Abstract
The antral follicle constitutes a complex and regulated ovarian microenvironment that influences oocyte quality. Oxidative stress is a cellular state that may play a role during folliculogenesis and oogenesis, although direct supporting evidence is currently lacking. We thus evaluated the expression of the three isoforms (SOD1, 2, 3) of the enzymatic antioxidant superoxide dismutase in all of the cellular (granulosa cells, cumulus cells, oocytes) and extracellular (follicular fluid) compartments of the follicle. Comparisons were performed in bovine ovaries across progressive stages of antral follicular development. Follicular fluid possessed increased amounts of SOD1, 2, 3 in small when compared to large antral follicles; concomitantly, total SOD activity was highest in follicular fluids from smaller diameter follicles. SOD1, 2, and 3 proteins were expressed in granulosa cells without any fluctuations with follicle sizes. All three SOD isoforms were present but distributed differently in oocytes from small, medium, or large antral follicles. Cumulus cells expressed high levels of SOD3, some SOD2, but no detectable SOD1. Our studies provide a temporal and spatial expression profile of the three SOD isoforms in the different compartments of the developing bovine antral follicles. These results lay the ground for future investigations into the potential regulation and roles of antioxidants during folliculogenesis and oogenesis.
INTRODUCTION
Good quality oocytes are a prerequisite to a multitude of clinical and agricultural applications. There is thus a critical need to identify and understand the cellular and molecular factors that influence oocyte and follicle health during antral folliculogenesis. The antral follicle constitutes the ovarian microenvironment within which the oocyte undergoes key developmental transitions, including preparatory events and eventually maturation at ovulation. It is while the antral follicle develops, grows, and differentiates that the oocyte acquires its meiotic and developmental competencies (Mermillod et al. 1999; Hendriksen et al. 2000; Sirard et al. 2006). The oocytes that are used for in vitro maturation (IVM) and later embryo production originate from antral follicles. These oocytes are of known heterogeneous quality, and developmental competence improves with advanced stages of antral folliculogenesis (Pavlok et al. 1992; Lonergan et al. 1994; Blondin & Sirard 1995; Hagemann et al. 1999; Lequarre et al. 2005). Much may thus be learnt from identifying the cellular and biochemical differences between follicles of varying sizes.
Several compartments ensure the normal functioning and development of antral follicles: three cellular ones (the oocyte, cumulus, and granulosa cells) and an acellular one (the follicular fluid). Much attention has focused on a variety of gene groups that modulate the antral follicle microenvironment, including endocrine and paracrine factors (Knight & Glister 2006; Webb & Campbell 2007). High steroidogenic and metabolic demands characterize the developing follicle (Boland et al. 1993; Fortune et al. 2001; Harris & Picton 2007), and as a result oxidative stress may ensue. Oxidative stress (OS) refers to an imbalance between the concentrations of pro- and anti-oxidants, thereby resulting in compromised cellular functions; sources of pro-oxidants are extensive, including their generation as byproducts of cellular respiration and steroid metabolism (Halliwell 1991). Previous findings support the relevance of OS to female reproduction, including endometriosis, polycystic ovarian syndrome, age-related fertility decline, early embryogenesis, and pregnancy (Tarin 1996; Guerin et al. 2001; Sabuncu et al. 2001; Agarwal et al. 2008; Ruder et al. 2008). However, there is a conspicuous lack of understanding when it comes to OS and normal ovarian function, especially with respect to folliculogenesis and oogenesis. Most studies in the ovary have focused on OS during ovulation, luteogenesis, luteal function, and luteolysis (Bertout et al. 2004; Sugino 2005); and follicular analyses of pro- and anti-oxidants have predominantly focused on human follicular fluid samples (Combelles et al. 2009).
The present study examines the enzymatic antioxidant superoxide dismutases (SOD) that convert the pro-oxidant superoxide into hydrogen peroxide. Three isoforms of SOD exist with varying structures, regulation, localizations, and functions (Johnson & Giulivi 2005). Cu, Zn-SOD (SOD1) is typically cytosolic and Mn-SOD (SOD2) mitochondrial, while extracellular SOD (SOD3) scavenges superoxide radicals in extracellular fluids and spaces (Fattman et al. 2003; Nozik-Grayck et al. 2005). Previous studies in the ovary have focused on SOD1 and SOD2, or total SOD enzymatic activity with neither a concomitant analysis of all three isoforms nor an evaluation of their cellular distributions within the developing antral follicle. Total SOD enzyme activity is present in bovine oocytes and cumulus cells obtained from 2–5 mm follicles (Cetica et al. 2001), with no consideration of the SOD isoforms and potential changes during later stages of antral folliculogenesis. Lonergan et al. (2003) documented the downregulation of SOD1 and SOD2 mRNAs in in vivo matured bovine oocytes when compared to in vitro matured and immature oocytes from 2–6 mm follicles. However, no comparison was made in oocytes derived from antral follicles larger than 6 mm, and analysis was limited to gene transcripts for unfertilized oocytes (Lequarre et al. 2001; Lonergan et al. 2003). In the rat, transcripts for the three SOD isoforms were measured in whole ovaries without an evaluation of individual follicles (Tilly & Tilly 1995). Bovine granulosa cells from dominant follicles showed changes in SOD1 and SOD2 mRNAs between days 4 and 8 of the first follicular wave with differences in SOD activity albeit no protein regulation (Valdez et al. 2005). In porcine (Peterson & Stevenson 1992) and goat (Behl & Pandey 2002) granulosa cells from follicles of increasing sizes, catalase activity augmented, thereby indicating the dynamic regulation of an antioxidant enzyme during antral folliculogenesis. In a pig model, follicular fluid SOD activity decreased with follicle growth (Basini et al. 2008) and in human, increased follicular fluid SOD activity was correlated with low fertilization rates (Sabatini et al. 1999). Lastly, mice with gene deletions for either SOD1 or its copper chaperone display reduced female fertility and notably abnormal follicular development (Ho et al. 1998; Matzuk et al. 1998; Wong et al. 2000). Taken together, previous studies point towards the need for a careful analysis of SOD proteins in the dynamic microenvironment of the developing antral follicle.
The vulnerability of growing antral follicles to OS remains to be tested. This is relevant given the changes in oxygen availability and metabolic activity that follicles and oocytes are subjected to whether under in vivo or in vitro conditions (Fischer et al. 1992; Sutton et al. 2003; Harris & Picton 2007). We predict that antral follicles are properly and dynamically equipped to withstand OS; more specifically, granulosa cells, follicular fluid, and the cumulus-oocyte-complex would provide protection by tightly and coordinately regulating the expression of the various SOD isoforms. Towards this goal, the total SOD enzymatic activity was measured in follicular fluid and the protein expression of SOD isoforms were analyzed in granulosa cells, follicular fluid, and cumulus-oocyte-complexes from bovine antral follicles containing oocytes of different developmental competencies.
MATERIALS AND METHODS
All reagents were obtained from Sigma-Aldrich (St. Louis, MO) unless specified otherwise.
Collection, categorization, and processing of bovine antral follicles, follicular fluid, granulosa cells, and cumulus-oocyte-complexes
Bovine ovaries were obtained from Champlain Beef slaughterhouse (Whitehall, NY) and transported to the laboratory in 0.9% NaCl, 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin B at 20°C. All samples were collected and processed within 2–4 hours of slaughter. As previously described, the routine assessment of corpus luteum morphology was used to determine the stage of the estrous cycle (Ireland et al., 1980). Antral follicles were dissected and stored in a Mimimum Essential Medium with Hanks Balanced Salt Solution (MEM-HBSS), 25 mM Hepes, 1 mM sodium pyruvate, 2 mM L-glutamine, 4 μg/ml bovine serum albumin (BSA), 100 U/ml penicillin, 100 μg/ml streptomycin, 50 μg/ml heparin). Obviously atretic follicles were avoided based on published morphological characteristics (Kruip & Dieleman 1982). Dissected follicles were grouped according to the following size categories: small (2–5 mm), medium (6–8 mm), and large (>8 mm). These size ranges were selected based on established key transitions in antral folliculogenesis and accompanying changes in the acquisition of oocyte developmental competence (Lonergan et al. 1994; Lequarre et al. 2005).
Follicular fluid was collected from each follicle by aspiration with an insulin syringe. Due to the low volume of removed follicular fluid from small follicles, samples were pooled (usually between 5 and 10 follicles) within a given ovary. No sample pooling was needed for the medium and large size categories. Samples were kept on ice until centrifugation for 4.5 minutes at 5400 g. The supernatant was evaluated for the presence of any residual cells under the microscope and then stored at −80°C. For follicular fluids to be analyzed by immunoblotting, protease inhibitor cocktail was added to each sample at a 1:100 dilution.
Granulosa cells were manually detached from the follicular wall with an inoculation loop and pooled within a size category. Granulosa cells were washed (by natural sedimentation, 2×10 minutes), and allowed to dissociate in MEM-HBSS without calcium and magnesium (MEM-HBSS-Ca2+-Mg2+) supplemented with 25 mM Hepes and 4 mg/ml BSA. The cell pellet was exposed for 5 seconds to a 100 μl of double-distilled water (for the lysis of erythrocytes) followed by immediate neutralization with 10 ml of supplemented MEM-HBSS-Ca2+-Mg2+ medium. Cells were then centrifuged at 800 g for a minute, and the resuspended pellet layered over a 50% Percoll gradient in supplemented MEM-HBSS-Ca2+-Mg2+. After a 10 minute spin, any remaining erythrocytes were separated from granulosa cells, which were retrieved from the Percoll-MEM-HBSS interface. The purified pellet of granulosa cells was centrifuged and washed in 200 μl of cold PBS at 800 g, 4°C for 3 minutes. The pellet was resuspended in 500 μl cold lysis buffer consisting of 2X RIPA buffer (0.2% SDS, 2% NP-40, 1% Na-deoxycholate, and 1X PBS), 100 μM PMSF, 100 μM Na-vanadate, 1X protease inhibitor cocktail, and 1X PBS. The suspension was passed 10 times through an insulin syringe and placed on a rotating platform for 30 minutes at 4°C. After a 16000 g, 4°C, 10 minute spin, the supernatant or whole cell lysate was frozen and stored at −80°C until use for immunoblotting.
Cumulus-oocyte-complexes (COCs) were released from sized follicles after bisection of the follicle with a scalpel. COC quality was scored based on morphological features of the oocyte and cumulus layers (Blondin & Sirard 1995). COCs were stripped manually of their outer cumulus layers with few cumulus cells kept attached to the zona pellucida. All partially denuded oocytes were fixed in freshly prepared 2% paraformaldehyde (PFA) in PBS, pH 7.4 for 15 minutes at room temperature (RT) in darkness with shaking. Following fixation, cells were extracted in 0.2% Triton-X100 in PBS for 30 minutes at RT with shaking. Samples were stored in a blocking WASH solution (0.2% Na-azide, 0.2% nonfat powdered milk, 2% normal donkey serum, 1% BSA, and 0.1 M glycine in PBS) at 4°C until labelling by immunocytochemistry.
Immunoblotting of follicular fluid and granulosa cell lysates
Each SOD isoform was detected and semi-quantified in follicular fluid and granulosa cells lysates using SDS-PAGE and immunoblotting. An identical amount of protein (10 μg for granulosa cell lysate and 30 μg for follicular fluid) was loaded per well, based on total proteins quantified with a bicinchoninic acid (BCA) assay (Pierce, Rockford, IL). Protein lysates were prepared with a 6X protein dye (0.5 M Tris pH 6.8, 29% glycerol, 10% SDS, 5% β-mercaptoethanol, 0.01% bromophenol blue) and boiled for 5 minutes. Samples were separated using a 15% acrylamide gel and proteins were transferred to PVDF membrane.
Equal loading of protein and effective transfer was verified by incubating membrane in Ponceau-S and visualized using Kodak 440 Gel Imager (Norwalk, CT). Membranes were blocked in 1X TBST (0.066M Tris, 0.054M NaCl, 0.1% Tween 20) with 5% powdered nonfat milk for one hour, and exposed for 2 hours at RT to working dilutions of primary antibody prepared in 1× TBST/5% nonfat milk. Working dilutions of primary rabbit antibodies were used as follows for follicular fluid and granulosa cell samples, respectively: anti-SOD1, 0.2 μg/ml and 0.1 μg/ml (Assay Designs, Ann Arbor, MI), SOD2, 0.5 μg/ml and 0.1 μg/ml (Millipore/Upstate Biotechnology, Billerica, MA), and SOD3, 1 μg/ml and 0.1 μg/ml (Millipore/Upstate Biotechnology, Billerica, MA). Controls were run for each antibody and blot using a non-specific rabbit IgG antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) at a working dilution identical to the one used for the test primary antibody, as well as a secondary only application. For granulosa cell samples, blots were also probed for β-actin (0.02 μg/ml) as a loading control. For follicular fluid samples, blots were also probed for mitochondrial and cytopkasmic markers to ensure the lack of contamination from cells or cell lysis: OxPhos Complex V inhibitor protein (mouse IgG, 0.2 μg/ml; Invitrogen, Carlsbad, CA), β-tubulin (mouse IgG, 0.01 μg/ml), and β-actin (0.02 μg/ml). After primary antibody exposure, three washes in 1X TBST were conducted with agitation for 10 minutes. A one-hour secondary exposure was then performed with a goat anti-rabbit secondary antibody conjugated to horseradish peroxidase (1:2000; Amersham/GE Healthcare, Piscataway, NJ). Membranes were subsequently washed as described before and then incubated 1 minute with ECL Western blotting detection solution (Pierce, Rockford, IL). Exposures were conducted to reach a linear range without signal saturation. Developed exposures were later quantified by digitizing blots and counting pixels from a set sized box over each signal band (Adobe Photoshop, San Jose, CA). For each blot, pixel data from follicular fluid or granulosa cells samples from small and medium follicles were arbitrarily normalized to pixel counts of samples from large follicle.
SOD enzyme activity assay in follicular fluid
Follicular fluid was evaluated for total superoxide dismutase activity using an assay kit from Dojindo Molecular Technology (Kurnamoto, Japan). The assay uses the activity of xanthine oxidase (XO), which produces the superoxide anion in the presence of oxygen. The water-soluble tetrazolium salt (WST-1 (2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium) reacts with the superoxide anion reducing it; WST-1 now forms a water-soluble formazan dye measurable with spectrophotometry. SOD competes with WST-1 for the superoxide anion produced by XO, and the percent inhibition of XO activity is a quantification of SOD activity. Standards spanning the range of 0.1 to 10 U/ml were prepared as serial dilutions from a 200 U/ml stock of bovine erythrocytes SOD. Initial testing showed the need to dilute follicular fluid samples from medium/large and small follicles 1:10 and 1:50, respectively. Reactions were initiated by adding 20 μl of the XO enzyme working solution to the WST reaction mixture. After incubating at 37°C for 20 minutes, samples were read at 450 nm with a Synergy HT spectrophotometric plate reader (BioTek, Winooski, VT). All standards, blanks, and samples were run in triplicates, and final SOD activity measurements are reported as U per ml of follicular fluid. Intra- and inter-assay coefficients of variation averaged 8.4% and 10.2%, respectively, and linearity of the assay for a range of erythrocyte SOD concentrations during the first 20 min of incubation is shown in Supplementary Figure 1.
Immunocytochemistry of oocytes and cumulus cells
Following fixation and blocking (see above), oocytes and cumulus cells were processed for immunocytochemistry with the aforementioned SOD antibodies. Working solutions of primary antibodies were prepared in WASH at the following optimized dilutions: SOD1, 2 μg/ml; SOD2, 5μg/ml; SOD3, 5 μg/ml. All incubations were undertaken in microwells of a 60-well plate, and routine labelling controls included a group of oocytes incubated with non-immune rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) at a same concentration as primary test antibodies as well as a secondary only group. After overnight incubation with the primary antibody (4°C, shaking) and three washes (with WASH, 10 minutes each at 37°C shaking), cells were incubated in a mixture of secondary Alexa Fluor 488 anti-rabbit (1:800 dilution of a 2 mg/ml stock; Invitrogen, Carlsbad, CA) and Texas-red phalloidin (1:20 dilution; Invitrogen, Carlsbad, CA) in WASH for 90 minutes at 37°C shaking. After a 10 minute wash, cells were exposed to DAPI (1 μg/ml) for 30 minutes at 37°C shaking. Following two final washes, oocytes and cumulus cells were mounted onto a glass slide in 50% glycerol:50% sodium azide-PBS and a coverslip added with minimal compression. Labelled samples were visualized and scored by conventional fluorescence microscopy with a Zeiss Axioscope 2 plus (Thornwood, NY). Imaging was undertaken by confocal laser microscopy with a Zeiss LSM 510 META. Fluorescent signals were captured after excitation with 405 nm, 488 nm, and 543 nm laser lines. Qualitative comparisons of labelling patterns and/or intensities in oocytes originating from small, medium, or large antral follicles were made only for oocytes that were collected, fixed, labelled, and imaged on a same day using identical conditions and acquisition parameters. Optical sections of 0.5 μm thickness were acquired and a total of 10 of them projected into a single image.
Statistical analysis
Numbers of samples and experimental replicates are provided below with each analysis. SOD activity in follicular fluid and signal intensity data obtained from immunoblotting experiments in granulosa cells or follicular fluids from small, medium, or large follicles were compared using a nonparametric Kruskal-Wallis test followed by Mann-Whitney tests for two independent samples (Statistics Package for Social Sciences 16.0, Chicago, IL). Statistical significance was accepted for p-values ≤ 0.05.
RESULTS
Presence and variation in the amounts of SOD isoforms in follicular fluids from progressive stages of antral development
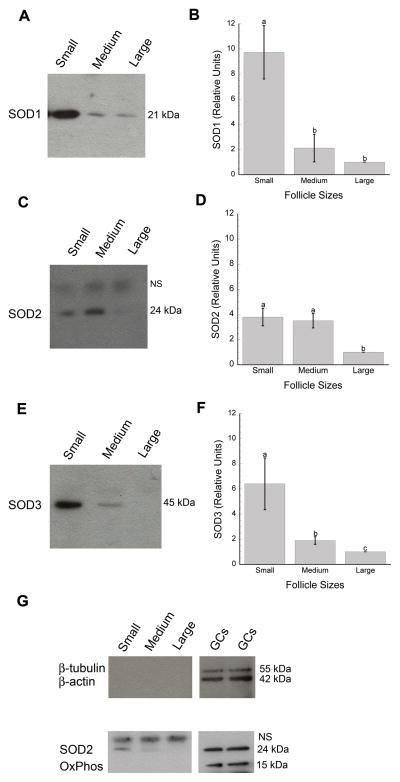
Immunoblotting detected the presence of all three SOD isoforms in bovine follicular fluids with a single and specific SOD1 band at 21 kDa (Figure 1A), SOD2 at 24 kDa (Fig. 1C), and SOD3 at 45 kDa (Fig. 1E) when compared to negative controls (for both control IgG and secondary only; data not shown). Quantification of the signal bands revealed significantly higher levels of each SOD type in follicular fluids from small when compared to large antral follicles (Fig. 1B, D, F). SOD1 and SOD3 expressions were more elevated in small than medium and large antral follicles (Fig. 1B, F) with SOD2 and SOD3 higher in medium than large follicles (Fig. 1F). These expression patterns were obtained from the following numbers of total blots and samples: 13 and 42 for SOD1; 9 and 27 for SOD2; and 11 and 33 for SOD3. No mitochondrial (OxPhos complex V inhibitor) or cytoplasmic (β-tubulin and β-actin) proteins were detected in follicular fluid samples (Fig. 1G).
Figure 1.
Expression of SOD1 (A–B), SOD2 (C–D), and SOD3 (E–F) proteins in follicular fluids from small (2–5 mm), medium (6–8 mm), and large (>8 mm) antral follicles. Panels A, C, and E show representative immunoblots for SOD1 (21 kDa), SOD2 (24 kDa), and SOD3 (45 kDa). SOD protein concentrations were quantified and reported as arbitrary units relative to a set value of 1 for samples from large follicles (B, D, F: means ± SD’s). Each SOD isoform showed higher protein expression in follicular fluid derived from small when compared to large antral follicles (B, SOD1: (a,b), p<0.001; D, SOD2: (a,b), p=0.001; F, SOD3: (a,c), p<0.001). SOD1 and SOD3 proteins were increased in follicular fluid from small when compared to medium antral follicles (B, F: (a,b), p<0.001). Follicular fluid from large follicles also contained less SOD2 (D: (a,b), p=0.001) and SOD3 (F: (b,c), p=0.002) than fluid from medium follicles. Control blots (G) supported the lack of cytosolic and mitochondrial contaminations with the probing of follicular fluid for β-tubulin/β-actin and OxPhos, respectively. The use of these markers was validated with granulosa cell lysates (GCs) as positive controls. (C, G): NS corresponds to a non-specific band at ~ 25 kDa based on rabbit IgG and secondary antibody alone negative controls.
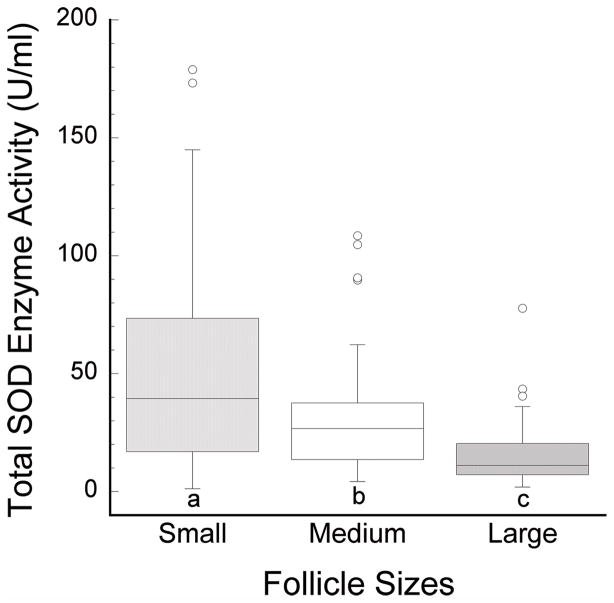
Variation in total SOD enzyme activity in follicular fluids originating from small, medium, and large antral follicles
The analysis of total SOD activity in follicular fluid showed levels that fluctuated with developmental stages of antral folliculogenesis (Figure 2). SOD activities were significantly higher in follicular fluids from small antral follicles (51.1±4.5 U/ml, n=88; mean±SD) than in those from medium antral follicles (29.9±2.3 U/ml, n=61); similarly, follicular fluids from medium antral follicles possessed elevated levels of SOD activity when compared with large follicles (14.8±1.1 U/ml, n=99; Figure 2). SOD activity levels were compared when expressed as units per ml, providing identical results and statistical significance as when units per mg of total protein were used. In line with this, total protein levels did not vary among follicular fluids from small (83.0±17.7 mg/ml, n=100; mean±SD), medium (84.3±17.7 mg/ml, n=65), and large (88.5±17.3 mg/ml, n=113) antral follicles (p>0.05). Over a span of 29 collections, a total of 116 ovaries from 72 animals were used for SOD activity assays yielding 88, 61, and 99 follicular fluid samples from small, medium, and large antral follicles, respectively.
Figure 2.
Total SOD enzyme activity (U/ml) in follicular fluid samples from small (2–5 mm), medium (6–8 mm), and large (> 8 mm) antral follicles. SOD activity is highest in follicular fluids from small antral follicles with greater concentrations in small than medium (a,b: p<0.05), and in medium than in large (b,c: p<0.0005) follicles.
SOD activity was also evaluated based upon estrous stage of the animal together with follicle size from which the follicular fluid was obtained (Supplementary Figure 2). Stages of the estrous cycle were estimated based on the morphology of the corpus luteum with stage I corresponding to days 1–4, II to days 5–10, III to days 11–17, and IV to days 18–20 (Ireland et al., 1980). Statistical analysis revealed no significant difference between estrous stages in follicular fluids derived from small, medium, or large follicles.
Expression of SOD isoforms in granulosa cells from small, medium, and large antral follicles
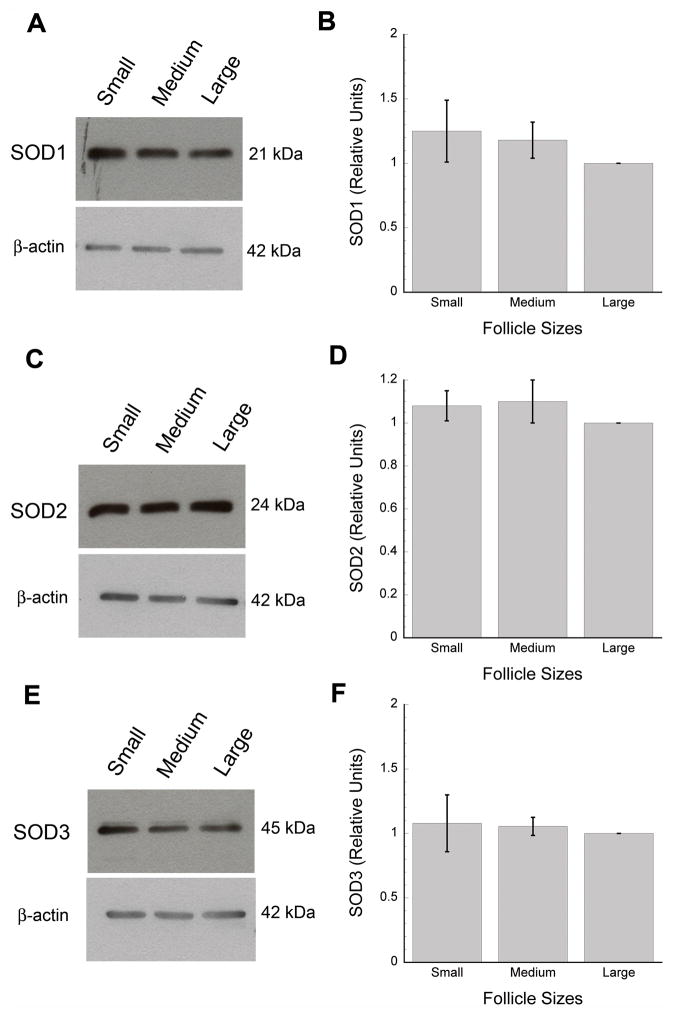
Granulosa cells express at the protein level all three types of SOD isoforms whether they originate from small, medium, or large antral follicles (Fig. 3A, C, E). Specificity of the single protein bands obtained with granulosa cell lysates were confirmed with routine negative controls (control rabbit IgG and secondary only; data not shown). Testing for SOD3 showed a second faint band at about 47 kDa, which also showed no sign of developmental regulation. Furthermore, the relative amounts of SOD1, 2, and 3 protein expression were comparable in cell lysates from small, medium, or large antral follicles (Fig. 3B, D, F; 12 collected sets of small, medium, and large samples or a total of 36 cell lysates for each SOD isoform). Equal protein loading was confirmed for each blot with β-actin expression (Fig. 3A, C, E).
Figure 3.
Expression of SOD1 (A–B), SOD2 (C–D), and SOD3 (E–F) proteins in pooled granulosa cell lysates from small (2–5 mm), medium (6–8 mm), and large (>8 mm) antral follicles. Panels A, C, and E show representative immunoblots for each SOD isoenzyme. SOD protein concentrations were quantified and reported as arbitrary units relative to a set value of 1 for samples from large follicles (in B, D, and F, Y-axis values are expressed as means ± SD’s). β-actin was used as a loading control for each SOD blot (A, C, E).
Expression and localization of SOD isoforms in oocytes and cumulus cells during antral folliculogenesis
An immunocytochemical approach demonstrated the spatially and temporally dynamic expression of each SOD isoform in intact germinal vesicle stage (prophase-I) oocytes as well as surrounding cumulus cells (Figures 4 and 5). 4″,6-Diamidino-2-phenylindole dihydrochloride (DAPI) and phalloidin counterlabels permitted evaluation of chromatin stages, nuclear status, and cell contours for the two somatic and germ cell compartments of the COC. While morphology was evaluated for each COC, no differences in any of the SOD labelling patterns were apparent across COC grades.
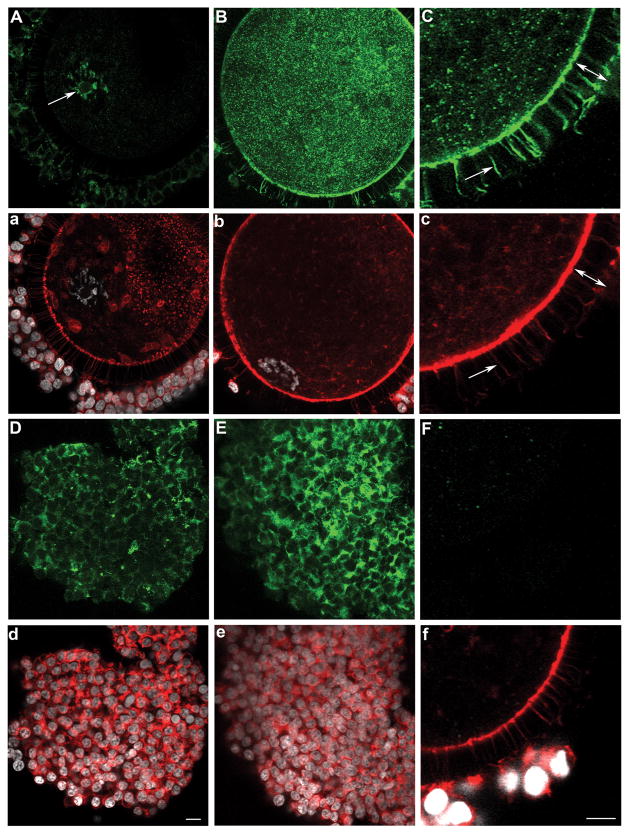
Figure 4.
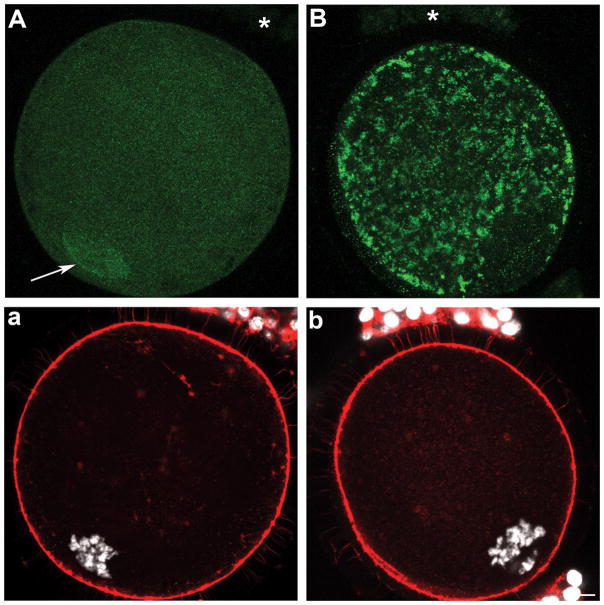
Expression and localization of SOD1 (A) and SOD2 (B) in oocytes and cumulus cells upon isolation from sized antral follicles. Panels a and b show corresponding DNA (white) and microfilaments (red). (A) A fine punctuate SOD1 labelling was localized throughout the ooplasm and nucleoplasm in oocytes from small, medium, and large antral follicles with an enhanced nuclear accumulation indicated by the arrow. SOD1 was not detectable in cumulus cells (A, asterisk). (B) SOD2 was restricted to the ooplasm of oocytes from small, medium, and large follicles, with a detectable but much reduced labelling in cumulus cells (asterisk). (b) Scale bar for all panels: 10 μm.
Figure 5.
Expression and localization of SOD3 in oocytes (A–B), trans-zonal projections (C), and cumulus cells (D–E) upon isolation from sized antral follicles. Corresponding microfilament (red) and DNA (white) stains are shown (a–f), and all processing and imaging conditions were kept identical across panels. SOD3 was organized as small aggregates (reminiscent of a vesicular labelling) in the oocyte cortex of oocytes from small (A), medium (C), and large (B) antral follicles. SOD3 was also abundant in cumulus cells and trans-zonal projections (C, c; arrow) with the width of the zona pellucida delineated by a double-ended arrow (C, c). All oocytes had nuclear SOD3, but ones from small (A, a) when compared to large (B, b) follicles showed more SOD3 in the nucleoplasm than ooplasm; SOD3 also co-localized (A, arrow) with chromatin (a). The use of control rabbit IgG antibody (F, f) confirmed the specificity of SOD labelling. (d) Scale bar for all panels except C, c, F, and f for which a scale bar is provided in f: 10μm.
At all stages of antral folliculogenesis (> 2 mm in diameters), oocytes display intracellular SOD1 proteins in a fine, dense, and punctate pattern throughout the volume of the oocyte (Fig. 4A, a; n=84, 42, and 61 oocytes from small, medium, and large antral follicle, respectively). This is in contrast to probing with secondary alone (n=46) or control rabbit IgG (n=42), which both yielded only background non-specific labelling (Fig. 5F, f). Interestingly, SOD1 positive foci were also present within the nucleoplasm of all oocytes, with some oocytes further displaying an accumulation of SOD1 within the nuclear confines (Fig. 4A, arrow; with an incidence of 79/84 or 94% of oocytes from small follicles when compared to 18/42 or 43% and 0/61 or 0% from medium and large antral follicles, respectively). The zona pellucida remained devoid of SOD1 across all size categories of antral follicles (Fig. 4A, a). Cumulus cells of the corona radiata also failed to demonstrate any reactivity to SOD1 antibodies whether they originated from small, medium, or large antral follicles (Fig. 4A, asterisk).
Oocytes and cumulus cells expressed SOD2 proteins at all of the analyzed stages of antral folliculogenesis (Fig. 4B; n=67, 48, and 52 oocytes from small, medium, and large antral follicle, respectively). SOD2 levels were elevated in comparison to control oocytes (Fig. 5F), with a strong and dense labelling pattern through the ooplasm with the exception of the nucleoplasm (Fig. 4B, b). Each cumulus cell showed a few specific foci that were positive for SOD2 in their cytoplasm (Fig. 4B, asterisk).
SOD3 was present not only in the oocyte cortex (Fig. 5A–B; n=81, 38, and 57 oocytes from small, medium, and large antral follicle, respectively) but also in cumulus cells (Fig. 5A–B, D–E). It was distributed in the cytoplasm of cumulus cells as well as in the extracellular spaces adjoining cells; furthermore, SOD3 labelling was characteristically strong in the oocyte and cumulus cell layer of COCs from large (Fig. 5B, E) when compared to small antral follicles (Fig. 5A, D). The zona pellucida was itself devoid of SOD3 (Fig. 5C, c, double-ended arrow: width of the zona pellucida), but the entire lengths of transzonal projections (TZPs) were positive for SOD3 as demonstrated by co-localization with actin microfilaments (Fig. 5C, c, arrow). TZPs all co-localized with SOD3 whether they originated from small, medium, or large antral follicles. The oocyte nucleoplasm always contained SOD3 (Fig. 5A–B); however, only in small oocytes was more SOD3 present in the nucleoplasm than in the surrounding ooplasm (Fig. 5A, a). SOD3 was also associated with the chromatin (Fig. 5A, arrow).
DISCUSSION
Our detailed spatial and temporal analysis of the three SOD isoforms revealed the expression of SOD1, 2, and 3 in the granulosa cells, follicular fluid, and cumulus-oocyte-complex of the developing antral follicle. In granulosa cells, there was no change in SOD expression as antral follicles increased in size, while follicular fluid showed highest SOD activity and SOD proteins in small when compared to medium and large follicles. COCs exhibited a compartmentalized and varying distribution of SOD1, 2, and 3 protein expression.
In light of the differential and prevalent expression of SOD isoenzymes in all compartments of the antral follicle, it may be speculated that antioxidants, and by inference pro-oxidants, play a role in follicle development. Reactive oxygen species (ROS) are not always detrimental to the cell as they can carry out functional roles at certain concentrations, notably during embryonic development (Covarrubias et al. 2008). In large diameter antral follicles, ROS may need to be above a certain threshold and keeping SODs at reduced concentrations within the follicular fluid milieu may provide the appropriate balance of superoxide anion and hydrogen peroxide for normal cell function. Of relevance is the documented need for free radicals at ovulation (Miyazaki et al. 1991). Given the follicular changes in oxygen availability (Fischer et al. 1992) and metabolic requirements (Harris & Picton 2007), it is likely that mechanisms exist to manage oxidative stress in antral follicles, and altered SOD levels may be one manifestation of this need.
Beyond physiological changes during follicular growth are selective pressures imposed on antral follicles since one will become dominant and others subordinate and destined for atresia. Follicular atresia involves apoptosis, a program of cell death during which ROS and antioxidants play a role (Ott et al. 2007; Covarrubias et al. 2008). This study only considered follicle size as a potential factor influencing antioxidant expression, but future work should test the involvement of oxidative stress during follicle selection and atresia. In the rat, exogenous SOD inhibits apoptosis in cultured rat follicles (Tilly & Tilly 1995) while in cows, no correlations were found between SOD1/SOD2 protein expression and granulosa cell apoptosis in the dominant follicle of the first follicular wave (Valdez et al. 2005). Nonetheless, SOD1/SOD2 mRNAs and SOD activity rose when nonviable granulosa cells also increased (Valdez et al. 2005). Lastly, it is conceivable that external factors may influence the oxidative stress status and in turn expression of SODs in the follicle. For our study, ovaries from adult (< 3 years old), cycling, non-pregnant animals were used without consideration of nutritional status and general or reproductive health; notably, infertility diagnosis appeared to influence SOD expression in human cumulus cells (Matos et al. 2009) and dietary supplements may impact ovarian function (Forges et al. 2007). Taken together, our work documents the dynamic expression of SOD isoforms during antral folliculogenesis and future studies will need to examine factors that may underlie any differences between animals and/or follicles.
Intra-follicular variations in antioxidants also prove relevant since not all follicular compartments exhibited similar changes in SODs. Indeed, SOD1, 2, 3 proteins and activity were elevated in follicular fluid from small follicles while SOD3 protein appeared most abundant in oocytes and cumulus cells from large relative to small follicles. Future work should thus examine follicular variances in not only the potential reliance of oocytes on exogenous sources of antioxidants but also the amount of oxidative stress that follicles are subject to. Our preliminary findings also support the need to quantify SOD protein and activity within single oocytes and cumulus cells of different follicular origins. Regardless, the bovine COC showed compartmentalization with respect to each SOD isoform and its cell types and regions. SOD2 is the predominant SOD expressed in the ooplasm while SOD3 is the main SOD type in the layer of cumulus cells and the only one located in TZPs. The localization of SOD3 in both cumulus cells and oocytes may reflect its intracellular processing, whether prior to exocytosis to impart oxidative protection (Tan et al. 2006) or upon endocytosis (Chu et al. 2006) as demonstrated in other cell systems. SOD1 showed an enhanced nuclear accumulation in oocytes from the majority of small and half of medium antral follicles. Previous reports in liver and brain cells documented the presence of SOD1 in the nucleoplasm (Slot et al. 1986; Moreno et al. 1997) but the functional significance of the observed change in localization during oogenesis is unknown. Similarly, SOD3 was present in the oocyte nucleus and a previous study in cultured somatic cells proposed a protective role for the regulated translocation of SOD3 to the nucleus (Ookawara et al. 2002). It may thus be relevant to test the potential role of SOD1 and SOD3 in the protection of genomic DNA and/or transcriptional regulation of redox-sensitive genes.
The distinct expression of each SOD isoform within the COC can be further rationalized: SOD1 neutralizes superoxide anions in the cytoplasm of cells, and in this case the oocyte is likely an abundant producer of ROS as it is metabolically active during the acquisition of developmental competence (Sutton et al. 2003); mammalian oocytes possess an abundance of mitochondria, the organelle where SOD2 is preferentially localized (Johnson & Giulivi 2005); and SOD3 acts in the extracellular matrix (Nozik-Grayck et al. 2005), a dynamically regulated and essential component of the developing COC. In addition, oocytes, or COCs as a whole developmental unit, may stockpile the various SOD isoforms for the upcoming events of oocyte maturation, fertilization, and early embryonic development. These processes occur precisely when the oocyte is faced with augmented oxidative challenges with the proposed burst of ROS at ovulation (Fujii et al. 2005) and the increase in oxygen tension within the oviduct (Fischer et al. 1992; Fischer & Bavister 1993). The hypothesis of antioxidant stockpiling by the oocyte was previously proposed based on the analysis of transcripts (Guerin et al. 2001); our study now define the intrinsic sources of SOD proteins that oocytes are equipped with prior to meiotic resumption. Also worthy of future investigation is the possibility that differences in the total SOD neutralizing capacity of COC reflect different vulnerability of COCs to oxidative stress, thereby influencing their subsequent developmental competence. In this vein, cumulus cells provide protection against ROS-induced apoptosis in porcine COCs during IVM (Tatemoto et al. 2000) and in the bovine during in vitro fertilization (Fatehi et al. 2005). Most recently, an analysis of human cumulus cells showed a positive relationship between total SOD activity and cycle success as defined by a live birth (Matos et al. 2009). Cell localization studies of the type undertaken here are insightful, notably in the case of SOD since the superoxide anion is largely produced in mitochondria and doesn’t diffuse readily through the plasma membrane, thereby mostly reacting within the cell where it is produced. The prevalence of SODs in all regions of the COC is thus not surprising, although the expression patterns of each isoform may point towards cell-type specific reliance on different protective mechanisms against superoxide. One must also consider the possibilities that the metabolite of SOD (hydrogen peroxide) plays a role in either the oocyte or cumulus cells, that SOD influences nitric oxide bioavailability (Nozik-Grayck et al. 2005), or that SOD possesses another, yet unknown, function.
As to the presence of SOD in follicular fluid, it may provide protection of the maturing pig oocytes against oxidative damage, in turn improving developmental competence (Tatemoto et al. 2004) while supplementation with exogenous SOD failed to influence bovine IVM (Ali et al. 2003). The stage is now set to examine the role of follicular fluid SOD isoforms on the prematuration of oocytes as the antral follicle grows. To our knowledge, this is the first report of SOD3 in follicular fluid, an expected association based on its known functions in body fluids (Nozik-Grayck et al. 2005). SOD2 is typically restricted to mitochondria although it is present in cerebrospinal fluid in bacterial meningitis (Hirose et al. 1995), one of its variant is secreted by a tumor cell line (Mancini et al. 2006), and one study detected SOD2 in human follicular fluid (Tamate et al. 1995). Importantly, we further demonstrated the lack of contaminants from cells or cell lysis within follicular fluid samples, and the functional significance of SOD2 in follicular fluid thus merits attention. Further studies should investigate potential releases of SOD2 and/or SOD1 in follicular fluid in responses to stress conditions. The presence of SOD1 in follicular fluid originates from the ability of SOD1 to be secreted in addition to its cytoplasmic functions (Mondola et al. 1996; Cimini et al. 2002). SOD1 was also previously reported in human follicular fluid (Carbone et al. 2003). Given the size of SOD proteins and their expression in granulosa and cumulus cells, this study indicates that the likely source of follicular fluid SODs is from follicular cells. Furthermore, protein expression did not change in granulosa cells while follicular fluid SOD proteins and activity diminished with increased follicle diameter. There may thus be mechanisms to control the secretion of SOD proteins from follicular cells and/or to alter the turnover of SOD proteins once in follicular fluid. With SODs exhibiting comparable patterns at the protein and enzyme activity levels, post-translational control likely doesn’t predominate in follicular fluid. However, post-translational regulation of SODs may operate in the cellular compartments of the follicle; of relevance are post-translational modifications of SOD isoforms (Johnson & Giulivi 2005; Nozik-Grayck et al. 2005). It must also be noted that data on SOD expression in granulosa cells were based on the pooling of cells from multiple follicles within a size category; thus, any intra-follicular wall or inter-follicular variances are not apparent.
During antral folliculogenesis, granulosa cells are steroidogenically active and SODs may play a role in controlling steroid production. SOD1 (indirectly via superoxide) inhibits progesterone production in rat luteal cells (Sugino et al. 1999) and in rat granulosa cells, SOD inhibited aromatase activity in vitro (LaPolt & Hong 1995). By analogy to the involvement of SOD and superoxide in corpus luteum steroidogenesis, SODs may thus provide a mechanism to regulate steroid output during follicular development. In turn SODs may be regulated by the steroid-rich environment of the antral follicle; notably, estradiol inhibited SOD1 and SOD2 mRNA expression in rat luteal cells (Sugino et al. 1998).
While this study lays the foundation for future work aimed at understanding the roles and regulation of SOD isoenzymes during antral folliculogenesis, consideration must also be given to (1) the dynamics of both membrane-impermeable superoxide (the free radical scavenged by SOD) and diffusible hydrogen peroxide (the ROS generated by SOD), and (2) the catalase and peroxidase enzymes that neutralize hydrogen peroxide. In the meantime, there are several potential applications of this novel knowledge on SOD expression during antral folliculogenesis. SODs may serve as biomarkers of follicle and in turn oocyte quality; indeed, it remains to be tested whether the concentrations and activity of SODs in follicular fluid can predict the developmental capacity of the oocyte originating from that follicle. Based on previous studies establishing the improved developmental competence of oocytes from increased size follicles, we hypothesize that lower SOD activity in follicular fluid corresponds to follicles containing oocytes of optimal quality. In such search for biomarkers, all follicular compartments should be examined since follicular fluid SOD activity was negatively correlated with oocyte fertilizability in human (Sabatini et al. 1999) but SOD activity was increased in human cumulus cells from successful cycles (Matos et al. 2009). Our findings also documented differences in SOD expression within the follicular microenvironment. Lastly, defining the physiologically relevant concentrations and proportions of each SOD isoform may contribute to the formulation of culture conditions for pre-maturation arrest or oocyte maturation.
Supplementary Material
Acknowledgments
We thank Dijana Poljak for her technical assistance with immunoblots. This work was supported by the National Institute of Child Health and Human Development (R15 HD05645-01), the Vermont Genetics Network through Grant Number P20 RR16462 from the INBRE program of the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and Middlebury College. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
Footnotes
Disclaimer. This is not the definitive version of record of this article. This manuscript has been accepted for publication in Reproduction, but the version presented here has not yet been copy edited, formatted or proofed. Consequently, the journal accepts no responsibility for any errors or omissions it may contain. The definitive version is available at http://dx.doi.org/10.1530/REP-09-0390 2010 Society for Reproduction and Fertility.
References
- Agarwal A, Gupta S, Sekhon L, Shah R. Redox considerations in female reproductive function and assisted reproduction: from molecular mechanisms to health implications. Antioxid Redox Signal. 2008;10:1375–1403. doi: 10.1089/ars.2007.1964. [DOI] [PubMed] [Google Scholar]
- Ali AA, Bilodeau JF, Sirard MA. Antioxidant requirements for bovine oocytes varies during in vitro maturation, fertilization and development. Theriogenology. 2003;59:939–949. doi: 10.1016/s0093-691x(02)01125-1. [DOI] [PubMed] [Google Scholar]
- Basini G, Simona B, Santini SE, Grasselli F. Reactive oxygen species and anti-oxidant defences in swine follicular fluids. Reprod Fertil Dev. 2008;20:269–274. doi: 10.1071/rd07147. [DOI] [PubMed] [Google Scholar]
- Behl R, Pandey RS. FSH induced stimulation of catalase activity in goat granulosa cells in vitro. Anim Reprod Sci. 2002;70:215–221. doi: 10.1016/s0378-4320(02)00006-4. [DOI] [PubMed] [Google Scholar]
- Bertout J, Mahutte NG, Preston SL, Behrman HR. Reactive oxygen species and ovarian function. In: Leung P, Adashi EY, editors. The Ovary. 2. San Diego: Elsevier Academic Press; 2004. pp. 353–368. [Google Scholar]
- Blondin P, Sirard MA. Oocyte and follicular morphology as determining characteristics for developmental competence in bovine oocytes. Mol Reprod Dev. 1995;41:54–62. doi: 10.1002/mrd.1080410109. [DOI] [PubMed] [Google Scholar]
- Boland NI, Humpherson PG, Leese HJ, Gosden RG. Pattern of lactate production and steroidogenesis during growth and maturation of mouse ovarian follicles in vitro. Biol Reprod. 1993;48:798–806. doi: 10.1095/biolreprod48.4.798. [DOI] [PubMed] [Google Scholar]
- Carbone MC, Tatone C, Delle Monache S, Marci R, Caserta D, Colonna R, Amicarelli F. Antioxidant enzymatic defences in human follicular fluid: characterization and age-dependent changes. Mol Hum Reprod. 2003;9:639–643. doi: 10.1093/molehr/gag090. [DOI] [PubMed] [Google Scholar]
- Cetica PD, Pintos LN, Dalvit GC, Beconi MT. Antioxidant enzyme activity and oxidative stress in bovine oocyte in vitro maturation. IUBMB Life. 2001;51:57–64. doi: 10.1080/15216540119253. [DOI] [PubMed] [Google Scholar]
- Chu Y, Piper R, Richardson S, Watanabe Y, Patel P, Heistad DD. Endocytosis of extracellular superoxide dismutase into endothelial cells: role of the heparin-binding domain. Arterioscler Thromb Vasc Biol. 2006;26:1985–1990. doi: 10.1161/01.ATV.0000234921.88489.5c. [DOI] [PubMed] [Google Scholar]
- Cimini V, Ruggiero G, Buonomo T, Seru R, Sciorio S, Zanzi C, Santangelo F, Mondola P. CuZn-superoxide dismutase in human thymus: immunocytochemical localisation and secretion in thymus-derived epithelial and fibroblast cell lines. Histochem Cell Biol. 2002;118:163–169. doi: 10.1007/s00418-002-0429-8. [DOI] [PubMed] [Google Scholar]
- Combelles CM, Gupta S, Agarwal A. Could oxidative stress influence the in-vitro maturation of oocytes? Reprod Biomed Online. 2009;18:864–880. doi: 10.1016/s1472-6483(10)60038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covarrubias L, Hernandez-Garcia D, Schnabel D, Salas-Vidal E, Castro-Obregon S. Function of reactive oxygen species during animal development: passive or active? Dev Biol. 2008;320:1–11. doi: 10.1016/j.ydbio.2008.04.041. [DOI] [PubMed] [Google Scholar]
- Fatehi AN, Roelen BA, Colenbrander B, Schoevers EJ, Gadella BM, Beverst MM, van den Hurk R. Presence of cumulus cells during in vitro fertilization protects the bovine oocyte against oxidative stress and improves first cleavage but does not affect further development. Zygote. 2005;13:177–185. doi: 10.1017/s0967199405003126. [DOI] [PubMed] [Google Scholar]
- Fattman CL, Schaefer LM, Oury TD. Extracellular superoxide dismutase in biology and medicine. Free Radic Biol Med. 2003;35:236–256. doi: 10.1016/s0891-5849(03)00275-2. [DOI] [PubMed] [Google Scholar]
- Fischer B, Bavister BD. Oxygen tension in the oviduct and uterus of rhesus monkeys, hamsters and rabbits. J Reprod Fertil. 1993;99:673–679. doi: 10.1530/jrf.0.0990673. [DOI] [PubMed] [Google Scholar]
- Fischer B, Kunzel W, Kleinstein J, Gips H. Oxygen tension in follicular fluid falls with follicle maturation. Eur J Obstet Gynecol Reprod Biol. 1992;43:39–43. doi: 10.1016/0028-2243(92)90241-p. [DOI] [PubMed] [Google Scholar]
- Forges T, Monnier-Barbarino P, Alberto JM, Gueant-Rodriguez RM, Daval JL, Gueant JL. Impact of folate and homocysteine metabolism on human reproductive health. Hum Reprod Update. 2007;13:225–238. doi: 10.1093/humupd/dml063. [DOI] [PubMed] [Google Scholar]
- Fortune JE, Rivera GM, Evans AC, Turzillo AM. Differentiation of dominant versus subordinate follicles in cattle. Biol Reprod. 2001;65:648–654. doi: 10.1095/biolreprod65.3.648. [DOI] [PubMed] [Google Scholar]
- Fujii J, Iuchi Y, Okada F. Fundamental roles of reactive oxygen species and protective mechanisms in the female reproductive system. Reprod Biol Endocrinol. 2005;3:43. doi: 10.1186/1477-7827-3-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerin P, El Mouatassim S, Menezo Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum Reprod Update. 2001;7:175–189. doi: 10.1093/humupd/7.2.175. [DOI] [PubMed] [Google Scholar]
- Hagemann LJ, Beaumont SE, Berg M, Donnison MJ, Ledgard A, Peterson AJ, Schurmann A, Tervit HR. Development during single IVP of bovine oocytes from dissected follicles: interactive effects of estrous cycle stage, follicle size and atresia. Mol Reprod Dev. 1999;53:451–458. doi: 10.1002/(SICI)1098-2795(199908)53:4<451::AID-MRD11>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Reactive oxygen species in living systems: source, biochemistry, and role in human disease. Am J Med. 1991;91:14S–22S. doi: 10.1016/0002-9343(91)90279-7. [DOI] [PubMed] [Google Scholar]
- Harris SE, Picton H. Metabolism of follicles and oocytes during growth and maturation. In: Tan SL, Chian RC, Buckett W, editors. In-vitro Maturation of Human Oocytes. 1. Oxon, U.K: Informa Healthcare; 2007. pp. 15–36. [Google Scholar]
- Hendriksen PJ, Vos PL, Steenweg WN, Bevers MM, Dieleman SJ. Bovine follicular development and its effect on the in vitro competence of oocytes. Theriogenology. 2000;53:11–20. doi: 10.1016/s0093-691x(99)00236-8. [DOI] [PubMed] [Google Scholar]
- Hirose Y, Mokuno K, Wakai M, Takahashi A, Hashizume Y, Yanagi T, Kato K. Elevated cerebrospinal fluid levels of manganese superoxide dismutase in bacterial meningitis. J Neurol Sci. 1995;131:51–57. doi: 10.1016/0022-510x(95)00040-9. [DOI] [PubMed] [Google Scholar]
- Ho YS, Gargano M, Cao J, Bronson RT, Heimler I, Hutz RJ. Reduced fertility in female mice lacking copper-zinc superoxide dismutase. J Biol Chem. 1998;273:7765–7769. doi: 10.1074/jbc.273.13.7765. [DOI] [PubMed] [Google Scholar]
- Johnson F, Giulivi C. Superoxide dismutases and their impact upon human health. Mol Aspects Med. 2005;26:340–352. doi: 10.1016/j.mam.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Knight PG, Glister C. TGF-beta superfamily members and ovarian follicle development. Reproduction. 2006;132:191–206. doi: 10.1530/rep.1.01074. [DOI] [PubMed] [Google Scholar]
- Kruip TA, Dieleman SJ. Macroscopic classification of bovine follicles and its validation by micromorphological and steroid biochemical procedures. Reprod Nutr Dev. 1982;22:465–473. doi: 10.1051/rnd:19820403. [DOI] [PubMed] [Google Scholar]
- LaPolt PS, Hong LS. Inhibitory effects of superoxide dismutase and cyclic guanosine 3′,5′-monophosphate on estrogen production in cultured rat granulosa cells. Endocrinology. 1995;136:5533–5539. doi: 10.1210/endo.136.12.7588305. [DOI] [PubMed] [Google Scholar]
- Lequarre AS, Feugang JM, Malhomme O, Donnay I, Massip A, Dessy F, Van Langendonckt A. Expression of Cu/Zn and Mn superoxide dismutases during bovine embryo development: influence of in vitro culture. Mol Reprod Dev. 2001;58:45–53. doi: 10.1002/1098-2795(200101)58:1<45::AID-MRD7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Lequarre AS, Vigneron C, Ribaucour F, Holm P, Donnay I, Dalbies-Tran R, Callesen H, Mermillod P. Influence of antral follicle size on oocyte characteristics and embryo development in the bovine. Theriogenology. 2005;63:841–859. doi: 10.1016/j.theriogenology.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Lonergan P, Gutierrez-Adan A, Rizos D, Pintado B, de la Fuente J, Boland MP. Relative messenger RNA abundance in bovine oocytes collected in vitro or in vivo before and 20 hr after the preovulatory luteinizing hormone surge. Mol Reprod Dev. 2003;66:297–305. doi: 10.1002/mrd.10357. [DOI] [PubMed] [Google Scholar]
- Lonergan P, Monaghan P, Rizos D, Boland MP, Gordon I. Effect of follicle size on bovine oocyte quality and developmental competence following maturation, fertilization, and culture in vitro. Mol Reprod Dev. 1994;37:48–53. doi: 10.1002/mrd.1080370107. [DOI] [PubMed] [Google Scholar]
- Mancini A, Borrelli A, Schiattarella A, Fasano S, Occhiello A, Pica A, Sehr P, Tommasino M, Nuesch JP, Rommelaere J. Tumor suppressive activity of a variant isoform of manganese superoxide dismutase released by a human liposarcoma cell line. Int J Cancer. 2006;119:932–943. doi: 10.1002/ijc.21904. [DOI] [PubMed] [Google Scholar]
- Matos L, Stevenson D, Gomes F, Silva-Carvalho JL, Almeida H. Superoxide dismutase expression in human cumulus oophorus cells. Mol Hum Reprod. 2009;15:411–419. doi: 10.1093/molehr/gap034. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Dionne L, Guo Q, Kumar TR, Lebovitz RM. Ovarian function in superoxide dismutase 1 and 2 knockout mice. Endocrinology. 1998;139:4008–4011. doi: 10.1210/endo.139.9.6289. [DOI] [PubMed] [Google Scholar]
- Mermillod P, Oussaid B, Cognie Y. Aspects of follicular and oocyte maturation that affect the developmental potential of embryos. J Reprod Fertil Suppl. 1999;54:449–460. [PubMed] [Google Scholar]
- Miyazaki T, Sueoka K, Dharmarajan AM, Atlas SJ, Bulkley GB, Wallach EE. Effect of inhibition of oxygen free radical on ovulation and progesterone production by the in-vitro perfused rabbit ovary. J Reprod Fertil. 1991;91:207–212. doi: 10.1530/jrf.0.0910207. [DOI] [PubMed] [Google Scholar]
- Mondola P, Annella T, Santillo M, Santangelo F. Evidence for secretion of cytosolic CuZn superoxide dismutase by Hep G2 cells and human fibroblasts. Int J Biochem Cell Biol. 1996;28:677–681. doi: 10.1016/1357-2725(96)00004-0. [DOI] [PubMed] [Google Scholar]
- Moreno S, Nardacci R, Ceru MP. Regional and ultrastructural immunolocalization of copper-zinc superoxide dismutase in rat central nervous system. J Histochem Cytochem. 1997;45:1611–1622. doi: 10.1177/002215549704501204. [DOI] [PubMed] [Google Scholar]
- Nozik-Grayck E, Suliman HB, Piantadosi CA. Extracellular superoxide dismutase. Int J Biochem Cell Biol. 2005;37:2466–2471. doi: 10.1016/j.biocel.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Ookawara T, Kizaki T, Takayama E, Imazeki N, Matsubara O, Ikeda Y, Suzuki K, Li Ji L, Tadakuma T, Taniguchi N, Ohno H. Nuclear translocation of extracellular superoxide dismutase. Biochem Biophys Res Commun. 2002;296:54–61. doi: 10.1016/s0006-291x(02)00804-5. [DOI] [PubMed] [Google Scholar]
- Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis. 2007;12:913–922. doi: 10.1007/s10495-007-0756-2. [DOI] [PubMed] [Google Scholar]
- Pavlok A, Lucas-Hahn A, Niemann H. Fertilization and developmental competence of bovine oocytes derived from different categories of antral follicles. Mol Reprod Dev. 1992;31:63–67. doi: 10.1002/mrd.1080310111. [DOI] [PubMed] [Google Scholar]
- Peterson SL, Stevenson PM. Changes in catalase activity and concentration during ovarian development and differentiation. Biochim Biophys Acta. 1992;1135:207–214. doi: 10.1016/0167-4889(92)90138-2. [DOI] [PubMed] [Google Scholar]
- Ruder EH, Hartman TJ, Blumberg J, Goldman MB. Oxidative stress and antioxidants: exposure and impact on female fertility. Hum Reprod Update. 2008;14:345–357. doi: 10.1093/humupd/dmn011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini L, Wilson C, Lower A, Al-Shawaf T, Grudzinskas JG. Superoxide dismutase activity in human follicular fluid after controlled ovarian hyperstimulation in women undergoing in vitro fertilization. Fertil Steril. 1999;72:1027–1034. doi: 10.1016/s0015-0282(99)00411-2. [DOI] [PubMed] [Google Scholar]
- Sabuncu T, Vural H, Harma M. Oxidative stress in polycystic ovary syndrome and its contribution to the risk of cardiovascular disease. Clin Biochem. 2001;34:407–413. doi: 10.1016/s0009-9120(01)00245-4. [DOI] [PubMed] [Google Scholar]
- Sirard MA, Richard F, Blondin P, Robert C. Contribution of the oocyte to embryo quality. Theriogenology. 2006;65:126–136. doi: 10.1016/j.theriogenology.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Slot JW, Geuze HJ, Freeman BA, Crapo JD. Intracellular localization of the copper-zinc and manganese superoxide dismutases in rat liver parenchymal cells. Lab Invest. 1986;55:363–371. [PubMed] [Google Scholar]
- Sugino N. Reactive oxygen species in ovarian physiology. Reproductive Medicine and Biology. 2005;4:31–44. doi: 10.1111/j.1447-0578.2005.00086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino N, Hirosawa-Takamori M, Zhong L, Telleria CM, Shiota K, Gibori G. Hormonal regulation of copper-zinc superoxide dismutase and manganese superoxide dismutase messenger ribonucleic acid in the rat corpus luteum: induction by prolactin and placental lactogens. Biol Reprod. 1998;59:599–605. doi: 10.1095/biolreprod59.3.599. [DOI] [PubMed] [Google Scholar]
- Sugino N, Takiguchi S, Kashida S, Takayama H, Yamagata Y, Nakamura Y, Kato H. Suppression of intracellular superoxide dismutase activity by antisense oligonucleotides causes inhibition of progesterone production by rat luteal cells. Biol Reprod. 1999;61:1133–1138. doi: 10.1095/biolreprod61.4.1133. [DOI] [PubMed] [Google Scholar]
- Sutton ML, Gilchrist RB, Thompson JG. Effects of in-vivo and in-vitro environments on the metabolism of the cumulus-oocyte complex and its influence on oocyte developmental capacity. Hum Reprod Update. 2003;9:35–48. doi: 10.1093/humupd/dmg009. [DOI] [PubMed] [Google Scholar]
- Tamate K, Sengoku K, Ishikawa M. The role of superoxide dismutase in the human ovary and fallopian tube. J Obstet Gynaecol. 1995;21:401–409. doi: 10.1111/j.1447-0756.1995.tb01029.x. [DOI] [PubMed] [Google Scholar]
- Tan RJ, Lee JS, Manni ML, Fattman CL, Tobolewski JM, Zheng M, Kolls JK, Martin TR, Oury TD. Inflammatory cells as a source of airspace extracellular superoxide dismutase after pulmonary injury. Am J Respir Cell Mol Biol. 2006;34:226–232. doi: 10.1165/rcmb.2005-0212OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarin JJ. Potential effects of age-associated oxidative stress on mammalian oocytes/embryos. Mol Hum Reprod. 1996;2:717–724. doi: 10.1093/molehr/2.10.717. [DOI] [PubMed] [Google Scholar]
- Tatemoto H, Muto N, Sunagawa I, Shinjo A, Nakada T. Protection of porcine oocytes against cell damage caused by oxidative stress during in vitro maturation: role of superoxide dismutase activity in porcine follicular fluid. Biol Reprod. 2004;71:1150–1157. doi: 10.1095/biolreprod.104.029264. [DOI] [PubMed] [Google Scholar]
- Tatemoto H, Sakurai N, Muto N. Protection of porcine oocytes against apoptotic cell death caused by oxidative stress during In vitro maturation: role of cumulus cells. Biol Reprod. 2000;63:805–810. doi: 10.1095/biolreprod63.3.805. [DOI] [PubMed] [Google Scholar]
- Tilly JL, Tilly KI. Inhibitors of oxidative stress mimic the ability of follicle-stimulating hormone to suppress apoptosis in cultured rat ovarian follicles. Endocrinology. 1995;136:242–252. doi: 10.1210/endo.136.1.7828537. [DOI] [PubMed] [Google Scholar]
- Valdez KE, Cuneo SP, Turzillo AM. Regulation of apoptosis in the atresia of dominant bovine follicles of the first follicular wave following ovulation. Reproduction. 2005;130:71–81. doi: 10.1530/rep.1.00430. [DOI] [PubMed] [Google Scholar]
- Webb R, Campbell BK. Development of the dominant follicle: mechanisms of selection and maintenance of oocyte quality. Soc Reprod Fertil Suppl. 2007;64:141–163. doi: 10.5661/rdr-vi-141. [DOI] [PubMed] [Google Scholar]
- Wong PC, Waggoner D, Subramaniam JR, Tessarollo L, Bartnikas TB, Culotta VC, Price DL, Rothstein J, Gitlin JD. Copper chaperone for superoxide dismutase is essential to activate mammalian Cu/Zn superoxide dismutase. Proc Natl Acad Sci U S A. 2000;97:2886–2891. doi: 10.1073/pnas.040461197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.