Abstract
In this study we have firstly compared a range of recombinant DNA poxvirus prime-boost immunisation strategies and shown that combined intramuscular (i.m.) 2×DNA-HIV/intranasal (i.n.) 2×FPV-HIV prime-boost immunisation can generate high-level of HIV-specific systemic (spleen) and mucosal (genito-rectal nodes, vaginal tissues and lung tissues) T cell responses and HIV-1 p24 Gag-specific serum IgG1, IgG2a and mucosal IgG, SIgA responses in vaginal secretions in BALB/c mice. Data indicate that following rDNA priming, two rFPV booster immunisations were necessary to generate good antibody and mucosal T cell immunity. This data also revealed that mucosal uptake of recombinant fowl pox (rFPV) was far superior to plasmid DNA. To further evaluate CD8+ T cell immunity, i.m. 2× DNA-HIV/i.n. 1× FPV-HIV immunisation strategy was directly compared with single shot poxvirus/poxvirus, i.n. FPV-HIV/i.m. VV-HIV immunisation. Results indicate that the latter strategy was able to generate strong sustained HIV-specific CD8+ T cells with higher avidity, broader cytokine/chemokine profiles and better protection following influenza-KdGag197–205 challenge compared to rDNA poxvirus prime-boost strategy. Our findings further substantiate the importance of vector selection/combination, order and route of delivery when designing effective vaccines for HIV-1.
Keywords: Poxvirus vectors, DNA vaccines, Mucosal immunity, T cell avidity
1. Introduction
The great majority of human pathogens are first encountered at the mucosae, leading to a renewed interest in developing vaccines that elicit mucosal immunity. Many HIV-1 “systemic vaccine trials” or vaccines delivered via the intramuscular route to the blood in humans have elicited poor outcomes [1,2], and there is now an increased awareness of the potential importance of inducing local antiviral immune responses at mucosal surfaces, particularly in the genital and rectal tissues, including the cervico-vaginal tissues in females which is the primary site of infection [3–6] and the gastrointestinal tract, a major reservoir of HIV virus replication with resultant CD4+ T cell depletion [7]. It is widely thought that vaccine-induced mucosal immunity requires that a vaccine be delivered to the mucosa, and that vaccines administered systemically do not generally induce good immune responses at mucosal sites [8].
To date a variety of plasmid DNA and/or recombinant virus heterologous prime-boost vaccine delivery strategies have been investigated as candidate vaccines for HIV-1. For safety reasons, the majority of studies have been based on virus vectors that are unable to replicate in the host. For example, avipox viruses such as fowl poxvirus (FPV) does not replicate in mammalian cells and has a highly restricted host range, although heterologous genes under the control of early promoters are expressed in mammalian cells, resulting in presentation of encoded vaccine antigen to the immune system [9–12]. To date many different heterologous prime-boost vaccine protocols have been explored, including non-replicating vaccinia virus (VV) and adenovirus (Ad) vectors. Protein antigens in combination with rDNA and/or recombinant virus vectors have also been used to augment antibody responses in prime-boost regimes [13–17]. Even though many of the rDNA prime-boost HIV-1 vaccines tested in animal models have shown promising results [18], human trials have generated poor outcomes [1,2,19,20]. It is now believed that this could be related, at least in part to sub-optimal doses of DNA vaccine. Interestingly, a recent phase I clinical trial using a 4 mg primary dose of DNA vaccine with NYVAC (New York vaccinia virus, a highly attenuated strain) in a prime-boost strategy reported good immunogenicity in humans and offers some optimism for the future [21].
A critical question that has not yet been clearly addressed is the generally poor immunogenicity of DNA vaccines in humans. Another major problem for the design of effective vaccines against HIV-1 and other intractable pathogens is our poor current understanding of the immunological correlates of protection. Does “quality” or “avidity” of immune responses matter? Functional T cell avidity, the ability of antigen-specific T cells to recognise and respond to antigen, is a key component that may underpin the effectiveness of T cells in clearing infection. By definition, low avidity T cells are incapable of effector function at low concentrations of antigen, while high avidity T cells can recognise low concentrations of antigen and appear to mediate increased functional activity [22,23].
In previous studies, macaques vaccinated with multiple HIV antigens via rDNA/rFPV prime boosting either systemically or via mucosal (intranasal) delivery of the FPV booster vaccine [24] showed that the mucosally immunised animals generated local T cell responses in cervico-vaginal tissues following pathogenic vaginal SHIV (simian human immunodeficiency virus) challenge with significant reduction in acute plasma viremia, in the absence of significant circulating SHIV T cell responses prior to challenge. Furthermore, we have shown that intranasal priming with FPV vectors followed by intramuscular boosting with VV vectors generates robust long-term systemic and mucosal T cell responses against HIV-1 vaccine antigens in BALB/c mice [25], which were also of higher avidity [26]. In this current study we have directly compared a systemic/mucosal heterologous rDNA/poxvirus prime-boost immunisation regime with poxvirus/poxvirus (rFPV/rVV) prime-boost immunisation and clearly shown that the latter strategy generates T cells with greater avidity, correlating with the weight loss profiles observed following influenza-KdGag197–205 mucosal challenge.
2. Materials and methods
2.1. Recombinant DNA or poxvirus vaccines
The DNA plasmid pHIS (DNA-HIV), recombinant fowl pox (FPV-HIV) and recombinant vaccinia virus (VV-HIV) vaccines expressing modified B clade gag and pol genes were prepared as described elsewhere [12,27,28] (Table 1).
Table 1.
Recombinant poxviruses used in this study [28].
| Recombinant | Insertion sites
|
|
|---|---|---|
| F region | TK-ORFX or TK | |
| FPV gag/pol (FPV-HIV) | B clade gag/pol(m) | |
| VV gag/pol (VV-HIV) | B clade gag/pol(m) | |
TK, thymidine kinase; ORFX, uncharacterised gene.
2.2. Immunisation of mice
Pathogen free 8–10 week old female BALB/c mice were obtained from the Animal Research Centre, Perth, Western Australia or the Animal Breeding Establishment, The John Curtin School of Medical Research (JCSMR). All animals were maintained and used in accordance with Institutional animal ethics guidelines. Mice (n = 4–5) were primed intramuscularly (i.m.) with 50 or 100 μg DNA-HIV in sterile phosphate-buffered saline (PBS), (50 μl/per quadriceps), or intranasally (i.n.) 20 μl per mouse complexed 1:3 with Lipofectamine transfection reagent (Invitrogen, Carlsbad, CA). Two doses were given at an interval of 4 weeks. 2–4 weeks following DNA priming, mice were boosted i.n., i.r. (intrarectally) or i.m with 5 ×106 or 107 pfu FPV HIV as indicated in Table 2. During i.m. delivery of rFPV, 50 μl per quadriceps and during i.n. or i.r delivery 20 μl rFPV per mouse were delivered after sonication of virus as indicated below (rFPV was not complexed with lipofectamine). Further groups of mice (n = 4–5) were primed and boosted with 1 ×107 pfu FPV-HIV followed by 1 ×107 pfu VV-HIV given 2 weeks apart using either i.n./i.m. (mucosal/systemic) or i.m./i.m. (pure systemic) immunisation routes as indicated in Table 2. Mice were immunised under mild methoxyfluorane anesthesia. Prior to each immunisation, FPV-HIV or VV-HIV vaccines were diluted in PBS and sonicated to obtain homogeneous viral suspensions. To evaluate protective immunity at 6 weeks after the final vaccine booster, mice were challenged mucosally (i.n.) with a dose (50 plaque forming units (PFU)) of influenza virus PR8 expressing the KdGag197–205 epitope of HIV in the neuraminidase stalk. This construct was created using reverse genetic technology as described elsewhere [29,30]. Body weight was monitored for 10 days after challenge.
Table 2.
Prime-boost vaccine strategies used in this study.
| Prime | Boost | |
|---|---|---|
| 1 | 2× i.n. DNA-HIV | 2× i.m. FPV-HIV |
| 2 | 2× i.n. DNA-control | 2× i.m. FPV-HIV |
| 3 | 2× i.m. DNA-HIV | 2× i.n. FPV-HIV |
| 4 | 2× i.m. DNA-control | 2× i.n. FPV-HIV |
| 5 | 2× i.m. DNA-HIV | 2× i.n. DNA-HIV |
| 6 | 2× i.m. DNA-HIV | 2× i.r. FPV-HIV |
| 7 | 2× i.m. DNA-HIV | 2× i.m. FPV-HIV |
| 8 | 2× i.m. DNA-HIV | i.m. FPV-HIV* |
| 9 | 2× i.m. DNA-HIV | i.n. FPV-HIV* |
| 10 | i.n. FPV-HIV* | i.m. VV-HIV* |
| 11 | i.m. FPV-HIV* | i.m. VV-HIV* |
All constructs encode HIV-1 subtype B gag/pol antigens, except DNA control. i.n., intranasal; i.m., intramuscular; i.r., intrarectal. Two DNA-HIV priming immunisations were performed in each case. In contrast as indicated some instances one (*) or two i.n. or i.m. FPV-HIV or VV-HIV immunisations were performed.
2.3. Preparation of lymphocytes
To measure T cell responses mice were sacrificed at different time intervals (4 weeks post 2nd DNA-HIV; 2–4 weeks post 1st FPV-HIV; 4,8 or 16 weeks post 2nd FPV-HIV or 2 weeks post VV-HIV, or 10 days post-challenge), spleen and genito-rectal nodes (iliac lymph nodes) were removed, and single cell suspensions were prepared in complete RPMI as described previously [25]. Splenocytes were treated with red cell lysis buffer to remove erythrocytes.
Single cell suspensions from mucosal tissues (i.e. vaginal, and lung) were prepared as follows. Tissue samples were collected in complete RPMI and were cut into small pieces and incubated at 37 °C with 2 mg/ml collagenase (Sigma), 2.4 mg/ml dispase (GIBCO) and 5 Units/ml DNAse (Calbiochem) in complete RPM for 1 h with gentle agitation and 5 ml of complete RPMI was added to each sample and was passed through 2 layers of sterile gauze to remove cell debris. Cells were then treated with red cell lysis buffer, washed twice with compete medium and particulate material was removed by passing though a cell stainer. Finally, cells were resuspended in complete medium.
2.4. Serum and lavage collection
Serum and vaginal lavages were collected from pre-immune mice, after 2 doses of DNA-HIV, after FPV-HIV the first booster, and after the 2nd FPV-HIV booster immunisations. Vaginal lavage fluids were collected by flushing the vagina with 40 μl of sterile PBS, then 1 μl of Phenyl methyl sulfonyl fluoride (Sigma) was added to each sample which was stored at −20 °C until use. Blood was collected by tail vein puncture and serum was separated by centrifugation and stored at −20 °C until assayed.
2.5. HIV-1 p24 Gag-specific serum enzyme-linked immunosorbent assay (ELISA)
ELISA was used to determine HIV-1 p24 Gag-specific IgG1 and IgG2a serum antibody titres. Falcon Microtest III plates (Becton Dickinson, Oxnard, CA) were coated with HIV-1 p24 Gag (kindly supplied by the NIH AIDS Research and Reference Reagent Program) or control protein at 1.5 μg/ml (50 μl/well) in borate buffer (Pierce) overnight at 4 °C. Plates were washed 5 times with 0.05% Tween20 in PBS (PBST), and non-specific binding sites were blocked by adding 5% skim milk/PBST (Diploma), at 200 μl/well for 2 h at 37 °C. Plates were then washed as before, and serum samples diluted in 5% skim milk/PBST were added in a volume of 50 μl to each well. Serum samples were diluted 2-fold from 1/50 to 1/400 for pre-immunisation samples, 1/200 to 1/25,600 for post-DNA and post-1st FPV samples, and 1/200 to 1/102,400 for post-2nd FPV samples. Plates were incubated for 1.5 h at 37 °C and washed as indicated with PBST. Secondary antibody, biotin-conjugated anti-mouse IgG1 or anti-mouse IgG2a (Southern Biotechnology Associates, Birmingham, AL) diluted to 1:1000 in 1% bovine serum albumin/PBST (Sigma) (BSA/PBST) was added to respective wells in a 50 μl volume, and incubated overnight at 4 °C. Plates were washed 5 times with PBST, and 50 μl of horseradish peroxidase-conjugated streptavidin (HRP-SA, Amersham Life Science) diluted 1:1000 in 1% BSA/PBST was added to each well. Plates were incubated at 37 °C for 1.5 h, washed 5 times with PBS and antibodies were detected using 0.01 mg/ml Tetramethyl–benzidine (TMB) (Sigma) substrate dissolved in dimethyl sulfoxide (Sigma) and diluted in TMB citrate/phosphate substrate buffer (Sigma). Colour development was stopped at 15 min by adding 50 μl/well of 1 M H2SO4 (Sigma). Optical densities (OD) in each well were read at the dual wavelengths of 450 nm and 690 nm. To determine endpoint titres, serum from unimmunised mice was titrated across an ELISA plate beginning at the same dilution as the samples. The OD values for each titration point were added together and the average and standard deviation were calculated. The endpoint titre was defined as the mean of the OD plus two standard deviations. The endpoint titre value was applied to each sample with the highest dilution being recorded as the reciprocal of the dilution and the endpoint after the control OD values were subtracted from the HIV-1 p24 Gag coated plates. Vaginal lavage was assayed for HIV-1 p24 Gag-specific IgG and IgA titres as for serum antibody titration, except that vaginal lavages were diluted 2-fold from 1/10 dilution to a 1/80 dilution for pre-immunised samples and from 1/10 to 1/1280 for post-immunisation samples.
2.6. IFN-γ and IL-2 ELISpot assay
IFN-γ or IL-2 HIV-specific T cell responses were measured by IFN-γ or IL-2 capture ELISpot assay as described previously [25,26]. Briefly, mouse anti- IFN-γ or IL-2 capture antibodies (BD PharMigen, San Diego, CA), were diluted to 5 μg/ml in PBS and 96-well Millipore PVDF plates were coated with 50 μl of the diluted antibodies. Splenocytes, lymphocytes from genito-rectal lymph nodes, or cells prepared from mucosal tissues (vaginal and lung) were added in duplicate or triplicate to appropriate wells at a final concentration of 2 ×105 cells per well in a final volume of 100 μl. For IFN-γ ELISpot, cells were stimulated for 20–24 h, and, in the case of IL-2 assays, for only 12–13 h, in the presence of immunodominant H-2Kd-CD8+ T cell epitope Gag 197–205 (AMQMLKETI). A single Gag 197–205 peptide (synthesised at the Bio-Molecular Resource Facility at JCSMR) or an HIV-specific 15-mer overlapping Gag peptide pool (kindly supplied by the NIH AIDS Research and Reference Reagent Program) was used in this study. ConA-stimulated cells (Sigma, USA) were used as positive controls and unstimulated cells as negative controls. Biotin labelled secondary antibody diluted 1:2000 in 1% BSA/PBS was added to each well, and plates were incubated at room temperature for 2 h. For both ELISpot assays, all steps were carried out as described previously [25]. Spot forming units (SFU) were counted using an ELISpot Bio Reader-4000 (BIOSYS, GmbH, Germany). Results are expressed as SFU per 106 T cells and represent mean values ±SD. Unstimulated cell counts were subtracted from stimulated before plotting the data. All instances the background SFU counts were extremely low.
2.7. CD4+ and CD8+ selection
Positive selections were performed using Dynal magnetic beads according to manufactures instructions to remove the CD4+ or CD8+ from sample. Post selection, flow through which were CD4-depleted or CD8-depleted were collected and cells stained with anti-CD8a FITC and/or anti-CD4 PerCp (BD PharMingen) and data acquired on a FACS Calibur (Becton-Dickenson) to determine the purity of the cell populations. Cells were then used for IFN-γELISpot assays as indicated above.
2.8. Intracellular cytokine staining (ICS)
2 ×106 lymphocytes were stimulated for 16 h in the presence of Gag 197–205 peptide and then for a further 5–6 h in the presence of brefeldin A, as described previously [25]. Following stimulation, cells were surface-stained with anti-CD8 Allophycocyanin or FITC and/or CD62L PerCpCy5.5 (BD PharMingen), then fixed and permeabilised before staining with anti-mouse CD107a-PE, IFN-γ–FITC and/or TNF-α-PE conjugates (BD PharMingen). 100,000 total gated events were acquired on a FACS Calibur flow cytometer (Becton-Dickinson), and results were analysed using Cell Quest Pro software. Unstimulated T cells counts were used as background controls, and were subtracted when plotting data.
2.9. Tetramer staining
Allophycocyanin-conjugated KdGag197–205 tetramers were synthesised at the Bio-Molecular Resource Facility at The John Curtin School of Medical Research. Tetramer staining was performed as described previously [25]. Briefly, 2–5 ×106 splenocytes or genito-rectal lymphocytes were stained with anti-CD8-FITCα antibody (BD PharMigen, San Diego, CA) and APC-conjugated KdGag197–205tetramer at room temperature for 40 min, protected from light, washed once with FACs buffer and were resuspended in 100 μl of FACS buffer containing 0.5% paraformaldehyde. Samples were acquired on a FACS Calibur (Becton-Dickinson) and analysed using Cellquest Pro software. Spleen and genito-rectal lymph node derived lymphocytes from unimmunised animals were used as background controls.
2.10. Tetramer dissociation assay
The tetramer dissociation assay was performed as described previously [26]. Briefly, 2 ×106 cells from each sample were aliquoted into a round-bottom 96 well plate and stained with FITC-anti CD8α and APC-Gag KdGag197–205 tetramer. Plates were configured to assess five time points per sample (0–60 min). 50 μg/ml of anti-H-2Kd competitive binding antibody (BD PharMigen, San Diego, CA) was added to each well to prevent dissociated tetramer from re-binding and plates were incubated at 37 °C, 5%CO2. At each time point, cells were transferred into ice-cold FACS buffer to stop the reaction, washed and resuspended in 100 μl of FACS buffer containing 0.5% paraformaldehyde. Samples were acquired on a FACs Calibur flow cytometer (Becton-Dickinson) and analysed using Cell Quest Pro software.
2.11. Cytokine antibody arrays
2 ×106 splenocytes were cultured in complete RPMI without IL-2 for 16 h h in the presence of H-2Kd binding Gag197–205 peptide as described in Ranasinghe and Ramshaw [31]. Supernatants were collected and cytokine antibody arrays were performed according to manufactures instructions (Ray Biotech Inc., USA). Cytokine expression was detected using chemiluminescence substrate. Protein expression signal intensities were calculated as a percentage absorbance, normalised against the positive controls on the membrane using Multi Gauge V3.0 software density linear calibration analysis (A –B/mm2; where A is the average absorbance of the cytokine, B is the average background absorbance, mm is the average area).
2.12. Statistics and analysis of data
SD or SEM was calculated and p-values determined using a two-tailed, two sample equal variance or unequal variance Student’s t-test. Except where stated, experiments have been repeated at least three times.
3. Results
3.1. Evaluating rDNA/rFPV prime-boost T cell responses generated with intranasal or intramuscular DNA priming
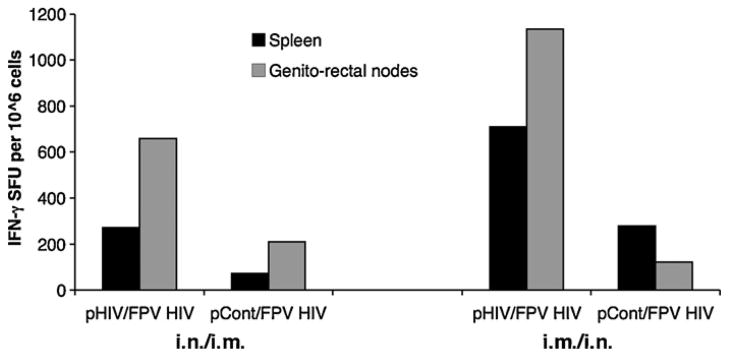
When mice were immunised either i.n./i.m. or i.m./i.n as indicated in Table 2, and HIV Gag-specific systemic (spleen) and mucosal (genito-rectal nodes) T cell responses were measured at 4 weeks following the 2nd FPV-HIV booster immunisation, results indicate that 2× i.m. rDNA/2× i.n. rFPV immunisation can generate heightened systemic and mucosal T cell responses compared to 2× i.n. rDNA/2× i.m. rFPV immunisation (Fig. 1). Data indicate that plasmid DNA uptake was more effective following i.m. delivery compared to mucosal delivery. Moreover, administration of control-DNA followed by i.n. or i.m. delivery of FPV-HIV vaccine did not generate good T cell responses to vaccine antigens (Fig. 1), this indicates that effective rDNA priming was pivotal for robust T cell immunity.
Fig. 1.
Route specific T cell immunity following 2× rDNA/2× rFPV prime-boost immunisation. Mice n = 4–5 per group were immunised i.n. with 50 μg of 2× DNA-HIV or 2× DNA-control complexed with lipofectamine and i.m. with 5 ×106 pfu FPV-HIV (left) or i.m. 2×DNA-HIV or 2×DNA-control prime (without lipofectamine) followed by i.n. FPV-HIV boost (right), These immunisations were performed 4 weeks apart as indicated in Table 2 (groups 1–4). At 4 weeks after the final boost, spleen (black bars) and genito-rectal node (grey bars) cells were stimulated with the 15-mer Gag peptide pool as in Section 2 and T cell responses were measured by IFN-γ ELIspot. Unstimulated cells from each sample were used as background controls and this value was subtracted from each sample before plotting the data. The data represent pooled values, and are representative of three experiments.
3.2. Evaluation of cellular and humoral immune responses following i.m. DNA-HIV prime and i.n. FPV-HIV boost immunisation
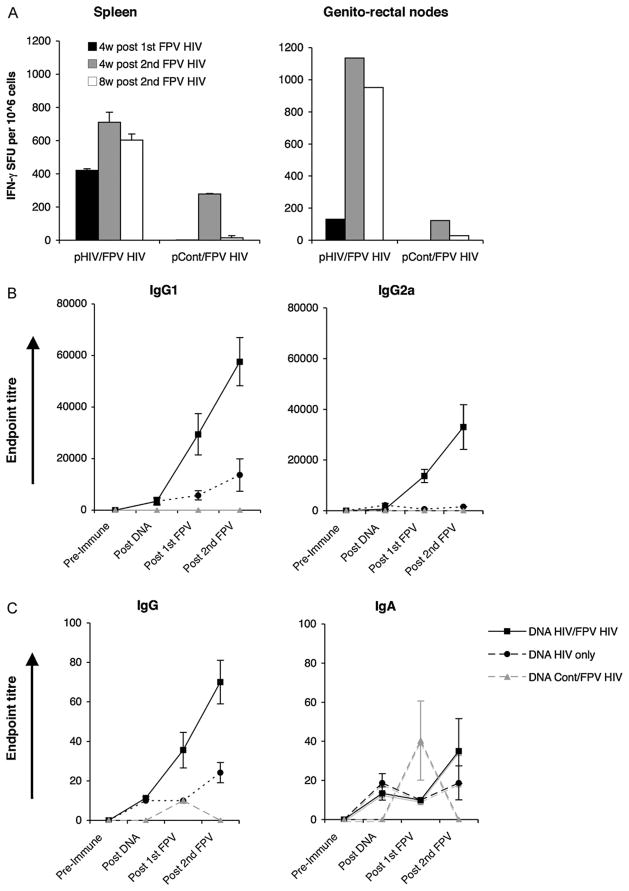
As i.m. delivery of DNA elicited stronger T cell responses, this delivery strategy was further evaluated in combination with i.n rFPV boosting and HIV Gag-specific systemic (spleen) and mucosal (genito-rectal nodes) T cell responses were measured at 4 weeks following the 1st and 2nd FPV-HIV and at 8 weeks following the 2nd FPV booster immunisation. IFN-γ ELISpot data indicate that two i.n. FPV-HIV booster immunisations were necessary following DNA priming in order to generate strong, sustained mucosal T cell responses, unlike systemic T cell immunity (Fig. 2A).
Fig. 2.
(A–C) Mucosal and systemic T cell and B cell responses following i.m. 2× DNA-HIV/i.n. 2× FPV-HIV prime-boost immunisation. Fig. 2A. Mice n = 4–5 per group were primed i.m. with 50 μg of 2×DNA-HIV and boosted i.n. with 5 ×106 pfu 2×FPV-HIV (left) or i.m. 2×DNA-control/i.n. 2×FPV-HIV, 4 weeks apart as indicated in Table 2 (groups 3 and 4). Mice were sacrificed at 4 weeks post 1st FPV-HIV boost (black), or at 4 (grey) or 8 (white) weeks post 2nd FPV-HIV boost respectively. Spleen (left) and genito-rectal node (right) cells were stimulated with 15-mer Gag peptide pool as indicated in Section 2 and T cell responses were measured by IFN-γ ELIspot. Unstimulated cells from each sample were used as background controls and were subtracted from each sample before plotting the data. The data represent pooled values, and are representative of three experiments. (B and C) Mice n = 15 per group were prime-boost immunised as indicated in Table 2 (groups 3–5). HIV-1 p24 Gag-specific serum IgG1 and IgG2A (B), and mucosal IgG and IgA (C) antibody responses were measured at pre-immunisation, at 4 weeks post 2nd DNA, at 4 weeks post 1st FPV-HIV and at 2nd FPV-HIV boost respectively. Control positive and negative sera were used in these assays. Y-axis shows endpoint titres calculated as in Section 2. The data represent mean ±SEM for 15 individual mice.
HIV-1 p24 Gag-specific serum IgG1 and IgG2a antibody responses and mucosal (vaginal lavage) IgG and IgA antibody responses were also evaluated at 4 weeks following the 2nd DNA dose, and at 4 weeks following the 1st and 2nd i.n. FPV-HIV immunisations. Data indicate that i.m. 2× DNA-HIV priming followed by i.n. 2× FPV-HIV booster immunisation generated strong, p24 Gag-specific IgG1 and IgG2a responses in serum and small but sustained IgG and IgA titres in genital secretions (Fig. 2B and C). Interestingly, a doubling of the levels of both systemic and mucosal antibody responses was observed following the 2nd i.n. FPV-HIV booster immunisation, while DNA-HIV vaccines given either twice or four times, did not induce measurable IgG2a antibody responses in serum, with slight serum IgG1 and mucosal IgG and IgA responses in vaginal lavage (Fig. 2C). 2× DNA-control vaccine followed by 2× FPV-HIV generated no antibody responses against p24 Gag protein in serum or mucosa, except for a small spike in IgA levels in vaginal lavage at 4 weeks following the 1st FPV-HIV booster immunisation which was not sustained (Fig. 2C).
3.3. Evaluation of total mucosal T cell responses following i.m. DNA-HIV priming followed by i.n., i.r. or i.m. FPV-HIV booster immunisation
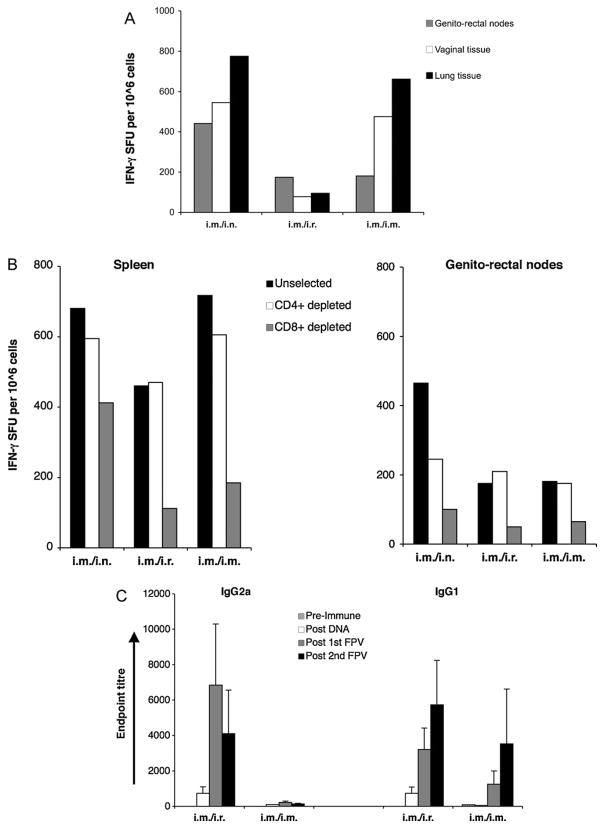
Mice were given 2× DNA-HIV i.m. followed by i.n., i.r. or i.m. doses of 2× FPV-HIV vaccine 3 weeks apart as indicated in Table 2. At 7–8 weeks after the 2nd FPV-HIV booster, mucosal T cell responses were measured in genito-rectal nodes, vaginal tissues and lung tissues. The results clearly indicated that i.m./i.n. prime-boosting generated robust, long-lasting IFN-γ producing T cell populations in all mucosal lymph nodes and tissues that were tested, with the strongest responses observed in lung tissues (Fig. 3A). In contrast, i.m./i.r, immunisation did not lead to high-level of T cell responses in any of the mucosal tissues, although i.m./i.m. immunisation generated T cell responses in vaginal and lung tissues at similar levels to i.m./i.n. immunisation.
Fig. 3.
(A) Mucosal memory T cell responses following different routes of FPV-HIV boosting. Mice (n = 10–15 per group) were primed i.m. with 50 μg of 2× DNA HIV and boosted i.n., i.r. or i.m. with 5 ×106 pfu 2× FPV-HIV 4 weeks later, as indicated in Table 2 (groups 3,6 and 7). Mice were sacrificed 7–8 weeks after the 2nd FPV-HIV boost and genito-rectal nodes (grey), vaginal tissues (white) and lung tissues (black) were harvested and single cell suspensions prepared as indicated in Section 2. Cells were stimulated with the 15-mer Gag peptide pool and T cell responses were measured by IFN-γ ELIspot. Unstimulated cells from each sample were used as background controls and were subtracted from each sample before plotting the data. The data represent pooled values, and are representative of two or three experiments. (B) CD4+ and CD8+ T cell responses following three 2× DNA-HIV/2× FPV-HIV immunisation strategies. Mice (n = 10–15 per group) were primed i.m. with 50 μg of 2× DNA-HIV and boosted i.n., i.r. or i.m. with 5 ×106 pfu 2× FPV-HIV 4 weeks apart as indicated in Table 2 (groups 3,6 and 7). Mice were sacrificed 7–8 weeks post-2nd FPV-HIV boost and spleen (left) and genito-rectal nodes (right) were harvested, single cell suspensions prepared, and CD4+ and CD8+ depletions performed using positive selection, as indicated in Section 2. Cells were stimulated with 15-mer Gag peptide pool and T cell responses were measured by IFN-γ ELIspot. Unselected cells (black) were compared with CD4+-depleted (white) and CD8+-depleted (grey) samples. Unstimulated cell responses from each sample were used as background controls and were subtracted from each sample before plotting the data (these ELIspot values were less than 25 SFU). The data represent pooled values, and are representative of two experiments. (C) Serum antibody responses following i.m. 2× DNA-HIV prime i.r or i.m 2× FPV-HIV boost immunisation. Mice (n = 10–15 per group) were primed i.m. with 50 μg of 2× DNA-HIV and boosted i.r or i.m. with 5 ×106 pfu 2× FPV-HIV 4 weeks apart as indicated in Table 2 (groups 6 and 7). HIV-1 p24 Gag-specific serum IgG1 (right) and IgG2A (left) antibody responses were measured pre-immunisation (striped), and at 4 weeks post-2nd DNA (white), 4 weeks post-1st FPV-HIV (grey) and 4 weeks post-2nd FPV HIV (black) boost. Control positive and negative sera were used in these assays. Y-axis shows endpoint titres calculated as in Section 2. The data represent mean ±SEM of 15 individual mice.
3.4. Evaluation of CD4+ and CD8+ T cell responses and antibody responses following i.m. 2× DNA-HIV prime followed by i.n., i.r. or i.m. 2× FPV-HIV booster immunisation
To evaluate the proportions of CD4+ and CD8+ systemic and mucosal T responses generated following each of these immunisation strategies, spleen and genito-rectal nodes were harvested and T cell populations were depleted either for CD4 + or CD8+ as described in Section 2. Flow through were collected (post selection CD8+ or CD4+ cell purity in supernatants were 95–96% respectively), stimulated with the 15-mer overlapping Gag peptide pool and T cell responses were assessed by IFN-γ ELISpot. Results indicate that the i.m./i.r. and i.m./i.m. prime-boost strategies generated good systemic responses, but relatively poor mucosal T cell immunity in genito-rectal nodes. Analyses of CD4-depleted and CD8-depleted T cell populations (supernatants collected after positive selection) indicated that the majority of the T cell response was skewed towards the CD8+ T cell subset (Fig. 3B). In contrast, i.m./i.n. immunisation appeared to generate almost similar levels of Gag-specific CD8+ and CD4+ T cell responses in both spleen and genito-rectal nodes (Fig. 3B). Out of the three immunisation strategies tested, i.m./i.n. prime-boosting appeared to generate the highest levels of mucosal T cell responses to vaccine antigens. Both i.m./i.r. and i.m./i.m. prime-boosting generated significantly lower anti-P24 Gag IgG1 and IgG2a antibody titres in serum (Fig. 3C) compared to i.m./i.n. immunisation (Fig. 2B), although a 2nd FPV-HIV immunisation markedly increased IgG1 responses in all three groups (Figs. 2C and 3C). Purely systemic immunisation (i.m./i.m.) generated the poorest IgG2a responses (endpoint titre < 500) compared to other strategies (Fig. 3C).
3.5. Comparison of heterologous DNA-HIV/FPV-HIV compared to poxvirus/poxvirus (FPV-HIV/VV-HIV) prime-boost immunisation
Next, we compared 2× DNA-HIV/FPV-HIV prime-boost immunisation with a purely poxvirus-based prime-boost regime. Mice were immunised either i.m./i.n. or i.m./i.m with 2× DNA-HIV/1× FPV-HIV or single shot FPV-HIV/VV-HIV as indicated in Table 2. (Note: We have previously shown that i.m VV-HIV/i.n. FPV-HIV does not generate good immunity hence this regime was not tested) [25]. In this study, doses of 100 μg of plasmid DNA per immunisation and/or 1 ×107 pfu of virus were used and all immunisations were performed at 2 weeks interval. (The rationale for using a high doses of rDNA (100 μg), was mainly due to low doses generating poor outcomes, particularly in humans [32], and a recent phase I clinical trial showing doses of 4 mg of rDNA prime/NYVAC-HIV boost strategy generating good immunogenecity in humans [21]. Furthermore, using a single shot of rFPV booster immunisation, albeit at a higher dose, was mainly due to repetitive immunisation with the same viral vector via the same delivery route in our hands has shown to reduce the avidity of vaccine-induced CTL (Ranasinghe unpublished observations).)
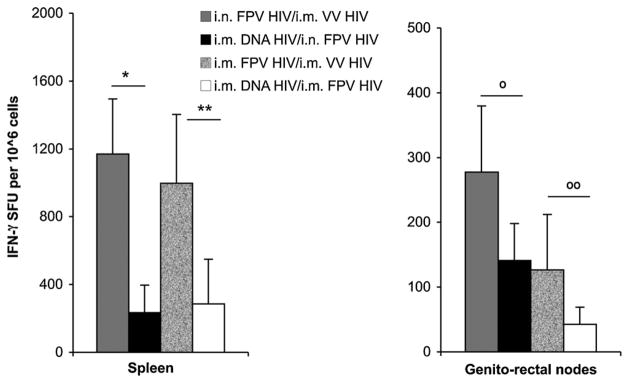
Two weeks following the booster immunisation HIV Gag-specific systemic (spleen) and mucosal (genito-rectal nodes) effector T cell responses were measured and IFN-γ ELISpot data clearly indicated that poxvirus-based prime-boost immunisation generated much greater Gag197–205-specific T cell responses than rDNA/rFPV prime-boost strategy (Fig. 4). The i.n. FPV-HIV/i.m. VV HIV immunisation generated the strongest mucosal and systemic CD8+ T cell responses compared to purely systemic poxvirus delivery as previously shown [25], with higher numbers of Gag197–205-specific systemic and mucosal T cells expressing IFN-γ generated in these mice compared to i.m. DNA-HIV/i.n FPV-HIV immunisation (spleen p = 0.015) (Fig. 4). In contrast, even though purely systemic poxvirus prime-boost immunisation generated significantly higher numbers of Gag197–205-specific T cell responses in spleen compared to i.m. DNA-HIV/i.m FPV-HIV immunisation (spleen p = 0.010), the level of mucosal responses in the genitor-rectal nodes in this group was not highly significant (p = 0.112) (Fig. 4). ELISpot results were also remarkably consistent with KdGag197–205-specific tetramer analysis, indicating that only the mucosal/systemic immunisation strategies were able to generate strong mucosal T cell responses in these experiments.
Fig. 4.
CD8+ T cell responses generated by 2× DNA-HIV/FPV-HIV and to FPV-HIV/VV-HIV immunisation strategies. Mice were: (a) primed i.m. with 100 μg of 2× DNA-HIV and boosted i.n. or i.m. with 1 ×107 pfu FPV-HIV, or (b) primed i.n. or i.m. with 1 ×107 pfu FPV-HIV and boosted with 1 ×107 pfu VV-HIV at 2-week intervals as indicated in Table 2 (groups 8–11). Mice were sacrificed 2 weeks post-boosting and spleen (left) and genito-rectal lymph nodes (right) cells were stimulated with immunodominant H-2Kd-binding AMQMLKETI Gag peptide. CD8+ T cell responses were measured by IFN-γ ELIspot. Unstimulated cells from each sample served as background controls and were subtracted from each sample before plotting the data. The data represent mean ±SD for 3–4 experiments (total n = 12 mice/group). Spleen samples *p = 0.005, **p = 0.038 and genito-rectal node samples °p = 0.078,°°p = 0.141 as determined using the Student’s t-test.
3.6. Evaluation of KdGag197–205-specific T cell avidity and cytokine/chemokine expression following DNA-HIV/FPV-HIV and FPV-HIV/VV-HIV immunisation strategies
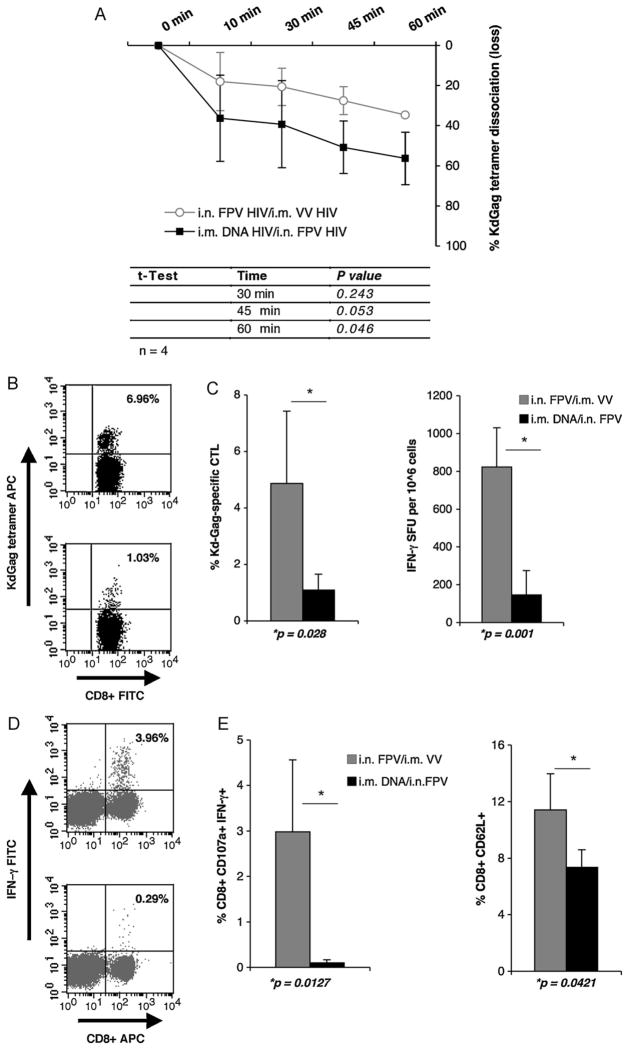
As it is established that high avidity T cells may offer better protection following an infection. To evaluate the avidity of KdGag197–205-specific CTL generated after i.m. DNA-HIV/i.n. FPV-HIV and i.n. FPV-HIV/i.m. FPV-HIV immunisations, splenocytes from immunised mice (n = 4) were used at 2 weeks following booster immunisation in a tetramer dissociation assay, as described elsewhere [26,31]. Splenocytes obtained from mice given i.m. DNA-HIV/i.n. FPV-HIV vaccines showed faster KdGag197–205-specific tetramer disassociation (i.e. were lower avidity) when compared to mice given i.n./i.m poxvirus mucosal/systemic prime-boost vaccine (Fig. 5A). These data demonstrate that poxvirus prime-boost immunisation can generate HIV-specific effector CTL of higher avidity than the DNA poxvirus/immunisation regime (rFPV/rVV vs rDNA/rFPV at 45 min p = 0.053, 60 min p = 0.046). Poxvirus prime-boost immunisation showed enhanced number of tetramer reactive CD8+ T cells (Fig. 5B) and IFN-γ SFU by ELIspot (Fig. 5C). Moreover, the KdGag197–205-specific CTL population generated also showed increased expression of CD107a (a marker correlating with CTL activity), IFN-γ (p = 0.0127) (Fig. 5D) and the CD62L memory marker (p = 0.042) (Fig. 5E).
Fig. 5.
(A) KdGag197–205-specific avidity of cost T cells generated following rDNA and pox-virus prime-boost immunisation. Mice were immunised: (a) i.m. 2× DNA-HIV/i.n. FPV-HIV (black line) or (b) i.n. FPV-HIV/i.m. VV-HIV (grey line) at 2 weeks intervals as indicated in Table 2 (groups 9 and 10). At 14 days following boosting, percentages of KdGag197–205 positive CD8+ T splenocyte dissociation were measured as described in Section 2. The data represent mean ± SD obtained with 4 mice per group. Tetramer loss p values were calculated at 30 min, 45 min and the 60 min time points using two-tailed, two-sample equal variance Student’s t-test and are shown in the bottom panel. The data are representative of at least three experiments. (B–E) HIV-specific effector CD8+ T cell responses following rDNA and poxvirus prime-boost immunisation. Mice were immunised i.m. 2×DNA-HIV/i.n. FPV-HIV (grey) or i.n. FPV-HIV/i.m. VV-HIV (black) at 2 weeks intervals as indicated in Table 2 (groups 9 and 10). 14 days later, KdGag197–205-specific effector T cell responses were measured by (B) tetramer staining (p = 0.028), (C) IFN-γ ELIspot (p = 0.001), (D) ICS of CD107a and IFN-γ (p = 0.0127), and (E) CD8α, CD62L staining (p = 0.0421) as described in Section 2. Representative FACS plots 5B and D, top indicate FPV-HIV/VV-HIV, bottom 2×DNA-HIV/FPV-HIV. All plots upper right quadrants indicate the percentage of tetramer reactive CD8+ T cells (5B) and percentage of CD8+ expressing IFN-γ (5D). Data represent mean +SD of 4 mice per group and p values were determined using two-tailed, two sample equal variance Student’s t-test. When plotting ELIspot and flow cytometry data (C–E), unstimulated cell responses from each sample were used as background controls and these values were subtracted from each sample (ELIspot values were less than 25 SFU). The data are representative of three experiments.
Cytokine antibody arrays indicated that 16 h following KdGag197–205-specific peptide stimulation rFPV/rVV immunised CD8+ splenocytes were able to express a greater range of cytokines and chemokines compared to DNA/poxvirus prime-boost immunisation. While IFN-γ expression was highest, the cytokines IL-3, IL-6 and chemokines GM-CSF, CCL3, CCL5 and CCL9 were also significantly up-regulated (showed over 3-fold increase) when compared to the DNA immunisation (Table 3).
Table 3.
Evaluation of cytokines/chemokines using antibody array.
| Cytokine/chemokine |
a FPV/VV % absorbance |
b DNA/FPV % absorbance |
a/b Fold increase |
|---|---|---|---|
| IFN-γ | 48.90 | 2.07 | 23.65 |
| IL-3 | 2.89 | 0.16 | 18.37 |
| IL-6 | 2.35 | 0.05 | 44.71 |
| GM-CSF | 1.07 | 0.24 | 4.42 |
| MIP-1-α (CCL-3) | 2.73 | 0.10 | 28.03 |
| RANTES (CCL-5) | 1.36 | 0.43 | 3.14 |
| MIP-1-γ (CCL-9) | 4.04 | 0.92 | 4.40 |
Fourteen days following i.n. FPV-HIV/i.m. VV-HIV (a) and i.m. 2× DNA-HIV/i.n. FPV-HIV (b) prime-boost immunisation, 2 ×106 splenocytes (from pooled spleens, n = 4) were cultured for 16 h in the presence immunodominant H-2Kd-binding AMQMLKETI Gag peptide. Supernatants were collected and antibody arrays were performed according to Ray Biotech Inc., USA instructions. Protein expression was calculated using Multi Gauge V3.0 soft wear density linear calibration analysis. Fold increases were calculated by diving the two % absorbance values (a/b). Protein increases over 3-fold are shown in this table.
3.7. Evaluating protective efficacy following DNA-HIV/FPV-HIV and FPV-HIV/VV-HIV immunisation strategies
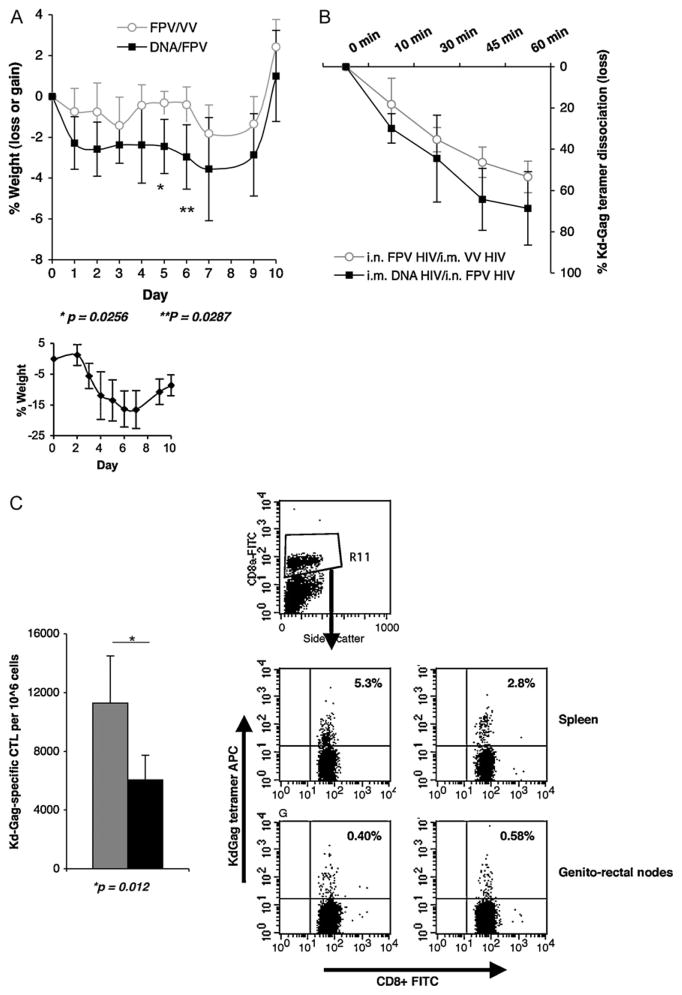
To evaluate the protective efficacy of mucosal/systemic prime-boosting, mice were challenged with 50 unites of influenza virus expressing the KdGag197–205 immunodominant epitope, 7 weeks following booster immunisation and body weights were monitored daily thereafter for 10 days. In these studies mice maintaining weight and not succumbing to flu infection is considered as a measure of protective immunity. Interestingly, i.m. 2× DNA-HIV/i.n. FPV-VV immunised mice lost more weight post-challenge compared to the i.n. FPV-HIV/i.m. VV-HIV. Mice in both vaccine groups recovered from infection and no differences in body weights were observed at 10 days (Fig. 6A). The control unimmunised, mice lost up to 20–24% of their body weight by day 7 and showed signs of some recovery by day 10 (Fig. 6A bottom graph). While the dissociation rate of CD8+ T splenocytes from mice given FPV-HIV/VV-HIV vaccines was slower compared to the 2×DNA-HIV/FPV-HIV immunised mice, no significant differences in T cell avidity were observed between the two groups following recovery (Fig. 6B), which is consistent with the weight profile observed at 10 days.
Fig. 6.
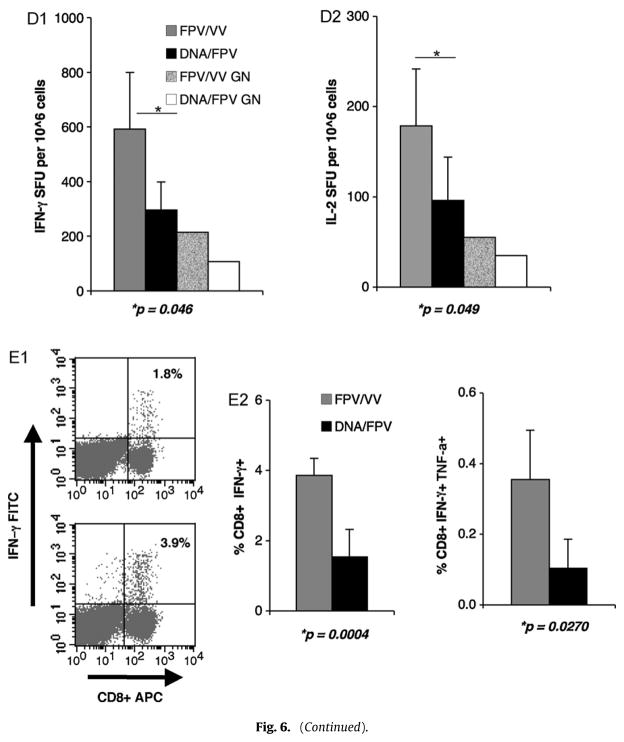
(A and B) Protective immunity and CTL avidity following PR8-KdGag197–205 challenge. BALB/c mice were immunised i.m. 2× DNA-HIV/i.n. FPV-HIV (grey line) or i.n. FPV-HIV/i.m. VV-HIV (black line) as indicated in Table 2 (groups 9 and 10). 6 weeks post-booster immunisation (top) or unimmunised (bottom) mice were challenged mucosally (i.n.) with 50 units influenza virus PR8 expressing KdGag197–205 epitope. (A) Body weight was monitored for 10 days and (B) avidity of KdGag197–205-specific T cells in spleen was also measured at 10 days following recovery, by tetramer dissociation assay, as described in Section 2. The data represent mean ±SD obtained with 5 mice per group and p values are calculated using two-tailed, two sample equal variance Student’s t-test. The data are representative of three experiments. (C–E) Memory CD8+ T cell responses following PR8-KdGag197–205 challenge. BALB/c mice were immunised i.m. 2× DNA-HIV/i.n. FPV-HIV (grey) or i.n. FPV-HIV/i.m. VV-HIV (black) as indicated in Table 2 (groups 9 and 10). 6 weeks after the booster immunisation, mice were challenged mucosally (i.n.) with 50 units influenza virus PR8 expressing the KdGag197–205 epitope. Following PR8-KdGag197–205 challenge, memory CD8+ T cell responses were measured by (C) KdGag197–205 tetramer staining (p = 0.012), (D1) IFN-γ ELIspot (p = 0.046), (D2) IL-2 ELIspot (p = 0.046), (E1) CD8+ IFN-γ+ ICS (p = 0.0004) and (E2) CD8+ IFN-γ+ TNF-α+ ICS (p = 0.0270) as described in Section 2. (C) KdGag197–205 tetramer staining data are represented as total number of KdGag197–205-specific CTL per 106 splenocytes and FACS plots (C) upper quadrants represent the percentage of CD8+ that are tetramer reactive, measured in spleen and genito-rectal nodes. IFN-γ ICS representative FACS plots (E1), top indicates 2× DNA-HIV/FPV-HIV and bottom FPV-HIV/VV-HIV, the upper right quadrant indicates the percentage CD8+ expressing IFN-γ. When plotting ELIspot and flow cytometry data, unstimulated cells from each sample were used as the background control and this value was subtracted from each sample. Data represent mean +SD of 5 mice per group and p values were determined using two-tailed, two sample equal variance Student’s t-test. The data are representative of three experiments.
The tetramer staining analysis indicated that rDNA/rFPV prime-boost immunisation generated lower numbers of KdGag197–205-specific CD8+ T cells compared to the FPV-HIV/VV-HIV (p = 0.012) (Fig. 6C). Similar results were obtained with ELISpot assays showing significantly higher numbers of Gag-specific systemic CD8+ T cells expressing IFN-γ (p = 0.046) and IL-2 (p = 0.049) compared to the i.m. DNA-HIV/i.n. FPV-HIV vaccine group (Fig. 6D1 and D2). Good mucosal responses were also observed in genio-rectal nodes (Fig. 6D1 and D2). Similarly, significantly higher systemic CD8+ IFN-γ+ (p = 0.0004) and CD8+ IFN-γ+ TNF-α+ (p = 0.027) T cells were alsoobservedfollowingi.n.FPV-HIV/i.m.VV-HIVimmunisationand challenge with influenza virus expressing KdGag197–205 (Fig. 6E1 and E2).
4. Discussion
The capacity of systemic rDNA/virus vector heterologous prime-boost immunisation to elicit strong, protective T cell-mediated immune responses against HIV or SIV challenge in non-human primates has been extensively studied [11,33–36]. However, following disappointing outcomes of HIV-1 rDNA/virus prime-boost phase I clinical trials, there is now a great push to improve the immunogenicity of DNA vaccines in humans and also to develop vaccines that generate good mucosal immunity, since mucosae are the primary sites of HIV infection. Here, we have performed a comprehensive study of rDNA and viral vector vaccine administration and evaluated the capacity of combined mucosal/systemic delivery routes to enhance both mucosal and systemic humoral and cell-mediated immunity to encoded HIV-1 vaccine antigens. In the second part of the study, we have mainly compared the best combined rDNA viral prime-boost immunisation strategy (rDNA/rFPV) to the best pox viral prime-boost immunisation strategy (rFPV/rVV) and evaluated both their protective efficacy and immunogenicity against an immunodominant KdGag197–205 epitope, including the avidity of vaccine-induced CD8+ T cells.
Delivery of rDNA vaccines i.n., either with or without lipid complexes, or i.m via needle delivery, has largely been ineffective at generating good mucosal immunity to encoded vaccine antigens [13], except after i.n. co-adminstration of cholera toxin has albeit with serious side effects [37,38]. We have previously tested i.n. delivery of lipid-complexed DNA followed by i.n. boosting with FPV HIV constructs and have found that this approach generated sub-optimal T cell immune responses in both mice and non-human primates (Ranasinghe and Ramsay unpublished observations) [24]. In the present study we compared different prime-boost immunisation strategies (i.n. 2× DNA-HIV/i.m. 2× FPV-HIV vs i.m. 2× DNA-HIV/i.n. 2× FPV-HIV) and have found that mucosal delivery of rFPV was greatly superior at generating strong sustained mucosal immune responses compared to i.n. DNA delivery. These data indicate that rFPV is an excellent and safe mucosal delivery vector similar to rMVA [39–41] or the poxvirus vector tiantan vaccinia (TiVV) [42] or NYVAC [43]. Our phase I human clinical trials have also clearly demonstrated that systemic delivery of rFPV was extremely safe in humans [1], although the effects of rFPV following mucosal delivery have not yet been clinically tested in humans. Current observations may be important for the development of clinical trials of poxvirus vector-based mucosal HIV-1 vaccines in the future.
In the current study, strong sustained memory mucosal T cell responses were observed in mucosal immune compartment (genito-rectal nodes, vaginal tissues and lung tissues), as well as in the systemic immune compartment (spleen) following i.m. 2× DNA-HIV/i.n. 2× FPV-HIV immunisation. These findings are also consistent with those of a recent i.n. TiVV-HIV-1 Gag prime/i.m. rDNA boost immunisation study demonstrating enhanced Gag-specific mucosal and systemic T and B cell immunity following i.m. rDNA delivery [42]. Surprisingly, only low-level mucosal T cell responses were observed following i.m. 2× DNA-HIV/i.r. 2× FPV-HIV immunisation, this could possibly be due to poor i.r. uptake of rFPV in mice unlike in macaques [24]. On the contrary, following i.m. 2×DNA-HIV/i.m. 2×FPV-HIV immunisation even though lower IFN-γSFU was observed in genito-rectal nodes, similar levels of IFN-γ SFU to i.m. 2×DNA-HIV/i.n. 2× FPV-HIV delivery was recorded in vaginal tissues, lung tissues and spleen. Few studies have shown that purely systemic vaccination can induce mucosal responses, but whether these responses are effective or sustained long term has been highly debated [44]. These studies further substantiate that the route of vaccine delivery can significantly influence the magnitude, immunodominance hierarchy and/or duration of resultant antibody and CTL responses [25,45,46].
Furthermore, the T cell depletion studies also demonstrated the influence of route of vaccine delivery on immunity. Interestingly, following i.m. 2× DNA-HIV/i.n. 2× FPV-HIV prime-boost immunisation enhanced systemic memory CD8+ T cell responses as well as CD4+ T cell responses, were detected compared to purely systemic (i.m./i.m.) or i.m./i.r immunisation strategies. The enhanced CD4+ T cell responses following i.m./i.n. delivery may also substantiate the elevated serum p24 Gag-specific IgG1 and IgG2a antibody levels that were observed following this vaccination. Surprisingly, low IgG2a serum antibody levels were observed following purely systemic routes of immunisation, and out of the three DNA immunisation strategies tested, only the i.m./i.n. combination generated measurable mucosal antibody responses (IgG and IgA) to p24 Gag. The reason why only the second i.n. rFPV booster immunisation (not the second i.r. or i.m. rFPV), enhanced the magnitude of antibody response, warrants further investigation. It is noteworthy that in previous studies, we have also found that single i.n. FPV-HIV/i.m. VV-HIV (poxvirus/poxvirus) prime-boost immunisation did not generate good p24 Gag-specific IgGantibody responses[25]. These current observations further indicate that the route of vaccine delivery and/or number of booster immunisations received can be critical factors when evaluating novel vector vaccine strategies for HIV-1.
Recently, we have shown that avidity of HIV-specific CD8+ T cells can also be modulated by the route of vaccine delivery [26]. Even though avidity of vaccine-induced T cell responses is an important factor in evaluating protective immunity [47] it is often overlooked when evaluating vaccine responses, possibly due to difficulties associated with assay techniques. Comparison of i.m. 2× DNA-HIV/i.n. FPV HIV delivery with poxvirus prime-boost immunisation, clearly showed that the latter approach generated enhanced systemic T cell immunity as measured by IFN-γ ELISpot and tetramer staining. The combined mucosal (i.n.) and systemic (i.m.) FPV-HIV/VV-HIV immunisation generated the most robust systemic and mucosal T cell responses against the KdGag197–205 epitope. Furthermore, when the avidities of vaccine-induced CD8+ T cells were compared following i.m. 2× DNA-HIV/i.n. FPV-HIV and i.n. FPV-HIV/i.m. VV-HIV immunisation strategies it was found that, the poxvirus/poxvirus prime-boost immunisation regime generated CTL of heightened avidity correlating with better protection against influenza virus- KdGag197–205 mucosal challenge. In an elegant study, Belyakov et al. have also shown that a mucosal peptide prime/poxvirus boost immunisation can induce greater number of high avidity mucosal CD8+ T cells that can control systemic dissemination of i.r. administered pathogenic SHIV in rhesus macaques and this protection correlated better with induction of mucosal CD8+ T cells than systemic CD8+ T cells [48]. These results are highly consistent with our earlier comparisons of T cell avidities resulting from combined mucosal/systemic poxvirus immunisation strategies, with purely systemic immunisation regimes, eliciting that the latter immunisation strategy generated CTL of lower avidity as measured by tetramer dissociation [26], or lower protection following influenza virus- KdGag197–205 mucosal challenge (Ranasinghe unpublished observations), similar to that observed here following i.m. 2× DNA-HIV/i.n. FPV-HIV immunisation. Interestingly, the magnitude of mucosal responses generated by this strategy was very similar to that of pure systemic (i.m./i.m.) FPV-HIV/VV-HIV delivery, although levels of systemic CD8+ T cell responses were greater in the latter case. These observations confirm that the magnitude of T cell responses as measured by IFN-γ production does not always correlate with T cell avidity or with protective efficacy [26,31], serving to underline a major caveat in the interpretation of vaccine studies.
Following combined mucosal/systemic prime-boost immunisation, the majority of Gag-specific IFN-γ producing CD8+ T cells were found to be CD107a-positive, suggestive of high levels of cytolytic activity. Furthermore, increased levels of CD62L expression indicated that a greater percentage of central memory CTL were generated following poxvirus prime-boosting compared to i.m. 2× DNA-HIV/i.n. FPV-HIV immunisation. More significant weight loss after challenge with influenza-KdGag197–205 virus was observed in mice vaccinated via rDNA/rFPV prime-boost compared to the i.n./i.m. poxvirus/poxvirus regime, especially at day 5 and 6 post-challenge (p = 0.0256 and p = 0.0287 respectively), although mice in both groups recovered by day 10. It is noteworthy that even the unimmunised mice, that lost up to 20–25% of their body weight, showed significant weight gain by day 9 post-challenge, a common finding in this influenza challenge model. Interestingly, CTL avidity curves (Figs. 5A and 6B) were also consistent with the protection data. Evaluation of T cell avidities at 5–6 days following influenza-KdGag197–205 virus challenge may reveal greater differences between the two vaccine groups and further studies have been designed to clarify this point. Previous studies have established that (i) high avidity CD8+ T cells are generated during early stages of pathogen infection but subsequently low-avidity CD8+ T cells can persist in chronic infections, and (ii) also T cell populations can be expanded following infection and that avidity modulation, either through infection or immunisation, may play an important role in the nature of the CD8+ T cell responses that are generated in vivo [49].
Antigen-specific polyfunctional CD4+ or CD8+ T cells that express IFN-γ, IL-2 and TNF-α are thought to be a hallmark of protective immunity [50–52]. Indeed, there are distinct differences in the potency of effector cells based on their polyfunctional cytokine secretion profiles [53]. However, the association of high avidity T cells with polyfunctional cytokine effector function is still unclear [54], although our studies indicate that these two functional activities may be closely related. Mice that received i.m. 2×DNA-HIV/i.n. FPV-HIV immunisation had reduced numbers of IFN-γ, IL-2 and TNF-α producing CD8+ T cells and also a reduction in total numbers of CD8+ T cells post challenge. Interestingly, antibody arrays also indicated that unlike rDNA/poxvirus prime-boost immunisation, the poxvirus/poxvirus prime-boost immunisation generated effector CD8+ T cells that expressed a wide range of cytokines and chemokines (IFN-γ, IL-3, IL-6, GM-CSF, CCL3, CCL5, and CCL9) 16 h following peptide stimulation. Our recent findings indicate that if profiles were measured at an earlier time point (4–5 h) a much broader cytokine profile would have been observed (i.e. IL-2 and TNF-α), as expression kinetics of different cytokines/chemokines are highly time dependent (Ranasinghe unpublished observations).
In conclusion, following rDNA vaccination in order to generate heightened mucosal T cell immunity and antibody responses two consecutive FPV-HIV booster immunisations were required. Out of the rDNA delivery strategies tested, i.m. 2× DNA-HIV/i.n. 2× FPV-HIV prime-boost immunisation generated the best mucosal and systemic cell-mediated and antibody responses to encoded vaccine antigens. However, the poxvirus/poxvirus prime-boost strategy (i.n. FPV-HIV/i.m. VV-HIV) elicited more robust and sustained effector/memory mucosal and systemic CD8+ T cell responses, with enhanced cytokine/chemokine profiles, T cell avidity and protective efficacy compared to the heterologous i.m. 2× DNA-HIV/i.n. FPV-HIV prime-boost immunisation. Hence, we believe that combined mucosal/systemic prime-boost immunisation strategies have considerable potential for the further development of HIV-1 vaccines.
Acknowledgments
The authors would like to thank the Bio-Molecular Resource Facility at The John Curtin School of Medical Research, The Australian National University for synthesising the HIV-specific tetramers. Rebecca Walker, Donna Woltring, Sherry Tu and Jill Medveczky for their technical assistance with the project. This work was supported by National Institutes of Health USA (NIH) HIV Vaccine Design and Development Team award N01-AI-05395 (AR, IR, DB) and R01-AI-058810 (AR); and the Australian National Health and Medical Research Council project grant award 525431 (CR).
References
- 1.Kelleher AD, Puls RL, Bebbington M, Boyle D, Ffrench R, Kent SJ, et al. A randomised, placebo-controlled phase I trial of DNA prime, recombinant fowlpox virus boost prophylactic vaccine for HIV-1. AIDS. 2006;20(2):294–7. doi: 10.1097/01.aids.0000199819.40079.e9. [DOI] [PubMed] [Google Scholar]
- 2.Sekaly RP. The failed HIV Merck vaccine study: a step back or a launching point for future vaccine development? J Exp Med. 2008;205(1):7–12. doi: 10.1084/jem.20072681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaul R, Plummer FA, Kimani J, Dong T, Kiama P, Rostron T, et al. HIV-1-specific mucosal CD8+ lymphocyte responses in the cervix of HIV-1-resistant prostitutes in Nairobi. J Immunol. 2000;164:1602–11. doi: 10.4049/jimmunol.164.3.1602. [DOI] [PubMed] [Google Scholar]
- 4.Kozlowski PA, Neutra MR. The role of mucosal immunity in prevention of HIV transmission. Curr Mol Med. 2003;3(3):217–28. doi: 10.2174/1566524033479852. [DOI] [PubMed] [Google Scholar]
- 5.Stevceva L, Strober W. Mucosal HIV vaccines: where are we now? Curr HIV Res. 2004;2(1):1–10. doi: 10.2174/1570162043485004. [DOI] [PubMed] [Google Scholar]
- 6.Belyakov IM, Ahlers JD, Berzofsky JA. Mucosal AIDS vaccines: current status and future directions. Expert Rev Vaccines. 2004;3(4 Suppl):S65–73. doi: 10.1586/14760584.3.4.s65. [DOI] [PubMed] [Google Scholar]
- 7.Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280(5362):427–31. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 8.Ogra PL, Faden H, Welliver RC. Vaccination strategies for mucosal immune responses. Clin Microbiol Rev. 2001;14(2):430–45. doi: 10.1128/CMR.14.2.430-445.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Somogyi P, Frazier J, Skinner MA. Fowlpox virus host range restriction: gene expression. DNA replication, and morphogenesis in nonpermissive mammalian cells. Virology. 1993;197(1):439–44. doi: 10.1006/viro.1993.1608. [DOI] [PubMed] [Google Scholar]
- 10.Leong KH, Ramsay AJ, Morin MJ, Robinson HL, Boyle DB, Ramshaw IA. Molecular approaches to the control of infectious diseases. In: Brown F, Chanock H, Norrby E, editors. Vaccine. Vol. 95. 1995. pp. 327–31. [Google Scholar]
- 11.Kent SJ, Zhao A, Best SJ, Chandler JD, Boyle DB, Ramshaw IA. Enhanced T-cell immunogenicity and protective efficacy of a human immunodeficiency virus type 1 vaccine regimen consisting of consecutive priming with DNA and boosting with recombinant fowlpox virus. J Virol. 1998;72(12):10180–8. doi: 10.1128/jvi.72.12.10180-10188.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyle DB, Anderson MA, Amos R, Voysey R, Coupar BE. Construction of recombinant fowlpox viruses carrying multiple vaccine antigens and immunomodulatory molecules. Biotechniques. 2004;37(1):104–6. 8–11. doi: 10.2144/04371RR02. [DOI] [PubMed] [Google Scholar]
- 13.Barnett SW, Rajasekar S, Legg H, Doe B, Fuller DH, Haynes JR, et al. Vaccination with HIV-1 gp120 DNA induces immune responses that are boosted by a recombinant gp120 protein subunit. Vaccine. 1997;15(8):869–73. doi: 10.1016/s0264-410x(96)00264-2. [DOI] [PubMed] [Google Scholar]
- 14.Caver TE, Lockey TD, Srinivas RV, Webster RG, Hurwitz JL. A novel vaccine regimen utilizing DNA, vaccinia virus and protein immunizations for HIV-1 envelope presentation. Vaccine. 1999;17(11–12):1567–72. doi: 10.1016/s0264-410x(98)00355-7. [DOI] [PubMed] [Google Scholar]
- 15.Otten G, Schaefer M, Greer C, Calderon-Cacia M, Coit D, Kazzaz J, et al. Induction of broad and potent anti-human immunodeficiency virus immune responses in rhesus macaques by priming with a DNA vaccine and boosting with protein-adsorbed polylactide coglycolide microparticles. J Virol. 2003;77(10):6087–92. doi: 10.1128/JVI.77.10.6087-6092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Epstein JE, Charoenvit Y, Kester KE, Wang R, Newcomer R, Fitzpatrick S, et al. Safety, tolerability, and antibody responses in humans after sequential immunization with a PfCSP DNA vaccine followed by the recombinant protein vaccine RTS, S/AS02A. Vaccine. 2004;22(13–14):1592–603. doi: 10.1016/j.vaccine.2004.01.031. [DOI] [PubMed] [Google Scholar]
- 17.Stambas J, Brown SA, Gutierrez A, Sealy R, Yue W, Jones B, et al. Long lived multi-isotype anti-HIV antibody responses following a prime-double boost immunization strategy. Vaccine. 2005;23(19):2454–64. doi: 10.1016/j.vaccine.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 18.Dale CJ, De Rose R, Wilson KM, Croom HA, Thomson S, Coupar BE, et al. Evaluation in macaques of HIV-1 DNA vaccines containing primate CpG motifs and fowlpoxvirus vaccines co-expressing IFNgamma or IL-12. Vaccine. 2004;23(2):188–97. doi: 10.1016/j.vaccine.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 19.Mwau M, Cebere I, Sutton J, Chikoti P, Winstone N, Wee EG, et al. A human immunodeficiency virus 1 (HIV-1) clade A vaccine in clinical trials: stimulation of HIV-specific T-cell responses by DNA and recombinant modified vaccinia virus Ankara (MVA) vaccines in humans. J Gen Virol. 2004;85(Pt 4):911–9. doi: 10.1099/vir.0.19701-0. [DOI] [PubMed] [Google Scholar]
- 20.Guimaraes-Walker A, Mackie N, McCormack S, Hanke T, Schmidt C, Gilmour J, et al. Lessons from IAVI-006, a phase I clinical trial to evaluate the safety and immunogenicity of the pTHr. HIVA DNA and MVA. HIVA vaccines in a prime-boost strategy to induce HIV-1 specific T-cell responses in healthy volunteers. Vaccine. 2008;26(51):6671–7. doi: 10.1016/j.vaccine.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 21.McCormack S, Stohr W, Barber T, Bart PA, Harari A, Moog C, et al. EV02: a phase I trial to compare the safety and immunogenicity of HIV DNA-C prime-NYVAC-C boost to NYVAC-C alone. Vaccine. 2008;26(25):3162–74. doi: 10.1016/j.vaccine.2008.02.072. [DOI] [PubMed] [Google Scholar]
- 22.Alexander-Miller MA, Leggatt GR, Sarin A, Berzofsky JA. Role of antigen CD8, and cytotoxic T lymphocyte (CTL) avidity in high dose antigen induction of apoptosis of effector CTL. J Exp Med. 1996;184(2):485–92. doi: 10.1084/jem.184.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.La Gruta NL, Turner SJ, Doherty PC. Hierarchies in cytokine expression profiles for acute and resolving influenza virus-specific CD8+ T cell responses: correlation of cytokine profile and TCR avidity. J Immunol. 2004;172(9):5553–60. doi: 10.4049/jimmunol.172.9.5553. [DOI] [PubMed] [Google Scholar]
- 24.Kent SJ, Dale CJ, Ranasinghe C, Stratov I, De Rose R, Chea S, et al. Mucosally-administered human-simian immunodeficiency virus DNA and fowlpoxvirus-based recombinant vaccines reduce acute phase viral replication in macaques following vaginal challenge with CCR5-tropic SHIV(SF162P3) Vaccine. 2005;23:5009–21. doi: 10.1016/j.vaccine.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 25.Ranasinghe C, Medveczky JC, Woltring D, Gao K, Thomson S, Coupar BEH, et al. Evaluation of fowlpox-vaccinia virus prime-boost vaccine strategies for high-level mucosal and systemic Iimmunity against HIV-1. Vaccine. 2006;24:5881–95. doi: 10.1016/j.vaccine.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 26.Ranasinghe C, Turner SJ, McArthur C, Sutherland DB, Kim JH, Doherty PC, et al. Mucosal HIV-1 pox virus prime-boost immunization induces high-avidity CD8+ T cells with regime-dependent cytokine/granzyme B profiles. J Immunol. 2007;178(4):2370–9. doi: 10.4049/jimmunol.178.4.2370. [DOI] [PubMed] [Google Scholar]
- 27.Boyle DB, Coupar BE, Both GW. Multiple-cloning-site plasmids for the rapid construction of recombinant poxviruses. Gene. 1985;35:169–77. doi: 10.1016/0378-1119(85)90169-6. [DOI] [PubMed] [Google Scholar]
- 28.Coupar BEH, Purcell DFJ, Thomson SA, Ramshaw IA, Kent SJ, Boyle DB. Fowlpox virus vaccines for HIV and SIV clinical and pre-clinical trials. Vaccine. 2006;24(9):1378–88. doi: 10.1016/j.vaccine.2005.09.044. [DOI] [PubMed] [Google Scholar]
- 29.Cukalac T, Moffat JM, Venturi V, Davenport MP, Doherty PC, Turner SJ, et al. Narrowed TCR diversity for immunised mice challenged with recombinant influenza A-HIV Env(311–320) virus. Vaccine. 2009;27(48):6755–61. doi: 10.1016/j.vaccine.2009.08.079. [DOI] [PubMed] [Google Scholar]
- 30.Sexton A, De Rose R, Reece JC, Alcantara S, Loh L, Moffat JM, et al. Evaluation of recombinant influenza virus-simian immunodeficiency virus vaccines in macaques. J Virol. 2009;83(15):7619–28. doi: 10.1128/JVI.00470-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ranasinghe C, Ramshaw IA. Immunisation route-dependent expression of IL-4/IL-13 can modulate HIV-specific CD8(+) CTL avidity. Eur J Immunol. 2009;39(7):1819–30. doi: 10.1002/eji.200838995. [DOI] [PubMed] [Google Scholar]
- 32.De Rose R, Sullivan MT, Dale CJ, Kelleher AD, Emery S, Cooper DA, et al. Dose–response relationship of DNA and recombinant fowlpox virus prime-boost HIV vaccines: implications for future trials. Hum Vaccine. 2006;2(3):134–6. doi: 10.4161/hv.2940. [DOI] [PubMed] [Google Scholar]
- 33.Amara RR, Villinger F, Altman JD, Lydy SL, O’Neil SP, Staprans SI, et al. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science. 2001;292(5514):69–74. doi: 10.1126/science.1058915. [DOI] [PubMed] [Google Scholar]
- 34.Horton H, Vogel TU, Carter DK, Vielhuber K, Fuller DH, Shipley T, et al. Immunization of rhesus macaques with a DNA prime/modified vaccinia virus Ankara boost regimen induces broad simian immunodeficiency virus (SIV)-specific T-cell responses and reduces initial viral replication but does not prevent disease progression following challenge with pathogenic SIVmac239. J Virol. 2002;76(14):7187–202. doi: 10.1128/JVI.76.14.7187-7202.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casimiro DR, Wang F, Schleif WA, Liang X, Zhang ZQ, Tobery TW, et al. Attenuation of simian immunodeficiency virus SIVmac239 infection by prophylactic immunization with dna and recombinant adenoviral vaccine vectors expressing Gag. J Virol. 2005;79(24):15547–55. doi: 10.1128/JVI.79.24.15547-15555.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Rose R, Batten CJ, Smith MZ, Fernandez CS, Peut V, Thomson S, et al. Comparative efficacy of subtype AE simian-human immunodeficiency virus priming and boosting vaccines in pigtail macaques. J Virol. 2007;81(1):292–300. doi: 10.1128/JVI.01727-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Imaoka K, Miller CJ, Kubota M, McChesney MB, Lohman B, Yamamoto M, et al. Nasal immunisation of nonhuman primates with simian immunodeficiency virus p55gag and cholera toxin adjuvant induces Th1/Th2 help for virus-specific immune responses in reproductive tissues. J Immunol. 1998;161:5952–8. [PubMed] [Google Scholar]
- 38.Hagiwara Y, Kawamura YI, Kataoka K, Rahima B, Jackson RJ, Komase K, et al. A second generation of double mutant cholera toxin adjuvants: enhanced immunity without intracellular trafficking. J Immunol. 2006;177(5):3045–54. doi: 10.4049/jimmunol.177.5.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gherardi MM, Esteban M. Recombinant poxviruses and mucosal vaccine vectors. J Gen Virol. 2005;86:2925–36. doi: 10.1099/vir.0.81181-0. [DOI] [PubMed] [Google Scholar]
- 40.Belyakov IM, Ahlers JD, Nabel GJ, Moss B, Berzofsky JA. Generation of functionally active HIV-1 specific CD8+ CTL in intestinal mucosa following mucosal, systemic or mixed prime-boost immunization. Virology. 2008;381(1):106–15. doi: 10.1016/j.virol.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramirez JC, Finke D, Esteban M, Kraehenbuhl JP, Acha-Orbea H. Tissue distribution of the Ankara strain of vaccinia virus (MVA) after mucosal or systemic administration. Arch Virol. 2003;148(5):827–39. doi: 10.1007/s00705-003-0006-z. [DOI] [PubMed] [Google Scholar]
- 42.Huang X, Liu L, Ren L, Qiu C, Wan Y, Xu J. Mucosal priming with replicative Tiantan vaccinia and systemic boosting with DNA vaccine raised strong mucosal and systemic HIV-specific immune responses. Vaccine. 2007;25(52):8874–84. doi: 10.1016/j.vaccine.2007.08.066. [DOI] [PubMed] [Google Scholar]
- 43.Corbett M, Bogers WM, Heeney JL, Gerber S, Genin C, Didierlaurent A, et al. Aerosol immunization with NYVAC and MVA vectored vaccines is safe, simple, and immunogenic. Proc Natl Acad Sci USA. 2008;105(6):2046–51. doi: 10.1073/pnas.0705191105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Belyakov IM, Ahlers JD. Comment on “trafficking of antigen-specific CD8+ T lymphocytes to mucosal surfaces following intramuscular vaccination”. J Immunol. 2009;182(4):1779. doi: 10.4049/jimmunol.0990002. [author reply −80] [DOI] [PubMed] [Google Scholar]
- 45.Lehner T, Wang Y, Cranage M, Bergmeier LA, Mitchell E, Tao L, et al. Protective mucosal immunity elicited by targeted iliac lymph node immunization with a subunit SIV envelope and core vaccine in macaques. Nat Med. 1996;2(7):767–75. doi: 10.1038/nm0796-767. [DOI] [PubMed] [Google Scholar]
- 46.Ito K, Shinohara N, Kato S. DNA immunization via intramuscular and intradermal routes using a gene gun provides different magnitudes and durations on immune response. Mol Immunol. 2003;39(14):847–54. doi: 10.1016/s0161-5890(03)00024-5. [DOI] [PubMed] [Google Scholar]
- 47.Alexander-Miller MA. High-avidity CD8+ T cells: optimal soldiers in the war against viruses and tumors. Immunol Res. 2005;31(1):13–24. doi: 10.1385/IR:31:1:13. [DOI] [PubMed] [Google Scholar]
- 48.Belyakov IM, Kuznetsov VA, Kelsall B, Klinman D, Moniuszko M, Lemon M, et al. Impact of vaccine-induced mucosal high-avidity CD8+ CTLs in delay of AIDS viral dissemination from mucosa. Blood. 2006;107(8):3258–64. doi: 10.1182/blood-2005-11-4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kroger CJ, Alexander-Miller MA. Cutting edge: CD8+ T cell clones possess the potential to differentiate into both high- and low-avidity effector cells. J Immunol. 2007;179(2):748–51. doi: 10.4049/jimmunol.179.2.748. [DOI] [PubMed] [Google Scholar]
- 50.Duvall MG, Jaye A, Dong T, Brenchley JM, Alabi AS, Jeffries DJ, et al. Maintenance of HIV-specific CD4+ T cell help distinguishes HIV-2 from HIV-1 infection. J Immunol. 2006;176(11):6973–81. doi: 10.4049/jimmunol.176.11.6973. [DOI] [PubMed] [Google Scholar]
- 51.Aagaard CS, Hoang TT, Vingsbo-Lundberg C, Dietrich J, Andersen P. Quality and vaccine efficacy of CD4+ T cell responses directed to dominant and subdominant epitopes in ESAT-6 from Mycobacterium tuberculosis. J Immunol. 2009;183(4):2659–68. doi: 10.4049/jimmunol.0900947. [DOI] [PubMed] [Google Scholar]
- 52.Ahmed N, Gottschalk S. How to design effective vaccines: lessons from an old success story. Expert Rev Vaccines. 2009;8(5):543–6. doi: 10.1586/erv.09.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferre AL, Hunt PW, Critchfield JW, Young DH, Morris MM, Garcia JC, et al. Mucosal immune responses to HIV-1 in elite controllers: a potential correlate of immune control. Blood. 2009;113(17):3978–89. doi: 10.1182/blood-2008-10-182709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harari A, Cellerai C, Enders FB, Kostler J, Codarri L, Tapia G, et al. Skewed association of polyfunctional antigen-specific CD8 T cell populations with HLA-B genotype. Proc Natl Acad Sci USA. 2007;104(41):16233–8. doi: 10.1073/pnas.0707570104. [DOI] [PMC free article] [PubMed] [Google Scholar]