Abstract
Background
Impairment of recollection memory is consistently reported in patients with major depressive disorder (MDD) and may reflect underlying functional hippocampal changes, particularly in those with extensive histories of illness. We hypothesized that relative to controls, patients with a protracted course of illness would show diminished hippocampal activation on functional magnetic resonance imaging (fMRI) during a recollection memory task.
Methods
Patients who experienced 3 or more previously treated depressive episodes were compared with age- and sex-matched controls. We acquired fMRI data while participants performed a recollection memory process dissociation task.
Results
Using bilateral regions of interest (ROIs) prescribed for the right and left hippocampal/ parahippocampal complex, we observed increased activation of the right hippocampal and left parahippocampal gyrus in controls compared with patients with MDD during recollection memory trials. Within-group comparisons revealed heightened engagement of the hippocampal head (R/L) for controls during recollection trials, and greater activation of the hippocampal body/tail (R/L) during the learn-list encoding period in both the MDD and control groups. Recollection memory performance was significantly correlated with changes in blood oxygen level–dependent signal during recollection trials in the ROIs of the right hippocampus and right hippocampal head.
Limitations
This study was limited by the inclusion of patients taking antidepressant medication, raising the possibility that the reported findings were treatment effects.
Conclusion
The findings of decreased recruitment of the right hippocampal and left parahippocampal gyrus in patients with MDD suggest that these regions may be sensitive to the impact of disease burden and repeated episodes of MDD. This attenuated activation may represent stable changes in hippocampal function that occur over the course of illness in patients with MDD. The findings from within-group comparisons show that the group differences in the activation of the right hippocampal head were driven by greater engagement of this region among controls during recollection memory performance. These results also associate recollection performance impairments in patients with MDD with diminished hippocampal engagement.
Introduction
Major depressive disorder (MDD) is associated with deficits in memory function. Of the discrete memory domains, recollection memory is the most impacted in patients with MDD. Dysfunction of effortful encoding and inefficiencies in the retrieval of specific facts or events appear to be part of a broad cognitive impairment that does not remit when a patient attains euthymia. Such deficits are considered reflective of disruptions in the functional networks that underlie depression and, more specifically, indicative of hippocampal involvement.
Hippocampal volume changes in patients with MDD have been extensively examined using magnetic resonance imaging (MRI), and most of the evidence identifies smaller hippocampal volumes in depressed patients compared with healthy controls.1 Despite the large number of anatomic MRI studies in patients with MDD, there is a striking paucity of research using functional imaging methods to study hippocampal function in patients with MDD. Only recently, one associative memory study2 failed to specifically show the involvement of the hippocampus, and instead noted activation changes in a number of other regions of the brain associated with memory. These findings contrast with those of a second recent study that solely examined the encoding period and identified hippocampal irregularities during an associative memory task in a small group of patients.3
Broadly surveying functional imaging studies in the psychiatric literature, only recently has hippocampal function been examined using functional magnetic resonance imaging (fMRI) in patients with disorders such as posttraumatic stress disorder (PTSD)4–6 or anxiety.7 Even in patients with schizophrenia, in whom there are clear disruptions of functional networks involving the temporal lobes, hippocampal function has been explored in only a handful of studies.8,9,10 Few data have emerged from the extant studies using fMRI methods to examine hippocampal function in patients with psychiatric illnesses describing the effect of course of illness or relevant clinical variables on determining hippocampal function during memory tasks.
Chronicity of illness may have an important role in determining hippocampal function in patients with MDD; volumetric differences have been most consistently reported for patients who are older (reviewed by McKinnon and colleagues1 and by Eker and Gonul11) and those who have experienced multiple previous episodes of depression.12,13 In the early stages of illness, hippocampal volume loss is not apparent, as studies have failed to identify this finding in first-episode patients in the absence of other risk factors, such as an early history of abuse or trauma. To date, few studies of patients with MDD have examined the impact of illness burden on hippocampal function. The goal of this study, therefore, was to use fMRI to examine hippocampal activation during a declarative memory task in a subset of patients with a known extensive history of illness.
The recollection memory process dissociation task from a previously described protocol14,15 was adapted for scanning. The process dissociation task was selected as it has been shown to distinguish recollection memory (hippocampus-dependent) from habit memory (hippocampus-independent) capacities16 and because it has been studied extensively in nonpsychiatric populations.17,18 We hypothesized that depressed patients would show deficits on hippocampus-dependent memory trials in comparison to healthy age- and sex-matched controls.
Methods
Participant selection
We recruited patients with MDD from the Mood Disorders Program at St. Joseph’s Centre for Mountain Health Services in Hamilton, Ont., and a group of controls were recruited from the community and group-matched based on age and sex. Patients with a history of head injury, neurologic illness, alcohol or substance abuse, electroconvulsive therapy or transcranial magnetic stimulation within the last 2 years were excluded from the study. Healthy controls were free from medication and had no current symptoms or history of a mental health disorder. A psychiatrist confirmed a primary diagnosis of nonpsychotic, unipolar MDD according to DSM-IV criteria,19 supplemented with a Structured Clinical Interview for DSM-IV.20 The MDD group comprised individuals who had extensive illness histories, having experienced 3 or more previous depressive episodes and/or an illness duration of 5 of more years. Psychiatric symptoms were assessed immediately before scanning using the Beck Depression Inventory (BDI)21 and the Hamilton Rating Scale for Depression (HAM-D).22 The Research Ethics Board of St. Joseph’s Health-care Hamilton approved this study, and all participants gave informed consent.
An index of medication load was calculated for each patient with MDD according to a previously established approach.23–26 Each antidepressant, anticonvulsant and anxiolytic medication and lithium were coded as no-dose, 1 and 2 for low doses, and 3 and 4 for high doses according to the rating system by Sackeim.23 Antipsychotics were converted to chlorpromazine hydrochloride dose equivalents27 and coded as no-dose, below the ED50 (effective dose-50; coded 1) or above the ED50 level (coded 2). A composite measure of medication load for each patient was produced by summing all individual codes across all medication categories.
Process dissociation task
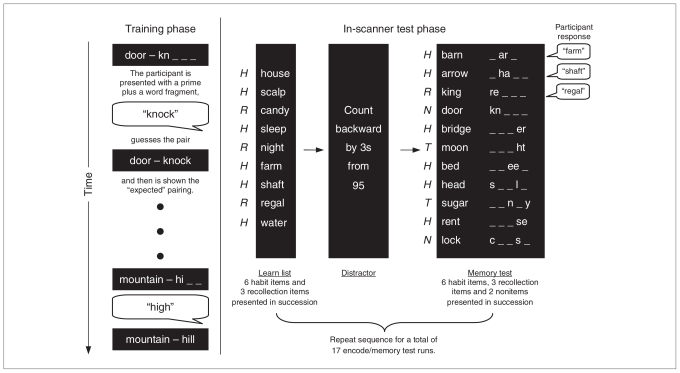
Participants completed the recollection memory process dissociation task, adapted for scanning from the previously described protocol (Fig. 1).14,15 Prior to scanning, the participants were trained on a series of stimulus word pairs. The pairs consisted of a single word prime (e.g., barn) matched with 2 possible associative pairs (e.g., yard or farm). The battery of word pairs consisted of 18 primes, each with 2 associated word pairs (see Appendix 1, available at www.cma.ca/jpn, for the full list). For each set of words in the series, 1 pair (e.g., barn farm) was presented at a higher frequency during training to produce habit memory for that pairing (e.g., barn yard appeared as the correct response in 67% of the trials, whereas the other choice, barn farm, appeared as the correct response 33% of the time). This was done to generate habit associations with the higher-frequency word pair. The particular word combinations presented as either high- or low-frequency pairs were equally distributed across all participants. Inside the scanner, participants were presented with 11-item word lists to be studied that were made up of habit word pairings and recollection word pairings (the low-frequency trained items). A total of 17 study lists were presented across 5 fMRI scans. Each study list contained 6 habit word pairs (high-frequency word match) and 3 recollection word pairs (low-frequency word match). We instructed participants to read the word pairs and remember them for the following memory test. Complete word pairs appeared on the screen for 1 second followed by a fixation point shown on the screen for 0.5 seconds before the next word pair. After the study list, a brief mathematical distractor task was presented. In the memory test list, participants were shown 11 word + word fragment combinations (e.g., barn _ar_) consisting of the 6 habit word pairs and 3 recollection word pairs from the study list. In addition, 2 word pairs that were not included in the study list were presented to assess a participant’s tendency to guess (nonitem trials). Participants were instructed to respond verbally and complete the word fragments with the words on the immediately preceding study list or to provide their best guess if they could not remember which word was presented in the study list. The paradigm was delivered as an event-related design with jittered interstimulus intervals with periods of 3, 6 or 12 seconds of a fixation point between test list items. This portion of the experiment was broken into 5 scan runs. With a total of 17 study lists/memory test series, participants were tested on a total of 102 habit trials, 51 recollection items and 34 nonitems. We obtained recollection scores by subtracting the recollection trial (when study-list pairs were the same as the low-frequency pair during training) probability from the habit trial (where study-list pairs were the same as the high-frequency pair during training) probability. An estimate of habit memory is obtained by the formula habit = low-frequency probability ÷ (1 – recollection).28
Fig. 1.
Procedures for the process dissociation task, before and during the functional magnetic resonance imaging data acquisition. Participant verbal responses are depicted in speech bubbles.
Image acquisition
The imaging session lasted about 1 hour and involved the acquisition of a scout image, 5 fMRI scans (as participants completed the process dissociation task) and 1 T1-weighted anatomic scan. Imaging was performed using a 3-T General Electric MRI scanner. Functional images were prescribed by visualizing the head of the hippocampus in the sagittal plane of the scout image, and then prescribed axially by centering the seventh slice of 13 axial slices on the head of the left hippocampus. We acquired blood oxygen level–dependent (BOLD) images with a temporal resolution of 3 seconds using an echo planar pulse sequence (echo time [TE] 43 ms, repetition time [TR] 3000 ms, matrix 128 × 64, flip angle 90°, 3-mm slice thickness). A 3-second gap in the scan sequence was built in after each TR, producing a silent period (no scanner noises) during which the participant could respond verbally. All verbal responses were recorded using an MR-compatible microphone fixed to the headcoil and connected to a Panasonic voice recorder located outside of the scanner. Stimuli were presented according to an event-related design with a jittered stimulus presentation. We obtained an anatomic scan in the sagittal orientation after the fMRI procedures. The scanning parameters for the anatomical image series were 3-dimensional (3-D) inversion recovery prepped, fast spoiled gradient recalled (SPGR) pulse sequence, sagittal plane, TR 10.8 ms, TE 2 ms, inversion time [TI] 400 ms, flip angle 20°, matrix 256 × 256, field of view [FOV] 24, slice thickness 1 mm, no skip, FOV 124 contiguous slices.
Statistical analysis
We compared age and memory performance between groups using 1-way analysis of variance. Acquired images were preprocessed and analyzed using Brain Voyager QX version 1.10.4 (Brain Innovation B.V.). The functional data sets were slice-time corrected, linear detrended, 3-D motion corrected, coregistered/ realigned and normalized to Talairach space.29 The product of the coregistration/realignment step was carefully inspected by overlaying the functional series on the native space anatomic scan and assessing dorsal–ventral placement by examining the anterior portions of the lateral ventrical, the fourth ventricle and juncture at the cerebellar tentorium. High-resolution T1-weighted 3-D anatomic MR data sets were transformed into Talairach space, used for coregistration and averaged to generate a composite image onto which functional activation results were projected. Bilateral regions of interest (ROIs) were prescribed for the hippocampal/parahippocampal complex on the summed anatomic images at the group level. The ROIs were drawn on sagittal slices in Mango (http://ric.uthscsa.edu/mango/index.html), first identifying the anteromedial aspects of the uncus and parahippocampal gyrus and then proceeding laterally in 1-mm increments to prescribe a region with a 2-mm extended boundary around the hippocampal complex. The ROIs were then checked in the coronal plane (anterior to posterior) to ensure continuity and consistency, and exported to BrainVoyager. We used an event-related model for each participant to examine the BOLD signal at every voxel. Using a random-effects multiple general linear model, we set recollection memory, habit memory, nonitem and study list presentations as the explanatory variables accounting for differences in BOLD signals within and between groups. Contrasts were corrected for multiple comparisons using the false discovery rate methodology set at 0.05,30 and the average statistical value for the resulting ROIs are reported.
Results
Participants
Twenty-two patients and 18 controls participated in this study. Clinical and demographic characteristics of participants can be found in Table 1. There were no significant differences in age between the MDD group (mean 44.9, standard deviation [SD] 11.3 yr) and controls (mean 42.1, SD 11.5 yr; F1,39 = 1.02, p = 0.31). The depressed patients included in the study had a mean illness duration of 20.1 (SD 12.8) years, a mean BDI score of 16.1 (SD 9.2) and a mean HAM-D score of 10.9 (SD 5.1). Eighty-six percent of the patients with depression were being treated with stable doses of antidepressant medications (selective serotonin reuptake inhibitor n = 13, serotonin-norepinephrine reuptake inhibitor n = 5, tetracyclic antidepressants n = 2, monoamine oxidase inhibitor n = 2, atypical antidepressants n = 2, tricyclic antidepressant n = 1), of whom 5 patients also received atypical antipsychotic medications as augmenting agents.
Table 1.
Clinical and demographic characteristics of patients with major depressive disorder and matched controls
| Group; mean (SD)* |
||
|---|---|---|
| Characteristic | MDD, n = 22 | Control, n = 18 |
| Age, yr | 44.9 (11.3) | 42.1 (11.5) |
| No. female | 15 | 13 |
| No. male | 7 | 5 |
| Education, yr | 15.5 (2.1) | 16.8 (3.7) |
| Duration of illness, yr | 20.1 (12.8) | |
| No. of episodes | 5.6 (2.4) | |
| Medication, no. | ||
| Antidepressants | 19 | |
| Antipsychotics | 5 | |
| Lithium | 2 | |
| Medication-free | 3 | |
| BDI score | 16.1 (9.2) | 0.94 (1.4) |
| HAM-D score | 10.9 (5.1) | |
Memory performance
Recollection memory was significantly impaired in the MDD group compared with controls (mean 0.52 [SD 0.19] v. 0.71 [SD 0.12]; F1,39 = 13.59, p < 0.001). There was an exceptionally low outlier in the MDD group; the recollection memory score was still significantly impaired after removal of this outlier (F1,38 = 13.61, p < 0.001). The MDD group also performed more poorly than controls in both habit memory trials (F1,39 = 0.25, p < 0.001) and did not differ in their tendency to guess on nonitem trials (F1,38 = 3.12, p = 0.58). Results of memory performance are reported in Table 2.
Table 2.
Memory performance across trial type during the process dissociation task
| Group; mean (SD)
|
|||
|---|---|---|---|
| Trial type | MDD, n = 22 | Control, n = 18 | p value |
| Recollection | 0.52 (0.19) | 0.71 (0.12) | 0.001 |
| Habit | 0.62 (0.13) | 0.82 (0.06) | 0.001 |
| Nonitem | 0.62 (0.10) | 0.64 (0.09) | 0.58 |
MDD = major depressive disorder; SD = standard deviation.
Imaging results
Functional imaging results
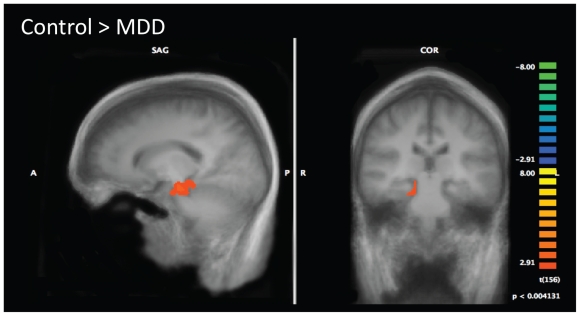
Bilateral ROIs were prescribed for the hippocampal/ parahippocampal complex on the summed anatomic images at the group level. Between-group activation patterns were determined for the contrast of each type of memory trial (recollection, habit and nonitem trials) against the baseline study list encoding period. Table 3 provides a full listing of results. We observed significant differences in activation between patients with MDD and controls during recollection memory trials, with controls showing greater activation of the right hippocampal and the left parahippocampal gyrus (Fig. 2). There were no differences between patients with MDD and controls in the activation patterns across habit trials or nonitem trials.
Table 3.
Activation patterns identified between and within groups during memory trials
| Trial; contrast | Talaraich coordinate
|
Region | Subregion | Brodmann area | t value | No. voxels | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Between-group contrast | ||||||||
| Recollection trials–study trials | ||||||||
| Control > MDD | 26 | −20 | −14 | Hippocampus | RHH | 3.679 | 1041 | |
| −27 | −25 | −16 | Hippocampus / parahippocampal gyrus | LHH | 35 | 3.334 | 26 | |
| Within-group contrasts | ||||||||
| Recollection trials–study trials | ||||||||
| MDD | 28 | −21 | −16 | Hippocampus | RHH | −3.393 | 5120 | |
| −26 | −19 | −2 | Parahippocampal gyrus | −3.211 | 3718 | |||
| −23 | −21 | −22 | Parahippocampal gyrus | 35 | −3.272 | 1083 | ||
| Control | 23 | −15 | −15 | Hippocampus / parahippocampal gyrus | RHH | 28 | 4.480 | 8829 |
| −21 | −12 | −15 | Hippocampus / parahippocampal gyrus | LHH | 28 | 4.291 | 5700 | |
| −22 | −31 | −2 | Hippocampus / parahippocampal gyrus | LHT | 27 | −3.928 | 2759 | |
| Habit trials–study trials | ||||||||
| MDD | 31 | −42 | −6 | Parahippocampal gyrus | Posterior | 19 | 3.594 | 1901 |
| 27 | −20 | −2 | Parahippocampal gyrus | Anterior | −3.345 | 229 | ||
| −29 | −36 | −6 | Hippocampus | LHT | 3.118 | 486 | ||
| Control | 21 | −15 | −15 | Hippocampus / parahippocampal gyrus | RHH | 28 | 3.776 | 3838 |
| −26 | −14 | −15 | Hippocampus | LHH | 3.278 | 3038 | ||
| Nonitem trials–study trials | ||||||||
| Control | 24 | −15 | −14 | Hippocampus | RHH | 4.517 | 10461 | |
| −26 | −18 | −15 | Hippocampus | LHH | 4.372 | 7599 | ||
LHH = left hippocampal head; LHT = left hippocampal tail; MDD = major depressive disorder; NA = not applicable; RHH = right hippocampal head.
Fig. 2.
Group differences in hippocampal activation during recollection memory trials. Controls show increased activation of the right hippocampus compared to patients with major depressive disorder (MDD). Statistical maps corrected for multiple comparisons are superimposed on averaged anatomical group images.
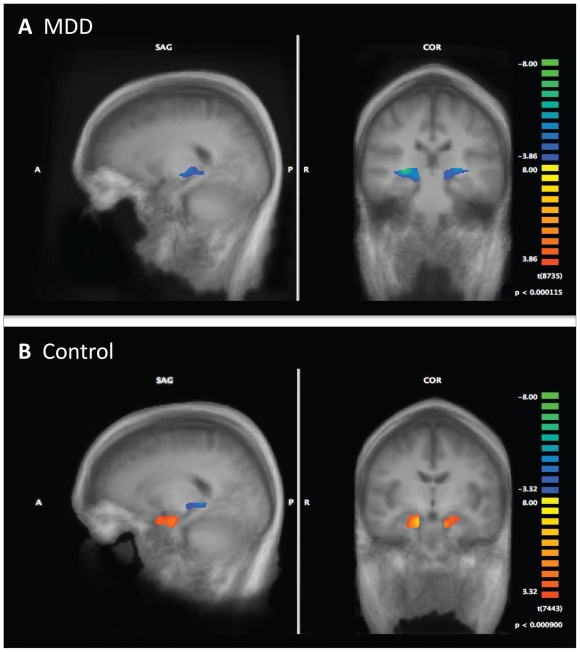
Examining the within-group activation patterns for the patients with MDD revealed heightened engagement of the hippocampal body/tail (R/L) during the learn-list encoding period as contrasted with recollection memory trials. Controls similarly engaged the left hippocampal body/tail during the encoding periods. However, in contrast to the MDD group, controls also showed heightened engagement of the right and left hippocampal heads during recollection trials. These findings indicate that the group differences in the activation of the right hippocampal head were driven by greater engagement of this region by controls during recollection memory performance (Fig. 3).
Fig. 3.
Within-group results for the recollection–encoding contrast. Areas highlighted in blue identify regions activated during encoding trials, whereas areas in red depict regions activated during recollection memory trials. Statistical maps corrected for multiple comparisons are superimposed on averaged anatomical group images. MDD = major depressive disorder.
Hippocampal head/body-tail signal correlations with performance
For controls, Pearson correlations revealed that recollection memory performance was significantly correlated with changes in BOLD signal (baseline corrected) during recollection trials in ROIs located in the right hippocampus (r18 = 0.57, p = 0.015) and the right hippocampal head (r18 = 0.48, p = 0.040). Controls who scored high in recollection performance were likely to exhibit a stronger BOLD response in the right hippocampus. We observed no significant correlations between recollection memory performance and hippocampal BOLD signal change for the MDD group. Correlations were found in patients with MDD between medication load and brain activation during recollection memory trials were significant in the left hippocampal head (r21 = −0.483, p = 0.027) and approached significance in the left hippocampal tail (r21 = −0.428, p = 0.05). These findings suggest that as medication load increased, activation in the left hippocampal head and tail was reduced during recollection memory trials.
Discussion
The aim of this study was to examine the functional capacity of the hippocampal region in patients with MDD who had extensive levels of illness burden using a recollection memory process dissociation task and fMRI. The focus on hippocampal function was founded in previous work linking illness chronicity to reductions of hippocampal volume and deficits in recollection memory in patients with MDD.1,28
Imaging studies of healthy participants have highlighted the importance of hippocampal recruitment for declarative memory. It has been suggested that the hippocampus plays a role in assessing novel items,31 information retrieval success,32 visual and spatial memory33 and recollection memory.34–36 Recently, a review of imaging studies examining memory in the medial temporal lobes found that hippocampal activation was most consistent with proposed memory models that link the hippocampus to recollection memory.37
In the present study, we found attenuated hippocampal activation during recollection memory trials and corresponding impairments in recollection memory performance in a group of patients who had histories of multiple episodes of depression contrasted with healthy matched controls. These results associate recollection performance impairments in patients with MDD with diminished hippocampal engagement. Moreover, our observation of a negative correlation between left hippocampal engagement and medication load suggests that patients who are the most ill show less engagement of the hippocampus during recollection memory.
The observation of group differences localized to the right hippocampus may be a function of the consistent (fixed) encoding and recollection demands across the 17 memory series used in our study. Recently, Ulrich and colleagues38 reported that activation of the left hippocampus is modulated by exponential increases in encoding demands, whereas the right hippocampus shows consistent strong engagement across all difficulty levels. The findings of attenuated hippocampal activation were examined further by analyzing the individual within-group patterns of activation for the same contrasts of interest (Table 3). We found that the controls showed robust hippocampal/parahippocampal activation bilaterally in response to recollection memory trials, whereas the MDD group showed diminished engagement of the hippocampal and parahippocampal gyrus. Given that the list-learning phase of the task was used as the contrast baseline for these group comparisons, these results suggest greater relative engagement of the hippocampus during encoding as opposed to the recollection periods in patients with MDD.
Examining the peak local maxima for activations, we found that the control group engaged the more anterior regions of the hippocampus during the recollection periods (Table 3).
Generally, it has been theorized that all subfields of the hippocampus play a role in both encoding and retrieval. Recently, however, studies have identified differential activity within the dentate gyrus and CA2/3 regions during encoding that contrasts with heightened CA1 and subiculum involvement during retrieval (see Carr and colleagues39 for a review). While these subfields are seen to traverse the (medial to lateral) dimension, there is an anteroposterior gradient in the proportional volume of each subfield in the head, body and tail of the hippocampus. For example, higher proportions of the CA1–3 and subiculum are found in the hippocampal head, whereas the hippocampal body includes the greatest proportion of the dentate gyrus (DG).40 Subregional dissociations have been revealed in recent high-resolution fMRI studies with differential engagement of the DG/CA2/3 regions at encoding and CA1/subiculum areas during retrieval.39,41–43 These data and the present findings of diminished engagement of the hippocampus during retrieval may have important implications for our understanding of the neuropathology of this disorder. In particular, CA1 neurons that project to the medial prefrontal cortices are localized primarily in the head of the hippocampus.44,45 Abnormalities in this area, therefore, appear consistent with network models that posit a disconnect between the hippocampus and frontal structures.46
In the extant literature there are 2 recent studies that have used fMRI to examine hippocampal function in patients with MDD. The first study used an associative face-occupation paradigm to examine hippocampal activity during memory encoding and retrieval.2 There were no significant differences in hippocampal activation between depressed participants and controls. However, the patients in this study did have increased parahippocampal gyrus activation during encoding and decreased activation in frontal and parietal regions without corresponding memory deficits.2 Werner and colleagues2 postulated that the small sample size (n = 11) and relatively young age of depressed patients (mean 37.18 yr) may have contributed to the lack of hippocampal findings. This study did not provide information on the number of episodes or illness duration of its study participants, and it will be important for future studies to examine patients with varying durations of illness as this may have accounted, at least in part, for the lack of impairment in this sample. In the second study by Fairhall and colleagues,3 a small group of patients and controls (8 per group) were scanned solely during the encoding period of an associative memory paradigm. These researchers found that MDD was associated with abnormal modulation of hippocampal activity during encoding. The present study extends these finding and suggests that such dysregulation impacts on subsequent retrieval processes in patients with MDD as well.
Limitations
The present study has several limitations. One is that it is difficult to ascertain with confidence the duration of illness or the precise number of depressive episodes that patients have experienced. Although we were confident that all patients with MDD had a significant past illness burden based on a known treatment history, even with life-charting methodology it can be difficult to ascertain the onset and duration of relatively mild episodes, particularly if patients have low-grade dysthymia or partial resolution of symptoms in the interepisode intervals. In addition, this study identified behavioural group effects for both recollection and habit memory, and yet only functional activation group differences during recollection performance. Previous behavioural research has associated MDD with recollection deficits and preserved habit memory.47 The current findings may reflect the examination of an enriched clinical group with high illness burden. In addition, modifications in the timing of the process dissociation task made necessary for the event-related fMRI paradigm may have impacted more heavily on the MDD group. Further work will be necessary to resolve these findings. Finally, because of the cross-sectional nature of the study design, it is impossible to confirm whether the past burden of illness in the MDD group led to or resulted in part from, the altered hippocampal activation that we observed in this group. Longitudinal functional imaging studies that repeat the same task in patients across various points in illness history are absent in the literature, likely because of the complexity involved in this approach.
Conclusion
We found attenuated activation in the hippocampus in patients with MDD who had a high past illness burden and who had impaired behavioural performance on the recollection component of the process dissociation task and during a hippocampus-dependent recollection memory task. These findings contribute to a nascent literature using fMRI to link behavioural deficits in recollection memory performance to attenuated activation of the hippocampus. This attenuated activation is not a function of current illness burden as patients were euthymic at the time of task completion; the attenuated activation may represent stable changes in hippocampal function that occur over the course of illness in patients with MDD.
Acknowledgement
This research was funded by a Young Investigator Award from the Ontario Mental Health Foundation awarded to G.B.C. Hall.
Footnotes
Competing interests: None declared for A.M.B. Milne. G.M. MacQueen declares that her institute has received grant funding from Astra Zeneca and that she has received consultancy and lecture fees from Astra Zeneca, Lilly, BMS, Pfizer, Lundbeck and Servier, lecture fees from CANMAT and both lecture fees and payment for development of educational presentations from the Canadian Psychiatric Association. G.B.C. Hall declares having received grant support from the Ontario Mental Health Foundation and student travel assistance to present at Human Brain Mapping 2008.
Contributors: G.M. MacQueen and G.B.C. Hall designed the study. A.M.B. Milne and G.B.C. Hall acquired the data. All authors analyzed the data, wrote the article and approved its publication.
References
- 1.McKinnon MC, Yucel K, Nazarov A, et al. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatry Neurosci. 2009;34:41–54. [PMC free article] [PubMed] [Google Scholar]
- 2.Werner NS, Meindl T, Materne J, et al. Functional MRI study of memory-related brain regions in patients with depressive disorder. J Affect Disord. 2009;119:124–31. doi: 10.1016/j.jad.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Fairhall SL, Sharma S, Magnusson J, et al. Memory related dysregulation of hippocampal function in major depressive disorder. Biol Psychol. 2010;85:499–503. doi: 10.1016/j.biopsycho.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 4.St Jacques PL, Botzung A, Miles A, et al. Functional neuroimaging of emotionally intense autobiographical memories in post-traumatic stress disorder. J Psychiatr Res. 2011;45:630–7. doi: 10.1016/j.jpsychires.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carrión VG, Haas BW, Garrett A, et al. Reduced hippocampal activity in youth with posttraumatic stress symptoms: an FMRI study. J Pediatr Psychol. 2010;35:559–69. doi: 10.1093/jpepsy/jsp112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Werner NS, Meindl T, Engel RR, et al. Hippocampal function during associative learning in patients with posttraumatic stress disorder. J Psychiatr Res. 2009;43:309–18. doi: 10.1016/j.jpsychires.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Onur OA, Schlaepfer TE, Kukolja J, et al. The N-methyl-D-aspartate receptor co-agonist D-cycloserine facilitates declarative learning and hippocampal activity in humans. Biol Psychiatry. 2010;67:1205–11. doi: 10.1016/j.biopsych.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 8.Wood FB, Flowers DL. Hypofrontal vs. hypo-Sylvian blood flow in schizophrenia. Schizophr Bull. 1990;16:413–24. doi: 10.1093/schbul/16.3.413. [DOI] [PubMed] [Google Scholar]
- 9.Ragland JD, Gur RC, Valdez JN, et al. Levels-of-processing effect on frontotemporal function in schizophrenia during word encoding and recognition. Am J Psychiatry. 2005;162:1840–8. doi: 10.1176/appi.ajp.162.10.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heckers S, Rauch SL, Goff D, et al. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat Neurosci. 1998;1:318–23. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- 11.Eker C, Gonul AS. Volumetric MRI studies of the hippocampus in major depressive disorder: meanings of inconsistency and directions for future research. World J Biol Psychiatry. 2010;11:19–35. doi: 10.1080/15622970902737998. [DOI] [PubMed] [Google Scholar]
- 12.Sheline YI, Sanghavi M, Mintun MA, et al. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19:5034–43. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colla M, Kronenberg G, Deuschle M, et al. Hippocampal volume reduction and HPA-system activity in major depression. J Psychiatr Res. 2007;41:553–60. doi: 10.1016/j.jpsychires.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 14.MacQueen GM, Campbell S, McEwen BS, et al. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci U S A. 2003;100:1387–92. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacQueen G, Parkin C, Marriott M, et al. The long-term impact of treatment with electroconvulsive therapy on discrete memory systems in patients with bipolar disorder. J Psychiatry Neurosci. 2007;32:241–9. [PMC free article] [PubMed] [Google Scholar]
- 16.Ruiz-Caballero JA, González P. Effects of level of processing on implicit and explicit memory in depressed mood. Motiv Emot. 1997;21:195–209. [Google Scholar]
- 17.Jacoby LL. Invariance in automatic influences of memory: toward a user’s guide for the process-dissociation procedure. J Exp Psychol Learn Mem Cogn. 1998;24:3–26. doi: 10.1037//0278-7393.24.1.3. [DOI] [PubMed] [Google Scholar]
- 18.Jacoby LL, Jennings JM, Hay JF. Dissociating automatic and consciously controlled processes: implications for diagnosis and rehabilitation of memory deficits. In: Herman D, McEvoy C, Hertzog C, et al., editors. Basic and applied memory research: theory in context. Vol. 1. New Hersey: Lawrence Erlbaum; 1996. pp. 161–93. [Google Scholar]
- 19.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington: The Association; 1994. [Google Scholar]
- 20.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders — Patient Edition (SCID-I/P. 2/2001 revision) New York (NY): Biomedics Research Department; 1998. [Google Scholar]
- 21.Beck AT, Beamesderfer A. Assessment of depression: the depression inventory. Mod Probl Pharmacopsychiatry. 1974;7:151–69. doi: 10.1159/000395074. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62(suppl 16):10–7. [PubMed] [Google Scholar]
- 24.Davis JM, Chen N. Dose response and dose equivalence of anti-psychotics. J Clin Psychopharmacol. 2004;24:192–208. doi: 10.1097/01.jcp.0000117422.05703.ae. [DOI] [PubMed] [Google Scholar]
- 25.Hassel S, Almeida JRC, Kerr N, et al. Elevated striatal and decreased dorsolateral prefrontal cortical activity to emotional stimuli in euthymic bipolar disorder: no associations with psychotropic medication load. Bipolar Disord. 2008;10:916–27. doi: 10.1111/j.1399-5618.2008.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phillips ML, Travis MJ, Fagiolini A, et al. Medication effects in neuroimaging studies of bipolar disorder. Am J Psychiatry. 2008;165:313–20. doi: 10.1176/appi.ajp.2007.07071066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Almeida JRC, Akkal D, Hassel S, et al. Reduced gray matter volume in ventral prefrontal cortex but not amygdala in bipolar disorder: significant effects of gender and trait anxiety. Psychiatry Res. 2009;171:54–68. doi: 10.1016/j.pscychresns.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacQueen GM, Galway TM, Hay J, et al. Recollection memory deficits in patients with major depressive disorder predicted by past depressions but not current mood state or treatment status. Psychol Med. 2002;32:251–8. doi: 10.1017/s0033291701004834. [DOI] [PubMed] [Google Scholar]
- 29.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York (NY): Thieme Medical Publishers; 1988. [Google Scholar]
- 30.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–8. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 31.Tulving E, Kapur S, Craik FI, et al. Hemispheric encoding/retrieval asymmetry in episodic memory: positron emission tomography findings. Proc Natl Acad Sci U S A. 1994;91:2016–20. doi: 10.1073/pnas.91.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nyberg L, McIntosh AR, Cabeza R, et al. General and specific brain regions involved in encoding and retrieval of events: what, where, and when. Proc Natl Acad Sci U S A. 1996;93:11280–5. doi: 10.1073/pnas.93.20.11280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bellgowan PS, Saad ZS, Bandettini PA. Understanding neural system dynamics through task modulation and measurement of functional MRI amplitude, latency, and width. Proc Natl Acad Sci U S A. 2003;100:1415–9. doi: 10.1073/pnas.0337747100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown MW, Aggleton JP. Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- 35.Rugg MD, Yonelinas AP. Human recognition memory: a cognitive neuroscience perspective. Trends Cogn Sci. 2003;7:313–9. doi: 10.1016/s1364-6613(03)00131-1. [DOI] [PubMed] [Google Scholar]
- 36.Yonelinas AP. Recognition memory ROCs for item and associative information: the contribution of recollection and familiarity. Mem Cognit. 1997;25:747–63. doi: 10.3758/bf03211318. [DOI] [PubMed] [Google Scholar]
- 37.Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn Sci. 2007;11:379–86. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Ulrich M, Jonas C, Gron G. Functional compensation of increasing memory encoding demands in the hippocampus. Neuroreport. 2010;21:59–63. doi: 10.1097/WNR.0b013e3283340d36. [DOI] [PubMed] [Google Scholar]
- 39.Carr VA, Rissman J, Wagner AD. Imaging the human medial temporal lobe with high-resolution fMRI. Neuron. 2010;65:298–308. doi: 10.1016/j.neuron.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malykhin NV, Lebel RM, Coupland NJ, et al. In vivo quantification of hippocampal subfields using 4.7 T fast spin echo imaging. Neuroimage. 2010;49:1224–30. doi: 10.1016/j.neuroimage.2009.09.042. [DOI] [PubMed] [Google Scholar]
- 41.Olsen RK, Nichols EA, Chen J, et al. Performance-related sustained and anticipatory activity in human medial temporal lobe during delayed match-to-sample. J Neurosci. 2009;29:11880–90. doi: 10.1523/JNEUROSCI.2245-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Viskontas IV, Carr VA, Engel SA, et al. The neural correlates of recollection: hippocampal activation declines as episodic memory fades. Hippocampus. 2009;19:265–72. doi: 10.1002/hipo.20503. [DOI] [PubMed] [Google Scholar]
- 43.Zeineh MM, Engel SA, Thompson PM, et al. Dynamics of the hippocampus during encoding and retrieval of face-name pairs. Science. 2003;299:577–80. doi: 10.1126/science.1077775. [DOI] [PubMed] [Google Scholar]
- 44.Barbas H, Blatt GJ. Topographically specific hippocampal projections target functionally distinct prefrontal areas in the rhesus monkey. Hippocampus. 1995;5:511–33. doi: 10.1002/hipo.450050604. [DOI] [PubMed] [Google Scholar]
- 45.Carmichael ST, Price JL. Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol. 1996;371:179–207. doi: 10.1002/(SICI)1096-9861(19960722)371:2<179::AID-CNE1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 46.Phillips ML, Drevets WC, Rauch SL, et al. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol Psychiatry. 2003;54:515–28. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 47.MacQueen GM, Galway TM, Hay J, et al. Recollection memory deficits in patients with major depressive disorder predicted by past depressions but not current mood state or treatment status. Psychol Med. 2002;32:251–8. doi: 10.1017/s0033291701004834. [DOI] [PubMed] [Google Scholar]