Abstract
A new class of fluorescent triazaborolopyridinium compounds was synthesized from hydrazones of 2-hydrazinylpyridine (HPY) and evaluated as potential dyes for live cell imaging applications. The HPY dyes are small, absorption/emission properties are tunable through variation of pyridyl or hydrazone substituents, and offer favorable photophysical characteristics featuring large Stokes shifts and general insensitivity to solvent or pH. The neutral charge, cell membrane permeability and favorable relative influences on the water solubility of HPY conjugates are complementary to existing fluorescent dyes, and offer advantages for the development of receptor-targeted small molecule probes. This potential was assessed through the development of a new class of cysteine-derived HPY-conjugate imaging agents for the kinesin spindle protein (KSP) that is expressed in the cytoplasm during mitosis, and is a promising chemotherapeutic target. Conjugates possessing the neutral HPY or charged AlexaFluor dyes that function as potent, selective allosteric inhibitors of the KSP motor were compared using biochemical and cell-based phenotypic assays, and live-cell imaging. These results demonstrate the effectiveness of the HPY dye moiety as components of probes for an intracellular protein target, and highlight the importance of dye structure in determining the pathway of cell-entry, and the overall performance of small molecule conjugates as imaging agents.
Introduction
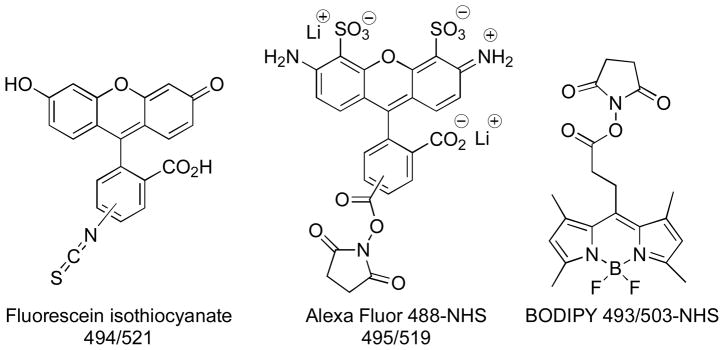
Fluorescent compounds serve as versatile tools for molecular and cellular imaging, flow cytometry, and a wide variety of applications in biology and biotechnology.1 The development of modular approaches, whereby different reporter groups can be conjugated to the targeting agent, is well suited for specific applications and benefits from the availability of structurally diverse fluorescent dyes with different spectroscopic properties. Biomolecules such as DNA, proteins and antibodies are routinely labeled with fluorescent dyes that match the required spectroscopic properties of the application, frequently involving derivatives such as fluorescein, rhodamine, and boron dipyrromethene (BODIPY) (Figure 1).2 Advances in chemical biology and molecular libraries screening approaches provide exceptional opportunities for the rapid identification of novel small molecule ligands with high affinity and selectivity for biological targets of interest, and the discovery of new fluorescent scaffolds.3 The structure and physicochemical properties of the dye-conjugate are key considerations for preservation of the targeting characteristics of the small molecule ligand conjugates. The development of agents for intracellular targets is faced with additional challenges, since access to the corresponding intracellular compartments must be achieved by simple permeation, active transport or endocytotic mechanisms. The large size, polycyclic aromatic structures and presence of charged functional groups in many fluorescent dyes present major challenges when applied to the development of imaging agents based on small molecules, where the physicochemical properties of the dye may dramatically alter the solubility, biodistribution and binding properties of the conjugate.4 The ideal reporter dye should have characteristics that include ease and versatility of methods for attachment to small molecule targeting agents, efficient cellular uptake, and lack of inherent biological activity or toxicity. The propensity of existing dyes to localize in specific sites or organelles is an additional factor that must be recognized and considered when designing dye-conjugates. Therefore, a significant need exists for the development of new small, neutral biocompatibile fluorescent cores that exhibit good aqueous solubility, membrane permeability, favorable photophysical properties, and versatile coupling chemistries that yield minimal perturbation of targeting properties. The spectroscopic characteristics must be compatible with the instrumentation used for detection, and the fluorescent output of the probe must be sufficient to permit the detection of the target at natural abundance levels.
Figure 1.
Structures of Representative Fluorescent Dyes
We have previously used metal-mediated coupling strategies to incorporate chelates derived from the 2-hydrazinylpyridine core into estrogen derivatives for the development of 99mTc-imaging agents, and became interested in the possibility of developing fluorescent dyes based on this heterocyclic scaffold.5 Hydrazines are versatile reagents in organic and aqueous media with rapid kinetics and favorable thermodynamics of hydrazone formation that are advantageous for bioorthogonal coupling strategies.6 SoluLink® offers proprietary technologies for bioconjugation using hydrazone formation with hydrazinylnicotinamide groups to connect proteins, DNA, antibodies and solid surfaces.7 The UV-traceable bis-aryl hydrazone chromophore provides a basis for quantitative determination of protein labeling using absorbance spectroscopy. Considering the versatile coupling chemistries associated with 2-hydrazinylpyridine and the promising photophysical properties associated with the extended π-systems that result from hydrazone formation, we explored strategies to construct fluorescent derivatives based on this scaffold. Herein, we report the synthesis of a new class of hydrazinylpyridine-derived hydrazones (HPY) that incorporate a rigid triazaborolopyridinium core structure. The photophysical properties and initial assessment of cellular permeability suggests the suitability of HPY dyes for use as imaging probes. This potential was used to develop a new class of cysteine-derived HPY-conjugate imaging agents that function as potent, selective inhibitors of the promising chemotherapeutic target kinesin spindle protein (KSP). Comparison of fluorescent HPY and charged AlexaFluor conjugate probes in biochemical and cell-based phenotypic assays, and live-cell imaging demonstrates the importance of dye structure in determining the pathway of cell-entry, and the overall performance of targeted imaging agents.
Results and Discussion
Synthesis of the Fluorescent Triazaborolopyridinium Core
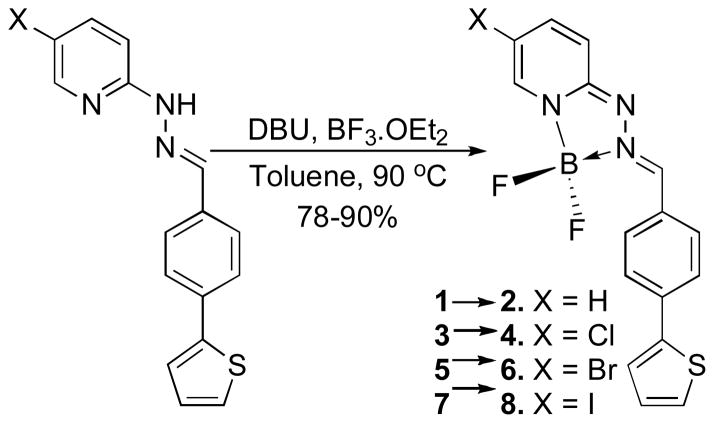
Hydrazin-2-yl-pyridine reacts rapidly and efficiently with aldehydes and ketones to form the corresponding hydrazones. Our initial studies included the representative hydrazones 1, 3, 5, 7 derived from 4-(thiophen-2-yl)benzaldehyde (Scheme 1). These compounds exhibited strong absorption maxima in the range of 354–360 nm with molar absorptivity constants (ε~38,000 M−1cm−1) (Supporting Information). However, these hydrazones exhibited weak fluorescence emission spectra λemi when excited at the λmax values with very low quantum yields (Φf = 6 x10−3). The emission was associated with relatively large Stokes shifts (~60 nm) but the low intensity precludes their use for most biological imaging applications. The simple benzaldehyde-derived hydrazone (9: Figure 2) exhibits shifts in the absorption and emission spectra to shorter wavelength (abs/emi = 328/397 nm) and reduced quantum yield (Φf = 2 x10−3), consistent with expectations for a small blue-shift due to loss of the extended conjugation of the 2-thiophenyl substituent.
Scheme 1.
Synthesis of Triazaborolopyridinium HPY Dyes
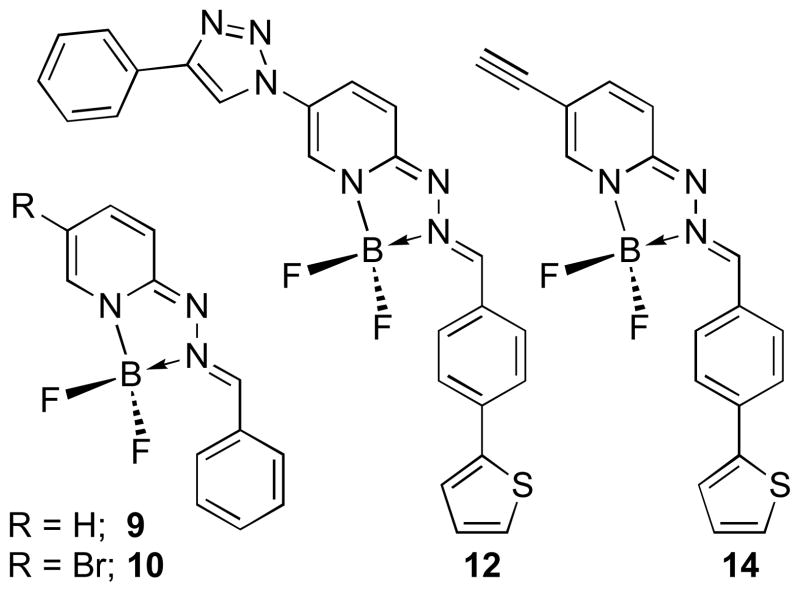
Figure 2.
Structures of 5-substituted HPY Dyes 9, 10, 12 and 14
We next considered the possible formation of cyclic systems incorporating the distal nitrogen atoms into a rigid scaffold with enforced planarity of the extended π-system, anticipating that this would enhance the resulting fluorescence emission properties. Considering the basicity of the pyridin-2-yl-hydrazone moiety, we investigated the formation of Lewis acid complexes with boron trifluoride diethyl etherate. The reaction conditions for cyclization to produce the triazaborolopyridinium dyes from the hydrazone precursors used BF3•OEt2 and 1,8-diazabicyclo[5.4.0]-undec-7-ene (DBU) as shown in Scheme 1, and provided yields ranging from 78–90%. The six-membered ring system of the well-known 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY) dyes are typically prepared from dipyrromethene intermediates under similar conditions,2 and structural elaboration of this scaffold has yielded new analogs with modified photophysical properties.8
The photophysical properties of a series of HPY dyes are summarized in Table 1. The HPY compounds derived from 4-(thiophen-2-yl)-benzaldehyde exhibit absorption spectra with λmax = 472–490 nm and molar absorptivities (ε) = 26,600–32,900 in CH3OH, and emission spectra λemi = 551–563 nm with characteristically large Stokes shifts (72–80 nm). These compounds exhibited high quantum yields with Φf = 0.69–0.95. Large stokes shifts facilitate signal detection and improve resolution by separating absorption and emission spectra, and are advantageous for imaging applications in which self-quenching must be avoided.8d Substituents on the 5-position of the pyridine ring were varied to identify related effects on the spectroscopic properties, since we anticipated that we would eventually use this position for attaching targeting agents. The 5-brominated compound 6 exhibited the highest absorptivity and quantum yield in comparison with the parent scaffold 2 and the chlorinated or iodinated halogen congeners 4 and 8, respectively (Scheme 1). The 1,2,3-triazole and ethyne derivatives 12 and 14 were selected to represent possible functional group substitutions on the pyridine backbone that could be elaborated to provide connecting linkages in small molecule probes and used for bioorthogonal labeling strategies (Figure 2).9 While these carbon-and nitrogen-linked derivatives produced minimal perturbation of the absorption and emission maxima, the resulting quantum yields were reduced nearly two-fold relative to the parent compound 2. Comparison of 6 with the simple benzaldehyde hydrazone derivative 10 reveals that the conjugated 2-thiophene group confers both longer wavelength absorption and increased quantum yield. Computational analysis of the structural effects exhibited by this series may help to identify the basis for the observed electronic trends, and would provide valuable insight for further optimization of photophysical properties.
Table 1.
Photophysical Properties of HPY Dyesa
| Dyea | λmax nm | ε M−1cm−1 | λemib nm | Φf | Solvent |
|---|---|---|---|---|---|
| 2 | 472 | 28 800 | 551 | 0.75 | MeOH |
| 4 | 491 | 26 800 | 563 | 0.69 | MeOH |
| 6 | 482 | 32 900 | 562 | 0.95 | MeOH |
| 8 | 490 | 26 600 | 562 | 0.71 | MeOH |
| 10 | 471 | 22 600 | 539 | 0.31 | MeOH |
| 12 | 491 | 33 800 | 572 | 0.37 | MeOH |
| 14 | 481 | 36 700 | 572 | 0.38 | MeOH |
| 16 | 403 | 8 500 | 493 | 0.21 | MeOH |
| 16 | 404 | 8 600 | 491 | 0.17 | 1%DMSO/PBSc |
| 16 | 408 | 9 300 | 497 | 0.22 | CH2Cl2 |
| 16 | 403 | 8 600 | 504 | 0.17 | Acetone |
| 16 | 406 | 9 800 | 500 | 0.07 | Toluene |
| 18 | 400 | 8 300 | 496 | 0.20 | MeOH |
| 21 | 403 | 12 000 | 492 | 0.21 | MeOH |
| 21 | 404 | 12 300 | 492 | 0.16 | 1%DMSO/PBSc |
| 21 | 406 | 11 200 | 497 | 0.20 | CH2Cl2 |
| 21 | 407 | 10 800 | 504 | 0.23 | Acetone |
| 21 | 410 | 10 600 | 501 | 0.51 | Toluene |
| 22 | 400 | 9 400 | 476 | 0.21 | MeOH |
20 μM samples were prepared.
Sample was excited at λmax.
pH 7.4.
A comparison of the structure and photophysical properties of the HPY dyes with the commercially available amine-reactive fluorescent dyes fluorescein-isothiocyanate (FITC), BODIPY 493/503 and AlexaFluor 488 provides a basis for considering the structural effects associated with small molecule conjugates, and their utility in live-cell imaging applications (Figure 1). These commercial dyes exhibit strong absorption and emission properties, and are available with reactive isothiocyanate or N-hydroxysuccinimidyl ester (NHS) groups to facilitate their conjugation to amines. The BODIPY and HPY dyes each incorporate a heterocyclic boron-containing ring system, however, they differ in their respective six-or five-membered ring sizes, and the inclusion of additional polar nitrogen atoms in the HPY dyes. Aza-dipyrromethene analogues incorporating a nitrogen in the backbone have been described recently.8e-e The BODIPY and HPY dyes are electronically neutral, and BODIPY dyes have demonstrated their ability to effectively cross cell membranes and preferentially interact with lipophilic components. While the relatively narrow emission bandwidth of the BODIPY dyes provides an intense signal, the small associated Stokes shift may require that they be excited or detected at suboptimal wavelengths, which limits the capacity to fully utilize their intense absorption/emission properties. In contrast, the HPY dyes exhibit much larger Stokes shift and increased resolution of absorption/emission spectra (see Supporting Figure 2.3.12). The FITC and AlexaFluor dyes present larger relative steric profiles and consist of a mixture of two regioisomers, although single isomer products are now commercially available. The AlexaFluor dyes are charged at physiological pH, due to the multiple anionic sulfate and carboxylate groups. The ionic charge increases water solubility, but reduces membrane permeability.
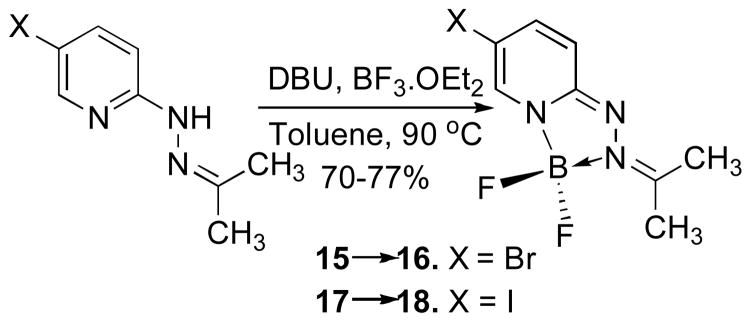
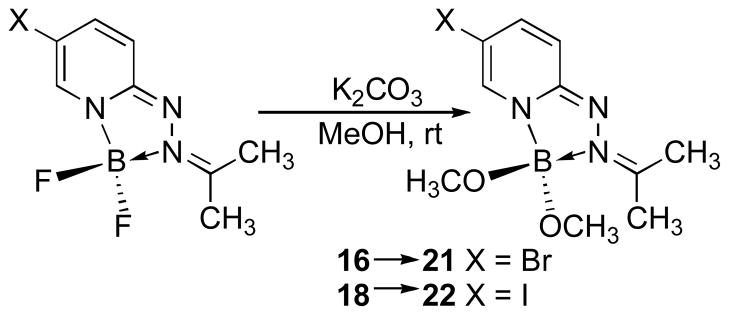
We were interested in optimizing the core structure of the HPY dyes for potential biological applications, intending to further reduce the steric profile and molecular weight, while also increasing water solubility without having to introduce charged groups that would compromise membrane permeability. We elected to eliminate the hydrophobic arylhydrazone group while retaining the 5-halogen substituent as a potential site for connecting linkages to biological targeting entities. The 2-hydrazinylpyridines react rapidly with acetone to form the corresponding hydrazone derivatives 15 and 17, which underwent cyclization to form the 5-bromo-and 5-iodo-substituted triazaborolopyridinium compounds 16 and 18 under similar conditions (Scheme 2). To provide an additional parameter for structural modification, we investigated the possibility of replacing the fluoride substituents attached to boron with alkoxide ligands, and found that this transformation occurred efficiently using mildly basic conditions in methanol to produce the stable bis-methoxide compounds 21 and 22 respectively (Scheme 3). A related example of this type of substitution has been reported for the six-membered BODIPY dyes, although more forcing conditions involving a stepwise procedure for fluoride activation with AlCl3 followed by alcohol substitution were required.10 The acetone-derived HPY compounds 16, 18, 21 and 22 were soluble in a variety of solvents, and comparison of the photophysical properties of derivatives 16 and 21 demonstrated only minor solvent-dependent variations in their absorption and emission properties in methanol, dichloromethane, acetone, toluene and aqueous 1% DMSO in PBS buffer solution at physiological pH 7.4 (Table 1). The acetone-derived HPY dyes required higher energy excitation (λmax = ~ 405 nm) in comparison with the arylhydrazone derivatives, attributed to the truncated π–system in these simple aliphatic derivatives. The smaller, acetone-derived hydrazone group was expected to be advantageous for increasing the relative water solubility of HPY-conjugates designed for biological applications. The absorbance/emission properties, and chemical stability of 16 were unaffected over a wide range of pH (4–9) in aqueous buffer solution, such that no decomposition in solution was evident after several days at ambient temperature.
Scheme 2.
Synthesis of HPY Dyes 16 and 18
Scheme 3.
Synthesis of HPY Dyes 21 and 22
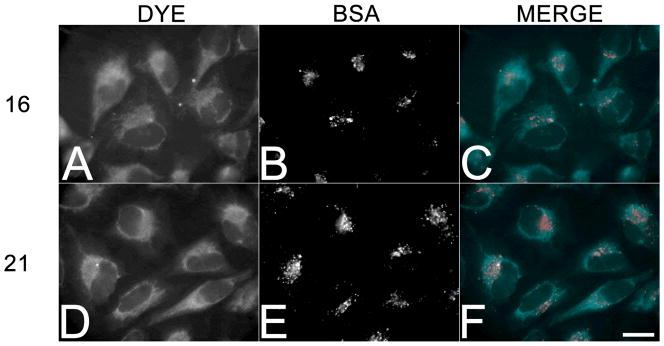
Considering the favorable characteristics of 16 and 21, we selected these dyes to evaluate their respective cell membrane permeability and imaging properties in HeLa cells (Figure 3). To visualize the staining by conventional epifluorescence microscopy, a custom filter set was assembled that closely matched the optimal excitation/emission spectra (395/500) for the two dyes in physiological buffers. Examination of cells incubated for 60 minutes in the presence of 20 μM 16 and 21 revealed that both dyes could be visualized throughout the cytoplasmic compartment, and were particularly enriched in intracellular membranes (Figure 3A and D). To distinguish between membrane permeability and uptake by endocytosis, 100 μg/mL AlexaFluor546-conjugated bovine serum albumin (BSA) was included to mark membrane compartments involved in endocytic trafficking (Figure 3B and E). As shown in the merged images (Figure 3C and F), the HPY dyes were distributed throughout membranous compartments that were distinct from those endosomal vesicles that contained the fluid-phase marker. The dyes 16 and 21 did not exhibit any obvious localization in specific organelles or other sites, and could be removed by washing free with buffered saline. Cells treated for up to 6 h with dye concentrations of 100 μM exhibited no toxicity or other obvious effects on cell viability or phenotypic changes. Coumarin based dyes also undergo uptake into cells without differential localization, however, sequestration in internal membranes is a common feature of many dyes such as coumarin 6, and can be washed away.4 In contrast, rhodamine dyes 123 and B are taken up into the mitochondria, cyanine dyes often localize in the nucleus, and BODIPY dyes typically localize in the endoplasmic reticulum. These imaging results from live cells demonstrate that the triazaborolopyridinium dyes were capable of entering mammalian cells in a pathway independent of endocytosis, suggesting that these dyes were membrane permeable, and suitable candidates for elaboration as probes for intracellular targets.
Figure 3.
Membrane permeability of 16 and 21 in living HeLa cells. HeLa cells were incubated in 20 μM 16 (A–C) or 21 (D–F) for 1 hour, washed free of the dye, and imaged live. Additionally, 100 μg/mL AlexaFluor546-labeled bovine serum albumin (BSA) was included as a marker for fluid phase endocytosis panels B and E. Bar, 20 μm.
Development of inhibitory KSP imaging probes
S-Trityl-L-cysteine is an allosteric small molecule inhibitor of the class 5 kinesin motor, Kinesin Spindle Protein (KSP).11 The tetrameric microtubule motor KSP plays a vital role in separating the spindle poles during mitosis, and when KSP is antagonized with small molecule inhibitors, cells form a characteristic monopolar spindle, resulting in mitotic arrest and eventual cell death by apoptosis.12 The development of small molecule inhibitors as probes have played an invaluable role in the study of the cytoskeleton.13 Fluorescent ATP-substrate analogues have been used to investigate kinesin motor function,14 but no examples of fluorescent inhibitors have been reported. Because KSP functions only in the cytoplasm during mitosis, an inhibitory dye conjugate would serve not only as a potential imaging probe, but would also provide independent measure of the membrane permeability and performance of the synthetic triazaborolopyridinium probes.
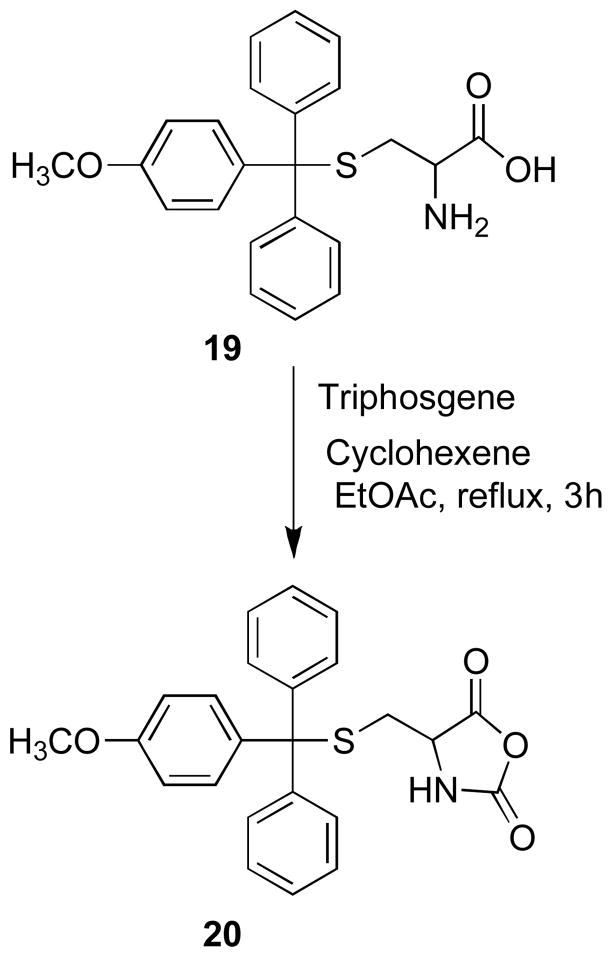
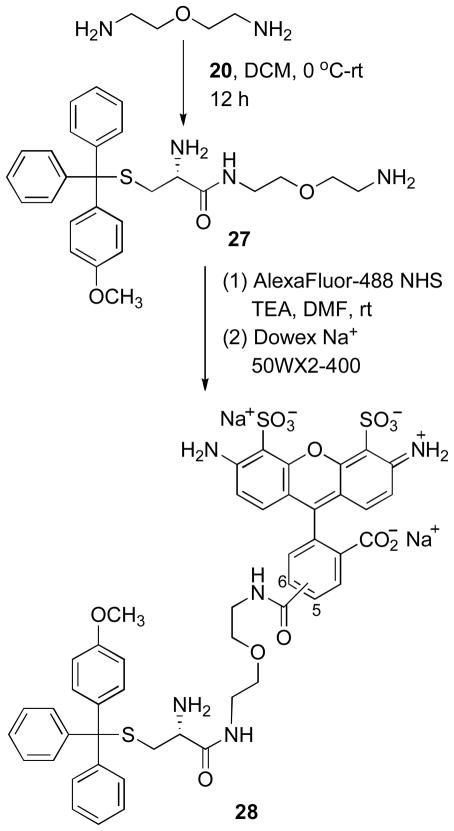
Structure-activity analyses have identified the triphenylmethyl-mercapatoethanamine moiety as the primary pharmacaphore in this class of KSP inhibitor, and the 4′-methoxy derivative 19 was found to exhibit increased potency in biochemical and cellular assays.11 Consideration of possible sites for the attachment of a dye to 19 requires that the resulting fluorescent conjugates retain the high affinity allosteric binding interaction. Docking studies have suggested that the carboxylic acid group projects outside of the hydrophobic binding pocket and is not required for binding, and amide derivatives were shown to retain inhibitory activity, therefore we selected this position for derivatization.11b The α-amino acid functionality of cysteine derivative 19 can be easily converted to an amine-reactive oxazolidine-2,5-dione group 20, which provides a viable synthetic route for developing carboxamide-linked selective fluorescent inhibitors of KSP (Scheme 4).
Scheme 4.
Synthesis of Oxazolidine-2,5-dione 20
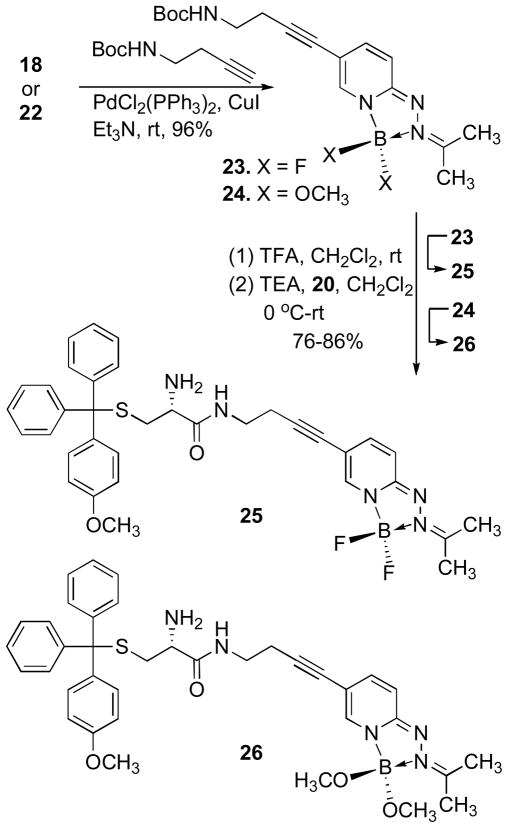
The iodide substituted difluoro-and bis-methoxy-HPY dyes 18 and 22 were converted to the amine-functionalized alkyne products 23 and 24 in excellent yields (≥95%) by Sonogashira coupling of tert-butyl-but-3-ynylcarbamate. Deprotection of the tBoc-group using TFA/CH2Cl2, followed by neutralization of the acidic reaction mixture with triethylamine provided the corresponding primary amines that were immediately coupled with oxazolidine-2,5-dione 20 to give the desired conjugates 25 and 26 (Scheme 5). No significant changes in the spectroscopic properties of conjugates 25 and 26 were evident in comparison to the representative HPY dyes 16 and 21 measured in 1% DMSO/PBS at pH-7.4 (Figure 4). These results demonstrate that the attachment of the triphenylmethyl substituent does not affect the photophysical properties or induce self-quenching, and suggests that the distance of separation and absence of extended conjugation in the aliphatic carboxamide linkage allow the HPY dyes to function effectively as a fluorescent reporter group in conjugates 25 and 26.
Scheme 5.
Synthesis of HPY-KSP Inhibitor Conjugates 25 and 26
Figure 4.
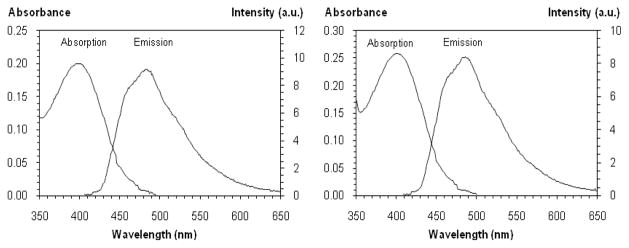
Absorption and emission spectra of (a) 25 (λmax = 400 nm, ε = 9 900, λemi = 489 nm, Φf = 0.31) and (b) 26 (λmax = 400 nm, ε = 12 900, λemi = 486 nm, Φf = 0.29). Samples (20 μM) were prepared in 1% DMSO/PBS, pH 7.4 at 25 °C and excited at 405 nm.
In order to compare the performance characteristics of the KSP-targeted HPY-conjugates with a representative analog containing a commercially available fluorescent dye, we synthesized the AlexaFluor488 analog 28 (Scheme 6). The amine-reactive oxazolidine-2,5-dione 20 was treated with excess 2,2′-oxy-bis-ethylamine to give the mono-carboxamide 27 in moderate yield. The activated N-hydroxysuccinimide ester of the AlexaFluor488 dye was coupled with the free amine of 27 to yield the desired conjugate 28. This product was purified by reverse phase chromatography followed by ion-exchange chromatography to provide the Na+ salt form. The observed regioselective carboxamide formation involving the sterically accessible terminal amine group was consistent with expectations for increased reactivity relative to the α-amine in 27. Acetylation of the α-amine group of 19, which is part of the pharmacophore, inactivates KSP inhibition.11 Our attempts to synthesize the analogous BODIPY-493/503 conjugates following a parallel synthetic route were unsuccessful due to major difficulties encountered in purification of the final products. These derivatives were very hydrophobic, amphiphilic compounds and tended to form intractable aggregates that were incompatible with normal or reverse phase chromatography purification methods, and this effort was abandoned.
Scheme 6.
Synthesis of AlexaFluor488-KSP Inhibitor Conjugate 28
Characterization of KSP Inhibition
The synthetic dye conjugates 25, 26 and 28 were evaluated for their ability to inhibit KSP using both an in vitro biochemical assay for microtubule-stimulated ATPase activity, as well as a cell-based assay for bipolar spindle assembly in HeLa cells (Table 2). Results of the biochemical assay using purified human KSP motor domain revealed that the original KSP inhibitor 19 exhibited a half maximal inhibitory concentration (IC50) of 0.27 μM, while the fluorescent HPY dye conjugates 25, 26 and AlexaFluor derivative 28 had similar IC50 values of 0.85, 0.60 and 0.73 μM, respectively. The IC50value of the mono-carboxamide derivative 27 was nearly identical to 28, demonstrating that the 2,2′-oxy-bis-ethylamine linkage itself had no distinct effect on KSP inhibition. These results show that each of the conjugates elicit similar inhibitory properties against the purified KSP motor, irrespective of the length of the carboxamide linkages used, or the differences in size and ionic charge of the dye structures.
Table 2.
IC50 Values for inhibition of microtubule-stimulated KSP ATPase activity and EC50 values for mitotic arrest in cell-based assays for compounds 19, 25–29a
| Compound | IC50 | EC50 |
|---|---|---|
| 19 | 0.27 | 0.23 |
| 25 | 0.85 | 1.13 |
| 26 | 0.60 | 1.03 |
| 27 | 0.75 | 0.58 |
| 28 | 0.73 | 5.40 |
| 29 | 7.8 | 49.00 |
Inhibitory concentrations are expressed in μM.
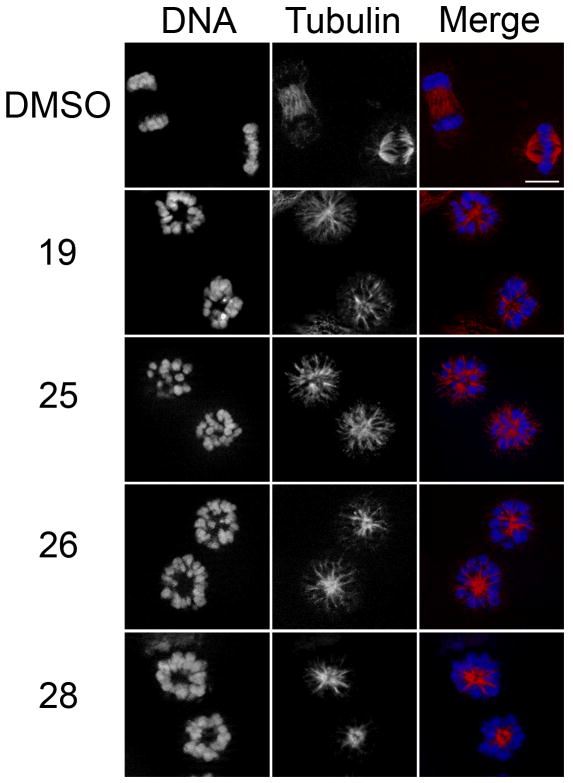
To determine the performance of the dye-conjugated KSP inhibitors in living cells, HeLa cells were incubated in the absence or presence of 10 μM compound (19, 25–28) for 4 h, and then fixed and probed for spindle organization using anti-tubulin antibodies. Whereas carrier control (DMSO)-treated cells exhibited normal bipolar spindle morphology (Figure 5), cells treated with 19, 25, 26 and 28 all exhibited dose-dependent formation of monopolar spindles consistent with compromised KSP activity. Comparison of the half maximal effective concentrations (EC50) for each conjugate revealed that while the HPY conjugates exhibited a 5-fold reduction in activity relative to the inhibitor 19 (Table 2), the AlexFluor conjugate 28 displayed a 23-fold decrease in inhibitory activity in the cell-based assay.
Figure 5.
Fluorescent cysteine conjugates retain KSP inhibitory activity. HeLa cells were incubated in the absence or presence of 10 μM 19, or conjugates 25, 26, and 28 for four hours, and then fixed and probed for tubulin (red) and DNA (blue) localization. Bar, 10 μm.
The observed differences in inhibitory properties of the probes in the cell-based assays can be attributed to structural differences in the attached dyes. The AlexaFluor488 dye is significantly larger than the HPY structure, however, the in vitro results demonstrated that there were no significant differences associated with this increased steric requirement (Table 2). The ionic charge associated with the sulfate and carboxylate groups of the AlexaFluor488 dye would, however, be expected to decrease the permeability across the membrane bilayer and would result in increased apparent EC50.
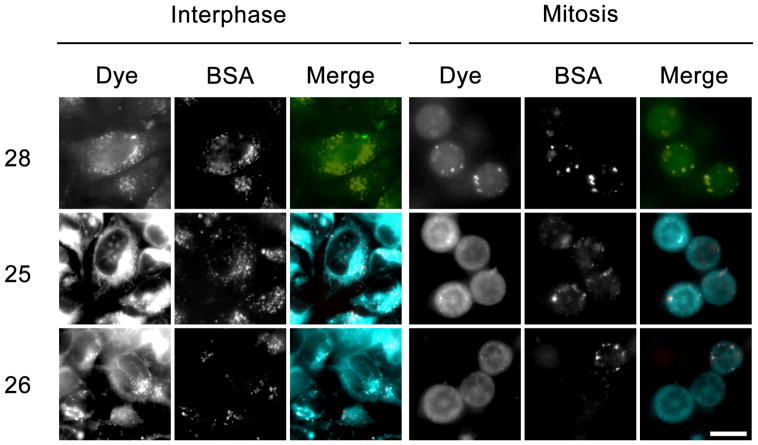
In order to further investigate the differences in KSP inhibition observed between the two classes of dye conjugates in the cell-based assay, the fluorescence imaging capabilities of the dye conjugates were employed to determine their subcellular distribution. HeLa cells were incubated with the dye conjugates 25, 26, and 28 for four hours, and then washed free of unbound dye and imaged live (Figure 6). Examination of both interphase (Left panels) and mitotic cells (Right panels) revealed differences in the localization patterns for the HPY and AlexaFluor conjugates. The HPY-conjugates displayed a cytoplasmic localization that mirrored the free dye (Figure 3). The presence of fluoride or alkoxide substituents on boron in 25 and 26 did not affect the imaging patterns, and suggests that both probes exhibit similar cellular properties. However, the AlexaFluor derivative 28 colocalized with the fluid-phase endocytosis marker (BSA) in both interphase and mitotic cells as shown in Figure 6. The observation that 28 retained KSP inhibitory activity in the cytoplasm was inconsistent with the imaging data that suggested the AlexFluor dye remained sequestered within endocytic compartments, and raised the possible scenario that the AlexaFluor conjugate 28 was not stable under the acidic conditions found in the endosomal compartment.
Figure 6.
Imaging of fluorescent cysteine conjugates in living cells. HeLa cells were treated with fluorescent conjugates of 10 μM 25, 26, and 28, and co-incubated with fluid-phase endocytosis marker AlexaFluor546-conjugated BSA for four hours, and then imaged using wide-field epifluorescence. Fluorescence was confined to punctate structures colocalizing with the endocytosis marker (BSA) in cells treated with 28, whereas, cells treated with conjugates 25 and 26 displayed diffuse fluorescence throughout the cytoplasm. Bar, 20 μm.
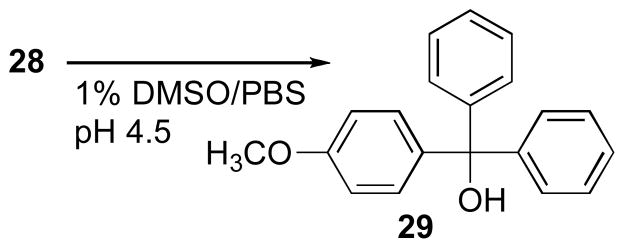
Molecules taken up by endocytosis encounter an increasingly acidic environment as they progress through the endosomal and lysosomal compartments. The stability of AlexaFluor conjugate 28 under conditions that resemble the acidic luminal pH of the mid-late endosome (pH 4.5) was evaluated by HPLC-MS (Supporting Information Figure 5.1). The S-trityl and carboxamide linkage in 28 are potential hydrolytic cleavage sites. Compound 28 underwent a time-dependent acid-catalyzed hydrolysis of the 4′-methoxytrityl group to produce the alcohol 29 that was characterized by HPLC retention time and identified by mass spectrometry in comparison with authentic material (Scheme 7). Compound 29 was evaluated individually in the biochemical and cell-based assays, revealing that this simple trityl alcohol moiety itself exhibited KSP inhibitory properties, although markedly less potent than any of the corresponding thioethanamine analogs. The poor water solubility of 29 contributes to the observation of higher EC50 values in the cell based assay, however the efficiency of endocytic uptake and delivery of the charged, acid-sensitive hydrolyzable probe 28 is evident from the higher relative potency. These results, in combination with the observed subcellular localization of the AlexaFluor fluorescence that was retained in the endocytic compartments, suggest the observed apparent KSP-inhibitory activity of conjugate 28 includes a contribution from the hydrolytic release of 29 in the acidic endosomes, which then diffuses into the cytoplasm where it inhibits KSP. In comparison, the HPY conjugates 25 and 26 are able to enter the cells via simple permeation, and exhibit potent inhibitory properties in both in vitro and cell-based assays.
Scheme 7.
Acid Stability Study of AlexaFluor488-KSP Inhibitor Conjugate 28
Consideration of dye structure is important for the design and performance of probes.4,15 The fluorescein group is often presumed to be innocuous and permeable, however associated effects on both transport pathway and subcellular localization have been observed in conjugates of cell-penetrating peptides.16 A BODIPY-androgen conjugate derived from dehydroepiandrosterone mimicked the steroid in functional assays and was effective for live cell imaging, but exhibited weak uniform staining of the cytoplasm that was not displaced by competition with excess steroids, and was metabolized differently.17 A recent investigation of a series of hydrophobic tamoxifen conjugates containing AlexaFluor, carboxyfluorescein, and BODIPY dyes revealed differences in binding affinities to estrogen receptors α and β, such that the large AlexaFluor conjugate exhibited the weakest binding, but all three antagonized ER-mediated transcription in the nucleus with similar inhibitory IC50 values.18 The AlexaFluor conjugate exhibited slower uptake than the neutral derivatives, which is consistent with reduced permeability at short time intervals due to the charged dye. However, expectations for nuclear localization of the dyes were not realized such that fluorescence was observed from the cytoplasm in all cases and excluded from the nucleus in ER-positive MCF7 cell lines. Attempts to identify possible cleavage products were unsuccessful, but were not ruled out due to the high probe concentrations and long incubation times involved, combined with the difficulties faced in quantifying subnanomolar concentrations of active probes. The results obtained in our investigation with AlexaFluor conjugate 28 illustrate an important caveat for interpretation of fluorescence localization and activity data, and suggests that endocytotic uptake can serve as a route for delivering intracellular probes possessing an acid-cleavable trityl linkage.
Conclusions
The triazaborolopyridinium HPY core represents a new class of fluorescent dyes that are readily synthesized from hydrazone precursors, and provide complementary structural and physicochemical properties that are well suited for use in the construction of small molecule probes. These neutral compounds are structurally related to the widely used BODIPY dyes and share favorable characteristics such as membrane permeability, and although they are not as bright, the HPY dyes exhibit larger Stokes shifts for better-resolved absorption and emission spectra. The small overall size and presence of three nitrogen atoms in the conjugated heterocyclic backbone of the HPY dyes are structural factors that contribute towards favorable water solubility. The HPY dyes demonstrated tunable absorption/emission properties with variation of the hydrazone substituent, but the individual photophysical properties were relatively insensitive to varying solvents from non-polar organic to aqueous media. The size and polarity of the hydrazone group can be easily varied, and the ability to exchange fluoride and alkoxide substituents on boron provides additional routes for structural optimization of HPY dyes for specific applications. The versatile chemistry associated with the HPY dye core structure should facilitate the construction of new fluorescent probe conjugates using a variety of synthetic strategies such as modification of the hydrazone, direct substitutions on the pyridine scaffold, or by incorporating linkages with appropriately matched reactive functional groups. In this study, we observed improved performance of the HPY-conjugated inhibitors in comparison with AlexaFluor conjugates. Because KSP inhibitors can be used to synchronize cell cultures at G2/M, conjugates such as 25 and 26 may have broad utility as reversible probes in cell cycle research. Synthetic and computational efforts designed to further optimize the photophysical properties of the HPY scaffold, expanded comparison of additional synthetic dye conjugates, and exploration of other targeted biological imaging applications are currently in progress.
Experimental Procedures
All compounds were synthesized in an efficient fume-hood. Commercially available solvents and reagents were purchased and used without further purification. AlexaFluor®488-NHS was purchased from Invitrogen. Preparative chromatography was performed by medium pressure column chromatography using AnaLogix SuperFlash or Sorbent Technologies RediSep C18 pre-packed columns. 1H NMR spectra were acquired at 300 MHz or 400 MHz, and 13C NMR were acquired at 75 MHz or 100 MHz spectrometers at ambient temperatures (20±2 °C). 1H NMR spectra in CDCl3 were referred to TMS, and C6F6 was used as an internal reference in 19F NMR spectra. Purity and the molecular weight of compounds were determined by Waters 2695 alliance HPLC/ESI-MS with C18 reverse phase column system eluting with 20–70% acetonitrile in water. High resolution mass spectra were obtained from the University of California at Riverside.
General Procedure A for Triazaborolopyridinium Cyclization
Boron trifluoride diethyl etherate (BF3·OEt2, 5.0 eq) was added drop-wise to the mixture of hydrazone (1.0 eq) and DBU (3.0 eq) in anhydrous toluene (0.02 M) at rt and heated at 90 °C for 1–6 h under an argon atmosphere. The reaction mixture was quenched with water (5.0 mL) and the product was extracted with CH2Cl2 (2x 50 mL). The combined organic layers were washed with water (3x 50 mL), dried over anhydrous Na2SO4 and evaporated under reduced pressure. The solid residue was purified by silica gel column chromatography using CH3OH/CH2Cl2 (0–1%) as the eluent.
General Procedure B for Methoxide Substitution
The fluorinated HPY dye (1.0 eq) was dissolved in methanol (2.0 M) and treated with K2CO3 (2.2 eq). The mixture was stirred for 2 h and diluted with CH2Cl2 (100 mL). The organic layer was washed with water (3× 50 mL) and dried over anhydrous Na2SO4. Volatiles were removed under reduced pressure and dried in vacuo. The solid residue was purified by reverse phase C18 column chromatography using CH3CN/H2O (20–50%) as the eluent.
3,3-Difluoro-2-(4-(thiophen-2-yl)benzylidene)-2,3-dihydro-[1,2,4,3]triazaborolo[4,5-a]pyridine-2-ium-3-uide (2)
The reaction was performed following the general procedure A for 5 h starting with 1 (0.056 g, 0.2 mmol). The product 2 (0.051 g, 78%) was obtained as a red solid. 1H NMR (400 MHz, DMSO-d6): δ8.67 (s, 1H), 8.05 (d, J = 8.48 Hz, 2H), 7.86 (d, J = 8.48 Hz, 2H), 7.74 (dd, J = 3.32, 1.06 Hz, 1H), 7.69 (dd, J = 5.02, 1.06 Hz, 2H), 7.52 (ddd, J = 8.25, 7.67, 1.68 Hz, 1H), 7.20 (dd, J = 5.02, 3.66 Hz, 1H), 6.70 (d, J = 8.88 Hz, 1H), 6.40 (dt, J = 6.49, 6.24, 0.86 Hz, 1H). 13C NMR (75 MHz, CDCl3):δ141.2, 138.9, 135.7, 133.2, 128.4, 127.2, 126.6, 126.0, 125.8, 125.3, 124.7, 123.5, 113.2, 110.2. 19F NMR (CDCl3): δ −150.33 (q, J = 28 Hz, 2F). FT-IR (KBr): 1641 (s), 1609 (w), 1491 (s), 1105 (m), 1023 (w) cm−1. HPLC/ESI-MS (m/z): calcd for C16H13BF2N3S [M+H]+ 328.09, found 328.19.
6-Chloro-3,3-difluoro-2-(4-(thiophen-2-yl)benzylidene)-2,3-dihydro-1,2,4,3]triazaborolo[4,5-a]pyridin-2-ium-3-uide (4)
The reaction was performed following the general procedure A for 3 h starting with 3 (0.063 g, 0.2 mmol). The product 4 (0.057 g, 78%) was obtained as a red solid. 1H NMR (300 MHz, CDCl3):δ8.33 (s, 1H), 7.98-7.94 (m, 2H), 7.75-7.71 (m, 2H), 7.46 (dd, J = 3.74, 1.14 Hz, 1H), 7.45 (s, 1H), 7.39 (dd, J = 5.06, 1.02 Hz, 1H), 7.29 (d, J = 2.31 Hz, 1H), 7.13 (dd, J = 5.10, 4.23 Hz, 1H), 6.60 (d, J = 9.64 Hz, 1H). 13C NMR (100 MHz, DMSO-d6): δ154.5, 144.2, 142.8, 140.6, 138.8, 134.2, 133.9, 128.6, 127.1, 126.1, 125.6, 124.2, 120.4, 108.6. 19F NMR (CDCl3): δ −150.4 (q, J = 28 Hz, 2F). FT-IR (KBr): 1645 (w), 1597 (w), 1491 (s), 1133 (m), 1059 (m) cm−1. HPLC/ESI-MS (m/z): calcd for C16H12BClF2N3S [M+H]+ 362.05, found 362.32.
6-Bromo-3,3-difluoro-2-(4-(thiophen-2-yl)benzylidene)-2,3-dihydro-[1,2,4,3]triazaborolo[4,5-a]pyridin-2-ium-3-uide (6)
The reaction was performed following the general procedure A for 3 h starting with 5 (0.085 g, 0.24 mmol). The product 6 (0.090 g, 90%) was obtained as a red solid. 1H NMR (300 MHz, CDCl3): δ8.42-8.39 (m, 2H), 7.78-7.75 (m, 2H), 7.67 (s, 1H), 7.50-7.45 (m, 3H), 7.40 (d, J = 5.12 Hz, 1H), 7.14 (dd, J = 4.90, 3.81 Hz, 1H), 6.77 (d, J = 9.51 Hz, 1H). 13C NMR (75 MHz, CDCl3): δ143.8, 143.0, 140.6, 138.1, 136.1, 133.5, 128.8, 128.5, 126.8, 126.0, 125.8, 124.9, 114.6, 102.7. 19F NMR (CDCl3):δ −149.4 (q, J = 28 Hz, 2F). FT-IR (KBr): 1641 (m), 1605 (w), 1526 (m), 1498 (s), 1048 (m) cm−1. HPLC/ESI-MS (m/z): calcd for C16H12BBrF2N3S [M+H]+ 406.00, found 406.04.
6-Bromo-3,3-difluoro-2-(4-(thiophen-2-yl)benzylidene)-2,3-dihydro-[1,2,4,3]triazaborolo[4,5-a]pyridin-2-ium-3-uide (8)
The reaction was performed following the general procedure A for 3 h starting with 7 (0.061 g, 0.15 mmol). The product 8 (0.054 g, 80%) was obtained as a red solid. 1H NMR (300 MHz, CDCl3): δ8.42 (m, 1H), 8.39 (m, 1H), 7.77 (m, 2H), 7.74 (m, 1H), 7.56 (ddd, J = 9.12, 1.77, 1.66 Hz, 1H), 7.50-7.47 (m, 2H), 7.39 (d, J = 4.98 Hz, 1H), 7.14 (ddd, J = 6.77, 3.74, 1.17 Hz, 1H), 6.68 (d, J = 9.39 Hz, 1H). 13C NMR (75 MHz, CDCl3): δ148.2, 143.0, 141.1, 140.5, 138.1, 133.6, 133.5, 128.8, 128.5, 126.8, 126.0, 125.8, 124.9, 115.0. 19F NMR (CDCl3):δ −149.4 (q, J = 28 Hz, 2F). FT-IR (KBr): 1634 (s), 1606 (m), 1493 (s), 1403 (m), 1147 (m) cm−1. HPLC/ESI-MS (m/z): calcd for C16H12BF2IN3S [M+H]+ 453.99, found 454.03.
2-Benzylidene-6-bromo-3,3-difluoro-2,3-dihydro-[1,2,4,3]triazaborolo[4,5-a]pyridin-2-ium-3-uide (10)
The reaction was performed following the general procedure A for 4 h starting with 9 (0.138 g, 0.5 mmol). The product 10 (0.133 g, 82%) was obtained as an orange solid. 1H NMR (400 MHz, DMSO-d6):δ8.57 (d, J = 7.28 Hz, 2H), 8.22 (s, 1H), 8.05 (s, 1H), 7.77 (dd, J = 9.14, 1.78 Hz, 1H), 7.62-7.57 (m, 3H), 6.94 (d, J = 9.48 Hz, 1H). 13C NMR (100 MHz, DMSO-d6): δ160.1,144.6, 141.9, 136.5, 133.0, 132.8, 130.0, 128.9, 114.4, 102.8. 19F NMR (DMSO-d6): δ −145.62 (q, J = 28 Hz, 2F). FT-IR (KBr): 1643 (m), 1608 (w), 1489 (s), 1405 (m), 1143 (m) cm−1. HPLC/ESI-MS (m/z): calcd for C12H10BBrF2N3 [M+H]+ 324.01, found 324.07.
3,3-Difluoro-6-(4-phenyl-1H-1,2,3-triazol-1-yl)-2-(4-(thiophen-2-yl)benzylidene)-2,3-dihydro-[1,2,4,3]triazaborolo[4,5-a]pyridin-2-ium-3-uide (12)
The reaction was performed following the general procedure A for 3 h starting with 11 (0.018 g, 0.043 mmol). The product 12 (0.018 g, 87%) was obtained as a red solid. 1H NMR (300 MHz, DMSO-d6):δ9.20 (s, 1H), 8.63 (s, 1H), 8.60 (s, 1H), 8.58 (d, J = 1.89 Hz, 1H), 8.21 (dd, J = 9.69, 2.34 Hz, 1H), 8.14 (s, 1H), 7.93-7.88 (m, 4H), 7.77 (dd, J = 3.66, 1.02 Hz, 1H), 7.71 (dd, J = 5.06, 1.17 Hz, 1H), 7.53-7.48 (m, 2H), 7.41-7.35 (m, 1H), 7.23-7.18 (m, 2H). 13C NMR (75 MHz, DMSO-d6): δ147.2, 142.3, 142.1, 137.8, 136.0, 134.1, 130.2, 129.2, 129.1, 128.9, 128.7, 128.5, 128.4, 128.2, 126.2, 125.5, 125.4, 123.5, 120.1, 114.1. 19F NMR (CDCl3):δ −148.4 (q, J = 28 Hz, 2F). FT-IR (KBr): 1655 (w), 1614 (m), 1519 (m), 1137 (w), 1025 (m) cm−1. HPLC/ESI-MS (m/z): calcd for C24H18BF2N6S [M+H]+ 471.14, found 471.41.
6-Ethynyl-3,3-difluoro-2-(4-(thiophen-2-yl)benzylidene)-2,3-dihydro-[1,2,4,3]triazaborolo[4,5-a]pyridin-2-ium-3-uide (14)
The reaction was performed following the general procedure A for 1 h starting with 9 (0.112 g, 0.37 mmol). The product 14 (0.107 g, 83%) was obtained as a red solid. 1H NMR (400 MHz, CDCl3):δ8.35 (s. 1H), 7.98-7.96 (m, 2H), 7.75-7.73 (m, 2H), 7.64 (m, 1H), 7.47 (dd, J = 3.72, 1.08 Hz, 1H), 7.40 (dd, J = 5.02, 1.07 Hz, 1H), 7.34 (dd, J = 9.38, 1.85 Hz, 1H), 7.14 (dd, J = 5.07, 3.62 Hz, 1H), 6.58 (d, J = 9.37 Hz, 1H), 3.05 (s, 1H). 13C NMR (75 MHz, CDCl3): δ153.4, 143.2, 142.9, 140.0, 138.2, 137.0, 133.6, 128.5, 126.9, 125,8, 125.0, 117.2, 117.2, 113.1, 79.3, 78.5. 19F NMR (CDCl3):δ −150.01 (q, J = 28 Hz, 2F). FT-IR (KBr): 2112 (m), 1644 (s), 1609 (w), 1509 (s), 1044 (m) cm−1. HPLC/ESI-MS (m/z): calcd for C18H13BF2N3S [M+H]+ 352.09, found 352.12.
6-Bromo-3,3-difluoro-2-(propan-2-ylidene)-2,3-dihydro-[1,2,4,3]triazaborolo[4,5-a]pyridin-2-ium-3-uide (16)
The reaction was performed following the general procedure A for 6 h using 15 (0.146 g, 0.64 mmol). The product 16 (0.107 g, 60%) was obtained as a greenish yellow solid. 1H NMR (300 MHz, DMSO-d6): δ7.82 (d, J = 1.32 Hz, 1H), 7.46 (dd, J = 9.69, 2.20 Hz, 1H), 6.69 (dd, J = 9.53 Hz, 1H), 2.36 (s, 3H), 2.32 (s, 3H). 13C NMR (75 MHz, DMSO-d6): δ166.3, 157.8, 142.8, 135.3, 113.6, 99.7, 21.2, 21.0. 19F NMR (DMSO-d6): δ −145.81 (q, J = 28 Hz, 2F). FT-IR (KBr): 1658 (w), 1625 (m), 1498 (s), 1154 (s), 1018 (m) cm−1. HRMS/ESI-TOF (m/z): calcd for C8H10BBrF2N3 [M+H]+ 276.0119, found 276.0114.
3,3-Difluoro-6-iodo-2-(propan-2-ylidene)-2,3-dihydro-[1,2,4,3]triazaborolo[4,5-a]pyridin-2-ium-3-uide (18)
The reaction was performed following the general procedure A for 4 h starting with 17 (0.235 g, 0.85 mmol). The product 18 (0.212 g, 77%) was obtained as a greenish yellow solid. 1H NMR (300 MHz, CDCl3): δ7.54(b s, 1H), 7.35 (dd, J = 9.40, 2.01, 1H), 6.41 (d, J = 9.54, 1H), 2.41 (s, 3H), 2.37 (s, 3H). 13C NMR (75 MHz, CDCl3): δ163.4, 158.6, 147.0, 140.3, 114.0, 66.6, 21.6, 21.5. 19F NMR (CDCl3): δ −149.38 (q, J = 28 Hz, 2F). FT-IR (KBr): 1658 (w), 1619 (m), 1501 (s), 1152 (s), 1014 (m) cm−1. HRMS/ESI-TOF (m/z): calcd for C8H10BF2IN3 [M+H]+ 323.9975, found 323.9979.
4-(((4-Methoxyphenyl)diphenylmethylthio)methyl)oxazolidine-2,5-dione (20)
Triphosgene (0.932 g, 3.15 mmol) was added to a mixture of 19 (0.589 g, 1.5 mmol) and cyclohexene (1.23 g, 15 mmol) in ethyl acetate (10 mL) at 0 °C. The reaction was heated at 85 °C for 3 h. The volatiles were removed in vacuo. The residue was dissolved in CH2Cl2 (20 mL) and washed with water (35 mL). The organic layer was dried over anhydrous Na2SO4 and concentrated under reduced pressure to isolate the product 20 (0.525 g, 84%) as a colorless solid. This compound was used directly in subsequent reactions, or could be stored at 5 °C under anhydrous conditions for up to 2 days. 1H NMR (300 MHz, CDCl3)δ7.53-7.08 (m, 12H), 6.93-6.73 (m, 2H), 5.15 (s, 1H), 3.80 (s, 3H), 3.58-3.51 (dd, J = 8.51, 3.96 Hz, 1H), 2.87-2.68 (m, 2H); 13C NMR (75 MHz, CDCl3)δ167.85, 158.43, 151.17, 144.00, 135.65, 130.58, 129.22, 128.24, 127.15, 113.50, 67.28, 56.53, 55.26, 33.27. FT-IR (KBr, cm−1): 3399 (s), 1860 (s), 1785 (m), 1601 (w), 1508 (w).
6-Bromo-3,3-dimethoxy-2-(propan-2-ylidene)-2,3-dihydro-[1,2,4,3]triazaborolo[4,5-a]pyridin-2-ium-3-uide (21)
The reaction was performed following the general procedure B starting with 16 (0.028 g, 0.1 mmol) and a reaction time of 3.5 h. The solid residue was purified by the reverse phase column chromatography using with CH3CN/H2O (50:50) to isolate the product 21 (0.023 g, 77%) as a greenish yellow solid. 1H NMR (300 MHz, CDCl3): δ7.37 (dd, J = 2.06, 0.74 Hz, 1H), 7.25 (dd, J = 9.53, 1.76 Hz, 1H), 6.48 (dd, J = 9.53, 0.73 Hz, 1H), 3.07 (s, 6H), 2.38 (s, 3H), 2.36 (s, 3H). 13C NMR (75 MHz, CDCl3): δ160.4, 159.0, 142.0, 135.9, 113.2, 100.1, 49.9, 21.3, 20.5. FT-IR (KBr): 1650 (m), 1619 (m), 1528 (m), 1500 (s), 1222 (m) cm−1. HRMS/ESI-TOF (m/z): calcd for C10H16BBrN3 [M+H]+ 300.0519, found 300.0513.
6-Iodo-3,3-dimethoxy-2-(propan-2-ylidene)-2,3-dihydro[1,2,4,3]triazaborolo[4,5-a]pyridin-2-ium-3-uide (22)
The reaction was performed following the general procedure B starting with 18 (0.097 g, 0.3 mmol) and a reaction time of 3.5 h. The solid residue was purified by reverse phase flash chromatography eluting with CH3CN/H2O (20:80) to isolate the product 22 (0.096 g, 90%) as a greenish yellow solid. 1H NMR (300 MHz, CDCl3): δ7.47 (dd, J = 2.00, 0.80 Hz, 1H), 7.35 (dd, J = 9.39, 2.06 Hz, 1H), 6.41 (dd, J = 9.39, 0.88 Hz, 1H), 3.07 (s, 6H), 2.38 (s, 3H), 2.36 (s, 3H). 13C NMR (75 MHz, CDCl3): δ160.3, 158.8, 146.3, 141.0, 113.7, 66.4, 50.0, 21.3, 20.5. FT-IR (KBr): 1651 (m), 1615 (m), 1523 (m), 1498 (s), 1219 (s) cm−1. HRMS/ESI-TOF (m/z): calcd for C10H16BBrN3O2 [M+H]+ 348.0375, found 348.0372.
6-(4-(tert-Butoxycarbonylamino)but-1-ynyl)-3,3-difluoro-2-(propan-2-ylidene)-2,3-dihydro-[1,2,4,3]triazaborolo[4,5-a]pyridin-2-ium-3-uide (23)
A mixture of 18 (0.134 g, 0.42 mmol), Boc-but-3-yn-1-amine (0.141 g, 0.83 mmol), PdCl2(PPh3)2 (14.6 mg, 2.07 ×10−2 mmol) and CuI (4.0 mg, 2.07×10−2 mmol) in Et3N (4 mL) was stirred for 2 h at ambient temperature under an argon atmosphere. Volatiles were removed in vacuo. The solid residue was dissolved in CH2Cl2 (10 mL) and filtered through a plug of celite. The filtrate was concentrated and the residue was purified by silica gel column chromatography using CH3OH/CH2Cl2 (0–0.5%) to isolate the product 23 (0.147 g, 96%) as a greenish yellow solid. 1H NMR (300 MHz, CDCl3): δ7.47 (s, 1H), 7.21 (dd, J = 9.30, 1.80 Hz, 1H), 6.51 (d, J = 9.39 Hz, 1H), 4.83 (bs, 1H), 3.32 (dt, J = 6.44, 6.31 Hz, 2H), 2.55 (t, J = 6.46, 2H), 2.42 (s, 3H), 2.37 (s, 3H), 1.46 (s, 9H). 13C NMR (75 MHz, CDCl3): δ163.1, 158.7, 155.7, 142.3, 138.2, 111.8, 104.3, 87.3, 79.5, 77.8, 39.5, 28.4, 21.5, 21.4, 21.0. 19F NMR (CDCl3): δ −149.94 (q, J = 28 Hz, 2F). FT-IR (Neat): 3300 (b), 2171 (w), 1650 (m), 1510 (s), 1110 (m) cm−1. HPLC/ESI-MS (m/z): calcd for C17H24BF2N4O2 [M+H]+ 365.20, found 365.30.
6-(4-(tert-Butoxycarbonylamino)but-1-ynyl)-3,3-dimethoxy-2-(propan-2-ylidene)-2,3-dihydro-[1,2,4,3]triazaborolo[4,5-a]pyridin-2-ium-3-uide (24)
The mixture of 22 (0.069 g, 0.2 mmol), Boc-but-3-yn-1-amine (0.068 g, 0.4 mmol), PdCl2(PPh3)2 (7.0 mg, 1.0×10−2 mmol) and CuI (1.9 mg, 1.0×10−2 mmol) in Et3N (4 mL) was stirred for 2 h at ambient temperature under argon atmosphere. Volatiles were evaporated under reduced pressure. The solid residue was dissolved in CH2Cl2 (10 mL) and filtered through a plug of celite. The filtrate was concentrated and the residue was purified by silica gel column chromatography using CH3OH/CH2Cl2 (0–1.0%) to isolate the product 24 (0.074 g, 95%) as a greenish yellow solid. 1H NMR (300 MHz, CDCl3): δ7.47 (d, J = 1.00 Hz, 1H), 7.21 (dd, J = 9.39, 1.91 Hz, 1H), 6.51 (dd, J = 9.39, 0.90 Hz, 1H), 4.85 (bs, 1H), 3.33 (dt, J = 6.30, 6.17 Hz, 2H), 3.06, (s, 6H), 2.57 (t, J = 6.60 Hz, 2H), 2.39 (s, 3H), 2.36 (s, 3H), 1.46 (s, 9H). 13C NMR (75 MHz, CDCl3): δ160.3, 158.9, 155.7, 141.6, 139.0, 111.5, 103.8, 87.2, 79.5, 78.2, 49.9, 39.5, 28.4, 21.3, 21.0, 20.5. FT-IR (Neat): 3259 (b), 2167 (w), 1630 (m), 1510 (s), 1141 (m) cm−1. HPLC/ESI-MS (m/z): calcd for C19H30BN4O2 [M+H]+ 389.24, found 389.16.
6-(4-(2-Amino-3-((4-methoxyphenyl)diphenylmethylthio)propanamido)but-1-ynyl)-3,3-difluoro-2-(propan-2-ylidene)-2,3-dihydro-[1,2,4,3]triazaborolo[4,5-a]pyridin-2-ium-3-uide (25)
Trifluoroacetic acid (TFA, 50 μL) was added drop-wise to compound 23 (0.026 g, 0.07 mmol) in methylene chloride (1.0 mL) and stirred for 3 h at ambient temperature under argon atmosphere. The mixture was diluted with CH2Cl2 (2 mL), cooled in an ice bath and neutralized by drop-wise addition of Et3N (90 μL). The cyclic anhydride oxazolidine-2,5-dione 20 (0.036 g, 0.089 mmol) in CH2Cl2 (2 mL) was added to the neutralized reaction mixture drop-wise over 15 min, and stirred for 3 h at 0 °C to rt under argon atmosphere. The reaction mixture was diluted with CH2Cl2 (100 mL). The organic layer was washed with saturated NH4Cl (2x 50 mL), sat. NaHCO3 (2x 50 mL), H2O (3x 50 mL) and dried over anhydrous Na2SO4. Volatiles were evaporated under reduced pressure and the solid residue was purified by silica gel column chromatography using CH3OH/CH2Cl2 (0–1%) to isolate the product 25 (0.039 g, 86%) as a greenish yellow solid. 1H NMR (300 MHz, CDCl3): δ7.45-7.28 (m, 10H), 7.23-7.14 (m, 4H), 6.81 (d, J = 9.10 Hz, 2H), 6.48 (d, J = 9.39 Hz, 1H), 3.78 (s, 3H), 3.76 (m, 1H), 3.43-3.28 (m, 2H), 3.06 (dd, J = 8.37, 3.96 Hz, 1H), 2.74 (dd, J = 12.60, 4.02 Hz, 1H), 2.62-2.50 (m, 3H), 2.42 (s, 3H), 2.37 (s, 3H), 1.60 (b s, 2H). 13C NMR (75 MHz, CDCl3): δ172.9, 163.1, 158.7, 158.2, 144.9, 144.7, 142.2, 138.2, 136.6, 130.7, 129.5, 127.9, 126.7, 113.2, 111.8, 104.3, 87.2, 77.9, 66.5, 55.2, 54.0, 37.9, 37.3, 21.5, 20.4. 19F NMR (CDCl3): −149.88 (q, J = 28 Hz, 2F). FT-IR (Neat): 3360 (b), 2120 (w), 1680 (m), 1510 (m), 1110 (m) cm−1. HRMS/ESI-TOF (m/z): calcd for C35H36BF2N5NaO2S [M+Na]+ 662.2543, found 662.2537.
6-(4-(2-Amino-3-((4-methoxyphenyl)diphenylmethylthio)propanamido)-but-1-ynyl)-3,3-dimethoxy-2-(propan-2-ylidene)-2,3-dihydro-[1,2,4,3]triazaborolo[4,5-a]pyridin-2-ium-3-uide (26)
Trifluoroacetic acid (TFA, 50 μL) was added drop-wise to compound 24 (0.027 g, 0.07 mmol) in CH2Cl2 (1.0 mL) and stirred for 3 h at rt under an argon atmosphere. The mixture was diluted with CH2Cl2 (2 mL), cooled in an ice bath and neutralized by drop-wise addition of Et3N (90 μL). The oxazolidine-2,5-dione 20 (0.038 g, 0.09 mmol) in CH2Cl2 (2 mL) was added to the neutralized reaction mixture drop-wise over 15 min and stirred for 3 h at 0 °C to rt under an argon atmosphere. The reaction mixture was diluted with CH2Cl2 (100 mL). The organic layer was washed with saturated NH4Cl (2x 50 mL), saturated NaHCO3 (2 × 50 mL), H2O (3 × 50 mL) and dried over anhydrous Na2SO4. Volatiles were evaporated under reduced pressure and the solid residue was purified by silica gel column chromatography using CH3OH/CH2Cl2 (0–1%) to isolate the product 26 (0.035 g, 76%) as a greenish yellow solid. 1H NMR (300 MHz, CDCl3): δ7.45-7.28 (m, 10H), 7.25-7.15 (m, 4H), 6.81 (d, J = 9.09 Hz, 2H), 6.49 (dd, J = 9.39, 0.90 Hz, 1H), 3.79 (s, 3H), 3.77-3.25 (m, 1H), 3.42-3.29 (m, 2H), 3.08-3.02 (m, 7H), 2.73 (dd, J = 12.75, 3.96 Hz, 1H), 2.61-2.51 (m, 3H), 2.39 (s, 3H), 2.39 (s, 3H), 1.60 (b s, 2H). 13C NMR (75 MHz, CDCl3): δ173.0, 160.4, 158.9, 158.2, 144.9, 144.7, 141.6, 139.0, 136.5, 130.8, 129.5, 127.9, 126.7, 113.2, 111.5, 103.8, 86.9, 78.4, 66.5, 55.2, 54.0, 49.9, 37.9, 37.4, 21.3, 20.4. FT-IR (KBr): 3270 (b), 2110 (w), 1689 (m), 1510 (s), 1140 (m) cm−1. HRMS/ESI-TOF (m/z): calcd for C37H43BN5O4S [M+H]+ 664.3123, found 664.3121.
2-Amino-N-(2-(2-aminoethoxy)ethyl)-3-((4-methoxyphenyl)diphenylmethylthio)propanamide (27)
The oxazolidine-2,5-dione 20 (0.105 g, 0.25 mmol) in CH2Cl2 (3 mL) was added dropwise to 2,2′-oxy-bis-ethylamine in CH2Cl2 (3 mL) at 0 °C for 30 min and the reaction mixture was allowed to stir for 12 h at rt. The reaction mixture was diluted with CH2Cl2 (25 mL) and washed with H2O. The organic layer was dried over anhydrous Na2SO4 and concentrated under reduced pressure. The crude residue was purified by silica gel column chromatography using CH3OH/CH2Cl2 (20:80) to provide the product 27 (0.067 g, 56%) as a colorless solid. 1H NMR (300 MHz, CDCl3)δ7.45-7.17 (m, 12H), 6.85-6.75 (m, 2H), 3.78 (s, 3H), 3.55-3.30 (m, 6H), 3.10-3.00 (dd, J = 8.37, 3.97 Hz, 1H), 2.85-2.78 (t, J = 5.06 Hz, 2H), 2.76-2.67 (dd, J = 12.62, 4.11 Hz, 1H), 2.60-2.50 (dd, J = 12.62, 8.36 Hz, 1H), 1.76 (s, 2H); 13 C NMR (75 MHz, CDCl3)δ173.0, 158.1, 144.9, 136.6, 130.7, 129.5, 127.9, 126.7, 113.2, 72.7, 69.5, 66.4, 55.2, 54.0, 41.5, 38.9, 37.5. FTIR (KBr, cm−1): 3391(s), 2954(m), 1658(s), 1384(m), 1250(m). HPLC-MS: Elution with CH3CN/H2O (20:80), exhibited a single peak at 4.43 min. HPLC/ESI-MS (m/z): calcd for C27H34N3O3S [M+H]+ 480.17; found 480.22.
Synthesis of AlexaFluor488 Conjugate (28)
To a mixture of 27 (1.0 mg, 0.002 mmol) and Et3N (5 μL) in dry DMF (0.25 mL) was added AlexaFluor®488-NHS (1.0 mg, 0.0016 mmol) in dry DMF (0.20 mL) at 0 °C over 15 min and stirred at rt for 12 h. Volatiles were removed in vacuo and the crude residue was purified by reverse phase C18 column using CH3CN/H2O (50:50). The fractions containing product were combined and concentrated in vacuo. A pipette column containing Dowex 50WX2-400 ion-exchange resin (1.0 g) was prepared by washing with sequential potions of H2O (2 mL), CH3OH (2 mL), H2O (2 mL), 2N NaOH (1 mL), and H2O (2 mL) respectively. The concentrate was dissolved in water (0.5 mL) and eluted through the column, fractions containing the Na+ salt of the product were combined and concentrated in vacuo to provide the product 28 (1.5 mg, 95%). HPLC/ESI-MS (m/z): calcd for C48H46N5O13S3 [M+H]+ 996.22, found 996.13.
Supplementary Material
Acknowledgments
Authors would like to thank Dr. Lori Wilmeth for her assistance in the cell culture experiments, and acknowledge funding from NM-INBRE P20 RR16480, R25 GM61222 and the Cowboys for Cancer Research Foundation.
Footnotes
Supporting Information Available: Experimental for synthetic and biological studies, compound identity and purifity characterization spectra, and complete reference 4. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Zhang J, Campbell RE, Ting AY, Tsien RY. Nat Rev Mol Cell Bio. 2002;3:906–918. doi: 10.1038/nrm976. [DOI] [PubMed] [Google Scholar]; (b) Lavis LD, Raines RT. ACS Chem Biol. 2008;3(3):142–155. doi: 10.1021/cb700248m. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Walter NG, Huang CY, Manzo AJ, Sobhy MA. Nature Methods. 2008;5(6):475–489. doi: 10.1038/nmeth.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Tinnefeld P, Sauer M. Angew Chem Int Ed. 2005;44:2642–2671. doi: 10.1002/anie.200300647. [DOI] [PubMed] [Google Scholar]; (e) Goncalves MST. Chem Rev. 2009;109:190–212. doi: 10.1021/cr0783840. [DOI] [PubMed] [Google Scholar]; (f) Kobayashi H, Ogawa M, Alford R, Choyke PL, Urano Y. Chem Rev. 2010;110:2620–2640. doi: 10.1021/cr900263j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loudet A, Burgess K. Chem Rev. 2007;107(11):4891–4932. doi: 10.1021/cr078381n. [DOI] [PubMed] [Google Scholar]
- 3.(a) Kim E, Koh M, Ryu J, Park SB. J Am Chem Soc. 2008;130:12206–12207. doi: 10.1021/ja8020268. [DOI] [PubMed] [Google Scholar]; (b) Abet V, Nuñez A, Mendicuti F, Burgos C, Alvarez-Builla J. J Org Chem. 2008;73:8800–8807. doi: 10.1021/jo801549u. [DOI] [PubMed] [Google Scholar]; (c) Ozhalici-Unal H, Pow CL, Marks SA, Jesper LD, Silva GL, Shank NI, Jones EW, Burnette JM, Berget PB, Armitage BA. J Am Chem Soc. 2008;130(38):12620–12621. doi: 10.1021/ja805042p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexander MD, et al. ChemBioChem. 2006;7:409–416. doi: 10.1002/cbic.200500466. [DOI] [PubMed] [Google Scholar]
- 5.(a) Ramesh C, Bryant B, Nayak T, Revankar CM, Anderson T, Carlson KE, Katzenellenbogen JA, Sklar LA, Norenberg JP, Prossnitz ER, Arterburn JB. J Am Chem Soc. 2006;128:14476–14477. doi: 10.1021/ja066360p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Nayak TK, Hathaway HJ, Ramesh C, Arterburn JB, Dai D, Sklar LA, Norenberg JP, Prossnitz ER. J Nuclear Med. 2008;49(6):978–986. doi: 10.2967/jnumed.107.048546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Dubs P, Bourel-Bonnet L, Subra G, Blanpain A, Melnyk O, Pinel AM, Gras-Masse H, Martinez J. J Comb Chem. 2007;9:973–981. doi: 10.1021/cc070033b. [DOI] [PubMed] [Google Scholar]; (b) Kurpiers T, Mootz HD. Angew Chem Int Ed. 2009;48:1729–1731. doi: 10.1002/anie.200805454. [DOI] [PubMed] [Google Scholar]; (c) Dilek O, Bane SL. Tetrahedron Lett. 2008;49:1413–1416. doi: 10.1016/j.tetlet.2007.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Tang W, Xiang Y, Tong A. J Org Chem. 2009;74:2163–2166. doi: 10.1021/jo802631m. [DOI] [PubMed] [Google Scholar]; (e) Dirksen A, Yegneswaran S, Dawson PE. Angew Chem Int Ed. 2010;49:2023–2027. doi: 10.1002/anie.200906756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.SoluLink®. http://www.solulink.com/productindex.php.
- 8.(a) Umezawa K, Nakamura Y, Makino H, Citterio D, Suzuki K. J Am Chem Soc. 2008;130:1550–1551. doi: 10.1021/ja077756j. [DOI] [PubMed] [Google Scholar]; (b) Zhang G, Chen J, Payne S, Kooi SE, Demas JN, Fraser CL. J Am Chem Soc. 2007;129:8942–8943. doi: 10.1021/ja0720255. [DOI] [PubMed] [Google Scholar]; (c) Gorman A, Killoran J, O’Shea C, Kenna T, Gallagher WM, O’Shea DF. J Am Chem Soc. 2004;126:10619–10631. doi: 10.1021/ja047649e. [DOI] [PubMed] [Google Scholar]; (d) Zhou Y, Xiao Y, Chi S, Qian X. Org Lett. 2008;10:633–636. doi: 10.1021/ol702963w. [DOI] [PubMed] [Google Scholar]; (e) Han J, Loudet A, Barhoumi R, Burghardt RC, Burgess K. J Am Chem Soc. 2009;131:1642–1643. doi: 10.1021/ja8073374. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Tasior M, O’Shea DF. Bioconjugate Chem. 2010;21:1130–1133. doi: 10.1021/bc100051p. [DOI] [PubMed] [Google Scholar]
- 9.(a) Chang PV, Prescher JA, Hangauer MJ, Bertozzi CR. J Am Chem Soc. 2007;129:8400–8401. doi: 10.1021/ja070238o. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Shao F, Weissleder R, Hilderbrand SA. Bioconjugate Chem. 2008;19:2487–2491. doi: 10.1021/bc800417b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Tsou LK, Zhang MM, Hang HC. Org Biomol Chem. 2009;7:5055–5058. doi: 10.1039/b917119n. [DOI] [PubMed] [Google Scholar]; (d) Jewett JC, Sletten EM, Bertozzi CR. J Am Chem Soc. 2010;132:3688–3690. doi: 10.1021/ja100014q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tahtaoui C, Thomas C, Rohmer F, Klotz P, Duportail G, Mély Y, Bonnet D, Hibert M. J Org Chem. 2007;72:269–272. doi: 10.1021/jo061567m. [DOI] [PubMed] [Google Scholar]
- 11.a) Debonis S, Skoufias DA, Lebeau L, Lopez R, Robin G, Margolis RL, Wade RH, Kozielski F. Mol Cancer Ther. 2004;3:1079–1090. [PubMed] [Google Scholar]; b) Debonis S, Skoufias DA, Indorato RL, Liger F, Marquet B, Laggner C, Joesph B, Kozielski F. J Med Chem. 2008;51:1115–1125. doi: 10.1021/jm070606z. [DOI] [PubMed] [Google Scholar]; (c) Ogo N, Oishi S, Matsuno K, Sawada J, Fujii N, Asai A. Bioorg Med Chem Lett. 2007;17:3921–3924. doi: 10.1016/j.bmcl.2007.04.101. [DOI] [PubMed] [Google Scholar]
- 12.(a) Brier S, Lemaire D, DeBonis S, Forest E, Kozielski F. Biochemistry. 2004;43:13072–13082. doi: 10.1021/bi049264e. [DOI] [PubMed] [Google Scholar]; (b) Skoufias DA, Debonis S, Saoudi Y, Lebeau L, Crevel I, Cross R, Wade RH, Hackney D, Kozielski F. J Biol Chem. 2006;281:17559–17569. doi: 10.1074/jbc.M511735200. [DOI] [PubMed] [Google Scholar]
- 13.Peterson JR, Mitchison TJ. Chem Biol. 2002;9:1275–1295. doi: 10.1016/s1074-5521(02)00284-3. [DOI] [PubMed] [Google Scholar]
- 14.Verbrugge S, Lechner B, Woehlke G, Peterman EJG. Biophysical Journal. 2009;97:173–182. doi: 10.1016/j.bpj.2009.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson I. Histochem J. 1998;30:123–140. doi: 10.1023/a:1003287101868. [DOI] [PubMed] [Google Scholar]
- 16.Puckett CA, Barton JK. J Am Chem Soc. 2009;131:8738–8739. doi: 10.1021/ja9025165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lemcke S, Hönnscheidt C, Waschatko G, Bopp A, Lütjohann D, Bertram N, Gehrig-Burger K. Mol Cell Endocrinol. 2010;314:31–40. doi: 10.1016/j.mce.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Rickert EL, Oriana S, Hartman-Frey C, Long X, Webb TT, Nephew KP, Weatherman RV. Bioconjugate Chem. 2010;21:903–910. doi: 10.1021/bc900461h. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.