Abstract
Background
Relatives of patients with major depressive disorder (MDD) and people who experienced early-life adversity are at risk for MDD. The aim of our study was to investigate whether unaffected first-degree healthy relatives (UHRs) of patients with MDD show changes in white matter fibre connections compared with healthy controls and whether there are interactions between early-life adversity and these microstructural changes.
Methods
Unaffected, healthy first-degree relatives of patients with MDD and healthy controls without any family history for a psychiatric disease underwent high angular resolution diffusion imaging with 61 diffusion directions. Data were analyzed with tract-based spatial statistics, and findings were confirmed with tractography.
Results
Twenty-one UHRs and 24 controls participated in our study. The UHRs showed greater fractional anisotropy than controls in the body and splenium of the corpus callosum, inferior fronto-occipital fasciculus (IFO), left superior longitudinal fasciculus (SLF) and right fornix. The UHRs who experienced more early-life adversity had greater fractional anisotropy than those with less early-life adversity in the splenium of the corpus callosum, fornix, IFO and SLF; in controls, early-life adversity was found to be associated with decreased fractional anisotropy in these fibre tracts.
Limitations
Studying participants’ strategies for coping with early-life adversity would have been helpful. Crossing fibres in tracts are a general limitation of the method used.
Conclusion
Altogether, our findings provide evidence for greater fractional anisotropy in UHRs and for interaction between early-life adversity and family risk on white matter tracts involved in cognitive–emotional processes. Whether stronger neural fibre connections are associated with more resilience against depression needs to be addressed in future studies.
Introduction
Mental disorders are a major cause of long-term disability and are a direct cause of mortality, with about 800 000 individuals dying from suicide every year worldwide and a high proportion of these deaths being related to major depressive disorder (MDD).1 Potent risk factors for MDD are neuroticism, sex and psychosocial adversity, whereby psychosocial adversity interacts with both neuroticism and with sex.2 Another strong risk factor is a family history of depression,3 and a review of twin studies found concordance rates of 0.23–0.67 for monozygotic twins and 0.14–0.43 for dizygotic twins,4 indicating the importance of genetic factors. A recent meta-analysis provided evidence that the serotonin transporter polymorphism 5-HTTLPR moderates the relation between stress, particularly early-life adversity, and depression.5 Because of the genetic background, assessment of first-degree relatives of patients with MDD may provide a powerful model in which to investigate biologic vulnerability.6
Detecting that neuroplasticity may play a core role in the pathophysiology of MDD has expanded our knowledge of the disease in recent years.7 This concept was supported by experimental studies that have shown that excessive cortisol secretion and excessive production of inflammatory cytokines, which can be triggered by inflammation or psychological stress, impair neuronal plasticity and neurogenesis in the hippocampus, a temporal lobe brain region involved in learning, memory and affect regulation.7 Family risk studies on the biologic background of vulnerability for MDD have determined that alterations in the stress–hormone axis, cognitive function, and structural changes like those in the hippocampus are present even before the onset of the disease. Healthy first-degree relatives of patients with depression have been shown to have higher cortisol levels in the morning than healthy participants who reported no affective episodes in a first-degree relative;8–10 however, this finding could not be replicated in a study comparing high-risk twins with low-risk twins.11 In the latter study, monozygotic high-risk twins had significantly higher evening cortisol levels than monozygotic low-risk twins.11 In line with the above-mentioned experimental research about the effect of cortisol on the hippocampus7 is the finding that small hippocampal and dorsolateral prefrontal cortex volumes were apparent before the manifestation of clinical symptoms of MDD in patients at family risk for MDD12 and that smaller hippocampal volumes were found in adolescents whose parents had MDD.13 Moreover, it has been reported that healthy twins who had a co-twin with a history of unipolar disorder performed worse than healthy low-risk twins on cognitive functions like declarative memory, executive function and language processing, suggesting that cognitive dysfunctions are present before the onset of the disease.14
Diffusion tensor imaging (DTI) is an important step forward for characterizing microstructural changes or differences. Previously, studies of white matter bundles were restricted to postmortem evaluation of fibre systems. Diffusion tensor imaging is sensitive to the properties of diffusion of water molecules, and, as such, this technique can be used to map and characterize the 3-dimensional diffusion of water as a function of spatial location.15 Since water molecules interact with tissue structure, DTI can help reveal the characteristics of the architectural organization of the brain. In a meta-analysis16 of whole-brain DTI studies of patients with MDD, we detected significantly decreased fractional anisotropy values in the superior longitudinal fasciculus (SLF) in patients with MDD compared with healthy controls. This effect was significantly more pronounced in studies that included only untreated patients than in those that included treated patients. Fractional anisotropy values in the SLF were also smaller in studies that included patients with a longer illness duration and more severe depression.16 Region of interest (ROI)–based DTI studies in patients with late-life depression have consistently reported reduced fractional anisotropy values in prefrontal brain regions.17–22 Reduced fractional anisotropy in the cingulate cortex has also been detected.19,23,24 In addition, Yang and colleagues22 and Murphy and colleagues19 reported a reduction in fractional anisotropy values in the parahippocampal gyrus. On the other hand, Lu and colleagues25 detected an increase in fractional anisotropy values in areas of the brain associated with mood regulation, such as the right superior frontal gyrus to right pallidum and left superior parietal gyrus to right superior occipital gyrus, by performing tractography analysis in patients with MDD.
A recent study using tract-based spatial statistics (TBSS) identified a reduction of fractional anisotropy in the SLF in 18 healthy adolescents at familial risk for unipolar depression compared with 13 healthy controls.26 Investigating unaffected first-degree relatives (UHRs) of adults with MDD, who are at a higher risk for MDD,27 with DTI is novel and might shed more light into underlying biologic substrates of vulnerability. The aim of the present study was to investigate microstructural changes in white matter tracts in UHRs compared with healthy controls without any family risk for psychiatric diseases by means of TBSS and tractography. It can be difficult to draw conclusions on the basis of whole-brain methods on specific tracts because of the high amount of fibre crossings in the brain. Here, tractography is a complementary method that allows the investigation of predefined fibre bundles. We expected that fractional anisotropy in frontotemporal regions would be reduced in UHRs compared with controls. We also investigated whether there are interactions between early-life adversity as a risk factor for MDD and the microstructural changes in white matter tracts.
Methods
Participants
The UHRs recruited for participation in this study were siblings or children of adult patients undergoing treatment for recurrent MDD in the mental health services department of the Adelaide and Meath Hospital, incorporating the National Children’s Hospital and St. James’s Hospital, which are teaching hospitals of Trinity College Dublin, Ireland. The diagnosis of the patients’ MDD was confirmed by psychiatric consultants based on DSM-IV criteria. We also recruited healthy controls without any family history of psychiatric disease from the local community via announcements. Groups were age- and sex-matched. Each participant was carefully screened and examined for medical conditions so that neither the controls nor the UHRs had a personal history of neurologic or psychiatric disorders (Axis I or Axis II), or a history of severe medical illness, head injury or substance abuse. No UHR was a relative of another UHR. We excluded UHRs when their first-degree family members had a comor-bid diagnosis in addition to MDD. The UHRs and controls were evaluated by a psychiatrist (A.C.) for any psychiatric conditions; this evaluation included the Structured Clinical Interview for DSM-IV Axis I (SCID-I)32 and Axis II (SCID-II)33 disorders. Demographic variables, inclusion and exclusion criteria were documented using a standardized questionnaire and through a structured interview with the psychiatrist. We used the Student t test to assess differences in demographic and clinical variables and the χ2 test for sex distribution.
We obtained written informed consent from all participants after providing a detailed description of the study, which was designed and performed in accordance with the ethical standards laid out by the Declaration of Helsinki and was approved by the ethics committee of the Adelaide and Meath Hospital.
Rating instruments
All study participants completed self- and observer-rated scales. The rating scales used were the Hamilton Rating Scale for Depression (HAM-D),28 Beck Depression Inventory (BDI),29 Childhood Trauma Questionnaire (CTQ),31 Beck Anxiety Inventory,30 Eysenck Personality Questionnaire34 and SCID-II. The CTQ was used to assess childhood stress. This questionnaire is a self-report instrument that assesses 5 types of childhood mistreatment: emotional, physical and sexual abuse, and emotional and physical neglect. Reliability and validity of the CTQ, including measures of convergent and discriminative validity from structured interviews, stability over time and corroboration, have been established.35 Owing to the small number of participants in each group who reported childhood trauma, we used median split as well as linear correlations for statistical analyses.
Diffusion tensor imaging
Magnetic resonance images were obtained using a 3-T Philips Achieva scanner. We also obtained high angular resolution diffusion images with 61 diffusion directions (field of view 200 × 257 × 126 mm, 60 slices, no gap, spatial resolution 1.8 × 1.8 × 2.1 mm, repetition time 12561 ms, echo time 59 ms, flip angle 90°, half k-space acquisition [half scan factor = 0.68], sensitivity encoding parallel imaging factor 2.5, β-values 0, 1200 s/mm2, with spectral presaturation inversion recovery fat suppression and dynamic stabilization in an image acquisition time of 15 min, 42 s).
Diffusion tensor imaging data preprocessing
Data were converted from a Philips PAR/REC format to NIfTI and B-matrix text-file formats using ExploreDTI. Thereafter, data were transferred to an ExploreDTI file and transferred to a voxel size of 2 × 2 × 2 mm. With our acquisition voxel size, there is no significant partial volume effect associated with this technique. Diffusion tensor estimation was linear. We applied motion correction to all data to adjust for movement during scanning using a cubic interpolation and restore function with the lowest speed but highest accuracy. Eddy currency correction was also used.36 To check the quality of the data, we first reviewed the DTI data by looping them. ExploreDTI also allowed us to look at the residuals and the outlier profiles, which were in order. Finally we checked the motion correction parameters. Movement during scanning was less than 2 mm in any direction and less than 3° rotation in sagittal, coronal or axial planes for all participants.
Tract-based spatial statistics
For TBSS implemented in FSL (www.fmrib.ox.ac.uk/fsl/), we extracted the fractional anisotropy and the mean, axial (λ1) and radial diffusivity (λ2 + λ3)/2) and fed them into the same FSL tools used in the subsequent steps described hereafter. The TBSS technique projects all participants’ fractional anisotropy data onto a mean fractional anisotropy tract skeleton before applying voxelwise cross-subject statistics. In brief, all participants’ fractional anisotropy data were aligned into a common space with the nonlinear registration tool FNIRT, which uses a B-spline representation of the registration warp field. The target template was the FMRIB58_FA standard space image. Next, we performed nonlinear and affine transformations to MNI152 space and then merged all images into a 4-dimensional image containing all participants. The mean of all images was created and fed into a script creating the mean skeletonized image. Each participant’s aligned fractional anisotropy data were then projected onto this skeleton, and the resulting data were fed into voxel-wise cross-subject statistics. We performed a voxelwise statistical analysis of the individual skeleton images of all participants derived in TBSS by means of threshold-free cluster enhancement using 5000 permutations for each test.37 Threshold-free cluster enhancement takes a raw statistic image and produces an output image in which the voxelwise values represent the amount of cluster-like local spatial support. Each new value of a voxel is given by the sum of the scores of all supporting sections. The output value is therefore a weighted sum of the local clustered signal, without the need for a hard cluster-forming thresholding. Threshold-free cluster enhancement has the advantage over cluster-based thresholding because it does not need arbitrary thresholds defined a priori that introduce instability in the overall processing chain. Furthermore, the amount of spatial smoothing is itself arbitrary, given that the expected signal extent is very rarely known in advance of the analysis.38
We also used a median split for childhood stress to test for interactions between childhood stress and diagnosis of MDD. We opted to use this procedure because both groups were healthy without significant differences in early-life adversity and because there were only a few participants who met the cut-off thresholds for traumatization. The statistical threshold was set at p < 0.05, fully corrected for multiple comparisons using threshold-free cluster enhancement across all white matter fibre bundles in the whole brain to find differences between UHRs and controls for fractional anisotropy and mean, radial and axial diffusivity (eigenvalue λ1). We extracted fractional anisotropy values from significant clusters for further graphic representation, and we identified areas of significant differences using the following FSL tools: the Harvard-Oxford Structural Atlas and the JHU ICBM-DTI and tractography atlases39 (www.fmrib.ox.ac.uk/fsl/data/atlas-descriptions.html#wm).
Tractography
All data were transformed into Montreal Neurological Institute (MNI) space. Seed point resolution was 2 × 2 × 2 mm with a seed fractional anisotropy threshold of 0.2. Deterministic tractography was applied with ExploreDTI.40 First, we conducted whole-brain tractography in each participant using a linear interpolation. Then, individual tracts that showed significant alterations in previous DTI studies of patients with MDD were isolated using protocols similar to the knowledge-based multiple region approach previously described for the association tracts.41 The protocols are described in detail below. The ExploreDTI software allows isolation of tracts passing through 2 ROIs (using the “AND” operator) or not passing through an ROI (using the “NOT” operator).
We used 2 ROIs per tract to extract the white matter fibre tracts uncinate fasciculus (UF), crus of the fornix, inferior fronto-occipital fasciculus (IFO) and SLF showing significance in the whole-brain TBSS analysis.
For the UF, which connects the anterior temporal lobe with the orbitofrontal and frontopolar cortices, we placed the ROIs on the most posterior coronal slice in which the temporal lobe is separated from the frontal lobe. The first ROI included the entire temporal lobe, and the second ROI was placed at the same coronal level and included the entire projections toward the frontal lobe. Moreover, we used an ROI with the “NOT” operator to avoid fibres from the IFO and the cingulum.
The crus of the fornix contain fibres from the hippocampus and the parahippocampal cortex. We placed the first ROI in the coronal section at the level of the middle hippocampal body, and the second ROI was placed where the body and crus of the fornix are clearly visible.
The SLF connects parts of the parietal lobule and posterior temporal lobe with the prefrontal lobe in a bidirectional way. For the SLF, we placed the first ROI at the level of the middle of the posterior limb of the internal capsule, and the second ROI was placed at the middle of the splenium. At these levels, the SLF is seen as a green triangular region lateral to the superior-to-inferior corticospinal blue fibres. We used ROIs with the “NOT” operator to avoid fibres crossing from the IFO and pyramidal tracts.42
The IFO connects the dorsolateral and premotor prefrontal cortices with the posterior part of the parietal, temporal and occipital lobes as well as the caudal cingulate cortex. We used 2 ROIs to isolate the IFO. For tractography of the IFO, we placed 2 ROIs along the course of the IFO in the coronal plane of the DTI images at the level of the anterior commissure and pontine crossing fibres, respectively.43
All ROIs were drawn on the coloured fractional anisotropy–weighted maps, and the investigator (T.F.) was blind to diagnosis. For all participants, we used the same numbers and locations of ROIs. We calculated interrater reliability after 2 raters independently performed tractography in 20 participants. Intraclass correlations were between 0.90 and 0.95 for mean fractional anisotropy values in the tracts. After performing the tractography for all tracts for all individuals, the mean fractional anisotropy and the axial and radial diffusivity were extracted and entered into SPSS software for further analysis. These parameters were subjected to an analysis of covariance with group (UHR, control) and early-life adversity (median split on CTQ total score) as fixed factors and with age and sex as covariates.
Results
Participants
We included 21 UHRs and 24 age- and sex-matched controls in our study. The demographic and clinical characteristics of participants are summarized in Table 1. The UHRs did not differ from controls in age, sex, education and weight. Sub-threshold depression scores derived from the HAM-D28 were significantly higher, and scores from the self-rating BDI29 tended to be significantly greater among UHRs than controls, but all values were still within the normal range (Table 1). Neuroticism and childhood trauma scores did not differ between the groups, and early-life adversity was not associated with higher depression scores. Based on the cut-off for traumatization only 6 of our participants with family history and 4 without family history reported childhood trauma.
Table 1.
Demographic and clinical characteristics of unaffected relatives of patients with major depressive disorder and healthy controls
| Group; mean (SD)* |
||||
|---|---|---|---|---|
| Characteristic | Control, n = 24 | UHR, n = 21 | t1,43 | p value |
| Age, yr | 34.7 (11.0) | 38.1 (14.5) | 0.92 | 0.37 |
| Sex,† female/male | 14/10 | 13/8 | 0.06 | 0.81 |
| Education, yr | 16.6 (2.4) | 15.9 (2.8) | 0.94 | 0.35 |
| Weight, kg | 71.5 (17.1) | 67.3 (13.4) | 0.90 | 0.37 |
| 21-item Hamilton Depression Scale28 score | 1.5 (1.7) | 4.0 (3.9) | 2.7 | 0.012 |
| Beck Depression Inventory29 score | 1.5 (2.1) | 3.7 (5.7) | 1.7 | 0.09 |
| Beck Anxiety Inventory30 score | 6.0 (5.2) | 5.9 (4.3) | 0.07 | 0.95 |
| Neuroticism score | 2.8 (3.0) | 3.4 (2.9) | 0.68 | 0.50 |
| Childhood Trauma Questionnaire31 score | 31.3 (6.8) | 31.3 (5.4) | 0.02 | 0.98 |
| Emotional neglect | 7.2 (2.9) | 7.4 (2.9) | 0.30 | 0.77 |
| Physical neglect | 6.2 (1.6) | 6.3 (1.8) | 0.15 | 0.88 |
| Sexual abuse | 5.6 (1.4) | 5.5 (1.2) | −0.15 | 0.88 |
| Emotional abuse | 6.5 (2.0) | 6.3 (1.9) | −0.29 | 0.77 |
| Physical abuse | 5.8 (1.7) | 5.7 (1.4) | −0.17 | 0.87 |
SD = standard deviation; UHR = unaffected relatives of patients with major depressive disorder.
Unless otherwise indicated.
χ2 test for sex differences between groups.
Tract-based spatial statistics
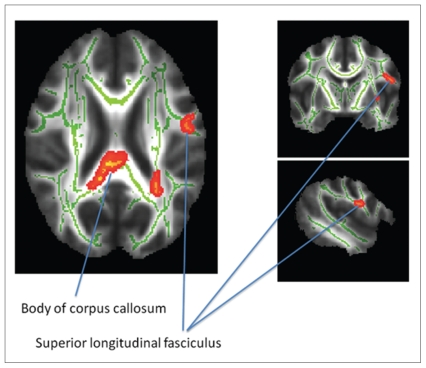
We detected significantly greater fractional anisotropy values in UHRs than controls in the posterior body and splenium of the corpus callosum, left SLF, left IFO, left external capsule and left anterior thalamic radiation (Fig. 1, Table 2). Mean radial or longitudinal diffusivity (λ1) did not differ significantly between the groups.
Fig. 1.
Comparison of fractional anisotropy in fibre tracts between UHRs and healthy controls. We found increased fractional anisotropy values in the posterior body of the corpus callosum (MNI coordinates x, y, z = 9, −30, 24) with extensions of the cluster into the splenium and the superior longitudinal fasciculus (MNI coordinates x, y, z = −48, −6, 21) in UHRs compared with controls (see also Table 2). Significant areas are highlighted in red–yellow (corrected for multiple comparisons) and displayed in radiologic convention: left is right and right is left. Tract names are derived from ICBM-DTI-81 white-matter labels and tractography atlas.39 MNI = Montreal Neurological Institute; UHR = unaffected first-degree relatives of patients with confirmed major depressive disorder.
Table 2.
Fractional anisotropy differences between unaffected relatives of patients with major depressive disorder and controls*
| MNI coordinates
|
|||||
|---|---|---|---|---|---|
| Region† | Voxels | p value‡ | x | y | z |
| Body–splenium of corpus callosum | 261 | 0.039 | 9 | −30 | 24 |
| Body of corpus callosum | 62 | 0.046 | −11 | −28 | 26 |
| Body of corpus callosum (left IFO, anterior thalamic radiation) | 158 | 0.041 | −27 | −45 | 21 |
| Left posterior corona radiate (left corticospinal tract) | 95 | 0.045 | −26 | −24 | 25 |
| Left post- and precentral gyrus (left SLF) | 71 | 0.045 | −48 | −6 | 21 |
| Left external capsule (left IFO) | 20 | 0.049 | −33 | −10 | 6 |
IFO = inferior fronto-occipital fasciculus; MNI = Montreal Neurological Institute; SLF = superior longitudinal fasciculus; UHR = unaffected relatives of patients with major depressive disorder.
The table shows regions with increased fractional anisotropy values for the UHR compared with the control group.
The Harvard-Oxford Structural Atlas and ICBM-DTI-81 white-matter labels atlas were used for region identification.39
The p values, family-wise error–corrected for multiple testing across the whole brain fibre tracts, are < 0.05 in all participants.
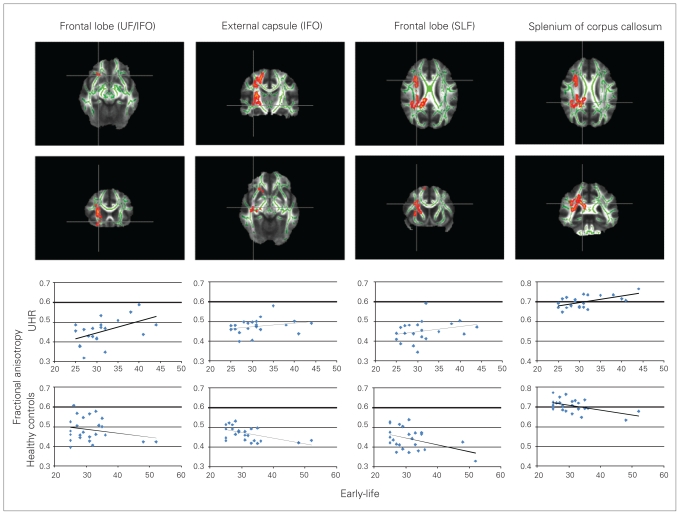
There were no significant main effects of early-life adversity (participants without childhood stress versus those with at least minor childhood stress). However, we detected a significant 2-way interaction between group (UHR, control) and early-life adversity for fractional anisotropy in 3 clusters (p < 0.05, corrected for multiple comparisons across the whole brain). The first large cluster was mainly in the right temporo-parietal white matter and involved the splenium and the body of the corpus callosum, right IFO and right SLF (k = 3612 voxels, MNI coordinates x, y, z = 32, −35, 4 for the most significant voxel; see Fig. 2 for individual coordinates). The second cluster was located in the right orbitofrontal cortex (right IFO; k = 41 voxels, MNI coordinates x, y, z = 25, 19, −16). The third cluster was in the right frontal cortex (right IFO, UF; k = 13 voxels, MNI coordinates x, y, z = 22, 22, −9). Analysis of the UHR and control groups separately showed that UHRs who reported at least minor childhood stress had greater fractional anisotropy than UHRs who reported no childhood stress. On the other hand, controls who reported having child-hood stress showed lower fractional anisotropy than controls who reported no childhood stress. Extracting the fractional anisotropy values from the seed voxel in each region allowed us to graphically depict these associations. Childhood stress correlated positively with fractional anisotropy in UHRs and negatively in controls, as shown in Figure 2.
Fig. 2.
Interaction between group (UHRs, control) and childhood stress. Depicted are significant areas in the frontal lobe (UF, IFO; most significant MNI coordinates x, y, z = 22, 22, −9), external capsule (IFO; MNI coordinates x, y, z = 32, −18, 4), frontal lobe (SLF; MNI coordinates x, y, z = 27, 9, 25) and the splenium of the corpus callosum (MNI coordinates x, y, z = 18, −43, 27) and are highlighted red–yellow (corrected for multiple comparisons) in axial (row 1) and coronal (row 2) slices. Images are displayed in the radiologic convention: left is right and right is left. The UHRs had greater fractional anisotropy with more early-life adversity, whereas controls had lower fractional anisotropy with more early-life adversity. Correlations between fractional anisotropy in the voxel of the peak coordinate and early-life adversity (Childhood Trauma Questionnaire31) are shown in row 3 for UHRs (UF/IFO: r = 0.47, p = 0.03, IFO: r = 0.37, p = 0.09, SLF: r = 0.34, p = 0.12, corpus callosum: r = 0.50, p = 0.02) and in row 4 for controls (UF: r = −0.11, p = 0.6, IFO: r = −0.57, p = 0.004, SLF: r = −0.33, p = 0.11, corpus callosum: r = −0.39, p = 0.06). Names of tracts that might be involved are derived from the JHU White-Matter Tractography Atlas.39 IFO = inferior fronto-occipital fasciculus; MNI = Montreal Neurological Institute; SLF = superior longitudinal fasciculus; UF = uncinate fasciculus; UHR = unaffected first-degree relatives of patients with confirmed major depressive disorder.
Tractography
To further explore the differences between groups, we performed deterministic tractography (Table 3). We detected greater mean fractional anisotropy values in the right fornix (F1,39 = 5.8, p = 0.020), right IFO (F1,39 = 4.0, p = 0.048) and left SLF (F1,39 = 5.7, p = 0.022) in UHRs compared with controls. No significant differences were found for the UF. No main effect of early-life adversity was detected; however, we found significant interactions between group and early-life adversity for fractional anisotropy in the right SLF (F1,39 = 5.2, p = 0.028). Here, UHRs tended to have greater fractional anisotropy in the right SLF when they had reported at least mild early-life adversity events (F1,17 = 3.2, p = 0.09). We observed a trend toward significant interactions for fractional anisotropy in the left fornix (F1,39 = 3.8, p = 0.06). In controls, fractional anisotropy in the left fornix was greater when they reported more early-life adversity (F1,20 = 3.7, p = 0.07). No significant effects were detected for radial, axial or mean diffusivity.
Table 3.
Fractional anisotropy values of the uncinate fasciculus, inferior fronto-occipital fasciculus, superior longitudinal fasciculus and fornix for healthy controls with and without a first-degree relative with major depressive disorder and with and without early childhood adversity
| Group; mean (SD) fractional anisotropy value
|
||||||
|---|---|---|---|---|---|---|
| Tractography | UHR with ELA | UHR without ELA | Control with ELA | Control without ELA | F1,39 | p value |
| Left UF | 0.442 (0.0138) | 0.439 (0.0153) | 0.436 (0.0172) | 0.443 (0.0163) | 0.2 | 0.66* |
| Right UF | 0.436 (0.0160) | 0.442 (0.0226) | 0.426 (0.0332) | 0.435 (0.0267) | 1.7 | 0.20* |
| Left IFO | 0.488 (0.0127) | 0.488 (0.0160) | 0.481 (0.0187) | 0.489 (0.0163) | 1.0 | 0.34* |
| Right IFO | 0.492 (0.0129) | 0.504 (0.0188) | 0.486 (0.0173) | 0.498 (0.0166) | 4.0 | 0.048* |
| Left fornix | 0.394 (0.0180) | 0.394 (0.200) | 0.389 (0.0166) | 0.403 (0.0178) | 3.7 | 0.06† |
| Right fornix | 0.414 (0.0257) | 0.425 (0.0217) | 0.410 (0.0149) | 0.405 (0.0228) | 5.8 | 0.020* |
| Left SLF | 0.461 (0.0179) | 0.476 (0.0177) | 0.452 (0.020) | 0.462 (0.0259) | 5.7 | 0.022* |
| Right SLF | 0.475 (0.0279) | 0.470 (0.0265) | 0.464 (0.0266) | 0.476 (0.0257) | 1.3 | 0.26† |
ELA = early-life adversity; IFO = inferior fronto-occipital fasciculus; NS = not significant; SD = standard deviation; SLF = superior longitudinal fasciculus; UF = uncinate fasciculus; UHR = unaffected first-degree relatives of patients with major depressive disorder.
UHR > control.
Post hoc: lower fractional anisotropy in controls with early-life adversity compared with controls without early-life adversity.
Discussion
Our study revealed that UHRs had greater fractional anisotropy than controls in the body and splenium of the corpus callosum. Moreover, greater fractional anisotropy was detected in the left external capsule and the pre– and postcentral lobes, changes that might be related to the IFO and SLF, respectively. The tractography analysis showed greater fractional anisotropy in the right IFO, left SLF and right fornix in UHRs compared with controls. A recent study by Huang and colleagues44 involving adolescents at familial risk for MDD reported lower fractional anisotropy in the left SLF, IFO, cingulum, UF and splenium of the corpus callosum. The mean age of participants in our study was more than 20 years older than that in the study by Huang and colleagues.44 Since adolescence is the peak risk period for the development of unipolar depressive disorder,45 it may be that the participants recruited in our study were those who did not experience a depressive episode, had a lower overall risk or were resilient. In fact, other risk factors like neuroticism or greater early-life adversity did not differ between UHRs and controls, suggesting that these other risk factors might not have been present in this population.
Lower fractional anisotropy in adolescents might reflect a greater vulnerability to depression, and greater fractional anisotropy in adults who did not become depressed might be related to resilience. Interestingly, increased fractional anisotropy in the affected fibre systems of the corpus callosum, IFO and SLF have been shown to be associated with better cognitive functions like executive functioning, working memory and attention processing.46 A recent magnetic resonance imaging study involving combat veterans with a self-reported history of blast-related concussion showed that 11 veterans without depression had greater fractional anisotropy values in the SLF than 11 veterans who had depression, a finding that might also support the idea that greater fractional anisotropy is associated with resilience against depression.47 In experimental studies, resilience to stress has been associated with the capacity to constrain stress-induced increases in corticotrophin-releasing hormone and corticosterone through an elaborated negative feedback system48,49 and via expression of brain-derived neurotrophic factor (BDNF).50,51 In turn, BDNF function has been found to be associated with structural brain changes.52 Hippocampal BDNF might be involved in the development of neural circuits that control adequate stress adaptations,51 and these circuits would involve the fornix. Our cross-sectional study allows us to only speculate about resilience. It would be highly important to investigate risk and protective factors longitudinally in future studies.
Executive and working memory functions served by the fornix, IFO, SLF and corpus callosum have been found to be altered in patients with MDD. The fornix connects the hippocampus to the septal region and mamillary bodies53 and is involved in functions for learning and memory.54 The IFO is involved in awareness and executive functions55 by connecting the dorsolateral and premotor prefrontal cortices to posterior parts of the parietal, temporal and occipital lobes as well as the caudal cingulate gyrus.56 In patients with MDD, alterations in emotional visual perception and reduced fractional anisotropy in the IFO have been reported.57–59 The SLF connects lateral parts of the inferior parietal lobule with the lateral inferior pre-frontal lobe in a bidirectional way60 and plays a role in the frontoparietal circuit involved in working memory.60,61
With respect to our second study objective, we found interactions between early-life adversity and differences between UHRs and controls mainly for fractional anisotropy in the right frontal and orbitofrontal lobes, likely involving the IFO, SLF and UF, as well as the splenium and genu of the corpus callosum in the TBSS analysis. Tractography also allowed us to observe such an interaction for the right SLF and the left fornix. In these tracts, greater fractional anisotropy was associated with more early-life adversity in UHRs, whereas in controls more early-life adversity was associated with lower fractional anisotropy. This interaction was seen in the correlation analysis and in the analysis of covariance, in which we used early-life adversity as a dichotomic factor (no early-life adversity, at least minor early-life adversity), indicating that even minor childhood events seem to have effects on brain fibre connections. In agreement with our finding of greater fractional anisotropy in participants with more early-life adversity is a study that reported greater fractional anisotropy in the left superior temporal gyrus in healthy participants with histories of exposure to parental verbal abuse compared with healthy controls.62 However, the findings of other previous DTI studies assessing effects of early-life adversity were inconsistent with this finding and showed that decreases of fractional anisotropy in the corpus callosum;63 left UF;64 and left cingulum, fornix and arcuate fasciculus65 were associated with childhood abuse. Individual differences in response to stress seem to implicate the BDNF system and a negative stress hormone feedback control.48 Thus, our finding of opposite effects of stress on fractional anisotropy in UHRs and controls might be in line with individual characteristics of the stress system, which in turn might be a marker for vulnerability.11
In agreement with our findings of right lateralized effects for stress are reports showing that the reaction of the brain structure to stress is lateralized in such a way that severe stress activates the right medial prefrontal cortex.66 Restraint stress also has been reported to have more severe effects on the right than the left prelimbic cortex within the prefrontal cortex.67 Interestingly, the effect of stress in the right hemisphere increases variance in white matter fractional anisotropy and might explain why the difference between UHRs and controls in our study was only observed in the right hemisphere when early-life adversity was additionally taken into account.
Limitations
Limitations of the present study are that early-life adversity was assessed retrospectively. It also has to be mentioned that abuse, particularly sexual abuse, remains underreported in healthy individuals.68 Nevertheless, we found significant effects of early-life adversity on DTI parameters even when most of the participants had little experience with early-life adversity. Another limitation is that the high-risk study design may have led to the selection of participants with a first-degree relative with MDD from treated populations, thereby selecting families with an increased rate of illness. To overcome this issue it would have been necessary to use register linkage, as performed previously in larger population-based studies.69 Moreover, we did not study participants’ strategies for coping with early-life adversity or their resilience behaviourally (e.g., with respect to current stressors), so we were not able to investigate the association between increased fractional anisotropy and coping mechanisms. White matter fibre crossing is always an issue in DTI studies, TBSS are limited to investigating local changes in white matter integrity, and interpreting differences in regions of crossing fibres can be complex.70 Considerable areas of fibre crossing exist, for example, in the centrum semiovale, UF and transpontine fibres, which have corresponding low fractional anisotropy and are difficult to investigate. Thus, it is difficult to relate significant areas back to specific fibre tracts. In the present study, we were able to observe changes in the IFO, SLF and fornix using tractography; however, evidence for changes in the UF could not be replicated with tractography. Fewer white matter crossings are seen in the corpus callosum;71 therefore, our results for the corpus callosum do not seem to be influenced by fibre crossings.
Conclusion
Effects of early-life adversity and family risk on fractional anisotropy values and volumes of frontotemporal fibre tracts were demonstrated in the present study. Our findings highlight the importance of stress–gene interactions. Whether the stronger fibre connections might be associated with resilience and might render participants more stable against environmental stressors needs further investigation in studies with longitudinal designs.
Acknowledgements
We thank Science Foundation Ireland for a fund to conduct the present research, awarded to T. Frodl (grant no. SFI/07/SK/B1214C Science Foundation Strokes Professorship Grant) as well as Health Research Board Ireland for funding the research Centre of Advanced Medical Imaging.
Footnotes
Competing interests: None declared; as above for T. Frodl.
Contributors: T. Frodl, A. Carballedo and J.F. Meaney designed the study. All authors acquired the data, which T. Frodl and J.F. Meaney analyzed. T. Frodl, D. Lisiecka and Y. Ferguson wrote the article, which T. Frodl, A. Carballedo, A.J. Fagan and J.F. Meaney reviewed. All authors approved publication.
References
- 1.Prince M, Patel V, Saxena S, et al. No health without mental health. Lancet. 2007;370:859–77. doi: 10.1016/S0140-6736(07)61238-0. [DOI] [PubMed] [Google Scholar]
- 2.Kendler KS, Kuhn J, Prescott CA. The interrelationship of neuroticism, sex, and stressful life events in the prediction of episodes of major depression. Am J Psychiatry. 2004;161:631–6. doi: 10.1176/appi.ajp.161.4.631. [DOI] [PubMed] [Google Scholar]
- 3.Williamson DE, Birmaher B, Axelson DA, et al. First episode of depression in children at low and high familial risk for depression. J Am Acad Child Adolesc Psychiatry. 2004;43:291–7. doi: 10.1097/00004583-200403000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000;157:1552–62. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- 5.Karg K, Burmeister M, Shedden K, et al. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Arch Gen Psychiatry. 2011;68:444–54. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sobczak S, Honig A, Nicolson NA, et al. Effects of acute trypto-phan depletion on mood and cortisol release in first-degree relatives of type I and type II bipolar patients and healthy matched controls. Neuropsychopharmacology. 2002;27:834–42. doi: 10.1016/S0893-133X(02)00345-7. [DOI] [PubMed] [Google Scholar]
- 7.Duman RS. Pathophysiology of depression: the concept of synaptic plasticity. Eur Psychiatry. 2002;17(Suppl 3):306–10. doi: 10.1016/s0924-9338(02)00654-5. [DOI] [PubMed] [Google Scholar]
- 8.Lupien SJ, King S, Meaney MJ, et al. Child’s stress hormone levels correlate with mother’s socioeconomic status and depressive state. Biol Psychiatry. 2000;48:976–80. doi: 10.1016/s0006-3223(00)00965-3. [DOI] [PubMed] [Google Scholar]
- 9.Halligan SL, Herbert J, Goodyer IM, et al. Exposure to postnatal depression predicts elevated cortisol in adolescent offspring. Biol Psychiatry. 2004;55:376–81. doi: 10.1016/j.biopsych.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 10.Mannie ZN, Harmer CJ, Cowen PJ. Increased waking salivary cortisol levels in young people at familial risk of depression. Am J Psychiatry. 2007;164:617–21. doi: 10.1176/ajp.2007.164.4.617. [DOI] [PubMed] [Google Scholar]
- 11.Vinberg M, Bennike B, Kyvik KO, et al. Salivary cortisol in unaf-fected twins discordant for affective disorder. Psychiatry Res. 2008;161:292–301. doi: 10.1016/j.psychres.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Amico F, Meisenzahl E, Koutsouleris N, et al. Structural MRI correlates for vulnerability and resilience to major depressive disorder. J Psychiatry Neurosci. 2011;36:15–22. doi: 10.1503/jpn.090186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen MC, Hamilton JP, Gotlib IH. Decreased hippocampal volume in healthy girls at risk of depression. Arch Gen Psychiatry. 2010;67:270–6. doi: 10.1001/archgenpsychiatry.2009.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christensen MV, Kyvik KO, Kessing LV. Cognitive function in unaffected twins discordant for affective disorder. Psychol Med. 2006;36:1119–29. doi: 10.1017/S0033291706007896. [DOI] [PubMed] [Google Scholar]
- 15.Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B. 1994;103:247–54. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- 16.Murphy M, Frodl T. Meta-analysis of diffusion tensor imaging (DTI) studies shows altered fractional anisotropy occurring in distinct brain areas in association with depression. [accessed 11 Oct. 2011];Biology of Mood & Anxiety Disorders. 2011 1:3. doi: 10.1186/2045-5380-1-3. Available: www.biolmoodanxietydisord.com/content/1/1/3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bae JN, MacFall JR, Krishnan KRR, et al. Dorsolateral prefrontal cortex and anterior cingulate cortex white matter alterations in late-life depression. Biol Psychiatry. 2006;60:1356–63. doi: 10.1016/j.biopsych.2006.03.052. [DOI] [PubMed] [Google Scholar]
- 18.Li L, Ma N, Li Z, et al. Prefrontal white matter abnormalities in young adult with major depressive disorder: a diffusion tensor imaging study. Brain Res. 2007;1168:124–8. doi: 10.1016/j.brainres.2007.06.094. [DOI] [PubMed] [Google Scholar]
- 19.Murphy CF, Gunning-Dixon FM, Hoptman MJ, et al. White-matter integrity predicts stroop performance in patients with geriatric depression. Biol Psychiatry. 2007;61:1007–10. doi: 10.1016/j.biopsych.2006.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nobuhara K, Okugawa G, Sugimoto T, et al. Frontal white matter anisotropy and symptom severity of late-life depression: a magnetic resonance diffusion tensor imaging study. J Neurol Neurosurg Psychiatry. 2006;77:120–2. doi: 10.1136/jnnp.2004.055129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimony JS, Sheline YI, D’Angelo G, et al. Diffuse microstructural abnormalities of normal appearing white matter in late life depression: a diffusion tensor imaging study. Biol Psychiatry. 2009;66:245–52. doi: 10.1016/j.biopsych.2009.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Q, Huang X, Hong N, et al. White matter microstructural abnormalities in late-life depression. Int Psychogeriatr. 2007;19:757–66. doi: 10.1017/S1041610207004875. [DOI] [PubMed] [Google Scholar]
- 23.Cullen KR, Klimes-Dougan B, Muetzel R, et al. Altered white matter microstructure in adolescents with major depression: a preliminary study. J Am Acad Child Adolesc Psychiatry. 2010;49:173–83. doi: 10.1097/00004583-201002000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor WD, MacFall JR, Boyd B, et al. One-year change in anterior cingulate cortex white matter microstructure: relationship with late-life depression outcomes. Am J Geriatr Psych. 2011;19:43–52. doi: 10.1097/JGP.0b013e3181e70cec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu C, Teng S, Wang P, et al. 2010 International Conference on Bioinformatics and Biomedical Technology (ICBBT) IEEE; 2010. A neuronal fiber tracking study for major depressive disorder using MR diffusion tensor imaging with fiber tractography; pp. 106–110. [Google Scholar]
- 26.Huang H, Fan X, Williamson DE, et al. White matter changes in healthy adolescents at familial risk for unipolar depression: a diffusion tensor imaging study. Neuropsychopharmacology. 2011;36:684–91. doi: 10.1038/npp.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joormann J, Gilbert K, Gotlib IH. Emotion identification in girls at high risk for depression. J Child Psychol Psychiatry. 2010;51:575–82. doi: 10.1111/j.1469-7610.2009.02175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamilton M. Standardised assessment and recording of depressive symptoms. Psychiatr Neurol Neurochir. 1969;72:201–5. [PubMed] [Google Scholar]
- 29.Beck AT, Steer RA, Brown GK. BDI-II Manual. San Antonio (TX): Harcourt Brace and Company; 1996. [Google Scholar]
- 30.Beck AT, Steer RA. Beck Anxiety Inventory Manual. San Antonio (TX): Psychological Corporation Harcourt Brace & Company; 1993. [Google Scholar]
- 31.Bernstein DP, Fink L, Handelsman L, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry. 1994;151:1132–6. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- 32.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP) New York (NY): Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 33.First MB, Gibbon M, Spitzer RL, et al. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II) Washington (DC): American Psychiatric Press, Inc; 1997. [Google Scholar]
- 34.Eysenck HJ, Eysenck SBG. Manual of the Eysenck Personality Scales (EPS Adult) London: Hodder and Stoughton; 1991. [Google Scholar]
- 35.Bernstein DP, Stein JA, Newcomb MD, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27:169–90. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- 36.Leemans A, Jones DK. The B-matrix must be rotated when correcting for subject motion in DTI data. Magn Reson Med. 2009;61:1336–49. doi: 10.1002/mrm.21890. [DOI] [PubMed] [Google Scholar]
- 37.Salimi-Khorshidi G, Smith SM, Nichols TE. Adjusting the effect of nonstationarity in cluster-based and TFCE inference. Neuroimage. 2011;54:2006–19. doi: 10.1016/j.neuroimage.2010.09.088. [DOI] [PubMed] [Google Scholar]
- 38.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 39.Wakana S, Caprihan A, Panzenboeck MM, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36:630–44. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leemans A, Jeurissen B, Sijbers J, et al., editors. ExploreDTI: a graphical toolbox for processing, analyzing, and visualizing diffusion MR data. 17th Annual Meeting of Intl Soc Mag Reson Med; Hawaii, USA. 2009. p. 3537. [Google Scholar]
- 41.Mori S, Kaufmann WE, Davatzikos C, et al. Imaging cortical association tracts in the human brain using diffusion-tensor-based axonal tracking. Magn Reson Med. 2002;47:215–23. doi: 10.1002/mrm.10074. [DOI] [PubMed] [Google Scholar]
- 42.Luck D, Buchy L, Czechowska Y, et al. Fronto-temporal disconnectivity and clinical short-term outcome in first episode psychosis: a DTI-tractography study. J Psychiatr Res. 2011;45:369–77. doi: 10.1016/j.jpsychires.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 43.Kvickström P, Eriksson B, van Westen D, et al. Selective frontal neurodegeneration of the inferior fronto-occipital fasciculus in progressive supranuclear palsy (PSP) demonstrated by diffusion tensor tractography. BMC Neurol. 2011;11:13. doi: 10.1186/1471-2377-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang H, Fan X, Williamson DE, et al. White matter changes in healthy adolescents at familial risk for unipolar depression: a diffusion tensor imaging study. Neuropsychopharmacology. 2011;36:684–91. doi: 10.1038/npp.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Co-morbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 46.Schmithorst VJ, Yuan W. White matter development during adolescence as shown by diffusion MRI. Brain Cogn. 2010;72:16–25. doi: 10.1016/j.bandc.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 47.Matthews SC, Strigo IA, Simmons AN, et al. A multimodal imaging study in U.S. veterans of Operations Iraqi and Enduring Freedom with and without major depression after blast-related concussion. Neuroimage. 2011;54(Suppl 1):S69–75. doi: 10.1016/j.neuroimage.2010.04.269. [DOI] [PubMed] [Google Scholar]
- 48.Sapolsky RM, Krey LC, McEwen BS. Glucocorticoid-sensitive hippocampal neurons are involved in terminating the adrenocortical stress response. Proc Natl Acad Sci U S A. 1984;81:6174–7. doi: 10.1073/pnas.81.19.6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry. 2004;161:195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- 50.Krishnan V, Han MH, Graham DL, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 51.Taliaz D, Loya A, Gersner R, et al. Resilience to chronic stress is mediated by hippocampal brain-derived neurotrophic factor. J Neurosci. 2011;31:4475–83. doi: 10.1523/JNEUROSCI.5725-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frodl T, Schule C, Schmitt G, et al. Association of the brain-derived neurotrophic factor Val66Met polymorphism with reduced hippocampal volumes in major depression. Arch Gen Psychiatry. 2007;64:410–6. doi: 10.1001/archpsyc.64.4.410. [DOI] [PubMed] [Google Scholar]
- 53.Concha L, Gross DW, Beaulieu C. Diffusion tensor tractography of the limbic system. AJNR Am J Neuroradiol. 2005;26:2267–74. [PMC free article] [PubMed] [Google Scholar]
- 54.D’Esposito M, Verfaellie M, Alexander MP, et al. Amnesia following traumatic bilateral fornix transection. Neurology. 1995;45:1546–50. doi: 10.1212/wnl.45.8.1546. [DOI] [PubMed] [Google Scholar]
- 55.Schmahmann JD, Pandya DN. The complex history of the fronto-occipital fasciculus. J Hist Neurosci. 2007;16:362–77. doi: 10.1080/09647040600620468. [DOI] [PubMed] [Google Scholar]
- 56.Kier EL, Staib LH, Davis LM, et al. MR imaging of the temporal stem: anatomic dissection tractography of the uncinate fasciculus, inferior occipitofrontal fasciculus, and Meyer’s loop of the optic radiation. AJNR Am J Neuroradiol. 2004;25:677–91. [PMC free article] [PubMed] [Google Scholar]
- 57.Kieseppä T, Eerola M, Mantyla R, et al. Major depressive disorder and white matter abnormalities: a diffusion tensor imaging study with tract-based spatial statistics. J Affect Disord. 2010;120:240–4. doi: 10.1016/j.jad.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 58.Cullen KR, Klimes-Dougan B, Muetzel R, et al. Altered white matter microstructure in adolescents with major depression: a preliminary study. J Am Acad Child Adolesc Psychiatry. 2010;49:173–83. doi: 10.1097/00004583-201002000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Phillips ML, Drevets WC, Rauch SL, et al. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol Psychiatry. 2003;54:515–28. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 60.Preuss TM, Goldman-Rakic PS. Connections of the ventral granular frontal cortex of macaques with perisylvian premotor and so-matosensory areas: anatomical evidence for somatic representation in primate frontal association cortex. J Comp Neurol. 1989;282:293–316. doi: 10.1002/cne.902820210. [DOI] [PubMed] [Google Scholar]
- 61.Makris N, Kennedy DN, McInerney S, et al. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex. 2005;15:854–69. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- 62.Tomoda A, Sheu YS, Rabi K, et al. Exposure to parental verbal abuse is associated with increased gray matter volume in superior temporal gyrus. Neuroimage. 2011;54(Suppl 1):S280–6. doi: 10.1016/j.neuroimage.2010.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paul R, Henry L, Grieve SM, et al. The relationship between early life stress and microstructural integrity of the corpus callosum in a non-clinical population. Neuropsychiatr Dis Treat. 2008;4:193–201. doi: 10.2147/ndt.s1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eluvathingal TJ, Chugani HT, Behen ME, et al. Abnormal brain connectivity in children after early severe socioemotional deprivation: a diffusion tensor imaging study. Pediatrics. 2006;117:2093–100. doi: 10.1542/peds.2005-1727. [DOI] [PubMed] [Google Scholar]
- 65.Choi J, Jeong B, Rohan ML, et al. Preliminary evidence for white matter tract abnormalities in young adults exposed to parental verbal abuse. Biol Psychiatry. 2009;65:227–34. doi: 10.1016/j.biopsych.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sullivan RM. Hemispheric asymmetry in stress processing in rat prefrontal cortex and the role of mesocortical dopamine. Stress. 2004;7:131–43. doi: 10.1080/102538900410001679310. [DOI] [PubMed] [Google Scholar]
- 67.Perez-Cruz C, Simon M, Czeh B, et al. Hemispheric differences in basilar dendrites and spines of pyramidal neurons in the rat pre-limbic cortex: activity- and stress-induced changes. Eur J Neurosci. 2009;29:738–47. doi: 10.1111/j.1460-9568.2009.06622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McBeth J, Morris S, Benjamin S, et al. Associations between adverse events in childhood and chronic widespread pain in adulthood: Are they explained by differential recall? J Rheumatol. 2001;28:2305–9. [PubMed] [Google Scholar]
- 69.Vinberg M, Mellerup E, Andersen PK, et al. Variations in 5-HTTLPR: relation to familiar risk of affective disorder, life events, neuroticism and cortisol. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:86–91. doi: 10.1016/j.pnpbp.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 70.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 71.Alexander AL, Lee JE, Lazar M, et al. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316–29. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]