Abstract
Eosinophils play important roles in regulation of cellular responses under conditions of homeostasis or infection. Intestinal infection with the parasitic nematode, Trichinella spiralis induces a pronounced eosinophilia that coincides with establishment of larval stages in skeletal muscle. We have shown previously that in mouse strains in which the eosinophil lineage is ablated, large numbers of T. spiralis larvae are killed by nitric oxide, implicating the eosinophil as an immune regulator. In this report, we show that parasite death in eosinophil-ablated mice correlates with reduced recruitment of IL-4+ T cells and enhanced recruitment of iNOS producing neutrophilsto infected muscle, as well as increased iNOS in local F4/80+CD11b+Ly6C+ macrophages. Actively growing T. spiralis larvae were susceptible to killing by NO in vitro, while mature larvae were highly resistant. Growth of larvae was impaired in eosinophil-ablated mice, potentially extending the period of susceptibility to the effects of NO and enhancing parasite clearance. Transfer of eosinophils into eosinophil-ablated ΔdblGATA mice restored larval growth and survival. Regulation of immunity was not dependent upon eosinophil peroxidase (EPO) or major basic protein 1 (MBP) and did not correlate with activity of the indoleamine 2,3-dioxygenase (IDO) pathway. Our results suggest that eosinophils support parasite growth and survival by promoting accumulation of Th2 cells and preventing induction of iNOS in macrophages and neutrophils. These findings begin to define the cellular interactions that occur at an extra-intestinal site of nematode infection in which the eosinophil functions as a pivotal regulator of immunity.
Keywords: Eosinophils, monocytes/macrophages, Parasitic-Helminth, Trichinella, nitric oxide
Introduction
Investigations of infections caused by helminths that are natural parasites of rodents have revealed a number of mechanisms of protective immunity. Studies of the intestine-dwelling nematodes Heligmosomoides polygyrus, Nippostrongylus brasiliensis, Trichuris muris and Trichinella sprialis have documented Th2-driven immune responses that incorporate production of IL-4, IL-5, IL-9, IL-10, and IL-13, as well as basophilia, eosinophilia, and alternative activation of macrophages (1). Parasite clearance from the intestine is abrogated in the absence of Stat6, IL-4 and/or IL-13, confirming the importance of these mediators; however, due to differences in habitats and life cycles, the specific effector mechanismthat clears worms from the intestine varies among infections. For example, mast cells are crucial to expulsion of intra-epithelial T. spiralis (2), but dispensable for clearance of T. muris and N. brasiliensis (3, 4) during primary infection. Among the cells that are prominent in immune responses to intestinal helminths, perhaps the most enigmatic is the eosinophil. Eosinophilia is a hallmark of nematode infection, yet infection of eosinophil-ablated mice with T. muris, S. mansoni or T. spiralis has failed to reveal a key role for eosinophils in clearance of intestinal worms (5–7).
Immune responses and mechanisms of helminth clearance from extra-intestinal sites have been less thoroughly studied in natural rodent hosts. It has been shown that clearance of Litomosoides sigmondontis is promoted by the presence of eosinophil granular proteins, MBP and EPO (8). Furthermore, that eosinophils are necessary for development of immunity that limits the early tissue migratory larval stage during secondary infections by N. brasiliensis (9). These findings support the paradigm of eosinophils as defenders against worm infection.
T. sprialis occupies both intestinal and extra-intestinal sites during the course of its life cycle. Adult worms in the intestine release newborn larvae (NBL) that migrate to skeletal muscle and initiate chronic infection. Arrival of NBL in muscle is coincident with an intestinal Th2 immune response that expels adult worms and induces prominent blood and tissue eosinophilia(7). Despite the magnitude of the local inflammatory response, intracellular muscle larvae mature to become infectious. We have shown previously that although eosinophil-ablated mice clear intestinal T. spiralis normally, immunity to the muscle stage of infection is impacted dramatically (7, 10). Muscle larvae die in large numbers (50–75%) coincident with enhanced IFN-γ and decreased IL-4 production in draining lymph nodes. In the absence of eosinophils, leukocytes at sites of infection produce inducible nitric oxide synthase (iNOS) and parasite survival improves when mice are treated with specific iNOS inhibitors. Introducing IL-10 deficiency into the PHIL background dramatically enhanced NO production and increased parasite killing to 90% or more. These observations suggest that eosinophils protect developing larvae against NO-mediated killing (7).
Here we extend our earlier findings by showing that accumulation of IL-4+ T cells to sites of infection is reduced in eosinophil-ablated mice and that this correlates with infiltration of iNOS+ neutrophils and inflammatory macrophages during a time at which the growing larva is vulnerable to the effects of NO. Restoring eosinophils to infected mice improved Th2 cell recruitment, parasite growth and survival, clearly implicating eosinophils as crucial to immune regulation that supports parasite survival.
Materials and Methods
Rats and mice
Adult Albino Oxford (AO) strain rats were produced and maintained in the Baker Institute Vivarium. IL-10−/−, PHIL and ΔdblGATA (C57BL/6background)(11) mice were bred at Cornell Transgenic Mouse Core Facility and progeny were transferred to the Baker Institute. PHIL and IL-10−/− mice were genotyped as described previously (7, 12). Eosinophil peroxidase (EPO−/−) and major basic protein 1 (MBP −/−)deficient mice were maintained as described (13, 14). C57BL/6NHsd mice(henceforth referred to as WT in the text) were purchased from Taconic. Animal care was in accordance with the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care and experiments were performed with the approval of the Institutional Animal Care and Use Committee of Cornell University.
Parasite and antigens
T. spiralis first-stage larvae (L1) and newborn larvae (NBL) were recovered from rats as described previously (15, 16). For synchronous infection, 20,000 NBL suspended in serum-free DMEM (Mediatech, Inc.) were delivered by retro-orbital injection. For oral infection, L1 were suspended in 2% nutrient broth (Difco)- 0.6% gelatin (Fisher Scientific) and doses of 300 L1 were administered by gavage. Mice were euthanized by CO2 inhalation at the times indicated in each experiment. Muscle larvae burdens were assessed 24 dpi or later in whole carcasses as described previously (15). In some experiments, larvae were recovered from diaphragms 12–18 dpi by digesting minced tissue for 15 min at 37°C in 5 mg/ml of collagenase I (Sigma). Somatic antigens from L1 were prepared as described previously(17).
Eosinophil transfer experiments
Eosinophils were obtained from the peritoneum(by lavage) and spleens of infected IL-5 transgenic mice 12–20 dpi. Cells were pooled and purified by either positive or negative MACS bead selection. For positive selection, eosinophils were enriched using PE-conjugated anti-Siglec-F antibody (BD) and anti-PE microbeads (Miltenyi Biotec), a procedure that yielded eosinophil preparations of >93% purity. For negative selection, contaminating cells were labeled with PE-conjugated rat anti-mouse CD90.2, B220, NK1.1, CD11c, F4/80, Ly-6G, and Ly-6C antibodies (eBioscience) and anti-PE microbeads (Miltenyi Biotec), yielding preparations that were 83% eosinophils. After washing twice in PBS, 5 × 106 purified cells were resuspended in 200 μl sterile PBS and injected intravenously into ΔdblGATA mice on alternative days for 10 days, as indicated in Fig. 7 A. Transfer of cells recovered by the two methods yielded similar results.
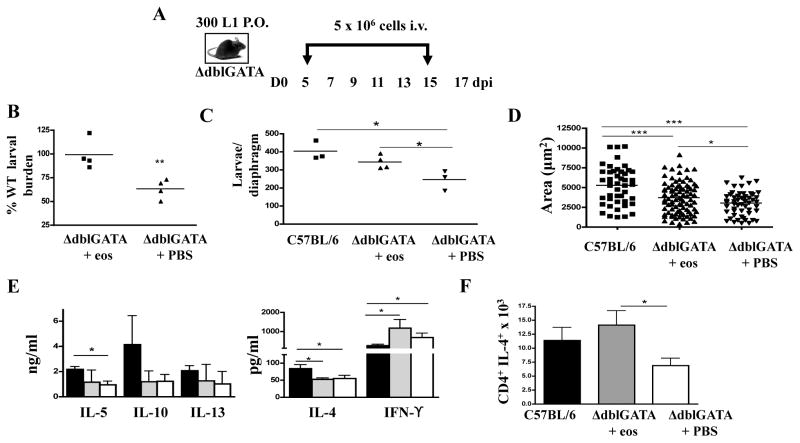
Fig. 7. The influence of eosinophil transfer on larval growth and survival.
(A) Design of experiments in which ΔdblGATA mice were given 5 × 106 eosinophilsor PBS every 48hrs between 5 and 15 dpi. (B) Parasite survival in ΔdblGATA mice following transfer of eosinophils (prepared by positive or negative selection, see text) or PBS. Values are expressed as a percentage of the burden in control C57BL/6 mice. Each point is a mean for that treatment group from one experiment (n=4 experiments). (C-F) Data collected from individual experiments performed with control C57BL/6 and ΔdblGATA mice that received PBS or negatively selected eosinophils. (C) Larval burdens 17 dpi in diaphragms. Reduction in larval burden in ΔdblGATA PBS recipients compared to C57BL/6 controls is similar to values we have reported previously (7). (D) Area of larvae recovered from diaphragms 17 dpi. Bars represent means from 75–90 larvae pooled from diaphragms of 3–4 mice.(E) Cytokines in antigen-stimulated cultures of CLN cells collected 17 dpi. (F) Mean number of CD4+IL-4+ cells in diaphragms of mice, 15 dpi. Panels C to F: Each data set was collected from two or three experiments with similar results. Values represent mean +/− SD, n = 3 – 4 mice. Significant differences were determined by ANOVA and Tukey’s test. *p<0.05, *** p<0.0001.
Histology and Immunohistochemistry
Histochemical staining and immunohistochemistry were performed as described previously(10). Leukocytes were recovered from diaphragms and cells were prepared for cytologic staining as previously described (15). Slides were stained with rabbit polyclonal anti-iNOS (NeoMarkers) and hematoxylin (Fisher) and differential counts were performed under 40X magnification using a BX51 microscope (Olympus).
Cytokine ELISA
Cells from cervical lymph nodes (CLN) were obtained and cultured as described previously (10). IL-4, IL-5, IL-10, IL-13 and IFN-γ were assayed in culture supernatants by ELISA as described previously (7).
Flow cytometry
Cells were recovered from individual diaphragms as described (10). For intracellular IL-4 detection, cells were cultured ex-vivo for 5 h with 250 ng/ml ionomycin (Sigma-Aldrich), 50 ng/ml PMA (Sigma-Aldrich), and 1 μg/ml Brefeldin A (BD Pharmingen). After a 15-min incubation with Fc block (eBioscience) and 10% normal mouse serum (NMS), cells were incubated for 15 min with FITC-conjugated anti-CD8 and PE-Cy7 conjugated anti-CD4 (eBiosicence). Samples were treated with fixation/permeabilization buffer (eBioscience) and permeabilized cells were stained using PE-conjugated anti-IFN-γ and APC-conjugated anti-IL-4 (eBiosicence). For intracellular iNOS detection, permeabilized diaphragm leukocytes were incubated with a rabbit polyclonal anti-iNOS (Neomarkers) followed by APC-conjugated goat anti-rabbit (IMGENEX). Cells were first stained for cell surface antigens as described above using PE-conjugated anti-CD11b, PE-Cy7 conjugated anti-F4/80, and FITC conjugated anti-Ly-6C (eBioscience). For basophil counts, cells isolated from CLN, mesenteric lymph node (MLN), and spleen (SPL) were stained with FITC-conjugated anti-CD49b(BioLegend), PE-conjugated anti-FcεR1-α (BioLegend) and APC-conjugated anti-c-kit(BioLegend).
Parasite measurements
Developing L1 were recovered by digesting minced diaphragms for 15 min at 37°C in 5mg/ml of collagenase I (Sigma). To prevent curling, larvae were treated with 70% ethanol at 560C and left overnight at room temperature. Straightened larvae were centrifuged and resuspended in 5% glycerol/70% ethanol to soften and clear them prior to preparation for cytospin. The cytospin slides were stained with HEMA-3 (Fisher Healthcare) and measurements were performed using 10x and 20x objectives on a BX51 microscope (Olympus) by fitting a polygon around the boundary of the larvae and computing the area (Microsuite Basic Olympus software). At least 20 larvae were measured per mouse and values are expressed in microns squared.
Quantitative RT-PCR
Total RNA was isolated from diaphragm tissue using TRIZOL reagent (Invitrogen) and cDNA was prepared using SuperScript III First-Strand cDNA Synthesis System (Invitrogen). A sample lacking reverse transcriptase served as negative control. qRT-PCR was performed for NOS2, ARG1, FIZZ-1, YM1, and GAPD Husing the following Taqman Gene Expression primers and probes: GAPDH, fwd 5′-TGTCAAGCTCATTTCCTGGTATGA-3′, rev 5′-CTTACTCCTTGGAGGCCATGTAG-3′, probe5′-TCCACCACCCTGTTGCTGTAGCCG-3′; YM1, fwd 5′-TTTGCTGGAATGCAGAATAATGAG-3′, rev 5′-CAATGCTTCATAGTCACGCAAGT-3′, probe 5′-TCACTTACACACATGAGCA-3′; ARG1, fwd 5′-AACGGGAGGGTAACCATAAGC-3′, rev 5′-TGATGCCCCAGATGGTTTTC-3′, probe 5′-ACTGACTACCTTAAACCAC-3′; NOS2, fwd 5′-CAGCTGGGCTGTACAAACCTTT-3′, rev 5′-CATTGGAAGTGAAGCGTTTCG-3′, probe 5′-CGGGCAGCCTGTGAGACCTTTGA-3′; FIZZ1, fwd 5′-TCCAGCTAACTATCCCTCCACTGT-3′, rev, 5′-GGCCCATCTGTTCATAGTCTTGA-3′, probe 5′-CGAAGACTCTCTCTTGCT-3′. Primers and probes for indoleamine 2,3-dioxygenase (IDO) were purchased from Applied Biosystems. qRT-PCR reactions were performed using the ABI PRISM 7500 Sequence Detection System and its analysis software, SDS 2.3 and RQ Manager (PE Applied Biosystems).
Measurement of IDO activity
IDO activity was measured by quantifying kynurenine (KYN)in culture supernatants of CLN and diaphragm cells that were recovered from infected WT, PHIL and ΔdblGATA mice and cultured as described previously(10).
Statistical analysis
All experiments were performed two to four times with similar results. Means ± SD were calculated from data collected from individual mice unless otherwise indicated. Significant differences were determined using Student’s t test or ANOVA with Tukey’s post-hoc test for multiple means. Statisticalanalysis was performed with GraphPad Prism 4 software.
Results
Cellular sources of iNOS in eosinophil-ablated mice
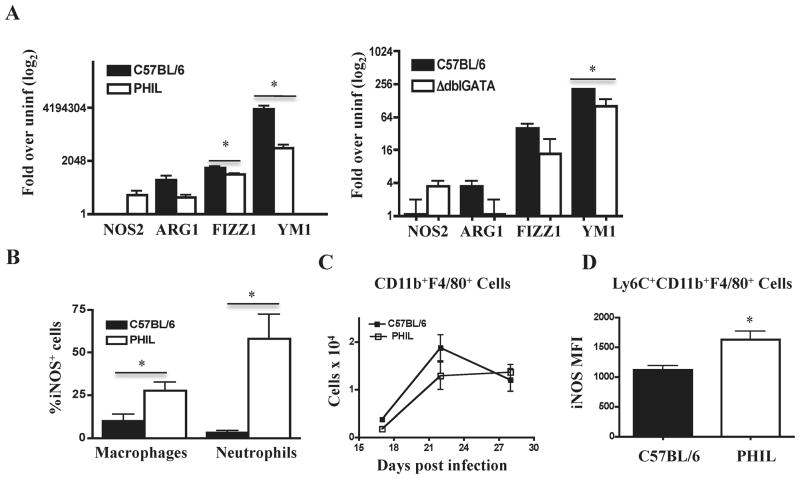
We have reported previously that iNOS contributes to clearance of muscle larvae in eosinophil-ablated mice (7). To better characterize the destructive immune response, we used qRT-PCR to document phenotypic changes in localmacrophages. We analyzed the expression of genes associated with alternatively activated (M2) macrophages (ARG1, YM1, and FIZZ1)and classically activated (M1) macrophages (NOS2) in diaphragms of infected WT, ΔdblGATA, and PHIL mice. Although all three strains dramatically upregulated M2 markers, eosinophil ablation was associated with a marked increase in NOS2 transcription and decreases in M2 marker expression at 17 dpi (Fig. 1 A). Immunohistological analysis of leukocytes recovered from diaphragms confirmed the presence of iNOS+ macrophages, but also revealed large numbers of iNOS+ neutrophils in PHIL mice (Fig. 1 B). Although the representation of iNOS+ cells among neutrophils was markedly greater than that of macrophages, the number of each cell type in infected muscle (7) is such that iNOS+ macrophages outnumbered neutrophils in diaphragms of eosinophil ablated mice by a ratio of 2:1. Overall, macrophage accumulation was not affected by eosinophil ablation (Fig. 1 C), but among phenotypically distinctmacrophage subsets, Ly-6C+ CD11b+ F4/80+ inflammatory macrophages produced significantly more iNOS in PHIL mice compared to WT (Fig. 1 D).
Fig. 1. Cellular sources of NOS2 in eosinophil deficient mice.
(A) Quantitative RT-PCR results for selected macrophage markers in diaphragms of PHIL, ΔdblGATA and WT mice, 17 days post infection (dpi). (B) Identification of iNOS+ cells by immunohistochemistry in cytospin preparations of diaphragm infiltrating cells collected 22 dpi. (C) Numbers of F4/80+CD11b+ macrophages in diaphragm leukocytes recovered from WT and PHIL mice. Cells were phenotyped by flow cytometry. (D) Mean fluorescence intensity for iNOS among Ly-6C+ CD11b+ F4/80+ inflammatory macrophages in PHIL and WT mice at 22dpi. Experiments were performed two to four times with similar results. Values represent means +/− SD, n = 3 -6 mice. Significant differences were determined by Student’s t test. *p<0.05.
Impact of eosinophil-deficiency on leukocyte recruitment
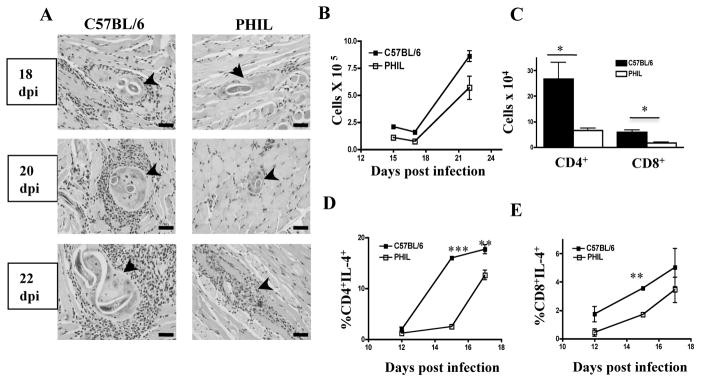
Histologic examination of tongues revealed that the cellular infiltrates around nurse cells in PHIL mice were reduced compared to WT(Fig. 2 A). Enumeration of leukocytes recovered from diaphragms confirmed a reduction in absolute cell numbers at sites of infection in PHIL mice (Fig. 2 B). Flow cytometric evaluation of T cell subsets documented fewer CD4+ and CD8+ T cells (Fig. 2 C) as well as significantly reduced frequencies of IL-4+ CD4+ and IL-4+ CD8+ T cells in diaphragms of PHIL mice (Fig. 2 D,E). The results suggest that eosinophils promote local recruitment of IL-4 producing T cells.
Fig. 2. Leukocyte accumulation and T cell responses in the absence of eosinophils.
(A) H&E stained sections of tongues collected from infected WT and PHIL mice. Arrows indicate nurse cells. (B) Number of leukocytes recovered from diaphragms of infected PHIL and WT mice. (C) Numbers of CD4+ and CD8+ T cells recovered from diaphragms of infected PHIL and WT mice at 15dpi and (D, E) frequency of IL-4 production by these cells 12–17 dpi. Surface phenotype and cytoplasmic cytokines wereassayed by flow cytometry. Experiments were performed two or three times with similar results. Values represent means+/− SD, n = 3 - 4 mice. Significant differences were determined by Student’s t test. *p<0.05; ** p < 0.001; ***p<0.0001.
Potential for IDOto regulate T cell responses
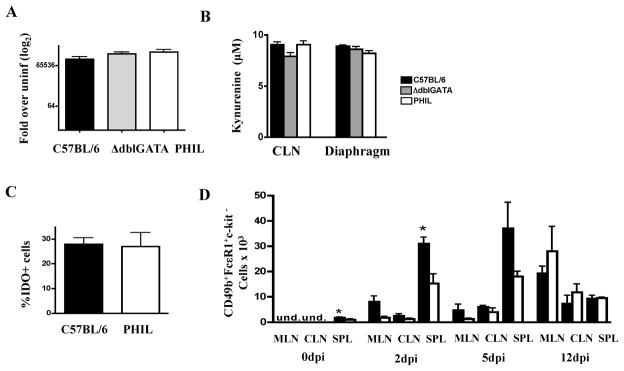
IDO mediates oxidation of tryptophan to KYN and has been implicated in regulating Th1 responses. Human eosinophils produce IDO in response to IFN-γ, thereby promoting apoptosis or inhibiting cellular proliferation of Th1 cells (18). We investigated whether IDO might be the link between eosinophils and reduced Th1 responses in WT mice by investigating gene expression and enzyme activity during infection. IDO gene expression was similar in infected muscles of PHIL, ΔdblGATA, and WT mice (Fig. 3 A). IDO enzymatic activity, measured by KYN production in cultures of antigen-stimulated CLN cells or diaphragm leukocytes, was similar across strains (Fig. 3 B). Immunohistochemical staining of diaphragm leukocytes showed that the percentages of IDO+ cells were similar between WT and PHIL mice (Fig. 3 C). Large mononuclear cells, but not eosinophils, were IDO+ in tissues of WT mice. The results do not support a role for IDO in eosinophil-dependent regulation of local T cell responses.
Fig. 3. IDO response and basophilia in eosinophil-ablated mice.
(A) IDO gene expression in diaphragms of infected ΔdblGATA, PHIL, and WT mice 15dpi.(B) KYN in cultures of CLN and diaphragm leukocytes collected 15dpi. (C) Detection of IDO indiaphragm leukocytes from infected WT and PHIL mice collected 12dpi. (D) Basophils in WT and ΔdblGATA lymphoid tissues. Basophils were identified as CD49b+FcεR1+c-kit− cells in the CLN, MLN, and spleen (SPL) in uninfected and infected mice 2, 5, and 12 dpi by flow cytometry. Experiments were performed two times with similar results. Values represent means +/− SD, n = 3 – 4 mice. Significant differences were determined by Student’s t test. *p<0.05.
Impact of eosinophil deficiency on basophilia
We examined the impact of eosinophil ablation on basophilia by counting basophils inMLN, CLN and spleen at 0, 2, 5 and 12 dpi(Fig. 3 D). Uninfected ΔdblGATA mice had significantly fewer basophils in the spleen and this trend was evident in the MLN, CLN and spleen on 2 and 5 dpi, although differences were statistically significant only in the spleen at 2 dpi. Numbers of basophils in all tissues were similar in the two mouse strains on 12 dpi, prior to the time at which parasite compromise and altered immunity is evident in eosinophil-ablated mice.
Eosinophil granular proteins do not influence survival of muscle larvae
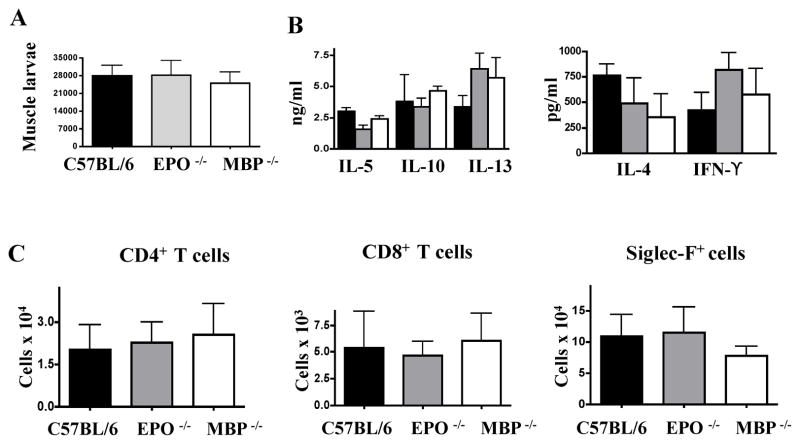
We investigated the impact of eosinophil granular proteins on the progression of infection and modulation of immune responses by infecting mice deficient in either EPO or MBP. Muscle larvae burdens in both strains were similar to WT (Fig. 4 A). Cytokine production by CLN cells in response to antigen re-stimulation was not altered (Fig. 4 B), nor was there an effect on the numbers of eosinophils, CD4+ or CD8+ cells at sites of infection (Fig. 4 C). Thus, MBP or EPO were dispensable for regulation of T cell responses and parasite survival.
Fig. 4. Effects of MBP and EPO on parasite survival andimmunity.
(A) Larval burdens in muscles of WT, EPO −/− and MBP −/− mice 28 dpi. (B) Cytokinesmeasured in antigen-stimulated cultures of CLN cells collected from WT, MBP−/−, and EPO −/− mice 17 dpi. (C) CD4+, CD8+, and Siglec-F+ cells recovered from diaphragms of infected WT, MBP −/−, and EPO −/− mice 17 dpi. Experiments were performed twice with similar results. Values represent means +/− SD, n = 3 – 4 mice. No significant differences were found.
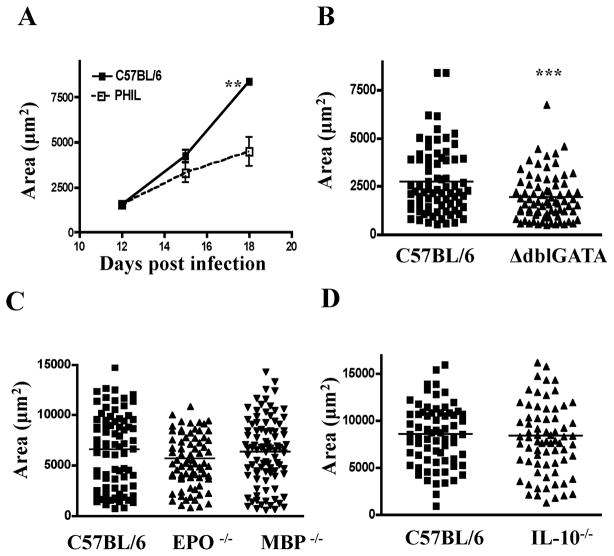
Larval growth is impaired in the absence of eosinophils
Microscopic examination of H & E stained tongue sections revealed that both nurse cells and larvae appeared to be smaller in PHIL versus WT mice (Fig. 2 A). To determine if larval growth was compromised in the absence of eosinophils, we measured the dimensions of larvae recovered from diaphragms between 12 and 18 dpi. The mean area of larvae was similar in PHIL versus WT mice on 12 dpi, but was reduced in PHIL mice on 15 and 18 dpi (Fig. 5 A). Similarly, a significant difference in larval area was detected in ΔdblGATA versus WT mice on 17 dpi (Fig. 5 B). Larvae grew normally in MBP−/− and EPO−/− mice (Fig. 5 C). In order to test whether iNOS activity, in the presence of eosinophils, would cause impaired larval growth, we infected IL-10−/− mice. This strain has dramatic, local iNOS+ cellular infiltrates and some reduction in larval burden(7, 10). Parasite growth was not altered by IL-10 deficiency (Fig. 5 D) indicating that nitrosative stress does not compromise parasite growth.
Fig. 5. Larval growth in eosinophil-ablated mice.
Estimated area of larvae recovered from(A) PHIL and WT mice between 12 and 18 dpi, (B) ΔdblGATA and WT mice 17dpi, (C) EPO−/−, MBP−/− and WTmice 18dpi, (D) IL-10 −/− and WT mice 18dpi. 25–30 larvae were evaluated per mouse. Values represent means +/− SD, n = 3 – 4 mice in A. Bars indicate the mean values from 75–90 larvae pooled from 3 mice in B, C and D. Experiments were performed two to four times with similar results. Significant differences were determined by Student’s t test (A, B, D) or by ANOVA and Tukey’s test (C). ** p < 0.001, *** p<0.0001.
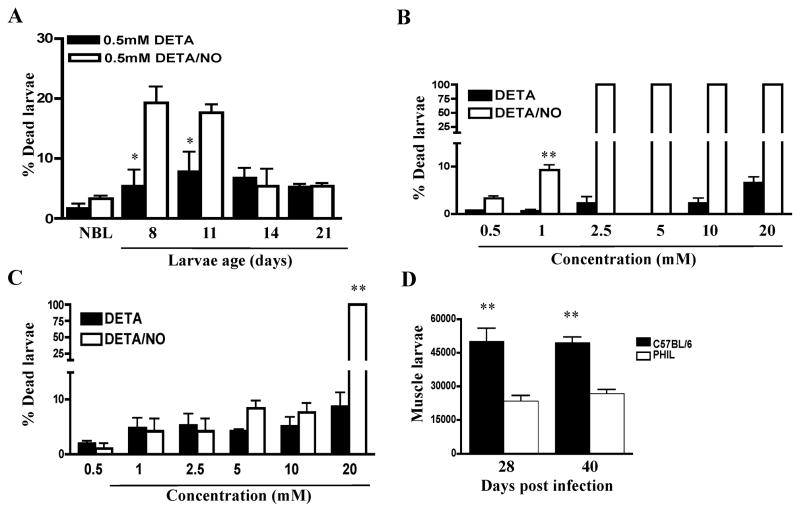
Developing T. spiralis larvae are susceptible to direct killing by NO
In order to determine whether death of larvae is the direct result of exposure to NO, we tested the larvicidal effect of NO on T. spiralis larvae in vitro. Larvae recovered from WT mice at different times post-infection were cultured for 48 h with the artificial NO donor DETA-NONOate or with the vehicle control DETA. In order to generate larvae that were at the same stage of development, mice were infected by intravenous injection of NBL to achieve synchronous muscle infections. The results show that NBL and muscle larvae up to 11 days of age were killed in significant numbers (Fig. 6 A and B), while relatively mature muscle larvae (14 days old or older) were resistant (Fig. 6 C). Note that due to the asynchronous nature of NBL production by adult worms in the intestine, newborn larvae colonize the muscle between 4 and 14 days post-oral infection(19). Thus, susceptible larvae would be present in the muscle between 4 and 28 days post-oral infection, with the proportion of susceptible larvae declining between 14 and 28 days. In support of the conclusion that only growing muscle larvae are susceptible to the effect of NO, we found that parasite clearance in PHIL mice is completed by 28 dpi, with no additional reduction in burden evident when estimated 40 dpi (Fig. 6 D).
Fig. 6. Susceptibility of muscle larvae to NO mediated killing.
Susceptibilities of different larval stages of T. spiralis to NO mediated killing were determined by culturing (A) 8–14 day old larvae isolated from C57BL/6 mice, (B) NBL, or (C) mature L1 (24 days old) with DETA/NO or DETA for 48 hrs in vitro. (D) Muscle burdens in PHIL and WT mice, 28 and 40 dpi. Experiments were performed twice with similar results. Values represent means +/− SD, n = 3 – 4. Significant differences were determined by Student’s t test. * p < 0.05; **p<0.001.
Adoptive transfer of eosinophils improved larvae growth and survival
In order to confirm the role of the eosinophil in parasite growth and retention, eosinophils isolated from infected IL-5Tg micewere transferred to infected ΔdblGATA mice (Fig. 7 A). Based on results of replicate experiments showing that transferred eosinophils extravasated in skeletal muscle and persisted there for 24 but not 48hrs (not shown), we designed the experimental protocol to incorporate cell transfers on alternate days between 5 and 15 dpi. Four experiments, conducted using positively selected (n=2) and negatively selected (n=2) eosinophils, yielded similar results and statistical analysis documented that the improvement in larval burdens in this group of experiments averaged 36% and was highly significant (p=0.005) (Fig. 7 B). One experiment performed with PHIL mice showed that transfer of positively selected eosinophils improved larval burdens by 42% (p=0.04). Results from a single experiment performed with negatively selected eosinophils in ΔdblGATA mice (Fig. 7 C-E) show that transfer of eosinophils improved larval burdens in the diaphragms of recipients (Fig. 7 C) and promoted a modest but significant enhancement of larval growth (Fig. 7D). Although cytokine responses in recall assays performed with CLN cells were not altered by eosinophil transfer (Fig. 7 E), Th2 accumulation at sites of infection improved markedly when eosinophils were transferred to ΔdblGATA mice (Fig. 7F).
Discussion
Host adaptation is highly evolved among parasitic worms and there is ample evidence that helminths manipulate the immune response in ways that prolong their survival in the host or promote their dispersal in the susceptible host population. The importance of obtaining a detailed and thorough understanding of the means by which nematodes interfere with immunity lies in the potential for such knowledge to inform the design of approaches to controlling infection and preventing disease. Our previously published findings revealed the potential for eosinophils to promote survival of T. spiralis in muscle. Specifically, we reported that when PHIL or ΔdblGATA mice are infected with T. spiralis, larvae are cleared from skeletal muscle by an iNOS-dependent mechanism (7). We show here that neutrophils and macrophages produce iNOS at sites of infection. Although markers of M2 macrophages were dramatically upregulated in diaphragm tissue of both eosinophil-ablated and WT mice, eosinophil-ablated mice upregulated NOS2 on day 17 post-infection and large numbers of iNOS positive neutrophils and macrophages were present among infiltrating cells. Among the macrophage/monocyte populations, Ly6C+CD11b+F4/80+ cells showed the highest levels of iNOS. This indicates that inflammatory monocytes recruited from blood, rather than tissue resident macrophages, were the primary sources of iNOS (20). Similar numbers of neutrophils and Ly6C+CD11b+F4/80+ monocytes/macrophages were present in diaphragms of WT mice, suggesting that the effect in eosinophil-ablated mice is not a defect in recruitment of such cells but rather the result of cytokines that influence the induction of NOS2 expression.
Taken together, the results support a model in which eosinophils act locally, either directly or indirectly, to prevent the development of iNOS producing neutrophils and macrophages capable of parasite killing. A direct effect of eosinophils on macrophage phenotype has been described recently. By virtue of their production of IL-4, eosinophils have been shown to promote alternative activation of macrophages in mouse adipose tissue, thereby promoting glucose tolerance and protecting against diet-induced obesity (21). Furthermore, a recent report has shown that M2 macrophages proliferatein the pleural cavities of mice infected with L. sigmodontis and that proliferation in situ is under the influence of IL-4 (22). Our findings are not incompatible with local proliferation of M2 macrophages in muscles of T. spiralis infected mice, making the potential influence of eosinophils on local macrophage populations of considerable interest.
Previous work did not determine whether NO-dependent clearance of T. spiralis resulted from direct toxicity to larvae or from nitrosative damage to nurse cells (7). Our results demonstrate that T. spiralis larvae are susceptible to direct killing by NO. Susceptibility is evident in newborns, but increases during the period of rapid growth between 4 and 14 days following invasion of muscle cells (23). Although NO has been implicated in helminth killing in mice vaccinated against Schistosoma mansoni or colonized with Brugia malayi (24, 25)this is the first demonstration that NO mediates killing of parasitic worms in response to infection in a natural host. NO produced by endothelial cells and macrophages kills S. mansoni larvae in vitro(26) and susceptibility is age-dependent; however, in contrast with T. spiralis, older S. mansoni larvae show greater susceptibility (27). In the B. malayi model, treatment of mice with an inhibitor of NO synthase abrogates resistance (24, 25). Therefore, it is apparent that in contrast with the well-established role for Th2 immune responses in expulsion of intestinal worms, reactive nitrogen species produced during Th1 immune responses can be effective in host defense against tissue-dwelling parasitic worms.
We found that T. spiralis larvae became remarkably resistant to NO as they approached maturity. Antioxidant enzymes likely afford protection to the parasite(28) and expressed sequence taganalysis predicted that mature T. spiralis muscle larvae transcribe three types of antioxidant genes, specifically, thioredoxin oxidase, peroxiredoxin, and glutathione peroxidase(29, 30). These genes are not transcribed in NBL, compatible with immature larvae being more vulnerable to oxidativeor nitrosative damage(29–31), and consistent with results of our in vitro and in vivo experiments. Eosinophils appear to protect larvae during a window of susceptibility to oxidative and nitrosative stress.
Prior to destruction of larvae in eosinophil-deficient mice, nurse cell development was impaired and larval growth was inhibited. We have not yet determined whether this inhibition is the result of an immune response that develops in the absence of eosinophils or it reflects the parasite’s dependence upon factors produced or induced by eosinophils. Parasite growth modulation by the immune system has been shown in other helminth infections. For example, T cells facilitate growth of S. mansoni by exerting non-cognate influence on MHC class II+ antigen presenting cells (32). In addition, development of the filarial nematode L. sigmodontisis transiently delayed in the absence of IL-5 or eosinophils, and B. malayi development improves in the presence of T cells and NK cells(33–35). Although the role of lymphocytes may vary across infections, the accumulating evidence supports a model in which innate immune cells influence the rate of growth and development of parasitic helminths.
We speculate that inhibition of larval growth would lengthen the period of susceptibility to NO-mediated killing and promote clearance of larvae. Consistent with this notion was the finding that larvae grew normally in IL-10 deficient mice, which demonstrate less dramatic parasite clearance but strong iNOS production (10). The mechanism(s) behind compromise of larval growth in eosinophil-ablated mice remains to be elucidated. Angiogenesis is a prominent feature of nurse cell development, during which infected myotubes develop a surrounding vascular network that is presumed to support the parasite (36). Eosinophils and M2 macrophages can promote angiogenesis (37–40). If vascularization is compromised in the absence of eosinophils, both nurse cell differentiation and parasite growth may be inhibited, a hypothesis that we are currently testing.
T. spiralis can live for years in skeletal muscles of its host (41). Prolonged survival requires that the worm suppress the host immune response or block its effects. Our data indicate that eosinophils directly or indirectly inhibit a Th1 immune response that induces the production of larvicidal NO; survival of the parasite is correlated with Th2 immunity. Eosinophils can promote Th2 responses by different mechanisms. It has been reported that human eosinophils expressing IDO catabolize tryptophan to KYN that subsequently causes apoptosis of Th1 cells (18). This phenomenon has not been documented in murine eosinophils; however, we hypothesized that mouse eosinophils might have a similar influence, possibly by promoting IDO production in other cells. We evaluated the numbers of IDO producing cells as well as IDO gene expression in diaphragms of WT, PHIL and ΔdblGATA mice. In addition, we measured KYN production by antigen stimulated CLN cells or leukocytes isolated from infected diaphragms. None of these measures was affected by eosinophil deficiency. Mononuclear cells rather than granulocytes were the dominant sources of IDO in all strains. These results do not support a eosinophil-dependent role for IDO in inhibiting a Th1 response in WT mice.
We found that T. spiralis infection progressed normally in the absence of the MBP or EPO, indicating that these granular proteins do not contribute to parasite growth and survival, or to immune modulation. Another granular protein, human eosinophil-derived neurotoxin (an orthologue of the mouse eosinophil-associated ribonuclease-2) has been shown to induce dendritic cell maturation and expansion of Th2 responses by virtue of its ability to activate TLR2 (42). We did not detect a difference in dendritic cell numbers or maturation (as evidenced by MHCII and CD86 expression) between WT and eosinophil-ablated mice in the CLN or diaphragm (data not shown). Our findings to date do not support a role for granular proteins in eosinophil-mediated regulation. Basophils produce IL-4 and have potential to influence immunity to T. spiralis(43). Eosinophil ablation in ΔdblGATA mice was associated with a modest but significant reduction in the number of basophils in the spleens of uninfected mice and infected mice at 2 dpi. It is not obvious that these differences would affect the immune response in skeletal muscle that develops 10 days later. Furthermore, improved growth and survival of larvae following transfer of eosinophils (between 5 and 15 dpi) reduces the likelihood that basophils have an important role in protecting larvae. Nevertheless, the data require further consideration of the potential influence of the basophil in larval survival and immune regulation.
Eosinophil transfer, during a limited but critical period of larval development, significantly improved growth and survival of larvae while simultaneously increasing Th2 cell migration to sites of infection. The results support the eosinophil as a regulatorof local immunity. Despite the local effect, the transfer protocol did not alter the cytokine response of CLN cells in antigen restimulation assays. It is possible that the delivery schedule or number of cells transferred was insufficient to have an effect in regional lymphoid tissue, or that the assay is not sufficiently sensitive to detect those effects. In murine models of allergic lung disease, local Th2 cytokine production is reduced in eosinophil-ablated mice, a result that derives from reduced T cell recruitment into the lung (44, 45). Similarly, mice deficient in the eosinophil chemotactic factors CCL11 (eotaxin-1) and CCL24 (eotaxin-2) show reduced cellular infiltrates and Th2 cytokine production in the lung(46) and supplementing ΔdblGATA mice with CCL11 enhances the Th2 response (44). Adoptive transfer of eosinophils or eosinophils and CD4 T cells reconstitutes disease in ΔdblGATA and PHIL mice, respectively (44, 45). Transfer of eosinophils deficient in IL-13 failed to restore disease, documenting IL-13 as a critical mediator of the regulatory effect of eosinophils in allergic airway disease (44, 47). Our previous studies showed that STAT6-deficient mice do not clear muscle larvae, while IL-10 deficient mice up-regulate NOS2 and clear parasites, suggesting that IL-10 may be more important than IL-4/IL-13 in protecting larvae against NO-mediated killing(10).
In addition to cytokines and granular proteins, eosinophils express MHC class II and present antigen, promoting Th2 differentiationin the context of helminth infection and allergic asthma (48, 49). Eosinophils in T. spiralis infected miceupregulate surface expression of MHCII and CD86 (Fabre and Appleton, unpublished observations), affording them the potential to influence the development of T cell subsets via antigen presentation and constitutive expression of IL-4(50).
Under homeostatic conditions, mouse eosinophils are present in the uterus, thymus, intestine, and mammary gland where they have been associated with processes of cellular growth and differentiation(51). The effect of eosinophils on macrophage phenotype in adipose tissue also occurs in the absence of infection (21). Neither IL-5 deficiency, nor over-expression of IL-5 impacts survival of T. spiralis muscle larvae (52–54), while ablation of the eosinophil lineage has a profound effect(7). These contrasting results indicate that eosinophilia is not necessary for eosinophil-mediated immune regulation during muscle infection by T. spiralis. Thus, innate eosinophil functions, rather than those promoted by adaptive immune cells, are likely to be central in this context.
Parasite protection by eosinophils may benefit the host by preserving the antigenic stimulus for a Th2 response that prevents re-infection of the intestine. This limits the risk of overburdening the host while at the same time reducing injury to skeletal muscle that is associated with parasite clearance. Dissection of the functional attributes of eosinophils, and identification of the cells with which they interact to exert their regulatory influence, will be crucial next steps in determining how these findings can be applied in developing new tools to prevent and control parasitic infections that continue to plague human and animal populations.
Acknowledgments
This work was supported by National Institute of Allergy and Infectious Diseases grant AI081043.
We thank Lisa Blum, Na Young Kim and Sara Cohen for technical assistance, Dr. Alison Humblesfor sharing the ΔdblGATA mice on the C57BL/6 background, and Dr. Avery Augustforhis help in obtaining the mice.
Abbreviations used in this article
- NBL
newborn larvae
- iNOS
inducible nitric oxide synthase
- DETA
diethylenetriamine
- IDO
indoleamine 2,3-dioxygenase
- KYN
kynurenine
- qRT-PCR
quantitative RT-PCR
- EPO
eosinophil peroxidase
- MBP
major basic protein 1
- CLN
cervical lymph node
- MLN
mesenteric lymph node
- dpi
days post infection
References
- 1.Maizels RM, Pearce EJ, Artis D, Yazdanbakhsh M, Wynn TA. Regulation of pathogenesis and immunity in helminth infections. J Exp Med. 2009;206:2059–2066. doi: 10.1084/jem.20091903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ha TY, Reed ND, Crowle PK. Delayed expulsion of adult Trichinella spiralis by mast cell-deficient W/Wv mice. Infect Immun. 1983;41:445–447. doi: 10.1128/iai.41.1.445-447.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koyama K, Ito Y. Mucosal mast cell responses are not required for protection against infection with the murine nematode parasite Trichuris muris. Parasite Immunol. 2000;22:13–20. doi: 10.1046/j.1365-3024.2000.00270.x. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell LA, Wescott RB, Perryman LE. Kinetics of expulsion of the nematode, Nippostrongylus brasiliensis, in mast-cell deficient W/WV mice. Parasite Immunol. 1983;5:1–12. doi: 10.1111/j.1365-3024.1983.tb00718.x. [DOI] [PubMed] [Google Scholar]
- 5.Svensson M, Bell L, Little MC, DeSchoolmeester M, Locksley RM, Else KJ. Accumulation of eosinophils in intestine-draining mesenteric lymph nodes occurs after Trichuris muris infection. Parasite Immunol. 2010:1365–3024. doi: 10.1111/j.1365-3024.2010.01246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swartz JM, Dyer KD, Cheever AW, Ramalingam T, Pesnicak L, Domachowske JB, Lee JJ, Lee NA, Foster PS, Wynn TA, Rosenberg HF. Schistosoma mansoni infection in eosinophil lineage-ablated mice. Blood. 2006;108:2420–2427. doi: 10.1182/blood-2006-04-015933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fabre V, Beiting DP, Bliss SK, Gebreselassie NG, Gagliardo LF, Lee NA, Lee JJ, Appleton JA. Eosinophil deficiency compromises parasite survival in chronic nematode infection. J Immunol. 2009;182:1577–1583. doi: 10.4049/jimmunol.182.3.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Specht S, Saeftel M, Arndt M, Endl E, Dubben B, Lee NA, Lee JJ, Hoerauf A. Lack of eosinophil peroxidase or major basic protein impairs defense against murine filarial infection. Infect Immun. 2006;74:5236–5243. doi: 10.1128/IAI.00329-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knott ML, Matthaei KI, Giacomin PR, Wang H, Foster PS, Dent LA. Impaired resistance in early secondary Nippostrongylus brasiliensis infections in mice with defective eosinophilopoeisis. Int J Parasitol. 2007;37:1367–1378. doi: 10.1016/j.ijpara.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Beiting DP, Gagliardo LF, Hesse M, Bliss SK, Meskill D, Appleton JA. Coordinated control of immunity to muscle stage Trichinella spiralis by IL-10, regulatory T cells, and TGF-beta. J Immunol. 2007;178:1039–1047. doi: 10.4049/jimmunol.178.2.1039. [DOI] [PubMed] [Google Scholar]
- 11.Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna EE, Ghiran S, Gerard NP, Yu C, Orkin SH, Gerard C. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305:1776–1779. doi: 10.1126/science.1100283. [DOI] [PubMed] [Google Scholar]
- 12.Lee JJ, Dimina D, Macias MP, Ochkur SI, McGarry MP, O’Neill KR, Protheroe C, Pero R, Nguyen T, Cormier SA, Lenkiewicz E, Colbert D, Rinaldi L, Ackerman SJ, Irvin CG, Lee NA. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004;305:1773–1776. doi: 10.1126/science.1099472. [DOI] [PubMed] [Google Scholar]
- 13.Denzler KL, Farmer SC, Crosby JR, Borchers M, Cieslewicz G, Larson KA, Cormier-Regard S, Lee NA, Lee JJ. Eosinophil major basic protein-1 does not contribute to allergen-induced airway pathologies in mouse models of asthma. J Immunol. 2000;165:5509–5517. doi: 10.4049/jimmunol.165.10.5509. [DOI] [PubMed] [Google Scholar]
- 14.Denzler KL, Borchers MT, Crosby JR, Cieslewicz G, Hines EM, Justice JP, Cormier SA, Lindenberger KA, Song W, Wu W, Hazen SL, Gleich GJ, Lee JJ, Lee NA. Extensive eosinophil degranulation and peroxidase-mediated oxidation of airway proteins do not occur in a mouse ovalbumin-challenge model of pulmonary inflammation. J Immunol. 2001;167:1672–1682. doi: 10.4049/jimmunol.167.3.1672. [DOI] [PubMed] [Google Scholar]
- 15.Beiting DP, Bliss SK, Schlafer DH, Roberts VL, Appleton JA. Interleukin-10 limits local and body cavity inflammation during infection with muscle-stage Trichinella spiralis. Infect Immun. 2004;72:3129–3137. doi: 10.1128/IAI.72.6.3129-3137.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crum ED, Despommier DD, McGregor DD. Immunity to Trichinella spiralis. I. Transfer of resistance by two classes of lymphocytes. Immunology. 1977;33:787–795. [PMC free article] [PubMed] [Google Scholar]
- 17.Appleton JA, Usack L. Identification of potential antigenic targets for rapid expulsion of Trichinella spiralis. Mol Biochem Parasitol. 1993;58:53–62. doi: 10.1016/0166-6851(93)90090-k. [DOI] [PubMed] [Google Scholar]
- 18.Odemuyiwa SO, Ghahary A, Li Y, Puttagunta L, Lee JE, Musat-Marcu S, Moqbel R. Cutting edge: human eosinophils regulate T cell subset selection through indoleamine 2,3-dioxygenase. J Immunol. 2004;173:5909–5913. doi: 10.4049/jimmunol.173.10.5909. [DOI] [PubMed] [Google Scholar]
- 19.Harley JP, Gallicchio V. Trichinella spiralis: migration of larvae in the rat. Exp Parasitol. 1971;30:11–21. doi: 10.1016/0014-4894(71)90064-6. [DOI] [PubMed] [Google Scholar]
- 20.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 21.Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, MacDonald AS, Allen JE. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 332:1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Despommier D, Aron L, Turgeon L. Trichinella spiralis: growth of the intracellular (muscle) larva. Exp Parasitol. 1975;37:108–116. doi: 10.1016/0014-4894(75)90058-2. [DOI] [PubMed] [Google Scholar]
- 24.Selkirk ME, V, Smith P, Thomas GR, Gounaris K. Resistance of filarial nematode parasites to oxidative stress. Int J Parasitol. 1998;28:1315–1332. doi: 10.1016/s0020-7519(98)00107-6. [DOI] [PubMed] [Google Scholar]
- 25.Rajan TV, Porte P, Yates JA, Keefer L, Shultz LD. Role of nitric oxide in host defense against an extracellular, metazoan parasite, Brugia malayi. Infect Immun. 1996;64:3351–3353. doi: 10.1128/iai.64.8.3351-3353.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oswald IP, Eltoum I, Wynn TA, Schwartz B, Caspar P, Paulin D, Sher A, James SL. Endothelial cells are activated by cytokine treatment to kill an intravascular parasite, Schistosoma mansoni, through the production of nitric oxide. PNAS. 1994;91:999–1003. doi: 10.1073/pnas.91.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmed SF, I, Oswald P, Caspar P, Hieny S, Keefer L, Sher A, James SL. Developmental differences determine larval susceptibility to nitric oxide-mediated killing in a murine model of vaccination against Schistosoma mansoni. Infect Immun. 1997;65:219–226. doi: 10.1128/iai.65.1.219-226.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dzik JM. Molecules released by helminth parasites involved in host colonization. Acta Biochim Pol. 2006;53:33–64. [PubMed] [Google Scholar]
- 29.Kazura JW, Meshnick SR. Scavenger enzymes and resistance to oxygen mediated damage in Trichinella spiralis. Mol Biochem Parasitol. 1984;10:1–10. doi: 10.1016/0166-6851(84)90013-6. [DOI] [PubMed] [Google Scholar]
- 30.Mitreva M, Appleton J, McCarter JP, Jasmer DP. Expressed sequence tags from life cycle stages of Trichinella spiralis: application to biology and parasite control. Vet Parasitol. 2005;132:13–17. doi: 10.1016/j.vetpar.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 31.Mitreva M, Jasmer DP, Appleton J, Martin J, Dante M, Wylie T, Clifton SW, Waterston RH, McCarter JP. Gene discovery in the adenophorean nematode Trichinella spiralis: an analysis of transcription from three life cycle stages. Mol Biochem Parasitol. 2004;137:277–291. doi: 10.1016/j.molbiopara.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 32.Lamb EW, Walls CD, Pesce JT, Riner DK, Maynard SK, Crow ET, Wynn TA, Schaefer BC, Davies SJ. Blood fluke exploitation of non-cognate CD4+ T cell help to facilitate parasite development. PLoS Pathog. 2010;6:e1000892. doi: 10.1371/journal.ppat.1000892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Babu S, Porte P, Klei TR, Shultz LD, Rajan TV. Host NK cells are required for the growth of the human filarial parasite Brugia malayi in mice. J Immunol. 1998;161:1428–1432. [PubMed] [Google Scholar]
- 34.Babu S, Shultz LD, Rajan TV. T cells facilitate Brugia malayi development in TCRalpha(null) mice. Exp Parasitol. 1999;93:55–57. doi: 10.1006/expr.1999.4438. [DOI] [PubMed] [Google Scholar]
- 35.Babayan SA, Read AF, Lawrence RA, Bain O, Allen JE. Filarial parasites develop faster and reproduce earlier in response to host immune effectors that determine filarial life expectancy. PLoS Biol. 2011;8:e1000525. doi: 10.1371/journal.pbio.1000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Despommier DD. Trichinella spiralis and the concept of niche. J Parasitol. 1993;79:472–482. [PubMed] [Google Scholar]
- 37.Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, Qian H, Xue XN, Pollard JW. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66:11238–11246. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 38.Horiuchi T, Weller PF. Expression of vascular endothelial growth factor by human eosinophils: upregulation by granulocyte macrophage colony-stimulating factor and interleukin-5. Am J Respir Cell Mol Biol. 1997;17:70–77. doi: 10.1165/ajrcmb.17.1.2796. [DOI] [PubMed] [Google Scholar]
- 39.Nissim Ben Efraim AH, Eliashar R, Levi-Schaffer F. Hypoxia modulates human eosinophil function. Clin Mol Allergy. 2010;8:10. doi: 10.1186/1476-7961-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puxeddu I, Alian A, Piliponsky AM, Ribatti D, Panet A, Levi-Schaffer F. Human peripheral blood eosinophils induce angiogenesis. Int J Biochem Cell Biol. 2005;37:628–636. doi: 10.1016/j.biocel.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 41.Despommier DD. How does Trichinella spiralis make itself at home? Parasitol Today. 1998;14:318–323. doi: 10.1016/s0169-4758(98)01287-3. [DOI] [PubMed] [Google Scholar]
- 42.Yang D, Chen Q, Su SB, Zhang P, Kurosaka K, Caspi RR, Michalek SM, Rosenberg HF, Zhang N, Oppenheim JJ. Eosinophil-derived neurotoxin acts as an alarmin to activate the TLR2-MyD88 signal pathway in dendritic cells and enhances Th2 immune responses. J Exp Med. 2008;205:79–90. doi: 10.1084/jem.20062027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Min B, Prout M, Hu-Li J, Zhu J, Jankovic D, Morgan ES, Urban JF, Jr, Dvorak AM, Finkelman FD, LeGros G, Paul WE. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. J Exp Med. 2004;200:507–517. doi: 10.1084/jem.20040590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walsh ER, Sahu N, Kearley J, Benjamin E, Kang BH, Humbles A, August A. Strain-specific requirement for eosinophils in the recruitment of T cells to the lung during the development of allergic asthma. J Exp Med. 2008;205:1285–1292. doi: 10.1084/jem.20071836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jacobsen EA, Ochkur SI, Pero RS, Taranova AG, Protheroe CA, Colbert DC, Lee NA, Lee JJ. Allergic pulmonary inflammation in mice is dependent on eosinophil-induced recruitment of effector T cells. J Exp Med. 2008;205:699–710. doi: 10.1084/jem.20071840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fulkerson PC, Fischetti CA, McBride ML, Hassman LM, Hogan SP, Rothenberg ME. A central regulatory role for eosinophils and the eotaxin/CCR3 axis in chronic experimental allergic airway inflammation. PNAS. 2006;103:16418–16423. doi: 10.1073/pnas.0607863103. [DOI] [PMC free article] [PubMed]
- 47.Walsh ER, Thakar J, Stokes K, Huang F, Albert R, August A. Computational and experimental analysis reveals a requirement for eosinophil-derived IL-13 for the development of allergic airway responses in C57BL/6 mice. J Immunol. 2011;186:2936–2949. doi: 10.4049/jimmunol.1001148. [DOI] [PubMed] [Google Scholar]
- 48.Wang HB, Ghiran I, Matthaei K, Weller PF. Airway eosinophils: allergic inflammation recruited professional antigen-presenting cells. J Immunol. 2007;179:7585–7592. doi: 10.4049/jimmunol.179.11.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Padigel UM, Hess JA, Lee JJ, Lok JB, Nolan TJ, Schad GA, Abraham D. Eosinophils act as antigen-presenting cells to induce immunity to Strongyloides stercoralis in mice. J Infect Dis. 2007;196:1844–1851. doi: 10.1086/522968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gessner A, Mohrs K, Mohrs M. Mast cells, basophils, and eosinophils acquire constitutive IL-4 and IL-13 transcripts during lineage differentiation that are sufficient for rapid cytokine production. J Immunol. 2005;174:1063–1072. doi: 10.4049/jimmunol.174.2.1063. [DOI] [PubMed] [Google Scholar]
- 51.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 52.Vallance BA, Matthaei KI, Sanovic S, Young IG, Collins SM. Interleukin-5 deficient mice exhibit impaired host defence against challenge Trichinella spiralis infections. Parasite Immunol. 2000;22:487–492. doi: 10.1046/j.1365-3024.2000.00328.x. [DOI] [PubMed] [Google Scholar]
- 53.Hokibara S, Takamoto M, Tominaga A, Takatsu K, Sugane K. Marked eosinophilia in interleukin-5 transgenic mice fails to prevent Trichinella spiralis infection. J Parasitol. 1997;83:1186–1189. [PubMed] [Google Scholar]
- 54.Herndon FJ, Kayes SG. Depletion of eosinophils by anti-IL-5 monoclonal antibody treatment of mice infected with Trichinella spiralis does not alter parasite burden or immunologic resistance to reinfection. J Immunol. 1992;149:3642–3647. [PubMed] [Google Scholar]