Abstract
Tissue macrophages play a critical role both in normal physiology as well in disease states. However, due to a lack of specific imaging agents, we continue to have a poor understanding of their absolute numbers, flux rates and functional states in different tissues. Here, we describe a new macrophage specific PET imaging agent, labeled with zirconium-89 (89Zr), that was based on a crosslinked, short-chain dextran nanoparticle (13 nm). Following systemic administration, the particle demonstrated a vascular half-life of 3.9 hours, and was found to locate primarily to tissue resident macrophages rather than to other white blood cells. Subsequent imaging of the probe using a xenograft mouse model of cancer allowed for quantitation of tumor associated macrophage (TAM) numbers, which are of major interest in emerging molecular targeting strategies. It is likely that the material described, which enables the visualization of macrophage biology in vivo, will likewise be useful for a multitude of human applications.
Keywords: Nanoparticles, Macrophage, 89Zr, PET imaging
Introduction
Macrophages are white blood cells that are produced by the differentiation of monocytes after their entry into tissues. While their primary role is to phagocytose pathogens and cellular debris, in response to tissue insults they can also act to stimulate the recruitment of lymphocytes and other immune cells. However, despite their known importance and widespread distribution throughout the body, little quantitative information exists regarding their total mass, relative numbers at different sites, distribution profiles across different diseases, or their mobilization and flux rates in both disease and normal physiologic conditions. Early landmark papers1 used macrophage specific antibodies (F4/80 in mice) to estimate macrophage numbers under resting conditions, or have attempted macrophage quantitation using other molecular markers (e.g. Mac-32, CD683, or PG-M14).
In previous work, our laboratory developed dextran coated nanomaterials that, upon systemic administration, were readily engulfed by mononuclear phagocytic cells. They could thus be used as imaging probes for examining the function of these cells in humans.5, 6 Importantly, we have now not only shown that dextran coated iron oxide nanoparticles have high macrophage avidity, but have also used them for magnetic resonance imaging (MRI), 5, 6 optical7 as well as positron emission tomography (PET) imaging.8, 9 While these multimodal nanoparticles were originally developed for MRI, using MRI to quantify exact nanoparticle numbers in vivo in different organs is often complicated, particularly in bone marrow and lung tissue. Radiolabeled nanomaterials have only recently been synthesized but have already shown potentially interesting clinical applications.8–13 In general, it is possible to administer radiolabeled nanomaterials at much lower concentrations than their magnetic (and fluorescent) counterparts. Furthermore, their pharmacokinetics can also be titrated by size and surface optimization, and can be matched with PET isotopes with the appropriate decay rate (e.g. 18F, 64Cu, 68Ga, 89Zr).
Given the absence of optimized macrophage specific nano-particulate imaging agents, our goal was to systematically develop such materials through an iterative synthesis and screening procedure.14 Here, we describe the characteristics and in vivo behavior of one lead compound identified from an initial larger screen. This nanoparticle is composed of several short (10Kd) dextran strands crosslinked into a 13 nm diameter nanoparticle. Since our aim is to ultimately use these materials clinically, we avoided the use of longer chain length linear dextrans as synthetic building blocks, due to their associated hypersensitivities.15 Rather, given their long clinical history,16 we chose to incorporate shorter dextrans into the nanoparticles. We subsequently showed that the described preparation is readily labeled with 89Zr (t1/2 = 78.4 h, β+ = 22.3%) via desferrioxamine, and can be used with PET to quantitate macrophages in vivo. Finally, in view of the recent interest regarding the role of tumor associated macrophages (TAM) in cancer,17, 18 we validated the agent by quantitating tumoral macrophages in a mouse model of cancer.
Materials and Methods
General
Dextran T10 was purchased from Amersham Biosciences AB (Sweden; Cat.# 17-0250-02; Lot# 298106). Amine-reactive VivoTag-680 was purchased from Perkin-Elmer. Desferrioxamine-thiocyanate was purchased from Macrocyclics. 89Zr was prepared following published procedures at Memorial Sloan-Kettering Cancer Center.19 All other reagents were purchased from Sigma-Aldrich and used without further purification. Pentynoyl-NHS ester was prepared as previously described.13
Synthesis
Dextran T10 (1.8 g) was dissolved in 9 mL of water at 60 °C. The solution was cooled to rt and stirred for 12 h then 5N aqueous NaOH (15 mL) and epichlorohydrin (6 mL) were added. The mixture was stirred at rt for 5 hours and ethylenediamine (26 mL) was added drop-wise to the reaction mixture, keeping the temperature below 20 °C. This mixture was allowed to stand at rt for 15 hours. The crude DNP-NH2 mixture was precipitated with ethanol (21 mL), centrifuged, and decanted. The pelleted DNP-NH2 was dissolved in 10 mL of water and dialyzed against 150 mM NaCl for several days until optical density (420 nm) of the dialyzing solution was lower than 0.02. Dextran concentration was determined employing a known sugar reducing quantitative colorimetric method in the presence of phenol and sulfuric acid.20 The percentage of dextran’s contribution to the total weight of the particle was subsequently determined by azeotropic distillation of water with acetonitrile, followed by lyophilization for several days. Amine concentration relative to dextran was quantified following the published procedure of Snyder and Sobocinski21 were 1.09, 0.93, and 1.17 μmol/mg Dextran for the three differently sized DNP preparations, 5, 6, and 13 nm, respectively.
Modification (succinylation, chelator, VT-680)
To a solution of DNP-NH2 (8.5 mg dextran) in 400 μL of 100 mM Na2CO3 (pH 8), VivoTag 680 (2.5 mM, 80 μL DMSO) was added, and the mixture was stirred at room temperature. After overnight stirring, the product DNP-VT was isolated using centrifugal membrane filter (10-kDa mwco, Amicon Ultra-0.5 mL, Millipore), washed with 300 μL MilliQ-water three times, then collected in 100 μL MilliQ-water solution. Then, the blue DNP-VT solution was reacted with desferrioxamine-p-SCN (25 mM, 80 μL DMSO) in 200 uL of 100 mM NaHCO3 (pH 9) at room temperature for 24 h. The reaction mixture was purified using PD-10 column (GE Healthcare) and concentrated using the centrifugal filter (10-kDa mwco) to give 250 μL of DNP-VT-Df conjugates. The DNP-VT-Df nanoparticles were further reacted with succinic anhydride (25 mM, 800 μL DMSO) at room temperature for 24 h, concentrated using the centrifugal filter (10-kDa mwco) to give fully capped DNP-VT-Df-S nanoparticles (14.4 mg).
Radiochemistry
Typically ~ 50 μg of desferrioxamine-conjugated DNP nanoparticles (either uncapped or capped) were mixed with 1 – 3 mCi of [89Zr]Zr oxalate in 100 μL of 1 M oxalic acid. The pH of reaction mixture was carefully adjusted to pH 7 by slow addition of 1 M Na2CO3 solution. Then, the solution was shaken at 25 °C for 1 h and purified using centrifugal membrane filter (10 kDa mwco). The radiolabeling yield was measured using the thin layer chromatography (ITLC SG (PALL Corporation), 50 mM EDTA (pH 7)). The radiochemical purity was further confirmed by Gel chromatography (Superdex 200 (GE healthcare); 50 mM phosphate buffer, 0.15 M NaCl, pH 7.0; 0.5 mL/min flow rate). The collected activity from gel-filtration column matched well with the injected activity demonstrating that no activity stuck to the column.
Size and charge measurement
Mean particle size was determined by dynamic laser light scattering using a Malvern Zetasizer NanoZS. The mean sizes for the three different DNP nanoparticle preparations were 4.6, 5.9, and 13.3 nm (referred to in the text as 5, 6 and 13 nm). The Zeta potential for the 10 kDa dextran starting material (Dextran T10) was measured to be −13.7 ± 0.5 while the measured Zeta potentials for the aminated 5, 6, and 13 nm DNP were −34.9 ± 1.2, +10.3 ± 1.2 and + 30.0 ± 1.0, respectively. After succinylation, the 13 nm DNP had a Zeta potential of −30.1 ± 1.0.
Transmission Electron Microscopy (TEM)
Ted Pella Ultrathin Holey carbon grids were plasma cleaned for 30 seconds before incubation on a drop of DNP sample for 2–3 minutes. After briefly blotting the edge of the grid to filter paper (Whatman No. 1), the grid was inverted onto a drop of 3% phosphotungstic acid (PTA) for approximately 1–2 minutes. The edge of the grid was blotted against filter paper and the grid was allowed to air dry before viewing in a JEOL 2100 TEM at 200 KeV. A negative control sample was prepared as above, omitting the first incubation step on the sample.
MALDI mass
Each DNP sample (1.0 μL, 1 mg/mL (w/v)) was mixed with 1.0 μL of the MALDI matrix 2,5-dihydroxybenzoic acid (DHB, Sigma; 200 mg/ml (w/v) in 80% acetonitrile, 0.1% TFA) on a 384-well 4800 MALDI target plate and air dried in the dark. After spots were completely dry (~15 min), they were immediately analyzed in an AB Sciex 4800 Plus MALDI TOF/TOF mass spectrometer using the linear high mass positive mode. Each spectrum is the average of 2000 laser shots (50 shots on 40 positions randomly throughout the dried spot). DHB matrix alone dried in parallel was used as a negative control.
Cells
CT-26 colon carcinoma cells and HT-1080 fibrosarcoma cells expressing H2B-RFP (ATCC) were grown in DMEM supplemented with 10% FBS, pen/strep and non-essential amino acids and were screened for pathogens and mycoplasma as previously described.22
Mice
All animal experiments were approved by Massachusetts General Hospital’s Institutional Review Committee. All mice were anesthetized (isoflurane 1.5%; O2 2 L/min) during pharmacokinetic and imaging studies with a gas delivery system. C57BL/6 mice (n = 18) received intravenous tail-vein injection of labeled nanoparticles (50 – 100 μCi). Particle size-dependent blood half-lives were measured by serial retro-orbital bleeds (n = 3/particle size; n = 9) and biodistribution studies (n = 3/particle size; n = 9) were determined at 24 hours after systemic injection.
BALB/c mice (n = 8) received subcutaneous injections with 106 colon carcinoma CT-26 cells (ATCC) into each flank, were allowed to expand and vascularize, and were imaged by PET-CT 10 days later (n = 6) and flow cytometry studies (n = 2). Following imaging, mice were euthanized, perfused with saline (20 mL) and tissues excised for biodistribution, autoradiograph and histology.
Nu/Nu mice (n = 3; Cox-7; Massachusetts General Hospital, Boston MA) were anesthetized for window implantation, cell injections and imaging by using an isoflurane vaporizer (Harvard Apperatus, Holliston, MA) at a flow rate of 2.0% isoflurane:2.0 L/min oxygen. Surgeries were performed under sterile conditions using a zoom stereo-microscope (Olympus SZ61). Titanium dorsal skinfold chambers (DSCs: APJ Trading Co, Inc.,Ventura CA) were implanted into the dorsal skin fold of Nu/Nu mice as described.22 Mice were anesthetized and ~2 x 106 HT-1080-H2B-RFP cells were injected into the subcutaneous layer in a DSC using a 0.5-mL insulin syringe (28½G, BD Biosciences, Chicago IL). After injection, a small volume of sterile saline was added to the DSC and it was closed with a fresh cover slip. HT-1080-H2B-RFP xenografts were allowed to expand 8 days before imaging to permit neovascularization. CLIO-FITC (0.4 mg Fe) was injected 24 hours before imaging to stain macrophages. The following day 7.5 nmol DNP-VT680 was injected via tail vein.
Intravital Microscopy
Intravital imaging was performed using a custom-built confocal Olympus FV1000 system based on a BX61-WI microscope running Fluoview 1000 version 2.1 software. Olympus objectives 20x XLUMPlanFL N (NA 1.0, water) and 60x LUMFL N (NA 1.10, water) were used for imaging. CLIO-FITC, H2B-RFP, and DNP-VT680 were excited using a 488 nm Argon ion laser-line, a 559 nm pumped solid-state laser, or a 635 nm diode laser in combination with a DM405/488/559/635 nm dichroic beam splitter. Emitted light was separated and collected with beam splitters SDM560 and SDM640 and band-pass filters BA505-540, BA575-620, and BA655-757. Channels were collected by sequential line-scanning to avoid bleed through of signal between channels. Image files were imported into ImageJ 1.45d (Rasband, 2004–2010) for processing and analysis. Co-localization was quantified using the ImageJ JaCoP plugin with all values set automatically by the program.
Flow cytometry
Established subcutaneous CT26 tumors were excised 24 h after injection of the 13 nm DNP nanoparticle. The tissue was cut up, and incubated in 10 mL RPMI 1640 (Gibco; Invitrogen) containing 0.2 mg/ml collagenase type I (Worthington Biochemical Corp.) for 1 hour at 37°C. Digestate was filtered through a sterile 40 μm porous nylon mesh (BD Falcon), and single cell suspensions were washed and resuspended in 1 mL PBS with 0.5% BSA. The cells were labeled with the following antibodies (all from BD Biosciences): PE-conjugated anti-CD90 (clone 53-2.1), PE-conjugated anti-B220 (clone RA3-6B2), PE-conjugated anti-CD49b (clone DX5), PE-conjugated anti-NK1.1 (clone PK136), PE-conjugated anti-Ly-6G (clone 1A8), PE-conjugated TER119 AB (clone Ter:119), APC-Cy7-conjugated anti-CD11b (clone M1/70), and PE-Cy7-conjugated anti-F4/80 (clone BM8). Mononuclear phagocytes were defined as CD11b+ Linlow (where Lin is B220/DX5/NK1.1/Ly6G/Ter119/CD90.2); neutrophils as CD11b+ Linhigh (Ly-6G+); and others as CD11b−. The labeled cells were analyzed by flow cytometry (LSRII; BD Biosciences). Data were analyzed with FlowJo v.8.8.7 (Tree Star, Inc.).
Histology
Thinly sliced tissue sections were frozen in OCT fluid and perserved (−80 °C) for histology analysis upon decay of 89Zr nuclide. Five μm sections were stained with hematoxyloin/eosin Immunohistochemistry staining for macrophages was performed using Mac-3 antibody (BD Pharmingen) and the reaction was visualized with a 3-amino-9-ethylcarbazole (AEC) substrate (DAKO). All sections were counterstained with Harris hematoxylin solution (Sigma). The images were captured using a digital slide scanner, NanoZoomer 2.0RS (Hamamatsu) and analyzed electronically. On adjacent sections, nanoparticle distribution was analyzed with an epifluorescence microscope (680nm), Nikon Eclipse 80i, equipped with a Cascade Model 512B camera (Roper Scientific) connected to a Macintosh workstation.
Autoradiography
Excised tissue sections were exposed to a phosphor imager plate and read with a Typhoon FLA9000 system (GE Healthcare) 4 hours later. Sliced sections were frozen in OCT fluid and preserved (−80 °C) for histology analysis upon decay of 89Zr nuclide. Visualization of scanned autoradiography images was performed using the program OsiriX (The OsiriX foundation, Geneva, Switzerland).
PET-CT imaging
All PET-CT images were acquired on a Siemens Inveon PET-CT. Each PET acquisition was ~60 minutes in duration. PET was reconstructed from 600 million coincidental, 511 keV photon counts on a series of LSO (lutetium oxyorthosilicate) scintillating crystal rings. Counts were rebinned in 3D by registering photons spanning no more than 3 consecutive rings, then reconstructed into sinograms by utilizing a high resolution Fourier Rebin algorithm. A reconstruction of sinograms yielded a 3D mapping of positron signal using a 2D filtered back-projection algorithm, with a Ramp filter at a Nyquist cutoff of 0.5. Image pixel size was anisotropic, with dimensions of 0.796 mm in the z direction and 0.861 mm in the x and y directions, for a total of 128 x 128 x 159 pixels. Calibration of PET signal preceded all scans and was done by scanning an 8.0 cm cylindrical phantom containing a known amount of 89Zr isotope. Data are expressed as standard uptake values (SUV), which normalize activity for body weight and injected activity. Mean injected activity was 100–150 μCi/mouse. CT images were reconstructed from 360 projections of X-rays with a cone beam angle of 9.3 degrees over 360 degrees perpendicular to the animal bed. 80 keV X-rays were transmitted from a 500 μA anode source, 347 mm from the center of rotation and recorded on a CCD detector, containing 2048 transaxial and 3072 axial pixels. Projections were calibrated using 70 dark and 70 light images, interpolated bi-linearly, processed through a Shepp-Logan filter, then reconstructed using a filtered back projection algorithm. Isotropic CT pixel size was 110.6 μm, with a total of 512 x 512 x 768 pixels. Scaling to Hounsfield Units, calibration was done using a 8.0 cm cylindrical phantom containing water prior to CT acquisition. During CT acquisition, iodine contrast was infused into the tail vein at a rate of 35 μl/min to enhance intravascular contrast. Projections were acquired at end expiration using a BioVet gating system (M2M Imaging, Cleveland, OH) and CT acquisition time was ~10 minutes. Reconstruction of data sets, PET-CT fusion and image analysis were done using IRW software (Siemens). Three-dimensional visualizations were produced with the DICOM viewer OsiriX (The OsiriX foundation, Geneva, Switzerland).
Results
Nanoparticle characterization
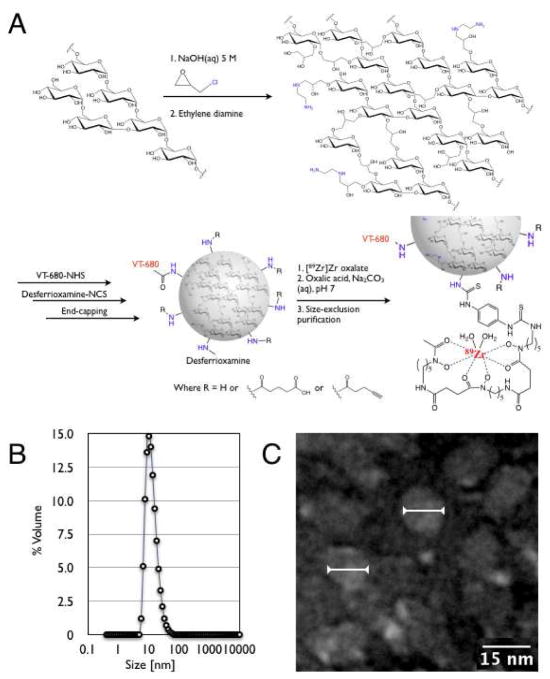
Crosslinked dextran nanoparticles (DNPs) were synthesized and aminiated following a modified literature procedure that uses epichlorohydrin as a crosslinking agent.14 Following work up, dialysis for several days provided DNPs in high purity. The size of the particles could then be reproducibly controlled as a function of the cross-linking reaction duration. Varying the duration of the reaction enabled synthesis of three preparations (mean diameters: 5, 6, and 13 nm), as measured by dynamic laser light scattering (DLS). Size distribution by volume data for these preparations is presented in Figure 1B and Figure SI.1 of the Supporting Information. The dextran concentration of these three preparations was determined by employing a sugar-reducing quantitative colorimetric method in the presence of phenol and sulfuric acid.20 The amine concentration relative to the dextran concentration was 1.09, 0.93, and 1.17 μmol/mg dextran respectively for the three different DNPs.21 Characterization of the 13 nm DNPs by matrix-assisted laser desorption/ionization (MALDI) mass spectrometry demonstrated a mean molecular weight of 80 kDa for these particles. Dextran’s weight contribution to the particles was determined to be 53%, 54% and 43% for the 5, 6, and 13 nm DNPs, respectively. The mean number of amine groups per 13 nm DNP particle was measured to be 40.
Figure 1.
(A) General synthesis and radiolabeling of dextran nanoparticles (DNPs). (B) Dynamic laser light scattering data for the 13 nm DNP preparation. C. Transmission electron microscopy image of several individual DNPs measuring 13 nm in diameter on average (arrows).
Particle size and morphology was further verified by transmission electron microscopy (TEM). TEM images (Figure 1) not only demonstrated the nanoparticles’ spherical shape and homogenous appearance, but also showed mean (narrow) diameters and overall sizes consistent with the DLS measurements. In negative controls, no globular-shaped nanoparticles were observed.
Modification of the particle surface for in vivo use was based on the relative modification of amines. The particles were first labeled with 1–2 fluorochromes (VT-680; Perkin Elmer) per nanoparticle to enable intravital microscopy, flow assisted cytometry and immunofluorescence histology. Secondly, ~20% of amines were capped with the zirconium chelator desferrioxamine, an FDA approved drug. Finally, to investigate the effects of pharmacokinetic modulation, all remaining amines were either left free or capped by reaction with an excess amount of succinic anhydride or pentynoyl-NHS ester. Following each modification step, DNPs were purified either by size exclusion chromatography or size exclusion membrane filtration. As expected, when DNP-NH2 was fully capped with succinic anhydride, a dramatic shift in zeta potential was observed from +30.0 to −30.1 ± 1.0, indicating a change of surface charge from positive to negative.
The desferrioxamine-conjugated DNPs were labeled with [89Zr]-Zr oxalate in quantitative yield within one hour, following a previously described procedure.23 Within 25 minutes at 25 °C, the radiochemical yield of DNPs with 89Zr was higher than 99.5%. The trace amount of free 89Zr ions was then removed by centrifugal membrane filtration. The radiochemical purity was 100%, and no remaining free 89Zr ions were detected on the high-performance liquid chromatography (HPLC) chromatogram after purification. The 89Zr-labeled DNPs did not show any sign of degradation, determined by ITLC, at 36 °C in phosphate buffered saline (PBS), for up to 5 days. While 18F is the most commonly PET tracer used in the clinical setting, the longer lived 89Zr was chosen for this work in anticipation nanoparticles would require longer circulation times for uptake into macrophages.
In vivo testing
To understand the effects of size and capping on pharmacokinetics, we performed blood half-life measurements. For the pentynoyl capped 5, 6, and 13 nm DNPs (Figure 2A), the blood half-life data fit a two-phase exponential decay model, with values of 0.2, 0.9, and 6.1 hours, respectively. The 5 and 6 nm preparations showed considerable renal excretion and much lower macrophage accumulation, and thus subsequent efforts focused solely on the 13 nm DNPs. To further determine the effects of surface modification on blood half-life we compared desferrioxamine labeled DNP with free amines, succinic anhydride or pentynoyl-NHS ester capping (Figure 2B). The two-phase blood half-lifes were 1.3 hours for the aminated, 3.9 hours for the succinylated, and 6.1 hours for the pentynoylated preparations. The above results demonstrate that blood half-life can be modulated based on particle size as well as surface modification chemistry. Reasoning that the succinylated 13 nm DNP had ideal pharmacokinetics for 89Zr, it was chosen for biodistribution experiments. Figure 2C summarizes the organ distribution 24 hours after injection of 100 μCi showing primary accumulation in lymph nodes (34 ± 16 % injected dose/gram tissue, %IDGT), liver (12 ± 2 %IDGT) blood (11 ± 1 %IDGT located primarily in circulating leukocytes, see below), and spleen (9 ± 1 %IDGT) with much lower accumulation in most other tissues including bone, kidneys and intestines. Adjusting for excretion (19.2 ± 0.8 %ID over 24 h) and the body mass of the main organs, the overall activity of the succinylated 13 nm DNP was 22 ± 3 % in liver, 19 ± 2 % in circulating cells, 2 ± 0.1 % in spleen and the remainder distributed in other organs.
Figure 2.
Pharmacokinetics of different DNP. (A) Effect of size on blood half-life. (B) Effect of surface modification on blood half-life. (C) Biodistribution plotted as tissue concentration 24 h after intravenous administration of succinylated 13 nm DNP. Insert: Distribution to major organs.
In vivo imaging
We next performed PET-CT imaging using the same 89Zr-DNP as above in a cancer mouse model. We used a syngeneic colon carcinoma (CT26) mouse model since it had been used extensively for investigations of tumor infiltrating host cells24, vaccines25 and other studies.26, 27 Figure 3 shows representative images from different mice bearing the xenografts. Tumoral uptake was surprisingly high (20 ± 5 %IDGT), surpassing concentrations of other reticucloendothelial system RES organs in some animals. To determine whether tumoral accumulation was due to cellular uptake or extraversion and interstitial accumulations we performed extensive correlative histology and flow cytometry studies.
Figure 3.
PET imaging of 89Zr-DNP in mice bearing bilateral flank tumors (24 h after administration). (A and B) two different animals showing similar distribution to all 4 tumors (coronal stacks). (C) Three-dimensional rendering of animal presented in A.
Harvested tumors were sliced into ~1 mm sections and imaged by autoradiography to juxtapose sections to Mac-3 (a macrophage specific stain) histological stain. Overall there was good agreement between “hot spots” and Mac3 positivity through the sections (Figure 4). Histology showed distinct uptake of fluorescent DNP in Mac3 positive cells and general lack of uptake in Mac3 negative cells (Figure 4).
Figure 4.
Autoradiography and histology of DNP distribution. (A) Autoradiography of 1 mm tumor and other tissue sections show predominant accumulation in tumors. (B) Co-localization of representative autoradiography sections with adjacent Mac3 immunohistology sections. (C) Distribution of fluorescent DNP to Mac3 positive cells.
To further investigate the pharmacokinetics and cellular uptake of the DNP we performed confocal intravital microscopy studies. H2B-RFP expressing HT1080 tumor cells were implanted in dorsal skinfold window-chambers and after 8 days of growth, animals received CLIO-FITC, a commonly used macrophage imaging agent.6 The following day, fluorescent DNP nanoparticles were administered systemically and animals were imaged over time. Figure 5A shows representative imaging at 3 hours post-injection demonstrating DNP (red) primarily in the vasculature with little cellular uptake or even interstitial accumulation. Tumor cells (blue) and tumor associated macrophages (TAMs, green) were clearly identified. At 24 hours post-injection, most of the DNP had localized exclusively to TAM with little compound remaining in vasculature or in tumor cells. The Pearson’s co-localization coefficient between DNP (red) and TAM (green) was 0.82 ± 0.07 (n = 5).
Figure 5.
Microscopy of DNP distribution in tumor microenvironment in live mouse. (A) 3 h after injection; (B) 24 h after injection. Initially the DNP is primarily seen in vasculature whereas it is almost exclusively co-localized (yellow color) with TAM at 24 h.
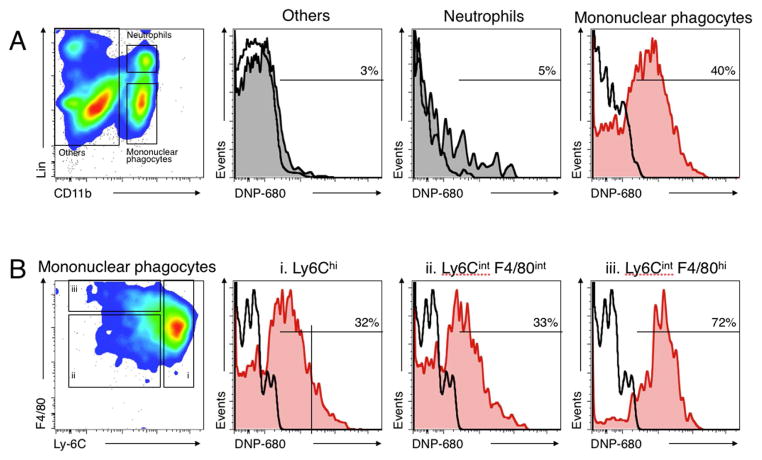
To augment the results of the IVM imaging, flow cytometry was performed on harvested tissues 24 h after administration of fluorescently labeled DNP. Presented in Figure 6, this data indicates that 40% of TAMs were positive for DNP, while only 5% of neutrophils and 3% of other lymphocytes were positive for DNP. Figure SI.3 (Supporting Information) includes FACS data analysis of DNP uptake in liver and kidney tissues. Here, 35 and 40% of macrophages are positive for DNP in the liver and kidney, respectively, and 7 and 9% of all other lymphocytes in these organs are positive for DNP. Together, this data and the IVM indicate strong specificity of DNP for macrophages.
Figure 6.
(A) Flow cytometric identification of mononuclear phagocytes (CD11b+ Linlow), neutrophils (CD11b+ Linhigh (Ly-6G+)), and other cells (CD11b−) in the tumor stroma (left). The three histograms on the top right show the percent of each population with detectable DNP-680 particles. Nearly all fluorescent DNP (DNP-680) is associated with mononuclear phagocytes; (B) Flow cytometric identification of mononuclear phagocyte subsets. CD11b+ Linlow cells were further divided into Ly6Chi (i) and Ly6Cint F4/80int (ii) monocyte-like cells and Ly-6Cint F4/80hi macrophages (left). The three histograms on the right show the percent of each population with detectable DNP-680 particles. DNP is associated with both monocyte subsets (Ly6Chi and Ly6Clo) as well as with macrophages (F4/80hi).
Discussion
We have synthesized a 13 nm nanoparticle comprised of short, crosslinked dextrans which were modified with desferrioxamine to chelate 89Zr for imaging of macrophages by PET. The nanoparticle was stable, biocompatible and accumulated specifically in tissue resident macrophages upon systemic administration. We show that cellular accumulation is complete within 24 hours after administration (vascular t1/2 3.9 hours), showing faster kinetics compared to larger dextran nanoparticles in clinical use (vascular t1/2 ~24–48 hours).6 We did not observe any adverse effects at the doses used and this is consistent with observations from dextran preparations in clinical use.28 It should be pointed out that the PET dose was <5% of the MR comparable dose of iron-oxide nanoparticles9 and could be further reduced with higher specific activity. Imaging and corroborative experiments in xenograft tumor models showed extensive accumulation in TAM and this should be particularly useful for prognostication of cancers29, 30 or therapy assessment.31, 32
Previously used dextran nanoparticles were primarily designed to have magnetic iron oxide cores and were naturally larger (20–200 nm). To determine the ideal size of dextran nanoparticles for PET applications, we initially performed a size optimization study leading up to the current preparation. Specifically, we synthesized analogous dextran nanoparticles of 5, 6, and 13 diameters (chosen from a larger size range study performed previously and ranging from 2 nm to 30 nm) and determined their pharmacokinetics, renal clearance rates and macrophage uptake in vivo. Perhaps as expected, the 5 nm nanoparticle showed considerable renal clearance whereas the 13 nm nanoparticle had highest macrophage uptake and was thus chosen for subsequent experiments. Leading up to this study we also compared the biological behavior of linear and cross-linked dextrans and found the latter to be better tolerated. While the current preparation indeed shows ideal imaging characteristics and clean macrophage uptake, based on the intravital microscopy and flow cytometry result, we anticipate a few changes for future clinical preparations. For one, we would anticipate using a 68Ga tracer to minimize radiation exposure and perhaps modulate the anticipated longer human blood half-life through modification of residuals amines with alternative capping agents.14
A number of prior studies have used alternative PET agents for imaging macrophages including 18FDG33, 34, ligands for the peripheral benzodiazepine receptor ligands (e.g. PK11195)35, 36 or magneto-optical nanoparticles.8, 9 Although commonly used, FDG is not macrophage specific as many non-phagocytic cells will also internalize glucose particularly cancer, neuronal, muscle cells as well as other leukocytes.37, 38 Peripheral benzodiazepine receptor ligands have been primarily used for neuroinflammation studies and few validation studies are available for peripheral macrophages. The receptor is now known to be the translocator protein (TSPO), an 18 kDa protein that has many functions and is found in diverse tissues including the heart, liver, adrenal, testis, brain, lymphatic systems in addition to leukocytes and macrophages.39 Therefore, any imaging agent targeting this receptor would not be specific for macrophages.
We anticipate that the developed macrophage selective imaging agents will be useful for a number of applications where quantitation of macrophages and inflammation levels are important. One of the most obvious applications is in cancer therapy where inflammation has been associated with inverse prognosis29 and where anti-macrophage strategies are now being employed to enhance therapeutic effects.32 Other applications include quantitation of inflammation in atherosclerosis and myocardial infarction in patients undergoing statin treatments. Macrophage imaging could be used as a surrogate of lesion activity. Finally, there are numerous other medical applications where foci of infection or inflammation require localization (e.g. neuroinflammation, Crohn’s disease, arthritis) or quantitation (e.g. macrophage activity in fat tissue of obese patients). We hope that the described material and future clinical derivatives fulfill the requirements of specificity and may be uniquely suited for clinical imaging.
Supplementary Material
Acknowledgments
We acknowledge the help of Dr. Rainer Kohler for help with intravital microscopy, Dr. Yvonne Fisher-Jeffes for review of the manuscript and Yoshiko Iwamoto for performing histology. We would also like to thank Nicholas Ramos (MSKCC) for 89Zr production. This work was funded in part by NIH grants P50 CA86355, U54 CA126515, U54 CA151884, HHSN268201000044C and U24 CA092782.
Footnotes
Supporting Information Available.
Experimental data regarding nanoparticle size distribution, intravital microscopy and flow cytometry analysis is presented. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Lee SH, Starkey PM, Gordon S. Quantitative analysis of total macrophage content in adult mouse tissues. Immunochemical studies with monoclonal antibody F4/80. J Exp Med. 1985;161:475–489. doi: 10.1084/jem.161.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ho MK, Springer TA. Tissue distribution, structural characterization, and biosynthesis of Mac-3, a macrophage surface glycoprotein exhibiting molecular weight heterogeneity. J Biol Chem. 1983;258:636–642. [PubMed] [Google Scholar]
- 3.Di Gregorio GB, Yao-Borengasser A, Rasouli N, Varma V, Lu T, Miles LM, Ranganathan G, Peterson CA, McGehee RE, Kern PA. Expression of CD68 and macrophage chemoattractant protein-1 genes in human adipose and muscle tissues: association with cytokine expression, insulin resistance, and reduction by pioglitazone. Diabetes. 2005;54:2305–2313. doi: 10.2337/diabetes.54.8.2305. [DOI] [PubMed] [Google Scholar]
- 4.Thiele J, Braeckel C, Wagner S, Falini B, Dienemann D, Stein H, Fischer R. Macrophages in normal human bone marrow and in chronic myeloproliferative disorders: an immunohistochemical and morphometric study by a new monoclonal antibody (PG-M1) on trephine biopsies. Virchows Arch A Pathol Anat Histopathol. 1992;421:33–39. doi: 10.1007/BF01607136. [DOI] [PubMed] [Google Scholar]
- 5.Gaglia JL, Guimaraes AR, Harisinghani M, Turvey SE, Jackson R, Benoist C, Mathis D, Weissleder R. Noninvasive imaging of pancreatic islet inflammation in type 1A diabetes patients. J Clin Invest. 2011;121:442–445. doi: 10.1172/JCI44339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harisinghani MG, Barentsz J, Hahn PF, Deserno WM, Tabatabaei S, van de Kaa CH, de la Rosette J, Weissleder R. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N Engl J Med. 2003;348:2491–2499. doi: 10.1056/NEJMoa022749. [DOI] [PubMed] [Google Scholar]
- 7.Leimgruber A, Berger C, Cortez-Retamozo V, Etzrodt M, Newton AP, Waterman P, Figueiredo JL, Kohler RH, Elpek N, Mempel TR, Swirski FK, Nahrendorf M, Weissleder R, Pittet MJ. Behavior of endogenous tumor-associated macrophages assessed in vivo using a functionalized nanoparticle. Neoplasia. 2009;11:459–68. doi: 10.1593/neo.09356. 2 p following 468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nahrendorf M, Keliher E, Marinelli B, Leuschner F, Robbins CS, Gerszten RE, Pittet MJ, Swirski FK, Weissleder R. Detection of macrophages in aortic aneurysms by nanoparticle positron emission tomography-computed tomography. Arterioscler Thromb Vasc Biol. 2011;31:750–757. doi: 10.1161/ATVBAHA.110.221499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nahrendorf M, Zhang H, Hembrador S, Panizzi P, Sosnovik DE, Aikawa E, Libby P, Swirski FK, Weissleder R. Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation. 2008;117:379–387. doi: 10.1161/CIRCULATIONAHA.107.741181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pressly ED, Rossin R, Hagooly A, Fukukawa K, Messmore BW, Welch MJ, Wooley KL, Lamm MS, Hule RA, Pochan DJ, Hawker CJ. Structural effects on the biodistribution and positron emission tomography (PET) imaging of well-defined (64)Cu-labeled nanoparticles comprised of amphiphilic block graft copolymers. Biomacromolecules. 2007;8:3126–3134. doi: 10.1021/bm700541e. [DOI] [PubMed] [Google Scholar]
- 11.Schipper ML, Cheng Z, Lee SW, Bentolila LA, Iyer G, Rao J, Chen X, Wu AM, Weiss S, Gambhir SS. microPET-based biodistribution of quantum dots in living mice. J Nucl Med. 2007;48:1511–1518. doi: 10.2967/jnumed.107.040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Almutairi A, Rossin R, Shokeen M, Hagooly A, Ananth A, Capoccia B, Guillaudeu S, Abendschein D, Anderson CJ, Welch MJ, Frechet JM. Biodegradable dendritic positron-emitting nanoprobes for the noninvasive imaging of angiogenesis. Proc Natl Acad Sci U S A. 2009;106:685–690. doi: 10.1073/pnas.0811757106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nahrendorf M, Keliher E, Marinelli B, Waterman P, Feruglio PF, Fexon L, Pivovarov M, Swirski FK, Pittet MJ, Vinegoni C, Weissleder R. Hybrid PET-optical imaging using targeted probes. Proc Natl Acad Sci U S A. 2010;107:7910–7915. doi: 10.1073/pnas.0915163107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weissleder R, Kelly K, Sun EY, Shtatland T, Josephson L. Cell-specific targeting of nanoparticles by multivalent attachment of small molecules. Nat Biotechnol. 2005;23:1418–1423. doi: 10.1038/nbt1159. [DOI] [PubMed] [Google Scholar]
- 15.Zinderman CE, Landow L, Wise RP. Anaphylactoid reactions to Dextran 40 and 70: reports to the United States Food and Drug Administration, 1969 to 2004. J Vasc Surg. 2006;43:1004–1009. doi: 10.1016/j.jvs.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Macdougall IC. Evolution of iv iron compounds over the last century. J Ren Care. 2009;35(Suppl 2):8–13. doi: 10.1111/j.1755-6686.2009.00127.x. [DOI] [PubMed] [Google Scholar]
- 17.Pittet MJ. Behavior of immune players in the tumor microenvironment. Curr Opin Oncol. 2009;21:53–59. doi: 10.1097/CCO.0b013e32831bc38a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coussens LM, Pollard JW. Leukocytes in mammary development and cancer. Cold Spring Harb Perspect Biol. 2011:3. doi: 10.1101/cshperspect.a003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holland JP, Sheh Y, Lewis JS. Standardized methods for the production of high specific-activity zirconium-89. Nucl Med Biol. 2009;36:729–739. doi: 10.1016/j.nucmedbio.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric Method for Determination of Sugars and Related Substances. Analytical Chemistry. 1956;28:350–356. [Google Scholar]
- 21.Snyder SL, Sobocinski PZ. An improved 2,4,6-trinitrobenzenesulfonic acid method for the determination of amines. Anal Biochem. 1975;64:284–288. doi: 10.1016/0003-2697(75)90431-5. [DOI] [PubMed] [Google Scholar]
- 22.Orth JD, Kohler RH, Foijer F, Sorger PK, Weissleder R, Mitchison TJ. Analysis of mitosis and antimitotic drug responses in tumors by in vivo microscopy and single-cell pharmacodynamics. Cancer Res. 2011;71:4608–4616. doi: 10.1158/0008-5472.CAN-11-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holland JP, Divilov V, Bander NH, Smith-Jones PM, Larson SM, Lewis JS. 89Zr-DFO-J591 for immunoPET of prostate-specific membrane antigen expression in vivo. J Nucl Med. 2010;51:1293–1300. doi: 10.2967/jnumed.110.076174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mempel TR, Pittet MJ, Khazaie K, Weninger W, Weissleder R, von Boehmer H, von Andrian UH. Regulatory T cells reversibly suppress cytotoxic T cell function independent of effector differentiation. Immunity. 2006;25:129–141. doi: 10.1016/j.immuni.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 25.Pedersen AE, Buus S, Claesson MH. Treatment of transplanted CT26 tumour with dendritic cell vaccine in combination with blockade of vascular endothelial growth factor receptor 2 and CTLA-4. Cancer Lett. 2006;235:229–238. doi: 10.1016/j.canlet.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 26.Yang XD, Ai W, Asfaha S, Bhagat G, Friedman RA, Jin G, Park H, Shykind B, Diacovo TG, Falus A, Wang TC. Histamine deficiency promotes inflammation-associated carcinogenesis through reduced myeloid maturation and accumulation of CD11b+Ly6G+ immature myeloid cells. Nat Med. 2011;17:87–95. doi: 10.1038/nm.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stangl S, Gehrmann M, Riegger J, Kuhs K, Riederer I, Sievert W, Hube K, Mocikat R, Dressel R, Kremmer E, Pockley AG, Friedrich L, Vigh L, Skerra A, Multhoff G. Targeting membrane heat-shock protein 70 (Hsp70) on tumors by cmHsp70.1 antibody. Proc Natl Acad Sci U S A. 2011;108:733–738. doi: 10.1073/pnas.1016065108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh A, Patel T, Hertel J, Bernardo M, Kausz A, Brenner L. Safety of ferumoxytol in patients with anemia and CKD. Am J Kidney Dis. 2008;52:907–915. doi: 10.1053/j.ajkd.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Steidl C, Lee T, Shah SP, Farinha P, Han G, Nayar T, Delaney A, Jones SJ, Iqbal J, Weisenburger DD, Bast MA, Rosenwald A, Muller-Hermelink HK, Rimsza LM, Campo E, Delabie J, Braziel RM, Cook JR, Tubbs RR, Jaffe ES, Lenz G, Connors JM, Staudt LM, Chan WC, Gascoyne RD. Tumor-associated macrophages and survival in classic Hodgkin's lymphoma. N Engl J Med. 2010;362:875–885. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 31.De Palma M, Lewis CE. Cancer: Macrophages limit chemotherapy. Nature. 2011;472:303–304. doi: 10.1038/472303a. [DOI] [PubMed] [Google Scholar]
- 32.DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, Junaid SA, Rugo HS, Hwang ES, Jirström K, West BL, Coussens LM. leukocyte Complexity Predicts Breast Cancer survival and Functionally regulates response to Chemotherapy. Cancer Discovery. 2011;1:52–64. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rogers IS, Tawakol A. Imaging of coronary inflammation with FDG-PET: feasibility and clinical hurdles. Curr Cardiol Rep. 2011;13:138–144. doi: 10.1007/s11886-011-0168-3. [DOI] [PubMed] [Google Scholar]
- 34.Galban CJ, Bhojani MS, Lee KC, Meyer CR, Van Dort ME, Kuszpit KK, Koeppe RA, Ranga R, Moffat BA, Johnson TD, Chenevert TL, Rehemtulla A, Ross BD. Evaluation of treatment-associated inflammatory response on diffusion-weighted magnetic resonance imaging and 2-[18F]-fluoro-2-deoxy-D-glucose-positron emission tomography imaging biomarkers. Clin Cancer Res. 2010;16:1542–1552. doi: 10.1158/1078-0432.CCR-08-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schweitzer PJ, Fallon BA, Mann JJ, Kumar JS. PET tracers for the peripheral benzodiazepine receptor and uses thereof. Drug Discov Today. 2010;15:933–942. doi: 10.1016/j.drudis.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 36.Chauveau F, Boutin H, Van Camp N, Dolle F, Tavitian B. Nuclear imaging of neuroinflammation: a comprehensive review of [11C]PK11195 challengers. Eur J Nucl Med Mol Imaging. 2008;35:2304–2319. doi: 10.1007/s00259-008-0908-9. [DOI] [PubMed] [Google Scholar]
- 37.Locasale JW, Vander Heiden MG, Cantley LC. Rewiring of glycolysis in cancer cell metabolism. Cell Cycle. 2010;9:4253. doi: 10.4161/cc.9.21.13925. [DOI] [PubMed] [Google Scholar]
- 38.Vander Heiden MG, Locasale JW, Swanson KD, Sharfi H, Heffron GJ, Amador-Noguez D, Christofk HR, Wagner G, Rabinowitz JD, Asara JM, Cantley LC. Evidence for an alternative glycolytic pathway in rapidly proliferating cells. Science. 2010;329:1492–1499. doi: 10.1126/science.1188015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapere JJ, Lindemann P, Norenberg MD, Nutt D, Weizman A, Zhang MR, Gavish M. Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci. 2006;27:402–409. doi: 10.1016/j.tips.2006.06.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.