Abstract
Within lymphopenic recipients, naïve T cells undergo proliferation that is induced by homeostatic mechanisms. Earlier studies have demonstrated that commensal antigens play a key role in inducing the proliferation. However, a relative contribution of endogenous self antigens in this process has not been formally investigated. In this study, we utilized a pharmacologic inhibitor that blocks T cell egress from the lymphoid tissues, antibiotics, and germ-free animals to examine the role of commensal and self antigens. The results suggest that T cell proliferation under lymphopenic conditions is a heterogeneous process triggered by both exogenous commensal and endogenous self antigens.
Keywords: homeostasis, T cell, commensal antigen, FTY720, self antigen, lymphopenia
Introduction
Understanding how naïve T cells are induced to proliferate under lymphopenic environments has been a topic of great interest. Using a germ-free (GF) SCID model, it was previously shown that both CD4 and CD8 T cell proliferation is significantly reduced in the absence of commensal microflora [1], which led to the conclusion that T cell proliferation is primarily induced by non-self-ligands, most likely derived from commensal microflora. Since MHC II expression by dendritic cells (DC) is essential for CD4 T cell proliferation to occur [2], commensal antigens presented by DC that reside in the gut draining mesenteric LN (mLN) are likely the major stimuli underlying this response. This notion is further supported by the fact that lymphopenic recipients that receive naïve non-regulatory CD4 T cells induce colitis, which is believed to be caused by uncontrolled T cell activation in response to commensal bacteria [3].
However, some level of T cell proliferation is still observed in GF SCID recipients following T cell transfer [1], raising a possibility that a gut antigen-independent mechanism might be operative in inducing the proliferation. Therefore, whether commensal antigens are the sole source of stimulation, or whether endogenous self antigens that are constantly presented to peripheral T cells as tonic signals are also involved in this process remains to be tested. Indeed, it has been proposed that activated T cells within lymphopenic environments can lead to autoimmune disorders such as diabetes, arthritis, or immune reconstitution inflammatory syndrome (IRIS) [4–6], further supporting the importance of self antigens in inducing the proliferation.
To better characterize T cell proliferation under lymphopenic settings, we took advantage of using S1P1 agonist, FTY720, and antibiotics to prevent activation induced T cell migration between lymphoid (and into non-lymphoid) tissues and to deplete commensal microflora, respectively. The approach revealed that although the mLN is the primary location where T cell proliferation takes place, a significant proliferation is still induced within the peripheral LN (pLN). In particular, treating recipients with both FTY720 and antibiotics demonstrated the contribution of non-mLN tissues in this process. Furthermore, transferring T cells into lymphopenic GF mice as well as the immunoscope analysis of memory phenotype CD4 T cells from neonatal GF mice strongly suggest the importance of self antigens. Therefore, T cell proliferation under lymphopenic conditions is a heterogeneous process induced by both exogenous commensal and endogenous self antigens.
Materials and methods
Mice
C57BL/6, B6 Ly5.1, B6 Thy1.1 and B6 TCRβ−/− mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Seven-day old germ-free (GF) or conventional Swiss Webster (SW) neonates with the dams were purchased from Taconic (Germantown, NY). They were maintained in flexible-film or semi-rigid isolators provided by Taconic. Adult GF mice in C57BL/6 background were bred and maintained at the Germ-free Animal Core Facility of the University of Michigan. GF mice were maintained in flexible film isolators and were checked weekly for germ-free status by aerobic and anaerobic culture. The absence of microbiota was verified by microscopic analysis of stained cecal contents to detect unculturable contamination. All animal procedures were conducted according to the guidelines of the Institutional Animal Care and Use Committee.
Cell sorting and adoptive transfer
LN naive T cells were obtained as follows. pLN (axillary, cervical, and inguinal LN) and mesenteric LN were pooled and the total T cells were purified by negative selection. CD44low naive T cells were further sorted using a FACSAria cell sorter (BD Bioscience, San Jose, CA). Sorted T cells were labeled with CFSE (Molecular Probe, Carlsbad, CA). 1 × 106 donor T cells were transferred i.v. into the recipients described throughout the study. In some experiment, SPF (Thy1.2+) and GF (Thy1.2+) mice were injected i.p. on Day 0 with cyclophosphamide (CTX) (4 mg/mouse) to induce transient state of lymphopenia [7]. 1×105 FACS sorted splenic Thy1.1 CD4 T cells were transferred into the CTX treated recipients. Two weeks later the recipients were sacrificed and the numbers of donor T cells were calculated from the spleen.
FACS analysis
Recipients were sacrificed at the indicated time points after donor T cell transfer. Peripheral LN, mesenteric LN, and the spleen were harvested, single cell suspension obtained, and stained with anti-CD4 (RM4–5), anti-CD8 (53–6.7), anti-CD122 (5H4), anti-CD127 (A7R34), anti-CD44 (IM7), CD62L (MEL-14), anti-CD45.1 (A20), or anti-Thy1.1 (HIS51) (all Abs were purchased from eBioscience). Cells were acquired using a FACSCalibur, and analyzed with FlowJo software.
Commensal microflora depletion
As described [8], 6–8 week old mice were treated with ampicillin (1g/L, Sigma), vancomycine (500mg/L), neomycin sulfate (1g/L), and metronidazole (1g/L) in drinking water for 4–5 weeks.
Splenectomy and mesenteric lymphadenectomy
Mice will be anesthesized with 50mg/kg Nembutal. 1.5~2cm left-sided incision between the last rib and the hip joint was made and the spleen was pulled onto the exterior surface of the peritoneum. The spleen was removed after appropriate blood vessel ligation. For mesenteric lymphadenectomy, mLN was microdissected along the length of the superior mesenteric artery to the aortic root. Sham animals underwent laparotomy without removing the spleen or the mLN.
Immunoscope analysis
Detailed experimental procedures and the primer sequences were previously reported [9]. In brief, total RNA was extracted from FACS sorted CD44bright CD4 T cells using the RNeasy procedure (QIAGEN Inc., Valencia, CA); cDNA was synthesized using oligo-(dT)12–18, Superscript reverse transcriptase (Invitrogen, Carlsbad, CA), and RNase Out (Invitrogen) in the provided buffer. PCR runs to saturation (40 cycles) were carried out in 25μl reaction volumes containing 0.6U of AmpliTaq GoldTM DNA polymerase (PE Applied Biosystems, Foster City, CA), 2.5mM MgCl2, 0.2mM dNTP, 0.25μM of each primer (BV and BC), and normalized amounts of cDNA template in the provided buffer. Amounts of cDNA encoding TCRβ chain were determined by real time quantitative PCR. Run-off reactions (5 cycles) were then performed on 2μl of PCR product with an internal BC-specific FAM-labeled primer at a final concentration of 0.1μM in 10μl final volume. 2μl volumes of run-off products were run on a 4.25% polyacrylamide 8M urea gel in an automated 377 DNA sequencer (PE Applied Biosystems), together with size standard. The intensity of the various bands was then recorded and analyzed with Immunoscope software.
Data analysis
Statistical significance was determined by the Student’s t-test using the Prism 4 (Graphpad Software, La Jolla, CA). P<0.05 was considered to indicate a significant difference.
Results
The contribution of LN tissues to proliferation of naïve T cells within lymphopenic settings
Following an initial contact between T cells and antigen-bearing APCs, immunological synapse forms and subsequent stable interaction may persist for up to 48 hours, after which T cells show signs of activation, dissociate from the APCs, and circulate [10]. Likewise, T cells that proliferate under lymphopenic conditions are expected to undergo prolonged interaction with MHC-expressing APCs, get activated, leave the initial activation sites, circulate, and traffic into different tissues. We recently reported that the LN entry is critical for the proliferation to occur as pertussis toxin pretreated naïve T cells failed to do so [2]. In order to locate sites where the initial activation takes place, we took an advantage of using the sphingosine 1-phosphate 1 (S1P1) receptor agonist, FTY720. FTY720 prevents lymphocyte egress from the lymphoid tissues, including the thymus and LN, through binding to and downregulating the S1P1 receptor [11, 12]. Thus, very few T cells are found in the circulation of mice treated with FTY720 [13]. It was shown that FTY720 treatment reduces the number of antigen-activated T cells, yet activation-induced proliferation was not impaired [14]. FACS sorted naïve T cells (containing both CD4 and CD8) were labeled with CFSE and adoptively transferred into T cell-deficient TCRβ−/− recipients. Starting 12 hours post cell transfer, mice received daily FTY720 treatments, which would allow many of the transferred cells to seek APCs that express the “antigens” before they are immobilized in the lymphoid tissues by FTY720. Mice were sacrificed 7 days after transfer and donor T cell proliferation determined by FACS analysis. Blood T cell levels of the treated recipients were analyzed prior to sacrifice to insure the effectiveness of FTY720 treatment. As shown in Figure 1A, FTY720 treatment dramatically increased both the proportions and the total numbers of donor CD4 and CD8 T cells in the LNs, especially mesenteric LN (mLN). By contrast, proliferating T cells accumulated in the spleen substantially decreased following the treatment (Figure 1A). Proliferation profiles determined by CFSE dilution further supported this finding. In the spleen, ~14 and ~35% of donor CD4 and CD8 T cells, respectively, remained undivided in FTY720 treated recipients, while the majority of donor T cells fully diluted the CFSE in the spleen of H2O treated recipients (Figure 1B). In the LNs, CFSE dilution was similar between the groups, indicating that signals that induce proliferation likely remain unchanged regardless of the treatment. In support of this, BrdU incorporation of the transferred cells in the LN was substantially elevated by FTY720 treatment, while that in the spleen was greatly reduced by the treatment (Figure 1C). Interestingly, BrdU+ donor T cells in the mLN remained unchanged regardless of the treatment. Therefore, these results demonstrate that in addition to mLN, naïve T cells appear to undergo substantial proliferation within the pLN. Furthermore, responding cells may escape from the spleen in presence of FTY, while they are confined within the LN. Alternatively, it is possible that there are less stimulatory antigens available in the spleen compared to those available in the LN tissues. Although it can be argued that a rise in T cell numbers in certain tissues may simply reflect different T cell sequestration or survival rather than proliferation, we estimated that T cells undergo more than 7 cell divisions within 7 days post transfer and that >20% of the T cells incorporate BrdU after a 24-hour BrdU pulse (Figure 1B and 1C). Therefore, differential accumulation of the transferred T cells is not likely due to different migration behavior and/or survival of T cells.
Figure 1. The effect of FTY720 treatment on the proliferation of adoptively transferred naive CD4 and CD8 T cells.

FACS sorted naive Ly5.1 T cells were transferred into TCRβ−/− mice. Mice were treated with FTY720 (0.5 mg/kg) or water for control group every day. (A) Total donor cell recovery, (B) CFSE profiles, and (C) BrdU incorporation of the transferred T cells were analyzed 7 days post transfer. Mice were injected with 1mg BrdU i.p 24 hr prior to sacrifice. Shown are the mean ± SD of individually tested mice (n =3). Each symbol shown in (A) represents individually tested recipients. *, p<0.05; **, p<0.01; ***, p<0.001.
TCR usage distribution under FTY720 treatment
If T cell proliferation is primarily induced by gut antigens, then it is possible that the clonality of proliferating T cells differs depending on the location where they are induced to proliferate. To examine if the repertoire of proliferating T cells is different under the influence of FTY720, we analyzed the TCRβ distribution of CD4 and CD8 T cells that proliferated in TCRβ−/− mice treated with either H2O or FTY720 as described above. Both CD4 and CD8 T cells proliferated within H2O treated TCRβ−/− recipients displayed highly diverse TCRβ expression. For CD4 T cells, although Vβ8.1/8.2 was the dominant Vβ chain, the TCR was evenly distributed throughout the remaining Vβs, Vβ3, Vβ4, Vβ5.1/5.2, Vβ10, Vβ11, Vβ12, and Vβ14. For CD8 T cells, Vβ5, Vβ7, and Vβ8.1/8.2 were the dominant Vβ chain (Figure 2A). Furthermore, the TCR distribution between the tissues was not dramatically different, except Vβ9 of CD8 T cells (black arrow in Figure 2A). Following FTY720 administration, however, we found that the TCR distribution of both CD4 and CD8 T cells was altered compared to that in H2O treated recipients. In case of CD4 T cells, Vβ5 and Vβ8.1/8.2 T cells became the dominant Vβs. However, no significant differences in TCR distribution between the tested tissues were noticed, except Vβ5 in mLN tissue. In case of CD8 T cells, Vβ5.1/5.2, Vβ6, and Vβ8.1/8.2 became the dominant TCR under the FTY720 (Figure 2A). Interestingly, when the TCR distribution of mLN CD4 and CD8 T cells was compared between H2O- and FTY720-treated recipients, the difference was relatively minor and not significant (Figure 2B). Thus, T cell proliferation within lymphopenic LN tissues is not an oligoclonal response. It should be pointed out that similar TCR distribution between the two settings does not necessarily indicate that the antigens that induce the proliferation are similar. Further study to compare the clonality of the proliferating cells will be necessary to better examine this issue. Moreover, antigens derived from even a single microorganism or a type of self antigen could induce very diverse response at the level of Vβ expression.
Figure 2. Treatment of FTY 720 does not alter the TCR repertoire of adoptively transferred naive CD4 and CD8 T cells.
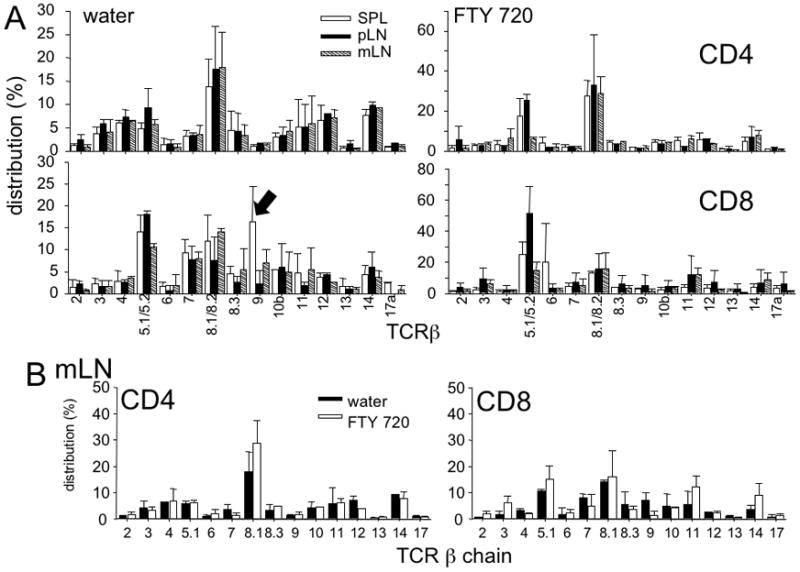
Sorted naive Ly5.1 T cells were transferred into TCRβ−/− recipients that were treated with FTY720 or water every day. TCR repertoire was examined 7 days post transfer. (A) The distribution of each Vβ expressing T cells was compared between different lymphoid tissues. (B) The distribution of each Vβ expressing T cells in mLN tissue was compared between FTY720- and water-treated recipients. Data shown are the mean ± SD of individually tested mice (n=3~4).
Roles of commensal bacteria in T cell endogenous proliferation
Antigens derived from the commensal bacteria were shown to be critical for inducing T cell proliferation in lymphopenic recipients [3]. Notably, fast T cell proliferation was substantially but not fully diminished in germ-free SCID recipients [1]. To closely analyze T cell proliferation/expansion responses within different lymphoid tissues, particularly mLN versus non-mLN, groups of TCRβ−/− mice received antibiotic water for 4 weeks prior to T cell transfer. Following transfer of naïve T cells, T cell proliferation was monitored. As shown in Figure 3A, the proportion of transferred CD4 T cells in the mLN and the spleen was dramatically reduced when recipients were antibiotics treated (Figure 3A). Interestingly, T cell levels in the pLNs were only slightly reduced by antibiotics treatment (Figure 3A). The total donor T cell recovery supported the findings (Figure 3B). CD4 T cell recovery was dramatically reduced especially in the spleen and mLN. The proportion of CD8 T cells was not different in antibiotic-treated and control mice, although the absolute numbers of CD8 T cells in the LN tissues were found reduced in antibiotics-treated recipients (Figure 3B). These results were further supported by CFSE profiles of the transferred T cells (Figure 3C). Therefore, consistent with the previous studies [1], diminution in the gut flora antigens substantially impairs lymphopenia-induced proliferation in most lymphoid tissues tested.
Figure 3. The effects of commensal microflora on the proliferation of adoptively transferred naïve CD4 and CD8 T cells.

CFSE labeled Thy1.1 naïve T cells were transferred TCRβ−/− mice as described above. (A) Donor T cells expansion, (B) the absolute numbers, and (C) CFSE profiles were analyzed 7 days post transfer. Recipient mice were treated with water or antibiotic water containing ampicillin, neomycin sulfate, vancomycine and metronidazole in drinking water for 4–5 weeks prior to T cell transfer. Each symbol shown in (B) represents individually tested recipients. Shown are the mean ± SD of individually tested mice (n =3). *, p<0.05; **, p<0.01; ***, p<0.001.
Finding sizeable T cell expansion in antibiotic treated recipients implies that a gut antigen-independent lymphopenia-mediated T cell proliferation may take place although it must be acknowledged that antibiotics-mediated depletion of gut microorganisms could be incomplete. To further examine the role of the gut flora in this process, we transferred naïve T cells into recipients that were treated with both antibiotics and FTY720. ~20% of CD4 T cells remained undivided in the spleen of treated recipients, while >96% of the transferred T cells in the LN tissues fully diluted their CFSE content (Figure 4A). Interestingly, ~50% of CD8 T cells remained undivided in the spleen of the treated recipients, while CD8 T cell proliferation in the LN tissues (both pLN and mLN) was comparable to control H2O-treated groups (Figure 4A). Consistent with these results, the total cell recovery was significantly reduced in the spleen; however, no difference was noticed in the LN tissues (Figure 4B). These results indicate that antibiotics treated recipients still support lymphopenia-induced proliferation that originates in the LN tissues, further suggesting that a substantial gut flora-independent component of T cell proliferation does exist. Notably, the treatment of antibiotics/FTY720 did not diminish T cell accumulation in the LN tissues, in contrast to significant reduction of T cell accumulation in the LN of mice treated with antibiotics alone (Figure 3B). Therefore, inhibiting activation-induced T cell egress from the pLN in these mice may be capable of promoting continuous expansion of T cells that is responsive to the remaining gut flora-independent (“self” or food) antigens. Although some adverse effect such as dehydration has recently been reported in mice administered with antibiotics [15], we did not notice any sign of dehydration in this experiment. Furthermore, our results also suggest that antibiotics do not appear to have a direct effect on T cell proliferation; however, altered mucosal T cell homeostasis such as cytokine expression has been reported [15].
Figure 4. The effects of both FTY720 and antibiotic treatment on the proliferation of adoptively transferred naïve T cells.

CFSE labeled Thy1.1 naive T cells were transferred TCRβ−/− mice. CFSE profiles (A) and the total donor cell recovery (B) were examined 7 days post transfer. Data shown are the mean ± SD of individually tested mice (n=3~4). *, p<0.05.
The contribution of spleen and of mLN to the T cell proliferation
Since T cell proliferation was significantly reduced in the spleen of mice treated with FTY720 plus antibiotics, we next tested if the spleen is dispensable for the naïve T cell proliferation. To this aim, splenectomy (Spx) was performed in TCRβ−/− mice prior to naïve T cell transfer and T cell proliferation was then examined. The degree of T cell expansion within the LN tissues of splenectomized TCRβ−/− recipients was almost identical to that of sham-splenectomized TCRβ−/− mice (Figure 5A). Furthermore, the total numbers of donor CD4 and CD8 T cells were comparable between splenectomized and control groups of recipients (Figure 5B). Splenectomy did not alter T cell proliferation pattern when determined by CFSE profiles (Figure 5C). The levels of BrdU+ donor T cells were also similar (data not shown). Therefore, the spleen is dispensable for naïve T cells to undergo proliferation under lymphopenic conditions.
Figure 5. The effect of splenectomy and lymphadenectomy on the proliferation of adoptively transferred naïve T cells.

Sorted naive Ly5.1 T cells were transferred into TCRβ−/− recipients that were either spenectomized (Spx) or mesenteric lymphadenectomized (Lnx). (A) Donor cells expansion and (B) the total donor cell recovery were examined 7 days post transfer. Results are representative of two independent experiments. Each symbol shown in (B) represents individually tested recipients. **, p<0.01; ***, p<0.001. (C) CFSE profiles of donor CD4 T cells from TCRβ−/− recipients with Spx or Lnx are shown. Proliferation of donor CD8 T cells was similar to that of CD4 T cells (data not shown).
Because depleting commensal microflora substantially impaired T cell proliferation in the mLN, we also examined the contribution of mLN by measuring T cell responses in TCRβ−/− recipients with mesenteric lymphadenectomy (Lnx) prior to transfer. To our surprise, T cell expansion (Figure 5A) as well as CFSE dilution (Figure 5C) was similar to that in control groups of recipients. In fact, donor cell recovery in the pLN of mice without mLN was significantly higher compared to sham control mice (Figure 5B). However, CFSE profiles suggest that increased T cells in pLN is not due to redistribution of proliferating T cells. Therefore, these results strongly suggest that T cell proliferation within non-mLN tissues is substantial in vivo.
Role of commensal microflora in T cell expansion and endogenous memory T cell generation
It was recently reported that commensal microflora promotes steady-state IL-7 production that is necessary for T cell survival [16]. To test whether T cell maintenance is affected by commensal microflora, FACS sorted Thy1.1 CD4 T cells were transferred into Thy1.2+ SPF or GF recipients that were injected with cyclophosphamide 2 days earlier. Cyclophosphamide induces transient state of lymphopenia and thus the treatment can induce “slow” homeostatic proliferation of transferred T cells [7]. When the recovery of transferred donor CD4 T cells was examined 2 weeks post transfer, we found that the recovery was slightly higher in cyclophosphamide-treated GF recipients, although the difference was not statistically significant (Figure 6A).
Figure 6. T cell expansion and differentiation under GF conditions.
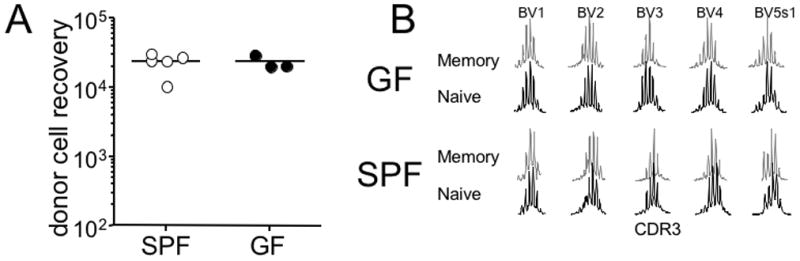
(A) FACS sorted Thy1.1 CD4 T cells were transferred into CTX-treated SPF or GF recipients as described in the Materials and Methods. The recipients were sacrificed 2 weeks post transfer, and the total donor T cell number was calculated from the spleen. Each symbol represents individually tested recipient. (B) CD44high memory phenotype CD4 T cells were isolated from 7-day old SPF or GF neonates. TCR repertoire distribution was determined using the immunoscope analysis as described in the Materials and Methods. ns, not significant. TCR repertoires shown are representative of three independent experiments.
Endogenous memory phenotype CD4 T cells in young mice are found to be generated from a robust proliferation as determined by the T cell receptor excision circle (TREC) numbers [17]. It was also suggested that this type of proliferation is a homeostasis-driven response induced by lymphopenic conditions imposed during neonatal period [17]. Moreover, the TCR distribution of these memory phenotype T cells of young mice was as diverse as those from adult mice [17]. To test whether the generation of endogenous memory phenotype CD4 T cells is dependent on commensal microflora, we isolated CD44high memory phenotype CD4 T cells from groups of SPF and GF neonatal mice and performed the immunoscope analysis. Of note, the lack of gut antigens did not alter the frequency or the distribution of CD44high memory phenotype CD4 T cells [18]. As shown in Figure 6B, TCR repertoire of both GF and SPF neonates was highly diverse. These results strongly suggest a dispensable contribution of commensal microflora-derived antigen in the maintenance as well as the generation of endogenous memory phenotype CD4 T cells.
Discussion
Our results demonstrate the importance of mLN tissue in inducing the proliferation of both CD4 and CD8 T cells. Thus, inhibiting activated T cell egress from the lymphoid tissues by downregulating S1P1 receptors leads to a significant accumulation of expanding T cells in the mLN. Consistent with this, treating recipients with antibiotics resulted in a substantial reduction of T cells accumulating in the mLN. Unexpectedly, however, we found essentially undiminished T cell proliferation in the pLN from the mice treated with FTY720. Interestingly, antibiotic treatment somewhat decreased the accumulation of T cells in the pLN. However, the decrease was not observed in mice treated with both FTY720 and antibiotics, indicating that stimulatory signals do exist in the pLN independent of commensal antigens. Although antibiotics treatment results in a substantial depletion of commensal microflora [8, 15], we cannot exclude the possibility that those T cells proliferating and accumulating under this condition are in response to antigens derived from the remaining commensal organisms. Yet, we found that proliferating T cells accumulated in GF mice with cyclophosphamide induced lymphopenia, implying that lymphopenia induced proliferation can occur in mice that lacked commensal flora. Furthermore, TCR repertoire analysis of endogenous memory phenotype CD4 T cells within neonatal mice that are generated by homeostatic mechanisms revealed a dispensable role of commensal microflora.
Our results imply that T cells can respond to both peptide/MHC complexes in which the peptides derived from commensal microflora and from endogenous “self” antigens under lymphopenic settings. Commensal antigens might still be dominant in inducing T cell proliferation, although relative contribution of self antigens to this process remains to be better defined. Gut associated inflammation such as colitis and systemic autoimmunity can arise from uncontrolled activation of T cells specific for commensal antigens and, presumably, for self antigens, respectively [3, 19, 20]. Therefore, the role of self antigens in triggering T cell proliferation and generating effector cells may be critical for the resulting autoimmune inflammation. Importantly, T cells proliferating in the absence (or marked diminution) of commensal antigens are as highly diverse in the TCR repertoire distribution as are those from SPF mice. Alternatively, the microbiota-independent responses might be induced by food antigens. Given the fact that TCR-MHCII interaction is essential for the proliferation to occur [2], self antigen(s) involved in tonic TCR signals to the naïve T cells might be responsible for the proliferation [21]. Mechanisms involved in converting tonic signals to proliferating signals under lymphopenic conditions remain to be determined.
It is interesting to note that T cell responses are not affected by the lack of spleen or mLN tissues. When commensal microflora dependent T cell responses are diminished by mesenteric lymphadenectomy, T cells entering the pLN and possibly reacting to self antigens might proliferate and expand better, which is likely to be mediated by increased availability of survival factors including IL-7 [22, 23]. Indeed, the overall T cell recovery from the pLN of mice with mesenteric lymphadenectomy was significantly elevated. It is possible that small lymph nodules or gut associated lymphoid tissues might still be functional in presenting antigens and inducing T cell activation after mesenteric lymphadenectomy. However, their contribution to the T cell responses examined in this study is likely to be minor.
Our results raise several interesting questions. First, what is the frequency of naïve T cells that respond to commensal vs. self antigens? We previously speculated that T cells that undergo proliferation in lymphopenic settings are in the range of 2~10% [24]. Although the actual specificity of these cells remains to be determined, it is reasonable to expect that T cells activated in these tissues may display different antigen specificity; that is, T cells proliferating within the mLN could be enriched in those activated by commensal antigens, while T cells proliferating within the pLN might be enriched for self antigen responding cells. Second, what are the antigen presenting cells in these tissues? We have recently demonstrated that CD11c+ dendritic cells are essential to induce T cell proliferation but that MHC II-abundant B cells are unable to do so [2]. Therefore, presentation of self antigens on these cells could be a default process and most of the commensal antigens are likely to be drained into the mLN and presented on the mLN resident DC. Alternatively, lamina propria DC may uptake commensal antigens, migrate into mLN, and present the antigens to T cells [25]. The contribution and the origin of DC in the different lymphoid tissues to the T cell proliferation will need more investigation. Third, is the specificity of naïve T cells different from other T cell subsets such as endogenous memory phenotype T cells or regulatory T (Treg) cells? Especially, Treg play important roles in limiting commensal microflora-induced intestinal inflammation [26]. Since the specificity of Treg could be important for the suppression [27], it is possible that Treg may react to gut antigens. In fact, inducible Treg cells are efficiently generated in the lamina propria [28]. Therefore, the use of FTY720 and/or antibiotics may be used to extend the current findings to different T cell subsets such as memory phenotype T cells or Treg cells, from which the mechanism underlying the generation of these cells could be identified.
Highlights.
We examined the contribution of commensal vs. non-commensal antigens to the T cell proliferation within lymphopenic recipients
Mesenteric LN is the dominant location where T cell proliferation occurs
T cell proliferation is pronounced within the peripheral LN upon commensal microflora depletion and FTY720 treatment
Spleen is dispensable for T cell proliferation
Gnotobiotic mice were used to demonstrate gut Ag-independent T cell proliferation
Acknowledgments
This study was supported by NIH grant AI074932 (B.M.) and the intramural research program of the NIAID/NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kieper WC, Troy A, Burghardt JT, Ramsey C, Lee JY, Jiang HQ, Dummer W, Shen H, Cebra JJ, Surh CD. Recent immune status determines the source of antigens that drive homeostatic T cell expansion. J Immunol. 2005;174:3158–3163. doi: 10.4049/jimmunol.174.6.3158. [DOI] [PubMed] [Google Scholar]
- 2.Do JS, Min B. Differential requirements of MHC and of DCs for endogenous proliferation of different T-cell subsets in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:20394–20398. doi: 10.1073/pnas.0909954106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feng T, Wang L, Schoeb TR, Elson CO, Cong Y. Microbiota innate stimulation is a prerequisite for T cell spontaneous proliferation and induction of experimental colitis. The Journal of experimental medicine. 2010;207:1321–1332. doi: 10.1084/jem.20092253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baccala R, Theofilopoulos AN. The new paradigm of T-cell homeostatic proliferation-induced autoimmunity. Trends Immunol. 2005;26:5–8. doi: 10.1016/j.it.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Khoruts A, Fraser JM. A causal link between lymphopenia and autoimmunity. Immunol Lett. 2005;98:23–31. doi: 10.1016/j.imlet.2004.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krupica T, Jr, Fry TJ, Mackall CL. Autoimmunity during lymphopenia: a two-hit model. Clin Immunol. 2006;120:121–128. doi: 10.1016/j.clim.2006.04.569. [DOI] [PubMed] [Google Scholar]
- 7.Bracci L, Moschella F, Sestili P, La Sorsa V, Valentini M, Canini I, Baccarini S, Maccari S, Ramoni C, Belardelli F, Proietti E. Cyclophosphamide enhances the antitumor efficacy of adoptively transferred immune cells through the induction of cytokine expression, B-cell and T-cell homeostatic proliferation, and specific tumor infiltration. Clin Cancer Res. 2007;13:644–653. doi: 10.1158/1078-0432.CCR-06-1209. [DOI] [PubMed] [Google Scholar]
- 8.Xiao H, Gulen MF, Qin J, Yao J, Bulek K, Kish D, Altuntas CZ, Wald D, Ma C, Zhou H, Tuohy VK, Fairchild RL, de la Motte C, Cua D, Vallance BA, Li X. The Toll-interleukin-1 receptor member SIGIRR regulates colonic epithelial homeostasis, inflammation, and tumorigenesis. Immunity. 2007;26:461–475. doi: 10.1016/j.immuni.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Pannetier C, Cochet M, Darche S, Casrouge A, Zoller M, Kourilsky P. The sizes of the CDR3 hypervariable regions of the murine T-cell receptor beta chains vary as a function of the recombined germ-line segments. Proc Natl Acad Sci U S A. 1993;90:4319–4323. doi: 10.1073/pnas.90.9.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stoll S, Delon J, Brotz TM, Germain RN. Dynamic imaging of T cell-dendritic cell interactions in lymph nodes. Science. 2002;296:1873–1876. doi: 10.1126/science.1071065. [DOI] [PubMed] [Google Scholar]
- 11.Sensken SC, Graler MH. Down-regulation of S1P1 receptor surface expression by protein kinase C inhibition. J Biol Chem. 2010;285:6298–6307. doi: 10.1074/jbc.M109.049692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 13.Chiba K, Yanagawa Y, Masubuchi Y, Kataoka H, Kawaguchi T, Ohtsuki M, Hoshino Y. FTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats. I. FTY720 selectively decreases the number of circulating mature lymphocytes by acceleration of lymphocyte homing. Journal of immunology. 1998;160:5037–5044. [PubMed] [Google Scholar]
- 14.Zhang Q, Chen Y, Fairchild RL, Heeger PS, Valujskikh A. Lymphoid sequestration of alloreactive memory CD4 T cells promotes cardiac allograft survival. J Immunol. 2006;176:770–777. doi: 10.4049/jimmunol.176.2.770. [DOI] [PubMed] [Google Scholar]
- 15.Hill DA, Hoffmann C, Abt MC, Du Y, Kobuley D, Kirn TJ, Bushman FD, Artis D. Metagenomic analyses reveal antibiotic-induced temporal and spatial changes in intestinal microbiota with associated alterations in immune cell homeostasis. Mucosal Immunol. 2010;3:148–158. doi: 10.1038/mi.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shalapour S, Deiser K, Sercan O, Tuckermann J, Minnich K, Willimsky G, Blankenstein T, Hammerling GJ, Arnold B, Schuler T. Commensal microflora and interferon-gamma promote steady-state interleukin-7 production in vivo. European journal of immunology. 2010;40:2391–2400. doi: 10.1002/eji.201040441. [DOI] [PubMed] [Google Scholar]
- 17.Min B, McHugh R, Sempowski GD, Mackall C, Foucras G, Paul WE. Neonates support lymphopenia-induced proliferation. Immunity. 2003;18:131–140. doi: 10.1016/s1074-7613(02)00508-3. [DOI] [PubMed] [Google Scholar]
- 18.Min B, Thornton A, Caucheteux SM, Younes SA, Oh K, Hu-Li J, Paul WE. Gut flora antigens are not important in the maintenance of regulatory T cell heterogeneity and homeostasis. Eur J Immunol. 2007;37:1916–1923. doi: 10.1002/eji.200737236. [DOI] [PubMed] [Google Scholar]
- 19.Surh CD, Sprent J. Homeostatic T cell proliferation: how far can T cells be activated to self-ligands? J Exp Med. 2000;192:F9–F14. doi: 10.1084/jem.192.4.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ochoa-Reparaz J, Mielcarz DW, Ditrio LE, Burroughs AR, Foureau DM, Haque-Begum S, Kasper LH. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J Immunol. 2009;183:6041–6050. doi: 10.4049/jimmunol.0900747. [DOI] [PubMed] [Google Scholar]
- 21.Hochweller K, Wabnitz GH, Samstag Y, Suffner J, Hammerling GJ, Garbi N. Dendritic cells control T cell tonic signaling required for responsiveness to foreign antigen. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:5931–5936. doi: 10.1073/pnas.0911877107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, Surh CD. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci U S A. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guimond M, Veenstra RG, Grindler DJ, Zhang H, Cui Y, Murphy RD, Kim SY, Na R, Hennighausen L, Kurtulus S, Erman B, Matzinger P, Merchant MS, Mackall CL. Interleukin 7 signaling in dendritic cells regulates the homeostatic proliferation and niche size of CD4+ T cells. Nat Immunol. 2009;10:149–157. doi: 10.1038/ni.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Min B, Paul WE. Endogenous proliferation: burst-like CD4 T cell proliferation in lymphopenic settings. Semin Immunol. 2005;17:201–207. doi: 10.1016/j.smim.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 25.Chieppa M, Rescigno M, Huang AY, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med. 2006;203:2841–2852. doi: 10.1084/jem.20061884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barnes MJ, Powrie F. Regulatory T cells reinforce intestinal homeostasis. Immunity. 2009;31:401–411. doi: 10.1016/j.immuni.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 27.Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nature immunology. 2002;3:756–763. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- 28.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]


