Abstract
(See the editorial commentary by Peters and Marston, on pages 166–8.)
Background. Understanding the impact of hepatitis B virus (HBV) in human immunodeficiency virus (HIV) coinfection has been limited by heterogeneity of HIV disease. We evaluated HBV coinfection and HIV-related disease progression in a cohort of HIV seroconverters.
Methods. Participants with HIV diagnosis seroconversion window of ≤3 years and serologically confirmed HBV infection (HB) status were classified at baseline into 4 HB groups. The risk of clinical AIDS/death in HIV seroconverters was calculated by HB status.
Results. Of 2352 HIV seroconverters, 474 (20%) had resolved HB, 82 (3%) had isolated total antibody to hepatitis B core antigen (HBcAb), and 64 (3%) had chronic HB. Unadjusted rates (95% confidence intervals [CIs]) of clinical AIDS/death for the HB-negative, resolved HB, isolated HBcAb, and chronic HB groups were 2.43 (2.15–2.71); 3.27 (2.71–3.84); 3.75 (2.25–5.25); and 5.41 (3.41–7.42), respectively. The multivariable risk of clinical AIDS/death was significantly higher in the chronic HB group compared to the HB-negative group (hazard ratio [HR], 1.80; 95% CI, 1.20–2.69); while the HRs were increased but nonsignificant for those with resolved HB (HR, 1.17; 95% CI, .94–1.46) and isolated HBcAb (HR, 1.14; 95% CI, .75–1.75).
Conclusions. HBV coinfection has a significant impact on HIV outcomes. The hazard for an AIDS or death event is almost double for those with chronic HB compared, with HIV-monoinfected persons.
Hepatitis B virus (HBV) is more common in human immunodeficiency virus (HIV)–infected individuals than in the general population owing to shared risk factors for viral acquisition [1–3]. Current evidence suggests that human immunodeficiency virus (HIV) infection has an adverse impact on HBV-related liver disease progression, with higher serum HBV DNA polymerase activity, lower rates of loss of serum hepatitis B e antigen, and increased risk of cirrhosis, liver-related mortality, and hepatocellular carcinoma at lower CD4 T-cell counts [1, 4–6]. HBV infection (HB) is more likely to be chronic in those with HIV infection [6]. The introduction of highly active antiretroviral therapy (HAART)–containing anti-HBV therapy may partially reconstitute HBV-specific CD4 and CD8 T-cell responses, the latter being critical for long-term control of HB in persons with resolving acute infection [7, 8] and improved HBV serologic outcome [9].
Clinical studies before the general availability of HAART that evaluated the impact of HB on HIV progression have shown inconsistent results [10–12]. Some studies found no differences in HIV progression between those with and those without chronic HB [1, 11, 13]. However, those studies were restricted by the heterogeneity of HIV disease as defined by CD4 cell count, unknown or long duration of HIV disease, population characteristics, and incomplete HBV seromarkers at study entry. Some investigators have attempted to adjust for this variability by CD4 cell count stratification, but they were still unable to detect an association [13]. Other studies have suggested that chronic HB may negatively impact HIV progression [14, 15]. However, studies were also restricted by an unknown duration of HIV infection. Therefore we sought to characterize the risk of HIV disease progression according to HB status at the time of HIV diagnosis in a large cohort with known and limited duration of HIV infection, free access to healthcare, minimal injection drug use (IDU), and long-term follow-up.
METHODS
Study Participants and Definitions
The US Military HIV Natural History Study (NHS) is a prospective multicenter continuous enrollment observational cohort of HIV-infected active duty military personnel and other beneficiaries (spouses, adult dependents, and retired military personnel), with >5200 HIV-infected participants from the Army, Navy/Marines, and Air Force enrolled since 1986. Participants are followed at 7 medical centers in the United States. Demographics, medical and medication histories, and standard laboratory studies are collected biannually, as described elsewhere [16]. In the NHS, dates of death are collected through the review of death certificates and medical records by study staff as well as by searching the Social Security Death Index database annually. Although the data are not captured in the NHS database, IDU has been reported to be very rare in this cohort [17]. All NHS participants provided informed consent, and approval for this research was obtained from the institutional review board at each participating site.
The HIV seroconversion (SC) window was defined as the time from last documented HIV seronegative date to the first documented HIV seropositive date, with the estimated SC date as the midpoint of the interval. NHS participants with a SC window ≤3 years were considered for these analyses. Screening for HB was performed in accordance with clinical standards of care and practice guidelines at the time, and included screening for hepatitis B surface antigen (HBsAg), total antibody to hepatitis B core antigen (HBcAb), and hepatitis B surface antibody (HBsAb). Those whose HB status within 2 years of the estimated SC date could not be determined were excluded from these analyses. The remaining participants were classified into 1 of 4 mutually exclusive groups determined by baseline HB status: (1) chronic HB: HBsAg reactivity on ≥2 separate occasions ≥6 months apart; (2) isolated HBcAb: HBcAb reactivity on ≥2 occasions without any other reactive HBV marker; (3) resolved HB: reactive for HBcAb and HBsAb concurrently; or (4) HB negative (both HBsAg and HBcAb negative). The initial HB panel was within 2 years of the estimated SC date for all participants classified as having HB and for the majority (91%) of those classified as HB negative; 155 persons whose first HB panel was negative but was conducted >2 years after the estimated SC date were assumed to be HB negative at baseline.
Hepatitis C virus (HCV) infection was defined as ≥1 positive HCV antibody test within 2 years of the estimated SC date. For those not classified as positive for HCV infection, a negative HCV antibody anytime after the HIV SC date was used to classify individuals as HCV antibody negative at baseline. Sexually transmitted infections were defined as a documented clinical history of gonorrhea, chlamydia, syphilis, or herpes simplex virus type 2 within 2 years after SC. HAART was defined as ≥2 nucleoside reverse-transcriptase inhibitors (NRTIs) in combination with ≥1 protease inhibitor or 1 nonnucleoside reverse-transcriptase inhibitor (NNRTI); or 1 NRTI in combination with ≥1 protease inhibitor and ≥1 NNRTI; or an abacavir- or tenofovir-containing regimen of ≥3 NRTIs [16]. AIDS-defining illnesses were defined using the 1993 Centers for Disease Control and Prevention classification excluding an isolated CD4 cell count <200 cells/μL [18].
Data Analysis
The 4 groups defined by baseline HB status were summarized with descriptive statistics. Medians were presented with interquartile ranges (IQRs) and were compared with Wilcoxon tests; differences in proportions were compared with χ2 tests. Characteristics were also compared between participants who were included and those who were excluded from our analyses. Participants were followed from the estimated time of HIV SC for the composite end point of an AIDS-defining illness or death from any cause. Among those without an event, data were censored on the date of last NHS study visit. The number of events, person-years at risk, and unadjusted rates per 100 person-years were calculated for the 4 baseline groups; rate ratios were estimated with Poisson regression models.
Kaplan-Meier curves and proportional hazards models were used to assess the risk of an AIDS-defining illness or death by baseline HB status, with time beginning at the estimated HIV SC date. For the proportional hazards models, delayed entry methods were used to account for the unobserved time from estimated HIV SC date to the documented date of HIV diagnosis. All proportional hazards models were stratified by era of HIV diagnosis (before 1996 vs later, determined by the general availability of HAART). Model 1 was not adjusted for any demographics or clinical characteristics. Backward selection methods, with forced inclusion of age, sex, and self-reported ethnicity, were used to determine the final multivariable (MV) models. Categories (including one for missing data) were used for CD4 cell count levels and HCV status. The final MV models (models 2 and 3) were both adjusted for age at HIV diagnosis, sex, race/ethnicity, year of HIV diagnosis, and HCV status at HIV diagnosis. MV model 2 was additionally adjusted for baseline CD4 cell count category, and MV model 3 was also adjusted for time-updated covariates for nadir CD4 cell count categories and use of antiretroviral therapy (ART) or HAART. Hazard ratios (HRs) are given with 95% confidence intervals (CIs).
Almost half (48%) of the eligible participants for this report had HIV infection diagnosed in the pre-HAART era (before 1996), yet the majority of events (57%) occurred during the HAART era (1996 or later). To assess the impact of confounding due to HAART on AIDS-defining illnesses or death, a series of sensitivity analyses were performed: model 4 considered the cohort diagnosed with HIV infection in the pre-HAART era and used all available follow-up time (including time on HAART); model 5 also considered the cohort diagnosed with HIV infection in the pre-HAART era but used only follow-up time before 1996; and model 6 considered only follow-up time during the HAART era by using delayed entry methods to start the time at risk as the maximum of 1 January 1996 or documented HIV diagnosis date. All sensitivity analyses (models 4–6) were adjusted for the same covariates as in MV model 3.
RESULTS
Of 5261 participants enrolled in the NHS, 2671 had an HIV SC window of ≤3 years. Of those, 2352 (88%) had HBV testing results available to determine baseline HB status and were included in these analyses. Compared to the participants in our analyses, the 12% excluded due to not having a categorizable HB status had an earlier year of HIV diagnosis, were more likely to self-report black race/ethnicity, and were less likely to have had prior HB vaccination. Among those included in the analyses, baseline HB status was classified as HB negative (n = 1732; 73.6%), resolved HB (n = 474; 20.2%), isolated HBcAb (n = 82; 3.5%), or chronic HB (n = 64; 2.7%). Characteristics at the time of HIV diagnosis are shown in Table 1. Age (overall median, 26.8 years; IQR, 23.2–332.5) differed significantly among the 4 baseline groups, as did the proportion male (n = 2229; 94.8% overall), the year HIV infection was diagnosed (overall median, 1996; IQR, 1991–2002), and the history of HBV vaccination (n = 527; 22.4%) or sexually transmitted infections (n = 849; 36.1%). Among those with a baseline CD4 cell count available, there were no differences between the groups (median, 507 cells/μL; IQR, 374–663). HIV RNA levels, which were generally not available before 1996, were available for 1411 participants (60%) and were similar among the groups (median, 4.4 log10 copies/mL; IQR, 3.7–4.9).
Table 1.
Baseline Characteristics by Hepatitis B Virus Infection Status at Diagnosis of HIV Infection
| Hepatitis B Status |
||||||
| Characteristic | Total Study (n = 2352) | HB Negative (n = 1732) | Resolved HB (n = 474) | Isolated HBcAb (n = 82) | Chronic HB (n = 64) | Pa |
| Age, median (IQR), years | 26.8 (23.2–32.5) | 26.1 (22.9–31.5) | 29.8 (25.0–35.2) | 29.8 (25.3–35.5) | 26.7 (25.0–34.5) | <.001 |
| Male, No. (%) | 2229 (94.8) | 1616 (93.3) | 470 (99.2) | 81 (98.8) | 62 (96.9) | <.001 |
| Race/ethnicity, No. (%) | .03 | |||||
| White | 995 (42.3) | 714 (41.2) | 219 (46.2) | 34 (41.5) | 28 (43.8) | |
| Black/African American | 1053 (44.8) | 770 (44.5) | 215 (45.4) | 41 (50.0) | 27 (42.2) | |
| Other | 304 (12.9) | 248 (14.3) | 40 (8.4) | 7 (8.5) | 9 (14.1) | |
| Prior hepatitis B vaccination, No. (%) | 527 (22.4) | 457 (26.4) | 60 (12.7) | 7 (8.5) | 3 (4.7) | <.001 |
| History of STI, No. (%) | 849 (36.1) | 579 (33.4) | 204 (43.0) | 40 (48.8) | 26 (40.6) | <.001 |
| Hepatitis C status, No. (%) | .16 | |||||
| Negative | 2251 (95.7) | 1667 (96.2) | 448 (94.5) | 75 (91.5) | 61 (95.3) | |
| Positive | 41 (1.7) | 25 (1.4) | 10 (2.1) | 4 (4.9) | 2 (3.1) | |
| Status unknown | 60 (2.6) | 40 (2.3) | 16 (3.4) | 3 (3.7) | 1 (1.6) | |
| Alanine aminotransferase | ||||||
| Measurement available, No. (%) | 1611 (68.5) | 1180 (68.1) | 339 (71.5) | 53 (64.6) | 39 (60.9) | |
| Level, median (IQR), U/L | 30.0 (22.0–43.0) | 30.0 (22.0–42.0) | 30.0 (22.0– 42.0) | 24.0 (17.0–38.0) | 43.0 (30.0–69.0) | <.001 |
| Year of HIV diagnosis, No. (%) | <.001 | |||||
| 1986–1990 | 499 (21.2) | 325 (18.8) | 126 (26.6) | 26 (31.7) | 22 (34.4) | |
| 1991–1995 | 627 (26.7) | 410 (23.7) | 155 (32.7) | 31 (37.8) | 31 (48.4) | |
| 1996–2000 | 451 (19.2) | 343 (19.8) | 97 (20.5) | 7 (8.5) | 4 (6.3) | |
| 2001–2005 | 436 (18.5) | 353 (20.4) | 65 (13.7) | 13 (15.9) | 5 (7.8) | |
| 2006–2009 | 339 (14.4) | 301 (17.4) | 31 (6.5) | 5 (6.1) | 2 (3.1) | |
| Calendar year, median (IQR) | 1996 (1991–2002) | 1998 (1992–2003) | 1993 (1990–2000) | 1992 (1989–1998) | 1992 (1989–1993) | <.001 |
| CD4 cell count, median (IQR) cells/mm3 | 507 (374–663) | 505 (372–661) | 521 (380–671) | 527 (390–670) | 491 (340– 631) | .51 |
| No. (%) by CD4 cell count | ||||||
| Missing count | 196 (8.3) | 172 (9.9) | 16 (3.4) | 4 (4.9) | 4 (6.3) | .002 |
| 0–199 cells/mm3 | 80 (3.4) | 61 (3.5) | 14 (3.0) | 3 (3.7) | 2 (3.1) | |
| 200–349 cells/mm3 | 383 (16.3) | 276 (15.9) | 81 (17.1) | 11 (13.4) | 15 (23.4) | |
| ≥350 cells/mm3, No. (%) | 1693 (72.0) | 1223 (70.6) | 363 (76.6) | 64 (78.0) | 43 (67.2) | |
| HIV RNA | ||||||
| HIV RNA measurement available, No. (%) | 1411 (60.0) | 1084 (62.6) | 262 (55.3) | 44 (53.7) | 21 (32.8) | |
| HIV RNA measurement, median (IQR), log10 copies/mL | 4.4 (3.7–4.9) | 4.4 (3.7–4.9) | 4.4 (3.8–4.8) | 4.2 (3.8–4.7) | 4.4 (3.9–4.7) | .94 |
Abbreviations: HB, hepatitis B virus infection; HBcAb, total antibody to hepatitis B core antigen; HIV, human immunodeficiency virus; IQR, interquartile range; STI, sexually transmitted infection
P values were calculated with χ2 tests for proportions and Wilcoxon rank sum tests for continuous variables.
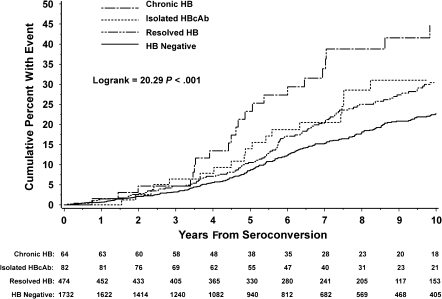
The Kaplan-Meier curve (Figure 1) suggests that those with chronic HB were at increased risk of an AIDS or death event (P < .001). During 16 946 person years of follow-up (range, June 1986 to January 2010), there were 469 AIDS-defining or death events (AIDS in 305, death in 164). Table 2 presents the number and unadjusted rate of AIDS or death events (per 100 person-years of follow-up) for the 4 baseline groups. Compared with those with no HB, the unadjusted rates were significantly higher for those with resolved HB (rate ratio [RR], 1.35; 95% CI, 1.09–1.66), those with isolated HBcAb (RR, 1.54; 95% CI, 1.02–2.34), and those with chronic HB (RR, 2.23; 95% CI, 1.51–3.28).
Figure 1.
AIDS or death events by hepatitis B virus infection (HB) status at seroconversion. The number of participants at risk of an AIDS or death event are provided for each HB group. Abbreviation: HBcAb, total antibody to hepatitis B core antigen.
Table 2.
Follow-up, Number of AIDS or Death Events, and Rates (per 100 Person-Years) by Hepatitis B Virus Infection Status at Time of HIV Infection
| Status | No. of Persons Followed Up | No. of Events | Years of Follow-up, Median (IQR) | Total Years of Follow-up | Rate (95% CI) | Rate Ratioa (95% CI) | P |
| HB Negative | 1732 | 288 | 5 (3–10) | 11 847 | 2.43 (2.15–2.71) | 1.0 | … |
| Resolved HB | 474 | 129 | 7 (4–12) | 3941 | 3.27 (2.71–3.84) | 1.35 (1.09–1.66) | .005 |
| Isolated HBcAb | 82 | 24 | 7 (4–10) | 640 | 3.75 (2.25–5.25) | 1.54 (1.02–2.34) | .04 |
| Chronic HB | 64 | 28 | 7 (4–12) | 517 | 5.41 (3.41–7.42) | 2.23 (1.51–3.28) | <.001 |
| Overall | 2352 | 469 | 6 (3–10) | 16 946 | 2.77 (2.52–3.02) | … | … |
Abbreviations: CI, confidence interval; HB, Hepatitis B virus infection; HBcAb, total antibody to hepatitis B core antigen; IQR, interquartile range.
Compared with HB negative.
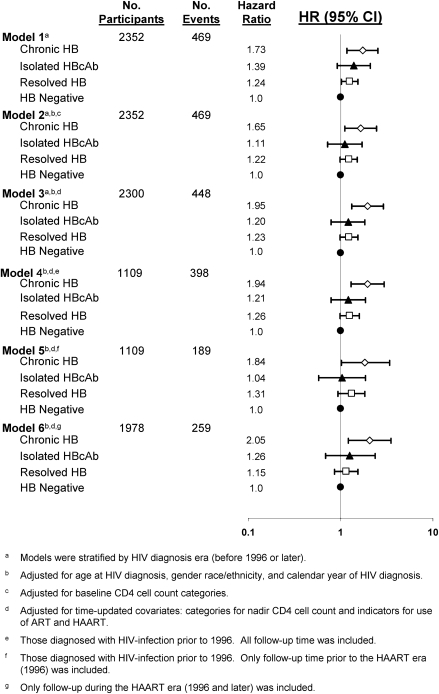
Results from proportional hazards models are shown in Table 3. Without any adjustment for possible confounders (model 1), those with chronic HB (HR, 1.73; 95% CI, 1.17–2.55) or resolved HB (HR, 1.24; 95% CI, 1.01–1.53) had increased risk of an AIDS or death event compared with those who were HB negative, whereas those with isolated HBcAb had increased risk that was not statistically significant (HR, 1.39; 95% CI, .91–2.10). With both of the final MV models (models 2 and 3), the HRs for all HB categories are similar to the model 1 results, but only those with chronic HB were at a significantly increased risk of an AIDS or death event. Comparing those with chronic HB with those who were HB negative, the HRs were similar for the 2 MV model (HR, 1.65 [95% CI, 1.11–2.45] in model 2, which included baseline CD4 cell count categories; HR, 1.80; 95% CI, 1.20–2.69 in model 3, with time-updated categories for nadir CD4 cell count and indicators for use of HAART and non-HAART ART).
Table 3.
Proportional Hazardsa Models for the Risk of AIDS or Death Event
| Model 1 |
Model 2 |
Model 3 |
||||
| Factors | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P |
| HB status | ||||||
| HB negative | 1.0 | 1.0 | ||||
| Resolved HB | 1.24 (1.01– 1.53) | .04 | 1.22 (.98–1.51) | .07 | 1.17 (.94, 1.46) | .16 |
| Isolated HBcAb | 1.39 (.91– 2.10) | .12 | 1.11 (.72–1.71) | .63 | 1.14 (.75, 1.75) | .54 |
| Chronic HB | 1.73 (1.17–2.55) | .006 | 1.65 (1.11–2.45) | .01 | 1.80 (1.20, 2.69) | .004 |
| Age at diagnosis of HIV infection (per 10-year increase in age.) | 0.93 (.80–1.09) | .38 | 1.03 (.88, 1.20) | .75 | ||
| Sex | ||||||
| Male | 1.46 (.92–2.33) | .11 | 1.28 (.80, 2.04) | .30 | ||
| Female | 1.0 | 1.0 | ||||
| Race/ethnicity | ||||||
| White | 1.0 | 1.0 | ||||
| Black | 0.84 (.69–1.02) | .08 | 0.79 (.65, .96) | .02 | ||
| Other | 0.98 (.71–1.35) | .89 | .91 (.66, 1.27) | .59 | ||
| Year of HIV diagnosis (per 1 year increase in calendar year of HIV diagnosis.) | 0.92 (.88, .95) | <.001 | .94 (.90, .98) | .002 | ||
| CD4 cell countb | ||||||
| Missing | 0.88 (.64–1.21) | .43 | NA | |||
| 0–199 cells/mm3 | 4.40 (2.89– 6.72) | <.001 | 9.46 (7.26, 12.33) | <.001 | ||
| 200–349 cells/mm3 | 1.66 (1.30– 2.12) | <.001 | 1.55 (1.16, 2.08) | .003 | ||
| ≥350 cells/mm3 | 1.0 | 1.0 | ||||
| Hepatitis C status | ||||||
| Unknown | 2.60 (1.76– 3.84) | <.001 | 3.38 (2.24, 5.10) | <.001 | ||
| Positive | 2.99 (1.66– 5.38) | <.001 | 2.56 (1.41, 4.63) | .002 | ||
| Negative | 1.0 | 1.0 | ||||
| ART status (time updated) | ||||||
| Off ART | … | 1.0 | ||||
| On non-HAART ART | … | .69 (.55, .87) | .002 | |||
| On HAART | … | .20 (.15, .27) | <.001 | |||
Italicized terms indicate the reference values. Abbreviations: ART, antiretroviral therapy; CI, confidence interval; HAART, highly active antiretroviral therapy; HB, hepatitis B virus infection; HBcAb, total antibody to hepatitis B core antigen; HIV, human immunodeficiency virus; HR, hazard ratio; NA, not applicable.
All models are stratified by era of HIV infection diagnosis (before 1996 vs later).
Model 2 uses baseline CD4 cell count; model 3, time-updated nadir CD4 cell count.
Other covariates that were associated with a significantly increased risk of an AIDS or death event in the MV models included lower CD4 cell count category (models 2 and 3) and HCV status (positive and unknown, models 2 and 3). Factors associated with a decreased risk of an AIDS or death event included black race/ethnicity (compared with white, model 3), later year of HIV diagnosis (models 2 and 3), and use of ART or HAART (model 3). Among those who were HB negative and those who had resolved HB, isolated HBcAb, or chronic HB, respectively, 31%, 25%, 21%, and 27% did not start ART before the event or censoring time; 86%, 81%, 68%, and 66% of those with any ART experience were receiving an HB-active drug (lamivudine, tenofovir, or emtricitabine) sometime during follow-up; and 71%, 64%, 62%, and 55% of those with any ART experience were receiving an HB-active drug at the time of the event or at the time of censoring. The median year of the event or censoring for the 4 groups was 2006, 2004, 2000 and 1999, respectively.
The results from the sensitivity analyses are similar to those from model 3, although the confidence intervals for models 5 and 6 are wider owing to the smaller number of events considered (Figure 2). With the sensitivity analysis models 4 and 6, those with chronic HB had a consistent and significantly increased risk of an AIDS or death event compared with those with no HB (HR, 1.81 [95% CI, 1.20–2.73] for model 4; and HR, 2.16 [95% CI, 1.26–3.70] for model 6). In Model 5, which censored everyone by 1 January 1996, the results were consistent but not significant (HR, 1.57; 95% CI, .85–2.90). Finally, in an additional model (not shown) similar to model 3 but also including baseline categories for HIV RNA levels (missing, <1000 copies/mL, and ≥1000 copies/mL), the results were consistent with all other models (HR, 1.17 [95% CI, .94–1.46] for resolved HB; HR, 1.12 [95% CI, .73–1.71] for isolated HBcAb; HR, 1.78 [95% CI, 1.19–2.66) for chronic HB).
Figure 2.
Risk of AIDS or death events by HB infection status at HIV seroconversion.
The proportional hazards models considered only hepatitis B status at baseline. Of note, 23 (1.3%) of the 1732 who were HB negative at baseline subsequently developed chronic HB, and 9 of those experienced an AIDS or death event after chronic HB. Similarly, 104 (6.0%) of those who were HB negative at baseline subsequently were determined to be HB positive (isolated HBcAb or resolved HB), and 18 of them experienced an AIDS or death event.
DISCUSSION
We found that HBV coinfection has a significant impact on HIV outcomes. When compared with individuals who were HB negative, those with chronic HB at the time of HIV diagnosis had a significantly higher risk of an AIDS or death event. Those with resolved HB and isolated HBcAb had an increased but nonsignificant risk, suggesting the possibility that HBV exposure is a surrogate of poorer outcome or, alternatively, that HB, even resolved HB or isolated HBcAb, may have a harmful effect on HIV disease. The increased risk of an AIDS or death event for those with chronic HB remained consistent across all time-to-event models considered, including an unadjusted model; MV models for the entire follow-up period adjusting for demographics, baseline, and time-updated CD4 cell count metrics, and time-updated indicators for the use of HAART and non-HAART ART; and a series of sensitivity analyses to account for possible confounding due to year of HIV diagnosis and the relative availability of HAART. We also found an increased risk of AIDS or death in the HAART era (model 6), despite the fact that the majority of participants received an HB-active drug as part of their regimen. Findings from the SMART study demonstrated that uncontrolled HBV replication resulting from discontinuation of HAART with HB-active agents was significantly associated with a faster CD4 decline, also suggesting a mechanism by which HB may contribute to HIV disease progression [19]. Our findings underscore the need to prevent HB in those with HIV and also in cohorts of HIV-negative individuals with risk factors for HIV acquisition.
Other factors associated with HIV outcome in our study, such as lower CD4 cell count (baseline and time-updated nadir) and no receipt of HAART, are well-established determinants of HIV disease progression and risk of death [20]. Although not the primary emphasis of our study, we found that individuals with HCV antibody at the time of HIV diagnosis and those with no HCV antibody results available had a significantly increased risk of AIDS or death, supporting similar findings in other studies [21–23, 24]. Controversy still surrounds the discussion of whether HCV coinfection affects HIV outcomes. Studies have reported HCV infection to be associated with poor HIV or immunologic outcomes [21–23], although a longitudinal study and meta-analysis of HCV coinfection in the pre-HAART era did not find an increased mortality [25, 26], and other studies in the HAART era have found no impact on immunologic and virologic outcomes following HAART initiation [4, 27]. In contrast to our study population, most prior studies evaluating HCV coinfection and risk of HIV-related disease progression were primarily in injection drug users [28, 29]. Although IDU is rare in our cohort and HCV RNA was not available to confirm that individuals were chronic HCV carriers, our findings are consistent with others that emphasize the importance of knowing an individual’s HCV status at the time of HIV infection [30] and the need for effective HCV prevention and treatment for HIV-infected adults.
HIV coinfection is known to influence the natural history and course of HB by impairing the quantity and quality of the innate and adaptive immune response to HB [31]. It is also known that highly productive and replicative infections such as those caused by HBV, HCV, and HIV induce impaired virus-specific adaptive immune responses [32]. Similar to HIV-1–induced impairment of HBV-specific CD4 and CD8 T-cell responses [8, 33, 34], HBV may lead to immunologic impairments that negatively influence the course or control of HIV disease. For example, Gomez-Gonzalo has shown that HBV X protein superinduces ongoing HIV-1 replication and HIV-1 long terminal repeat transcription by synergizing with Tat protein and with T-cell activation signals that may contribute to a faster progression to AIDS in HIV/HBV-coinfected individuals [35]. Alternatively, rather than HBV directly affecting HIV pathogenesis, developing chronic HB may be a marker for increased risk of HIV-related disease progression. Previous studies have shown that genetic factors associated with HIV-related disease progression are also associated with HB outcome. Specifically, alterations in chemokine receptor 5 are associated with progression to AIDS as well as the risk of developing chronic HB [36–39]. Additionally, regulatory T-cell function has been shown to be associated with ineffective immune responses to both HBV and HIV, suggesting similar functional immune impairments may play a role in both infections [40–43]. From these observations, it is clear that further research is needed to elucidate the potential mechanisms and bidirectional interactions of HBV and HIV.
The NHS cohort provides a unique opportunity to understand the impact of HBV coinfection on HIV outcomes in a relatively homogeneous group of HIV seroconverters. The low use of injection drugs in this cohort, as well as open access to medical care, vaccinations, and medications in the military healthcare system, also help reduce potential confounding from such factors [17]. However, these same features may limit external generalizability. In addition, given the limited number of women in our cohort, the study findings may not be generalizable to women. Therefore, in our cohort, and probably for other HIV-infected persons with similar characteristics, chronic HB seems to be associated with an increased risk of HIV-related disease progression. However, whether such associations are overwhelmed by other factors such as IDU in other populations remains to be determined. There are other limitations to our results. As with any cohort study, we were unable to determine whether HB directly caused an increased risk of AIDS or death or served as a marker of other factors that we were unable to assess. Furthermore, the differential distribution of participant study enrollment and the number of clinical events in the pre-HAART and HAART eras may have confounded results. However, to account for this we performed several sensitivity analyses that were all remarkably consistent. We used baseline HB status to group participants and did not allow for changing HB status during follow-up. However, our use of baseline HB status probably underestimated the true risk of AIDS or death for those with chronic HB, because, of those who were classified as HB negative at baseline, 127 subsequently developed HB (23 chronic and 104 isolated core or resolved), and 27 of those experienced an AIDS or death event after HB. The interpretation of data regarding HCV status were also limited, because confirmatory HCV RNA results were unavailable for the majority of those who were anti-HCV positive. Finally, we were unable to adjust for HIV RNA in the MV models considering all available follow-up time, because the test was not available before 1996 and was thus unavailable for 40% of participants at the time of HIV diagnosis. However, in a model that used categories for baseline HIV RNA (including a category for missing), the HRs for risk by hepatitis group were consistent with the results from all other models.
Our study adds further insight into the understanding of the complex interactions between HB and HIV infection, and demonstrates the associations between HB status at the time of HIV diagnosis and subsequent HIV outcomes, with chronic HB status associated with an increased risk of AIDS events and mortality and a trend of association between resolved HB and isolated HBcAb and HIV outcomes. This association warrants further investigation to evaluate whether HB is a surrogate of poorer outcome or whether it has a direct harmful impact on HIV disease progression. Our findings may have implications for many aspects of HBV coinfection, including early diagnosis and, foremost, prevention of HB. Insights into the pathogenesis and potential immune dysfunction behind the higher risk for AIDS events and death are needed and may help reduce the excess morbidity and mortality associated with HBV coinfection in those with HIV infection.
Notes
Acknowledgments.
The authors would like to thank Eric Seaberg for his valuable early methods discussions. We would also like to express our gratitude to the current members of Infectious Disease Clinical Research Program HIV Working Group and the long line of military HIV researchers who have supported the HIV NHS, and to the research coordinators and support staff for their countless hours of work. Most importantly, we would like to thank the patients for their participation, without which this research would not have been possible.
Financial support.
This work was supported by the Infectious Disease Clinical Research Program (IDCRP; www.idcrp.org), a Department of Defense program executed through Uniformed Services University of the Health Sciences. This project has been funded in whole, or in part, with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH), under Inter-Agency Agreement Y1-AI-5072. The IDCRP reviewed the study design, collected the data and provided salary support to investigators (M. L. L., K. H. H., M. P. R., N. F. C., A. C. W., A. G., and B. K. A.). The analyses, conclusions, and decision to submit the manuscript are the independent work and decision of the authors.
The content of this publication is the sole responsibility of the authors and does not necessarily reflect the views or policies of the NIH or the Department of Health and Human Services, the Department of Defense, or the Departments of the Army, Navy, or Air Force. Mention of trade names, commercial products, or organizations does not imply endorsement by the US government.
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Appendix
Infectious Disease Clinical Research Program (IDCRP) HIV Working Group Collaborators
H. Chun, Department of Defense HIV/AIDS Prevention Program, Naval Health Research Center, San Diego, CA; B. Agan, W. Bradley, M. Kortepeter, and G. Macalino, Uniformed Services University of the Health Sciences, Bethesda, MD; C. Decker, A. Ganesan, T. Warkentien, and T. Whitman, National Naval Medical Center, Bethesda; S. Fraser, J. Hartzell, R. Ressner, P. Waterman, A. Weintrob, G. Wortmann, and M. Zapor, Walter Reed Army Medical Center, Washington, DC; G. Hsue and A. Johnson, Tripler Army Medical Center, Honolulu, HI; S. Banks, T. Lalani, and J. Maguire, Naval Medical Center, Portsmouth, VA; L. Eberly, A. Lifson, K. Hullsiek, and M. Roediger, University of Minnesota, Minneapolis; C. Eggleston, L. Jagodzinski, R. O'Connell, and S. Peel, Walter Reed Army Institute of Research, Silver Spring, MD; M. Landrum, J. Okulicz, and S. Merritt, San Antonio Military Medical Center, Fort Sam Houston, TX; M. Polis, J. Powers, and E. Tramont, National Institute of Allergy and Infectious Diseases, Bethesda; M. Bavaro, N. Crum-Cianflone, and M. Linfesty, Naval Medical Center, San Diego.
References
- 1.Gilson RJC, Hawkins AE, Beecham MR, et al. Interactions between HIV and hepatitis B virus in homosexual men: effects on the natural history of infection. AIDS. 1997;11:597–606. doi: 10.1097/00002030-199705000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Kellerman SE, Hanson DL, McNaghten AD, Fleming PL. Prevalence of chronic hepatitis B and incidence of acute hepatitis B infection in human immunodeficiency virus-infected subjects. J Infect Dis. 2003;188:571–7. doi: 10.1086/377135. [DOI] [PubMed] [Google Scholar]
- 3.Weber R, Sabin CA, Friis-Moller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166:1632–41. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 4.Mathews G, Bhagani S. The epidemiology and natural history of HIV/HBV and HCV co-infections. J HIV Ther. 2003;8:77–84. [PubMed] [Google Scholar]
- 5.Thio CL, Seaberg EC, Skolasky R, Jr, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS) Lancet. 2002;360:1921–6. doi: 10.1016/s0140-6736(02)11913-1. [DOI] [PubMed] [Google Scholar]
- 6.Thio CL. Hepatitis B and human immunodeficiency virus co-infection. Hepatology. 2009;49(5 Suppl):S138–45. doi: 10.1002/hep.22883. [DOI] [PubMed] [Google Scholar]
- 7.Lascar RM, Gilson RJ, Lopes AR, Bertoletti A, Maini MK. Reconstitution of hepatitis B virus (HBV)-specific T cell responses with treatment of human immunodeficiency virus/HBV co-infection. J Infect Dis. 2003;188:1815–19. doi: 10.1086/379896. [DOI] [PubMed] [Google Scholar]
- 8.Lascar RM, Lopes AR, Gilson RJ, et al. Effect of HIV infection and antiretroviral therapy on hepatitis B virus (HBV)-specific T cell responses in patients who have resolved infection. J Infect Dis. 2005;191:1169–79. doi: 10.1086/428502. [DOI] [PubMed] [Google Scholar]
- 9.Landrum ML, Fieberg AM, Chun HM, et al. The effect of human immunodeficiency virus on hepatitis B virus serological status in co-infected adults. PLoS One. 2010;5:e8687. doi: 10.1371/journal.pone.0008687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eskild A, Magnus P, Petersen G, et al. Hepatitis B antibodies in HIV-infected homosexual men are associated with more rapid progression to AIDS. AIDS. 1992;6:571–4. doi: 10.1097/00002030-199206000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Scharschmidt BF, Held MJ, Hollander HH, et al. Hepatitis B in patients with HIV infection: relationship to AIDS and patient survival. Ann Intern Med. 1992;117:837–8. doi: 10.7326/0003-4819-117-10-837. [DOI] [PubMed] [Google Scholar]
- 12.Sinicco A, Raiteri R, Sciandra M, et al. Co-infection and superinfection of hepatitis B virus in patients infected with human immunodeficiency virus: no evidence of faster progression to AIDS. Scand J Infect Dis. 1997;29:111–15. doi: 10.3109/00365549709035869. [DOI] [PubMed] [Google Scholar]
- 13.Solomon RE, Van Raden M, Kaslow RA, et al. Association of hepatitis B surface antigen and core antibody with acquisition and manifestations of human immunodeficiency virus type 1 (HIV-1) infection. Am J Public Health. 1990;80:1475–8. doi: 10.2105/ajph.80.12.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenspan D, Greenspan JS, Overby G, et al. Risk factors for rapid progression from hairy leukoplakia to AIDS: a nested case-control study. J Acquir Immune Defic Syndr. 1991;4:652–8. [PubMed] [Google Scholar]
- 15.Ockenga J, Tillmann HL, Trautwein C, Stoll M, Manns MP, Schmidt RE. Hepatitis B and C in HIV-infected patients. Prevalence and prognostic value. J Hepatol. 1997;27:18–24. doi: 10.1016/s0168-8278(97)80274-7. [DOI] [PubMed] [Google Scholar]
- 16.Chun HM, Fieberg AM, Hullsiek KH, et al. The epidemiology of hepatitis B virus infection in a U.S. cohort of HIV-infected individuals during the last 20 years. Clin Infect Dis. 2010;50:426–36. doi: 10.1086/649885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brodine SK, Starkey MJ, Shaffer RA, et al. Diverse HIV-1 subtypes and clinical, laboratory and behavioral factors in a recently infected US military cohort. AIDS. 2003;17:2521–7. doi: 10.1097/00002030-200311210-00016. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41 http://www.cdc.gov/mmwr/preview/mmwrhtml/00018871.htm. Accessed 4 January 2008. [PubMed] [Google Scholar]
- 19.Dore GJ, Soriano J, Rockstroh J, et al. Frequent hepatitis B virus rebound among HIV-hepatitis B virus-coinfected patients following antiretroviral therapy interruption. AIDS. 2010;24:857–65. doi: 10.1097/QAD.0b013e328334bddb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.SMART Study Group, El-Sadr WM, Grund B, et al. Risk for opportunistic disease and death after reinitiating continuous antiretroviral therapy in patients with HIV previously receiving episodic therapy: a randomized trial. Ann Intern Med. 2008;149:289–99. doi: 10.7326/0003-4819-149-5-200809020-00003. [DOI] [PubMed] [Google Scholar]
- 21.Körner C, Tolksdorf F, Schulte D, et al. Hepatitis C coinfection enhances sensitization of CD4 (+) T-cells towards Fas-induced apoptosis in viraemic and HAART-controlled HIV-1-positive patients. Antivir Ther. 2011;16:1047–55. doi: 10.3851/IMP1882. [DOI] [PubMed] [Google Scholar]
- 22.Kovacs A, Karim R, Mack WJ, et al. Activation of CD8 T cells predicts progression of HIV infection in women coinfected with hepatitis C virus. J Infect Dis. 2010;201:823–34. doi: 10.1086/650997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.d'Arminio MA, Cozzi-Lepri A, Castagna A, et al. Risk of developing specific AIDS-defining illnesses in patients coinfected with HIV and hepatitis C virus with or without liver cirrhosis. Clin Infect Dis. 2009;49:623–5. doi: 10.1086/603557. [DOI] [PubMed] [Google Scholar]
- 24.Potter M, Odueyungbo A, Yang H, et al. Impact of hepatitis C viral replication on CD4+ T-lymphocyte progression in HIV-HCV co-infection before and after antiretroviral therapy. AIDS. 2010;24:1857–65. doi: 10.1097/QAD.0b013e32833adbb5. [DOI] [PubMed] [Google Scholar]
- 25.Dorrucci M, Pezzotti P, Phillips AN, Lepri AC, Rezza G. Co-infection of hepatitis C virus with human immunodeficiency virus and progression to AIDS. Italian Seroconversion Study. J Infect Dis. 1995;172:1503–8. doi: 10.1093/infdis/172.6.1503. [DOI] [PubMed] [Google Scholar]
- 26.Chen TY, Ding EL, Seage GR, Kim AY. Meta-analysis: increased mortality associated with hepatitis C in HIV-infected persons is unrelated to HIV disease progression. Clin Infect Dis. 2009;49:1605–15. doi: 10.1086/644771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fuping G, Wei LV, Yang H, et al. Impact of hepatitis C virus co-infection on HAART in HIV-infected individuals: multicentric observation cohort. J Acquir Immune Defic Syndr. 2010;54:137–42. doi: 10.1097/QAI.0b013e3181cc5964. [DOI] [PubMed] [Google Scholar]
- 28.Sulkowski MS, Thomas DL. Hepatitis C in the HIV-infected person. Ann Intern Med. 2003;138:197–207. doi: 10.7326/0003-4819-138-3-200302040-00012. [DOI] [PubMed] [Google Scholar]
- 29.Rockstroh JK, Mocroft A, Soriano V, et al. Influence of hepatitis C virus infection on HIV-1 disease progression and response to highly active antiretroviral therapy [published online ahead of print 11 August 2005] J Infect Dis. 2005;192:992–1002. doi: 10.1086/432762. [DOI] [PubMed] [Google Scholar]
- 30.The Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) Study Group. Factors associated with specific causes of death amongst HIV-positive individuals in the D:A:D study. AIDS. 2010;24:1537–48. doi: 10.1097/QAD.0b013e32833a0918. [DOI] [PubMed] [Google Scholar]
- 31.Puoti M, Torti C, Bruno R, Filice G, Carosi G. Natural history of chronic hepatitis B in co-infected patients. J Hepatol. 2006;44(1 Suppl):S65–70. doi: 10.1016/j.jhep.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 32.Frebel H, Richter K, Oxenius A. How chronic viral infections impact on antigen-specific T-cell responses. Eur J Immunol. 2010;40:654–63. doi: 10.1002/eji.200940102. [DOI] [PubMed] [Google Scholar]
- 33.Colin JF, Cazals-Hatem D, Loriot MA, et al. Influence of human immunodeficiency virus infection on chronic hepatitis B in homosexual men. Hepatology. 1999;29:1306–10. doi: 10.1002/hep.510290447. [DOI] [PubMed] [Google Scholar]
- 34.Chang JJ, Wightman F, Bartholomeusz A, et al. Reduced hepatitis B virus (HBV)-specific CD4+ T-cell responses in human immunodeficiency virus type 1-HBV-coinfected individuals receiving HBV-active antiretroviral therapy. J Virol. 2005;79:3038–51. doi: 10.1128/JVI.79.5.3038-3051.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gomez-Gonzalo M, Carretero M, Rullas J, et al. The hepatitis B virus X protein induces HIV-1 replication and transcription in synergy with T-cell activation signals. J Biol Chem. 2001;276:35435–43. doi: 10.1074/jbc.M103020200. [DOI] [PubMed] [Google Scholar]
- 36.Thio CL, Thomas DL, Carrington M. Chronic viral hepatitis and the human genome. Hepatology. 2000;31:819027. doi: 10.1053/he.2000.4316. [DOI] [PubMed] [Google Scholar]
- 37.Thio CL, Astemborski J, Bashirova A, et al. Genetic protection against hepatitis B virus conferred by CCR5Delta32: evidence that CCR5 contributes to viral persistence. J Virol. 2007;81:441–5. doi: 10.1128/JVI.01897-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahn SH, Kim do Y, Chang HY, et al. Association of genetic variations in CCR5 and its ligand, RANTES with clearance of hepatitis B virus in Korea. J Med Virol. 2006;78:1564–71. doi: 10.1002/jmv.20739. [DOI] [PubMed] [Google Scholar]
- 39.Carrington M, Dean M, Martin MP, O’Brien SJ. Genetics of HIV-1 infection: chemokine receptor CCR5 polymorphism and its consequences. Hum Mol Genet. 1999;8:1939–45. doi: 10.1093/hmg/8.10.1939. [DOI] [PubMed] [Google Scholar]
- 40.Wang LW, Chen H, Gong ZJ. High mobility group box-1 protein inhibits regulatory T cell immune activity in liver failure patients with chronic hepatitis B. Hepatobiliary Pancreat Dis Int. 2010;9:499–507. [PubMed] [Google Scholar]
- 41.Li Q, Skinner PJ, Ha SJ, et al. Visualizing antigen-specific and infected cells in situ predicts outcomes in early viral infection. Science. 2009;323:1726–9. doi: 10.1126/science.1168676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manigold T, Racanelli V. T-cell regulation by CD4 regulatory T cells during hepatitis B and C virus infections: facts and controversies. Lancet Infect Dis. 2007;7:804–13. doi: 10.1016/S1473-3099(07)70289-X. [DOI] [PubMed] [Google Scholar]
- 43.Stoop JN, van der Molen RG, Baan CC, et al. Regulatory T cells contribute to the impaired immune response in patients with chronic hepatitis B virus infection. Hepatology. 2005;41:771–8. doi: 10.1002/hep.20649. [DOI] [PubMed] [Google Scholar]