppl):8546. 2008. [Google Scholar]
- 32.Richardson P, Lonial S, Jakubowiak A, Krishnan A, Wolf J, Densmore J, et al. Multi-Center Phase II Study of Perifosine (KRX-0401) Alone and in Combination with Dexamethasone (dex) for Patients with Relapsed or Relapsed/Refractory Multiple Myeloma (MM): Promising Activity as Combination Therapy with Manageable Toxicity. ASH Annual Meeting Abstracts. 2007 November 16;110(11):1164. 2007. [Google Scholar]
- 33.Richardson P, Wolf JL, Jakubowiak A, Zonder JA, Lonial S, Irwin DH, et al. Perifosine in Combination with Bortezomib and Dexamethasone Extends Progression-Free Survival and Overall Survival in Relapsed/Refractory Multiple Myeloma Patients Previously Treated with Bortezombib: Updated Phase I/II Trial Results; ASH Annual Meeting Abstracts; 2009. Nov 20, p. 1869. 2009. [Google Scholar]
- 34. NCT01002248: Assessment of Efficacy and Safety of Perifosine, Bortezomib and Dexamethasone in Multiple Myeloma Patients. [last accessed March 5, 2010]; Available at http://clinicaltrials.gov/ct2/show/NCT01002248.
- 35. NCT00391560: Phase II Study of Perifosine in Patients With Refractory and Relapsed Leukemia. [last accessed March 5, 2010]; Available at http://clinicaltrials.gov/ct2/show/NCT00391560.
- 36.Vogelzang NJ, Hutson TE, Samlowski W, Somer B, Richey S, Alemany C, et al. Phase II study of perifosine in metastatic renal cell carcinoma (RCC) progressing after prior therapy (Rx) with a VEGF receptor inhibitor. J Clin Oncol (Meeting Abstracts) 2009 May 20;27(15S):5034. 2009. [Google Scholar]
- 37.Cho DC, Figlin RA, Flaherty KT, Michaelson D, Sosman JA, Ghebremichael M, et al. A phase II trial of perifosine in patients with advanced renal cell carcinoma (RCC) who have failed tyrosine kinase inhibitors (TKI) J Clin Oncol (Meeting Abstracts) 2009 May 20;27(15S):5101. 2009. [Google Scholar]
- 38.Chee KG, Longmate J, Quinn DI, Chatta G, Pinski J, Twardowski P, et al. The AKT inhibitor perifosine in biochemically recurrent prostate cancer: a phase II California/Pittsburgh cancer consortium trial. Clin Genitourin Cancer. 2007 Dec;5(7):433–437. doi: 10.3816/CGC.2007.n.031. [DOI] [PubMed] [Google Scholar]
- 39.AEterna Zentaris Press Release. Æterna Zentaris Discloses Preliminary Phase 2 Trial Results for Perifosine in Combination with Radiotherapy for Non Small Cell Lung Cancer. [last accessed March 5, 2010];2009 June 8; Available at http://www.aeternazentaris.com/en/page.php?p=60&q=316.
- 40.Vukelja S, Richards D, Campos LT, Bedell C, Hagenstad C, Hyman W, et al. Randomized phase II study of perifosine in combination with capecitabine versus capecitabine alone in patients with second- or third-line metastatic colon cancer. J Clin Oncol (Meeting Abstracts) 2009 May 20;27(15S):4081. 2009. [Google Scholar]
- 41. NCT01097018: A Phase III Randomized Study to Assess the Efficacy and Safety of Perifosine Plus Capecitabine Versus Placebo Plus Capecitabine in Patients With Refractory Advanced Colorectal Cancer. [last accessed April 4, 2010]; Available at http://www.clinicaltrials.gov.
- 42.Chee KG, Lara PN, Longmate J, Twardowski P, Quinn DI, Chatta G, et al. The AKT inhibitor perifosine in biochemically recurrent, hormone-sensitive prostate cancer (HSPC): A phase II California Cancer Consortium trial. J Clin Oncol (Meeting Abstracts) 2005 June 1;23(16_suppl):4642. 2005. [Google Scholar]
- 43.Marsh RdW, Lima CMR, Levy DE, Mitchell EP, Rowland KMJ, Benson ABI. A Phase II Trial of Perifosine in Locally Advanced, Unresectable, or Metastatic Pancreatic Adenocarcinoma. American Journal of Clinical Oncology. 2007;30(1):26–31. doi: 10.1097/01.coc.0000251235.46149.43. [DOI] [PubMed] [Google Scholar]
- 44.Ernst D, Eisenhauer E, Wainman N, Davis M, Lohmann R, Baetz T, et al. Phase II Study of Perifosine in Previously Untreated Patients with Metastatic Melanoma. Investigational New Drugs. 2005;23(6):569–575. doi: 10.1007/s10637-005-1157-4. [DOI] [PubMed] [Google Scholar]
- 45.Argiris A, Cohen E, Karrison T, Esparaz B, Mauer A, Ansari R, et al. A phase II trial of perifosine, an oral alkylphospholipid, in recurrent or metastatic head and neck cancer. Cancer Biol Ther. 2006 Jul;5(7):766–770. doi: 10.4161/cbt.5.7.2874. [DOI] [PubMed] [Google Scholar]
- 46.Hirai H, Sootome H, Nakatsuru Y, Miyama K, Taguchi S, Tsujioka K, et al. An allosteric Akt inhibitor, MK-2206 enhanced anti-tumor efficacy by standard of care agents or molecular targeted drugs in vitro and in vivo; AACR Meeting Abstracts; 2009. p. 3707. [DOI] [PubMed] [Google Scholar]
- 47.Lu W, Defeo-Jones D, Davis LJ, Hang G, Tammam JG, Hatch H, et al. In vitro and in vivo antitumor activities of MK-2206, a new allosteric Akt inhibitor; AACR Meeting Abstracts; 2009. p. 3714. [Google Scholar]
- 48.Trucksis M, Friedman E, Taylor A, Delgado L, Reynders T, DeSmet M, et al. A phase I single-rising dose study evaluating the safety, tolerability, pharmacokinetics and pharmacodynamics of an oral akt inhibitor in healthy male volunteers; AACR Meeting Abstracts; 2009. p. 3604. [Google Scholar]
- 49.Tolcher AW, Yap TA, Fearen I, Taylor A, Carpenter C, Brunetto AT, et al. A phase I study of MK-2206, an oral potent allosteric Akt inhibitor (Akti), in patients (pts) with advanced solid tumor (ST) J Clin Oncol (Meeting Abstracts) 2009 May;27(15S):3503. 2009. [Google Scholar]
- 50.Cappuzzo F, Magrini E, Ceresoli GL, Bartolini S, Rossi E, Ludovini V, et al. Akt phosphorylation and gefitinib efficacy in patients with advanced non-small-cell lung cancer. J Natl Cancer Inst. 2004 Aug 4;96(15):1133–1141. doi: 10.1093/jnci/djh217. [DOI] [PubMed] [Google Scholar]
- 51.Yap TA, Patnaik A, Fearen I, Olmos D, Papadopoulos K, Tunariu N, et al. First-in-class phase I trial of a selective Akt inhibitor, MK2206 (MK), evaluating alternate day (QOD) and once weekly (QW) doses in advanced cancer patients (pts) with evidence of target modulation and antitumor activity. J Clin Oncol (Meeting Abstracts) 2010 May 20;28(15_suppl):3009. [Google Scholar]
- 52. NCT00460278: Study of XL418 in Adults With Solid Tumors. [last accessed February 25, 2010]; Available at http://clinicaltrials.gov/ct2/show/NCT00460278.
- 53.Gallay N, Dos Santos C, Cuzin L, Bousquet M, Simmonet Gouy V, Chaussade C et al. The level of AKT phosphorylation on threonine 308 but not on serine 473 is associated with high-risk cytogenetics and predicts poor overall survival in acute myeloid leukaemia. Leukemia. 2009 Jun;23(6):1029–1038. doi: 10.1038/leu.2008.395. [DOI] [PubMed] [Google Scholar]
- 54.Blackwell KL, Burstein HJ, Storniolo AM, Rugo H, Sledge G, Koehler M, et al. Randomized Study of Lapatinib Alone or in Combination With Trastuzumab in Women With ErbB2-Positive, Trastuzumab-Refractory Metastatic Breast Cancer. J Clin Oncol. 2010 March 1;28(7):1124–1130. doi: 10.1200/JCO.2008.21.4437. [DOI] [PubMed] [Google Scholar]
- 55.Hirai H, Sootome H, Nakatsuru Y, Miyama K, Taguchi S, Tsujioka K, et al. MK-2206, an Allosteric Akt Inhibitor, Enhances Antitumor Efficacy by Standard Chemotherapeutic Agents or Molecular Targeted Drugs In vitro and In vivo. Molecular Cancer Therapeutics. 2010 July;9(7):1956–1967. doi: 10.1158/1535-7163.MCT-09-1012. [DOI] [PubMed] [Google Scholar]
- 56.Yoon H, Kim DJ, Ahn EH, Gellert GC, Shay JW, Ahn C-H, et al. Antitumor activity of a novel antisense oligonucleotide against Akt1. Journal of Cellular Biochemistry. 2009;108(4):832–838. doi: 10.1002/jcb.22311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rexahn Pharmaceuticals Press Release. [last accessed March 5, 2010];US Patent Granted for Rexahn Pharmaceuticals Leading Cancer Candidate. 2006 November 6; Available at http://www.rexahn.com/media.php?name=rexahn_media&page=3.
- 58.Marshall J, Posey J, Hwang J, Malik S, Shen R, Kazempour K, et al. A phase I trial of RX-0201 (AKT anti-sense) in patients with an advanced cancer. J Clin Oncol (Meeting Abstracts) 2007 June 20;25(18_suppl):3564. 2007. [Google Scholar]
- 59. NCT01028495: A Safety and Efficacy Study of RX-0201 Plus Gemcitabine in Metastatic Pancreatic Cancer. [last accessed March 5, 2010]; Available at http://clinicaltrials.gov/ct2/show/NCT01028495.
- 60.Handrick R, Rübel A, Faltin H, Eibl H, Belka C, Jendrossek V. Increased cytotoxicity of ionizing radiation in combination with membrane-targeted apoptosis modulators involves downregulation of protein kinase B/Akt-mediated survival-signaling. Radiotherapy and Oncology. 2006;80(2):199–206. doi: 10.1016/j.radonc.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 61.Jendrossek V, Handrick R. Membrane targeted anticancer drugs: potent inducers of apoptosis and putative radiosensitisers. Curr Med Chem Anticancer Agents. 2003 Sep;3(5):343–353. doi: 10.2174/1568011033482341. [DOI] [PubMed] [Google Scholar]
- 62.Kugler W, Erdlenbruch B, Otten K, Jendrossek V, Eibl H, Lakomek M. MAP kinase pathways involved in glioblastoma response to erucylphosphocholine. Int J Oncol. 2004 Dec;25(6):1721–1727. [PubMed] [Google Scholar]
- 63.Takai N, Ueda T, Nasu K, Narahara H. Erucylphosphocholine shows a strong anti-growth activity in human endometrial and ovarian cancer cells. Gynecologic Oncology. 2008;111(2):336–343. doi: 10.1016/j.ygyno.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 64.Bidyasar S, Kurzrock R, Falchook GS, Naing A, Wheler JJ, Durand J, et al. A first-in-human phase I trial of PBI-05204 (oleandrin), an inhibitor of Akt, FGF-2, NF-Kb, and p70S6K in advanced solid tumor patients. J Clin Oncol (Meeting Abstracts) 2009 May 20;27(15S):3537. 2009. [Google Scholar]
- 65.Rhodes N, Knick V, McConnell R, Lansing T, Rusnak D, Duckett D, et al. GSK690693, a pan-AKT kinase inhibitor has potent anti-tumor activity and shows additive effect with lapatinib; AACR Meeting Abstracts; 2007. Apr 14, p. 279. 2007. [Google Scholar]
- 66.Kumar R, Rhodes N, Knick V, Eberwein D, Duckett D, Zhang S, et al. GSK690693, a pan-AKT kinase inhibitor with potent pharmacodynamic and antitumor activity in vivo; AACR Meeting Abstracts; 2007. Apr 14, p. 277. 2007. [Google Scholar]
- 67.Rhodes N, Heerding DA, Duckett DR, Eberwein DJ, Knick VB, Lansing TJ, et al. Characterization of an Akt Kinase Inhibitor with Potent Pharmacodynamic and Antitumor Activity. Cancer Res. 2008 April 1;68(7):2366–2374. doi: 10.1158/0008-5472.CAN-07-5783. 2008. [DOI] [PubMed] [Google Scholar]
- 68.Altomare DA, Zhang L, Deng J, Di Cristofano A, Klein-Szanto AJ, Kumar R, et al. GSK690693 Delays Tumor Onset and Progression in Genetically Defined Mouse Models Expressing Activated Akt. Clinical Cancer Research. 2010 January 15;16(2):486–496. doi: 10.1158/1078-0432.CCR-09-1026. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. NCT00493818: Open-Label Study to Investigate the Safety, Tolerability, PK, and Pharmacodynamics of the AKT Inhibitor GSK690693. [last accessed February 26, 2010]; Available at http://clinicaltrials.gov/ct2/show/NCT00493818.
- 70.LoRusso P, Hurwitz H, Chiorean E, Jewell R, Lampkin T, Bachman K, et al. AKT inhibitor GSK690693: Preliminary results from the first time in human study; AACR Meeting Abstracts; 2008. Apr 12, p. LB-68. 2008. [Google Scholar]
- 71.Crouthamel M-C, Kahana JA, Korenchuk S, Zhang S-Y, Sundaresan G, Eberwein DJ, et al. Mechanism and Management of AKT Inhibitor-Induced Hyperglycemia. Clinical Cancer Research. 2009 January 1;15(1):217–225. doi: 10.1158/1078-0432.CCR-08-1253. 2009. [DOI] [PubMed] [Google Scholar]
- 72. NCT00920257: A Phase I, Open-Label, Two-Stage Study to Investigate the Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of the Oral AKT Inhibitor GSK2141795 in Subjects With Solid Tumors or Lymphomas. [last accessed December 22, 2009]; doi: 10.1007/s10637-018-0591-z. Available at http://www.clinicaltrials.gov. [DOI] [PMC free article] [PubMed]
- 73.Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004 Apr 23;304(5670):554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 74.Amzel LM, Huang C-H, Mandelker D, Lengauer C, Gabelli SB, Vogelstein B. Structural comparisons of class I phosphoinositide 3-kinases. Nat Rev Cancer. 2008;8(9):665–669. doi: 10.1038/nrc2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sarker D, Reid AHM, Yap TA, de Bono JS. Targeting the PI3K/AKT Pathway for the Treatment of Prostate Cancer. Clinical Cancer Research. 2009 August 1;15(15):4799–4805. doi: 10.1158/1078-0432.CCR-08-0125. 2009. [DOI] [PubMed] [Google Scholar]
- 76.Liu D, Hou P, Liu Z, Wu G, Xing M. Genetic Alterations in the Phosphoinositide 3-Kinase/Akt Signaling Pathway Confer Sensitivity of Thyroid Cancer Cells to Therapeutic Targeting of Akt and Mammalian Target of Rapamycin. Cancer Res. 2009 September 15;69(18):7311–7319. doi: 10.1158/0008-5472.CAN-09-1077. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cully M, You H, Levine AJ, Mak TW. Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer. 2006;6(3):184–192. doi: 10.1038/nrc1819. [DOI] [PubMed] [Google Scholar]
- 78.Suzuki H, Freije D, Nusskern DR, Okami K, Cairns P, Sidransky D, et al. Interfocal heterogeneity of PTEN/MMAC1 gene alterations in multiple metastatic prostate cancer tissues. Cancer Res. 1998 Jan 15;58(2):204–209. [PubMed] [Google Scholar]
- 79.Yoshimoto M, Cunha IW, Coudry RA, Fonseca FP, Torres CH, Soares FA, et al. FISH analysis of 107 prostate cancers shows that PTEN genomic deletion is associated with poor clinical outcome. Br J Cancer. 2007;97(5):678–685. doi: 10.1038/sj.bjc.6603924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mao JH, Kim IJ, Wu D, Climent J, Kang HC, DelRosario R, et al. FBXW7 targets mTOR for degradation and cooperates with PTEN in tumor suppression. Science. 2008 Sep 12;321(5895):1499–1502. doi: 10.1126/science.1162981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sargent DJ, Conley BA, Allegra C, Collette L. Clinical Trial Designs for Predictive Marker Validation in Cancer Treatment Trials. J Clin Oncol. 2005 March 20;23(9):2020–2027. doi: 10.1200/JCO.2005.01.112. 2005. [DOI] [PubMed] [Google Scholar]
- 82.Holland WS, Mack PC, Tepper CG, Gandara DR, Lara P., Jr Combined mTOR and AKT inhibition in renal cell carcinoma (RCC) J Clin Oncol (Meeting Abstracts) 2010 May 20;28(15_suppl):e15041. [Google Scholar]
- 83.Barrios C, Liu M-C, Lee S, Vanlemmens L, Ferrero J-M, Tabei T, et al. Phase III randomized trial of sunitinib versus capecitabine in patients with previously treated HER2-negative advanced breast cancer. Breast Cancer Research and Treatment. 2010;121(1):121–131. doi: 10.1007/s10549-010-0788-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Burstein HJ, Elias AD, Rugo HS, Cobleigh MA, Wolff AC, Eisenberg PD, et al. Phase II Study of Sunitinib Malate, an Oral Multitargeted Tyrosine Kinase Inhibitor, in Patients With Metastatic Breast Cancer Previously Treated With an Anthracycline and a Taxane. J Clin Oncol. 2008 April 10;26(11):1810–1816. doi: 10.1200/JCO.2007.14.5375. 2008. [DOI] [PubMed] [Google Scholar]
- 85.Mayer EL, Dhakil S, Patel T, Sundaram S, Fabian C, Kozloff M, et al. SABRE-B: an evaluation of paclitaxel and bevacizumab with or without sunitinib as first-line treatment of metastatic breast cancer. Annals of Oncology. 2010 May 23;:2010. doi: 10.1093/annonc/mdq260. [DOI] [PubMed] [Google Scholar]
- 86.Cho DC, Cohen MB, Panka DJ, Collins M, Ghebremichael M, Atkins MB, et al. The Efficacy of the Novel Dual PI3-Kinase/mTOR Inhibitor NVP-BEZ235 Compared with Rapamycin in Renal Cell Carcinoma. Clinical Cancer Research. 2010 July 15;16(14):3628–3638. doi: 10.1158/1078-0432.CCR-09-3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brachmann SM, Hofmann I, Schnell C, Fritsch C, Wee S, Lane H, et al. Specific apoptosis induction by the dual PI3K/mTor inhibitor NVP-BEZ235 in HER2 amplified and PIK3CA mutant breast cancer cells. Proceedings of the National Academy of Sciences. 2009 December 29;106(52):22299–22304. doi: 10.1073/pnas.0905152106. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Burris H, Rodon J, Sharma S, Herbst RS, Tabernero J, Infante JR, et al. First-in-human phase I study of the oral PI3K inhibitor BEZ235 in patients (pts) with advanced solid tumors. J Clin Oncol (Meeting Abstracts) 2010 May 20;28(15_suppl):3005. [Google Scholar]
- 89.Iglehart JK. Prioritizing Comparative-Effectiveness Research -- IOM Recommendations. N Engl J Med. 2009 July 23;361(4):325–328. doi: 10.1056/NEJMp0904133. 2009. [DOI] [PubMed] [Google Scholar]