Abstract
Many bacteria-associated polysaccharides induce long-lived antibody responses that protect against pathogenic microorganisms. The maintenance of polysaccharide-specific antibody titers may be due to long-lived plasma cells or ongoing antigen-driven B cell activation due to polysaccharide persistence. BALB/c and VHJ558.3 transgenic (TG) mice respond to α 1→3-dextran (DEX) by generating a peak anti-DEX response at 7 days, followed by maintenance of serum antibody levels for up to 150 days. Analysis of the cellular response to DEX identified a population of short-lived, cyclophosphamide sensitive DEX-specific plasmablasts in the spleen, and a quiescent, cyclophosphamide resistant DEX-specific antibody-secreting population in the bone marrow. BrdU pulse-chase experiments demonstrated the longevity of the DEX-specific antibody-secreting population in the bone marrow. Splenic DEX-specific plasmablasts were located in the red pulp with persisting DEX-associated CD11c+ dendritic cells 90 days after immunization, whereas DEX was not detected in the bone marrow after 28 days. Selective depletion of short-lived DEX-specific plasmablasts and memory B1b B cells using cyclophosphamide and anti-CD20 treatment had a minimal impact on the maintenance of serum anti-DEX antibodies. Collectively, these findings demonstrate that the maintenance of serum polysaccharide-specific antibodies is the result of continuous antigen-driven formation of short-lived plasmablasts in the spleen and a quiescent population of antibody-secreting cells maintained in the bone marrow for a long duration.
Introduction
Plasma cells are the terminal differentiated progeny of B lymphocytes activated by antigen or mitogens. It is becoming increasingly clear that plasma cells are not only the end stage of B cell differentiation, but also constitute a separate cell compartment accounting for serologic memory to protein and viral-based vaccines (1, 2).
Plasma cell differentiation is driven by the increased expression of Blimp-1, which is associated with plasmablasts exiting cell cycle (3, 4), chemokine changes promoting their migration into the bone marrow (5-7), and down regulation of co-stimulatory molecules along with their surface Ig (1, 4). Mature plasma cells can be divided into short and long-lived populations. Short-lived plasma cells can be generated by both T cell dependent and independent mechanisms, while long-lived plasma cell development has mostly been studied in antibody responses dependent upon T cell help (8).
Maintenance of both plasmablasts and short-lived plasma cells appears to depend upon ongoing inflammatory conditions (9), whereas long-lived plasma cells are maintained under non-inflammatory conditions in the bone marrow (1, 2). It has been clearly shown in humans and in mice that long-lived plasma cells (1, 2) are quiescent, persistent and produce antibody in the absence of antigen leading some to coin the term ‘plasma cell memory’ to describe their function (10). More recently it has been shown that homeostasis of long-lived plasma cells is not dependent upon memory B cells indicating that this population constitutes an independent compartment responsible for serologic memory (11).
In mice and humans the persistence of polysaccharide-specific antibody production in the spleen (12-15) has led to the suggestion that polysaccharides, like T cell dependent antigens have the ability to generate long-lived plasma cells (9). However, it is unclear whether plasmablasts generated in response to polysaccharide antigens possess the capacity to migrate into the bone marrow and become long-lived plasma cells similar to their T cell dependent counterparts (16).
Alternatively, maintenance of anti-polysaccharide antibody serum antibody titers may result from continuous antigen-dependent stimulation of B cells. It is known that bacteria-associated polysaccharides persist in tissues of mice and humans for long periods of time after bacterial infection or deliberate immunization with polysaccharide. This persistence may result from their polymeric nature and absence of host glycolytic enzymes capable of degrading them (17-20). Antibody secreting cells generated in response to the synthetic polysaccharide NP-Ficoll are actively dividing within the spleen even at late stages in the persisting antibody response (14, 21) arguing for an important role for NP-Ficoll persistence in driving an ongoing antibody response (19).
A recent report showed that mice immunized with type 3 pneumococcal polysaccharide (PSIII) generated a functionally distinct population of radiation resistant plasma cells responsible for maintenance of polysaccharide-specific antibody titers independent of memory B1b B cells. These plasma cells provided serologic protection against Streptococcus pneumoniae infection and appeared to persist in the bone marrow for the duration of antibody production analyzed (22). These findings have been complemented by a recent publication demonstrating a role for IgM producing, bone marrow antibody-secreting cells in providing long term protection to Ehrlichia muris infection (23). However, it is not entirely clear from these studies whether the maintenance of long-lived IgM dominated antibody responses to polysaccharides and bacterial outer membrane proteins results from a continual response to persistent antigen or is established in a manner similar to the conventional long-lived plasma cell pool considered to be independent of persisting antigen stimulation.
To examine the role of polysaccharide persistence and turn-over of polysaccharide-specific plasma cells in long-lived antibody production we characterized cellular aspects of the antibody response to epitopes formed by α 1→3-glucosidic linkages in bacteria and α 1→3-dextran (DEX), a large molecular weight glucan. This or a similar epitope is present on a variety of clinically relevant organisms including the opportunistic pathogen Enterobacter cloacae (24), fungi including the dimorphic fungal pathogen Histoplasma capsulatum (25) and the commensal fungus Aspergillus fumigatus (Dizon and Kearney, unpublished results), as well as the epiphytic bacterial species Leuconostoc mesenteroides (26). Both purified DEX and DEX-expressing E. cloacae induced prolonged production of IgM, DEX-specific antibodies, which was largely independent of persisting DEX-driven plasmablast generation occurring in the spleen. Instead the response was maintained by a novel population of cyclophosphamide and anti-CD20 resistant antibody-secreting cells that persisted for a lengthened duration in the bone marrow throughout the antibody response. These results show that the long-lived antibody response observed in these experiments is partly attributed to the generation of a quiescent, antigen independent antibody-secreting population capable of maintenance of long lasting IgM specific antibody response to polysaccharide antigens.
Materials and Methods
Animals
VH J558 transgenic mice were generated as described previously (27) and backcrossed onto the C57BL/6 background for more than 10 generations. Female BALB/c, Athymic BALB/c Foxn1nu/J and C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). All mice were bred and housed in specific pathogen free facilities according to rules and regulations established by the University of Alabama at Birmingham animal resource program.
Immunizations
8 to 16 wk old VHJ558 transgenic and 8 to 16 week old female BALB/c or athymic BALB/c mice were immunized intravenously at day 0 with either 50 ug of purified α 1→3-dextran (B1355S), 108 heat killed, paraformaldehyde fixed Enterobacter cloacae strain MK7 (24) or DEX-negative strain cBAN (a gift from A.J. MacPherson, University of Bern, 3013 Bern, Switzerland). Mice were injected IP with 35mg/kg of cyclophosphamide (Sigma-Aldrich, St. Louis, MO) (28) on days 31-34 post E. cloacae strain MK7 immunization. At 30 days post E. cloacae strain MK7 immunization mice were given weekly injections of anti-mouse CD20 (a gift from Dr. Robert Dunn, Biogen Idec, San Diego, CA) or an IgG2a isotype matched control at a concentration of 20 mg/kg for 3 consecutive weeks. At appropriate time points mice were sacrificed and blood and tissues were harvested for ELISA, ELISpot, FACS, and histological analysis.
Flow cytometry
FITC labeled, anti-CD4, anti-Ki67, PE-labeled anti-mouse IgM, anti-Mac 1, anti-CD19, anti-CD80, anti-CD86, anti-MHCII, rat IgG2aK, biotin labeled anti-Mac-1, anti-CD11c, anti-CD16/CD32, Annexin V Cy5, 7AAD, and PE-Cy7 labeled anti-B220 were purchased from Pharmingen (San Diego, CA). Goat anti-mouse IgM Cy5 was purchased from Jackson Immunoresearch Inc. (West Grove, PA). Goat anti-mouse IgM PE and purified goat anti-mouse IgM, which was conjugated to Pacific Blue (Invitrogen Corporation, Carlsbad, CA), were purchased from SBA (Birmingham, AL). PE anti-CD21 (clone 7G6) was described previously (29). Anti-DEX (IgMλ, clone 1-21), anti-λ, (clone JC5-1), TD6-4 and EB3-7-2, both specific for the anti-DEX idiotype (30) were all generated in our laboratory and directly conjugated to Alexa 488, 647, and Pacific Blue dyes (Molecular Probes, Invitrogen Corporation, Carlsbad, CA). α 1→3-dextran (gift from Dr. Allene Jeanes) was conjugated to Alexa 488 (Molecular Probes, Invitrogen Corporation, Carlsbad, CA). Heat killed, paraformaldehyde fixed Enterobacter cloacae strains MK7 (DEX+) and cBAN (DEX−) were conjugated to Alexa 647 dye (Molecular Probes, Invitrogen Corporation, Carlsbad, CA). All 5-7 color FACS analysis were performed on a BD LSRII flow cytometer (BD Biosciences, Mountain View, CA) and analyzed using FlowJo software (Tree Star, Inc, Ashland, OR).
Dex Elisa
Sera collected from VHJ558 transgenic and BALB/c mice immunized with each antigen were assayed for the presence of α 1→3-DEX specific antibodies using a previously established protocol (24). Briefly, serum diluted 1/1000 was incubated for 2 hrs at 37°C on 96 well EIA/RIA plates (Costar, Corning, NY) previously coated overnight at 4°C with 1 ug/ml of purified α 1→3-dextran. Plates were washed and developed with goat anti-mouse λ AP for 2 hours at 37°C. Concentrations of DEX-specific antibodies were quantified by ELISA using purified J558 Id+ IgM antibody (clone 1-21) as a reference standard.
ELISpot Analysis
Numbers of DEX-specific antibody secreting cells (ASC's) in the spleen and bone marrow encompassing cells prepared from two femurs and tibia per mouse utilized in these experiments were determined by the following method. (i) First purified DEX or goat anti-mouse IgG, IgA, or IgM antibody was coated overnight at 4°C on EIA/RIA plates with high binding, certified surface chemistry (Costar, Corning, NY) at a concentration of 2 ug/ml and (ii) then blocked for 1hr at 37°C with 1% gelatin solution prepared in PBS. (iii) Single cell suspensions were prepared in RPMI (Gibco, Invtrogen Corporation, Carlsbad, CA) supplemented with 10% FCS (Hyclone Laboratories Inc, Logan, Utah) from individual BALB/c mice sacrificed at 0, 7, 14, 28, 60 days after immunization with each antigen as described above. (iv) Large numbers ranging from 10 – 15 × 106 bone marrow or splenic cells were added to the first well and then diluted 5-fold in RPMI-C with 5% FCS to generate quantifiable spots. (v) Plates were then incubated overnight at 37°C and then (vi) washed with 1% Tween in PBS 3X the following day to remove cells. After washing (vii) plates were incubated with either anti-λ, anti-IgA, anti-IgG1, anti-IgG2a, anti-IgG2b, anti-IgG3, or anti-IgM alkaline phosphatase conjugates purchased from SBA (Birmingham, AL) diluted 1/500 with 1% Tween in PBS at 37°C for 2 hours. Plates were (viii) washed with 1% Tween in PBS 3X and (viiii) developed for 4 to 12 hours at 37°C in substrate buffer pH 10.25 containing 1M 2-amino-2-methyl-1-propanol (AMP) (Sigma-Aldrich, St. Louis, MO), 0.1% Triton-X405 (Fluka, Sigma-Aldrich), 0.01g/ml of 5-bromo-4-chloro-3-indolyl-phosphate (BCIP) (Sigma-Aldrich, St. Louis, MO). (x) The next day plates were washed in dH20, dried and the spots were counted. Total numbers of DEX-specific spots were calculated by multiplying the spot frequency by total cell numbers.
Immunofluorescence analysis of tissue sections
Tissue sections were processed and viewed as described previously (31). Frozen sections were stained with anti-Moma-1 (Rat, IgG2aκ, a gift from Dr. George Kraal, VU University, Amsterdam, The Netherlands) and developed with goat anti-rat IgG Alexa 350 or 647 (Molecular Probes, Invitrogen Corporation, Carlsbad, CA), then blocked with normal rat serum (Peel-Freeze, Rogers, AR), and stained with Alexa 555 labeled TD6-4, which is specific for the anti-DEX idiotype (30), and CD4 FITC. To determine DEX persistence in BALB/c mice frozen tissue sections were stained with Alexa 488 labeled DEX-specific, IgMλ (clone 1-21) and anti-CD19 PE. Frozen sections from BALB/c mice immunized 90 days prior with DEX were stained with anti-DEX 488, goat anti-lambda PE, and either biotinylated anti-CD11c or anti-Mac-1, which were developed with SA-Alexa 350. To detect dividing plasmablasts in E. cloacae immunized TG mice, frozen sections were stained with anti-Ki67 FITC (Pharmingen, BD, San Diego, CA) and TD6-4 Alexa 555.
Statistics
Data from three or more groups were analyzed by a one-way ANOVA test for data with normal distribution and Kruskal-Wallis test for data that did not distribute normally. Data with two groups were analyzed by a two-tailed unpaired t test to determine if statistically significant differences existed. Statistically significant results were determined by a p value of <0.01 (*) or <0.01 (**) or <0.001 (***).
BrdU pulse-chase assay
BALB/c mice were immunized i.v. with E. cloacae as above and were subsequently fed BrdU (Sigma Aldrich, St. Louis, MO) in their drinking water at a concentration of 1 mg/mL for seven days. Mice were then sacrificed 28 days after E. cloacae injection and incorporation of BrdU in DEX-specific ASCs was evaluated by flow cytometry using the BD BrdU APC kit (San Diego, CA).
Results
The DEX-specific antibody response is long lived
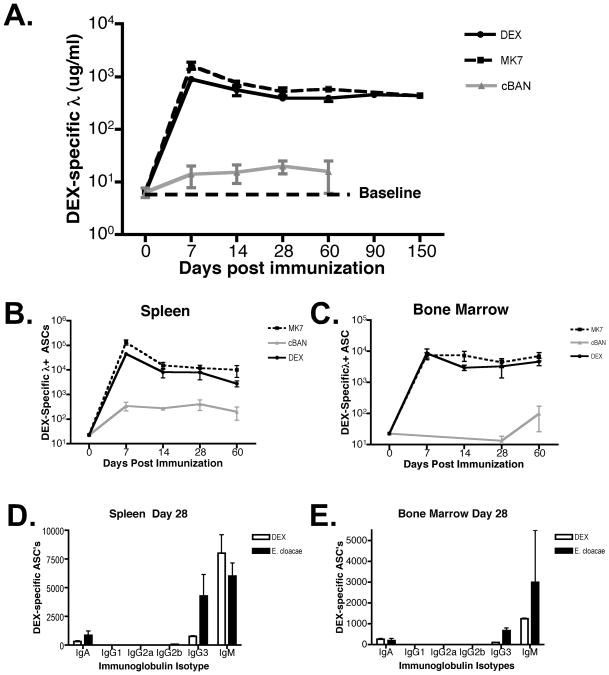
To determine the kinetics of the DEX-specific antibody response BALB/c mice were immunized with soluble DEX, DEX-expressing E. cloacae strain MK7 or the DEX-negative strain cBAN. DEX-specific serum antibody titers and total numbers of ASC's were determined by ELISA and ELISpot analysis, respectively, at 0, 7, 14, 28, 60, 90, and 150 days after immunization. Mice challenged with soluble DEX or E. cloacae, strain MK7, but not strain cBAN generated DEX-specific antibody (Figure 1A) and DEX-specific ASC's that were stable in number for at least 150 days (Figure 1B, C and data not shown). VHJ558 transgenic mice (TG) generated a similar antibody response to these forms of DEX that persisted throughout the duration of analysis (data not shown). Ig isotype switching in DEX-specific antibody-secreting cells (ASCs) 28 days after DEX or E. cloacae strain MK7 challenge of BALB/c mice was limited to IgG3 and IgA isotypes as determined by ELISpot consistent with patterns seen in the peak serum primary antibody responses. However the proportion of IgG3 in the spleen was higher at 28 days in E. cloacae strain MK7 vs. DEX challenged mice (Figure 1D).
Figure 1. DEX-specific antibody production in BALB/c mice is long-lived.
(A) Concentration of serum DEX-specific λ+ antibodies in BALB/c mice immunized with DEX, E. cloacae strain MK7 (DEX positive) and strain cBAN (DEX negative). The dotted line defines baseline DEX-specific antibodies in the serum of naïve BALB/c mice. (B) Total numbers of λ+ DEX-specific ASCs in BALB/c mice immunized with purified DEX, E. cloacae strain MK7 or strain cBAN in the (B) spleen and (C) bone marrow. The number of DEX-specific ASCs in the bone marrow was calculated from total BM cells harvested from two femur and tibia per mouse. 10 mice per group were utilized for data derived from experiments A-C. DEX-specific ASCs display restricted isotype usage, 28 days after immunization with DEX or E. cloacae strain MK7 in the (D) spleen and (E) bone marrow. The data from D and E is derived from two independent experiments involving 5 mice per group.
Isotype switching to IgG3 and IgA in response to DEX has been largely characterized as T cell independent (24). Examination of the expression of germinal center B cell antigens Fas receptor and GL-7 in BALB/c mice 14 days after immunization with E. cloacae strain MK7 confirmed that responding DEX-specific J558 idiotype+ B cells did not express either cell surface antigen at 14 days or at any other point in the response to E. cloacae strain MK7 in both BALB/c and TG mice (Figure S1A-B and data not shown). The ability to mount an effective DEX-specific antibody response in the absence of T cells was confirmed by immunizing athymic BALB/c mice with E. cloacae strain MK7. Both BALB/c and athymic BALB/c mice produced equivalent amounts of IgM DEX-specific antibodies in response to E. cloacae strain MK7 immunization (Figure S1C). Furthermore, titers of serum DEX-specific IgG3 and IgA antibodies were equivalent in BALB/c and athymic mice indicating that these responses are also T cell independent (data not shown).
These findings show that DEX-specific antibody production in response to either purified DEX or E. cloacae strain MK7 was sustained over 150 days by ASCs in the bone marrow and spleen of both BALB/c and TG mice in the absence of T cells.
DEX-specific IgM secreting cells maintain a plasmablast surface phenotype throughout the response to DEX
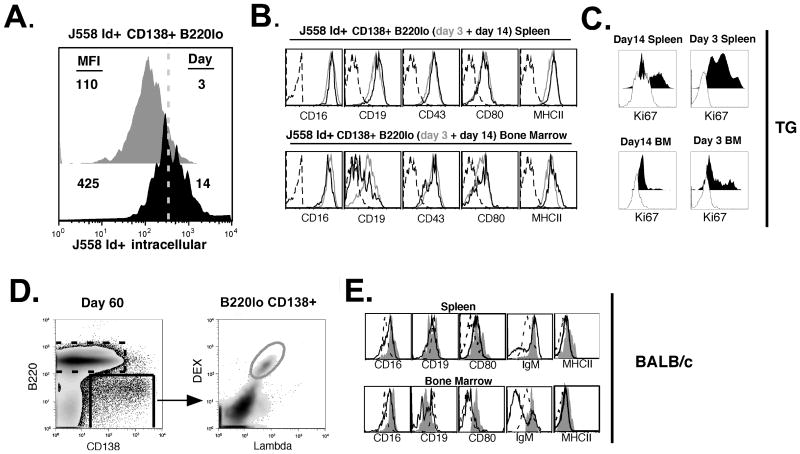
The prolonged DEX-specific antibody response could be mediated by: i) continuous generation of plasmablasts/plasma cells in response to persisting antigen; and/or ii) long-lived plasma cells. To determine the phenotype of DEX-specific plasma cells we compared intracellular IgM expression and the cell surface markers expressed on J558 Id+ CD138+ B cells at days 3 and 14 after immunization of TG mice. Consistent with a transition from plasmablasts to more mature antibody secreting cells, splenic J558 Id+ CD138+ B cells displayed greater intracellular IgM expression at 14 days compared to 3 days post-immunization with E. cloacae strain MK7 (Figure 2A). However, at each time point, these cells, isolated from the spleen or bone marrow, maintained high levels of MHCII, CD80, CD43 and CD16. The only exception was that the majority of J558 Id+ CD138+ B cells isolated from the bone marrow 14 days after E. cloacae strain MK7 challenge expressed lower levels of CD19 (Figure 2B) and Ki67 (Figure 2C). The equivalent population in the spleen expressed much higher frequency of Ki67+ cells (Figure 2C) with the plasmablast surface phenotype maintained in both Ki67+ and Ki67 negative subpopulations (data not shown).
Figure 2. Mature DEX-specific plasma cells in VHJ558.3 TG and BALB/c mice maintain a plasmablast phenotype.
(A) Comparison of DEX-specific CD138+ B cells isolated from TG mice shows that larger amounts of intracellular DEX-specific IgM are found at 14 days (black histogram) after E. cloacae strain MK7 immunization than at 3 days (grey histogram). (B) Cell surface phenotypes of DEX-specific CD138+ cells detected with antibodies specific for CD16, CD19, CD43, CD80, and MHCII at 3 days (grey histogram) and 14 days (black histogram) after immunization, compared to an isotype matched control (dashed black histogram). (C) Ki67 expression by DEX-specific CD138+ cells 14 days and 3 days after immunization in the spleen (top panel) and the bone marrow (lower panel). Dashed histogram is that of the negative control sample. (D) Gating strategy to analyze DEX-specific λ+ CD138+ B cells generated in the spleen and bone marrow of BALB/c mice in response to E. cloacae strain MK7 immunization. (E) Phenotypes of B220 lo CD138+ λ+ DEX-binding B cells (solid grey histogram) at 60 days post immunization of BALB/c mice compared to bulk B220lo CD138+ B cells (dashed black histogram), and bulk B220+ CD138- B cells (black histogram). Data shown is representative of 5 VHJ558.3 TG and 5 BALB/c mice.
To extend these findings, we examined the cell surface phenotype of DEX-specific CD138+ B cells isolated from the bone marrow and spleens of BALB/c mice 2 months after a single immunization with E. cloacae strain MK7 (Figure 2D). Even at this late time point the majority of DEX-specific CD138+ B cells retained surface expression of IgM (≥ 80%) and displayed a plasmablast cell surface phenotype similar to J558 Id+ CD138+ B cells isolated from spleen and bone marrow of TG mice (Figure 2E). The IgM negative DEX-specific CD138+ B cell expressed either surface IgA or IgG3 (data not shown) correlating with the isotypes detected by ELISpot and serum analyses.
An issue of concern in this flow cytometry based analysis in BALB/c mice is the possibility of skewing our analysis to immature DEX-specific Ig secreting cells maintaining surface Ig and missing those which have down regulated surface Ig expression. To ensure that the frequency of plasma cells determined by FACS correlated with the number of ASCs measured by ELISpots, we compared the numbers of DEX+ λ+ CD138+ B cells with DEX-specific ASCs. This comparison showed no statistical differences between the two populations indicating that secretory DEX-specific plasma cells retained surface immunoglobulin (Figure S2A). Intracellular staining with anti-J558 Id+ (TD6-4) to identify anti-DEX secreting plasma cells in the spleen and bone marrow confirmed their retention of surface immunoglobulin and CD138 expression (Figures S2B and C).
These findings suggest that long lasting production of anti-DEX antibodies is maintained by DEX-specific IgM secreting cells within the bone marrow and spleen that retain expression of surface molecules present on activated B cells and plasmablasts, despite displaying high levels of intracellular immunoglobulin. Because of the difficulty of assigning these two mature stages of B cell differentiation to a plasmablast or a plasma cell, we will refer to these cells in this manuscript as antibody-secreting cells (ASCs).
DEX-specific plasmablasts proliferate in the spleen
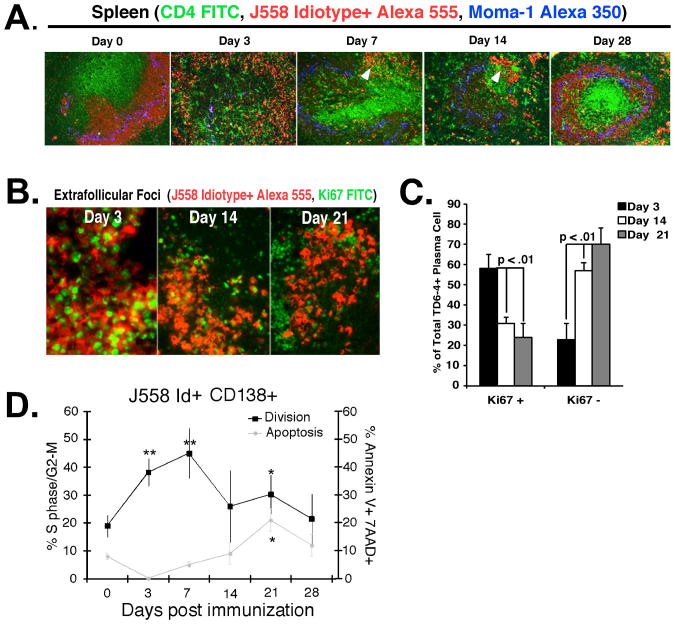
To reconcile the plasmablast-like phenotype of DEX-specific ASCs with the longevity of the antibody response, we characterized the cellular turnover of DEX-specific plasmablasts in TG mice immunized with E. cloacae strain MK7. As in other MZ B cell dominated responses, antigen responsive J558 Id+ B cells moved from the MZ to the T-B border after IV immunization (Figure 3A), giving rise to peak numbers of Ki67+ plasmablasts at 3 days (Figures 3B and C). From days 7-14 plasmablasts matured to bright IgM staining cells in the extrafollicular foci and were maintained concurrent with recovery of the MZ B cell compartment at 28 days post E. cloacae strain MK7 immunization (Figure 3A). 60 – 70% of J558 Id+ cells at 14 and 21 days post-immunization were negative for Ki67 expression (Figure 3B and C). This transition from Ki67+ to Ki67 negative IgM secreting cells was accompanied by an increased rate of apoptosis (Figure 3D). These findings demonstrate a concurrent decrease, though not absence of, cell division and an increase in intracellular IgM expression in splenic J558 Id+ plasmablasts beginning 1 week post E. cloacae strain MK7 immunization.
Figure 3. Extrafollicular J558 Id+ CD138+ early proliferation is balanced by apoptosis later in the response to E. cloacae.
(A) Spleen sections from VHJ558 TG mice immunized with E. cloacae strain MK7 were harvested at 0, 3, 7, 14, and 28 days after immunization and stained with antibodies specific for DEX-specific B cells bearing the J558 idiotype (red), CD4+ T cells (green), and metallophilic macrophages (blue) revealing the presence of J558 idiotype high plasma cells within extrafollicular foci in the red pulp (white arrows). (B) Many more extrafollicular J558 Id+ CD138+ B cells express Ki67 at 3 days compared to 21 days after immunization with E. cloacae. Sections of spleen from VHJ558 TG mice immunized with E. cloacae strain MK7 and stained with Ki67-FITC (green) and anti-J558 Id (red). (C) Ki67+ J558 Id+ plasmablasts in extrafollicular foci at 3, 14, and 21 days after E. cloacae strain MK7 immunization. Numbers were calculated by counting Ki67 staining cells in 5 individual extrafollicular foci per 3 mice. (D) DEX-specific plasmablast homeostasis is balanced by proliferation and apoptosis. Analysis of division and apoptosis of J558 Id+ CD138 + B cells using intracellular PI cell to determine cycle status (black line; left y axis) and surface annexin V+/7AAD+ staining to determine late apoptotic cells (grey line; right y axis). Data shown is from 3 independent experiments (n= 3 mice per experiment). Asterisks show statistical significance in comparison to the day 0 time point.
Accumulation of a quiescent population of DEX-specific ASCs in the bone marrow in response to E. cloacae strain MK7 challenge
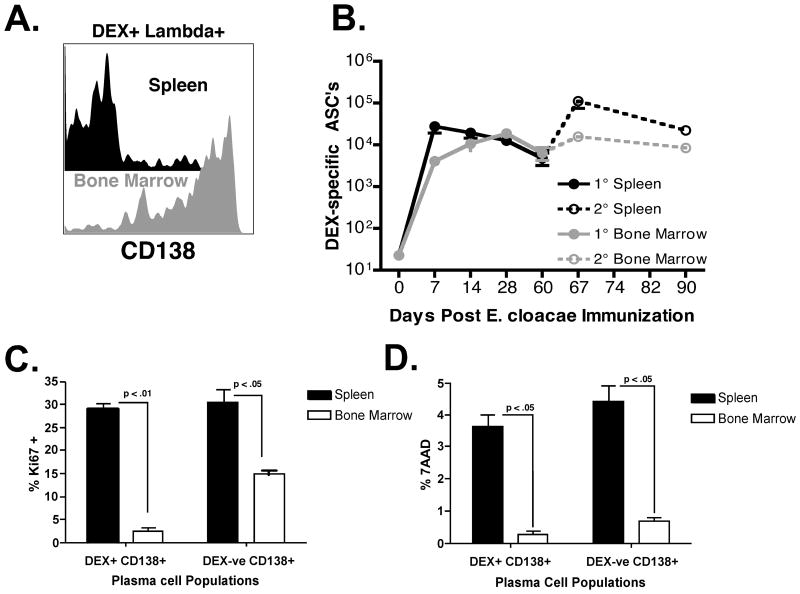
Initial analysis of DEX-specific ASC numbers by FACS and ELISpot demonstrated an accumulation of DEX-specific ASCs over 28 days within the bone marrow in contrast to the sharp reduction seen in the spleen of BALB/c mice a week post immunization (Figure 1). CD138 expression on DEX+ λ+ B cells in the spleen and bone marrow 60 days post immunization was examined to determine the relative proportions of DEX-specific ASCs present in each tissue. A minor population of DEX+ λ+ B cells in the spleen continued to express CD138 (∼15 – 30%), which contrasted with bone marrow DEX+ λ+ B cells, which maintained expression of high levels of CD138 (>80%) confirming selective accumulation of DEX-specific CD138+ B cells in the bone marrow versus the spleen (Figure 4A). DEX-specific ASC numbers in the bone marrow remained stable throughout the duration of the primary and secondary antibody response to E. cloacae strain MK7 (Figure 4B). This contrasted with the expansion and contraction of DEX-specific ASCs in the spleen (Figure 4B) consistent with previous studies indicating that this is a major site for naïve and memory DEX-driven B cell expansion occurring during after primary and secondary E. cloacae strain MK7 challenge (27).
Figure 4. A quiescent population of DEX+ λ+ CD138+ ASCs accumulate in the bone marrow and display increased intracellular immunoglobulin expression.
(A) The expression of CD138 on surface positive DEX+ λ+ B cells in the spleen (black histogram) and bone marrow (grey histogram) 60 days after E. cloacae strain MK7 immunization. Data is representative of 5 mice. (B) DEX-specific λ+ ASC numbers were measured in the spleen after primary (black line, closed circles, E. cloacae strain MK7 injected on day 0) and secondary (dashed black line, open circles, E. cloacae strain MK7 re-challenge on day 60) and bone marrow after primary (grey line, closed circles, E. cloacae strain MK7 injected on day 0) and secondary (dashed grey line, open circles, E. cloacae strain MK7 injected on day 60) immunization; n= 5 mice per time point. (C) Proportions of Ki67 positive DEX+ λ+ CD138+ and DEX negative CD138+ B cells in the spleen (black bars) and bone marrow (white bars) 60 days post E. cloacae strain MK7 immunized BALB/c mice. (D) Proportions of 7AAD positive DEX+ λ+ CD138+ and DEX negative CD138+ B cells in the spleen (black bars) and bone marrow (white bars) in 60 days post E. cloacae strain MK7 immunized BALB/c mice. Proportions shown for (C) and (D) were calculated from 5 mice.
The accumulation and maintenance of a stable pool of DEX-specific IgM secreting ASCs in the bone marrow suggested that there were differences in population turnover between the bone marrow and splenic ASC populations. We therefore compared the proliferation and life span of DEX+ λ+ CD138+ B cells 60 days post E. cloacae strain MK7 immunization of BALB/c mice. Approximately 30% of splenic DEX+ CD138+ B cells were Ki67+, whereas >95% of DEX+ CD138+ B cells in the bone marrow were Ki67 negative. These results combined with the stability of the DEX-specific ASC population size in the bone marrow indicate that the majority of DEX+ CD138+ B cells in the bone marrow were not proliferating (Figures 4C and data not shown). Analysis of apoptosis using a comparison of the frequency of late apoptotic cells by 7AAD staining showed that there were ∼10 fold more DEX-specific ASCs in the spleen displaying evidence of late apoptosis than those residing in the bone marrow (Figure 4D).
Collectively our data show that E. cloacae strain MK7 immunization establishes a continuous pool of proliferating DEX-specific plasma cell progenitors in the spleen and a quiescent pool of DEX-specific plasma cells in the bone marrow that develop early and are maintained throughout the antibody response.
The bone marrow harbors a signficant population of long-lived DEX-specific ASCs 28 days after E. cloacae immunization
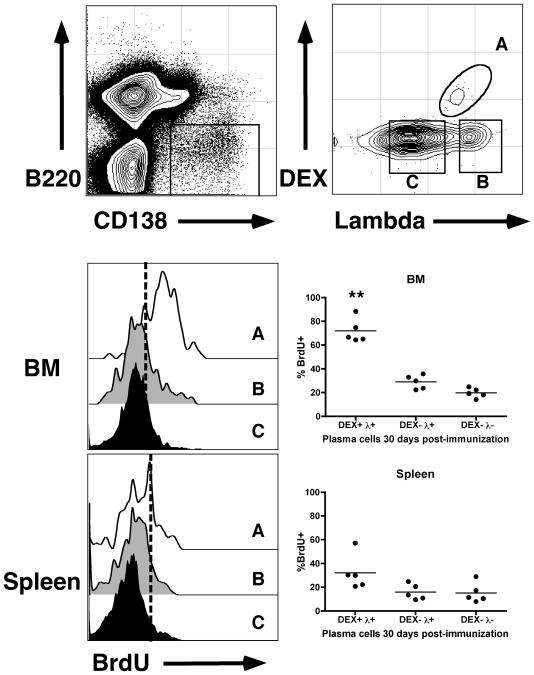
The prolonged capacity of DEX-specific ASCs in the bone marrow to generate antibody suggested that they are long-lived. To demonstrate the prolonged life span of DEX-specific ASCs we performed BrdU pulse-chase experiments. E. cloacae-immunized mice were pulsed with BrdU in their drinking water during the peak of the response (for 7 days after immunization) and then switched to normal drinking water for 3 more weeks. Flow cytometry analysis showed that a significantly larger proportion of DEX-specific ASCs in the bone marrow labeled with BrdU compared to those in the spleen (Figure 5). These results extend our findings that DEX-specific ASCs in the bone marrow are quiescent and have a low turnover rate in comparison to those in the spleen that are continously responding to persisting antigen.
Figure 5. DEX-specific ASCs in the bone marrow are long-lived.
Immunized BALB/c mice were fed BrdU in drinking water for 7 days after injection. 4 weeks after injection, mice were sacrificed and levels of BrdU incorporation were determined. Top panel shows the electronic gates used to determine DEX-specific ASCs (Gate A), DEX negative Lambda light chain + ASCs (Gate B) and DEX and Lambda light chain double negative ASCs (Gate C). Middle and lower panels show representative histograms and percent BrdU staining positively in each of the three populations A, B and C in the bone marrow and spleen respectively. Dashed lines in the histograms determine background BrdU staining based on negative controls. Asterisks show statistical significance in comparison to DEX+ λ+ ASCs in the spleen.
DEX persists in the splenic red pulp in association with CD11c+ dendritic cells and antigen specific plasma cells
The phenotype and turnover of spleen DEX-specific ASCs in immunized BALB/c and TG mice suggested that persisting antigen may be responsible for driving the production of dividing plasmablasts. We therefore examined the persistence of DEX in the spleen and bone marrow by FACS and histological analyses. As expected, DEX localized in the MZ associated with MZ B cells and MZ macrophages 30 minutes after i.v. immunization of BALB/c mice (Figure 6A). DEX was later found predominantly in the red pulp where it remained detectable for the duration (7 – 90 days) of the antibody response (Figure 6A). At 90 days after injection, DEX was localized in the extrafollicular foci (Figure 6B) in association with CD11c+ dendritic cells and DEX-specific ASCs (blue green stain) (Figure 6C, arrows). In contrast, at 28 days post injection DEX was not detected in the bone marrow compared to the spleen (data not shown). DEX persistence after E. cloacae strain MK7 immunization was not observed using similar detection methods. However, Alexa 647 labeled bacteria were detected in the spleen and bone marrow for up to at least 5 days post immunization in a pattern similar to purified DEX localization at this time point (data not shown).
Figure 6. DEX is associated with MZ macrophages early and later with CD11c+ dendritic cells and J558 Id+ plasma cells at extrafollicular sites after I.V. injection of BALB/c mice.
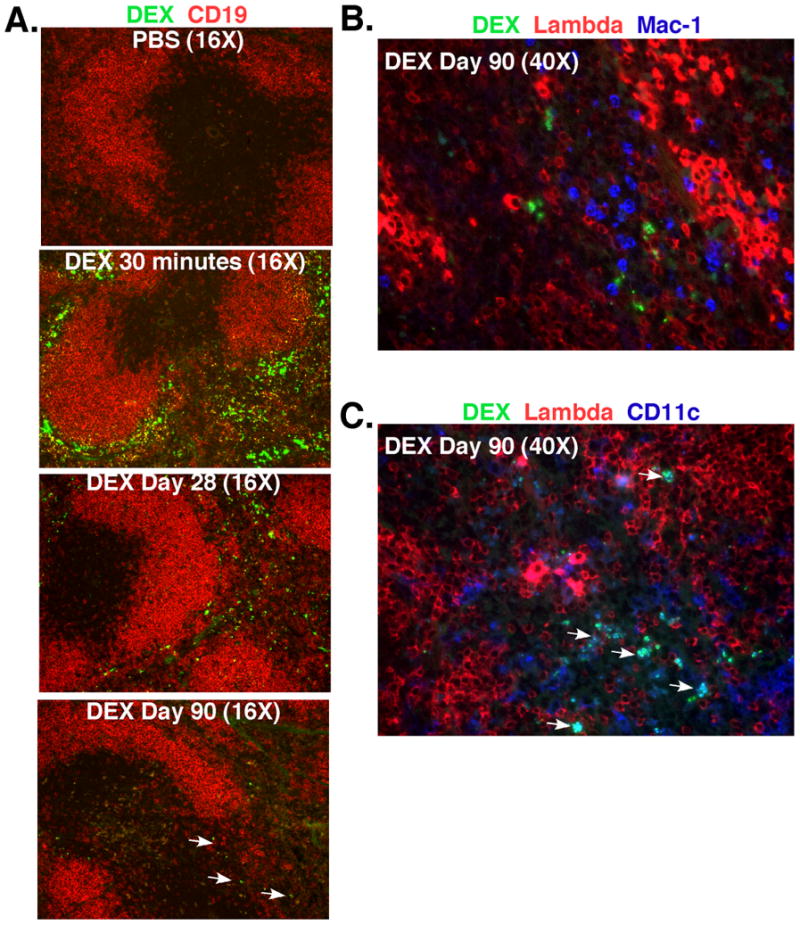
(A) DEX (green) is located in the splenic MZ containing CD19+ B cells (red) at 30 minutes and later at 28-90 days DEX is found predominantly in the red pulp. White arrows indicate the presence of DEX in BALB/c mice 90 days post injection. (B+ C) DEX (green) does not associate with Mac-1+ neutrophils (blue) distinguished by their multi-lobed nuclei, but associates with CD11c+ DCs (blue/blue green) and λ+plasma cells (bright red) in BALB/c mice at 90 days after immunization with DEX. White arrows indicate the association of DEX (green) with CD11c+ dendritic cells (blue). Each image shown is representative of 3 mice per time point.
Although it was not possible to demonstrate antigen persistence after E. cloacae compared to DEX immunization, the kinetics and magnitude of the antibody responses were identical, strongly suggesting a role for antigen persistence within the spleen in the prolonged antibody responses observed in each case.
Resistance of bone marrow DEX-specific ASCs to cyclophosphamide induced cell death
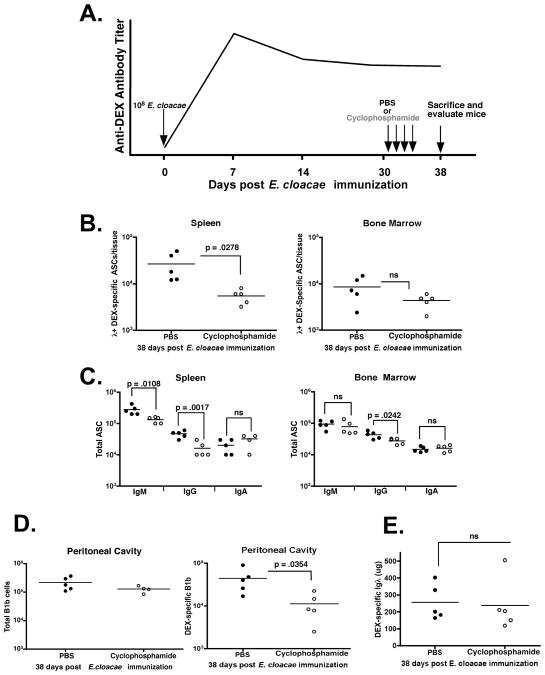
Cyclophosphamide, which depletes plasmablasts and short-lived plasma cells was used to determine the relationship between DEX-specific ASCs in the spleen and bone marrow (28, 32, 33). BALB/c mice were immunized with E. cloacae strain MK7 and then given IP injections of cyclophosphamide at 35 kg/mg body weight on days 30-33 post E. cloacae strain MK7 immunization (28). Four days after the last cyclophosphamide injection (day 38 post-immunization) DEX-specific and total ASCs in the bone marrow and spleen were quantified according to the protocol described in figure 7A. Splenic DEX-specific ASCs in cyclophosphamide-treated mice were reduced by 75% compared to PBS injected mice, while the numbers of DEX-specific ASCs present in the bone marrow were not significantly affected (Figure 7B). Cyclophosphamide treatment also decreased the number of IgM and IgG ASCs in the spleen and IgG ASCs in the bone marrow (Figure 7C). These results indicate that cyclophosphamide impacted the homeostasis of both antigen specific and total Ig secreting ASCs in the spleen but, with the exception of IgG, not the bone marrow.
Figure 7. DEX-specific ASCs in the bone marrow are resistant to cyclophosphamide and maintain serum anti-DEX antibody titers.
(A) Protocol for the treatment of BALB/c mice immunized with 108 E. cloacae strain MK7 and injected I.P. with either 35mg/kg of cyclophosphamide or PBS on days 31-35 post E. cloacae strain MK7 immunization. (B) Numbers of λ DEX-specific ASCs in the spleen of cyclophosphamide (open circles) and PBS (closed circles) treated mice at 38 days post E. cloacae strain MK7 immunization. (C) Total numbers of IgM, IgG, and IgA ASCs in the spleen and bone marrow in PBS and cyclophosphamide treated mice showing significant reductions in IgM and IgG ASCs in the spleen and IgG ASCs in the bone marrow (p < 0.05). (D) The numbers of DEX-specific IgM+ Mac1+ B1b B cells in PBS and cyclophosphamide injected mice. (E). Titers of serum DEX-specific λ+ antibodies in mice injected with PBS or cyclophosphamide 38 days post E. cloacae strain MK7 immunization (4 days post cyclophosphamide injection). Data shown is representative from 2 independent experiments (n= 5 mice per group)
Because B1b B cells respond vigorously upon recall secondary challenge (27) we determined the effects of cyclophosphamide on their homeostasis. Cyclophosphamide treatment reduces the number of DEX-specific, but not total, B1b B cells in the peritoneal cavity by greater than 50% indicating that both de novo plasma cell generation in the spleen and the DEX-specific memory B cell compartment were affected by cyclophosphamide treatment, whereas, DEX-specific ASCs in the bone marrow were unaffected. Despite significant reductions in the aforementioned populations, anti-DEX antibody titers were not significantly different in both PBS and cyclophosphamide treated mice suggesting that antibody produced by the bone marrow DEX-specific ASCs was sufficient to maintain prolonged anti-DEX antibody levels (figure 7D).
These findings indicate that the bone marrow contains a significant reservoir of cyclophosphamide resistant, ASCs with the potential to contribute to the maintenance of serologic memory to DEX.
DEX-specific ASCs are maintained despite anti-CD20 mediated B cell depletion
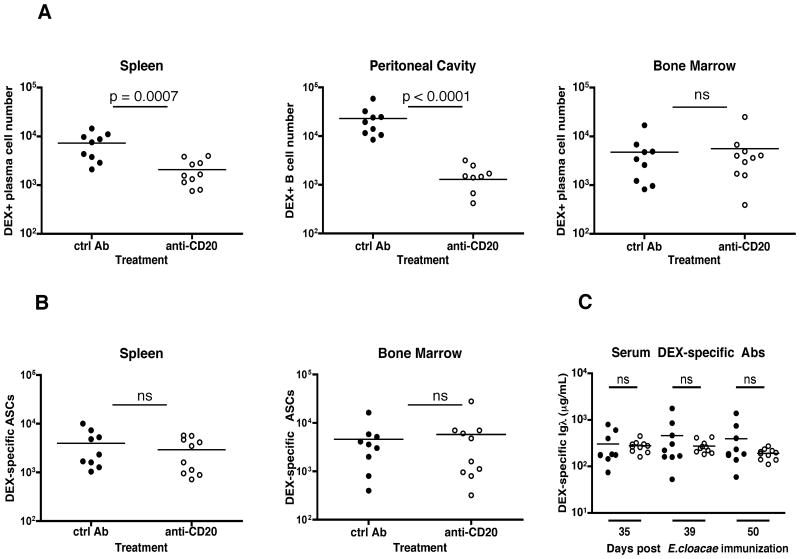
To confirm the long-lived nature of the DEX-specific ASCs that accumulate in the bone marrow BALB/c mice were treated with an anti-CD20 monoclonal antibody to deplete mature B cells, including memory B cells, plasmablasts and short-lived plasma cells (11, 34, 35). Mice were injected weekly for 3 weeks beginning 30 days after E. cloacae strain MK7 immunization and were then sacrificed one week after the last anti-CD20 injection (50 days post E. cloacae strain MK7 immunization). Anti-CD20 treatments resulted in > 100-fold decrease in all splenic B cell populations and a 10-fold decrease in the bone marrow and peritoneal cavity B cells (Fig S3). In agreement with our data from cyclophosphamide-treated mice, the numbers of DEX-specific ASCs (B220 lo CD138+), as determined by flow cytometry, were significantly reduced in the spleen but not in the bone marrow of treated mice (Figure 8A). DEX-specific B cells in the peritoneal cavity were also reduced upon treatment (Figure 8A). In contrast, the numbers of DEX-specific ASCs were unchanged in the spleen and the bone marrow upon anti-CD20 treatment (Figure 8B). Serum levels of DEX-specific antibodies were maintained at similar levels following anti-CD20 injections in comparison to the control group, indicating that anti-DEX antibodies are maintained after anti-CD20 treatment (Figure 8C).
Figure 8. DEX-specific ASCs in the bone marrow are resistant to anti-CD20 mediated depletion and maintain serum anti-DEX antibody titers.
A protocol similar to that described in Figure 7A was used with the exception that mice received anti-CD20 or IgG2a controls at days 37, 44 and 51 after injection with E. cloacae strain MK7. (A) Total cell numbers of DEX+ λ+ CD138+ cells in the spleen, and bone marrow, and DEX+ λ+ B cells in the peritoneal cavity as determined by flow cytometry. (B) Total numbers of DEX+ λ+ ASCs in the spleen and bone marrow as determined by ELISpot assay. (C) Titers of serum DEX+ λ+ antibodies were measured at the indicated time points after immunization with E. cloacae strain MK7. Closed circles: Rat IgG2a control antibody-treated mice; open circles: anti-CD20 antibody-treated mice. Data shown was calculated from 2 independent experiments (n= 5 mice per group).
These results highlight the resilience of DEX-specific ASCs in the bone marrow, which are maintained despite B cell depletion by anti-CD20 antibody.
Discussion
In these studies we have demonstrated that challenge with either DEX or E. cloacae strain MK7 leads to a long-lasting DEX-specific antibody response. This response is driven by: 1) the continual formation of short-lived plasmablasts stimulated by persisting antigen in the spleen and 2) the formation of a quiescent population of ASCs that persists in the bone marrow throughout the antibody response to DEX.
In addition to analyzing the kinetics of anti-DEX ASC production, we investigated the effects of persisting polysaccharide on the population turnover of IgM secreting cells in the bone marrow and spleen. It has been known for many years that polysaccharides introduced by bacterial infections or deliberate injection of synthetic polysaccharides persist in various tissue compartments (17-20). In our experiments, DEX was associated early with MZ macrophages and later with dendritic cells in the splenic red pulp. Thus, residual DEX may continually interact with antigen specific B cells driving prolonged plasmablast generation. Similar findings were reported in the primary antibody response to phosphorylcholine on Streptococcus pneumoniae and NP-Ficoll (36, 37). Despite the similar capacity of E. cloacae strain MK7 to induce long-lived DEX-specific antibody production, DEX associated epitopes on E. cloacae strain MK7 were not detectable in the lymphoid tissues of mice injected with bacteria after 48 hours. However, there was clearly continued de novo plasma cell generation in the spleen as evidenced by Ki67 expression. The inability to visualize DEX after i.v. bacterial immunization may be due to its retention in a form that is not recognized by our detection methods. Similar findings were reported by Schwab et al, attributing a role for neutrophils in breaking down streptococcal wall components into smaller molecules undetectable by conventional fluorescence microscopy (38).
As demonstrated in other models, IgM secreting cells residing in the bone marrow are the source of prolonged antibody production in response to DEX (23). These cells do not represent a pre-plasmablast population as they express CD138 as well as high intracellular levels of Ig, unlike pre-plasmablasts. From a physiological perspective, these cells resemble long-lived plasma cells as they home to the bone marrow, do not cycle or undergo apoptosis while maintaining serum antibody levels to DEX. The differences between this long-lived ASC population and the classic T-dependent plasma cells have not been described. It is possible that the surface phenotype that they acquire is a result of the nature of the antigen, the nature of the T cell independent response and the fact that MZ and B1b B cells are the primary sources of IgM secreting cells that respond to this particular antigen.
DEX-specific IgM secreting cells within the bone marrow and spleen displayed a plasmablast cell surface phenotype differing only in the lower levels of CD19 expression on ASCs residing in the bone marrow. Down regulation of CD19 is a direct result of Blimp-1-mediated repression of the transcription factor Pax5, which controls CD19 expression and other molecules associated with the B cell lineage and is evidence for mature plasma cell commitment (39). It is possible that down regulation of CD19 limits the signaling capacity of the BCR given the role of CD19 in modulating B cell signaling threshold (40).
Although “classic” long-lived plasma cells and the long-lived ASCs described here share some characteristics, it is not clear if retention of surface IgM and co-stimulatory molecules are functional in this quiescent state. There are few studies on the nature of long-lived IgM secreting plasma cells found in the spleen and bone marrow of mice in a T cell independent response. Recent findings by Racine et al. demonstrate the long-term persistence of IgM ASCs localized in the bone marrow, which maintain serum IgM antibodies against outer membrane proteins of Ehrlicia muris (23). It has also been shown that B1 B cells homing to the bone marrow provide an important source of serum IgM and IgA influenza specific antibodies (41). In humans, similar to our findings in mouse bone marrow, IgM and MHC Class II are expressed on long-lived plasma cell subsets in the spleen and bone marrow. These human bone marrow plasma cell populations also expressed lower levels of CD19 compared to spleen counterparts (42). Therefore it is likely that IgM secreting cells in the bone marrow represent a population of ASCs with a unique lifespan and specificities for bacterial and viral antigens that maintain serologic memory in the form of antigen specific IgM isotype antibodies. It would be of interest to test the resilience of this ASC population in BCMA-deficient animals or after neutralization of BAFF and APRIL, where long-lived plasma cells fail to develop (43).
Our previous work has demonstrated a role for peritoneal B1b cells in the generation of B cell memory to DEX (27). Upon re-challenge, we speculate that, similar to T-dependent long lived ASCs, T-independent long lived ASCs we have described have the potential to provide prompt protection against rapidly proliferating and invasive pathogens at the time when memory B1b cells have yet to respond and contribute to the recall response. One way to dissect the individual contribution of antigen-specific B1b and long-lived ASCs in the recall response to T-independent antigens in future work would be to re-challenge mice whose antigen-specific B1b cells have been depleted by cyclophosphamide treatment.
To confirm the longevity of DEX-specific ASCs in the bone marrow we used cyclophosphamide and an anti-CD20 monoclonal antibody to deplete plasmablasts and short-lived plasma cells (28, 32, 33). In agreement with previous studies, both treatments depleted splenic DEX-specific ASCs, but not those present in bone marrow after E. cloacae strain MK7 immunization. The inability of cyclophosphamide to deplete DEX-specific ASCs present in the bone marrow was likely due to their exit from cell cycle, which may render antigen specific plasma cells resistant to cyclophosphamide (28). Furthermore, their maintenance, after both treatments, provided evidence that their homeostasis was partially independent of B1b B memory and ongoing antigen-driven plasmablast generation occurring in the spleen given the significant level of depletion of these populations after these cyclophosphamide and anti-CD20 treatment. These findings are supported by recent evidence demonstrating engraftment of long-lived IgM secreting cells in the bone marrow in response to pneumococcal capsular polysaccharide after treatment with gamma irradiation (22). Likewise, a similar ASC population, responsive to Ehrlicia muris outer membrane protein, is maintained after doxycycline treatment (23).
It was of interest that there were less DEX-specific plasmablasts, as measured by flow cytometry, than ASCs, as measured by ELISpot assay, in the spleen of anti-CD20 treated mice versus control. It is possible that anti-CD20-mediated B cell depletion reduces competition and promotes the survival of ASCs so that they attain a more mature and surface IgM negative phenotype, thereby making them undetectable by flow cytometry. It is also possible that anti-CD20 treatment is more efficient in depleting other B cell subtypes that compete for bone marrow-derived stromal factors such CXCL12, which is required for plasma cell survival.
Collectively, these findings demonstrate a role for bacterial polysaccharide in establishing continuous generation of polysaccharide-specific, short-lived plasmablasts in the spleen and a quiescent population in the bone marrow that displays characteristics of long-lived plasma cells. The phenotypic dissimilarities including maintenance of surface IgM and co-stimulatory molecules highlight differences of these populations compared to “classical” plasma cells derived from T-cell dependent germinal centers. This study and future work will provide pertinent information regarding the design and implementation of vaccines constructed to elicit long-lasting antibody levels to bacterial polysaccharides.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the invaluable technical help of Lisa Jia, Brian Dizon, and Jeffrey Sides.
Footnotes
This work was supported by Award Numbers RO1AI014782-29, RO1AI090742 and UO1AI090902 from the National Institute of Allergy and Infectious Diseases.
References
- 1.Manz RA, Lohning M, Cassese G, Thiel A, Radbruch A. Survival of long-lived plasma cells is independent of antigen. Int Immunol. 1998;10:1703–1711. doi: 10.1093/intimm/10.11.1703. [DOI] [PubMed] [Google Scholar]
- 2.Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8:363–372. doi: 10.1016/s1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- 3.Angelin-Duclos C, Cattoretti G, Lin KI, Calame K. Commitment of B lymphocytes to a plasma cell fate is associated with Blimp-1 expression in vivo. J Immunol. 2000;165:5462–5471. doi: 10.4049/jimmunol.165.10.5462. [DOI] [PubMed] [Google Scholar]
- 4.Kallies A, Hasbold J, Tarlinton DM, Dietrich W, Corcoran LM, Hodgkin PD, Nutt SL. Plasma cell ontogeny defined by quantitative changes in blimp-1 expression. J Exp Med. 2004;200:967–977. doi: 10.1084/jem.20040973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hauser AE, Debes GF, Arce S, Cassese G, Hamann A, Radbruch A, Manz RA. Chemotactic responsiveness toward ligands for CXCR3 and CXCR4 is regulated on plasma blasts during the time course of a memory immune response. J Immunol. 2002;169:1277–1282. doi: 10.4049/jimmunol.169.3.1277. [DOI] [PubMed] [Google Scholar]
- 6.Muehlinghaus G, Cigliano L, Huehn S, Peddinghaus A, Leyendeckers H, Hauser AE, Hiepe F, Radbruch A, Arce S, Manz RA. Regulation of CXCR3 and CXCR4 expression during terminal differentiation of memory B cells into plasma cells. Blood. 2005;105:3965–3971. doi: 10.1182/blood-2004-08-2992. [DOI] [PubMed] [Google Scholar]
- 7.Odendahl M, Mei H, Hoyer BF, Jacobi AM, Hansen A, Muehlinghaus G, Berek C, Hiepe F, Manz R, Radbruch A, Dorner T. Generation of migratory antigen-specific plasma blasts and mobilization of resident plasma cells in a secondary immune response. Blood. 2005;105:1614–1621. doi: 10.1182/blood-2004-07-2507. [DOI] [PubMed] [Google Scholar]
- 8.Crotty S, Kersh EN, Cannons J, Schwartzberg PL, Ahmed R. SAP is required for generating long-term humoral immunity. Nature. 2003;421:282–287. doi: 10.1038/nature01318. [DOI] [PubMed] [Google Scholar]
- 9.MacLennan IC, Toellner KM, Cunningham AF, Serre K, Sze DM, Zuniga E, Cook MC, Vinuesa CG. Extrafollicular antibody responses. Immunol Rev. 2003;194:8–18. doi: 10.1034/j.1600-065x.2003.00058.x. [DOI] [PubMed] [Google Scholar]
- 10.Tokoyoda K, Hauser AE, Nakayama T, Radbruch A. Organization of immunological memory by bone marrow stroma. Nat Rev Immunol. 2010;10:193–200. doi: 10.1038/nri2727. [DOI] [PubMed] [Google Scholar]
- 11.Ahuja A, Anderson SM, Khalil A, Shlomchik MJ. Maintenance of the plasma cell pool is independent of memory B cells. Proc Natl Acad Sci U S A. 2008;105:4802–4807. doi: 10.1073/pnas.0800555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen PZ, Kabat EA. Persistence of circulating antibodies in human subjects immunized with dextran, levan and blood group substances. J Immunol. 1958;80:495–500. [PubMed] [Google Scholar]
- 13.Benner R, van Oudenaren A. Antibody formation in mouse bone marrow. IV. The influence of splenectomy on the bone marrow plaque-forming cell response to sheep red blood cells. Cell Immunol. 1975;19:167–182. doi: 10.1016/0008-8749(75)90201-4. [DOI] [PubMed] [Google Scholar]
- 14.Garcia de Vinuesa C, O'Leary P, Sze DM, Toellner KM, MacLennan IC. T-independent type 2 antigens induce B cell proliferation in multiple splenic sites, but exponential growth is confined to extrafollicular foci. Eur J Immunol. 1999;29:1314–1323. doi: 10.1002/(SICI)1521-4141(199904)29:04<1314::AID-IMMU1314>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 15.Howard JG, Courtenay BM, Vicari G. Influence of molecular structure of the tolerogenicity of bacterial dextrans. III. Dissociation between tolerance and immunity to the alpha1--6- and alpha1--3-linked epitopes of dextran B1355. Immunology. 1975;29:611–619. [PMC free article] [PubMed] [Google Scholar]
- 16.Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KG, Dorner T, Hiepe F. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat Rev Immunol. 2006;6:741–750. doi: 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]
- 17.Felton LD. The significance of antigen in animal tissues. J Immunol. 1949;61:107–117. [PubMed] [Google Scholar]
- 18.Grinsell M, Weinhold LC, Cutler JE, Han Y, Kozel TR. In vivo clearance of glucuronoxylomannan, the major capsular polysaccharide of Cryptococcus neoformans:a critical role for tissue macrophages. J Infect Dis. 2001;184:479–487. doi: 10.1086/322787. [DOI] [PubMed] [Google Scholar]
- 19.Humphrey JH. Tolerogenic or immunogenic activity of hapten-conjugated polysaccharides correlated with cellular localization. Eur J Immunol. 1981;11:212–220. doi: 10.1002/eji.1830110310. [DOI] [PubMed] [Google Scholar]
- 20.Moreno C, Hale C, Hewett R, Esdaile J. Induction and persistence of B-cell tolerance to the thymus-dependent component of the alpha(1 leads to 6) glucosyl determinant of dextran. Recovery induced by treatment with dextranase in vivo. Immunology. 1981;44:517–527. [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu MC, Toellner KM, Vinuesa CG, Maclennan IC. B cell clones that sustain long-term plasmablast growth in T-independent extrafollicular antibody responses. Proc Natl Acad Sci U S A. 2006;103:5905–5910. doi: 10.1073/pnas.0601502103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taillardet M, Haffar G, Mondiere P, Asensio MJ, Gheit H, Burdin N, Defrance T, Genestier L. The thymus-independent immunity conferred by a pneumococcal polysaccharide is mediated by long-lived plasma cells. Blood. 2009;114:4432–4440. doi: 10.1182/blood-2009-01-200014. [DOI] [PubMed] [Google Scholar]
- 23.Racine R, McLaughlin M, Jones DD, Wittmer ST, MacNamara KC, Woodland DL, Winslow GM. IgM production by bone marrow plasmablasts contributes to long-term protection against intracellular bacterial infection. J Immunol. 2011;186:1011–1021. doi: 10.4049/jimmunol.1002836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kearney JF, McCarthy MT, Stohrer R, Benjamin WH, Jr, Briles DE. Induction of germ-line anti-alpha 1-3 dextran antibody responses in mice by members of the Enterobacteriaceae family. J Immunol. 1985;135:3468–3472. [PubMed] [Google Scholar]
- 25.Rappleye CA, Eissenberg LG, Goldman WE. Histoplasma capsulatum alpha-(1,3)-glucan blocks innate immune recognition by the beta-glucan receptor. Proc Natl Acad Sci U S A. 2007;104:1366–1370. doi: 10.1073/pnas.0609848104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilham CA, Alexander BH, Jeanes A. Heterogeneity in dextran preparations. Arch Biochem Biophys. 1955;59:61–75. doi: 10.1016/0003-9861(55)90463-x. [DOI] [PubMed] [Google Scholar]
- 27.Foote JB, Kearney JF. Generation of B cell memory to the bacterial polysaccharide alpha-1,3 dextran. J Immunol. 2009;183:6359–6368. doi: 10.4049/jimmunol.0902473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luger EO, Fokuhl V, Wegmann M, Abram M, Tillack K, Achatz G, Manz RA, Worm M, Radbruch A, Renz H. Induction of long-lived allergen-specific plasma cells by mucosal allergen challenge. J Allergy Clin Immunol. 2009;124:819–826. e814. doi: 10.1016/j.jaci.2009.06.047. [DOI] [PubMed] [Google Scholar]
- 29.Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14:617–629. doi: 10.1016/s1074-7613(01)00129-7. [DOI] [PubMed] [Google Scholar]
- 30.Kearney JF, Pollok BA, Stohrer R. Analysis of idiotypic heterogeneity in the anti-alpha 1-3 dextran and anti-phosphorylcholine responses using monoclonal anti-idiotype antibodies. Ann N Y Acad Sci. 1983;418:151–170. doi: 10.1111/j.1749-6632.1983.tb18063.x. [DOI] [PubMed] [Google Scholar]
- 31.Oliver AM, Martin F, Kearney JF. IgMhighCD21high lymphocytes enriched in the splenic marginal zone generate effector cells more rapidly than the bulk of follicular B cells. J Immunol. 1999;162:7198–7207. [PubMed] [Google Scholar]
- 32.Hoyer BF, Moser K, Hauser AE, Peddinghaus A, Voigt C, Eilat D, Radbruch A, Hiepe F, Manz RA. Short-lived plasmablasts and long-lived plasma cells contribute to chronic humoral autoimmunity in NZB/W mice. J Exp Med. 2004;199:1577–1584. doi: 10.1084/jem.20040168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ndungu FM, Cadman ET, Coulcher J, Nduati E, Couper E, Macdonald DW, Ng D, Langhorne J. Functional memory B cells and long-lived plasma cells are generated after a single Plasmodium chabaudi infection in mice. PLoS Pathog. 2009;5:e1000690. doi: 10.1371/journal.ppat.1000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DiLillo DJ, Hamaguchi Y, Ueda Y, Yang K, Uchida J, Haas KM, Kelsoe G, Tedder TF. Maintenance of long-lived plasma cells and serological memory despite mature and memory B cell depletion during CD20 immunotherapy in mice. J Immunol. 2008;180:361–371. doi: 10.4049/jimmunol.180.1.361. [DOI] [PubMed] [Google Scholar]
- 35.Huang H, Benoist C, Mathis D. Rituximab specifically depletes short-lived autoreactive plasma cells in a mouse model of inflammatory arthritis. Proc Natl Acad Sci U S A. 2010;107:4658–4663. doi: 10.1073/pnas.1001074107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balazs M, Martin F, Zhou T, Kearney J. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity. 2002;17:341–352. doi: 10.1016/s1074-7613(02)00389-8. [DOI] [PubMed] [Google Scholar]
- 37.Garcia De Vinuesa C, Gulbranson-Judge A, Khan M, O'Leary P, Cascalho M, Wabl M, Klaus GG, Owen MJ, MacLennan IC. Dendritic cells associated with plasmablast survival. Eur J Immunol. 1999;29:3712–3721. doi: 10.1002/(SICI)1521-4141(199911)29:11<3712::AID-IMMU3712>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 38.Schwab JH, Ohanian SH. Degradation of streptococcal cell wall antigens in vivo. J Bacteriol. 1967;94:1346–1352. doi: 10.1128/jb.94.5.1346-1352.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Calame K. Transcription factors that regulate memory in humoral responses. Immunol Rev. 2006;211:269–279. doi: 10.1111/j.0105-2896.2006.00377.x. [DOI] [PubMed] [Google Scholar]
- 40.Buhl AM, Pleiman CM, Rickert RC, Cambier JC. Qualitative regulation of B cell antigen receptor signaling by CD19: selective requirement for PI3-kinase activation, inositol-1,4,5-trisphosphate production and Ca2+ mobilization. J Exp Med. 1997;186:1897–1910. doi: 10.1084/jem.186.11.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi YS, Baumgarth N. Dual role for B-1a cells in immunity to influenza virus infection. J Exp Med. 2008;205:3053–3064. doi: 10.1084/jem.20080979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ellyard JI, Avery DT, Phan TG, Hare NJ, Hodgkin PD, Tangye SG. Antigen-selected, immunoglobulin-secreting cells persist in human spleen and bone marrow. Blood. 2004;103:3805–3812. doi: 10.1182/blood-2003-09-3109. [DOI] [PubMed] [Google Scholar]
- 43.Elgueta R, de Vries VC, Noelle RJ. The immortality of humoral immunity. Immunol Rev. 2010;236:139–150. doi: 10.1111/j.1600-065X.2010.00924.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.