Abstract
Background. The biggest challenge in human immunodeficiency virus type 1 (HIV-1) prevention in Africa is the high HIV-1 burden in young women. In macaques, proinflammatory cytokine production in the genital tract is necessary for target cell recruitment and establishment of simian immunodeficiency virus (SIV) infection following vaginal inoculation. The purpose of this study was to assess if genital inflammation during early HIV-1 infection predisposes women to rapid disease progression.
Methods. Inflammatory cytokine concentrations were measured in cervicovaginal lavage (CVL) from 49 women 6, 17, 30, and 55 weeks after HIV-1 infection and from 22 of these women before infection. Associations between genital inflammation and viral load set point and blood CD4 cell counts 12 months after infection were investigated.
Results. Elevated genital cytokine concentrations 6 and 17 weeks after HIV-1 infection were associated with higher viral load set points and, to a lesser extent, with CD4 depletion. CVL cytokine concentrations during early infection did not differ relative to preinfection but were elevated in women who had vaginal discharge, detectable HIV-1 RNA in their genital tracts, and lower blood CD4 counts.
Conclusion. Genital inflammation during early HIV-1 infection was associated with higher viral load set point and CD4 depletion, which are markers of rapid disease progression. Strategies aimed at reducing genital inflammation during early HIV-1 infection may slow disease progression.
In sub-Saharan Africa, which has the highest prevalence of human immunodeficiency virus type 1 (HIV-1) worldwide, most new infections occur by sexual transmission to women [1]. The genital mucosa is the initial site of viral replication following vaginal transmission of HIV-1 in women and simian immunodeficiency virus (SIV) in rhesus macaques [2, 3]. In macaques, vaginal inoculation with SIV is followed by proinflammatory cytokine production and recruitment of CD4+ T cells that are necessary for local viral expansion and dissemination to the systemic compartment [4–6]. Proinflammatory cytokine expression in the genital mucosa correlates with viral replication and approaches baseline as peak SIV viremia declines [6]. HIV-1 infection may likewise be accompanied by an early inflammatory cascade in the genital tract that is associated with viral replication in this compartment. HIV-1 has been shown to directly induce inflammatory cytokine production via Toll-like receptor 7 and 8 activation [7]. Elevated concentrations of inflammatory cytokines in turn may upregulate HIV-1 replication by recruiting and activating target cells and through NF-κB activation [4, 8–11].
Several studies have shown that cervicovaginal proinflammatory cytokines are upregulated in women with early or chronic HIV-1 infection compared with HIV-uninfected women [10, 12–16]. However this upregulation may be attributed to the high frequency of sexually transmitted infections (STIs) or bacterial vaginosis (BV) in these individuals rather than HIV-1 itself [17]. For example, BV was associated with increased concentrations of proinflammatory interleukin (IL)–1β, whereas chronic HIV-1 infection was not [18]. HIV-1 shedding, which is associated with STIs [19], may induce further inflammatory cytokine production.
Plasma cytokine concentrations during early HIV-1 infection are predictive of plasma viral load set points and CD4 depletion [20], and treatment with cytokines such as IL-12p70 and IL-15 during acute SIV infection is associated with altered disease course in macaques [21–23]. Several studies have suggested that cytokine responses in the genital tract during the early stages of HIV-1 infection may likewise be associated with disease progression. In macaques, induction of inflammatory cytokines and immune cell influx into the genital tract prior to vaginal SIV inoculation was associated with increased plasma viral load set points [24]. This suggests that preexisting genital inflammation in humans may similarly influence HIV-1 disease progression. Zara et al [14] demonstrated that upregulation of IL-1β in the genital tracts of HIV-1–infected women was associated with increased plasma viral loads. Recently, we reported that elevated proinflammatory cytokines in cervicovaginal lavage (CVL) correlated with lower blood CD4+ T-cell counts during early HIV-1 infection [15].
In this study, the relationships between genital cytokine concentrations during early HIV-1 infection and plasma viral load set point and blood CD4+ T-cell counts 12 months postinfection were investigated.
MATERIALS AND METHODS
Study Participants
Forty-nine South African women recently infected with HIV-1 subtype C were recruited as part of the CAPRISA Acute Infection Study [25]. Each woman provided informed consent and then attended regular evaluations of HIV-1 disease status. Time of infection was defined as the midpoint between the last HIV-1 antibody negative test result and the first HIV-1 antibody positive test result, or as 14 days prior to a positive RNA polymerase chain reaction (PCR) assay on the same day as a negative HIV-1 enzyme immunoassay. This study was approved by the University of Kwazulu-Natal and University of Cape Town ethics committees.
Cytokine Measurements
CVL samples were collected, as described elsewhere [15], at 5 time points: 22 women were assessed preinfection (36 weeks preinfection, range, 2–92), 39 were assessed 6 weeks postinfection (range, 1–13), 32 were assessed at 17 weeks postinfection (range, 14–23), 39 were assessed at 30 weeks postinfection (range, 24–36), and 40 were assessed at 55 weeks postinfection (range, 50–62). Eighteen of the 22 women who were assessed preinfection had matching 6-week postinfection CVL samples available. CVLs were prefiltered using 0.2 μm Costar Spin-X cellulose acetate filters (Sigma) and the supernatant was stored at −80°C. Concentrations of IL-1α, IL-1β, IL-2, IL-6, IL-7, IL-8, IL-10, IL-12p40, IL-12p70, IL-15, interferon (IFN)–α, eotaxin, fractalkine, granulocyte colony-stimulating factor (G-CSF), granulocyte macrophage (GM)–CSF, monocyte chemotactic protein 1, macrophage inflammatory protein (MIP)–1α, MIP-1β, MIP-3α, RANTES, soluble CD40 ligand (sCD40L), and tumor necrosis factor (TNF)–α were measured using Human Cytokine LINCOplex premixed kits (LINCO Research), Milliplex kits (Millipore), and enzyme-linked immunosorbent assay (R&D Systems). The sensitivity of these kits ranged between 0.05 and 18.33 pg/mL. Multiplex data were collected using a Bio-Plex Suspension Array Reader (Bio-Rad Laboratories), and a 5 PL regression formula was used to calculate cytokine concentrations from the standard curves (Bio-Plex Manager software, version 4). Cytokine concentrations below the lower limit of detection were reported as the midpoint between the lowest concentration measured for each cytokine and zero.
Clinical Characteristics
Blood was collected by venipuncture into acetate citrate dextran vacutainer tubes. Absolute blood CD4+ T-cell counts were measured using a FACSCalibur flow cytometer. Plasma HIV-1 RNA concentrations were quantified using the COBAS AMPLICOR HIV-1 Monitor v1.5 or COBAS Ampliprep/COBAS TaqMan 48 Analyzer (Roche Diagnostics). CVL viral loads were determined using Nuclisens Easyq HIV-1 (version 1.2). As viral load set point after 3 months postinfection is predictive of time to AIDS [26], the average viral load measurement of 3 consecutive visits overlying 12 months postinfection (range, 37–69) was used to assess disease progression. Additionally, associations between cytokines and (1) average CD4+ T-cell measurements of the same 3 visits and (2) CD4+ T-cell loss between preinfection and 12 months postinfection were investigated.
Participants were screened for Chlamydia trachomatis, Neisseria gonorrhoeae, and herpes simplex virus type 2 (HSV-2) in cervical swab samples by PCR and Trichomonas vaginalis by Diamond’s culture and PCR. Agents of BV were assessed by Gram staining.
Statistical Analyses
Statistical analyses were performed using GraphPad Prism software, version 5 (GraphPad) and Stata, version 10 (StataCorp). Distribution of variables was assessed by Shapiro-Wilk and Shapiro-Francia tests. The χ2 test was used to compare proportions. Mann-Whitney U and Wilcoxon signed-rank tests were used for unmatched and matched comparisons, respectively. Spearman rank test was used for correlations. The relationships between cytokine concentrations and markers of disease progression were evaluated using multivariate linear regression. Variables were log-transformed, and cytokines were standardized to allow for direct comparison of β-coefficients. Mixed-effects logistic regression was used to assess the change in HIV-1 shedding over time. P values <.05 were considered significant. P values were adjusted using false discovery rate step-down procedure in order to reduce false-positive results when multiple comparisons were made [27].
RESULTS
Forty-nine women recently infected with HIV-1 were recruited and followed longitudinally. Their median age was 24 years (range, 18–59). Most women (98%) were unmarried, 20% had multiple partners, and 32% reported using injectable hormonal contraceptives. The median CD4+ T-cell count and viral load measurements at 6 weeks postinfection were 524 cells/μL and 56 500 copies/mL, respectively (Table 1). The median CD4+ T-cell loss during the first 12 months of HIV-1 infection was 486 cells/μL, while the median set-point viral load was 39 783 copies/mL. STIs and BV were prevalent in this cohort, both pre– and post HIV-1 infection.
Table 1.
Clinical Characteristics of Study Participants
| CD4+ T-Cell Counts (Cells/μL) | Cells/μL, Median (IQR) | No. |
| Preinfection CD4+ T-cell count | 975 (860–1149) | 22 |
| Six-week postinfection CD4+ T-cell count | 524 (379–685) | 48 |
| CD4+ T-cell count set point (average of 3 visits overlying 12 months postinfection) | 408 (339–551) | 46 |
| CD4+ T-cell loss (preinfection minus 12-month postinfection CD4+ T-cell count) | 486 (254–653) | 22 |
| Plasma viral loads (copies/mL) | Copies/mL, Median (IQR) | No. |
| Six-week postinfection plasma viral load | 56 500 (14 200–370 500) | 49 |
| Plasma viral load set point (average of 3 visits overlying 12-months postinfection) | 39 783 (7248–102 208) | 46 |
| Sexually transmitted infections | Preinfection, No./Total (%) | Early infection, No./Total (%) |
| Prevalence of active STIs (women with laboratory-diagnosed STIa) | 8/22 (36.3) | 17/44 (38.6) |
| Bacterial vaginosis (women with Gram stain positive for BV) | 15/22 (68.2) | 34/44 (77.4) |
| Vaginal discharge (women with visible discharge) | 3/22 (13.7) | 8/49 (16.3) |
| Genital ulcer (women with visible genital ulcer[s]) | 0/22 (0) | 6/49 (12.2) |
| Multiple HIV-1 variant transmission | Dual, No./Total (%) | Heterogenous, No./Total (%) |
| Participants with multiple transmitted variants | 4/45 (8.9) | 7/45 (15.6) |
Abbreviations: BV, bacterial vaginosis; HIV-1, human immunodeficiency virus type 1; IQR, interquartile range; STI, sexually transmitted infection.
Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis, herpes simplex virus type 2.
Cytokine Concentrations in the Genital Tract of Women Recently Infected With HIV-1 Were Not Significantly Elevated Compared With Preinfection
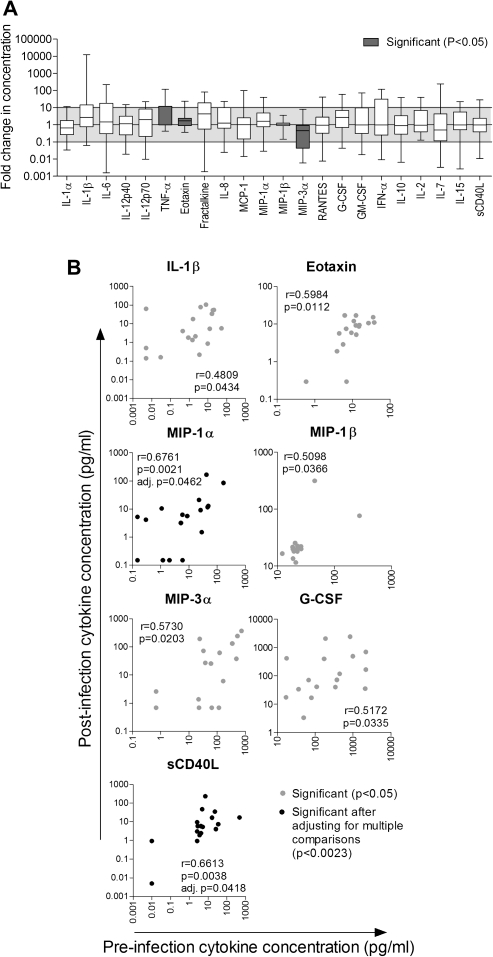
The concentrations of 22 cytokines in CVL from women prior to HIV-1 infection (median, 36 weeks preinfection) were compared with those of the same women during early HIV-1 infection (median, 6 weeks postinfection) for whom samples at both time points were available (n = 18). In matched samples, TNF-α and eotaxin concentrations were higher in CVLs from early HIV-1 infection compared with preinfection, whereas MIP-3α concentrations were lower (P < .05; Figure 1A). However, these changes were not significant after adjustment for multiple comparisons. Furthermore, in an unmatched analysis including all women who had either preinfection samples (n = 22) or 6-week postinfection samples available (n = 39), only MIP-3α concentrations were lower in postinfection CVLs, before adjusting for multiple comparisons. These data indicate that, in contrast to previous reports that have compared genital cytokine concentrations in HIV-1 infected women to unmatched HIV-uninfected women [10, 12–16], the women in this study did not have significantly altered CVL cytokine concentrations during early HIV-1 infection relative to preinfection samples from women who later became infected with HIV-1.
Figure 1.
Comparison of cervicovaginal lavage (CVL) cytokine concentrations in women (n = 18) before infection (median, 36 weeks preinfection) and during early human immunodeficiency virus type 1 (HIV-1) infection (median, 6 weeks postinfection). A, Fold changes in cytokine concentrations following HIV-1 infection are shown as box-and-whisker plots; error bars indicate the range. Wilcoxon signed-rank test was used to compare cytokine concentrations in CVL from the same women pre– and post HIV-1-infection. Changes in cytokine concentrations that were significant before adjusting for multiple comparisons and are indicated by gray bars (P < .05). B, Spearman correlations between cytokine concentrations measured in pre- and postinfection CVL. MIP-1β was no longer correlated between time points following exclusion of outliers. Gray dots indicate cytokines that correlated significantly before adjusting for multiple comparisons (P < .05). Black dots indicate cytokines that remained significantly correlated after adjustment (P < .0023). G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte macrophage-colony stimulating factor; IFN, interferon; IL, interleukin; MCP, monocyte chemotactic protein; MIP, macrophage inflammatory protein; sCD40L, soluble CD40 ligand; TNF, tumor necrosis factor.
Interestingly, the concentrations of 7 of 22 cytokines measured in CVLs correlated between pre– and post HIV-1 infection (Figure 1B). MIP-1α and sCD40L remained significantly correlated after adjusting for multiple comparisons. Following evaluation of cytokine concentrations in longitudinal samples (17, 30, and 55 weeks postinfection), it was found that 11 of 20 cytokines correlated between at least 3 of the 5 time points. After adjusting for multiple comparisons, IL-1α, IL-β, IL-6, IL-8, MIP-1α, G-CSF, and GM-CSF were significantly correlated across ≥3 time points. These findings suggest that the relative degrees of cervicovaginal inflammation in individual women remained relatively constant during the study period.
Genital Tract Inflammation During Early HIV-1 Infection Was Not Associated With Multivariant HIV-1 Transmission
Abrahams et al [29] have reported that in this cohort of women, 22% were infected with >1 HIV-1 genetic variant. To investigate the relationship between genital tract inflammation and transmission of multiple HIV-1 variants, cytokine concentrations at 6 weeks postinfection were compared in women with multiple (n = 10) or single (n = 25) transmitted variants. No differences in cervicovaginal cytokines were found between women infected with multiple or single variants (data not shown), suggesting that genital inflammation was not associated with the break in HIV-1 transmission bottleneck in this cohort.
Cervicovaginal Inflammation During Early HIV-1 Infection Was Associated With STIs
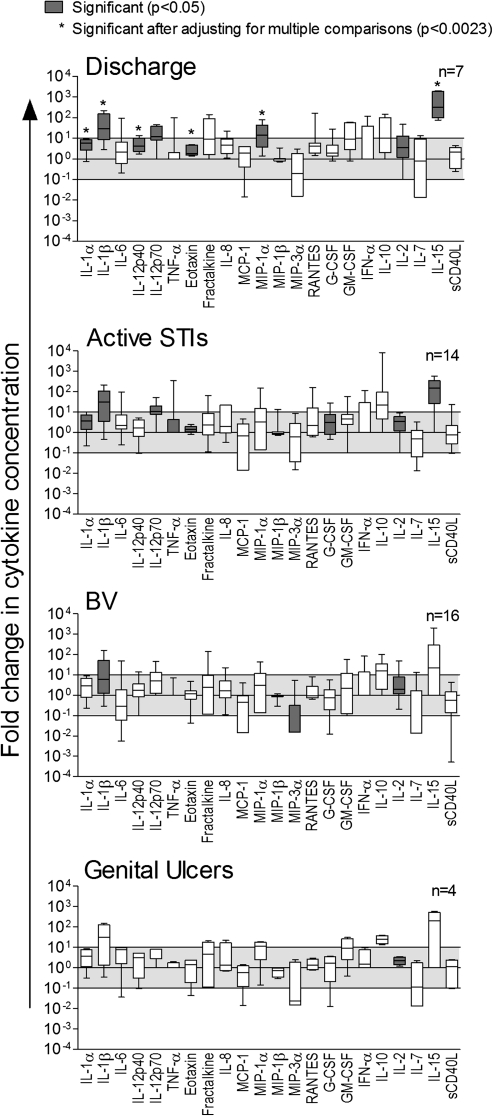
Cytokine concentrations in CVL from women with early HIV-1 infection (6 weeks postinfection) who tested positive for ≥1 STI or had symptoms of STIs were compared with those of women who did not have an STI or BV (Figure 2). Women who had ≥1 active STI (C. trachomatis, N. gonorrhoeae, T. vaginalis, or HSV-2; n = 14) tended to have elevated cytokine concentrations; however, these changes were not significant after adjusting for multiple comparisons. Abnormal vaginal discharge, which may be caused by other factors in addition to STIs, including BV, Candida albicans, allergic reaction, irritation, or physiologic changes [28], was associated with elevated concentrations of proinflammatory IL-1α, IL-1β, IL-12p40, eotaxin, MIP-1α, and T-cell homeostatic IL-15, after adjustment for multiple comparisons.
Figure 2.
Fold change in cytokine concentrations in cervicovaginal lavage (CVL) samples from women who had ≥1 sexually transmitted infection (STI) or bacterial vaginosis (BV) compared with the median concentrations in women who did not have an STI. Cytokine concentrations were measured in CVL samples from women infected with human immunodeficiency virus type 1 (HIV-1) (median, 6 weeks postinfection) who had no STI (n = 7), women who had visible vaginal discharge (n = 7) or genital ulceration (n = 4), women who had ≥1 laboratory-diagnosed active STIs (Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis, or herpes simplex virus type 2 [HSV-2]; n = 14) and women who had BV (n = 16). Mann-Whitney U test was used to compare cytokine concentrations in CVL from women with STIs, BV, or symptoms to women with no STIs and/or BV. Fold changes in cytokine concentrations are shown as box-and-whisker plots; error bars indicate the range. Gray bars indicate cytokines that were significantly different before adjusting for multiple comparisons (P < .05). Stars indicate cytokines that remained significant after adjustment (P < .0023).
Genital Tract Inflammation During HIV-1 Infection Was Associated With HIV-1 Shedding
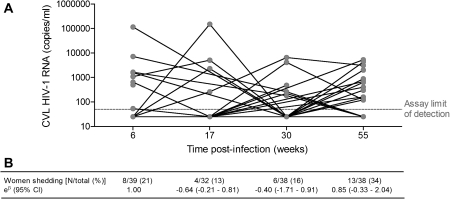
HIV-1 viral loads were measured in CVL samples collected at 6, 17, 30, and 55 weeks postinfection as an indicator of HIV-1 shedding. CVL viral loads and proportions of women with detectable HIV-1 RNA did not differ significantly between time points (Figure 3). CVL viral loads did not correlate between time points, indicating that different women were shedding HIV-1 at different times. Cytokine concentrations in CVL correlated with CVL viral loads at all time points, and women who had detectable HIV-1 RNA in their genital tracts had higher cytokine concentrations than did women who were not shedding HIV-1 (Table 2). Preinfection cytokine concentrations did not differ between women who were shedding and women who were not shedding HIV-1 6 weeks postinfection. Additionally, lower blood CD4 counts and/or higher plasma viral loads were associated with CVL viral loads at each time point. The prevalence of STIs did not differ between women with detectable HIV-1 RNA levels in their genital tracts [3 of 7 (42.9%)] and women who were not shedding [11 of 31 (35.5%)]. Furthermore, there were no significant differences in occurrence of BV, vaginal discharge, and genital ulcers in women shedding HIV-1 compared with women who were not shedding.
Figure 3.
Human immunodeficiency virus type (HIV-1) RNA concentrations in cervicovaginal lavage (CVL) at 6, 17, 30, and 55 weeks postinfection. A, HIV-1 viral loads in CVL from each woman are indicated by gray dots. Lines connect RNA concentrations in CVL from the same women at different time points. No significant Spearman correlations were found between HIV-1 RNA levels at each time point, indicating that different women were shedding at each time point. B, Proportion of women who had detectable HIV-1 RNA at each time point. Mixed-effects logistic regression was used to determine whether the proportion of women shedding HIV-1 at 17, 30, and 55 weeks postinfection differed relative to 6 weeks postinfection; eβ indicates the change in number of women shedding at each time point relative to 6 weeks postinfection (reference time point). No significant differences were found.
Table 2.
Spearman Rank Correlations Between CVL HIV-1 RNA Concentrations and Blood CD4 Counts, Plasma Viral Loads, and CVL Cytokine Concentrations
| 6 Weeks Postinfection |
17 Weeks Postinfection |
30 Weeks Postinfection |
55 Weeks Postinfection |
|||||
| ρ (P Value) | ρ (P Value) | ρ (P Value) | ρ (P Value) | |||||
| Blood CD4+ T-cell count | −0.485 | (.002) | −0.305 | (.090) | −0.428 | (.007) | −0.047 | (.782) |
| Plasma viral load | 0.430 | (.006) | 0.533 | (.002) | 0.314 | (.055) | 0.371 | (.022) |
| IL-1α | 0.236 | (.147) | 0.154 | (.401) | 0.260 | (.114) | 0.337 | (.039)a |
| IL-1β | 0.355 | (.026)a | 0.478 | (.006)b | 0.207 | (.212) | 0.513 | (.001)b |
| IL-6 | 0.427 | (.007)a | 0.446 | (.011)b | 0.261 | (.113) | 0.338 | (.038)a |
| IL-12p40 | 0.159 | (.333) | 0.211 | (.247) | 0.294 | (.073) | 0.175 | (.293) |
| IL-12p70 | 0.325 | (.044) | 0.204 | (.263) | 0.177 | (.287) | 0.027 | (.873) |
| TNF-α | 0.388 | (.015)a | 0.382 | (.031)a | 0.415 | (.010)a | 0.309 | (.059) |
| Eotaxin | 0.216 | (.186) | 0.227 | (.211) | −0.038 | (.821) | 0.473 | (.003)b |
| Fractalkine | 0.163 | (.321) | 0.405 | (.022)a | −0.186 | (.264) | 0.331 | (.042)a |
| IL-8 | 0.323 | (.045) | 0.279 | (.122) | 0.393 | (.015)a | 0.437 | (.006)b |
| MCP-1 | 0.419 | (.008)a | 0.487 | (.005)b | 0.099 | (.555) | 0.432 | (.007)b |
| MIP-1α | 0.155 | (.345) | 0.385 | (.030)a | 0.059 | (.726) | 0.128 | (.445) |
| MIP-1β | 0.186 | (.258) | 0.570 | (.001)b | 0.087 | (.604) | 0.314 | (.055) |
| RANTES | 0.490 | (.002)a | 0.531 | (.002)b | 0.267 | (.106) | 0.549 | (.0004)b |
| G-CSF | 0.310 | (.055) | 0.457 | (.009)b | 0.198 | (.234) | 0.379 | (.019)a |
| GM-CSF | 0.090 | (.585) | 0.537 | (.002)b | 0.315 | (.054) | 0.077 | (.645) |
| MIP-3α | 0.226 | (.207) | ND | ND | ND | |||
| IFN-α | 0.212 | (.201) | ND | ND | ND | |||
| IL-10 | 0.378 | (.018)a | 0.247 | (.173) | 0.038 | (.821) | −0.160 | (.336) |
| IL-2 | 0.385 | (.015)a | 0.534 | (.002)b | 0.137 | (.413) | −0.134 | (.421) |
| IL-7 | 0.260 | (.110) | 0.119 | (.517) | 0.126 | (.450) | −0.057 | (.736) |
| IL-15 | 0.382 | (.016)a | 0.220 | (.226) | 0.375 | (.020)a | −0.013 | (.939) |
| sCD40L | 0.331 | (.040)a | 0.309 | (.085) | 0.218 | (.188) | 0.123 | (.462) |
Significant associations are shown in bold (P < .05). Associations that were significant after adjusting for multiple comparisons are underlined.
Abbreviations: CVL, cervicovaginal lavage; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte macrophage colony-stimulating factor; HIV -1, human immunodeficiency virus type 1; IFN, interferon; IL, interleukin; MCP, monocyte chemotactic protein; MIP, macrophage inflammatory protein; ND, not done; sCD40L, soluble CD40 ligand; TNF, tumor necrosis factor.
Cytokine concentration significantly higher in women who had detectable HIV-1 RNA in their genital tracts compared with women who had undetectable HIV-1 RNA concentrations (P < .05).
Cytokine concentration significantly higher in women who had detectable HIV-1 RNA after adjusting for multiple comparisons.
Elevated Genital Tract Cytokine Concentrations During Early HIV-1 Infection Correlated With Lower Blood CD4+ T-Cell Counts
We have previously reported that cervicovaginal IL-1β, IL-6, and IL-8 concentrations during early HIV-1 infection were associated with lower blood CD4+ T-cell counts at these early time points [15]. In this study, it was similarly found that higher concentrations of IL-6 (ρ = −0.455, adjusted P = .030), TNF-α (ρ = −0.498, adjusted P = .017), RANTES (ρ = −0.460, adjusted P = .026), and IL-10 (ρ = −0.503, adjusted P = .027) in CVL correlated with lower blood CD4 counts during early HIV-1 infection. Only weak associations between 17-, 30-, and 55-week cytokine concentrations and concurrent CD4 cell counts were found, and these were not significant after adjusting for multiple comparisons, indicating that the relationship was restricted to early HIV-1 infection.
Despite finding a significant correlation between (1) plasma viral load and HIV-1 shedding and (2) genital inflammation and HIV-1 shedding, few associations were found between genital cytokine concentrations and plasma viral loads measured at the same time points. This suggests that changes in inflammatory cytokine production in the genital tract during early HIV-1 infection are largely independent of systemic viral load.
Cervicovaginal Inflammation During Early HIV-1 Infection Was Associated With CD4+ T-Cell Depletion During the First Year of Infection
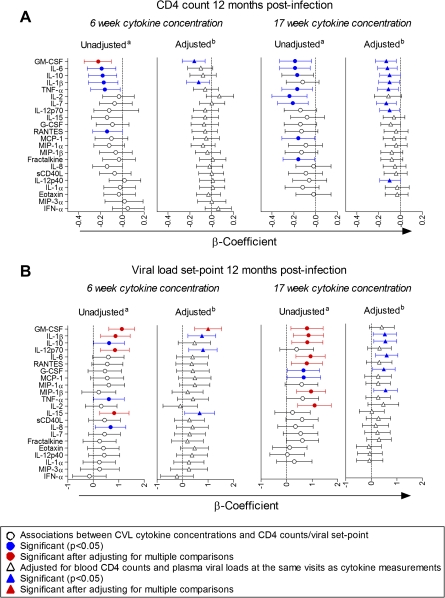
To determine whether genital cytokine concentrations during HIV-1 infection were associated with disease progression, associations between cytokines and (1) average blood CD4 counts measured at 3 consecutive visits overlying 12 months postinfection and (2) CD4 loss between preinfection and 12 months postinfection were investigated. Although higher concentrations of several cytokines at multiple time points were associated with lower CD4 counts 12 months postinfection before adjusting for multiple comparisons, only GM-CSF concentrations 6 weeks postinfection remained significantly associated after adjustment (Figure 4A). No significant relationship between preinfection cytokine concentrations and CD4 counts 12 months postinfection was found, although relatively few CVL samples (22 of 49) from preinfection time points were available for this analysis.
Figure 4.
Linear regression was used to assess relationships between cytokine concentrations in cervicovaginal lavage (CVL) from women 6 weeks (n = 37) and 17 (n = 31) weeks postinfection and average blood CD4 counts of 3 consecutive visits overlying the 12-month postinfection time point (A) and viral load set points (B). Viral loads, CD4 counts, and cytokine concentrations were log-transformed, and cytokine concentrations were standardized to allow for direct comparison between β-coefficients. β-Coefficients that were generated by univariate regression are indicated by circles and show the relationship between each cytokine and 12-month CD4 counts or viral load set points. β-Coefficients that indicate the relationships between cytokine concentrations and 12-month CD4 counts or viral load set points, following adjustment for CD4 counts and viral loads at the same visits as cytokine measurements using multivariate regression, are represented by triangles. Error bars indicate 95% confidence intervals. Cytokines were ranked according to the strength of their associations with 12-month CD4 counts or viral load set point. Significant associations are shown in blue (P < .05). Associations that were significant after adjusting for multiple comparisons are shown in red. G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte macrophage-colony stimulating factor; IFN, interferon; IL, interleukin; MCP, monocyte chemotactic protein; MIP, macrophage inflammatory protein; sCD40L, soluble CD40 ligand; TNF, tumor necrosis factor.
Blood CD4 counts and plasma viral loads during early HIV-1 infection were also associated with 12 month postinfection CD4 counts (β = .70 [95% CI, .46 to .95] and β = −.07 [95% CI, –.12 to –.02], respectively). HIV-1 disease status could predispose individuals to higher incidence of STIs and genital shedding of HIV-1 [30, 31], thereby exacerbating genital inflammation, which would therefore be indirectly rather than directly associated with disease progression. Multivariate regression was thus used to adjust for CD4 counts and viral loads measured at the same time points as cytokine concentrations. Again, there was a strong trend toward an association between higher cytokine concentrations and lower 12-month CD4 counts.
Finally, associations between preinfection (n = 22) and 6-week postinfection (n = 18) cytokine levels and blood CD4+ T-cell loss during the first 12 months of HIV-1 infection were investigated in a subset of women for whom preinfection CD4 count measurements were available. CD4 depletion during the first year of infection was associated with higher preinfection cervicovaginal concentrations of RANTES (ρ = 0.46, P = .03) and 6-week postinfection concentrations of IL-1β (ρ = 0.53, P = .02) and GM-CSF (ρ = 0.62, P = .006). However, these associations were not upheld after adjusting for multiple comparisons, which may be due to the relatively small sample sizes available for this analysis.
Genital Inflammation During Early HIV-1 Infection Was Associated With Higher Viral Load Set Point
It was found that the concentrations of several inflammatory and T-cell homeostatic cytokines in CVL during early HIV-1 infection (6 and 17 weeks postinfection) were associated with higher plasma viral load set point (Figure 4B), even though these cytokines did not correlate with viral loads measured at the same time points as cytokine measurements. No associations between cytokine concentrations at later time points postinfection (30 and 55 weeks postinfection) and viral load set point were significant after adjusting for multiple comparisons. Additionally, although cytokine concentrations did not differ between preinfection and early infection time points, no significant associations between preinfection cytokine concentrations and viral load set point were found. Elevated GM-CSF, IL-1β, IL-12p70, and IL-15 concentrations at 6 weeks postinfection and GM-CSF, IL-1β, IL-10, IL-6, RANTES, MIP-1β, and IL-2 concentrations 17 weeks postinfection were associated with higher viral load set point. Following adjustment for CD4 counts and viral loads measured at the same time points as cytokine measurements, elevated GM-CSF concentrations 6 weeks postinfection remained significantly associated with higher viral load set point. This suggests that the relationship between this inflammatory cytokine and disease progression is at least partly independent of early infection systemic disease state.
DISCUSSION
Previous studies have shown that women with early and chronic HIV-1 infection have elevated genital inflammatory cytokine concentrations relative to unmatched HIV-uninfected women [10, 12–16]. This study is the first to compare cytokine concentrations in cervicovaginal samples from the same women preinfection and during early HIV-1 infection. It was found that inflammatory cytokine levels preinfection were correlated with those postinfection and in fact were not significantly elevated shortly following HIV-1 infection (6 weeks postinfection). However, genital cytokine concentrations were higher in women who had vaginal discharge and in women who were shedding HIV-1 in their genital secretions. In addition, cytokine concentrations were elevated in women who had lower blood CD4+ T-cell counts 6 weeks postinfection [15]. Elevated cervicovaginal cytokines during early HIV-1 infection (6 and 17 weeks postinfection) were associated with higher plasma viral load set point, which is predictive of time to AIDS [26]. Additionally, higher GM-CSF concentrations 6 weeks postinfection were associated with lower CD4 counts 12 months postinfection.
When genital cytokine concentrations were compared in the same women preinfection and at several time points during the first year of HIV-1 infection, some women maintained higher levels of genital inflammation over time, while others had consistently low cytokine concentrations. STI prevalence did not differ between pre– and post HIV-1 infection; thus, the cause of sustained inflammation may be STI recurrence. Mitchell et al [18] demonstrated that genital inflammatory cytokines were not elevated in chronically HIV-1 infected women relative to uninfected women but rather were associated with BV. The higher frequency of STIs in HIV-1 infected women compared with uninfected women [17] may account for previous findings that genital inflammatory cytokines are higher in women with early HIV-1 infection. It is possible that elevated genital inflammatory cytokine responses, similar to those reported shortly after SIV infection of macaques [6], may have subsided by 6 weeks postinfection. Alternatively, cervicovaginal inflammatory cytokine concentrations may increase over time during HIV-1 infection and may thus be higher in chronically infected women relative to those with early infection [12].
Genital HIV-1 RNA concentrations correlated not only with higher levels of genital inflammation but also with lower blood CD4 counts and higher plasma viral loads at the same time points. Although these findings may suggest that high systemic viral loads drive HIV-1 shedding, which in turn induces genital inflammation, which is thus indirectly associated with disease progression, shedding during early HIV-1 infection was not associated with 12-month CD4 counts or viral load set point. Additionally, genital inflammation during early HIV-1 infection was only weakly associated with concurrent plasma viral load before adjusting for multiple comparisons but was strongly associated with plasma viral loads at subsequent time points. It was also found that, although CVL cytokine levels correlated positively over time, different women were shedding at different time points during HIV-1 infection. Therefore, shedding was not the cause of sustained cervicovaginal inflammation that was observed in this cohort, and genital inflammation may rather facilitate HIV-1 shedding in individuals who have high plasma viral loads by recruiting HIV-1-infected cells to the genital tract and by promoting viral replication.
It was found that elevated CVL cytokine concentrations 6 weeks postinfection were associated with lower blood CD4 counts at the same time point. Blood CD4 depletion may reflect CD4 depletion in the genital tract. This would contribute to genital inflammation by inducing T-cell homeostatic cytokine production [32], which in turn induces inflammatory cytokine production [33, 34]. In support, T-cell homeostatic cytokines IL-2 and IL-15 were inversely associated with blood CD4 counts before adjusting for multiple comparisons. Therefore, STIs and other factors that are associated with vaginal discharge are likely to be major contributors to cervicovaginal inflammation during early HIV-1 infection, with inflammation exacerbated by HIV-1 replication in the genital tract and the homeostatic response to CD4 depletion. A recent macaque study demonstrated the importance of proinflammatory cytokine production in the genital tract in establishment of productive SIV infection following vaginal inoculation [4]. Wang et al [24] further showed that induction of inflammatory cytokine responses in the genital tracts of macaques prior to vaginal inoculation with SIV was associated with increased plasma viral load set point, suggesting that genital cytokine concentrations at the time of infection may influence disease progression. Similar to macaque studies, it was found in this study that higher levels of cervicovaginal proinflammatory and T-cell homeostatic cytokines during early HIV-1 infection were associated with more rapid HIV-1 disease progression. Additionally, the findings of this study suggest that the association between genital inflammation during early infection and disease progression is partly independent of blood CD4 counts and plasma viral loads measured at the same time points as cytokine concentrations. Although the earliest time point included in this study (6 weeks postinfection) was past viral dissemination to blood, preinfection genital cytokine concentrations correlated with those measured during the first year of HIV-1 infection. Therefore, genital inflammation 6 weeks postinfection may reflect inflammation at the time of infection. Although preinfection genital inflammation may be associated with HIV-1 disease progression, this could not be investigated here as the number of women for whom preinfection samples were available was small (22 of 49 women). Cytokine concentrations measured 6 weeks postinfection (range, 1–13) may, however, be a closer representation of the level of genital inflammation present at the time of HIV-1 infection than were preinfection cytokine concentrations that were measured 36 weeks prior to infection (range, 2–92). Higher concentrations of cervicovaginal inflammatory cytokines at the time of HIV-1 transmission may favor disease progression by recruiting and activating CD4+ T cells for HIV-1 infection and directly promoting viral replication [4, 9–11]. These findings suggest that the inflammatory environment in the genital tracts of women who become infected with HIV-1 through sexual transmission may influence disease outcome and that strategies to reduce genital inflammation may slow disease progression.
Notes
Acknowledgments.
The authors would like to acknowledge the following people for their contribution to this work: the Centre for the AIDS Programme of Research in South Africa (CAPRISA), and especially the members of the Acute Infection Study Team; and the participants of the Acute Infection Study, without whom this work would not have been possible.
Financial support.
This work was supported by the Comprehensive International Program of Research on AIDS of the Division of AIDS, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), and US Department of Health and Human Services (DHHS) (grant number U19 AI51794); the Center for HIV-AIDS Vaccine Immunology via the NIAID, NIH, and the US DHHS (grant number AI51794); the Poliomyelitis Research Foundation of South Africa; the Wellcome Trust Intermediate Fellowship in Infectious Diseases (to J. P.); the Columbia University–Southern African Fogarty AIDS International Training and Research Programme, and the Fogarty Ellison Programme funded by the Fogarty International Center, NIH (grant number D43TW00231 to L. R. and L. B.); and the South African Medical Research Council (to L. R.).
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.United Nations Programme on HIV/AIDS report on the global AIDS epidemic 2010. http://www.unaids.org. Accessed 11 March 2011. [Google Scholar]
- 2.Pope M, Haase AT. Transmission, acute HIV-1 infection and the quest for strategies to prevent infection. Nat Med. 2003;9:847–52. doi: 10.1038/nm0703-847. [DOI] [PubMed] [Google Scholar]
- 3.Miller CJ, Li Q, Abel K, et al. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J Virol. 2005;79:9217–27. doi: 10.1128/JVI.79.14.9217-9227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Q, Estes JD, Schlievert PM, et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458:1034–8. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haase AT. Early events in sexual transmission of HIV and SIV and opportunities for intervention. Annu Rev Med. 2011;62:127–39. doi: 10.1146/annurev-med-080709-124959. [DOI] [PubMed] [Google Scholar]
- 6.Abel K, Rocke DM, Chohan B, Fritts L, Miller CJ. Temporal and anatomic relationship between virus replication and cytokine gene expression after vaginal simian immunodeficiency virus infection. J Virol. 2005;72:12164–72. doi: 10.1128/JVI.79.19.12164-12172.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meier A, Alter G, Frahm N, et al. MyD88-dependent immune activation mediated by HIV-1-encoded TLR ligands. J Virol. 2007;81:8180–91. doi: 10.1128/JVI.00421-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gumbi PP, Nkwanyana NN, Bere A, et al. Impact of mucosal inflammation on cervical human immunodeficiency virus (HIV-1)-specific CD8 T-cell responses in the female genital tract during chronic HIV infection. J Virol. 2008;82:8529–36. doi: 10.1128/JVI.00183-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swingler S, Mann A, Jacque JM, et al. HIV-1 Nef mediates lymphocyte chemotaxis and activation by infected macrophages. Nat Med. 1999;5:997–1003. doi: 10.1038/12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nkwanyana NN, Gumbi PP, Roberts L, et al. Impact of HIV infection and inflammation on composition and yield of cervical mononuclear cells in the female genital tract. Immunology. 2009;128:e746–57. doi: 10.1111/j.1365-2567.2009.03077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osborn L, Kunkel S, Nabel GJ. Tumor necrosis factor-alpha and interleukin-1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc Natl Acad Sci U S A. 1989;86:2336–40. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bélec L, Gherardi R, Payan C, et al. Proinflammatory cytokine expression in cervicovaginal secretions of normal and HIV-infected women. Cytokine. 1995;7:568–74. doi: 10.1006/cyto.1995.0077. [DOI] [PubMed] [Google Scholar]
- 13.Crowley-Nowick PA, Ellenberg JH, Vermund SH, Douglas SD, Holland CA, Moscicki AB. Cytokine profile in genital tract secretions from female adolescents: impact of human immunodeficiency virus, human papillomavirus, and other sexually transmitted pathogens. J Infect Dis. 2000;181:939–45. doi: 10.1086/315311. [DOI] [PubMed] [Google Scholar]
- 14.Zara F, Nappi RE, Brerra R, Migliavacca R, Maserati R, Spinillo A. Markers of local immunity in cervico-vaginal secretions of HIV infected women: implications for HIV shedding. Sex Transm Infect. 2004;80:108–12. doi: 10.1136/sti.2003.005157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bebell LM, Passmore JS, Williamson C, et al. Relationship between levels of inflammatory cytokines in the genital tract and CD4+ cell counts in women with acute HIV-1 infection. J Infect Dis. 2008;198:710–14. doi: 10.1086/590503. [DOI] [PubMed] [Google Scholar]
- 16.Guha D, Chatterjee R. Cytokine levels in HIV infected and uninfected Indian women: correlation with other STAs. Exp Mol Pathol. 2009;86:65–8. doi: 10.1016/j.yexmp.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Fennema JS, van Ameijden EJ, Coutinho RA, van den Hoek AA. HIV, sexually transmitted diseases and gynaecologic disorders in women: increased risk for genital herpes and warts among HIV-infected prostitutes in Amsterdam. AIDS. 1995;9:1071–8. [PubMed] [Google Scholar]
- 18.Mitchell CM, Balkus J, Agnew KJ, et al. Bacterial vaginosis, not HIV, is primarily responsible for increased vaginal concentrations of proinflammatory cytokines. AIDS Res Hum Retroviruses. 2008;24:667–71. doi: 10.1089/aid.2007.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson LF, Lewis DA. The effect of genital tract infections on HIV-1 shedding in the genital tract: a systematic review and meta-analysis. Sex Transm Dis. 2008;35:946–59. doi: 10.1097/OLQ.0b013e3181812d15. [DOI] [PubMed] [Google Scholar]
- 20.Roberts L, Passmore JS, Williamson C, et al. Plasma cytokine levels during acute HIV-1 infection predict HIV disease progression. AIDS. 2010;24:819–31. doi: 10.1097/QAD.0b013e3283367836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ansari AA, Mayne AE, Sundstrom JB, et al. Administration of recombinant rhesus interleukin-12 during acute simian immunodeficiency virus (SIV) infection leads to decreased viral loads associated with prolonged survival in SIVmac251-infected rhesus macaques. J Virol. 2002;76:1731–43. doi: 10.1128/JVI.76.4.1731-1743.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mueller YM, Do DH, Altork SR, et al. IL-15 treatment during acute simian immunodeficiency virus (SIV) infection increases viral set-point and accelerates disease progression despite the induction of stronger SIV-specific CD8+ T cell responses. J Immunol. 2008;180:350–60. doi: 10.4049/jimmunol.180.1.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okoye A, Park H, Rohankhedkar M, et al. Profound CD4+/CCR5+ T cell expansion is induced by CD8+ lymphocyte depletion but does not account for accelerated SIV pathogenesis. J Exp Med. 2009;206:1575–88. doi: 10.1084/jem.20090356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Abel K, Lantz K, Krief AM, McChesney MB, Miller CJ. The Toll-like receptor 7 (TLR7) agonist, imiquimod, and the TLR9 agonist, CpG ODN, induce antiviral cytokines and chemokines but do not prevent vaginal transmission of simian immunodeficiency virus when applied intravaginally to rhesus macaques. J Virol. 2005;79:14355–70. doi: 10.1128/JVI.79.22.14355-14370.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Loggerenberg F, Mlisana K, Williamson C, et al. Establishing a cohort at high risk of HIV infection in South Africa: challenges and experiences of the CAPRISA 002 acute infection study. PLoS One. 2008;3:e1954. doi: 10.1371/journal.pone.0001954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyles RH, Munoz A, Yamashita TE, et al. Natural history of human immunodeficiency virus type 1 viremia after seroconversion and proximal to AIDS in a large cohort of homosexual men. J Infect Dis. 2000;181:872–80. doi: 10.1086/315339. [DOI] [PubMed] [Google Scholar]
- 27.Columb MO, Sagadai S. Multiple comparisons. Curr Anaesth Crit Care. 2006;17:233–6. [Google Scholar]
- 28.Mitchell H. Vaginal discharge—causes, diagnosis and treatment. BMJ. 2004;328:1306–8. doi: 10.1136/bmj.328.7451.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abrahams MR, Anderson JA, Giorgi EE, et al. Quantitating the multiplicity of infection with human immunodeficiency virus type 1 subtype C reveals a non-Poisson distribution of transmitted variants. J Virol. 2009;83:3556–67. doi: 10.1128/JVI.02132-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghys PD, Diallo MO, Ettiègne-Traoré V, et al. Genital ulcers associated with human immunodeficiency virus–related immunosuppression in female sex workers in Abidjan, Ivory Coast. J Infect Dis. 1995;172:1371–4. doi: 10.1093/infdis/172.5.1371. [DOI] [PubMed] [Google Scholar]
- 31.Ghys PD, Fransen K, Diallo MO, et al. The associations between cervicovaginal HIV shedding, sexually transmitted diseases and immunosuppression in female sex workers in Abidjan, Côte d’Ivoire. AIDS. 1997;11:F85–93. doi: 10.1097/00002030-199712000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Catalfamo M, Mascio MD, Hu Z, et al. HIV infection-associated immune activation occurs by two distinct pathways that differentially affect CD4 and CD8 T cells. Proc Natl Acad Sci U S A. 2008;105:19851–6. doi: 10.1073/pnas.0810032105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alderson MR, Tough TW, Ziegler SF, Grabstei KH. Interleukin 7 induces cytokine secretion and tumoricidal activity by human peripheral blood monocytes. J Exp Med. 1991;173:923–30. doi: 10.1084/jem.173.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Damås JK, Wæhre T, Yndestad A, et al. Interleukin-7–mediated inflammation in unstable angina: possible role of chemokines and platelets. Circulation. 2003;107:2670–6. doi: 10.1161/01.CIR.0000070542.18001.87. [DOI] [PubMed] [Google Scholar]