Abstract
Amphotericin B (AmB), is a highly effective antileishmanial agent used as first-line treatment in different formulations in visceral leishmaniasis endemic areas of Bihar, India. However, parenteral infusion, prolonged hospitalization, and toxicity are major hurdles. Our previous work demonstrated the efficacy and stability of functionalized carbon nanotubes as a delivery mechanism for AmB. In this study, using the hamster model, we have shown that this novel formulation of AmB can be administered orally, resulting in 99% inhibition of parasite growth following a 5-day course at 15 mg/kg body weight.
Visceral leishmaniasis (VL) is a life-threatening parasitic disease caused by obligate intramacrophage protozoa of the genus Leishmania. The World Health Organization (WHO) estimates the annual death toll to be 50 000 with 500 000 new cases each year. In humans, VL is always fatal if left untreated, and treatment options are limited. The drugs available are mostly parenteral and have significant toxicities. This problem is further compounded in the endemic areas of India where widespread resistance to pentavalent antimonial has persisted for the last 2 decades [1].
A polyene antibiotic, amphotericin B (AmB) is the most active antileishmanial agent and has been used as first-line treatment in antimony-resistant areas because it has an almost 100% cure rate in the endemic area of Bihar, India [2]. It has to be given over 30–40 days, necessitating prolonged hospitalization. Adverse drug reactions are another major limiting factor in the treatment of VL [3]. Development of lipid formulations of AmB, especially liposomal, has alleviated this problem. This strategy targets the drug to the intramacrophage organisms, thus increasing the bioavailability and reducing toxicity in VL patients [2]. However, the prohibitive cost of these liposomal formulations, despite preferential WHO pricing, has put them beyond the reach of most patients in the endemic areas of VL, which represent the poorest areas of the world [4].
Conventional AmB exhibits low solubility and instability at gastric pH and is unable to penetrate the brush border membrane of the small intestine. Different vehicles have been explored, but only cochleates containing AmB have shown significant efficacy when delivered orally in a mouse model of systemic aspergillosis [5]. For the treatment of VL, a novel lipid-based oral AmB formulation has been developed, which overcomes the barriers preventing oral absorption. Oral lipid AmB formulations in BALB/c mice at 10 and 20 mg/kg twice daily for 5 days resulted in 99.5% and 99.8% inhibition of liver parasitemia, respectively [6]. Nanovectors have previously been explored as a delivery system for AmB in the treatment of VL: in an oral nanosuspension form, AmB significantly reduced the hepatic parasitic burden in a murine VL model but was without curative effect, showing superior drug intake over conventional oral AmB [7]. In our previously published work, functionalized carbon nanotubes (f-CNTs) have been demonstrated to be an effective nanovector for AmB via the intraperitoneal route in a hamster VL model [8]. Functionalized CNTs are emerging as a new family of nanovectors for the delivery of different types of therapeutic molecules [9] given their capacity to interact with macromolecules such as proteins and oligosaccharides [10, 11]. Covalent modification by the organic functionalization of end groups and side walls of f-CNTs allows for a dramatic increase of the solubility of functionalized carbon nanotubes in a range of solvents, including water [12]. Water-soluble carbon nanotubes interact with mammalian calls, leading to their cytoplasmic translocation [11]. These properties led us to believe that f-CNTs may be an effective vehicle for oral administration of AmB. In this study, we examined the leishmanicidal efficacy of oral f-CNT-AmB in the treatment of Leishmania donovani in an established hamster model of infection.
Materials and Methods
Ethics Statement.
Syrian golden male hamsters were maintained in accordance with the Committee for the Purpose of Control and Supervision of Experiments on Animals, Delhi, India. Studies in L. donovani–infected hamsters were carried out in accordance with protocols approved by the Central Animal Ethics Committee (CAEC) at the Institute of Medical Sciences, Banaras Hindu University (CAEC number Dean/10–11/185).
Animals and Parasites.
Mesocricetus auratus, the Syrian golden male hamsters (4–6 weeks old) were used as an animal model for in vivo study. L. donovani LEM138 (MHOM/IN/00/DEVI) stationary-stage promastigotes were used for in vivo work. The parasites were grown in a biphasic medium and cultured in a supplemented Roswell Park Memorial Institute 1640 medium (HyClone) as described previously [13].
Drug Formulation.
The AmB drug as a powder (Bharat Serum and Vaccines Ltd, Mumbai, India) was attached to f-CNTs by a chemical synthesis process as described previously [8]. Miltefosine (Paladin Labs, Canada) and liposomal-AmB (L-AmB, AmBisome; Gilead Sciences) were used as control drugs for animal treatment.
In Vivo Evaluation on Hamster Model.
Thirty-five male hamsters (35–45 g) were infected by intracardiac injection with 1 × 108 promastigotes of LEM138 L. donovani. The patency of infection was checked by analyzing Giemsa-stained, spleen-dabbed glass slides from 2–3 hamsters randomly selected 30 days after inoculation. Hamsters were anaesthetized by injecting thiopentone (Thiosol e sodium; Neon Laboratories Ltd, India) at 80 mg/kg intraperitoneally. The experiment was repeated with 30 male hamsters for reproducibility. The formulated drug f-CNT-AmB was reconstituted in 1 × phosphate buffered saline (PBS) at 1, 2, and 4 mg/mL (injected volume ranged from 400 to 750 μL as per animal weight). The hamsters were split into groups of 5–6 and administered either oral f-CNT-AmB at 5, 10, and 15 mg/kg body weight for 5 consecutive days using oral catheters, intraperitoneal AmB attached to carbon nanotubes, intraperitoneal AmBisome at single dose of 5 mg/kg body weight, or oral miltefosine at 5 mg/kg body weight daily for 5 days via catheters. The control group was given PBS orally.
Animals were sacrificed on day 37 after infection to calculate drug efficiency. The weight of the spleen was taken immediately after autopsy, and dabbed imprints on glass slides were prepared. The effect of formulated drug on hamster spleen tissue cicatrization has been investigated by optical microscopy. The parasitic infection was monitored microscopically by using Giemsa-stained imprints. The percentage of inhibition (PI) and Leishman-Donovan units were calculated using the number of amastigotes and macrophages and the following formulas [8, 14]:
 |
where PP is the number of amastigotes per 500 nuclei in the spleen before treatment, PT is the number of amastigotes per 500 nuclei after treatment, PS is the percentage of suppression of parasite replication, and PC is the number of amastigotes per 500 nuclei in spleen tissue after treatment in the control group. The Leishman-Donovan unit indicates the number of amastigotes per 500 nuclei × tissue weight (mg).
Results
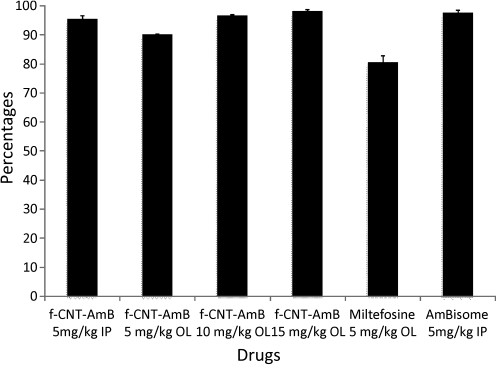
The experimental observation from both in vivo experiments was collated, and the mean value was taken for analysis. Dose response data are reported in Table 1 and Figure 1. There is a linear relationship between the PI of parasite load and oral dose of f-CNT: dosing at 5, 10, and 15 mg/kg body weight daily for 5 days resulted in 90.2% ± 0.9%, 96.5% ± 0.4%, and 98.2% ± 0.5% inhibition in spleen parasitemia, respectively, compared with a control group that received PBS. These data demonstrate that at 15 mg/kg orally, f-CNT AmB had a similar efficacy to intraperitoneal L-AmB at 5 mg/kg. In this model, at the lowest dose of 5 mg/kg, f-CNT has a significantly greater potency than 5 mg/kg miltefosine, a licensed oral agent. Assessment of effectiveness of intraperitoneal AmBisome resulted in a 97.6% ± 0.9% inhibition of spleen amastigote parasites when it was given as a single dose at 5 mg/kg body weight, whereas intraperitoneal f-CNT-AmB has shown a 95.5% ± 1.2% inhibition at a single dose of 5 mg/kg body weight. Miltefosine has shown a 80.6% ± 2.3% inhibition in the spleen parasites when it was given orally to hamsters at a single dose of 5 mg/kg body weight.
Table 1.
Results of In Vivo Efficacy Studies in Syrian Golden Hamsters
| f-CNT-AmB |
Miltefosine | AmBisome | ||||||
| Drug, Route, and Dose | 30 Days Postinfection (n = 7) | Control (n = 8) | IP 5 mg/kg (n = 11) | OL 5 mg/kg (n = 8) | OL 10 mg/kg (n = 7) | OL 15 mg/kg (n = 8) | OL 5 mg/kg (n = 8) | IP 5 mg/kg (n = 11) |
| Amastigotes/500 nuclei | 580.8 ± 26.6 | 615.5 ± 15.2 | 26.2 ± 7.3 | 57.7 ± 5.8 | 20.2 ± 2.2 | 10.8 ± 2.9 | 113.6 ±13.8 | 13.8 ± 5.3 |
| % inhibition in spleen | … | … | 95.5 ± 1.2 | 90.2 ± 0.2 | 96.6 ± 0.4 | 98.2 ± 0.5 | 80.6 ± 2.3 | 97.6 ± 0.9 |
| % suppression in parasite replication | … | … | 95.7 ± 1.2 | 99.9 ± 0.0 | 99.9 ± 0.0 | 99.9 ± 0.0 | 99.9 ± 0.0 | 97.7 ± 0.8 |
| Parasite burden, LDUa | 1225.3 ± 63.1 | 1583.5 ± 44.6 | 32.2 ± 9.2 | 71.1 ± 9.2 | 34.3 ± 7.1 | 14.9 ± 6.7 | 188.9 ± 88.9 | 16.4 ± 6.3 |
Data are expressed as mean ± standard deviation.
Abbreviations: AmB, amphotericin B; f-CNT, functionalized carbon nanotubes; IP, intraperitoneal; LDU, Leishman-Donovan unit; OL, oral.
LDU = number of amastigotes per 500 nuclei × tissue weight (mg).
Figure 1.
Comparison of activity of oral amphotericin B attached to functionalized carbon nanotubes with established treatments in Leishmania donovani–infected hamsters. Animals were intracardially infected and treated after 30 days as per the described protocol. The parasitic burden was estimated by counting the number of amastigotes per 500 splenic nuclei. The percentage inhibition was derived from the ratio between untreated and treated splenic parasite burdens. Each bar represents the mean result of 2 independent experiments. The unpaired t test shows nonsignificant differences between AmBisome and f-CNT-AmB (15 mg/kg body weight). Data are expressed as mean ± SD.
Abbreviations: AmB, amphotericin B; f-CNT, functionalized carbon nanotubes; IP, intraperitoneal; OL, oral.
Discussion
The development of nanomaterials for biomedical and biotechnological applications is an area of research that holds great promise and intense interest [9]. The potential use of carbon nanotubes to nanomedicine is their use in the therapeutic field. In this study, we have demonstrated that the formulation of AmB attached to carbon nanotubes has a beneficial effect on controlled drug delivery, through the oral route, with the potential for eradication of amastigotes from the hamster spleen.
Amphotericin attached to carbon nanotubes has previously shown good antileishmanial activity, with minimum toxicity, when it was given intraperitoneally to L. donovani–infected hamsters. In the present study, we found that this formulation through the oral route has shown good antileishmanial activity in hamster spleen tissues. Furthermore, in our previous work, AmB nanosuspension proved to be stable, with durable antileishmanial activity, indicating good shelf-life characteristics. With respect to pharmacoeconomics, this drug delivery system for AmB is easy to prepare and is cost effective. Further optimization of the formulation may be required to improve its dosage efficacy—instability in the high electrolyte and acid pH of the gut may be leading to reduced delivery of AmB to its target. Pharmacokinetic studies are planned. However, this study and previous work [8] clearly demonstrate that f-CNT as a drug delivery system for orally administrated AmB has significant potential for further development. Carbon nanotubes have not been approved for use in humans; it is at the proof-of-concept research stages. The length of carbon nanotubes is in the micrometer range, which may create a problem in biodegradation. Also, we have checked its toxicity during intraperitoneal administration: there are chances that it may easily filtrate out through the kidney, but extensive toxicity studies are needed in animal models before we can perform human safety and efficacy studies.
Notes
Acknowledgments.
V. K. P. is thankful to Indian Council of Medical Research New Delhi for providing a Senior Research Fellowship. We thank Dr Meghan Rose Perry for helpful comments and suggestions in the preparation of the manuscript.
Financial support.
This work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (TMRC grant No. 1P50AI074321), and DST (UNANST:BHU).
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Sundar S, More DK, Singh MK, et al. Failure of pentavalent antimony in visceral leishmaniasis in India: report from the center of the Indian epidemic. Clin Infect Dis. 2000;31:1104–7. doi: 10.1086/318121. [DOI] [PubMed] [Google Scholar]
- 2.Sundar S, Chakravarty J, Agarwal D, Rai M, Murray HW. Single-dose liposomal amphotericin B for visceral leishmaniasis in India. N Engl J Med. 2010;362:504–12. doi: 10.1056/NEJMoa0903627. [DOI] [PubMed] [Google Scholar]
- 3.Thakur CP, Singh RK, Hassan SM, Kumar R, Narain S, Kumar A. Amphotericin B deoxycholate treatment of visceral leishmaniasis with newer modes of administration and precautions: a study of 938 cases. Trans R Soc Trop Med Hyg. 1999;93:319–23. doi: 10.1016/s0035-9203(99)90037-8. [DOI] [PubMed] [Google Scholar]
- 4.Boelaert M, Meheus F, Sanchez A, et al. The poorest of the poor: a poverty appraisal of households affected by visceral leishmaniasis in Bihar, India. Trop Med Int Health. 2009;14:639–44. doi: 10.1111/j.1365-3156.2009.02279.x. [DOI] [PubMed] [Google Scholar]
- 5.Delmas G, Park S, Chen ZW, et al. Efficacy of orally delivered cochleates containing amphotericin B in a murine model of aspergillosis. Antimicrob Agents Chemother. 2002;46:2704–7. doi: 10.1128/AAC.46.8.2704-2707.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wasan KM, Wasan EK, Gershkovich P, et al. Highly effective oral amphotericin B formulation against murine visceral leishmaniasis. J Infect Dis. 2009;200:357–60. doi: 10.1086/600105. [DOI] [PubMed] [Google Scholar]
- 7.Golenser J, Domb A. New formulations and derivatives of amphotericin B for treatment of leishmaniasis. Mini Rev Med Chem. 2006;6:153–62. doi: 10.2174/138955706775476037. [DOI] [PubMed] [Google Scholar]
- 8.Prajapati VK, Awasthi K, Gautam S, et al. Targeted killing of Leishmania donovani in vivo and in vitro with amphotericin B attached to functionalized carbon nanotubes. J Antimicrob Chemother. 2010;66:874–9. doi: 10.1093/jac/dkr002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prato M, Kostarelos K, Bianco A. Functionalized carbon nanotubes in drug design and discovery. Acc Chem Res. 2008;41:60–8. doi: 10.1021/ar700089b. [DOI] [PubMed] [Google Scholar]
- 10.Chen RJ, Zhang Y, Wang D, Dai H. Noncovalent sidewall functionalization of single-walled carbon nanotubes for protein immobilization. J Am Chem Soc. 2001;123:3838–9. doi: 10.1021/ja010172b. [DOI] [PubMed] [Google Scholar]
- 11.Pantarotto D, Briand JP, Prato M, Bianco A. Translocation of bioactive peptides across cell membranes by carbon nanotubes. Chem Commun (Camb) 2004;1:16–17. doi: 10.1039/b311254c. [DOI] [PubMed] [Google Scholar]
- 12.Georgakilas V, Kordatos K, Prato M, Guldi DM, Holzinger M, Hirsch A. Organic functionalization of carbon nanotubes. J Am Chem Soc. 2002;124:760–1. doi: 10.1021/ja016954m. [DOI] [PubMed] [Google Scholar]
- 13.Maurya R, Mehrotra S, Prajapati VK, Nylen S, Sacks D, Sundar S. Evaluation of blood agar microtiter plates for culturing Leishmania parasites to titrate parasite burden in spleen and peripheral blood of patients with visceral leishmaniasis. J Clin Microbiol. 2010;48:1932–4. doi: 10.1128/JCM.01733-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manandhar KD, Yadav TP, Prajapati VK, et al. Antileishmanial activity of nano-amphotericin B deoxycholate. J Antimicrob Chemother. 2008;62:376–80. doi: 10.1093/jac/dkn189. [DOI] [PubMed] [Google Scholar]