Abstract
Background. The penetration of T cells and trypanosomes into the brain parenchyma is a major pathogenetic event in African trypanosomiasis.
Methods. The role of innate immune responses in the penetration of T cells and Trypanosoma brucei brucei into the brain was studied in knockout mice by using double immunofluorescent staining and real-time polymerase chain reaction.
Results. We demonstrate that Toll-like receptor (TLR)–MyD88–mediated signaling is required for T-cell and parasite penetration into the brain and microglial activation, besides controlling parasitemia and antigen-specific T-cell activation. Among different TLR-deficient mice studied, TLR9 mediated parasitemia control and T-cell penetration into the brain. TLR-MyD88 signals increased levels of interferon (IFN) β and tumor necrosis factor (TNF) α transcripts in the brains of infected mice and both TNF-α and IFN-α/β, receptors promoted T-cell and trypanosoma infiltration into the brain parenchyma. Both resident and infiltrating inflammatory cells in the brain controlled parasite densities in a TLR2- and TLR9-MyD88–mediated manner. However, neither IFN-α/β nor TNF-α contributed to parasite control in the brain.
Conclusions. Our data indicate that innate immune TLR signals stimulate the expression of TNF-α and IFN-α/β that initiate brain invasion of T cells and trypanosomes, and control T. brucei brucei load in the brain by molecules distinct from these.
Infections with subspecies of the extracellular parasite Trypanosoma brucei cause African trypanosomiasis, a disease that affects both humans (sleeping sickness) and animals. During the early stage of human African trypanosomiasis the parasites invade the hemolymphatic system, and during the late meningoencephalitic stage severe signs of nervous system involvement are observed [1–3]. In a mouse model of the disease, T. brucei brucei penetrate the blood-brain barrier (BBB) at a late stage and can enter the brain parenchyma [4]. Within the CNS, activation of white blood cell infiltrates and resident cells probably leads to the nervous system disease [5].
The innate immune system has evolved several strategies of self-nonself discrimination that are based on the recognition of molecular patterns demarcating infectious nonself. Different Toll-like receptors (TLRs), by recognizing diverse pathogen-associated molecular patterns of microbes, activate innate immunity and may initiate the subsequent development of adaptive immunity. TLR agonists stimulate the secretion of proinflammatory cytokines and type I interferons (IFN-α/β) that are involved not only in protection against infections but also in infection-mediated pathology. The development of adaptive immune responses is at least in part mediated through the ability of the innate receptor signaling to activate and stimulate the migration of antigen-presenting cells into the lymph nodes [6]. The binding of TLRs (except TLR3) by their corresponding ligands results in the recruitment of the intracellular adaptor molecule MyD88. Myd88–/– animals are highly susceptible to infection with a wide variety of different pathogens, including infection with T. brucei brucei [7–9].
We have shown elsewhere that the T-cell–derived cytokine IFN-γ, as well as the IFN-inducible chemokine CXCL10, promote the penetration of T cells and parasites in the brain [4, 10], suggesting also that parasites follow T cells during their brain invasion across the BBB. Despite the accepted view that signaling from specific innate immune receptors are required to activate and determine the quality of T-cell responses, the role of innate immunity in T-cell–mediated central nervous system (CNS) diseases, such as sleeping sickness, is poorly understood.
In the current study, we investigated whether signals emanating from TLR control the accumulation of T cells and parasites in the brain parenchyma. We demonstrate that TLR signaling regulates the penetration of T cells and parasites across the BBB and controls survival of the latter in the brain parenchyma through distinct mechanisms.
MATERIALS AND METHODS
Mice, Parasites, and Infection
Mice deficient in MyD88, both interleukin (IL) 1 receptor (R) and IL-18R, TLR2, TLR4, TLR9, IFN-α/βR, interferon regulatory factor-3 (IRF3), and tumor necrosis factor (TNF) receptor 1 (R1) were generated by homologous recombination in embryonic stem cells (see supplemental information). Tlr2−/−/Tlr9−/− were generated by cross-breeding of Tlr2−/− and Tlr9−/− mice. All strains were back-crossed on a C57BL/6 background, and C57BL/6 mice were used as wild-type (WT) controls. All experiments were authorized by the Stockholm animal research ethical committee. Mice (8–12 weeks old) were infected by intraperitoneal injection with 2 × 103 parasites of a pleomorphic stabilate of T. brucei brucei, AnTat 1.1E (obtained from ITG).
Real-Time Polymerase Chain Reaction
Gene transcripts were quantified in brains from uninfected and infected WT and knockout mice by real-time polymerase chain reaction, as described elsewhere [4]. The primer sequences are listed in Table S1.
Immunohistochemical Techniques
To examine passage of trypanosomes across the BBB, sections of nonperfused, fresh-frozen brains at a level of the lateral ventricles containing the choroid plexus and the septal nuclei were cut, mounted, fixed, and immunostained with anti-AnTat 1.1 VSG (ITG), anti-CD4, or anti-CD8 to determine parasites or T-cell presence together with antiglucose transporter 1 labeling brain endothelial cells, as described elsewhere [11]. Brain sections were also stained with anti–Iba-1 (Wako Pure Chemical Industries) up-regulated in activated microglia, with anti–β-amyloid precursor protein [12] to find signs of neurodegeneration or with anti-CD54 (Intercellular adhesion molecule 1 [ICAM-1]) antibodies (KAT1; eBiosciences).
Measurement of IFN-γ in Mouse Serum
The levels of IFN-γ were determined in serum samples from uninfected and infected WT and Myd88−/− mice. The concentrations of IFN-γ were measured using a solid phase sandwich enzyme-linked immunosorbent assay (OptiEIA; BD-Pharmingen), with a detection limit of 5 pg/mL.
T-Cell Restimulation Assay
Mouse bone marrow–derived dendritic cells (BMDCs) were differentiated using granulocyte-macrophage colony-stimulating factor, as described elsewhere [13]. Magnetic bead-selected CD90+ T spleen cells (106 cells/ mL) from T. brucei brucei–infected WT, Myd88−/−, or Tlr2/9−/− mice were cocultured with lysates of T. brucei brucei and WT BMDCs (5 × 105 cells/ mL) for 72 hours [14]. IFN-γ levels in culture supernatant were determined by enzyme-linked immunosorbent assay (BD Pharmingen). Controls included cultures lacking dendritic cells, T cells, or antigen for each genotype used.
Bone Marrow Radiation Chimeric Mice
Bone marrow (BM) cells from Myd88−/− and WT mice were harvested by flushing with cold phosphate-buffered saline through the BM cavities, and red blood cells were lysed. To create BM chimeras, WT and Myd88−/− mice were irradiated with 900 cGy and 4 hours later inoculated intravenously with 107 BM cells from WT or Myd88−/− mice. Six weeks after reconstitution, mice were infected intraperitoneally with 2 × 103 T. brucei brucei. As a control for the efficacy of reconstitution, irradiated Myd88−/− and WT mice were inoculated with 107 BM cells from gfp-tagged WT mice, and the number of CD3+ and CD19+/GFP+ splenocytes was analyzed 6 weeks after inoculation. Two months after reconstitution, the BM marker GFP was detectable in almost all blood cells (data not shown).
RESULTS
Role of MyD88 in T-Cell and Parasite Accumulation in the Brain of T. brucei brucei–Infected Mice
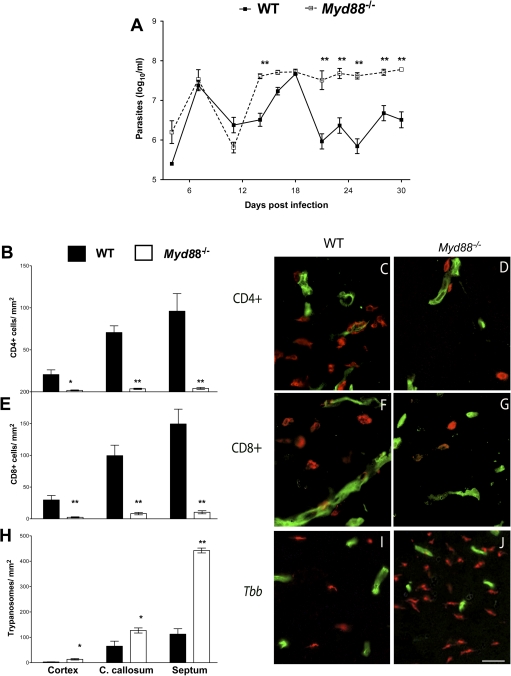
The role of MyD88-mediated responses in the outcome of the encephalitic phase of the murine infection with T. brucei brucei was first studied. Myd88−/− mice showed higher levels of parasitemia during infection (Figure 1A) and died earlier than WT mice (data not shown). We then measured the densities of T cells and T. brucei brucei in the brains of infected WT and Myd88–/– mice. A reduced density of CD4+ and CD8+ T cells in the cerebral cortex, corpus callosum, and septal nuclei of Myd88−/− mice at 30 days after infection, compared with WT controls, was observed by double immunolabeling of T cells and cerebral endothelial cells (Figure 1B–G). On the other hand, the density of trypanosomes in the brain was significantly increased in Myd88−/− mice compared with infected WT mice (Figure 1H–J).
Figure 1.
Parasitemia, CD4+ and CD8+ T cells, and trypanosome accumulation in the brain parenchyma of infected wild-type (WT) and Myd88−/− mice. A, Levels of parasitemia. Each point represents the mean log10 parasites per milliliter ± standard error of measurement (SEM) obtained from 9 or 10 animals per group. **P < .01 (analysis of variance; significant differences compared with infected WT animals). B, E, H, Mean numbers (± SEM) of CD4+ (B), CD8+ T cells (E), and Trypanosoma brucei brucei (H) per mm2 in the cortex, corpus callosum (C. callosum), and septal nuclei of mice at 30 days post infection (4 animals per group). *P < .05, **P < .01 (unpaired t test; significant differences compared with WT mice at same postinfection time point). C–D, F–G, I–J, Immunofluorescence images from the corpus callosum of mice at 30 days post infection (red, CD4+ [C, D] and CD8+ [F, G] cells and T. brucei brucei [I, J]; green, cerebral endothelial cells). In WT mice, CD4+ (C) and CD8+ (F) T cells, and parasites (I) are observed in the parenchyma. Note decreased CD4+ and CD8+ T-cell accumulation and increased trypanosome density in the brain parenchyma of Myd88−/− compared with WT mice (D, G, J) (scale bar, 50 μm).
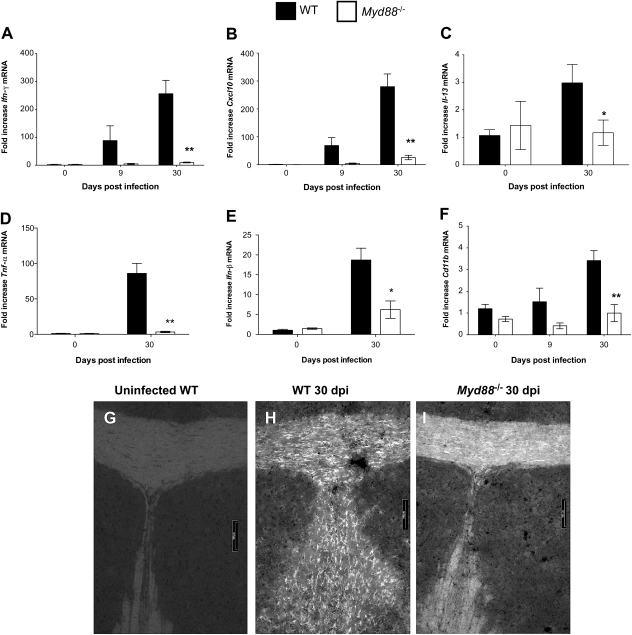
Infection of WT mice with T. brucei brucei increased the cerebral levels of Ifn-γ and Cxcl10 messenger RNA (mRNA) (Figure 2A and 2B). Levels of these transcripts were strikingly lower in brains from infected Myd88−/− mice than in infected WT mice (Figure 2A and 2B). Transcripts of IL-13, secreted primarily during Th2 responses [15], the proinflammatory Tnf-α (Figure 2C), and Ifn-α/β were found to be increased in brains of T. brucei brucei–infected WT mice, whereas they were reduced in brains of infected Myd88−/− mice, compared with infected WT mice (Figure 2C–E).
Figure 2.
Levels of Ifn-γ, Cxcl-10, Il-13, Tnf-α, Ifn-β, and Cd11b messenger RNA (mRNA) and microglia activation in Trypanosoma brucei brucei–infected wild-type (WT) and Myd88−/− mice. A–F, Relative expression of Ifn-γ (A), Cxcl-10 (B), Il-13 (C), Tnf-α (D), Ifn-β (E), and Cd11b (F) mRNA in the brains of uninfected or infected WT and Myd88−/− mice. All values are normalized with respect to Hprt mRNA levels. Each bar represents mean ± standard error of measurement (SEM) of the values obtained from 4 animals. *P < .05; **P < .01 (unpaired t test; significant differences between WT and Myd88−/− mice killed at same postinfection time point). G–I, Activated microglia (as detected by Iba-1 immunostaining) in the corpus callosum of WT (G, H) or Myd88−/− (I) mice before infection (G) or 30 days after infection (H, I). Robust staining of microglia is observed in the corpus callosum of infected WT mice (H) (scale bar, 100 μm).
CD11b, a subunit of Mac 1 or the complement receptor 3 (CR3) found in monocytes, macrophages, and neutrophils, is increased in microglia during activation [16]. We observed increased levels of Cd11b mRNA in brains of infected WT mice but not in Myd88−/− mice (Figure 2F). Increased activation of microglia, as visualized by Iba-1 immunolabeling, was also observed in the corpus callosum and the septal nuclei of infected WT mice compared with Myd88−/− mice (Figure 2G–I and data not shown). No signs of neurodegeneration were observed in either WT or Myd88−/− infected mice using immunostaining of the β-amyloid precursor protein (data not shown).
Next, we asked whether the low T-cell numbers in the brains of infected Myd88−/− mice were related to changes in numbers or activation of peripheral T cells. WT and Myd88−/− mice infected with T. brucei brucei showed splenomegaly and increased numbers of splenocytes, with preferential increase of non-T cells (data not shown). Increased levels of CXC3R, the receptor of CXCL10, were observed in CD4+ and CD8+ spleen cells from infected WT and Myd88−/− mice, although the expression of CXCR3 was slightly lower in CD4+, but not in CD8+, T cells from infected Myd88−/− mice, compared with WT mice (data not shown). Thus, the decreased number of T cells in the brains of Myd88−/− mice is not due to reduced T-cell numbers or activation in immune organs.
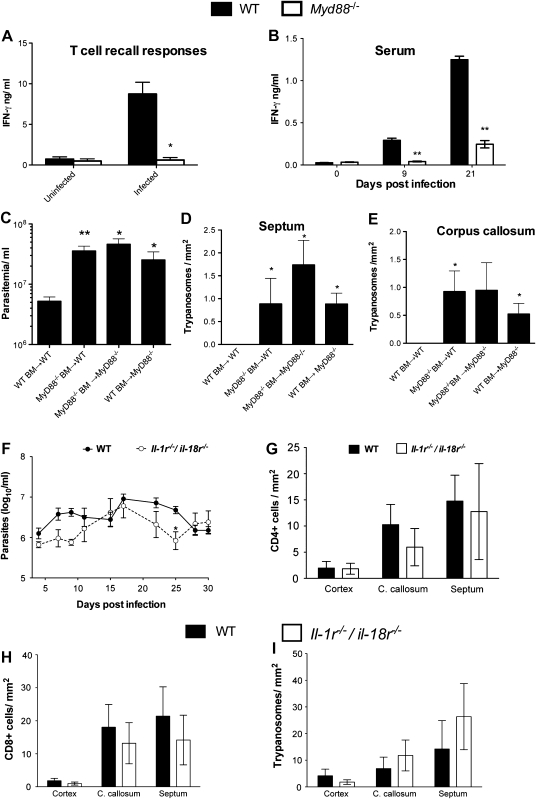
To determine whether MyD88 is required for specific T-cell priming during infection with T. brucei brucei, we measured the recall IFN-γ responses of isolated splenic T cells from T. brucei brucei–infected WT mice or Myd88−/− mice. T cells were coincubated with BMDCs from WT animals with or without the presence of parasite lysates. T cells from infected Myd88–/–animals showed reduced T-cell responses to trypanosomal antigens, indicating that MyD88 is required for priming adaptive immune responses during infection (Figure 3A). Because T cells are a major source of IFN-γ, we then investigated whether there were differences in serum levels of IFN-γ between infected WT and myd88−/− mice. Serum samples from T. brucei brucei–infected MyD88−/− mice showed lower IFN-γ titers than those from WT controls (Figure 3B).
Figure 3.
MyD88 regulates the recall T-cell responses in the periphery, serum levels of interferon (IFN) γ, and parasitotoxic responses in the brain parenchyma. A, T-cell recall responses. Purified splenic T cells from wild-type (WT) or Myd88−/− mice infected with Trypanosoma brucei for 25 days or not infected were cocultured with syngeneic bone marrow (BM)–derived dendritic cells in the presence of T. brucei brucei lysates. IFN-γ was assayed by enzyme-linked immunosorbent assay in culture supernatants 72 hours after incubation. The means ± standard error of measurement (SEM) from triplicate cultures per condition are depicted. The experiments were performed twice with similar results. *P < .05 (unpaired t test; significant differences between WT and Myd88−/− cells). B, Serum IFN-γ levels after T. brucei brucei infection. Each bar represents mean ± SEM of values obtained from 3 or 4 animals per group and time point. **P < .01 (unpaired t test; significant differences compared with uninfected animals). C–E, Parasitemia (C) and trypanosome density in the septum (D) and corpus callosum (E) of radiation chimeric mice as measured 15 days post infection with T. brucei brucei. Mean parasitemia and parasite density (± SEM) in brain sections of 5 mice per group. Differences compared with WT BM→WT sham chimeras are significant (*P < .05, **P < .01; unpaired t test). F, Levels of parasitemia in infected WT and Il-1r−/−/Il-18r−/− mice. Each point represents mean log10 parasites per milliliter (± SEM) obtained from 5–9 animals per group. *P < .05 (2-way analysis of variance; significant differences compared with infected WT animals). G–I, Mean numbers (± SEM) of CD4+ T cells (G), CD8+ T cells (H), and T. brucei brucei (I) per square millimeter in the cerebral cortex, corpus callosum (C. callosum), and septal nuclei of infected WT and Il-1r−/−/Il-18r−/− mice at 30 days post infection (4 animals per group).
We hypothesized that the increased numbers of parasites in the brains of myd88−/− mice was due to a lack of activation of MyD88-dependent parasitotoxic responses by cells in the CNS. To determine whether hematopoietic or CNS-resident cells, for example, microglia, contributes to MyD88-mediated parasite growth control, BM radiation chimeric mice were generated. Myd88−/− BM→WT and WT BM→Myd88−/− radiation chimeric mice showed increased parasitemia (Figure 3C) and numbers of parasites in the brain (Figure 3D and 3E) compared with WT BM→WT sham chimeras. Thus, both hematopoietic and nonhematopoietic resident cells control the growth of T. brucei brucei in the brain of infected mice. Irradiated and sham chimeric mice showed increased susceptibility to infection, as indicated by decreased survival time.
Role of TLRs in T-Cell and Parasite Accumulation in the Brain During T. brucei brucei Infection
MyD88 mediates signaling by IL-1 and IL-18 receptors (R) and all TLRs (except TLR3) [17]. We inquired whether or not IL-1R or IL-18R signaling plays a role in the outcome of T. brucei brucei infection. WT and Il-1r−/−/Il-18r−/− showed similar parasitemia levels (Figure 3F). The densities of CD4+ and CD8+ T cells and parasites in the brain parenchyma were similar WT and Il-1r−/−/Il-18r−/− mice (Figure 3G–I), indicating that TLRs, rather than IL-1R and/or IL-18R, play a role in the pathogenesis of the encephalitic phase of infection.
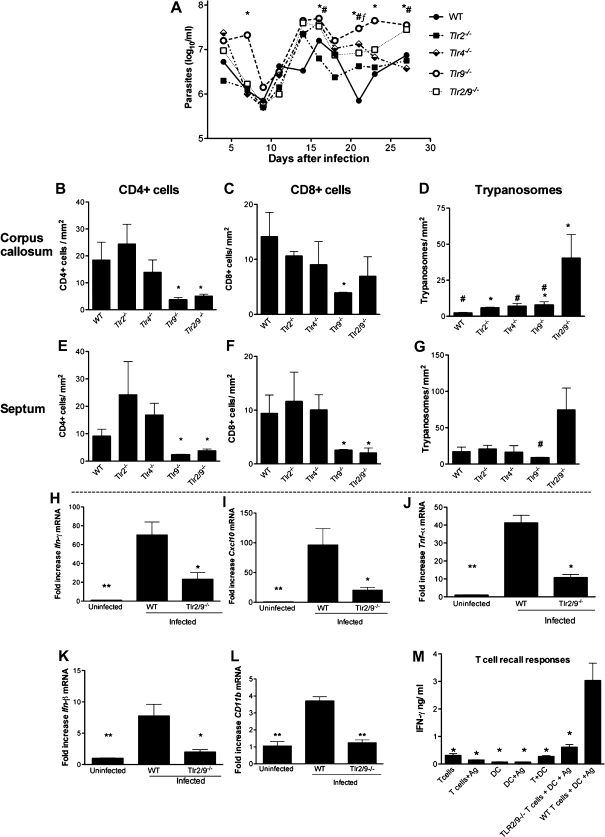
To identify the TLR required for the control of parasite and T-cell accumulation in the brain parenchyma, different TLR knockout mice were used. Tlr2−/−/Tlr9−/− (Tlr2/9−/−) and Tlr9−/− mice (but not Tlr2−/− and Tlr4−/− mice) showed elevated parasitemia levels compared with WT mice (Figure 4A). The densities of CD4+ and CD8+ T cells in the brain parenchyma of infected Tlr2−/− and Tlr4−/− mice were similar to those in infected WT mice, whereas infected Tlr9−/− and Tlr2/9−/− mice showed lower densities (Figure 4B, 4C, 4E and 4F). On the other hand, higher numbers of trypanosomes were found in the brains of Tlr2/9−/− mice, in comparison with WT or Tlr9−/− mice (Figure 4D and 4G). Ifn-γ, cxcl10, cd11b, tnf-α, and ifn-β transcript levels in the brains of tlr2/9−/− mice were all diminished compared with infected WT controls (Figure 4H–L).
Figure 4.
Role of Toll-like receptor (TLR) in the control of parasitemia and T-cell and parasite densities in the brains of Trypanosoma brucei brucei–infected mice. A, Levels of parasitemia in infected wild-type (WT), Tlr2−/−, Tlr4−/−, Tlr9−/−, and Tlr2/9−/− mice. Each point represents mean log10 parasites per milliliter ± standard error of measurement (SEM) for 4 animals per group. *P < .05 (analysis of variance [ANOVA]; significant differences between WT and Tlr9−/− mice); #P < .05 (ANOVA; significant differences between WT and Tlr2/9−/− mice); ƒP < .05 (ANOVA; significant differences between WT and Tlr4−/− mice ). B–G, Mean number (± SEM) of CD4+ (B, E) and CD8+ (C, F) T cells and T. brucei brucei (D, G) per square millimeter in the corpus callosum (C. callosum) (B–D) and septal nuclei (E–G) of infected WT, Tlr2−/−, Tlr4−/−, Tlr9−/−, and Tlr2/9−/− mice at 27 days post infection (3 or 4 animals per group). *P < .05 (unpaired t test; significantly different from WT mice at same postinfection time point); #P < .05 (unpaired t test; significant differences in T. brucei brucei density compared with tlr2/9−/− mice). H–L, Relative expression of Ifn-γ (H), Cxcl-10 (I), Tnf-α (J), Ifn-β (K), and Cd11b (L) messenger RNA (mRNA) in the brains of uninfected WT controls and WT and Tlr2/9−/− mice at 27 days post infection. Each bar represents the mean ± SEM of values obtained from 4 animals. *P < .05 and **P < .01 (unpaired t test; significantly different from infected WT mice). M, Purified splenic T cells from WT or Tlr2/9−/− mice infected or not infected with T. brucei brucei for 25 days were cocultured with syngeneic bone marrow–derived dendritic cells (DCs) in the presence of T. brucei brucei lysates. Interferon (IFN) γ was assayed by enzyme-linked immunosorbent assay in culture supernatants 72 hours after incubation; mean values (± SEM) from triplicate wells are presented. The experiment shown was performed twice, with similar results. *P < .05 (unpaired t test; significantly different from WT T cells + DCs + antigen).
We then asked whether T cells from T. brucei brucei–infected tlr2/9−/− mice show defective recall responses to T. brucei brucei antigens. Spleen T cells from infected WT or Tlr2/9−/−mice were coincubated with WT BMDCs, with or without T. brucei brucei lysates. T cells from Tlr2/9−/− animals showed diminished T. brucei brucei–stimulated IFN-γ secretion (Figure 4M).
Roles of IFN-α/β Signaling in T-Cell and Parasite Accumulation in the Brain
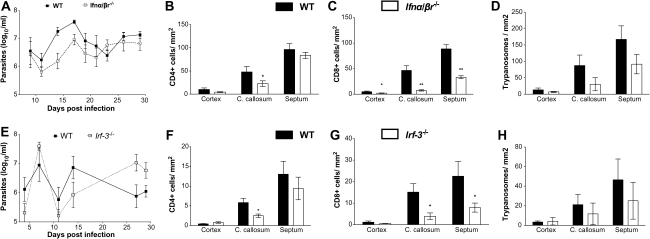
Because IFN-β transcripts were increased in the brains of T. brucei brucei–infected mice in a MyD88-dependent manner, we investigated whether IFN-α/β also played a role in T-cell and T. brucei brucei accumulation in the brain. Ifn-α/βr−/− mice showed no increased parasitemia compared with WT mice (Figure 5A). A reduced density of CD8+ T cells was found in the cerebral cortex, corpus callosum, and septal nuclei of Ifn-α/βr–/– mice, compared with infected WT mice, whereas CD4+ T cells were reduced only in the corpus callosum (Figure 5B and 5C). In contrast to Myd88−/− and Tlr2/9−/− mice, densities of trypanosomes in the parenchyma of Ifn-α/βr−/− were not increased compared with WT mice (Figure 5D).
Figure 5.
Role of interferon (IFN) α/β and IRF3 in T-cell and parasite infiltration in the brains of Trypanosoma brucei brucei–infected mice. A, levels of parasitemia in infected wild-type (WT) and Ifn-α/β r−/− mice. Each point represents the mean log10 parasites per milliliter ± standard error of measurement (SEM) obtained from 5 or 6 animals per group. B–D, Mean numbers (± SEM) of CD4+ (B) and CD8+ (C) T cells and T. brucei brucei (D) per square millimeter in the cortex, corpus callosum (C. callosum), and septal nuclei of infected WT and Ifn-α/β r−/− mice at 30 days post infection (5 or 6 animals per group). *P < .05, **P < .01 (unpaired t test; significantly different from WT mice). E, Levels of parasitemia in infected WT and Irf3−/− mice. Each point represents the mean log10 parasites per milliliter (± SEM) obtained from 6 animals per group. F–H, Mean numbers (± SEM) of CD4+ (F) and CD8+ (G) T cells and T. brucei brucei (H) per square millimeter in the cerebral cortex, corpus callosum, and septal nuclei of infected WT and Irf3−/− mice at 30 days post infection (4–6 animals per group). *P < .05 (unpaired t test; significantly different from WT mice).
Although most TLRs signal via the MyD88 adaptor, TLR3 signals exclusively through a MyD88-independent pathway in which TIR-domain-containing adapter-inducing interferon-β (TRIF) is the adaptor. TRIF mediates phosphorylation and translocation of IRF3 into the nucleus. IRF3 mediates IFN-α/β release via other innate receptors as well [6]. We therefore asked whether the IRF3-mediated pathway might, along with the MyD88-mediated one, control the outcome of infection with T. brucei brucei. Similar parasitemia levels were recorded in irf3−/− and WT mice throughout infection (Figure 5E). A reduced density of CD8+ T cells was found in the corpus callosum and septal nuclei of Irf3−/− mice, compared with WT mice, whereas CD4+ T cells were reduced only in the corpus callosum (Figure 5F and 5G). The density of trypanosomes in the parenchyma of Irf3−/− and WT mice was similar (Figure 5H). Altogether, Ifn-α/βr−/− and Irf3−/− mice showed a similar outcome of infection with T. brucei brucei.
Role of TNF-α in T-Cell and Parasite Penetration Into the Brain
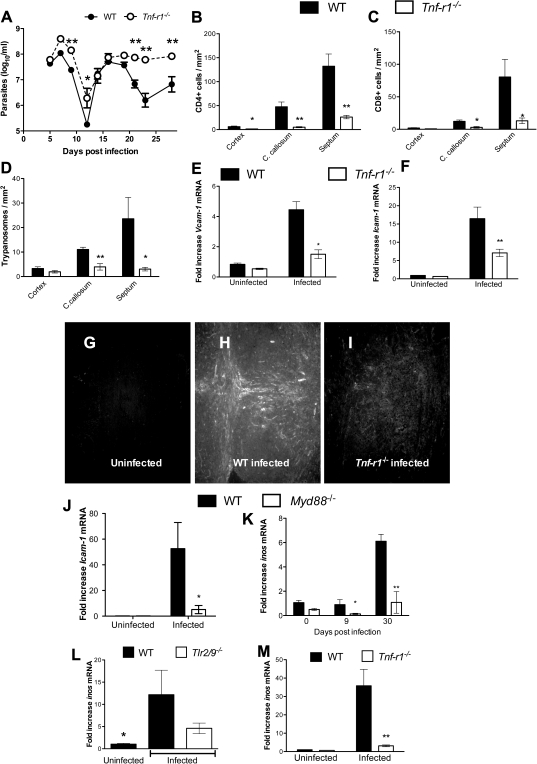
TNF-α might be secreted after TLR signaling and has been shown to reduce the parasite load in nonneural tissues in mouse models of infection [18] but has also been associated with immunopathology [19, 20]. It is not known whether the proinflammatory cytokine TNF-α affects the T-cell penetration and trypanosome load in the brain. Because T. brucei brucei stimulates the cerebral expression of TNF-α in mice in a manner mediated by MyD88 and TLR2/9, the role of TNFR1, involved in most TNF effects, was studied [21]. Tnfr1−/− mice showed increased levels of parasitemia after the second peak, compared with WT mice (Figure 6A). Moreover, decreased numbers of CD4+ and CD8+ T cells were detected in the different brain regions of Tnfr1−/− compared with WT mice at 27 days post infection (Figure 6B and 6C). However, the number of parasites in the brains of Tnfr1−/− mice was lower than in WT mice (Figure 6D).
Figure 6.
Tumor necrosis factor (TNF) α mediates T-cell densities in the brains of Trypanosoma brucei brucei–infected mice. A, Levels of parasitemia in infected wild-type (WT) and Tnfr1−/− mice. Each point represents the mean log10 parasites per milliliter ± standard error of measurement (SEM) obtained from 6 animals per group. *P < .05, **P < .01 (analysis of variance; significantly different from infected WT animals). B–D, Mean numbers (± SEM) of CD4+ (B) and CD8+ (C) T cells and T. brucei brucei (D) per square millimeter in the cerebral cortex, corpus callosum (C. callosum), and septal nuclei of infected Tnfr1−/− and WT mice at 27 days post infection (5 animals per group). *P < .05, **P < .01 (Student t test; significantly different from WT mice at same postinfection time point). E–F, J, Relative expression of Icam-1 (E, J) and Vcam-1 (F) messenger RNA (mRNA) in the brains of Tnfr1 (E, F), Myd88−/− (J), or WT mice at 27 days (Tnfr1−/−) or 30 days (Myd88−/−) post infection. Each bar represents mean ± SEM of the values obtained from 5 animals. *P < .5, **P < .01 (unpaired t test; significantly different from WT group at same time point). G–I, ICAM-1 staining in the corpus callosum and septum of WT (G, H) and TNF R1−/− (I) mice before (G) or 27 days after (H, I) infection. In infected WT mice, robust staining of endothelial cells with ICAM-1 is observed (H) in the corpus callosum and septum. ICAM-1–labeled cells could be observed in brains from T. brucei brucei–infected Tnfr1−/− mice but not from uninfected controls. K–M, Relative expression of Inos mRNA in the brains of Myd88−/− (K), Tlr2/9−/− (L), and Tnfr1−/− (M) and WT mice 27 days after infection with T. brucei brucei. K, M, *P < .05 (unpaired t test; significantly different from WT mice at same postinfection time point). L, *P < .05 (unpaired t test; significantly different from infected WT mice).
TNF-α enhances the adhesion of leukocytes to the endothelium through increased expression of adhesion molecules such as ICAM-1 or Vascular cell adhesion protein 1 (VCAM-1), thereby contributing to inflammation [22, 23]. We found that infection with T. brucei brucei strongly stimulated expression of ICAM-1 and VCAM-1 in brains of mice at the mRNA and protein levels. Icam-1 and Vcam-1 mRNA levels increased less in infected Tnfr1−/− mice than in WT mice (Figure 6E and 6F). Strikingly, increased ICAM-1 immunostaining was observed in brain vessels from infected WT mice compared with infected Tnfr1−/− or uninfected WT mice (Figure 6G–I). The levels of Icam-1 mRNA were decreased in brains of Myd88−/− mice compared with infected WT controls (Figure 6J).
The inducible nitric oxide synthase (iNOS) has been shown to mediate the control of T. brucei brucei parasitemia levels. We therefore hypothesized that iNOS is also involved in the intracerebral control of parasite load. Inos mRNA levels were increased in brains of T. brucei brucei- infected WT mice. Brains from Myd88−/− and Tlr2/9−/− mice showed lower Inos levels compared with infected WT mice (Figure 6K and 6L). However, the level of Inos mRNA was also reduced in brains from Tnfr1−/− mice despite the fact that these mice showed fewer parasites in the brain (Figure 6M).
DISCUSSION
We investigated the role of MyD88-dependent innate immune responses in the regulation of parasite and T-cell infiltration into the brain parenchyma, which are major pathogenic events in infections with T. brucei. We found that TLRs rather than IL-1R and IL-18R signals controlled the outcome of T. brucei brucei infection. At an initial phase of infection, T. brucei–specific T cells are activated in secondary lymphoid organs in a TLR- and MyD88-dependent manner before they invade the CNS.
TLR9, but not TLR2 or TLR4, promoted T-cell accumulation in the brain parenchyma. The administration of CpG-containing oligonucleotides, agonists of TLR9, has been shown to increase resistance of mice to Trypanosoma brucei rhodesiense [24]. Trypanosomal CpGs potentiate Th1-adaptive responses and macrophage and B-cell activation [7, 24, 25]. Note that MyD88 deficiency resulted in a robust inhibition of T-cell activation and T-cell penetration into the brain, whereas TLR-9–deficient mice showed comparatively less inhibition, suggesting that also other TLRs contribute to T-cell activation and extravasation into the brain during African trypanosomiasis.
MyD88 signaling may stimulate IFN-α/β secretion [6]. We show that the TLR-MyD88 pathway accounts at least in part for the IFN-β induction in the brain during T. brucei brucei infection. IFN-α/β increased invasion of T cells and trypanosomes into the brain. IFN-α/β has been shown elsewhere to regulate the outcome of infection with T. brucei rhodesiense [26]. IFN-α/β can enhance cross-priming of CD8+ T cells to parasite antigens and mediate Th1 activation by stimulating the antigen-presenting ability of dendritic cells [27, 28]. IFN-α/β in the brain might also stimulate CXCL10 secretion by endothelial cells and astrocytes resulting in T-cell and trypanosome passage across the BBB [10]. The recognition of parasite antigens by T cells in the brain parenchyma can then stimulate IFN-γ secretion followed by a further increase in CXCL10 levels to accelerate the process [29]. Accordingly, we have observed lower levels of CXCL10 mRNA in brains from T. brucei brucei–infected Ifn-α/βr−/− and Ifn-γ−/ mice, compared with WT mice [10].
We observed that IRF3-deficient mice had a partial inhibition of invasion of T cells into the brain. IRF3 is activated by either TLR4, which we found to have no effect, or by TLR3, which recognizes double-stranded RNA (dsRNA), found in some viruses. TLR3 has also been shown to recognize dsRNA from nonviral pathogens [30]. Because African trypanosomes display endogenous RNA interference and thereby contain dsRNA [31], we are now investigating whether TLR3 recognizes trypanosomal molecules and regulate T-cell and parasite neuroinvasion. However, IRF3-mediated IFN-α/β secretion may also be triggered by trypanosomes in a TLR-independent manner, as shown for the intracellular T. cruzi [32]. Altogether, our data suggest that IFN-α/β secretion, mediated by both MyD88 and IRF3, can contribute to T-cell and trypanosoma neuroinvasion.
In agreement with previous reports on Tnf-α−/− mice [18], TNFR1 was required for the control of parasitemia. Brains from Tnfr1−/− mice showed lower T-cell and parasite numbers than WT controls, strongly suggesting a role for TNF-α in their penetration through the BBB. TNF-α could facilitate T-cell penetration by increasing expression of adhesion molecules on endothelial cells [33]. We showed here that infection with T. brucei induces the expression of ICAM-1 and VCAM-1 in brain endothelial cells in a TNFR1-dependent manner. Leakiness of inter–endothelial cell tight junctions [34] or stimulation of matrix metalloproteases activities that open the parenchymal basement membranes could be also regulated by TNF-α [35, 36].
In addition to its role in invasion across the BBB, we also demonstrate that TLR2/9- MyD88/-mediated signaling participates in an intracerebral control of parasite load in the brain (Figure 7). TLR2 and TLR9 has been shown to cooperate in the control of infections by intracellular pathogens, such as Trypanosoma cruzi [14], M. tuberculosis [37], herpes simplex virus [38], adenovirus, and pneumococcal infections [39, 40]. Highly purified glycosylphosphatidylinositol anchors from T. cruzi parasites are potent activators of TLR2 [41]. Whether the membrane-bound variant surface glycoprotein of African trypanosomes can play a similar role and cooperate with the trypanosomal CpG agonists of TLR9 remains to be investigated.
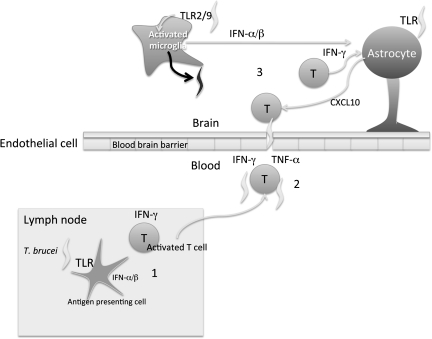
Figure 7.
Model of Toll-like receptor (TLR)/MyD88 regulation of cerebral T-cell infiltration and parasite load in the brains of Trypanosoma brucei–infected mice. In an initial phase T. brucei–specific CD4+ and CD8+ lymphocytes are activated in secondary lymphoid organs in a TLR2 and TLR9 and MyD88-dependent manner (1). Interferon (IFN) α/β might participate in T-cell activation via dendritic cell (DC) activation. Activated T cells will subsequently invade the central nervous system (CNS) in a manner dependent on tumor necrosis factor α (TNF-α), IFN-γ, and IFN-α/β. Infection-induced MyD88 signals stimulate TNF-α secretion that mediates the expression of adhesion molecules on brain endothelial cells. Parasites probably follow T cells in penetration into the brain (2). Once in the CNS, T cells will probably contribute to signs of disease. TLR will induce IFN-α/β expression that, together with IFN-γ, will stimulate CXCL10 secretion by astrocytes, resulting in further recruitment of T cells and parasites into the brain [10]. In the brain, TLR2 and TLR9 synergistically stimulate an innate immune control of parasite levels that does not require TNF-α or IFN-α/β (3).
Such immune parasite control in the brain is not dependent on T cells or IFN-γ, because brains of Rag1−/−, Ifn-γ−/−, or Ifn-γR−/− mice all show diminished parasite loads compared with controls, although all such mice showed enhanced parasite numbers in the bloodstream [4, 10]. TNFR1 and IFN-α/βR signals were also redundant for controlling the parasite load in the brain in the present study. Both hematopoietic and nonhematopoietic cells are involved in such innate immune control of parasite densities. CNS resident microglial cells could account for the nonhematopoietic control of parasites in the brain. Microglia were activated in a MyD88-mediated manner during T. brucei brucei infection. Microglia have been shown to secrete proinflammatory cytokines in response to TLR agonists [42]. We showed that T. brucei infection stimulates iNOS expression via TLR-MyD88–mediated signals. One possible mechanism for control of trypanosome load in the brain could therefore be that parasites binding to activated microglial cells or macrophages might be killed by NO secreted by these cells [43, 44]. However, because iNOS mRNA was diminished in infected Tnfr1−/− mice, showing lower parasite density in the brain, nitric oxide alone is less likely to mediate T. brucei control in the brain. The MyD88-mediated mechanisms controlling parasite levels in the brain therefore remain to be defined. Reactive oxygen species, antimicrobial peptides, parasitotoxic neuropeptides, prostanoids, or indoleamine dioxygenase could be candidates for such activity [45–48]. Although our data suggest that intracerebral innate immune responses control parasite levels, these mechanisms may not completely eliminate the trypanosomes, which can persist in the brain after suboptimal chemotherapy [49].
In summary, we demonstrate that during infection, TLR-MyD88 signaling is required for the activation of antigen-specific T cells and T-cell penetration into the brain, which might pave the way for the trypanosomes. TLR-MyD88 stimulated both TNF-α and IFN-α/β that regulated the penetration of T cells into the brain parenchyma. In addition, using a different molecular mechanism, TLR-MyD88–mediated innate immune responses exert control of the parasite load in the brain (Figure 7).
Supplementary data
Supplementary materials are available at The Journal of Infectious Diseases online (http://www.oxfordjournals.org/our_journals/jid/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments.
We thank Margareta Widing for excellent technical help with histological sections. Yohannes Woubishet is acknowledged for help with animal experimentation. We thank Frank Brombacher, University of Cape Town, South Africa, and Søren Riis Paludan, Aarhus University, Denmark for their critical comments on the manuscript.
Financial support.
This work was supported by funds from the Karolinska Institute, the Swedish International Development Agency, the Wellcome Trust (WT089992MA), US NIH Fogerty (1R21NS064888-01A1), and the Swedish Research Council.
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Dumas M, Bisser S. Clinical aspects of human African trypanosomiasis. In: Dumas M, Bouteille B, Buguet A, editors. Progress in human African trypanosomiasis, sleeping sickness. Paris: Springer; 1999. pp. 215–33. [Google Scholar]
- 2.Kibona SN, Matemba L, Kaboya JS, Lubega GW. Drug-resistance of Trypanosoma B. rhodesiense isolates from Tanzania. Trop Med Int Health. 2006;11:144–55. doi: 10.1111/j.1365-3156.2005.01545.x. [DOI] [PubMed] [Google Scholar]
- 3.Kristensson K, Mhlanga JD, Bentivoglio M. Parasites and the brain: neuroinvasion, immunopathogenesis and neuronal dysfunctions. Curr Top Microbiol Immunol. 2002;265:227–57. doi: 10.1007/978-3-662-09525-6_12. [DOI] [PubMed] [Google Scholar]
- 4.Masocha W, Robertson B, Rottenberg ME, Mhlanga J, Sorokin L, Kristensson K. Cerebral vessel laminins and IFN-gamma define Trypanosoma brucei brucei penetration of the blood-brain barrier. J Clin Invest. 2004;114:689–94. doi: 10.1172/JCI22104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kristensson K, Nygard M, Bertini G, Bentivoglio M. African trypanosome infections of the nervous system: parasite entry and effects on sleep and synaptic functions. Prog Neurobiol. 2010;91:152–71. doi: 10.1016/j.pneurobio.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–20. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 7.Drennan MB, Stijlemans B, Van den Abbeele J, et al. The induction of a type 1 immune response following a Trypanosoma brucei infection is MyD88 dependent. J Immunol. 2005;175:2501–9. doi: 10.4049/jimmunol.175.4.2501. [DOI] [PubMed] [Google Scholar]
- 8.Feng CG, Scanga CA, Collazo-Custodio CM, et al. Mice lacking myeloid differentiation factor 88 display profound defects in host resistance and immune responses to Mycobacterium avium infection not exhibited by Toll-like receptor 2 (TLR2)- and TLR4-deficient animals. J Immunol. 2003;171:4758–64. doi: 10.4049/jimmunol.171.9.4758. [DOI] [PubMed] [Google Scholar]
- 9.Rothfuchs AG, Trumstedt C, Wigzell H, Rottenberg ME. Intracellular bacterial infection-induced IFN-gamma is critically but not solely dependent on Toll-like receptor 4-myeloid differentiation factor 88-IFN-alpha beta-STAT1 signaling. J Immunol. 2004;172:6345–53. doi: 10.4049/jimmunol.172.10.6345. [DOI] [PubMed] [Google Scholar]
- 10.Amin DN, Rottenberg ME, Thomsen AR, et al. Expression and role of CXCL10 during the encephalitic stage of experimental and clinical African trypanosomiasis. J Infect Dis. 2009;200:1556–65. doi: 10.1086/644597. [DOI] [PubMed] [Google Scholar]
- 11.Mulenga C, Mhlanga JD, Kristensson K, Robertson B. Trypanosoma brucei brucei crosses the blood-brain barrier while tight junction proteins are preserved in a rat chronic disease model. Neuropathol Appl Neurobiol. 2001;27:77–85. doi: 10.1046/j.0305-1846.2001.00306.x. [DOI] [PubMed] [Google Scholar]
- 12.Mankowski JL, Queen SE, Tarwater PM, Fox KJ, Perry VH. Accumulation of beta-amyloid precursor protein in axons correlates with CNS expression of SIV gp41. J Neuropathol Exp Neurol. 2002;61:85–90. doi: 10.1093/jnen/61.1.85. [DOI] [PubMed] [Google Scholar]
- 13.Yang T, Stark P, Janik K, Wigzell H, Rottenberg ME. SOCS-1 protects against Chlamydia pneumoniae-induced lethal inflammation but hampers effective bacterial clearance. J Immunol. 2008;180:4040–9. doi: 10.4049/jimmunol.180.6.4040. [DOI] [PubMed] [Google Scholar]
- 14.Bafica A, Santiago HC, Goldszmid R, Ropert C, Gazzinelli RT, Sher A. Cutting edge: TLR9 and TLR2 signaling together account for MyD88-dependent control of parasitemia in Trypanosoma cruzi infection. J Immunol. 2006;177:3515–19. doi: 10.4049/jimmunol.177.6.3515. [DOI] [PubMed] [Google Scholar]
- 15.Minty A, Chalon P, Derocq JM, et al. Interleukin-13 is a new human lymphokine regulating inflammatory and immune responses. Nature. 1993;362:248–50. doi: 10.1038/362248a0. [DOI] [PubMed] [Google Scholar]
- 16.Reid DM, Perry VH, Andersson PB, Gordon S. Mitosis and apoptosis of microglia in vivo induced by an anti-CR3 antibody which crosses the blood-brain barrier. Neuroscience. 1993;56:529–33. doi: 10.1016/0306-4522(93)90353-h. [DOI] [PubMed] [Google Scholar]
- 17.Fitzgerald KA, Chen ZJ. Sorting out Toll signals. Cell. 2006;125:834–6. doi: 10.1016/j.cell.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 18.Magez S, Radwanska M, Beschin A, Sekikawa K, De Baetselier P. Tumor necrosis factor alpha is a key mediator in the regulation of experimental Trypanosoma brucei infections. Infect Immun. 1999;67:3128–32. doi: 10.1128/iai.67.6.3128-3132.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sileghem M, Flynn JN, Logan-Henfrey L, Ellis J. Tumour necrosis factor production by monocytes from cattle infected with Trypanosoma (Duttonella) vivax and Trypanosoma (Nannomonas) congolense: possible association with severity of anaemia associated with the disease. Parasite Immunol. 1994;16:51–4. doi: 10.1111/j.1365-3024.1994.tb00304.x. [DOI] [PubMed] [Google Scholar]
- 20.Okomo-Assoumou MC, Daulouede S, Lemesre JL, N'Zila-Mouanda A, Vincendeau P. Correlation of high serum levels of tumor necrosis factor-alpha with disease severity in human African trypanosomiasis. Am J Trop Med Hyg. 1995;53:539–43. doi: 10.4269/ajtmh.1995.53.539. [DOI] [PubMed] [Google Scholar]
- 21.Peschon JJ, Torrance DS, Stocking KL, et al. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J Immunol. 1998;160:943–52. [PubMed] [Google Scholar]
- 22.Osborn L, Hession C, Tizard R, et al. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989;59:1203–11. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- 23.Bevilacqua MP, Stengelin S, Gimbrone MA, Jr, Seed B. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989;243:1160–5. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- 24.Harris TH, Mansfield JM, Paulnock DM. CpG oligodeoxynucleotide treatment enhances innate resistance and acquired immunity to African trypanosomes. Infect Immun. 2007;75:2366–73. doi: 10.1128/IAI.01649-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shoda LK, Kegerreis KA, Suarez CE, et al. DNA from protozoan parasites Babesia bovis, Trypanosoma cruzi, and T. brucei is mitogenic for B lymphocytes and stimulates macrophage expression of interleukin-12, tumor necrosis factor alpha, and nitric oxide. Infect Immun. 2001;69:2162–71. doi: 10.1128/IAI.69.4.2162-2171.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopez R, Demick KP, Mansfield JM, Paulnock DM. Type I IFNs play a role in early resistance, but subsequent susceptibility, to the African trypanosomes. J Immunol. 2008;181:4908–17. doi: 10.4049/jimmunol.181.7.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Bon A, Etchart N, Rossmann C, et al. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat Immunol. 2003;4:1009–15. doi: 10.1038/ni978. [DOI] [PubMed] [Google Scholar]
- 28.Le Bon A, Schiavoni G, D'Agostino G, Gresser I, Belardelli F, Tough DF. Type i interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity. 2001;14:461–70. doi: 10.1016/s1074-7613(01)00126-1. [DOI] [PubMed] [Google Scholar]
- 29.Thomsen AR. Lymphocytic choriomeningitis virus-induced central nervous system disease: a model for studying the role of chemokines in regulating the acute antiviral CD8+ T-cell response in an immune-privileged organ. J Virol. 2009;83:20–8. doi: 10.1128/JVI.00682-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aksoy E, Zouain CS, Vanhoutte F, et al. Double-stranded RNAs from the helminth parasite Schistosoma activate TLR3 in dendritic cells. J Biol Chem. 2005;280:277–83. doi: 10.1074/jbc.M411223200. [DOI] [PubMed] [Google Scholar]
- 31.Ullu E, Tschudi C, Chakraborty T. RNA interference in protozoan parasites. Cell Microbiol. 2004;6:509–19. doi: 10.1111/j.1462-5822.2004.00399.x. [DOI] [PubMed] [Google Scholar]
- 32.Chessler AD, Ferreira LR, Chang TH, Fitzgerald KA, Burleigh BA. A novel IFN regulatory factor 3-dependent pathway activated by trypanosomes triggers IFN-beta in macrophages and fibroblasts. J Immunol. 2008;181:7917–24. doi: 10.4049/jimmunol.181.11.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Minagar A, Alexander JS. Blood-brain barrier disruption in multiple sclerosis. Mult Scler. 2003;9:540–9. doi: 10.1191/1352458503ms965oa. [DOI] [PubMed] [Google Scholar]
- 34.Zeni P, Doepker E, Schulze-Topphoff U, Huewel S, Tenenbaum T, Galla HJ. MMPs contribute to TNF-alpha-induced alteration of the blood-cerebrospinal fluid barrier in vitro. Am J Physiol Cell Physiol. 2007;293:C855–64. doi: 10.1152/ajpcell.00470.2006. [DOI] [PubMed] [Google Scholar]
- 35.Hanemaaijer R, Koolwijk P, le Clercq L, de Vree WJ, van Hinsbergh VW. Regulation of matrix metalloproteinase expression in human vein and microvascular endothelial cells. Effects of tumour necrosis factor alpha, interleukin 1 and phorbol ester. Biochem J. 1993;296:803–9. doi: 10.1042/bj2960803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnatty RN, Taub DD, Reeder SP, et al. Cytokine and chemokine regulation of proMMP-9 and TIMP-1 production by human peripheral blood lymphocytes. J Immunol. 1997;158:2327–33. [PubMed] [Google Scholar]
- 37.Bafica A, Scanga CA, Feng CG, Leifer C, Cheever A, Sher A. TLR9 regulates Th1 responses and cooperates with TLR2 in mediating optimal resistance to Mycobacterium tuberculosis. J Exp Med. 2005;202:1715–24. doi: 10.1084/jem.20051782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sorensen LN, Reinert LS, Malmgaard L, Bartholdy C, Thomsen AR, Paludan SR. TLR2 and TLR9 synergistically control herpes simplex virus infection in the brain. J Immunol. 2008;181:8604–12. doi: 10.4049/jimmunol.181.12.8604. [DOI] [PubMed] [Google Scholar]
- 39.Appledorn DM, Patial S, McBride A, et al. Adenovirus vector-induced innate inflammatory mediators, MAPK signaling, as well as adaptive immune responses are dependent upon both TLR2 and TLR9 in vivo. J Immunol. 2008;181:2134–44. doi: 10.4049/jimmunol.181.3.2134. [DOI] [PubMed] [Google Scholar]
- 40.Lee KS, Scanga CA, Bachelder EM, Chen Q, Snapper CM. TLR2 synergizes with both TLR4 and TLR9 for induction of the MyD88-dependent splenic cytokine and chemokine response to Streptococcus pneumoniae. Cell Immunol. 2007;245:103–10. doi: 10.1016/j.cellimm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Campos MA, Almeida IC, Takeuchi O, et al. Activation of Toll-like receptor-2 by glycosylphosphatidylinositol anchors from a protozoan parasite. J Immunol. 2001;167:416–23. doi: 10.4049/jimmunol.167.1.416. [DOI] [PubMed] [Google Scholar]
- 42.Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916–24. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- 43.Wei G, Bull H, Zhou X, Tabel H. Intradermal infections of mice by low numbers of African trypanosomes are controlled by innate resistance but enhance susceptibility to reinfection. J Infect Dis. 2011;203:418–29. doi: 10.1093/infdis/jiq051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amrouni D, Gautier-Sauvigne S, Meiller A, et al. Cerebral and peripheral changes occurring in nitric oxide (NO) synthesis in a rat model of sleeping sickness: identification of brain iNOS expressing cells. PLoS One. 2010;5:e9211. doi: 10.1371/journal.pone.0009211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McGwire BS, Olson CL, Tack BF, Engman DM. Killing of African trypanosomes by antimicrobial peptides. J Infect Dis. 2003;188:146–52. doi: 10.1086/375747. [DOI] [PubMed] [Google Scholar]
- 46.Gobert AP, Semballa S, Daulouede S, et al. Murine macrophages use oxygen- and nitric oxide-dependent mechanisms to synthesize S-nitroso-albumin and to kill extracellular trypanosomes. Infect Immun. 1998;66:4068–72. doi: 10.1128/iai.66.9.4068-4072.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Figarella K, Rawer M, Uzcategui NL, et al. Prostaglandin D2 induces programmed cell death in Trypanosoma brucei bloodstream form. Cell Death Differ. 2005;12:335–46. doi: 10.1038/sj.cdd.4401564. [DOI] [PubMed] [Google Scholar]
- 48.Delgado M, Anderson P, Garcia-Salcedo JA, Caro M, Gonzalez-Rey E. Neuropeptides kill African trypanosomes by targeting intracellular compartments and inducing autophagic-like cell death. Cell Death Differ. 2009;16:406–16. doi: 10.1038/cdd.2008.161. [DOI] [PubMed] [Google Scholar]
- 49.Jennings FW, Hunter CA, Kennedy PG, Murray M. Chemotherapy of Trypanosoma brucei infection of the central nervous system: the use of a rapid chemotherapeutic regimen and the development of post-treatment encephalopathies. Trans R Soc Trop Med Hyg. 1993;87:224–6. doi: 10.1016/0035-9203(93)90502-h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.