Abstract
Mental illnesses, such as bipolar disorder, attention-deficit/hyperactivity disorder, depression and schizophrenia are a major public health concern worldwide. Several pharmacologic agents acting on monoamine neurotransmission are used for the management of these disorders. However, there is still little understanding of the ultimate molecular mechanisms responsible for the therapeutic effects of these drugs or their relations with disease etiology. Here I provide an overview of recent advances on the involvement of the signalling molecules Akt and glycogen synthase kinase-3 (GSK3) in the regulation of behaviour by the monoamine neurotransmitters dopamine (DA) and serotonin (5-HT). I examine the possible participation of these signalling molecules to the effects of antidepressants, lithium and antipsychotics, as well as their possible contribution to mental disorders. Regulation of Akt and GSK3 may constitute an important signalling hub in the subcellular integration of 5-HT and DA neurotransmission. It may also provide a link between the action of these neurotransmitters and gene products, like disrupted in schizophrenia 1 (DISC1) and neuregulin (NRG), that are associated with increased risk for mental disorders. However, changes in Akt and GSK3 signalling are not restricted to a single disorder, and their contribution to specific behavioural symptoms or therapeutic effects may be modulated by broader changes in biologic contexts or signalling landscapes. Understanding these interactions may provide a better understanding of mental illnesses, leading to better efficacy of new therapeutic approaches.
Introduction
Neurotransmission mediated by the monoamines dopamine (DA) and serotonin (5-HT) is a major target for psychiatric drugs. Reuptake inhibitors that elevate synaptic 5-HT levels are commonly used for the treatment of major depression and other mood disorders,1 whereas medications blocking 5-HT2A receptors have antipsychotic effects.2 Likewise, the first generation of antipsychotic drugs, such as haloperidol and chlorpromazine, are potent blockers of D2-class DA receptors,3,4 whereas psychostimulants, like amphetamine and methylphenidate, that are used for the treatment of attention-deficit/hyperactivity disorder (ADHD) elevate DA tones.
Most DA neurons in the brain have their cell bodies in the substantia nigra pars compacta and ventral tegmental area that project to the striatum, nucleus accumbens and frontal cortex. Most 5-HT neurons are located in the raphe nuclei and send projections to multiple brain regions, including the striatum, hippocampus and frontal cortex.
Multiple control mechanisms regulate the activity of monoaminergic synapses. Briefly, DA and 5-HT are synthesized in presynaptic neurons, stored into vesicles by the vesicular monoamine transporter and released to the synapse in response to action potentials.5 In the adult brain, the rate-limiting enzymes for the synthesis of DA and 5-HT are tyrosine hydroxylase and tryptophan hydroxylase 2 (Tph2), respectively.6–9 Following release, neurotransmitter molecules stimulate postsynaptic receptors. The duration of this stimulation and concentration of neurotransmitters at the synapse are tightly regulated by transporters, such as the DA transporter (DAT) and the 5-HT transporter, that reuptake neurotransmitters from the extracellular space to the cytoplasm of presynaptic neurons where they will be stored into vesicles.5,10 The intensity of neurotransmitter release and synthesis are also regulated by autoreceptors on presynaptic neurons. There are 6 DA receptors and more than 15 5-HT receptors (Table 1). With the exception of 1 class of 5-HT receptors (5-HT3 receptors), all of these are 7-transmembrane domain proteins coupled to heterotrimeric G proteins. These G protein–coupled receptors (GPCRs) exert most of their known actions on signalling by modulating the production of different second-messenger molecules (Table 1).
Table 1.
Dopamine and serotonin receptor classes
| Receptor class | Receptors | Coupled to | Localization |
|---|---|---|---|
| Dopamine | |||
| D1 class | D1 and D5 | GαS(cAMP↑) | Postsynaptic |
| D2 class | D2L, D2S, D3, D4 | Gαi/o(cAMP↓) β arrestin-2 (D2, D3) |
Pre-and postsynaptic |
| 5-HT1 | 5-HT1A–F, 5-HT5 | Gαi/o(cAMP↓) | Pre-and postsynaptic |
| 5-HT2 | 5-HT2A–C | Gαq | Postsynaptic and non-neuronal |
| 5-HT3 | 5-HT3A–C | Ion channel | Postsynaptic and non-neuronal |
| 5-HT others | 5-HT4, 5-HT6, 5-H7 | GαS(cAMP↑) | Postsynaptic and non-neuronal |
Although understanding the effects of psychoactive drugs on DA and 5-HT neurotransmission has allowed the development of drugs targeting key molecules, such as mono-amine transporters, more selectively,12 there is still little information concerning the involvement of these neurotransmitter systems in the etiology of mental disorders. For example, several genetic studies have focused on the role of DA receptor dysfunction in human disorders.13–18 However, as noted in 2000 by Wong and colleagues,19 “while there are some evidences that polymorphisms and mutations in [DA] receptors can alter functional activity and pharmacological profiles, no conclusive data link these gene variants to drug response or disease.” Unfortunately, this situation has not changed much over the past 10 years.
Instead of establishing a clear link with monoamine neurotransmission, genetic studies of mental disorders conducted over the last decade identified several polymorphisms in genes, such as disrupted in schizophrenia 1 (DISC1)20 and neuregulin 1 (Nrg1),21 that are not obviously related to DA or 5-HT neurotransmission. This suggests that drugs acting on 5-HT or DA neurotransmission may exert their therapeutic effects in mental disorders by “normalizing” cell signalling mechanisms that are also affected by genetic or environmental factors in people with these disorders. Recent lines of evidence from my colleagues and I, as well as other groups, have indicated that both DA and 5-HT exert part of their actions on behaviour by modulating the activity of glycogen synthase kinase-3 (GSK3) and signalling molecules, such as Akt, that are involved in its regulation. Interestingly, this signalling pathway is also regulated by different types of psychiatric drugs, including antipsychotics, antidepressants and lithium.22 Furthermore, several proteins encoded by genes associated with mental disorders affect the activity of this signalling pathway. In this brief review, I provide an overview of research by my colleagues and I on the characterization of the regulation of Akt and GSK3 signalling by DA and 5-HT. I also examine the possible role of these molecules as subcellular integrators for the effects of DA and 5-HT in mental disorders and the action of psychiatric drugs.
The Akt–GSK3 signalling pathway
Glycogen synthase kinase-3 and Akt, also known as protein kinase B, are serine threonine kinases that were initially identified as playing a role in the regulation of glycogen synthesis in response to insulin receptor stimulation.23–25 Over the years, these molecules were shown to be involved in a host of normal and pathologic processes, including the regulation of glycogenesis, cellular proliferation, programmed cell death, embryogenesis and circadian entrainment.26–29
There are 3 isoforms of Akt that are encoded by separate human genes, AKT1 (position 14q32.32), AKT2 (position 19q13.1-q13.2) and AKT3 (position 1q44). All of these isoforms are activated in response to phosphoinositide-3 kinase (Pi3K)–mediated signalling following the stimulation of various cell surface receptors like GPCRs and receptor tyrosine kinases (e.g., the brain-derived neurotrophic factor receptor TrkB and the Nrg1 receptor ErbB). Activation of Pi3K signalling results in the activation of Akt1 following its phosphorylation on the Thr308 and Ser473 residues.30 Akt is also negatively regulated by protein phosphatases, notably protein phosphatase 2A (PP2A) that can inactivate Akt in vivo.31–33
Glycogen synthase kinase-3 isoforms are among the most extensively studied substrates of Akt. There are 2 isoforms of GSK3 (GSK3α and GSK3β) that are encoded by different human genes, GSK3A (position 19q13.2) and GSK3B (position 3q13.3).34 These proteins are constitutively active serine/threonine kinases that are negatively regulated by Akt and other serine/threonine kinases through phosphorylation of serine residues on their amino-terminal domain — Ser21 for GSK3α and Ser9 for GSK3β.28,35
Regulation of Akt and GSK3 by DA
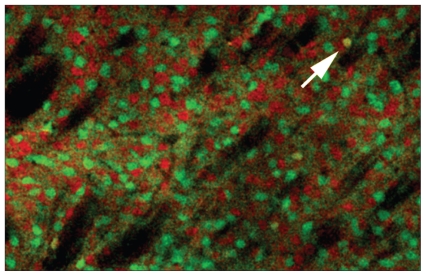
The various functions of DA receptors have been primarily associated with the regulation of cyclic adenosine monophosphate (cAMP) and protein kinase A (PKA) via G protein–dependent signalling (Table 1).11 The D1 class receptors are mostly coupled to Gαs and stimulate cAMP production and the activity of PKA. In contrast, D2 class receptors are coupled to Gαi/o to inhibit the production of cAMP and thus diminish PKA activity.36–39 There is evidence that some neuronal populations can coexpress both receptor types with an effect on signalling responses.40–42 However, several independent studies using whole-gene regulated fluorescent reporters in bacterial artificial chromosome transgenic mice have shown that striatal neurons appear to express preferentially either D1 or D2 receptors (Fig. 1).43–45
Fig. 1.
Segregation of D1 and D2 dopamine (DA) expression in the dorsal striatum. Confocal microscopy images of striatal neurons in double bacterial artificial chromosome transgenic mice expressing the green fluorescent protein reporter gene under the control of the D2 DA receptor gene promoter and the (red) tomato fluorescent protein reporter gene under the control of the DA receptor gene promoter. The arrow points to a cell that expresses both reporter genes.
Studies by my colleagues and I of cell signalling in response to elevated DA identified a reduction of Akt phosphorylation/activity along with concomitant activation of both GSK3α and GSK3β in the striatum of mice lacking the DAT (DAT knockout mice).46,47 Administration of amphetamine, methamphetamine or the nonselective DA receptor agonist apomorphine to normal mice also results in an inhibition of Akt activity and concomitant activation of GSK3, whereas striatal DA depletion has the opposite effect, thus firmly establishing the regulation of the Akt–GSK3 pathway by DA.31,46,48,49 Further characterization of these signalling responses using selective D1 and D2 receptor antagonists,46 the antipsychotic haloperidol50,51 or mice lacking different subtypes of DA receptors have shown that Akt, GSK3α and GSK3β are regulated by D2 receptors.52 More specifically, D2 receptors appear to be essential for the inhibition of striatal Akt by DA in mice. Interestingly, mice lacking the D3 receptor display a reduced sensitivity of Akt-mediated signalling to dopaminergic drugs but retain the action of these drugs on Akt at higher doses. This suggests that D3 receptors also participate in the regulation of Akt–GSK3 signalling potentially by enhancing the D2 receptor–mediated DA response.52
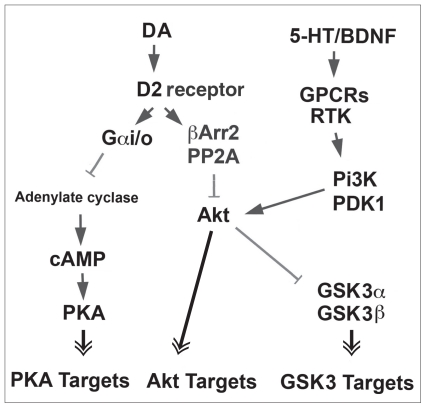
In the mouse striatum neither Akt nor GSK3 is affected by a direct modulation of cAMP levels, indicating that this signalling pathway is not controlled by canonical D2 receptor signalling46 (Fig. 2). Instead, behavioural and biochemical evidence have revealed a role for β-Arrestin-2 (βArr2), a multi-functional scaffolding/adaptor protein generally involved in GPCR desensitization in the regulation of the Akt–GSK3 pathway by D2 receptors.
Fig. 2.
Signalling networks regulated by dopamine (DA) in neurons responding to D2-class agonists. Regulation of Gαs–cAMP–PKA and βArr2–Akt–GSK3 signalling by D2 receptors. The action of other neurotransmitters, growth factors and neurotrophin has been included to illustrate the role of many of these intermediates as signal integrators. Single arrows: activation. Grey lines: inhibition. Double arrows: actions that can either be activatory or inhibitory in function of specific substrates. 5-HT = serotonin; βArr2 = β-Arrestin-2; BDNF = brain-derived neurotrophic factor; cAMP = cyclic adenosine monophosphate; GPCR = G protein–coupled receptor; GSK3 = glycogen synthase kinase-3; PDK1 = 3-phosphoinositide-dependent kinase-1; Pi3K = phosphoinositide-3 kinase; PKA = protein kinase A; PP2A = protein phosphatase 2A; RTK = receptor tyrosine kinase.
Following GPCR stimulation, G protein–mediated signalling is inactivated by mechanisms that result in receptor de-sensitization, internalization and termination of G protein–mediated signalling. G protein–coupled receptor activation induces the phosphorylation of the receptors and the recruitment of arrestins.53–55 Most mammalian tissues express 2 arrestins, β–Arrestin-1 and βArr2.53 The interaction of arrestins with GPCRs is followed by the recruitment of an endocytic complex, which results in the internalization of receptors.53,55–57 However, the role of arrestins in GPCR functions is not limited to desensitization. It has become apparent that in addition to activating G proteins, GPCRs can also elicit cellular responses mediated by the formation of signalling protein complexes held together by arrestins.58–60
Research by my colleagues and I has shown that when administered to βArr2 knockout mice, the DA drugs amphetamine and apomorphine fail to reduce Akt phosphorylation as they do in wild-type animals.31 Similarly, mice lacking both βArr2 and the DAT do not display an inhibitory action of excessive DA on Akt phosphorylation. This demonstrates that D2 receptors regulate Akt through βArr2.31 Further characterization of the mechanism by which βArr2 regulates Akt in response to DA showed that stimulation of D2 receptors causes the formation of a protein complex composed at least of Akt, βArr2 and PP2A, which facilitates the de-phosphorylation and/or deactivation32 of Akt by PP2A in response to DA (Fig. 3A).31,46 This reduction of Akt activity leads to an activation of GSK3 in response to D2 receptor stimulation (Fig. 2).
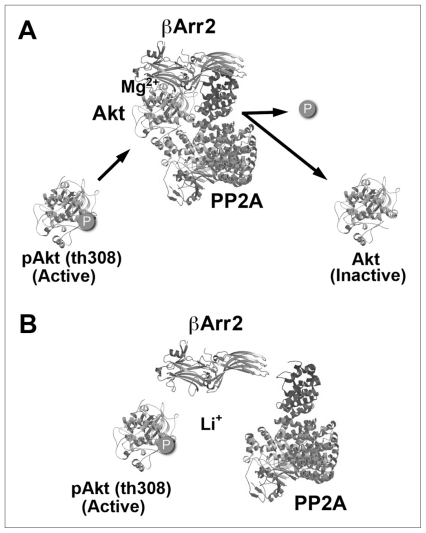
Fig. 3.
Model for arrestin-dependent inhibition of Akt and its regulation by lithium (Li+). (A) Under basal conditions, Akt phosphorylation/activation is the result of an equilibrium between activation of Akt by phosphorylation and its inactivation by protein phosphatase 2A (PP2A)–mediated dephosphorylation. Dephosphorylation of Akt is facilitated by formation of the magnesium (Mg2+)-dependent scaffolding of Akt and PP2A by β-Arrestin-2 (βArr2). (B) By displacing magnesium, lithium destabilizes the signalling complex formed by Akt, βArr2 and PP2A, thereby enhancing Akt activity by reducing its dephosphorylation.
Regulation of behaviour by the D2 receptor–βArr2–Akt–GSK3 signalling pathway
There are several lines of evidence pointing toward a role of βArr2, Akt and GSK3 in the regulation of DA-dependent behaviours. Under basal conditions,54,61 βArr2 knockout mice are less active than wild-type littermates when placed in a novel environment, a behaviour that is mediated in part by DA.31,62–64 Outside of this habituation period, βArr2 knockout mice are not overtly impaired in terms of DA functions, as would be the case for mice with more severe disruption of DA neurotransmission.11,65,66 However, a lack of βArr2 affects behavioural responsiveness to different drugs acting on DA receptor functions. Mice lacking βArr2 have reduced responses to the D1 and D2 receptor agonist apomorphine and to the DA-dependent actions of amphetamine and morphine.31,54,64 Furthermore, mice lacking both βArr2 and the DAT display a reduction of the typical novelty-induced hyperactivity phenotype of DAT knockout mice.31
In addition to behavioural deficits in βArr2 knockout mice, several other observations support the involvement of the βArr2–Akt–GSK3 pathway in the regulation of DA-related behaviours. Pharmacologic GSK3 inhibitors can reduce hyperactivity both in DAT knockout mice and in animals treated with amphetamine.46,67,68 Furthermore, observations of genetically engineered animals lacking one functional allele of Gsk3b69 revealed that a 50% reduction of brain GSK3β protein levels46 reduces behavioural responsiveness to amphetamine.46 Conversely, mutant mice lacking inhibitory phosphorylation sites on both GSK3α and GSK3β are hyperresponsive to amphetamine,70 whereas transgenic mice overexpressing GSK3β develop a hyperactivity phenotype reminiscent of hyperdopaminergic DAT knockout mice.71 Finally, Akt1 knockout mice show enhanced disruption of sensorimotor gating by amphetamine in prepulse inhibition tests.50 Disruption of sensorimotor gating by amphetamine is commonly used as a behavioural paradigm to model psychosis in rodents, and this effect can be blocked by antipsychotics.72 Since Akt1 is inhibited following the stimulation of D2 receptors, the increased behavioural effect of amphetamine in Akt1 knockout mice supports the involvement of Akt inhibition in DA-related behavioural responses. However, DA regulates more than locomotion and sensorimotor gating.73 Further detailed characterization of DA-related behaviours in more specific tests in rodents or nonhuman primates may thus be warranted to fully understand the contribution of βArr2, Akt and GSK3 in the expression of DA-associated behaviours.
Regulation of GSK3 by 5-HT
Investigations of drosophila have shown that the fly ortholog of the 5-HT1A receptors regulates shaggy, the ortholog of GSK3, in the insect brain.74 Regulation of shaggy by 5-HT1A receptors in the fly is important for the control of circadian rhythms. In mice, different classes of drugs acting on 5-HT neurotransmission, such as selective serotonin reuptake inhibitors (SSRIs), monoamine oxidase (MAO) inhibitors and tricyclic antidepressants, inhibit GSK3β by increasing its phosphorylation in the frontal cortex, hippocampus and striatum.75–77 In addition, mice with about 80% reduction in 5-HT synthesis resulting from the expression of a Tph2 R439H loss-of-function mutant (equivalent to the human R441H Tph2 variant9,78) display increased GSK3 activity in the frontal cortex.79
Two classes of serotonin receptors, 5-HT1 and 5-HT2, appear to be involved and play opposing roles in regulating GSK3β activity.75,76 Administration of the 5-HT1 agonist 8-OH-DPAT or of the 5-HT2 antagonist LY53857 to wild-type mice both result in increased GSK3β phosphorylation in the brain.76 This suggests that 5-HT can inhibit GSK3β by acting through 5-HT1 receptors, whereas activation of 5-HT2 receptors would result in GSK3β activation. However, it should be mentioned that the 5-HT2A agonist DOI and the 5-HT1A antagonist WAY100635 did not affect GSK3β activity in the brain,76 thus leaving the question of the relative contribution of different 5-HT receptors to the regulation of GSK3β only partially addressed. There are also contradictory observations concerning the involvement of Akt in the regulation of GSK3 by 5-HT receptors. Some studies reported this regulation mechanism to be independent from Akt following the administration of SSRIs.47,75 In contrast, a more recent study has indicated that administration of d-fenfluramine, which increases brain serotonergic tones, activates Akt in the mouse brain.80 One possible explanation for these discrepancies is that older studies monitored the phosphorylation of Ser473 as an indicator of Akt activity, whereas the more recent study followed the phosphorylation of the Thr308 residue of Akt. Since the phosphorylation of Akt on Thr308 appears to be critical for the regulation of GSK3 by Akt,81 it is possible that the more recent study may be more representative of the role of Akt in the regulation of GSK3 by 5-HT. However further studies of the mechanisms through which 5-HT regulates Akt and GSK3 activity in vivo may be needed to firmly establish this possibility.
A role for GSK3 in the regulation of behaviour by 5-HT
There is also evidence for a role of GSK3 in the regulation of 5-HT–mediated behaviours. Mice treated with GSK3 inhibitors67 or different genetically modified mice with altered GSK3 function display behavioural changes that are reminiscent of normal animals following antidepressant treatment. Glycogen synthase kinase-3β haploinsuficient mice have been shown to exhibit an antidepressant-like response in the Porsolt forced swimming test and the tail suspension test.63,82,83 Furthermore, mutant mice lacking GSK3α and GSK3β inhibitory phosphorylation sites display a general reduction of anxiety and increased exploratory behaviour in several tests that are generally used to evaluate the effects of drugs acting on 5-HT neurotransmission, indicating that regulation of GSK3 amino-terminal domain phosphorylation by Akt may play a role in the regulation of these behaviours.84
To assess the contribution of GSK3β to 5-HT–regulated behaviours, mice expressing the R439H Tph2 loss-of-function mutation were bred with haploinsufficient GSK3β animals69 to produce mice carrying a different allelic combination of the wild-type and mutant Tph2 gene and with mice of the same Tph2 genotypes with reduced GSK3β expression. Mice from these different genotypes were evaluated in several behavioural tests designed to analyze 5-HT–mediated emotional states in rodents. Results from these experiments showed that a reduction of about 50% in the expression of GSK3β (about 25% of total GSK3) is sufficient to rescue behavioural deficits induced by reduced 5-HT synthesis in the tail suspension test and in a test used to measure anxiety-like behaviours in mice.79 Interestingly, the reduction of GSK3β expression also curbed the display of aggressive behaviours in 5-HT–depleted mice,79 suggesting a role of GSK3 in the regulation of social behaviours and aggression by 5-HT. That being said, 5-HT and GSK3 have both been implicated in the regulation of several other behavioural manifestations beyond the regulation of mood, anxiety and social interactions. Mice expressing the R439H Tph2 variant with normal or reduced expression of GSK3β should provide an interesting tool to clarify the roles played by GSK3 in the regulation of 5-HT–related behaviours in the future. However, this model will need to be combined with other pharmacologic and genetic engineering approaches to firmly establish a link of causality between changes in GSK3 activity and behavioural modifications resulting from changes in 5-HT neurotransmission.
Akt and GSK3 signalling in the actions of antipsychotics
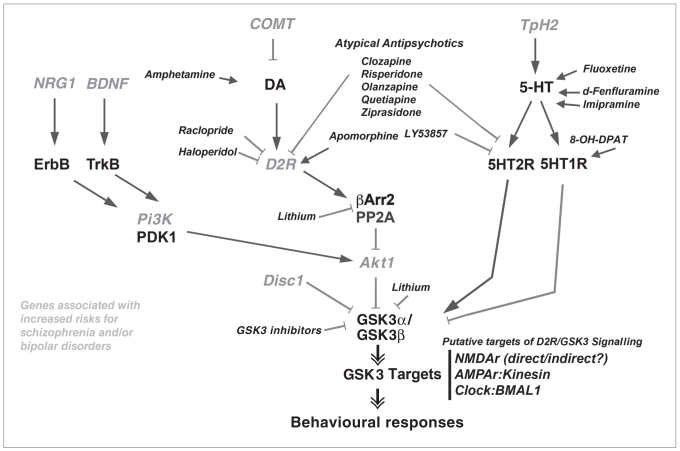
Several psychoactive drugs have been shown to modulate the activity of the Akt–GSK3 signalling pathway (Fig. 4).22 Drugs like SSRIs and MAO inhibitors that elevate 5-HT synaptic transmission have been shown to inhibit GSK3 in vivo.75,76 Conversely, drugs that elevate DA neurotransmission (e.g., amphetamine) reduce the inhibitory phosphorylation of GSK3α and GSK3β and therefore increase the activity of these kinases.46,70,85 By blocking D2 receptors, classic antipsychotics can prevent the inhibition of Akt and concomitant activation of GSK3 by DA. Such regulation of Akt and GSK3 activity has been reported in mice after chronic treatment with haloperidol.50
Fig. 4.
Regulation of Akt–GSK3 signalling by psychoactive drugs and gene products associated with mental disorders. Proteins are the product of genes associated with increased risk for schizophrenia and/or bipolar disorders. Behavioural changes in dopaminergic responses have been reported in Akt1 knockout and β-Arrestin-2 (βArr2) knockout mice and in GSK3β haploinsuficient mice. Single arrows = activation; solid lines = inhibition; double arrows = actions that can either be activatory or inhibitory in function of specific substrates. 5-HT = serotonin; BDNF = brain-derived neurotrophic factor; BMAL1 = brain and muscle Arnt-like protein-1; Clock = circadian locomotor output cycles kaput; COMT = catechol-O-methyltransferase; DA = dopamine; Disc1 = disrupted in schizophrenia 1; GSK3 = glycogen synthase kinase-3; NMDA = N-methyl-d-aspartate; NRG1 = neuregulin 1; PDK1 = 3-phosphoinositide-dependent kinase-1; Pi3K = phosphoinositide-3 kinase; PP2A = protein phosphatase 2A; TPH2 = tryptophan hydroxylase 2.
Acute and chronic treatments with atypical antipsychotics, including clozapine, olanzapine, quetiapine, ziprasidone and risperidone, have also been shown to result in an inhibition of GSK3 in the rodent brain.75,86 The effect of atypical anti-psychotics on GSK3 activity can be explained in part by the D2 receptor antagonist action of atypical antipsychotics. An elegant study conducted using bioluminescence energy transfer in cell lines has suggested that although the effects of different atypical antipsychotics on G protein–mediated D2 receptor signalling may differ, all of these drugs share the common property of blocking the recruitment of βArr2 to this GPCR.87 Unfortunately, this study did not directly address the effect of these different drugs on the βArr2-mediated regulation of Akt and GSK3 by DA in the brain, and a few alternative mechanisms may also explain the modulation of GSK3 activity by these drugs in vivo. For instance, in addition to their effects on D2 receptors, atypical antipsychotics are also antagonists of 5-HT2A receptors12 and may interfere with the regulation of GSK3 by 5-HT.75,77 In addition, a handful of studies also suggest a role for the disheveled Wnt signalling pathway in the regulation of GSK3 activity by antipsychotics.51,88 Therefore, there may be more than 1 mechanism through which antipsychotic drugs affect GSK3 activity in a given cell or in different neuronal populations. Further characterizations of the role of these mechanisms and of possible functional cross-talk between them at the cellular and system levels may thus be important to understand the possible implication of GSK3-mediated signalling in the effects of these drugs.
Akt and GSK3 signalling in the actions of lithium
Akt and GSK3 have also been associated with the action of the mood stabilizer lithium (Fig. 4). Lithium concentrations of 2 mM are known to inhibit GSK3β in vitro.89,90 However, these lithium concentrations are higher than the 0.5–1.5 mM lithium serum concentrations used for the treatment of bipolar disorders.91 In the mouse brain, both acute and chronic lithium treatments inhibit GSK3 indirectly by activating Akt and increasing the regulatory amino-terminal domain phosphorylation of GSK3.46,92 We have shown that in the mouse striatum, this indirect effect of lithium on the activity of GSK3 can result from the disruption of a signalling complex composed of Akt, βArr2 and PP2A.63 This protein complex mediates, among other potential roles, some of the D2 receptor signalling functions (Fig. 2, Fig. 3B).31,63 When administered to βArr2 knockout mice, lithium does not affect Akt–GSK3 signalling as it does in normal animals. Furthermore, several behavioural effects of chronic lithium treatments in tests used to model mania or antidepressant drug effects in rodents are abolished in mice lacking βArr2.63
It has long been suggested that lithium may exert some of its pharmacologic effects by competing with magnesium, which acts as a cofactor for several enzymes, including kinases such as GSK3.93,94 The molecular mechanism through which lithium interferes with the formation of the Akt–βArr2–PP2A signalling complex appears to involve an interference with a biologic function of magnesium ions (Fig. 3).63 Initial investigations by my colleagues and I have shown that increased concentrations of magnesium, but not of other monovalent or divalent cations, can prevent the disruption of preassembled Akt–βArr2–PP2A complexes by lithium in vitro.63 Furthermore, magnesium salts were shown to be required for the interaction between recombinant Akt1 and βArr2, whereas addition of lithium disrupted this same interaction in the presence of magnesium.63 This suggests that magnesium contributes to the interaction of Akt and βArr2 and that lithium competition with magnesium can affect not only enzymatic activity but also the stability of higher-order protein complexes, thus providing a potential mechanism for the destabilization of the Akt–βArr2–PP2A complex by lithium.
Regulation of Akt–GSK3 signalling in psychiatric disorders
There is converging evidence indicating that genetic risk factors for mental illnesses may affect Akt–GSK3 signalling. Significant association of AKT1 haplotypes with schizophrenia and/or bipolar disorder has been reported in several independent cohorts.22,50,95–98 Lymphocytes and postmortem brain samples from patients with schizophrenia have also been reported to have reduced Akt protein levels compared with those of controls.50 In contrast, the direct association of GSK3A and GSK3B with psychiatric disorders is less clear. There is no clinical evidence demonstrating an association between GSK3A and psychiatric disorders. As for GSK3B, isolated reports have indicated an association between a –50T/C polymorphism in the GSK3B promoter and responsiveness to lithium therapy or the occurrence of psychotic symptoms in patients with mood disorders.22,96 Recent evidence also indicates that temporal lobe grey matter volume in people with schizophrenia is associated with a GSK3B polymorphism.99
Although the evidence for a direct role of GSK3A or GSK3B mutations is scarce, there are several indications that the regulation of GSK3 is altered in psychiatric disorders. Reduced levels of phosphorylated GSK3β have been found in postmortem frontal cortex samples from some individuals with schizophrenia.50,100 These variations in phosphorylation can be caused in part by reduced Akt expression. However, other products from genes associated with mental disorders are also involved in the regulation of GSK3. Genetic variants of TPH2, DISC1 and NRG1 have been associated with the pathogenesis of schizophrenia and/or bipolar disorder101,102 and can affect the Akt–GSK3 signalling pathway in various experimental models.79,103–105 In addition, significant epistatic interaction has been found between an AKT1 variant and a functional polymorphism (Val158Met) in the catechol-O-methyltransferase (COMT) gene that is associated with schizophrenia,106 therefore suggesting complex interactions between genetic variants associated with mental disorders and proteins involved in Akt–GSK3 signalling.
Conclusion
Research conducted by my colleagues and I, as well as other groups, has uncovered several biochemical, genetic, behavioural and pharmacologic evidence for a contribution of the Akt–GSK3 signalling pathway in the treatment and etiology of psychiatric disorders. However, our understanding of this role is certainly far from complete. For instance, there is still little knowledge about the exact nature of the neural circuits involved in the regulation of behaviour by these kinases. The possible involvement of Akt and GSK3 in signalling evoked by the monoamines norepinephrine and histamine or by active monoamine degradation products such as 3-methoxytyramine107 has also remained unexplored. Furthermore, the molecular mechanisms through which this pathway exerts its various actions in the brain are not well understood beyond the simple control of GSK3 activity.
Several recent research efforts have established a link between ionotropic glutamate receptor functions and GSK3 activity in the hippocampus and frontal cortex.108–110 This is particularly intriguing since altered glutamate receptor functions have long been suspected to play a role in mental disorder pathology, mostly in schizophrenia.73,111 However, it would be premature to conclude that the role of Akt–GSK3 signalling is restricted to the regulation of glutamate receptor functions. Over the last 5 years, Akt and GSK3 have also been associated with several other mechanisms that can potentially be involved in schizophrenia, depression or bipolar disorders. Among these, it is of interest that Akt is involved in the regulation of the mammalian target of rapamycin112 and of the forkhead box transcription factors.80 Furthermore, there is strong evidence for a role of GSK3 in the regulation of circadian rhythm, epigenetic gene regulation29 and 5-HT1B receptor cell surface trafficking.113,114
Another intriguing observation is that changes in the regulation of Akt and GSK3 activity so far are similar across different drug categories. Overall, antipsychotics, antidepressants acting on 5-HT neurotransmission and lithium have all been reported to activate Akt and inhibit GSK3 in vivo. Interestingly, the mood stabilizers valproate and lamotrigine have also been reported to inhibit GSK3 under certain conditions, but the mechanisms underlying this effect and their behavioural significance are not clear.91,115 Furthermore, direct inhibition of GSK3 isoforms, either through pharmacologic or genetic means, has been shown to have effects that are similar to some of those of antidepressants, lithium or anti-psychotics in different behavioural tests conducted in rodents.22,68,82 Yet these drugs do not share the same clinical profile in humans. It is possible that inhibition of GSK3 in specific neuronal populations in response to a given drug could be associated with distinct behavioural outcomes. Activation of Akt and inhibition of GSK3 may also represent core effects for some shared action of psychoactive drugs, which may explain their increased efficacy in the context of combination therapies.22,91,116,117 In this case, a modulation of Akt–GSK3 signalling may contribute to the effect of a drug while not being its exclusive component. The complete clinical profile of a given drug would then result not from the inhibition or activation of a single signalling pathway but from a more complex modulation of the overall cell signalling landscape118,119 in different neuronal populations.
A similar observation can be made for the potential contribution of Akt and GSK3 to the etiology of mental disorders. Several studies suggest that activation of GSK3 can be a shared outcome of several susceptibility genes for mental disorders. However, whereas dysregulation of these genes can lead to a reduced expression of Akt or affect the inhibition of GSK3 by DISC1, the disease specificity of such changes is unclear (e.g., association with schizophrenia, bipolar disorders and depression in the case of DISC1120 and with bipolar disorders and schizophrenia in the case of AKT197), whereas their contribution to disease development is not always confirmed across studies.120,121 It is a truism that several factors must simultaneously contribute to psychiatric illnesses.122–124 It can be postulated that a modulation of GSK3 activity may represent a core risk factor that increases the probability for a psychiatric illness in a given individual under certain genetic and environmental conditions. An analogous situation exists in cancer biology where “double hit” or “multiple hit” models predict that accumulation of germinal and somatic mutations in some genes regulating cell proliferation and survival is ultimately responsible for disease progression.125,126 In these models, the gain or loss of function of a single signalling protein contributes to disease causation in a nonobligatory and nonexclusive fashion. Similar types of models involving the contribution of various genetic and environmental “hits” have also been put forward for schizophrenia.122,123 Therefore, in the present state of knowledge, it is probable that an activation of GSK3 is one of several factors that can contribute to mental disorders. However, it is not possible at the moment to conclude that the deregulation of a given signalling protein or pathway is essential or sufficient for the occurrence of a specific psychiatric disease.
Whereas many questions remain to be answered about the role of Akt and GSK3 in mental disorders, it remains important that existing data strongly indicate that regulation of Akt and GSK3 constitute an important signalling hub in the sub-cellular integration of 5-HT and DA neurotransmission with the functions of genes that are associated with mental disorders. Understanding these interactions may provide a better understanding of mental illnesses in general, leading to new therapeutic approaches having superior efficacy and lesser side effects.
Acknowledgements
This work was supported by the Canadian Institutes of Health Research (CIHR; grant NSA 93798) and a Natural Sciences and Engineering Research Council of Canada discovery grant. J.-M. Beaulieu holds a Canada Research Chair in Molecular Psychiatry and is a National Alliance for Research on Schizophrenia and Depression (NARSAD) Vital Projects Fund, Inc. Investigator.
Footnotes
Competing interests: J.-M. Beaulieu declares grants to his institution from the Canadian Institutes of Health Research, the NARSAD and the Natural Sciences and Engineering Research Council of Canada.
References
- 1.Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–54. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- 2.Kapur S, Remington G. Atypical antipsychotics: new directions and new challenges in the treatment of schizophrenia. Annu Rev Med. 2001;52:503–17. doi: 10.1146/annurev.med.52.1.503. [DOI] [PubMed] [Google Scholar]
- 3.Seeman P, Weinshenker D, Quirion R, et al. Dopamine supersensitivity correlates with D2High states, implying many paths to psychosis. Proc Natl Acad Sci U S A. 2005;102:3513–8. doi: 10.1073/pnas.0409766102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Snyder SH. The dopamine hypothesis of schizophrenia: focus on the dopamine receptor. Am J Psychiatry. 1976;133:197–202. doi: 10.1176/ajp.133.2.197. [DOI] [PubMed] [Google Scholar]
- 5.Torres GE, Gainetdinov RR, Caron MG. Plasma membrane monoamine transporters: structure, regulation and function. Nat Rev Neurosci. 2003;4:13–25. doi: 10.1038/nrn1008. [DOI] [PubMed] [Google Scholar]
- 6.Nagatsu T, Levitt M, Udenfriend S. Tyrosine hydroxylase. The initial step in norepinephrine biosynthesis. J Biol Chem. 1964;239:2910–7. [PubMed] [Google Scholar]
- 7.Zhang X, Beaulieu JM, Sotnikova TD, et al. Tryptophan hydroxylase-2 controls brain serotonin synthesis. Science. 2004;305:217. doi: 10.1126/science.1097540. [DOI] [PubMed] [Google Scholar]
- 8.Walther DJ, Peter JU, Bashammakh S, et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Beaulieu JM, Gainetdinov RR, et al. Functional polymorphisms of the brain serotonin synthesizing enzyme tryptophan hydroxylase-2. Cell Mol Life Sci. 2006;63:6–11. doi: 10.1007/s00018-005-5417-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gainetdinov RR, Caron MG. Monoamine transporters: from genes to behavior. Annu Rev Pharmacol Toxicol. 2003;43:261–84. doi: 10.1146/annurev.pharmtox.43.050802.112309. [DOI] [PubMed] [Google Scholar]
- 11.Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- 12.Roth BL, Sheffler DJ, Kroeze WK. Magic shotguns versus magic bullets: selectively non-selective drugs for mood disorders and schizophrenia. Nat Rev Drug Discov. 2004;3:353–9. doi: 10.1038/nrd1346. [DOI] [PubMed] [Google Scholar]
- 13.Parsons MJ, Mata I, Beperet M, et al. A dopamine D2 receptor gene-related polymorphism is associated with schizophrenia in a Spanish population isolate. Psychiatr Genet. 2007;17:159–63. doi: 10.1097/YPG.0b013e328017f8a4. [DOI] [PubMed] [Google Scholar]
- 14.Bertram L. Genetic research in schizophrenia: new tools and future perspectives. Schizophr Bull. 2008;34:806–12. doi: 10.1093/schbul/sbn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rondou P, Haegeman G, Van Craenenbroeck K. The dopamine D4 receptor: biochemical and signalling properties. Cell Mol Life Sci. 2010;67:1971–86. doi: 10.1007/s00018-010-0293-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coghill D, Banaschewski T. The genetics of attention-deficit/hyperactivity disorder. Expert Rev Neurother. 2009;9:1547–65. doi: 10.1586/ern.09.78. [DOI] [PubMed] [Google Scholar]
- 17.Gizer IR, Ficks C, Waldman ID. Candidate gene studies of ADHD: a meta-analytic review. Hum Genet. 2009;126:51–90. doi: 10.1007/s00439-009-0694-x. [DOI] [PubMed] [Google Scholar]
- 18.Le Foll B, Gallo A, Le Strat Y, et al. Genetics of dopamine receptors and drug addiction: a comprehensive review. Behav Pharmacol. 2009;20:1–17. doi: 10.1097/FBP.0b013e3283242f05. [DOI] [PubMed] [Google Scholar]
- 19.Wong AH, Buckle CE, Van Tol HH. Polymorphisms in dopamine receptors: What do they tell us? Eur J Pharmacol. 2000;410:183–203. doi: 10.1016/s0014-2999(00)00815-3. [DOI] [PubMed] [Google Scholar]
- 20.Millar JK, Thomson PA, Wray NR, et al. Response to Amar J. Klar: The chromosome 1;11 translocation provides the best evidence supporting genetic etiology for schizophrenia and bipolar affective disorders. Genetics. 2003;163:833–5. doi: 10.1093/genetics/163.2.833. author reply 837–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall D, Gogos JA, Karayiorgou M. The contribution of three strong candidate schizophrenia susceptibility genes in demographically distinct populations. Genes Brain Behav. 2004;3:240–8. doi: 10.1111/j.1601-183X.2004.00078.x. [DOI] [PubMed] [Google Scholar]
- 22.Beaulieu JM, Gainetdinov RR, Caron MG. Akt/GSK3 signaling in the action of psychotropic drugs. Annu Rev Pharmacol Toxicol. 2009;49:327–47. doi: 10.1146/annurev.pharmtox.011008.145634. [DOI] [PubMed] [Google Scholar]
- 23.Embi N, Rylatt DB, Cohen P. Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. Eur J Biochem. 1980;107:519–27. [PubMed] [Google Scholar]
- 24.Alessi DR, Andjelkovic M, Caudwell B, et al. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–51. [PMC free article] [PubMed] [Google Scholar]
- 25.Cross DA, Alessi DR, Cohen P, et al. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–9. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 26.Woodgett JR. Physiological roles of glycogen synthase kinase-3: potential as a therapeutic target for diabetes and other disorders. Curr Drug Targets Immune Endocr Metabol Disord. 2003;3:281–90. doi: 10.2174/1568008033340153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen P, Goedert M. GSK3 inhibitors: development and therapeutic potential. Nat Rev Drug Discov. 2004;3:479–87. doi: 10.1038/nrd1415. [DOI] [PubMed] [Google Scholar]
- 28.Cohen P, Frame S. The renaissance of GSK3. Nat Rev Mol Cell Biol. 2001;2:769–76. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- 29.Borrelli E, Nestler EJ, Allis CD, et al. Decoding the epigenetic language of neuronal plasticity. Neuron. 2008;60:961–74. doi: 10.1016/j.neuron.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scheid MP, Woodgett JR. PKB/AKT: functional insights from genetic models. Nat Rev Mol Cell Biol. 2001;2:760–8. doi: 10.1038/35096067. [DOI] [PubMed] [Google Scholar]
- 31.Beaulieu JM, Sotnikova TD, Marion S, et al. An Akt/beta-Arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–73. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 32.Andjelkovi M, Jakubowicz T, Cron P, et al. Activation and phosphorylation of a pleckstrin homology domain containing protein kinase (RAC-PK/PKB) promoted by serum and protein phosphatase inhibitors. Proc Natl Acad Sci U S A. 1996;93:5699–704. doi: 10.1073/pnas.93.12.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ugi S, Imamura T, Maegawa H, et al. Protein phosphatase 2A negatively regulates insulin’s metabolic signaling pathway by inhibiting Akt (protein Kinase B) activity in 3T3-L1 adipocytes. Mol Cell Biol. 2004;24:8778–89. doi: 10.1128/MCB.24.19.8778-8789.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woodgett JR. Judging a protein by more than its name: GSK-3. Sci STKE. 2001;2001:re12. doi: 10.1126/stke.2001.100.re12. [DOI] [PubMed] [Google Scholar]
- 35.Stambolic V, Woodgett JR. Mitogen inactivation of glycogen synthase kinase-3 beta in intact cells via serine 9 phosphorylation. Biochem J. 1994;303:701–4. doi: 10.1042/bj3030701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kebabian JW, Calne DB. Multiple receptors for dopamine. Nature. 1979;277:93–6. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- 37.Kebabian JW, Greengard P. Dopamine-sensitive adenyl cyclase: possible role in synaptic transmission. Science. 1971;174:1346–9. doi: 10.1126/science.174.4016.1346. [DOI] [PubMed] [Google Scholar]
- 38.Enjalbert A, Bockaert J. Pharmacological characterization of the D2 dopamine receptor negatively coupled with adenylate cyclase in rat anterior pituitary. Mol Pharmacol. 1983;23:576–84. [PubMed] [Google Scholar]
- 39.Missale C, Nash SR, Robinson SW, et al. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 40.Perreault ML, Hasbi A, Alijaniaram M, et al. The dopamine D1–2 receptor heteromer localizes in dynorphin/enkephalin neurons: increased high affinity state following amphetamine and in schizophrenia. J Biol Chem. 2010 doi: 10.1074/jbc.M110.159954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rashid AJ, So CH, Kong MM, et al. D1–D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc Natl Acad Sci U S A. 2007;104:654–9. doi: 10.1073/pnas.0604049104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang ZW, Burke MW, Calakos N, et al. Confocal analysis of cholinergic and dopaminergic inputs onto pyramidal cells in the prefrontal cortex of rodents. Front Neuroanat. 2010;4:21. doi: 10.3389/fnana.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shuen JA, Chen M, Gloss B, et al. Drd1a-tdTomato BAC transgenic mice for simultaneous visualization of medium spiny neurons in the direct and indirect pathways of the basal ganglia. J Neurosci. 2008;28:2681–5. doi: 10.1523/JNEUROSCI.5492-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valjent E, Bertran-Gonzalez J, Herve D, et al. Looking BAC at striatal signaling: cell-specific analysis in new transgenic mice. Trends Neurosci. 2009;32:538–47. doi: 10.1016/j.tins.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 45.Bateup HS, Svenningsson P, Kuroiwa M, et al. Cell type-specific regulation of DARPP-32 phosphorylation by psychostimulant and antipsychotic drugs. Nat Neurosci. 2008;11:932–9. doi: 10.1038/nn.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beaulieu JM, Sotnikova TD, Yao WD, et al. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc Natl Acad Sci U S A. 2004;101:5099–104. doi: 10.1073/pnas.0307921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beaulieu JM, Sotnikova TD, Gainetdinov RR, et al. Paradoxical striatal cellular signaling responses to psychostimulants in hyper-active mice. J Biol Chem. 2006;281:32072–80. doi: 10.1074/jbc.M606062200. [DOI] [PubMed] [Google Scholar]
- 48.Chen PC, Lao CL, Chen JC. Dual alteration of limbic dopamine D1 receptor-mediated signalling and the Akt/GSK3 pathway in dopamine D3 receptor mutants during the development of methamphetamine sensitization. J Neurochem. 2007;100:225–41. doi: 10.1111/j.1471-4159.2006.04203.x. [DOI] [PubMed] [Google Scholar]
- 49.Bychkov E, Ahmed MR, Dalby KN, et al. Dopamine depletion and subsequent treatment with L-DOPA, but not the long-lived dopamine agonist pergolide, enhances activity of the Akt pathway in the rat striatum. J Neurochem. 2007;102:699–711. doi: 10.1111/j.1471-4159.2007.04586.x. [DOI] [PubMed] [Google Scholar]
- 50.Emamian ES, Hall D, Birnbaum MJ, et al. Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nat Genet. 2004;36:131–7. doi: 10.1038/ng1296. [DOI] [PubMed] [Google Scholar]
- 51.Roh MS, Seo MS, Kim Y, et al. Haloperidol and clozapine differentially regulate signals upstream of glycogen synthase kinase 3 in the rat frontal cortex. Exp Mol Med. 2007;39:353–60. doi: 10.1038/emm.2007.39. [DOI] [PubMed] [Google Scholar]
- 52.Beaulieu JM, Tirotta E, Sotnikova TD, et al. Regulation of Akt signaling by D2 and D3 dopamine receptors in vivo. J Neurosci. 2007;27:881–5. doi: 10.1523/JNEUROSCI.5074-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shenoy SK, Lefkowitz RJ. Multifaceted roles of beta-Arrestins in the regulation of seven-membrane-spanning receptor trafficking and signalling. Biochem J. 2003;375:503–15. doi: 10.1042/BJ20031076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gainetdinov RR, Premont RT, Bohn LM, et al. Desensitization of G protein-coupled receptors and neuronal functions. Annu Rev Neurosci. 2004;27:107–44. doi: 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- 55.Lohse MJ, Benovic JL, Codina J, et al. beta-Arrestin: a protein that regulates beta-adrenergic receptor function. Science. 1990;248:1547–50. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- 56.Ferguson SS, Downey WE, III, Colapietro AM, et al. Role of beta-Arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science. 1996;271:363–6. doi: 10.1126/science.271.5247.363. [DOI] [PubMed] [Google Scholar]
- 57.Laporte SA, Miller WE, Kim KM, et al. beta-Arrestin/AP-2 interaction in G protein-coupled receptor internalization: identification of a beta-Arrestin binging site in beta 2-adaptin. J Biol Chem. 2002;277:9247–54. doi: 10.1074/jbc.M108490200. [DOI] [PubMed] [Google Scholar]
- 58.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-Arrestins. Science. 2005;308:512–7. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 59.Luttrell LM, Ferguson SS, Daaka Y, et al. Beta-Arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–61. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- 60.Luttrell LM, Roudabush FL, Choy EW, et al. Activation and targeting of extracellular signal-regulated kinases by beta-Arrestin scaffolds. Proc Natl Acad Sci U S A. 2001;98:2449–54. doi: 10.1073/pnas.041604898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bohn LM, Lefkowitz RJ, Gainetdinov RR, et al. Enhanced morphine analgesia in mice lacking beta-Arrestin 2. Science. 1999;286:2495–8. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- 62.Gainetdinov RR, Wetsel WC, Jones SR, et al. Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science. 1999;283:397–401. doi: 10.1126/science.283.5400.397. [DOI] [PubMed] [Google Scholar]
- 63.Beaulieu JM, Marion S, Rodriguiz RM, et al. A beta-Arrestin 2 signaling complex mediates lithium action on behavior. Cell. 2008;132:125–36. doi: 10.1016/j.cell.2007.11.041. [DOI] [PubMed] [Google Scholar]
- 64.Bohn LM, Gainetdinov RR, Sotnikova TD, et al. Enhanced rewarding properties of morphine, but not cocaine, in beta(Arrestin)-2 knock-out mice. J Neurosci. 2003;23:10265–73. doi: 10.1523/JNEUROSCI.23-32-10265.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou QY, Palmiter RD. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell. 1995;83:1197–209. doi: 10.1016/0092-8674(95)90145-0. [DOI] [PubMed] [Google Scholar]
- 66.Sotnikova TD, Beaulieu JM, Barak LS, et al. Dopamine-independent locomotor actions of amphetamines in a novel acute mouse model of Parkinson disease. PLoS Biol. 2005;3:e271. doi: 10.1371/journal.pbio.0030271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gould TD, Einat H, Bhat R, et al. AR-A014418, a selective GSK-3 inhibitor, produces antidepressant-like effects in the forced swim test. Int J Neuropsychopharmacol. 2004;7:387–90. doi: 10.1017/S1461145704004535. [DOI] [PubMed] [Google Scholar]
- 68.Kalinichev M, Dawson LA. Evidence for antimanic efficacy of glycogen synthase kinase-3 (GSK3) inhibitors in a strain-specific model of acute mania. Int J Neuropsychopharmacol. 2011 Jan 6;:1–17. doi: 10.1017/S1461145710001495. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 69.Hoeflich KP, Luo J, Rubie EA, et al. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature. 2000;406:86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- 70.Polter A, Beurel E, Yang S, et al. Deficiency in the inhibitory serine-phosphorylation of glycogen synthase kinase-3 increases sensitivity to mood disturbances. Neuropsychopharmacology. 2010;35:1761–74. doi: 10.1038/npp.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Prickaerts J, Moechars D, Cryns K, et al. Transgenic mice overexpressing glycogen synthase kinase 3beta: a putative model of hyperactivity and mania. J Neurosci. 2006;26:9022–9. doi: 10.1523/JNEUROSCI.5216-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Powell SB, Geyer MA. Overview of animal models of schizophrenia. Curr Protoc Neurosci. 2007;Chapter 9(Unit 9.24) doi: 10.1002/0471142301.ns0924s39. [DOI] [PubMed] [Google Scholar]
- 73.Carlsson A. Perspectives on the discovery of central monoaminergic neurotransmission. Annu Rev Neurosci. 1987;10:19–40. doi: 10.1146/annurev.ne.10.030187.000315. [DOI] [PubMed] [Google Scholar]
- 74.Yuan Q, Lin F, Zheng X, et al. Serotonin modulates circadian entrainment in Drosophila. Neuron. 2005;47:115–27. doi: 10.1016/j.neuron.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 75.Li X, Rosborough KM, Friedman AB, et al. Regulation of mouse brain glycogen synthase kinase-3 by atypical antipsychotics. Int J Neuropsychopharmacol. 2007;10:7–19. doi: 10.1017/S1461145706006547. [DOI] [PubMed] [Google Scholar]
- 76.Li X, Zhu W, Roh MS, et al. In vivo regulation of glycogen synthase kinase-3beta (GSK3beta) by serotonergic activity in mouse brain. Neuropsychopharmacology. 2004;29:1426–31. doi: 10.1038/sj.npp.1300439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Beaulieu JM. Not only lithium: regulation of glycogen synthase kinase-3 by antipsychotics and serotonergic drugs. Int J Neuropsychopharmacol. 2007;10:3–6. doi: 10.1017/S1461145706006857. [DOI] [PubMed] [Google Scholar]
- 78.Zhang X, Gainetdinov RR, Beaulieu JM, et al. Loss-of-function mutation in tryptophan hydroxylase-2 identified in unipolar major depression. Neuron. 2005;45:11–6. doi: 10.1016/j.neuron.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 79.Beaulieu JM, Zhang X, Rodriguiz RM, et al. Role of GSK3beta in behavioral abnormalities induced by serotonin deficiency. Proc Natl Acad Sci U S A. 2008;105:1333–8. doi: 10.1073/pnas.0711496105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Polter A, Yang S, Zmijewska AA, et al. Forkhead box, class O transcription factors in brain: regulation and behavioral manifestation. Biol Psychiatry. 2009;65:150–9. doi: 10.1016/j.biopsych.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jacinto E, Facchinetti V, Liu D, et al. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–37. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 82.O’Brien WT, Harper AD, Jove F, et al. Glycogen synthase kinase-3beta haploinsufficiency mimics the behavioral and molecular effects of lithium. J Neurosci. 2004;24:6791–8. doi: 10.1523/JNEUROSCI.4753-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Beaulieu JM, Zhang X, Rodriguiz RM, et al. Reply to Belmaker et al: GSK3β haploinsufficiency results in lithium-like effects in the forced swim test. Proc Natl Acad Sci U S A. 2008;105:E24. [Google Scholar]
- 84.Ackermann TF, Kempe DS, Lang F, et al. Hyperactivity and enhanced curiosity of mice expressing PKB/SGK-resistant glycogen synthase kinase-3 (GSK-3) Cell Physiol Biochem. 2010;25:775–86. doi: 10.1159/000315097. [DOI] [PubMed] [Google Scholar]
- 85.Beaulieu JM, Gainetdinov RR, Caron MG. The Akt-GSK-3 signaling cascade in the actions of dopamine. Trends Pharmacol Sci. 2007;28:166–72. doi: 10.1016/j.tips.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 86.Alimohamad H, Rajakumar N, Seah YH, et al. Antipsychotics alter the protein expression levels of beta-catenin and GSK-3 in the rat medial prefrontal cortex and striatum. Biol Psychiatry. 2005;57:533–42. doi: 10.1016/j.biopsych.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 87.Masri B, Salahpour A, Didriksen M, et al. Antagonism of dopamine D2 receptor/beta-Arrestin 2 interaction is a common property of clinically effective antipsychotics. Proc Natl Acad Sci U S A. 2008;105:13656–61. doi: 10.1073/pnas.0803522105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sutton LP, Honardoust D, Mouyal J, et al. Activation of the canonical Wnt pathway by the antipsychotics haloperidol and clozapine involves dishevelled-3. J Neurochem. 2007;102:153–69. doi: 10.1111/j.1471-4159.2007.04527.x. [DOI] [PubMed] [Google Scholar]
- 89.Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci U S A. 1996;93:8455–9. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol. 1996;6:1664–8. doi: 10.1016/s0960-9822(02)70790-2. [DOI] [PubMed] [Google Scholar]
- 91.Beaulieu JM, Caron MG. Looking at lithium: molecular moods and complex behaviour. Mol Interv. 2008;8:230–41. doi: 10.1124/mi.8.5.8. [DOI] [PubMed] [Google Scholar]
- 92.De Sarno P, Li X, Jope RS. Regulation of Akt and glycogen synthase kinase-3 beta phosphorylation by sodium valproate and lithium. Neuropharmacology. 2002;43:1158–64. doi: 10.1016/s0028-3908(02)00215-0. [DOI] [PubMed] [Google Scholar]
- 93.Birch NJ. Lithium and magnesium-dependent enzymes [letter] Lancet. 1974;2:965–6. doi: 10.1016/s0140-6736(74)91187-8. [DOI] [PubMed] [Google Scholar]
- 94.Ryves WJ, Harwood AJ. Lithium inhibits glycogen synthase kinase-3 by competition for magnesium. Biochem Biophys Res Commun. 2001;280:720–5. doi: 10.1006/bbrc.2000.4169. [DOI] [PubMed] [Google Scholar]
- 95.Lai WS, Xu B, Westphal KG, et al. Akt1 deficiency affects neuronal morphology and predisposes to abnormalities in prefrontal cortex functioning. Proc Natl Acad Sci U S A. 2006;103:16906–11. doi: 10.1073/pnas.0604994103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Freyberg Z, Ferrando SJ, Javitch JA. Roles of the Akt/GSK-3 and Wnt signaling pathways in schizophrenia and antipsychotic drug action. Am J Psychiatry. 2010;167:388–96. doi: 10.1176/appi.ajp.2009.08121873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Karege F, Perroud N, Schurhoff F, et al. Association of AKT1 gene variants and protein expression in both schizophrenia and bipolar disorder. Genes Brain Behav. 2010;9:503–11. doi: 10.1111/j.1601-183X.2010.00578.x. [DOI] [PubMed] [Google Scholar]
- 98.Magno LA, Miranda DM, Neves FS, et al. Association between AKT1 but not AKTIP genetic variants and increased risk for suicidal behavior in bipolar patients. Genes Brain Behav. 2010;9:411–8. doi: 10.1111/j.1601-183X.2010.00571.x. [DOI] [PubMed] [Google Scholar]
- 99.Benedetti F, Serretti A, Colombo C, et al. A glycogen synthase kinase 3-beta promoter gene single nucleotide polymorphism is associated with age at onset and response to total sleep deprivation in bipolar depression. Neurosci Lett. 2004;368:123–6. doi: 10.1016/j.neulet.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 100.Karege F, Perroud N, Burkhardt S, et al. Alteration in kinase activity but not in protein levels of protein kinase B and glycogen synthase kinase-3beta in ventral prefrontal cortex of depressed suicide victims. Biol Psychiatry. 2007;61:240–5. doi: 10.1016/j.biopsych.2006.04.036. [DOI] [PubMed] [Google Scholar]
- 101.Ross CA, Margolis RL, Reading SA, et al. Neurobiology of schizophrenia. Neuron. 2006;52:139–53. doi: 10.1016/j.neuron.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 102.Serretti A, Mandelli L. The genetics of bipolar disorder: genome ‘hot regions,’ genes, new potential candidates and future directions. Mol Psychiatry. 2008;13:742–71. doi: 10.1038/mp.2008.29. [DOI] [PubMed] [Google Scholar]
- 103.Keri S, Seres I, Kelemen O, et al. The relationship among neuregulin 1-stimulated phosphorylation of AKT, psychosis proneness, and habituation of arousal in nonclinical individuals. Schizophr Bull. 2011;37:141–7. doi: 10.1093/schbul/sbp063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kéri S, Beniczky S, Kelemen O. Suppression of the P50 evoked response and neuregulin 1-induced AKT phosphorylation in first-episode schizophrenia. Am J Psychiatry. 2010;167:444–50. doi: 10.1176/appi.ajp.2009.09050723. [DOI] [PubMed] [Google Scholar]
- 105.Sei Y, Li Z, Song J, et al. Epistatic and functional interactions of catechol-o-methyltransferase (COMT) and AKT1 on neuregulin1-ErbB signaling in cell models. PLoS ONE. 2010;5:e10789. doi: 10.1371/journal.pone.0010789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Meyer-Lindenberg A, Nichols T, Callicott JH, et al. Impact of complex genetic variation in COMT on human brain function. Mol Psychiatry. 2006;11:867–77. 797. doi: 10.1038/sj.mp.4001860. [DOI] [PubMed] [Google Scholar]
- 107.Sotnikova TD, Beaulieu JM, Espinoza S, et al. The dopamine metabolite 3-methoxytyramine is a neuromodulator. PLoS ONE. 2010;5:e13452. doi: 10.1371/journal.pone.0013452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li YC, Xi D, Roman J, et al. Activation of glycogen synthase kinase-3 beta is required for hyperdopamine and D2 receptor-mediated inhibition of synaptic NMDA receptor function in the rat prefrontal cortex. J Neurosci. 2009;29:15551–63. doi: 10.1523/JNEUROSCI.3336-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Karam CS, Ballon JS, Bivens NM, et al. Signaling pathways in schizophrenia: emerging targets and therapeutic strategies. Trends Pharmacol Sci. 2010;31:381–90. doi: 10.1016/j.tips.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Du J, Wei Y, Liu L, et al. A kinesin signaling complex mediates the ability of GSK-3beta to affect mood-associated behaviors. Proc Natl Acad Sci U S A. 2010;107:11573–8. doi: 10.1073/pnas.0913138107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mohn AR, Gainetdinov RR, Caron MG, et al. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell. 1999;98:427–36. doi: 10.1016/s0092-8674(00)81972-8. [DOI] [PubMed] [Google Scholar]
- 112.Li N, Lee B, Liu RJ, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–64. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen L, Salinas GD, Li X. Regulation of serotonin 1B receptor by glycogen synthase kinase-3. Mol Pharmacol. 2009;76:1150–61. doi: 10.1124/mol.109.056994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen L, Zhou W, Chen P, et al. Glycogen synthase kinase-3beta is a functional modulator of serotonin 1B receptors. Mol Pharmacol. 2011;79:974–86. doi: 10.1124/mol.111.071092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li X, Jope RS. Is glycogen synthase kinase-3 a central modulator in mood regulation? Neuropsychopharmacology. 2010;35:2143–54. doi: 10.1038/npp.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dé Montigny C, Grunberg F, Mayer A, et al. Lithium induces rapid relief of depression in tricyclic antidepressant drug non-responders. Br J Psychiatry. 1981;138:252–6. doi: 10.1192/bjp.138.3.252. [DOI] [PubMed] [Google Scholar]
- 117.Valenstein M, McCarthy JF, Austin KL, et al. What happened to lithium? Antidepressant augmentation in clinical settings. Am J Psychiatry. 2006;163:1219–25. doi: 10.1176/ajp.2006.163.7.1219. [DOI] [PubMed] [Google Scholar]
- 118.Costanzo M, Baryshnikova A, Bellay J, et al. The genetic landscape of a cell. Science. 2010;327:425–31. doi: 10.1126/science.1180823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Beaulieu JM. Morphine-induced mu-opioid receptor internalization: a paradox solved in neurons. J Neurosci. 2005;25:10061–3. doi: 10.1523/JNEUROSCI.3914-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chubb JE, Bradshaw NJ, Soares DC, et al. The Drosoph Inf ServC locus in psychiatric illness. Mol Psychiatry. 2008;13:36–64. doi: 10.1038/sj.mp.4002106. [DOI] [PubMed] [Google Scholar]
- 121.Toyota T, Yamada K, Detera-Wadleigh SD, et al. Analysis of a cluster of polymorphisms in AKT1 gene in bipolar pedigrees: a family-based association study. Neurosci Lett. 2003;339:5–8. doi: 10.1016/s0304-3940(02)01428-3. [DOI] [PubMed] [Google Scholar]
- 122.Bayer TA, Falkai P, Maier W. Genetic and non-genetic vulnerability factors in schizophrenia: the basis of the “two hit hypothesis. J Psychiatr Res. 1999;33:543–8. doi: 10.1016/s0022-3956(99)00039-4. [DOI] [PubMed] [Google Scholar]
- 123.Maynard TM, Sikich L, Lieberman JA, et al. Neural development, cell-cell signaling, and the “two-hit” hypothesis of schizophrenia. Schizophr Bull. 2001;27:457–76. doi: 10.1093/oxfordjournals.schbul.a006887. [DOI] [PubMed] [Google Scholar]
- 124.Maziade M, Roy MA, Chagnon YC, et al. Shared and specific susceptibility loci for schizophrenia and bipolar disorder: a dense genome scan in Eastern Quebec families. Mol Psychiatry. 2005;10:486–99. doi: 10.1038/sj.mp.4001594. [DOI] [PubMed] [Google Scholar]
- 125.Knudson AG., Jr Mutation and cancer: statistical study of retino-blastoma. Proc Natl Acad Sci U S A. 1971;68:820–3. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Devilee P, Cleton-Jansen AM, Cornelisse CJ. Ever since Knudson. Trends Genet. 2001;17:569–73. doi: 10.1016/s0168-9525(01)02416-7. [DOI] [PubMed] [Google Scholar]