Abstract
Confirming clinical evidence, we recently demonstrated that a blunt chest trauma considerably impaired fracture healing in rats, possibly via the interaction of posttraumatic systemic inflammation with local healing processes, the underlying mechanisms being unknown. An important trigger of systemic inflammation is the complement system, with the potent anaphylatoxin C5a. Therefore, we investigated whether the impairment of fracture healing by a severe trauma resulted from systemically activated complement.
Rats received a blunt chest trauma and a femur osteotomy stabilized with an external fixator. To inhibit the C5a dependent posttraumatic systemic inflammation, half of the rats received a C5aR-antagonist intravenously immediately and 12 h after the thoracic trauma. Compared to the controls (control peptide), the treatment with the C5aR-antagonist led to a significantly increased flexural rigidity (three-point-bending test), an improved bony bridging of the fracture gap, and a slightly larger and qualitatively improved callus (μCT, histomorphometry) after 35 days.
In conclusion, immunomodulation by a C5aR-antagonist could abolish the deleterious effects of a thoracic trauma on fracture healing, possibly by influencing the function of inflammatory and bone cells locally at the fracture site. C5a could possibly represent a target to prevent delayed bone healing in patients with severe trauma.
Keywords: Fracture healing, blunt chest trauma, complement
Introduction
A severe trauma such as a blunt chest trauma is considered a potent initiator of a systemic inflammatory response, being characterized by a strong systemic activation of the complement and coagulation cascades, and the release of pro-inflammatory cytokines and prostanoids1–3. It was reported that fracture healing was delayed and more non-unions occurred in severely injured patients4,5. In confirming the clinical evidence, we recently demonstrated experimentally in rats that a blunt chest trauma, which induced a posttraumatic systemic inflammation, considerably impaired fracture healing. This suggests that the systemic inflammatory response disturbs the local inflammatory and regeneration processes in bone, the underlying mechanisms, however, remaining unknown6.
A powerful trigger of the posttraumatic systemic inflammation is the complement system1,7,8. The complement cascade, consisting of over 30 proteins, is an important component of the innate immunity and can be activated by four pathways, the classical, the lectin, the alternative and the extrinsic pathways. In all cases the activation pathways lead to the production of the important anaphylatoxin C5a9,10. In trauma victims, systemic C5a immediately increased within minutes and was strongly correlated with injury severity11,12. C5a induces for example the migration of phagocytes, degranulation of mast cells, systemic cytokine release, respiratory burst induction and the regulation of apoptosis in inflammatory cells, thus acting at the very first line of defense in the posttraumatic systemic inflammatory response1. The excessive activation of complement, however, can also cause harmful effects, for example immunoparalysis and organ dysfunction12,13. Due to its strong pro-inflammatory character, C5a is regarded as being the most hazardous molecule in the over-activated complement cascade1,13,14. Therefore, the question arises as to whether the posttraumatic systemic activation of C5a contributes to delayed fracture healing observed clinically4,5 and experimentally6 after severe injury.
This assumption is also strengthened by a recent study by our group demonstrating for the first time that the cellular receptor for C5a, C5aR, was locally expressed in a distinct spatial and temporal pattern in the fracture callus of rats not only by inflammatory cells but also by osteoblasts, chondroblasts and osteoclasts in zones of intramembranous and enchondral bone formation15. Furthermore, in vitro studies revealed that in osteoblasts C5aR activation could induce cell migration15 and cytokine release16, and also modulate osteoclast formation17. This suggests that the anaphylatoxin C5a could potentially influence the fine local inflammatory balance of the bone healing process by acting on inflammatory cells as well as on osteoblasts and osteoclasts.
Therefore, in this study we addressed the question, whether the impairment of fracture healing by a severe trauma resulted from systemically activated complement. Based on our previous work6 we hypothesized that the systemic administration of a C5aR-antagonist would at least partly abolish the deleterious effect of a blunt chest trauma on bone healing in a rat model. The C5aR-antagonist was applied after the thoracic trauma to prevent the immediate C5a-dependent systemic inflammation. The fracture healing outcome was investigated after 35 days.
Methods
Animal experiment
The animal experiment was performed according to international regulations for the care and use of laboratory animals, and approved by the local ethical committee (Regierungspräsidium Tübingen, Germany). Sixteen male Wistar rats (weight 400–450 g; age 10–12 weeks) received a blunt chest trauma combined with a femur osteotomy that was stabilized with an external fixator. Then the animals received either a C5aR-antagonist (n=8) or a control peptide (control group, n=8).
Surgery and blunt chest trauma
Surgery was performed as described previously6,18. Briefly, a standardized osteotomy gap of 1 mm was created at the mid-shaft of the right femur and fixated with a custom-made external fixator. The offset of the fixator block was 6 mm, resulting in an axial stiffness of 119 N/mm6. Immediately after surgery the rats received an additional blunt chest trauma under general anesthesia using a blast wave generator as previously described in detail1,19. This model allows a bilateral, isolated lung contusion by the application of a standardized single blast wave centered on the middle of the thorax and induces a reproducible transient systemic inflammation1,6. An analgesic (20 mg/kg, Tramal®, Gruenenthal GmbH, Aachen, Germany) was administered subcutaneously during the operation and was diluted in the drinking water (25 mg/l) for the first 3 days following surgery. Each animal was individually housed, given unrestricted access to food and monitored daily for infection and mobility.
C5aR-antagonist
Immediately after the blunt chest trauma, one group received a C5aR-antagonist ([Ac-F[OPdChaWR]; PMX-53) at a dosage of 1 mg/kg intravenously into the penis vein20,21. The injection was repeated 12 h after the trauma to prevent the C5a-dependent systemic inflammation, which was detectable during the first 12–24 hours after the blunt chest trauma in rats1,6. Control animals received a peptide ([Ac-F[OPdChaAdR]) with two changed amino acids, which does not have antagonistic activity and thus does not develop any biological effect at the same concentration and at the same time points22.
Biomechanical testing
After 35 days the rats were sacrificed and the operated as well as the contralateral intact femora were explanted. Biomechanical testing was performed using a non-destructive, three-point bending test, as described previously6. Briefly, after removing the fixators, the distal end of each bone was potted in a cylinder using polymethylmethacrylate (Technovit® 3040, Heraeus Kulzer GmbH, Wertheim, Germany) and fixed in a hinge joint whereas the proximal end of the femur rested on the bending support. A quasistatic load was applied in a three-point bending mode with a materials testing machine (1454, Zwick GmbH, Ulm, Germany) using a 500 N load cell (System-Technik GmbH, Germany) and the flexural rigidity (EI) was calculated from the slope of the force deflection curve. The absolute values of the operated femora were related to the contralateral values of the un-operated femora to eliminate individual differences.
Micro-computed tomography
The femora were scanned using a μCT scanning device (skyscan 1172, Kontich, Belgium), operating at a peak voltage of 50 kV and 200 μA at a resolution of 15 μm. The mineralized callus within the former osteotomy gap was segmented and the total tissue volume and the bone volume fraction (BV/TV) were calculated by global thresholding to distinguish between mineralized and non-mineralized tissue23. The maximum moment of inertia was calculated based on the tissue area on the transversal slices in the fracture gap. The apparent modulus of elasticity was calculated as the flexural rigidity divided by the maximum moment of inertia24. According to the standard clinical evaluation of x-rays the number of bridged cortices per callus were evaluated in two planes at right angles to one another by using an CT analyzing software (Data viewer, Skyscan, Kontich, Belgium)25. The distal pin hole served as orientation for the exact positioning of the specimens. At least three bridged cortices per callus were considered as a “healed fracture”. Two observers evaluated the cortical bridging independently in a blinded fashion.
Histomorphometry
After fixating the femora in buffered 4% formaldehyde they were dehydrated with ethanol (40–100%) and embedded in methyl methacrylate (Merck KGaA, Darmstadt, Germany). 70 μm longitudinal sections were prepared, which were cut in anterior-posterior direction of the right femur. The pin holes guaranteed the standardized orientation of the sections. Then the sections were stained with Paragon (Toluidin blue and Fuchsin; both Waldeck GmbH & Co KG, Münster, Germany), which stains fibrous tissue in blue, cartilage tissue in purple and mineralized matrix in white-yellow. In the former osteotomy gap the newly formed tissue was evaluated by using a light microscope (Leica DMI6000B, Heerbrugg, Switzerland) at a 5-fold magnification. The amount of bone, cartilage and fibrous tissue was assessed by circumscribing the corresponding areas with image analysis software (Leica MMAF 1.4.0 Imaging System, Leica, Heerbrugg, Switzerland powered by MetaMorphR).
Statistical analysis
Results are presented as medians and interquartile ranges (IR). For statistical analysis, the software PASW Statistics 18.0 (SPSS Inc., Chicago, USA) was used. Differences between groups regarding flexural rigidity, μCT-parameters and histomorphometrical data were calculated using a Mann-Whitney U test, whereas differences between groups regarding the number of bridged cortices were calculated using the Fisher exact test. The level of significance was p < 0.05.
Results
Biomechanical testing
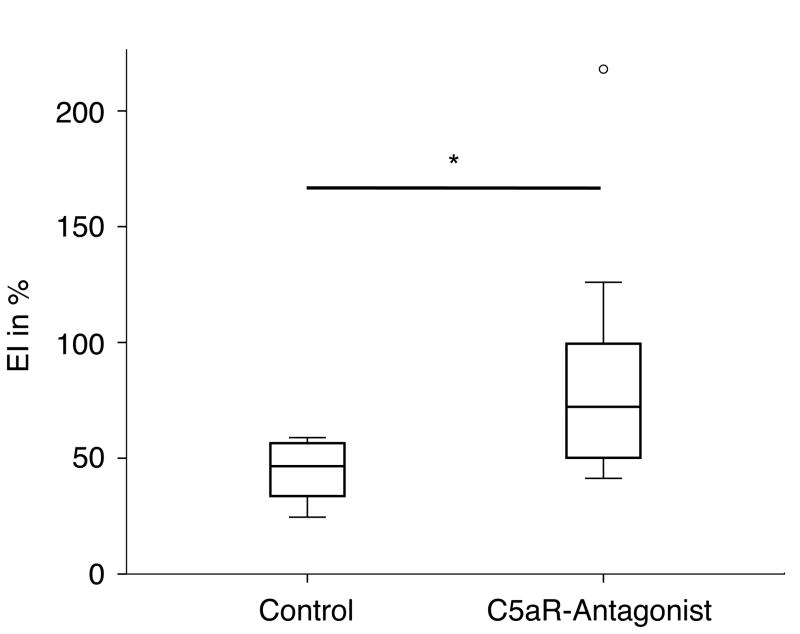
The treatment of the animals with the C5aR-antagonist after blunt chest trauma significantly increased the flexural rigidity (Ctrl: EI=46,54% (IR: 27,44); C5aR-Ag: EI=72,18% (IR: 66,50)) of the callus by about 55% compared to the control group, which received the control peptide (Figure 1).
Figure 1.
Flexural rigidity (EI) of the fracture callus of rats without (Control) or with treatment with a C5aR-antagonist immediately and 12 h after the blunt chest trauma. * = p < 0.05
Micro-computed tomography
The application of the C5aR-antagonist led to a tendency for higher total callus volume, maximum moment of inertia and apparent modulus of elasticity. None of the parameters showed statistical significance compared to the control group (Table 1). The results might indicate a somewhat larger and qualitatively superior callus in the rats treated with the C5aR-antagonist. Furthermore, the fracture callus of rats which received the C5aR-antagonist showed considerably more bridged cortices compared to the control group, even though this was not statistically significant (Table 2).
Table 1.
μ-computed tomography analysis of the calli of rats without (Control) or with treatment with a C5aR-antagonist after blunt chest trauma
| Measure | Control | C5aR-Antagonist |
|---|---|---|
| Total callus volume (mm3) | 15.72 (IR: 6,35) | 16.47 (IR: 7.75) |
| BV/TV (%) | 85.55 (IR: 11.15) | 82.26 (IR: 21.89) |
| Maximum moment of inertia (mm4) | 33.32 (IR: 26.60) | 41.12 (IR: 36.93) |
| Apparent modulus of elasticity (MPa) | 2469.98 (IR: 5357,93) | 6214.25 (IR: 6470.93) |
Tab. 2.
Number of bridged cortices of the calli evaluated by μ-computed tomography in two planes of rats without (Control) or with treatment with a C5aR-antagonist.
| Number of bridged cortices | Clinical fracture healing outcome | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | not healed | healed | ||
| Group | Group | |||||||
| Control | 1 | 2 | 0 | 0 | 5 | Control | 3 | 5 |
| C5aR Antagonist | 0 | 0 | 0 | 1 | 7 | C5aR Antagonist | 0 | 8 |
Histomorphometry
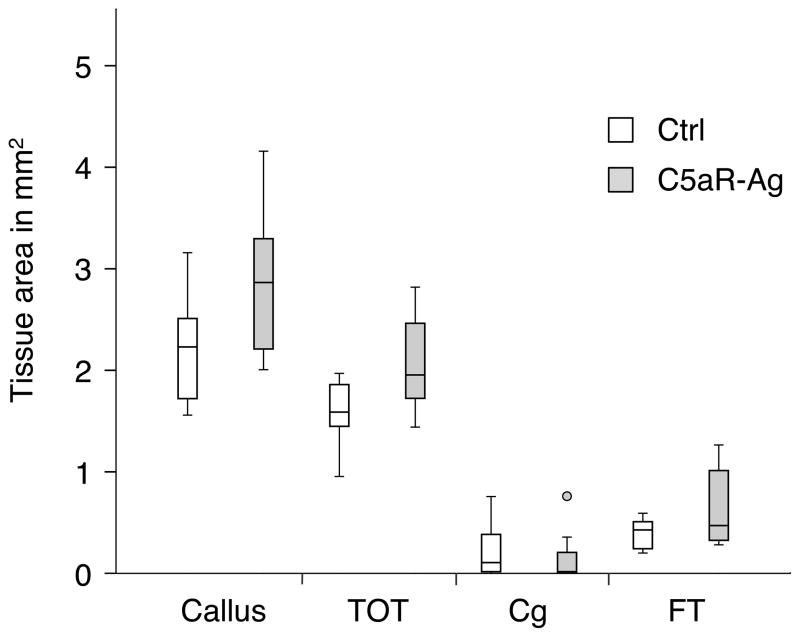
The histomorphometrical results confirmed the results of the μCT analysis showing a slightly increased callus with more bone in the C5aR-antagonist treated group (Figure 2).
Figure 2.
Absolute amounts of osseous tissue (TOT), cartilage tissue (Cg) and fibrous tissue (FT) within the callus of rats without (Ctrl) or with treatment with a C5aR-antagonist.
Discussion
This study demonstrated that systemically activated complement significantly contributes to the impairment of bone healing observed after severe trauma. Our results revealed that the application of a C5aR-antagonist during the initial phase of the posttraumatic inflammatory response abolished the deleterious effect of a blunt chest trauma on fracture healing in a rat model. This was reflected by a considerably improved flexural rigidity of the fracture callus, a higher bony bridging between the fracture fragments and a slightly larger and qualitatively improved callus formation in the rats treated with the C5aR-antagonist.
In a previous study we showed that a blunt chest trauma significantly impaired fracture healing in the same rat model6. The flexural rigidity of the healed femora was reduced by approximately 60% and a smaller callus with an inferior quality was formed compared to the control group, which did not receive a thoracic trauma6. We proposed that the complex systemic inflammatory response induced by the thoracic trauma disturbed fracture healing locally, the underlying mechanisms remaining unknown6. The rat blunt chest trauma model used in the present study is well-established and induces a reproducible and transient systemic inflammatory response1,26. Reflecting clinical data in polytraumatic patients12,13, Flierl et al. recently showed that the complement system, especially C5a, triggered the complex systemic inflammatory response in this experimental model by enhancing the systemic cytokine release as well as disturbing the neutrophil function. Neutrophils displayed an enhanced chemotactic activity, phagocytosis and production of reactive oxygen species followed by prolonged functional defects1. Furthermore, the systemic administration of an anti-C5a antibody immediately after the blunt chest trauma decreased the systemic cytokine release as well as the number of circulating neutrophils, and enhanced neutrophil function. This suggests that antagonizing excessive C5a might improve the outcome of a blunt chest trauma1. To investigate whether the systemic C5a increase is also responsible for the deleterious effects of the blunt chest trauma on bone healing we applied a specific C5aR-antagonist20,21. Blocking of C5aR completely abolished the negative impact of the thoracic trauma on fracture healing, suggesting that C5a was one of the main players in this scenario. The flexural rigidity of the callus increased significantly in the C5aR-antagonist treated group and nearly reached the levels of the intact contralateral femur. The biomechanical results correlated with an improved bony bridging of the fracture gap. Furthermore, the callus of the C5aR-antagonist treated group and the apparent modulus of elasticity, describing the mechanical quality of the newly formed callus24, were slightly increased. Histomorphometry also indicated a slightly increased amount of newly formed bone in the C5aR-antagonist treated group. The radiological and histological results revealed that the predominant tissue in the fracture callus in both groups was newly formed bone, indicating that the healing process had widely progressed after a period of 35 days. Nevertheless, the differences between the C5aR-antagonist treated and the control group in the biomechanical outcome were still considerable. In ongoing studies earlier investigation time points are included to evaluate differences in callus composition during the course of healing.
At present little is known about the role of C5a in fracture healing. It is well known that complement is locally activated after tissue injury, and is important for an adequate and effective inflammation, for example by increasing vascular permeability, recruitment of leukocytes, lymphocyte activation, opsonization of pathogens, and clearance of necrotic and apoptotic tissues at the site of injury27,28. Local activation of complement might, therefore, play an essential role in bone regeneration, especially in the fracture hematoma and the early stages of bone healing where inflammatory cells are predominant29,30. This was confirmed by a recent study of our group demonstrating that C5aR was abundantly expressed by these cells, but intriguingly also by osteoblasts, chondroblasts and osteoclasts in zones of intramembranous and enchondral ossification15. This suggests that C5a may be essential for regular regeneration processes during all stages of fracture healing.
After a blunt chest trauma systemic C5a is increased very rapidly and transiently1. Accordingly, we applied the C5aR-antagonist during the first hours after the blunt chest trauma and were able to significantly improve the fracture healing outcome after 35 days, indicating that C5a triggers determining effects in the very early healing phase. Systemically generated C5a causes a cascade of events, which could interact in several ways with the local fine-tuned inflammatory balance of bone healing. Because C5a activates the endothelium and enhances cell migration1,31,32, it may increase the number of inflammatory cells, such as macrophages and polymorphnuclear neutrophils in the fracture hematoma. C5a influences the function of leukocytes by inducing cytokine release as well as the production of proteases and reactive oxygen species33,34. This was confirmed by Flierl et al. in their rat model of blunt chest trauma1. An increased number and changed activity of neutrophils could, therefore, enhance and/or prolong the inflammatory phase of fracture healing. This was confirmed by studies reporting improved fracture healing after the depletion of neutrophils35 or disturbed healing by the application of zymosan, which stimulates the generation of reactive oxygen species by leukocytes36. C5a can prime macrophages to a more pro-inflammatory phenotype, leading to an increased secretion of cytokines in response to a second inflammatory stimulus34. An increased release of inflammatory cytokines from macrophages has been shown to provoke delayed fracture healing37. Furthermore, C5a could act as a potent inhibitor of angiogenesis by pushing macrophages towards an angiogenesis-inhibitory phenotype22, therefore possibly generating negative effects on bone regeneration. C5a might not only influence inflammatory cells but also osteoblast progenitors and osteoblasts as well as osteoclasts. It is a chemotactic factor for mesenchymal stem cells and osteoblasts, suggesting that it may modulate the recruitment of these cells to the site of injury15,38. It was reported that osteoblast-like osteosarcoma cells (MG-63) expressed functional C5aR and responded to C5a by releasing IL-616. C5a appears also to increase osteoclast formation directly by binding to C5aR on osteoclast precursorsor and indirectly by increasing the expression of receptor activator of nuclear factor-kB (RANKL) and IL-6 in osteoblasts17, which could in turn stimulate osteoclast formation and activity39. Taken together, systemic C5a could trigger a number of events in inflammatory cells as well as osteoblast and osteoclasts in the early phase of fracture healing, which might lead to a prolonged and/or increased inflammatory phase, and as a consequence to disturbed fracture healing. As outlined before, complement might be essential for regular regeneration processes during all stages of fracture healing. Therefore, it could be speculated that blocking complement during the whole healing period might have negative effects. Currently, further studies are ongoing in our group to prove this hypothesis.
In conclusion, our results demonstrate that the increase of C5a during the posttraumatic systemic inflammation considerably accounts for the deleterious effects of a blunt chest trauma on fracture healing and that immunomodulation by a C5aR-antagonist in the acute posttraumatic phase could abolish this effect, possibly via influencing the function of inflammatory and bone cells contributing to the early phase of fracture healing. Therefore, C5a could possibly represent a target to prevent delayed bone healing in patients with severe trauma4,5.
Acknowledgments
This study was funded by the German Research Foundation (KFO 200) and by National Institutes of Health grants AI068730 (to JDL). The authors appreciate the technical assistance of Uwe Wolfram, Ursula Maile and Marion Tomo. Each author in this manuscript has not and will not receive benefits in any form from a commercial party related directly or indirectly to the content of this manuscript. None of the authors have any conflicts of interest.
References
- 1.Flierl MA, Perl M, Rittirsch D, et al. The role of C5a in the innate immune response after experimental blunt chest trauma. Shock. 2008;29:25–31. doi: 10.1097/shk.0b013e3180556a0b. [DOI] [PubMed] [Google Scholar]
- 2.Gebhard F, Pfetsch H, Steinbach G, et al. Is interleukin 6 an early marker of injury severity following major trauma in humans? Arch Surg. 2000;135:291–295. doi: 10.1001/archsurg.135.3.291. [DOI] [PubMed] [Google Scholar]
- 3.Strecker W, Gebhard F, Rager J, et al. Interleukin-6 (IL-6) - An early marker of chest trauma. European Journal of Trauma. 2002;28:75–84. [Google Scholar]
- 4.Bhandari M, Tornetta P, 3rd, Sprague S, et al. Predictors of reoperation following operative management of fractures of the tibial shaft. J Orthop Trauma. 2003;17:353–361. doi: 10.1097/00005131-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Karladani AH, Granhed H, Karrholm J, Styf J. The influence of fracture etiology and type on fracture healing: a review of 104 consecutive tibial shaft fractures. Arch Orthop Trauma Surg. 2001;121:325–328. doi: 10.1007/s004020000252. [DOI] [PubMed] [Google Scholar]
- 6.Recknagel S, Bindl R, Kurz J, et al. Experimental blunt chest trauma impairs fracture healing in rats. J Orthop Res. 2010 doi: 10.1002/jor.21299. [DOI] [PubMed] [Google Scholar]
- 7.Donnelly TJ, Meade P, Jagels M, et al. Cytokine, complement, and endotoxin profiles associated with the development of the adult respiratory distress syndrome after severe injury. Crit Care Med. 1994;22:768–776. doi: 10.1097/00003246-199405000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Zilow G, Joka T, Obertacke U, et al. Generation of anaphylatoxin C3a in plasma and bronchoalveolar lavage fluid in trauma patients at risk for the adult respiratory distress syndrome. Crit Care Med. 1992;20:468–473. doi: 10.1097/00003246-199204000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Ehrnthaller C, Ignatius A, Gebhard F, Huber-Lang M. New Insights of an Old Defense System: Structure, Function, and Clinical Relevance of the Complement System. Mol Med. 2010 doi: 10.2119/molmed.2010.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albers S, Burk AM, Rittirsch D, et al. Impairment of the Complement Function After Multiple Trauma in Humans. Shock. 2006;26 (Suppl 1):14. [Google Scholar]
- 12.Hecke F, Schmidt U, Kola A, et al. Circulating complement proteins in multiple trauma patients--correlation with injury severity, development of sepsis, and outcome. Crit Care Med. 1997;25:2015–2024. doi: 10.1097/00003246-199712000-00019. [DOI] [PubMed] [Google Scholar]
- 13.Ganter MT, Brohi K, Cohen MJ, et al. Role of the alternative pathway in the early complement activation following major trauma. Shock. 2007;28:29–34. doi: 10.1097/shk.0b013e3180342439. [DOI] [PubMed] [Google Scholar]
- 14.Gerard C. Complement C5a in the sepsis syndrome--too much of a good thing? N Engl J Med. 2003;348:167–169. doi: 10.1056/NEJMcibr022995. [DOI] [PubMed] [Google Scholar]
- 15.Ignatius A, Ehrnthaller C, Brenner RE, et al. The anaphylatoxin receptor C5aR is present during fracture healing in rats and mediates osteoblast migration in vitro. J Trauma. 2010 doi: 10.1097/TA.0b013e3181f8aa2d. ACCEPTED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pobanz JM, Reinhardt RA, Koka S, Sanderson SD. C5a modulation of interleukin-1 beta-induced interleukin-6 production by human osteoblast-like cells. J Periodontal Res. 2000;35:137–145. doi: 10.1034/j.1600-0765.2000.035003137.x. [DOI] [PubMed] [Google Scholar]
- 17.Tu Z, Bu H, Dennis JE, Lin F. Efficient osteoclast differentiation requires local complement activation. Blood. 2010;116:4456–4463. doi: 10.1182/blood-2010-01-263590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Claes L, Blakytny R, Gockelmann M, et al. Early dynamization by reduced fixation stiffness does not improve fracture healing in a rat femoral osteotomy model. J Orthop Res. 2009;27:22–27. doi: 10.1002/jor.20712. [DOI] [PubMed] [Google Scholar]
- 19.Knoferl MW, Liener UC, Seitz DH, et al. Cardiopulmonary, histological, and inflammatory alterations after lung contusion in a novel mouse model of blunt chest trauma. Shock. 2003;19:519–525. doi: 10.1097/01.shk.0000070739.34700.f6. [DOI] [PubMed] [Google Scholar]
- 20.Crane JW, Buller KM. Systemic blockade of complement C5a receptors reduces lipopolysacharride-induced responses in the paraventricular nucleus and the central amygdala. Neurosci Lett. 2007;424:10–15. doi: 10.1016/j.neulet.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 21.Huber-Lang MS, Riedeman NC, Sarma JV, et al. Protection of innate immunity by C5aR antagonist in septic mice. FASEB J. 2002;16:1567–1574. doi: 10.1096/fj.02-0209com. [DOI] [PubMed] [Google Scholar]
- 22.Langer HF, Chung KJ, Orlova VV, et al. Complement-mediated inhibition of neovascularization reveals a point of convergence between innate immunity and angiogenesis. Blood. 2010;116:4395–4403. doi: 10.1182/blood-2010-01-261503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morgan EF, Mason ZD, Chien KB, et al. Micro-computed tomography assessment of fracture healing: relationships among callus structure, composition, and mechanical function. Bone. 2009;44:335–344. doi: 10.1016/j.bone.2008.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Claes L, Blakytny R, Besse J, et al. Late dynamization by reduced fixation stiffness enhances fracture healing in a rat femoral osteotomy model. J Orthop Trauma. 2011;25:169–174. doi: 10.1097/BOT.0b013e3181e3d994. [DOI] [PubMed] [Google Scholar]
- 25.Claes LE, Cunningham JL. Monitoring the mechanical properties of healing bone. Clin Orthop Relat Res. 2009;467:1964–1971. doi: 10.1007/s11999-009-0752-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seitz DH, Perl M, Liener UC, et al. Inflammatory Alterations in a Novel Combination Model of Blunt Chest Trauma and Hemorrhagic Shock. J Trauma. 2010 doi: 10.1097/TA.0b013e3181d7693c. [DOI] [PubMed] [Google Scholar]
- 27.Ward PA. The dark side of C5a in sepsis. Nat Rev Immunol. 2004;4:133–142. doi: 10.1038/nri1269. [DOI] [PubMed] [Google Scholar]
- 28.Rutkowski MJ, Sughrue ME, Kane AJ, et al. The complement cascade as a mediator of tissue growth and regeneration. Inflamm Res. 2010;59:897–905. doi: 10.1007/s00011-010-0220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Einhorn TA. The science of fracture healing. J Orthop Trauma. 2005;19:S4–6. doi: 10.1097/00005131-200511101-00002. [DOI] [PubMed] [Google Scholar]
- 30.Chung R, Cool JC, Scherer MA, et al. Roles of neutrophil-mediated inflammatory response in the bony repair of injured growth plate cartilage in young rats. J Leukoc Biol. 2006;80:1272–1280. doi: 10.1189/jlb.0606365. [DOI] [PubMed] [Google Scholar]
- 31.Marder SR, Chenoweth DE, Goldstein IM, Perez HD. Chemotactic responses of human peripheral blood monocytes to the complement-derived peptides C5a and C5a des Arg. J Immunol. 1985;134:3325–3331. [PubMed] [Google Scholar]
- 32.Foreman KE, Vaporciyan AA, Bonish BK, et al. C5a-induced expression of P-selectin in endothelial cells. J Clin Invest. 1994;94:1147–1155. doi: 10.1172/JCI117430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sacks T, Moldow CF, Craddock PR, et al. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978;61:1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riedemann NC, Guo RF, Bernacki KD, et al. Regulation by C5a of neutrophil activation during sepsis. Immunity. 2003;19:193–202. doi: 10.1016/s1074-7613(03)00206-1. [DOI] [PubMed] [Google Scholar]
- 35.Grogaard B, Gerdin B, Reikeras O. The polymorphonuclear leukocyte: has it a role in fracture healing? Arch Orthop Trauma Surg. 1990;109:268–271. doi: 10.1007/BF00419942. [DOI] [PubMed] [Google Scholar]
- 36.Gokturk E, Turgut A, Baycu C, et al. Oxygen-free radicals impair fracture healing in rats. Acta Orthop Scand. 1995;66:473–475. doi: 10.3109/17453679508995590. [DOI] [PubMed] [Google Scholar]
- 37.Grundnes O, Reikeraas O. Effects of macrophage activation on bone healing. J Orthop Sci. 2000;5:243–247. doi: 10.1007/s007760050159. [DOI] [PubMed] [Google Scholar]
- 38.Schraufstatter IU, Discipio RG, Zhao M, Khaldoyanidi SK. C3a and C5a are chemotactic factors for human mesenchymal stem cells, which cause prolonged ERK1/2 phosphorylation. J Immunol. 2009;182:3827–3836. doi: 10.4049/jimmunol.0803055. [DOI] [PubMed] [Google Scholar]
- 39.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]