Abstract
Background. Activated transfected killer (ATAK) cells are immortal phagocytes transfected with a luminescence reporter that effectively treat lethal infections in neutropenic mice. Their in vivo trafficking, lifespan, and immunogenicity are unknown.
Methods. Mice were made neutropenic; infected or not with Staphylococcus aureus, Acinetobacter baumannii, Candida albicans, or Aspergillus fumigatus; and treated intraperitoneally with ATAK cells. Cell trafficking and lifespan were assessed by in vivo imaging and reverse transcription–polymerase chain reaction.
Results. In uninfected neutropenic mice, ATAK cells spread from the mesentery into visceral organs on days 1–3. Splenic accumulation of ATAK cells increased at day 1 after infection with S. aureus and A. baumannii, and kidney accumulation increased in mice infected with C. albicans. Lung accumulation was seen at day 3 in mice infected by inhalation with A. fumigatus. By day 8, coincident with increasing anti-ATAK antibodies, luminescence signal was lost and there was no detectable mRNA transcription from ATAK cells.
Conclusions. ATAK cells accumulated in target organs with distinct profiles, depending on the microbial etiology of infection. Finally, generation of an anti-ATAK immune response may provide an important safety mechanism that helps clear the cells from the host as the marrow recovers.
Duration of neutropenia is one predictor of survival for neutropenic patients with bacterial and fungal infections [1–3]. Therefore, exogenous replacement of phagocytes is a promising means to treat such infections by shortening the duration of neutropenia [4]. Unfortunately, although neutrophil transfusions have shown promising results [5, 6], daunting technical difficulties have prevented their general availability. For example, harvesting sufficient neutrophils to mediate a protective effect (≥1 × 1011 neutrophils/day in infected patients) [7–9] is difficult to achieve [4, 10]. In addition, ex vivo neutrophils undergo rapid apoptosis and very quickly lose their ability to chemotax to and kill microorganisms [10]. This loss of microbicidal activity is particularly severe for killing fungal pathogens, such as Candida, compared with smaller bacterial organisms [11]. Therefore, despite promising results of neutrophil transfusion at major transplant centers [12], they remain experimental after >50 years of study.
Activated transfected killer (ATAK) cells have been developed as a technology to enhance feasibility and general availability of neutrophil transfusions [13–15]. ATAK cells are based on activation and differentiation of HL-60 myeloid cell line cells toward mature neutrophils. When differentiated, the cells kill microbes at 25%–50% of the efficiency as freshly harvested neutrophils [13, 14]. ATAK cells have been transfected with an inducible suicide trap to enable their in vivo purging when the host immune system recovers from chemotherapy, thereby preventing engraftment of the allogeneic cells. The cells have also been transfected with a luminescence reporter system to enable real-time monitoring of the cell fate in vivo in treated hosts. ATAK cells have been previously shown to improve the survival of infected, neutropenic mice and to result in enhanced clearance of microbes from tissues in infected neutropenic mice [13–15].
The presence of the luminescence reporter and unique, cell-specific genetic signatures enabled precise monitoring of ATAK cell location and duration of viability in vivo. The current study was conducted to define the extent of tissue accumulation of these professional phagocytes in mice infected intravenously with a yeast (Candida albicans) or archetypal gram-positive (Staphylococcus aureus) or gram-negative (Acinetobacter baumannii) bacterial pathogens, or through inhalation with a mold (Aspergillus fumigatus), and to define the immunogenicity and lifespan of ATAK cells in vivo after treatment.
MATERIALS AND METHODS
Cells and Culture
HL-60 cells (American Type Culture Collection) were cultured and activated as described elsewhere [13, 14]. In brief, cells were activated for 3 days by incubation in the presence of 1.3% (v/v) dimethyl sulfoxide (DMSO) and 2.5 μM retinoic acid (RA). For harvesting, cells were centrifuged at 250g, washed in phosphate-buffered saline (PBS), and resuspended at the appropriate concentration. In some experiments, HL-60 cells were irradiated with ∼2000 ± 300 rads of gamma rays by exposure to cesium chloride (Cs-137; JL Shepherd & Associates irradiator Model 143–45) in the blood bank at Harbor–University of California Los Angeles (UCLA) Medical Center, as described elsewhere [14, 15].
C. albicans SC5314 [13, 16], a well-characterized clinical isolate that is highly virulent in animal models, was serially passaged 3 times in yeast peptone dextrose broth (Difco) and washed twice with PBS. S. aureus LAC (USA300) is a highly virulent, community-acquired, methicillin-resistant strain that is virulent in animal models [17, 18]. A. baumannii HUMC1 is a clinical bloodstream isolate from a patient with bacteremic ventilator-associated pneumonia that is carbapenem resistant. S. aureus was grown overnight in tryptic soy broth and A. baumannii in Lysogeny broth, both at 37°C with shaking. Bacteria were passaged to mid-log growth and rinsed in PBS, and final inocula for infection were prepared in PBS. Inocula of A. fumigatus AF293 (a generous gift of P. Magee) were prepared by growth on Sabouraud dextrose agar plates for 2 weeks at 37°C. Conidia were collected by flooding the plates with sterile PBS containing 0.2% (vol/vol) Tween 80. Infectious inocula were prepared by counting in a hemacytometer.
In Vivo and Ex Vivo Experiments
Male Balb/c mice (18–20 g) were obtained from Jackson Laboratories. Mice were made neutropenic by a single intraperitoneal injection of cyclophosphamide (230 mg/kg), resulting in 7 days of neutropenia, as described elsewhere [13, 14, 19]. For the aspergillosis model, a second dose of cyclophosphamide was administered on day 3 after infection. In addition, for the aspergillosis model, cortisone acetate (250 mg/kg; Sigma-Aldrich) was given by subcutaneous injection with both doses of cyclophosphamide. For the aspergillosis model, mice were treated daily with 5 mg in 0.2 mL ceftazidime subcutaneously to prevent bacterial superinfection. Mice were infected via the tail vein with 0.2 mL PBS containing blastoconidia of C. albicans (104 inoculum), S. aureus (105 inoculum), or A. baumannii (105 inoculum) ∼32 hours after cyclophosphamide injection, as described elsewhere [16]. The inhalational model of aspergillosis was used as described elsewhere [20, 21]. In brief, mice in the aerosolization chamber were exposed for 1 hour to A. fumigatus AF293 conidia aerosolized with a small-particle nebulizer (Hudson Micro Mist; Hudson RCI). Immediately after exposure, 3 mice were euthanized and lungs were homogenized and quantitatively cultured to confirm the infectious inoculum.
After activation of HL-60 cells for 3 days in DMSO/RA, infected neutropenic mice were treated with 1.5 × 107 ATAK cells (∼7.5 × 108 cells/kg) or placebo in 0.25 mL PBS, administered intraperitoneally ∼1 hour after infection. Whole mouse imaging was performed using an In Vitro Imaging System (IVIS; Caliper Life Sciences). For each time point, mice were treated with 3.5 mg/kg coelenterazine in 5% ethanol/PBS administered intraperitoneally. Two hours later, the mice were anesthetized using inhaled isofluorane, and luminescence was measured using the IVIS system. Several mice per group at each time point were euthanized so that organs could be harvested for analysis by IVIS. Because absolute IVIS photon counts vary based on the gain of input signal for each measurement, daily measurements were normalized to control (saline-injected) mice.
All procedures involving mice were approved by the Los Angeles Biomedical Research Institute Animal Care and Use Committee, following the National Institutes of Health guidelines for animal housing and care.
Reverse Transcription–Polymerase Chain Reaction
Total RNA was prepared from mice whole tissue with an RNA isolation kit (Ambion). The RNA was reverse transcribed with oligo(dT) primer using the SuperScript First-Strand Synthesis System (Invitrogen) to generate first-strand cDNA. The products were used in polymerase chain reaction (PCR) to detect the expression of thymidine kinase (TK) and glyceraldehyde-3-phosphate dehydrogenase (G3PDH). The sequences of the PCR primers are as follows: 5′-CATGCCTTATGCCGTGAC-3′ and 5′-TCCAGGATAAAGACGTGC-3′ for 597bp TK and 5′-AGGTCGGTGTGAACGGATTTG-3′ and 5′-CATGTAGGCCATGAGGTCCAC-3′ for mouse 980bp G3PDH. The PCR products were separated on a 1.5% agarose gel containing 0.1 μg/mL ethidium bromide.
Enzyme-Linked Immunosorbent Assay
To generate whole membrane preparations from HL-60 cells, 107 cells/mL were lysed by sonication with a protease inhibitor cocktail (Sigma). The lysate was centrifuged at 35 000g for 30 minutes, and the supernatant containing membrane proteins was collected.
Enzyme-linked immunosorbent assay (ELISA) was conducted by a modification of our previously described methods [18, 22]. In brief, ELISA plates were coated with 25 μg/mL of HL-60 cell membrane proteins, blocked, and exposed to serial dilutions of mouse serum. Negative control wells received an irrelevant isotype control monoclonal antibody. Goat antimouse secondary antibody conjugated with horseradish peroxidase was added, followed by washing and incubation with o-phenylenediamine to generate a colorometric reaction. The reaction was terminated after 20 minutes with sulfuric acid. The ELISA titer was taken as the reciprocal of the last serum dilution with a positive optical density (OD) reading (ie, >OD of negative control samples + [SD × 2]).
Flow Cytometry
ATAK cells were placed on ice and incubated for 1 hour with immune (from ATAK-exposed mice) or control (from untreated mice) serum samples. The cells were rinsed, fixed with Perm Buffer (BD Pharmingen), and stained with fluorescein isothiocyanate goat antimouse immunoglobin G secondary antibody (Sigma-Aldrich). Cells were quantified using flow cytometry, with gating by size and density.
ATAK Cell Viability
ATAK cells were induced after 3 days of activation in DMSO and RA, as described above. Cells were rinsed and transferred into fresh media (lacking DMSO and RA) and with either serum from Balb/c mice rendered neutropenic but not treated with ATAK cells (control) or from Balb/c mice 1–2 months after the mice had been made neutropenic and treated with ATAK cells (immune serum). At 1 and 24 hours, aliquots were taken from the wells and the cells counted with trypan blue. Percent viability was calculated for each well, and the percent viability of each individual well was normalized to the median viability in the control wells.
Statistics
Comparisons were made using Student t test. P values <.05 were considered to be statistically significant.
RESULTS
ATAK Cell Organ Accumulation and Lifespan in Uninfected Mice
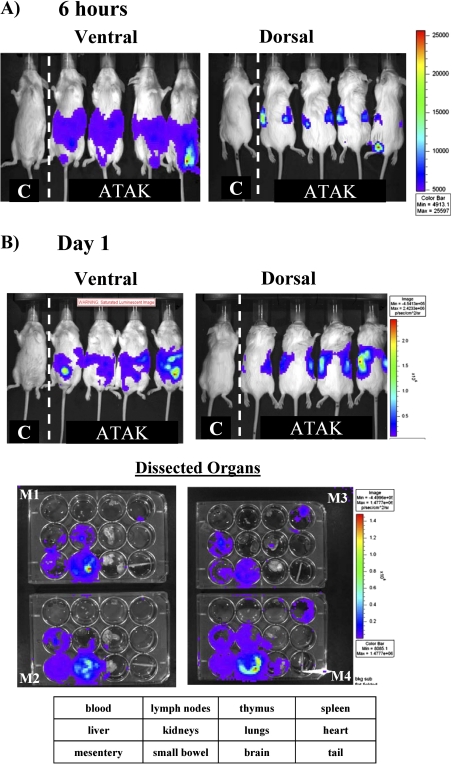
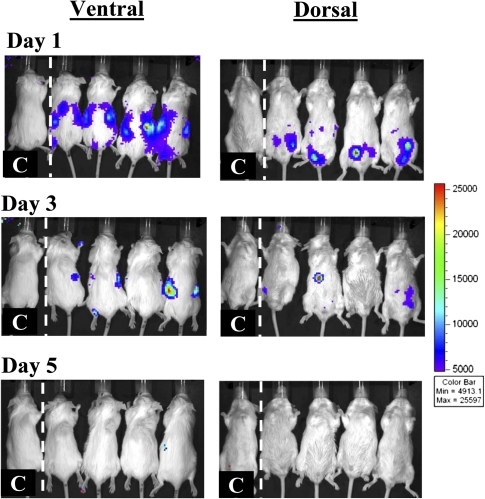
To determine how long ATAK cells survive in vivo in neutropenic mice, ATAK cells transfected with Renilla luciferase [15] were administered intraperitoneally into neutropenic, uninfected mice. Some mice were treated with ATAK cells that had been irradiated to further inhibit replication, and other mice were treated with nonirradiated ATAK cells [14, 15]. Coelenterazine substrate for luciferase was administered intraperitoneally at serial time points, and the mice were imaged by IVIS (Calipers Life Science). At 6 hours after intraperitoneal treatment of uninfected mice, the majority of the cells remained in the peritoneum, but luminescence signal was already found in the retroperitoneal compartment, indicative of trafficking of the cells out of the peritoneum and into kidneys (Figure 1A). By 24 hours after ATAK cell administration (day 1), cells had spread out of the peritoneum and concentrated most heavily in the mesentery around the small bowel (Figure 1B). Livers, kidneys, and spleens were also infiltrated by the ATAK cells, reflective of passage from the lymph drainage of the peritoneum into the systemic circulation. Luminescence was not detected in blood, likely because of the low density of circulating cells per volume of blood.
Figure 1.
In vivo monitoring of luminescent ATAK cell distribution in uninfected, neutropenic mice serially after treatment. Mice were made neutropenic with cyclophosphamide and treated once intraperitoneally with ATAK cells. The same 4 ATAK-treated mice were serially imaged after treatment. Two control untreated mice were also serially imaged, a representative one of which is shown in each picture. At each time point, 4 mice treated with ATAK cells were euthanized, and organs were dissected, plated, and imaged by IVIS. A, Six hours after treatment. B, One day after treatment. C, Three days after treatment. D, Five days after treatment. E, Eight days after treatment.
Abbreviations: ATAK, activated transfected killer.
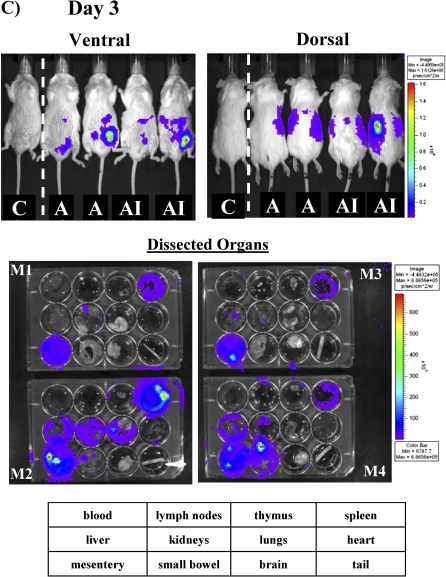
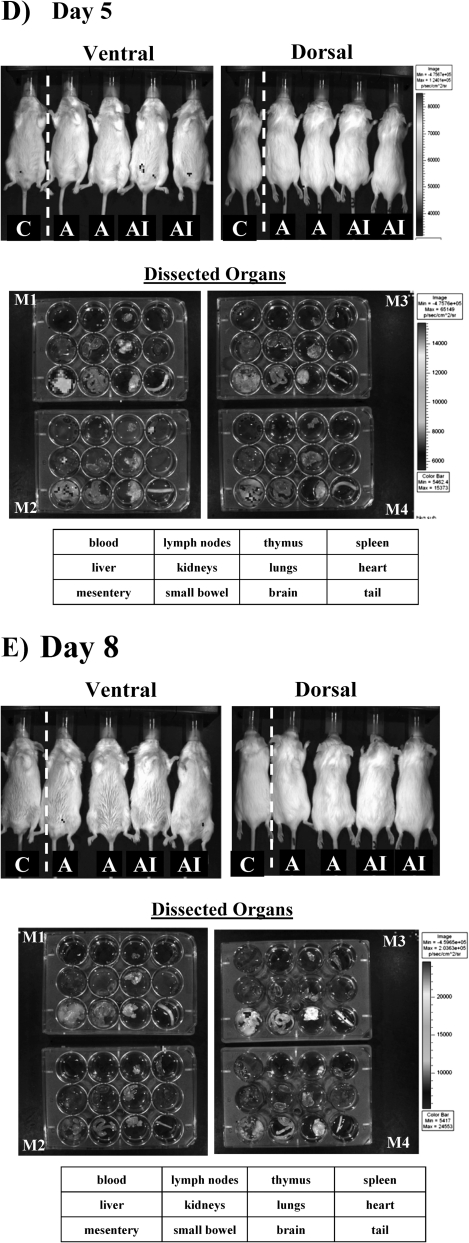
By day 3 after treatment of uninfected mice, cells had infiltrated more significantly into the spleen and also began to spread into the lungs (Figure 1C). The diffuse abdominal signal present at 6 hours and day 1, reflecting a reservoir of cells remaining in the peritoneum, had substantially reduced by day 3, as cells continued to egress into systemic circulation. By day 5, cell signal waned, both in whole mouse imaging and individual organ imaging (Figure 1D). By day 8, the day after neutrophil recovery begins in this model [13–15], there was no detectable signal left in any of the mice or dissected organs (Figure 1E). Of note, the organ trafficking of the cells and their decay rate were not altered by low-dose irradiation of the cells (data not shown). Thus, by luminescence signal, ATAK cells distributed widely into tissues after intraperitoneal administration and signal began to wane dramatically by day 5.
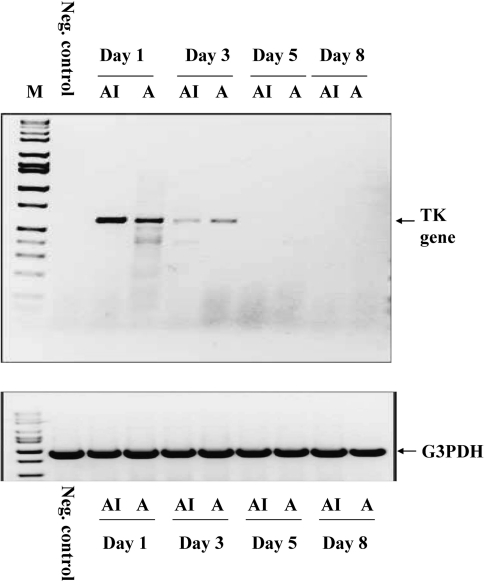
As a second method to confirm the luminescence results, reverse-transcription PCR (RT-PCR) was used to detect mRNA transcript from a sequence uniquely found in ATAK cells and not in the mouse genome. The herpes simplex virus thymidine kinase (HSV TK) sequence contained in the transfected ATAK cells was used. One mouse per time point, treated in parallel with the luminescence experiment, was euthanized and whole body homogenized and had RNA extracted. RT-PCR was conducted in treated mice and in a mouse that had not been treated as a negative control. Strong bands were detected at days 1 and 3 after treatment (Figure 2). However, by day 5, no mRNA was detected, indicating no active transcription of HSV TK. Again, no difference between irradiated and nonirradiated ATAK cells was found with respect to RT-PCR signal. Thus, the RT-PCR results paralleled the luminescence results.
Figure 2.
RT-PCR detection of ATAK cells serially after treatment. Untreated (negative control) or ATAK cell–treated neutropenic mice were euthanized at serial time points after treatment (4 per group). The mice were homogenized in a blender. RT-PCR targeting the Herpes Simplex Virus Thymidine Kinase (HSV TK) sequence was used to detect viable cells. A indicates ATAK; AI, irradiated ATAK.
Abbreviations: RT-PCR, reverse transcription–polymerase chain reaction; ATAK, activated transfected killer.
ATAK Cell Trafficking in Infected Mice
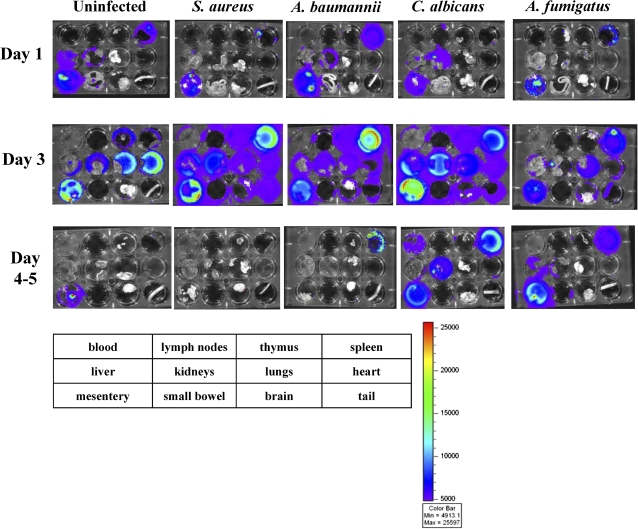
To determine the impact of infection on ATAK cell trafficking, mice were rendered neutropenic and infected with sublethal intravenous inocula of S. aureus, A. baumannii, or C. albicans. In addition, mice were infected via inhalation with A. fumigatus to define ATAK cell trafficking during an infectious model in which the organism was not administered intravenously. Compared with uninfected control mice and mice infected with any other organism, at day 1 after infection, there was enhanced splenic uptake of ATAK cells in mice infected with A. baumannii (Figure 3 and Table 1). In contrast, in mice infected with C. albicans, the primary uptake of ATAK cells was in the kidneys (the primary target organ of C. albicans infection in this model [13, 14, 23, 24]), even as early as day 1 after infection. Mice infected with C. albicans had lower splenic uptake by day 1 after infection but increased splenic uptake by day 3 after infection (Table 1). Mice infected with A. fumigatus had similar ATAK cell trafficking as uninfected mice on day 1 after infection.
Figure 3.
Ex vivo monitoring of luminescent ATAK cells in infected mice serially after treatment. Mice were rendered neutropenic with cyclophosphamide and treated once intraperitoneally with ATAK cells. The mice were infected intravenously with sublethal inocula of S. aureus LAC, A. baumannii HUMC1, or C. albicans SC5314 or through lung inhalation of A. fumigatus AF293. At each time point, organs were harvested from 2 mice per group, plated, and imaged by IVIS. The experiment was repeated once (2–4 mice total per group).
Abbreviation: ATAK, activated transfected killer.
Table 1.
Mean ± SD Relative Quantitative Luminescence (Normalized to Control) in Individual Organs in Mice (2–5 per Group) Treated Without or With ATAK Cells in the Absence or Presence of Infection
| Variable | ATAK | ATAK + S. aureus | ATAK + A. baumannii | ATAK + C. albicans | ATAK + A. fumigatus |
| Spleen | |||||
| Day 1a | 30 ± 0 | 7 ± 3 | 99 ± 0 | 8 ± 0 | 27 ± 18 |
| Day 3a | 11 ± 6 | 77 ± 47 | 36 ± 38 | 92 ± 11 | 29 ± 23 |
| Day 5 | 3 ± 2 | 1 ± 0 | 1 ± 0 | 29 ± 39 | 38 ± 19 |
| Liver | |||||
| Day 1 | 14 ± 9 | 4 ± 0 | 9 ± 0 | 15 ± 0 | 4 ± 2 |
| Day 3 | 9 ± 10 | 14 ± 6 | 8 ± 6 | 17 ± 23 | 8 ± 5 |
| Day 5 | 2 ± 1 | 1 ± 0 | 1 ± 0 | 2 ± 0 | 5 ± 5 |
| Kidneys | |||||
| Day 1a | 5 ± 3 | 1 ± 0 | 15 ± 0 | 198 ± 0 | 15 ± 11 |
| Day 3a | 8 ± 6 | 29 ± 14 | 8 ± 5 | 47 ± 30 | 9 ± 5 |
| Day 5a | 1 ± 0 | 1 ± 0 | 1 ± 0 | 15 ± 7 | 1 ± 0 |
| Lungs | |||||
| Day 1 | 1 ± 1 | 1 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 |
| Day 3a | 5 ± 10 | 5 ± 1 | 7 ± 3 | 5 ± 3 | 14 ± 6 |
| Day 5 | 1 ± 0 | 1 ± 1 | 1 ± 0 | 1 ± 0 | 1 ± 0 |
| Heart | |||||
| Day 1 | 1 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 |
| Day 3a | 1 ± 1 | 5 ± 2 | 5 ± 2 | 11 ± 9 | 6 ± 3 |
| Day 5 | 1 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 |
Abbreviation: ATAK, activated transfected killer.
P < .05 by analysis of variance.
By day 3 in uninfected control mice, the primary ATAK signal was seen in the heart and mesentery. In contrast, mice infected intravenously with all the organisms had ATAK cell signal in the spleen above the level in uninfected mice. By day 3, kidney signal was highest in mice infected with C. albicans, although S. aureus–infected mice also had high kidney signal. Only mice infected in the lung with A. fumigatus had ATAK signal in the lung above uninfected mouse background. Again, by days 4–5, ATAK signal waned in all organs. However, in mice infected with C. albicans, ATAK signal persisted in the spleen and kidneys through day 5 after infection. Signal was not detectable in any mice by day 8 after infection. Thus, infection with distinct organisms altered accumulation profiles of the ATAK cells in vivo.
Host Immune Response to ATAK Cells
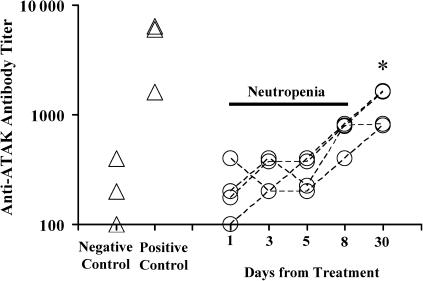
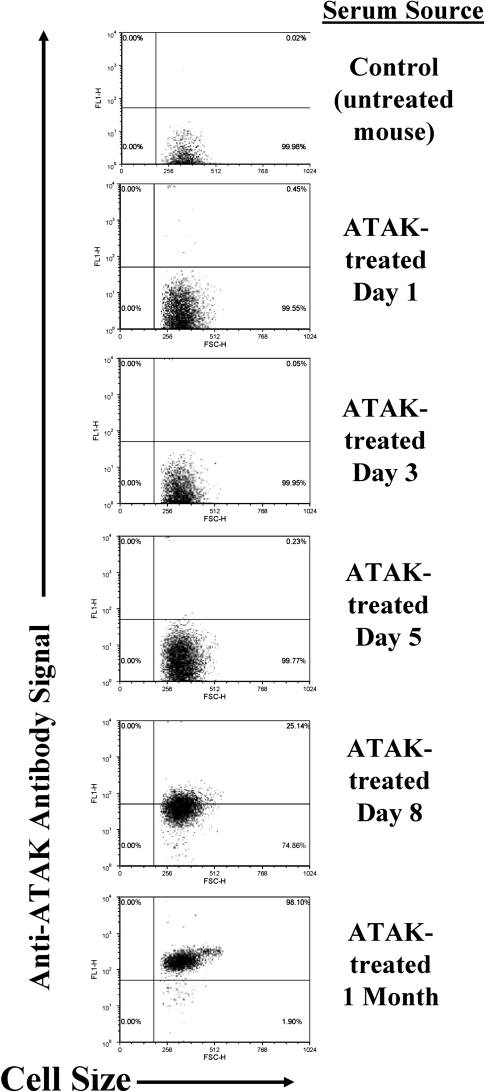
To determine whether neutropenic mice mounted host responses to ATAK cells, both healthy (control) and neutropenic mice had blood samples obtained at baseline and were treated with ATAK cells. Blood samples were serially drawn from individually marked mice after ATAK cell treatment. Nonneutropenic mice experienced a >10-fold increase in anti-ATAK antibodies 2 weeks after treatment (Figure 4). Neutropenic mice experienced no increase in antibody titers during the period of neutropenia. By day 8 after treatment (the day after neutrophil recovery in the model), paired antibody titers began to increase in the ATAK-treated mice, and they were significantly elevated by day 30 after infection (Figure 4). Similarly, after flow cytometry of intact ATAK cells stained with immune versus control serum, anti-ATAK cell antibodies were first detectable on day 8 after infection and had substantially increased in staining intensity by 30 days after infection (Figure 4).
Figure 4.
Neutropenic mice treated with ATAK cells generate anti-ATAK cell antibodies coinciding with the resolution of neutropenia. Anti-ATAK cell membrane antibody titers measured by ELISA from paired serum samples from neutropenic mice serially bled after treatment with ATAK cells (open circles) or from healthy mice before (negative control) and after (positive control) treatment with ATAK cells (open triangles). *P < .05 (vs day 1). Flow cytometry of ATAK cells stained with serum from untreated mice (control) or mice treated with ATAK cells (immune). Representatives of 3 experiments are shown.
Abbreviations: ATAK, activated transfected killer; ELISA, enzyme-linked immunosorbent assay.
To determine the in vivo relevance of these anti-ATAK antibodies, mice were again made neutropenic, treated once with ATAK cells, and then allowed to recover from neutropenia. One month later, the mice were made neutropenic again and treated a second time with ATAK cells. Luminescence signal was clearly detectable outside the peritoneum by day 1 after infection (Figure 5). Although such signal remained detectable in most mice by day 3, the signal was 94% lower in mice after repeat administration of ATAK cells than in mice treated with the first dose of ATAK cells (mean ± SD whole mouse luminescence, 2.6 × 106 ± 4.5 × 106 vs 4.1 × 107 ± 3.8 × 107; P = .03) (Figure 5 and Figure 1B). As previously described, no signal was detectable in mice by day 5 after treatment.
Figure 5.
In vivo monitoring of luminescent signal in uninfected, neutropenic mice treated for a second time with ATAK cells. Mice were made neutropenic with cyclophosphamide and treated once intraperitoneally with ATAK cells. One month after marrow recovery, the mice were rendered neutropenic again and retreated intraperitoneally with ATAK cells. The same 4 mice were serially imaged after treatment. One control mouse was also serially imaged.
Abbreviation: ATAK, activated transfected killer.
To determine whether immune serum samples had direct impact on cell viability, ATAK cells were cultured in the presence of serum samples from mice that had not been previously exposed to ATAK cells (control serum) or in the presence of serum samples obtained 1–2 months after treatment with ATAK cells (immune serum). No effect on cell viability was seen at 1 hour of incubation; however, immune serum reduced viability of ATAK cells by 50% after 24 hours of incubation (Figure 6).
Figure 6.
Immune serum reduced viability of ATAK cells after 24 hours of culture. ATAK cells were cultured in the presence of nonimmune serum (control) or in immune serum. Viability in each well was normalized to the median viability in the control wells. Median and interquartile ranges are shown. *P < .01 (vs all other groups).
Abbreviation: ATAK, activated transfected killer.
DISCUSSION
Administration of luminescence-tagged ATAK cells enabled definition of phagocyte trafficking profiles during infection with 4 distinct pathogens in neutropenic mice. As early as 1 day after infection, substantial recruitment to spleen had already occurred in mice infected with A. baumannii or S. aureus. In contrast, ATAK cells preferentially trafficked to the kidney in mice infected with C. albicans and to lungs in mice infected with A. fumigatus through inhalation. Thus, pathogens with distinct tissue tropisms (eg, A. baumannii and S. aureus vs C. albicans vs A. fumigatus) mediated differential tissue accumulation by the ATAK cells.
The kidneys are the primary target organ of C. albicans in neutropenic mice, and in untreated neutropenic mice, the organism burden in the kidneys increases over the first 3 days after infection [13, 19, 24, 25]. Similarly, the spleen is a primary target organ of S. aureus infection in neutropenic mice [26, 27]. Thus, ATAK cells initially preferentially trafficked to the primary target organs of infecting pathogens. Whether the spread of ATAK cells outside the primary target organs and into other organs during subsequent days reflects clearance of the primary burden of organism from the primary site, replication of organisms in secondary tissues subsequently, or other factors is not yet clear.
The waning of luminescence signal in infected or uninfected mice by days 5–8 was concordant with the loss of active mRNA transcription by the ATAK cells. The disappearance of viable ATAK cells from circulation and from tissue was coincident with marrow recovery and appearance of specific anti-ATAK cell antibodies. Anti-ATAK cell antibodies began to increase by day 4 after infection, had significantly increased by day 8 after infection, and were substantially elevated by 1 month after infection. The peak of the antibody response was blunted in the neutropenic mice compared with healthy mice exposed to ATAK cells. Nevertheless, survival of ATAK cells by day 3 after treatment was substantially reduced after re-exposure of the mice, and the immune serum reduced viability of ATAK cells after 24 hours of culture in vitro. Clearance of the ATAK cells by the host immune system is an additional safety layer to ensure that cells do not permanently engraft, and we have previously found no evidence of engraftment by extensive histopathological evaluation and long-term clinical follow-up [15]. Nevertheless, after re-exposure, ATAK cells persisted and were able to traffic out of the peritoneum by 24 hours after retreatment. Thus, immediate rejection of the ATAK cells did not occur in vitro or in vivo after retreatment.
In the initial set of experiments, more luminescence was seen in the small bowel than in repeat experiments. This luminescence is most likely to the result of residual omentum that remained attached to the small bowel in the initial experiment. Alternatively, because the ATAK cells were administered intraperitoneally, it is possible that microperforation of the small bowel by the administering needle could have seeded the bowel with ATAK cells in the initial but not the follow-up experiment. There was also increased ATAK cell signal in the heart in uninfected mice during the repeat experiment that was not seen during the initial experiment. The reason for this discrepancy is not clear.
In summary, ATAK cells accumulate in infected tissues in response to a diverse group of pathogens, concordant with their functions as a neutrophil surrogate. Luminescent ATAK cells enabled real-time monitoring of phagocyte trafficking to target organs of infection in neutropenic mice. Distinct pathogens induced different organ trafficking profiles for the phagocytes. The potential breadth of responsiveness to other pathogens merits continued investigation to define any possible limitations. After a single treatment, the cells persisted for approximately the duration of neutropenia, before being rejected as the host marrow recovered. Generation of an immune response to the ATAK cells provides an important safety mechanism to help prevent engraftment of the cells in the host. These collective results underscore the need for continued translational development of ATAK technology as a potential future treatment for life-threatening infections in neutropenic hosts.
Notes
Financial support.
This work was supported by National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases (NIAID) (R01 AI081719 and AI072052).
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Malik IA, Moid I, Aziz Z, Khan S, Suleman M. A randomized comparison of fluconazole with amphotericin B as empiric anti-fungal agents in cancer patients with prolonged fever and neutropenia. Am J Med. 1998;105:478–83. doi: 10.1016/s0002-9343(98)00326-x. [DOI] [PubMed] [Google Scholar]
- 2.Nucci M, Pulcheri W, Spector N, et al. Fungal infections in neutropenic patients. A 8-year prospective study. Rev Inst Med Trop Sao Paulo. 1995;37:397–406. doi: 10.1590/s0036-46651995000500004. [DOI] [PubMed] [Google Scholar]
- 3.Lehrnbecher T, Groll AH, Chanock SJ. Treatment of fungal infections in neutropenic children. Curr Opin Ped. 1999;11:47–55. doi: 10.1097/00008480-199902000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Hubel K, Dale DC, Engert A, Liles WC. Current status of granulocyte (neutrophil) transfusion therapy for infectious diseases. J Infect Dis. 2001;183:321–8. doi: 10.1086/317943. [DOI] [PubMed] [Google Scholar]
- 5.Alavi JB, Root RK, Djerassi I, et al. A randomized clinical trial of granulocyte transfusions for infection in acute leukemia. N Eng J Med. 1977;296:706–11. doi: 10.1056/NEJM197703312961302. [DOI] [PubMed] [Google Scholar]
- 6.Herzig RH, Herzig GP, Graw RG, Jr, Bull MI, Ray KK. Successful granulocyte transfusion therapy for gram-negative septicemia. A prospectively randomized controlled study. N Eng J Med. 1977;296:701–5. doi: 10.1056/NEJM197703312961301. [DOI] [PubMed] [Google Scholar]
- 7.Athens JW, Haab OP, Raab SO, et al. Leukokinetic studies. XI. Blood granulocyte kinetics in polycythemia vera, infection, and myelofibrosis. J Clin Invest. 1965;44:778–88. doi: 10.1172/JCI105190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marsh JC, Boggs DR, Cartwright GE, Wintrobe MM. Neutrophil kinetics in acute infection. J Clin Invest. 1967;46:1943–53. doi: 10.1172/JCI105684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dancey JT, Deubelbeiss KA, Harker LA, Finch CA. Neutrophil kinetics in man. J Clin Invest. 1976;58:705–15. doi: 10.1172/JCI108517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huestis DW, Glasser L. The neutrophil in transfusion medicine. Transfusion. 1994;34:630–46. doi: 10.1046/j.1537-2995.1994.34794330020.x. [DOI] [PubMed] [Google Scholar]
- 11.Glasser L. Effect of storage on normal neutrophils collected by discontinuous-flow centrifugation leukapheresis. Blood. 1977;50:1145–50. [PubMed] [Google Scholar]
- 12.Safdar A, Hanna HA, Boktour M, et al. Impact of high-dose granulocyte transfusions in patients with cancer with candidemia: retrospective case-control analysis of 491 episodes of Candida species bloodstream infections. Cancer. 2004;101:2859–65. doi: 10.1002/cncr.20710. [DOI] [PubMed] [Google Scholar]
- 13.Spellberg BJ, Collins M, French SW, Edwards JE, Jr, Fu Y, Ibrahim AS. A phagocytic cell line markedly improves survival of infected neutropenic mice. J Leukoc Biol. 2005;78:338–44. doi: 10.1189/jlb.0205072. [DOI] [PubMed] [Google Scholar]
- 14.Spellberg BJ, Collins M, Avanesian V, et al. Optimization of a myeloid cell transfusion strategy for infected neutropenic hosts. J Leukoc Biol. 2006;81:632–41. doi: 10.1189/jlb.0906549. [DOI] [PubMed] [Google Scholar]
- 15.Lin L, Ibrahim AS, Baquir B, et al. Safety and efficacy of activated transfected killer cells for neutropenic fungal infections. J Infect Dis. 2010;201:1708–17. doi: 10.1086/652496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spellberg BJ, Johnston D, Phan QT, et al. Parenchymal organ, and not splenic, immunity correlates with host survival during disseminated candidiasis. Infect Immun. 2003;71:5756–64. doi: 10.1128/IAI.71.10.5756-5764.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spellberg B, Ibrahim AS, Yeaman M, et al. The anti-fungal rAls3p-N vaccine protects mice against the bacterium Staphylococcus aureus. Infect Immun. 2008;76:4574–80. doi: 10.1128/IAI.00700-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin L, Ibrahim AS, Xu X, et al. Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog. 2009;5:e1000703. doi: 10.1371/journal.ppat.1000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van t Wout JW, Mattie H, van Furth R. Comparison of the efficacies of amphotericin B, fluconazole, and itraconazole against a systemic Candida albicans infection in normal and neutropenic mice. Antimicrob Agents Ch. 1989;33:147–51. doi: 10.1128/aac.33.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheppard DC, Rieg G, Chiang LY, Filler SG, Edwards JE, Jr, Ibrahim AS. Novel inhalational murine model of invasive pulmonary aspergillosis. Antimicrob Agents Ch. 2004;48:1908–11. doi: 10.1128/AAC.48.5.1908-1911.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rieg G, Spellberg B, Schwartz J, et al. Antifungal prophylaxis is effective against murine invasive pulmonary aspergillosis. Antimicrob Agents Ch. 2006;50:2895–6. doi: 10.1128/AAC.00299-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin L, Ibrahim AS, Baquir B, Avanesian V, Fu Y, Spellberg B. Immunological surrogate marker of rAls3p-N vaccine-induced protection against Staphylococcus aureus. FEMS Immunol Med Mic. 2009;55:293–5. doi: 10.1111/j.1574-695X.2008.00531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spellberg B, Ibrahim AS, Edwards JE, Jr, Filler SG. Mice with disseminated candidiasis die of progressive sepsis. J Infect Dis. 2005;192:336–43. doi: 10.1086/430952. [DOI] [PubMed] [Google Scholar]
- 24.Spellberg BJ, Ibrahim AS, Avenissian V, et al. The anti–Candida albicans vaccine composed of the recombinant N terminus of Als1p reduces fungal burden and improves survival in both immunocompetent and immunocompromised mice. Infect Immun. 2005;73:6191–3. doi: 10.1128/IAI.73.9.6191-6193.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Deventer AJ, Goessens WH, van Belkum A, van Etten EW, van Vliet HJ, Verbrugh HA. PCR monitoring of response to liposomal amphotericin B treatment of systemic candidiasis in neutropenic mice. J Clin Microbiol. 1996;34:25–8. doi: 10.1128/jcm.34.1.25-28.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hegde SS, Difuntorum S, Skinner R, Trumbull J, Krause KM. Efficacy of telavancin against glycopeptide-intermediate Staphylococcus aureus in the neutropenic mouse bacteraemia model. J Antimicrob Chemoth. 2009;63:763–6. doi: 10.1093/jac/dkp001. [DOI] [PubMed] [Google Scholar]
- 27.Hegde SS, Skinner R, Lewis SR, Krause KM, Blais J, Benton BM. Activity of telavancin against heterogeneous vancomycin-intermediate Staphylococcus aureus (hVISA) in vitro and in an in vivo mouse model of bacteraemia. J Antimicrob Chemoth. 2010;65:725–8. doi: 10.1093/jac/dkq028. [DOI] [PubMed] [Google Scholar]