Abstract
The current study tested the association between fear and perception in spider phobic individuals (n = 57) within the context of a treatment outcome study. Participants completed 5 post-treatment Behavioral Approach Tasks (BATs) in which they encountered a live spider and were asked to provide spider size estimates. Consistent with predictions, results indicated that high levels of fear were associated with magnified perception of phobic stimuli. Specifically, we found a significant positive correlation between size estimates and self-reported fear while encountering spiders. Together with previous findings, these results further support the notion that fear is involved in the encoding and processing of perceptual information.
Keywords: Phobias, Cognitive Bias, Perceptual Distortion
1. Introduction
The cognitive biases that characterize phobic individuals have garnered considerable research interest. Studies have consistently found attentional biases toward threat in phobics compared to controls, pointing toward a hyperactive threat detection system (e.g., Cisler et al., 2007; Mogg & Bradley, 2006). Interpretational biases have also been demonstrated, indicating a heightened propensity in phobics to interpret ambiguous stimuli as threatening (e.g., Riskind et al., 1995; Teachman & Woody, 2003). Evidence for memory biases in phobics has been quite mixed; most studies have failed to find evidence for a bias (see Coles & Heimberg, 2002), although some recent studies have indeed found biases (e.g., Cody & Teachman, 2010; Reinecke et al., 2010).
Only recently, however, have researchers begun to investigate the possibility that phobias may not only involve distorted cognitive processing of threat stimuli, but also distorted encoding and processing of perceptual information related to threat. This line of research reflects the view that phobic individuals may see, or report having seen, threatening objects as much larger or more extreme than they actually are. In accord with oft-reported anecdotes of rats the size of small children or snakes as long as fire-hoses, fear may alter the processing of visual information.
This connection between fear and visual perception is not only supported by these anecdotal claims, but also by neurobiological findings. The amygdala – which is crucially implicated in the phobic response – has considerable reciprocal connections with the visual cortex (see Phelps, 2004; Amaral et al., 1992). Importantly, the amygdala not only receives sensory input, but also feeds back to visual cortices to direct further perceptual processing, based on determinations of the emotional salience of stimuli (see Phelps, 2004; Vuilleumier & Driver, 2007). In the case of threatening stimuli, the strength of amygdala activation has been correlated with activation in the visual cortex (Larson et al., 2006; Ahs et al., 2008). These findings suggest that once the amygdala detects threat, it recruits perceptual resources to alter further visual processing (see Phelps, 2004).
Research in the field of visual perception has also supported a link between fear and visual perception. Several studies have implicated the role of non-visual factors on visual perception. These studies have demonstrated that individuals differ in perceptions of slant, distance, and height based on factors such as environmental context, the presence of chronic pain, and glucose consumption (Witt et al, 2007; Witt et al., 2009; Schnall et al., 2010). Importantly, an individual s level of fear has also been shown to influence visual perception. For example, individuals standing on a skateboard at the top of a hill (which was shown to provoke fear) estimated the hill as steeper than individuals standing on a wooden box (Stefanucci et al., 2008).
Researchers have recently begun to translate these theoretical formulations into clinical investigations; two studies have examined the possibility of biased threat perception in phobic individuals. Notably, Teachman et al. (2008) tested for a perceptual bias in acrophobia (height fear) by using a visual matching task, in which participants looked over a two-story balcony and directed an experimenter to stand an equivalent horizontal distance from them as they were from the ground. Individuals high in acrophobia overestimated height of the balcony more than individuals low in acrophobia, with a between groups difference of nearly 5 feet. Height estimates, however, were not significantly related to self-reported anxiety following height exposures.
Building on this work, Clerkin et al. (2009) had participants estimate the height of a balcony using the same visual matching task, but added an imagery induction (instructing participants to imagine leaning over the edge, losing balance, and falling). As predicted, there was a main effect for imagery, with individuals receiving the imagery induction overestimating the height of the balcony more than controls. There was also an imagery by height status interaction, with high and low fear individuals differing in the extent to which they overestimated the balcony in the imagery condition versus the control condition (high fear individuals showed a greater difference across conditions). In this study, height estimates were also significantly related to state measures of anxious cognitions and bodily sensations, but only when those measured were administered following the height exposure. Self-reported anxiety during the actual experience did not correlate with height estimation. Furthermore, the main finding from Teachman et al. (2008) was not replicated; there was no main effect for height status. That is, high fear individuals did not offer larger average height estimates than low fear individuals.
Thus, while both studies have lent some support to the hypothesized connection between fear and perceptual bias, some uncertainty remains. Specifically, although theoretical work (in neurobiology and visual perception) would predict that an individual s fear level while encountering the phobic stimulus likely has a greater impact on perception than their trait-level fear, neither of the previous studies have found strong relations between measures administered during the phobic encounter and perceptual bias. While certain findings (like the relation between imagery group and height overestimation) point toward this connection between in-the-moment fear and perceptual bias, the fact that none have been found introduces a degree of ambiguity to the formulation.
In the current study, we sought to clarify some of this ambiguity by testing the relation between fear level and spider size estimation in spider phobia. To do so, we collected data from spider phobic individuals within the context of a treatment outcome study and examined the association between fear and the ratio of a participant s spider size estimates relative to objective measures (henceforth labeled size ratio ). Consistent with theoretical work on the relation between fear and perception, we predicted that elevated subjective units of distress (SUDs) ratings during the spider encounter would predict overestimation of spider size.
2. Method
2.1. Participants
Participants were 57 undergraduate students from the Ohio State University and individuals from the Columbus, Ohio metro area. Students were recruited from undergraduate psychology courses and received partial course credit for their participation. Community volunteers responded to flyers seeking individuals who were very afraid of spiders and received $60 for their participation. All participants met diagnostic criteria for specific phobia of spiders, as assessed by the Structured Clinical Interview for DSM-IV Axis I Disorders. Interviews were administered by clinical psychology doctoral students trained to criterion levels of inter-rater agreement. The mean age of the sample was 19.3 years (SD = 2.6) and 80% were female. Approximately 80% of the participants were White, 11% African-American or Black, 4% Asian or Asian-American, 2% Hispanic or Latino/Latina, 2% Native Hawaiian or other Pacific Islander, and 2% other.
2.2. Spiders
The spiders used in the study were tarantulas from one of two closely related genera: Grammostola (e.g., Grammostola pulchripes, the Chaco Golden Knee Tarantula) and Brachypelma (e.g., Brachypelma smithi, The Mexican Red-Knee Tarantula). Members of these genera were used because of their reputation for docile temperament and because of the distinct differences in sizes and appearance across spiders. Each participant interacted with five distinct varieties of tarantulas, varying in size from small (e.g., 2 cm) to large (e.g., 15 cm.).
2.3. Measures
2.3.1. Fear during spider encounter
Throughout their encounters with spiders, participants were asked for SUDs ratings on a scale of 0 to 100. These verbal self-reports were used as an index of state anxiety during the spider encounter. Following each encounter, participants completed the Intertrial Questionnaire (IQ), a 16 item self-report measure with a scale of 0 to 8 (Rowe & Craske, 1998). Answers to the first IQ item (“What was the most anxiety/fear you felt during this task – how high did your anxiety/fear go?) were used as written self-reports of peak anxiety during the task.
Self-reported physiological arousal and fearful cognitions experienced during the spider encounters were assessed by subsequent items of the IQ. These items correspond to the 13-item DSM-IV checklist of panic symptoms (American Psychiatric Association, 1994). Participants are asked, for example, how much they experienced heart palpitations, sweating, dizziness, and fear of losing control during the encounter. The measure showed strong reliability in our sample (Cronbach s alpha = 0.92).
2.3.2. Thoughts about fear reduction and future spider encounters
After having completed the IQ, participants filled out the Metacognition Questionnaire (MCQ), a 4 item self report measure with a scale of 0 to 8 (Rowe & Craske, 1998). The 4 items ask participants to rate (1) the extent to which their fear has decreased, (2) the permanence of this reduction, (3) their level of fear if confronted with a spider outside of the experiment, and (4) their level of fear if asked to repeat the task in a few weeks.
2.3.3. Spider size estimation and bias
Participants estimated size of spiders by drawing a single line on a large (8x5 inch) index card indicating the length of the spider from the tips of its front legs to the tips of its back legs. Prior to the size estimation the spider tank was covered (such that the spider could no longer be viewed). The size ratio1 was calculated by dividing the participant s size estimate by an objective measure of the spider s actual size. Actual size measurements were conducted weekly by three independent raters while viewing the spiders in clear plastic containers (i.e., 16 oz. deli cups). Raters were instructed to measure spiders in their most elongated position in order to produce the most conservative (i.e., largest possible) estimate of the spiders true size. A spider s actual size for a given session was calculated as the average measurement across all three raters for the week in which a session was conducted. Raters were very consistent in their measurements (Cronbach s alpha = 0.98).
2.3.4. Spider fear
Spider fear was measured by the Fear of Spiders Questionnaire (FSQ), an 18 item self-report measure. Szymanski and O'Donohue (1995) demonstrated that the FSQ has good psychometric properties and is sensitive to differences between phobics and nonphobics.
2.4. Procedure
Three sessions were held over the course of 8 weeks. In each session, participants completed one or more Behavioral Approach Tasks (BATs), in which they encountered a live spider in an uncovered glass tank. Participants began 12 feet from the tank (Step 1) and were asked to approach the spider. Once standing next to the tank (Step 2), they were asked to guide the spider around the tank by touching it first with an 8-inch probe for two minutes (Step 3), then with a 5.5 inch probe for two minutes (Step 4). SUDS ratings were made prior to each of the four steps and again after completion of Step 4. Following each BAT, the spider tank was covered by a white sheet. Participants then filled out the IQ and MCQ (which typically took approximately 2 minutes) and were asked to estimate the spider s size. In session 1, participants approached one spider. In session 2, participants completed a standardized exposure therapy protocol (Rowe & Craske, 1998) facilitated by clinical psychology doctoral students. Participants then approached both a familiar and a novel spider. In session 3, participants approached a familiar spider and two novel spiders. At the end of each session, participants completed the FSQ.
3. Results
3.1. Overview of data analysis
Data were analyzed for all 5 post-treatment BATs. The pre-treatment BAT was not included because, in contrast to post-treatment sessions, participants did not have an expectation that they would be asked for a size estimate of the spider. Thus, participants may have approached the task in systematically different ways (e.g., differing in the degree to which they attended to the spider s size) in the pre- vs. post-treatment sessions. Additionally, since all participants met diagnostic criteria for specific phobia of spiders, the range of pre-treatment Subjective Unit of Distress (SUDS) ratings was severely restricted. Although it was excluded from correlational analyses, it is important to note that a one-sample t-test demonstrated that the average size ratio for the pre-treatment BAT (M = 1.09, SD = .32), was significantly greater than 1.0, t (56) = 2.10, p = .04.
Size estimates were only analyzed for BATs in which the participant attempted to approach the spider. Out of 285 total BATs (57 participants each completed 5 BATs), on only 6 occasions did a participant refuse to approach ( 2% of all BATs). No participants refused to approach more than once (i.e., all participants completed at least 4 BATs). For these participants, average size ratio and fear were computed across all BATs in which they actually approached the spider.
3.2. Size ratio as a function of fear during BATs
To evaluate the relationship between size ratio and fear during the phobic encounter, we averaged participants verbally reported peak SUDs ratings (i.e., the highest of the five SUDS ratings made during each BAT) and spider size ratio across BATs. A preliminary examination of the data revealed one significant outlier. Regression diagnostics revealed that it had an undue impact on the estimated correlation (e.g., Studentized deleted residual = 3.76, p < .001). Consequently, we report correlations reflecting both the full sample and in a reduced sample with the outlier dropped.
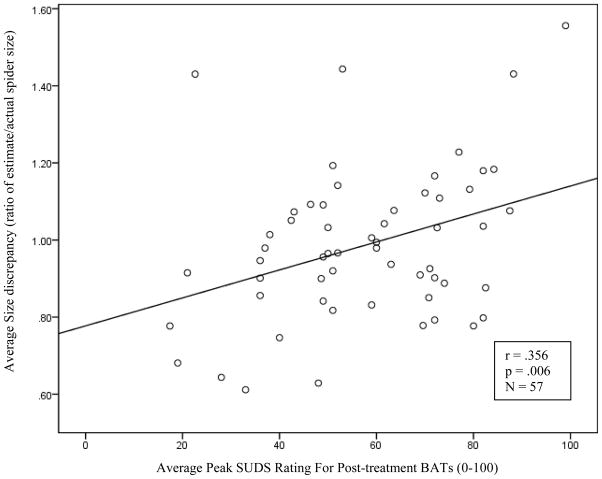
Consistent with predictions, average peak SUDs ratings significantly correlated with average spider size ratio across post-treatment encounters (rFull Sample = 0.36, p = .006; rReduced Sample = 0.46, p < .001). To ensure that this correlation was not primarily due to a participant s tendency to consistently estimate the size of a familiar spider (i.e., always draw a line of the same length after viewing a particular spider), we also tested the relation between peak SUDs and size ratio for the 3 novel spiders only, with similar results (rFull Sample = 0.37, p = .005; rReduced Sample = .47, p < .001)2.
As shown in Table 1, similar results were found when assessing the relationship between size ratio and participants written reports of anxiety during spider encounters (responses to the question: “What was the most anxiety/fear you felt during this task how high did your anxiety/fear go?”) and their reports of physiological arousal during the BAT.
Table 1.
Pearson correlation coefficients among size ratio and anxiety/fear measures.
| Peak Anxiety (SUDS) | Peak Anxiety (IQ 1) | Physiologic arousal (IQ 4-16) | Spider Fear (FSQ) | |
|---|---|---|---|---|
| Size ratio (all post-treatment BATs) | .36** [.46**] |
.31* [.37**] |
.25 [.30*] |
.19 [.24] |
| Size ratio (novel spider BATs only) | .37** [.47**] |
.34* [.38**] |
.29* [.34*] |
.15 [.20] |
Note: Measures reported in this table refer to averages across post-treatment BATs (row 1) and across BATs with novel spiders only (row 2). The top correlations in each cell are based on the entire sample (N=57) while the bottom correlations (in brackets) refer to the partial sample with 1 excluded outlier (N=56).
p < .05 level
p < .01 level
3.3. Size ratio as a function of trait fear
We hypothesized that size ratio would be more related to state measures of fear during the phobic encounter than to trait measures of spider fear. To test this hypothesis, we first examined the relationship between participants average post-treatment FSQ scores and average size ratio. Consistent with predictions, there was a marginal yet non-significant association between average FSQ scores across both post-treatment sessions and average spider size ratio across post-treatment spider encounters (r = 0.19, p = .16). Similar results were observed in relation to novel spider encounters (r = 0.15, p = .26).
We also tested whether trait fear (as measured by the FSQ) accounted for significant unique variance in size ratio for novel spiders over and above SUDS ratings during BATs. When FSQ scores were added to a regression model containing average peak SUDs ratings, they failed to account for significant additional variance (srFSQ = −.05, p = .66; srSUDS = .32, p = .004). Thus, it is clear that average FSQ scores do not make significant unique contributions toward predicting average size ratio (t = 0.27, p = .79). These results imply that subjective fear in the moment is a stronger predictor of size ratio than a more global measure of spider fear.
3.4. Overall size ratio
The average size ratio ratios across post-treatment BATs and for novel spiders were .987 and 1.011, respectively. In contrast to the pre-treatment size ratio, neither of these ratios was significantly different from 1.0 (t = 0.51, p = 0.61 and t = 0.35, p = 0.73, respectively), implying that participants estimates, on average, and independent raters measurements tended to agree regarding the length of the spider.
4. Discussion
These results suggest that high levels of fear are associated with magnified perception of phobic stimuli. Together with the findings in Teachman et al. (2008) and Clerkin et al. (2009), these results further support the notion that fear is involved in the encoding and processing of perceptual information. The current study adds to this formulation by providing evidence for a link between size estimation and fear during a phobic encounter. To our knowledge, this is the first finding of this nature in the literature.
This evidence for a link between fear and size discrepancy, however, does not necessarily imply a purely perceptual bias in which fearful individuals actually see spiders as larger than they actually are. In fact, our procedure, in which participants estimated the spider s size when the spider was no longer in view and after having completed two short questionnaires, potentially captures both biased encoding of and memory for perceptual information. As such, we cannot assess the relative extent to which these biases contributed to the observed size discrepancy.
Furthermore, even if evidence for size bias at encoding was found, the bias may not be wholly perceptual in nature. Instead, there are various ways in which cognitive factors, like attention and memory, may have figured crucially into observed size discrepancies. For instance, one s attentional allocation (either directly toward the spider or away from it) could impact the amount and quality of size information perceived and consequently influence one s estimate. That is, fearful individuals may be biased in part because they spend less time directly looking at the spider and thus rely on inferences from their level of fear (e.g., “I don t remember its size very well, but I was terrified, so it must have been huge!”).
This notion, however, runs counter to a number of findings that show enhanced attentional capture and processing in response to emotional stimuli (e.g., Ohman, Flykt, and Esteves, 2001). According to this view, fearful individuals should actually spend more time with their attention locked in on the spider, potentially resulting in a more accurate size estimate. A possible resolution to these contradictory viewpoints may involve understanding the degree to which the size of the spider is attentionally salient. It could be that fearful individuals do indeed show enhanced attention when viewing the spider, but that this enhanced attention is directed to aspects other than the form of the spider. If the primary source of threat is the spider s movement (e.g., “It could scurry up my arm!”), attention may be devoted to scanning for movement of the spider and monitoring its distance, potentially precluding the encoding of size information. Unfortunately, the data in this study cannot resolve this issue; future research is needed to shed light on the role of attention on size estimation.
Another cognitive factor that may influence size estimation is memory. Given the fact that size estimates were made with the spider tank covered, a memory bias could have led to a magnified representation of the spider in fearful individuals. For phobics, it might indeed be adaptive to exaggerate the size and consequent danger of the phobic stimulus to make future encounters less likely. This postulation, however, potentially contradicts findings that point toward enhanced memory for threat-relevant stimuli in phobics (e.g., Reinecke et al., 2010) and positive associations between amygdala response at encoding and success of future recollection (e.g., Cahill et al., 2003). But while these findings indicate that phobics may be better at noting the presence or absence of fearful stimuli, it could be that the representation of perceptual elements of these stimuli in memory is distorted. It could also be that ex consequentia reasoning (“If I feel this anxious, the spider must have been huge”) could impact representations of the spider (Arntz, Rauner, & Van den Hout, 1995). Future studies should test these and other potential mediators of size estimation bias; existing research has only pointed toward to the existence of a bias, not mechanisms that might produce it.
Future research is also necessary to investigate the extent to which this size estimation bias figures into the maintenance of fear and anxiety. It is possible that a tendency to magnify phobic stimuli leads to increased fear and subsequent avoidance. Such a possibility would seem to be consistent with the fact that Clerkin et al. (2009) found balcony height estimates to be correlated and post-exposure ratings of anxiety but not with anxiety during the actual experience. The notion that biased perceptions might lead to increased fear is also supported by research on false-feedback. For example, Ehlers, Margraf, Rogh, Taylor, and Birbaumer (1988) found that panic disorder patients who were misled to believe their heart rates had increased reported increased anxiety and physiological arousal compared to control patients. That is, the experimental inducement of perceptual distortions has been shown to lead to heightened fear responses.
While size estimation bias may increase fear, making phobics aware of this bias may have some therapeutic value. For instance, showing a phobic individual a clear difference between what they perceive and what actually exists could support the more general goal in cognitive-behavioral approaches of encouraging patients to view their thoughts and perceptions as guesses subject to investigation, as opposed to absolute facts. Indeed, perceptual feedback – in the form of heart rate and skin conductance biofeedback – has been shown to reduce anxiety symptoms in patients with animal phobia and panic disorder (Nunes & Marks, 1975; Gilbert, 1986). Researchers have suggested that this feedback may help patients become aware of their tendencies to misperceive threat-relevant stimuli. As a result, patients may reduce their likelihood of responding to these misperceptions with anxious thoughts and behaviors (Story & Craske, 2008).
While it may seem difficult to make phobics aware of their tendency to overestimate the size of spiders, given the fact that the average size ratio across post-treatment sessions did not differ from 1.0, a few points should be mentioned. First, the average size ratio in the pre-treatment BAT was significantly different from 1.0, implying a propensity to magnify spider size in non-treated phobics. Second, it should be noted that raters were instructed to measure spiders in their most elongated position in order to produce the largest possible (i.e., most conservative) estimate of the spiders true size. In most cases, participants did not view spiders in this fully elongated position; instead, the spiders often responded to being placed in the tank by adopting a hunched posture, which, given the length of the spiders legs, could result in the spider appearing as little as half the size as it would appear in an elongated position. Thus, it is quite possible that, despite an average size ratio of approximately 1.0, participants on average actually overestimated the size of the spider when compared to its fully extended length. Similarly, participants with bias ratios less than 1.0 may not have been underestimating the size of the spider; instead they may have been accurately assessing the apparent size of the spider as presented. At the very least, we suggest that our calculation of bias is likely a lower bound of the degree of actual bias.
4.1. Limitations and conclusions
A limitation of this study is that data were only collected from individuals with spider phobia who had undergone treatment. Thus, although data were analyzed only for post-treatment BATs (in which participants varied considerably in spider fear; see Figure 1), results may not generalize outside of the context of treatment. Future work should test the relation between fear and size estimation outside of a treatment context with participants from the full range of spider fear. Another limitation is that this study is correlational, thus prohibiting an understanding of fear s causal role in size overestimation. Finally, since our size estimation procedure potentially includes biases at encoding as well as during processing and recall, we cannot assess the relative extent to which these processes contribute to overall size bias. Despite these limitations, this study provides an empirical basis for the role of fear in the encoding and processing of perceptual information related to threat. Moreover, it is the first study of its kind to demonstrate a link between perceptual bias and fear during a phobic encounter. Taken together with past investigations, these results further support the notion that individuals magnify phobic stimuli when in fearful state. Future research will be necessary to understand how specific cognitive and perceptual factors might play a role in this bias and the ways in which perceptual biases relate to the maintenance and treatment of phobias.
Figure 1.
Scatterplot of the association between average size ratio and average peaks SUDS rating across all post-treatment BATs.
Highlights.
Test of association between fear and perception in spider phobics
Phobics encountered 5 spiders, reported fear, and estimated size of spiders
Significant correlation between size estimates and self-reported fear
High levels of fear associated with magnified perception of spiders
Acknowledgments
This research was supported by Grant MH38832 from the National Institute of Mental Health. The authors thank Anna Levin and Salene Wu for their contributions to the study.
Footnotes
A ratio (as opposed to a difference score) was used so that each size estimate would receive equal weight when averaged. Otherwise, estimates for larger spiders would disproportionately contribute to overall size estimation. Even when difference scores are used as the DV, statistically similar findings were obtained.
Size discrepancy was highly reliable across post-treatment sessions (Cronbach s alpha = .86) and for novel spiders (.85). Peak SUDS ratings were also highly reliable across post-treatment sessions (Cronbach s alpha = .89) and for novel spiders (.81)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahs F, Pissiota A, Michelgard A, Frans O, Furmark T, Appel L, Fredrikson M. Disentangling the web of fear: Amygdala reactivity and functional connectivity in spider and snake phobia. Psychiatry Research: Neuroimaging. 2009;172:103–108. doi: 10.1016/j.pscychresns.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Behniea H, Kelly JL. Topographic organization of projections from the amygdala to the visual cortex in the macaque monkey. Neuroscience. 2003;118(4):1099–1120. doi: 10.1016/s0306-4522(02)01001-1. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: 1994. [Google Scholar]
- Arntz A, Rauner M, Van den Hout M. “If I feel anxious, there must be danger”: Ex-consequentia reasoning in inferring danger in anxiety disorders. Behaviour Research and Therapy. 1995;33(8):917–925. doi: 10.1016/0005-7967(95)00032-s. [DOI] [PubMed] [Google Scholar]
- Cahill L, Kilpatrick L. Amygdala modulation of parahippocampal and frontal regions during emotionally influenced memory storage. NeuroImage. 2003;20:2091–2099. doi: 10.1016/j.neuroimage.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Cisler JM, Ries BJ, Widner RL., Jr Examining information processing biases in spider phobia using the rapid serial visual presentation paradigm. Journal of Anxiety Disorders. 2007;21:977–990. doi: 10.1016/j.janxdis.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Clerkin EM, Cody MW, Stefanucci JK, Profitt DR, Teachman BA. Imagery and fear influence height perception. Journal of Anxiety Disorders. 2009;23:381–386. doi: 10.1016/j.janxdis.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cody MW, Teachman BA. Post-event processing and memory bias for performance feedback in social anxiety. Journal of Anxiety Disorders. 2010;24:468–479. doi: 10.1016/j.janxdis.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin. 1992;112(1):155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Coles ME, Heimberg RG. Memory biases in the anxiety disorders: Current status. Clinical Psychology Review. 2002;22:587–627. doi: 10.1016/s0272-7358(01)00113-1. [DOI] [PubMed] [Google Scholar]
- Ehlers A, Margraf J, Roth WT, Taylor CB, Birbaumer N. Anxiety induced by false heart rate feedback in patients with panic disorder. Behavior Research and Therapy. 1988;26:1–11. doi: 10.1016/0005-7967(88)90028-9. [DOI] [PubMed] [Google Scholar]
- Gilbert C. Skin conductance feedback and panic attacks. Biofeedback and Self-Regulation. 1986;11:251–254. doi: 10.1007/BF01003484. [DOI] [PubMed] [Google Scholar]
- Judd CM, McClelland GH, Ryan CS. Data analysis: A model comparison approach. 2. New York: Routledge; 2009. [Google Scholar]
- Larson CL, Schaefer HS, Siegle GJ, Jackson CAB, Anderle MJ, Davidson RJ. Fear is fast in phobic individuals: Amygdala activation in response to fear-relevant stimuli. Biological Psychiatry. 2006;60:410–417. doi: 10.1016/j.biopsych.2006.03.079. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. Time course of attentional bias for fear-relevant pictures in spider-fearful individuals. Behaviour Research and Therapy. 2006;44:1241–1250. doi: 10.1016/j.brat.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Nunes JS, Marks IM. Feedback of true heart rate during exposure in vivo. Archives of General Psychiatry. 1975;32:933–936. doi: 10.1001/archpsyc.1975.01760250125014. [DOI] [PubMed] [Google Scholar]
- Ohman A, Flykt A, Esteves F. Emotion drives attention: detecting the snake in the grass. Journal of Experimental Psychology. 2001;130(3):466–478. doi: 10.1037/0096-3445.130.3.466. [DOI] [PubMed] [Google Scholar]
- Phelps E. The human amygdala and awareness: Interactions between emotion and cognition. In: Gazzaniga MS, editor. The Cognitive Neurosciences III. Cambridge: The MIT Press; 2004. pp. 1005–1015. [Google Scholar]
- Reinecke A, Becker ES, Rinck M. Visual working memory and threat monitoring: Spider fearfuls show disorder-specific change detection. Behaviour Research and Therapy. 2010;48(8):770–778. doi: 10.1016/j.brat.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Riskind JH, Moore R, Bowley L. The looming of spiders: The fearful perceptual distortion of movement and menace. Behaviour Research and Therapy. 1995;33:171–178. doi: 10.1016/0005-7967(94)e0023-c. [DOI] [PubMed] [Google Scholar]
- Rowe MK, Craske MG. Effects of an expanding-spaced vs massed exposure schedule on fear reduction and return of fear. Behaviour Research and Therapy. 1998;36:701–717. doi: 10.1016/s0005-7967(97)10016-x. [DOI] [PubMed] [Google Scholar]
- Schnall S, Zadra JR, Proffitt DR. Direct evidence for the economy of action: Glucose and the perception of geographical slant. Perception. 2010;39(4):464–82. doi: 10.1068/p6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanucci JK, Proffitt DR, Clore GL, Parekh N. Skating down a steeper slope: fear influences the perception of geographical slant. Perception. 2008;37:321–323. doi: 10.1068/p5796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Story TJ, Craske MG. Responses to false physiological feedback in individuals with panic attacks and elevated anxiety sensitivity. Behavior Research and Therapy. 2008;46:1001–1008. doi: 10.1016/j.brat.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Szymanski J, O’Donohue W. Fear of Spiders Questionnaire. Journal of Behavioral Therapy & Experimental Psychiatry. 1995;26:31–34. doi: 10.1016/0005-7916(94)00072-t. [DOI] [PubMed] [Google Scholar]
- Teachman BA, Stefanucci JK, Clerkin EM, Cody MW, Proffitt DR. A new mode of fear expression: Perceptual bias in height fear. Emotion. 2008;8:296–301. doi: 10.1037/1528-3542.8.2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teachman BA, Woody SR. Automatic processing in spider phobia: Implicit fear associations over the course of treatment. Journal of Abnormal Psychology. 2003;112:100–109. [PubMed] [Google Scholar]
- Vuilleumier P, Driver J. Modulation of visual processing by attention and emotion: Windows on causal interactions between human brain regions. Philosophical Transaction of the Royal Society of Biological Sciences. 2007;362:837–855. doi: 10.1098/rstb.2007.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt JK, Proffitt DR. Perceived slant: A dissociation between perception and action. Perception. 2007;36:249–257. doi: 10.1068/p5449. [DOI] [PubMed] [Google Scholar]
- Witt JK, Linkenauger SA, Bakdash JZ, Augustyn JS, Cook A, Proffitt DR. The long road of pain: chronic pain increases perceived distance. Experimental Brain Research. 2009;192:145–148. doi: 10.1007/s00221-008-1594-3. [DOI] [PMC free article] [PubMed] [Google Scholar]