Abstract
Due to their unique hierarchical structure and composition, tendons possess characteristic biomechanical properties, including high mechanical strength and viscoelasticity, which enable them to carry and transmit mechanical loads (muscular forces) effectively. Tendons are also mechano-responsive by adaptively changing their structure and function in response to altered mechanical loading conditions. In general, mechanical loading at physiological levels is beneficial to tendons, but excessive loading or disuse of tendons is detrimental. This mechano-adaptability is due to the cells present in tendons. Tendon fibroblasts (tenocytes) are the dominant tendon cells responsible for tendon homeostasis and repair. Tendon stem cells (TSCs), which were recently discovered, also play a vital role in tendon maintenance and repair by virtue of their ability to self-renew and differentiate into tenocytes. TSCs may also be responsible for chronic tendon injury, or tendinopathy, by undergoing aberrant differentiation into non-tenocytes in response to excessive mechanical loading. Thus, it is necessary to devise optimal rehabilitation protocols in order to enhance tendon healing while reducing scar tissue formation and tendon adhesions. Moreover, along with scaffolds that can mimic tendon matrix environments and platelet-rich plasma (PRP), which serves as a source of growth factors, TSCs may be the optimal cell type for enhancing repair of injured tendons.
Keywords: Tendon, biomechanics, mechanobiology, tendon stem cells, rehabilitation
I. Introduction
Tendons are soft connective tissues that are composed of closely packed, parallel collagen fiber bundles. Tendons play an essential role in the musculoskeletal system by transferring tensile loads from muscle to bone so as to enable joint motions and stabilize joints. Two types of tendons can be categorized, including tendons that mainly function to transmit loads (e.g., patellar and Achilles tendons) and those that mainly transmit motions (e.g., flexor tendons).
Tendons in vivo are subjected to large mechanical loads, and as a result, tendons are frequently injured. Each year in the U.S. alone, there are 16.4 million tendon and ligament injuries, at least 100,000 of which involve Achilles tendons [1]. Examples of commonly injured tendons include the rotator cuff, finger flexor tendons, patellar tendons, and Achilles tendons. Tendon injury may not only result in the deprivation of mobility or abnormal joint kinematics, but may also cause damage to tissues adjacent to the joint, such as cartilage, which may eventually lead to morbidity, pain, and osteoarthritis. For example, injury-related swelling and increased local pressure in a tendon may prevent blood flow in its adjacent area.
Tendon injuries are generally divided into two types, acute and chronic injury. Lacerations and ruptures are two common acute tendon injuries, and both often occur in athletic settings. Chronic tendon injury is often referred to as tendinopathy. Tendinopathy is manifested by pain and inflammation, and at advanced stages, it is also associated with the formation of damaging lipids, proteoglycans, and calcified tissues in tendon lesions [2]. In the USA alone, tendinopathy affects millions of people in both occupational and athletic settings [3]. Despite its prevalence, the precise pathogenic mechanisms of tendinopathy remain unclear and, as a result, current treatments of tendinopathy are largely empirical and less effective [4].
Once injured, tendon undergoes a slow, spontaneous healing process, and surgical intervention is sometimes required to facilitate this process. However, tendon healing often results in the formation of scar tissue, or disorganized matrix made largely of dense collagenous fibers, and hence have inferior mechanical properties compared to intact tendons. Consequently, repaired tendons risk re-injuries at the repair site [5]. Another problem with tendon healing is the formation of adhesions, caused by tissue scarring around the tendon sheath in injured flexor tendons, resulting in limited finger functions. Tendon adhesions are a common problem in healing tendons [6]. Thus, restoration of normal structure and function to injured tendons is one of the most challenging areas in orthopedic/sports medicine [7].
As mechanical load-bearing tissues, tendons have unique biomechanical characteristics. Moreover, as live biological tissues, tendons are mechano-responsive, meaning they alter their structure and biological behaviors in response to the various mechanical loading conditions placed on them [8]. In this mini review, we will introduce the basic concepts of tendon biomechanics and mechanobiology. Readers who are interested in a more in-depth discussion of this topic should consult many excellent reviews (e.g. [8–10]). We will also discuss tendon mechanobiology with a focus on recent advancements in the subject.
II. Tendon Structure, Composition, and Mechanical Properties
At the insertion point, i.e., the junction between tendon and bone, a tendon merges with the periosteum, the thin membrane covering the bone. At the other end, the tendon merges with fascia, the thin membrane covering the muscle. Ultrastructurally, tendons have a hierarchy of fibrillar arrangement which is sequentially composed of collagen molecules, fibrils, fibers, fascicles (or fiber bundles), and the tendon unit [8]. The tendon units are surrounded by epitenon, which functions to reduce friction with adjacent tissues. Such a hierarchical structure aligns all structural levels parallel to the long axis of the tendon, making it ideal for carrying and transmitting large tensile mechanical loads.
The key to the tendons’ tensile strength is collagen [11]. Type I collagen accounts for about 70–80% of the dry weight of normal tendons. In addition to type I collagen, many other types of collagen, including III, V, IX, X, XI XII, are also present in tendons, albeit in minor amounts. However, these collagen types have important functions. For example, type V collagen together with type I collagen serves as a regulator of collagen fibril diameter [12]; type III collagen is beneficial in the healing of tendons due to its ability to form rapid crosslinks so as to stabilize the repair site [13]; type XII collagen, on the other hand, provides lubrication between collagen fibers [14]. In addition to collagen, many proteoglycans (e.g., aggrecan and decorin) and glycoproteins (e.g., tenascin-C, fibronectin, and elastin) also have important functions in tendons [15, 16]. Aggrecan holds water and resists compression, and decorin facilitates fibrillar slippage; on the other hand, tenascin-C, fibronectin, and elastin function to enhance mechanical stability, facilitate tendon healing, and allow tendons to return to their pre-stretched lengths following physiological loading (the collagen configuration recovery), respectively.
In addition to the structural components above, there are many types of cells in tendons, including tenocytes, chondrocytes, synovial cells, and vascular cells. However, the vast majority of cells in tendon tissues are tenocytes. These cells are fibroblast-like and are interspersed between collagen fiber bundles and aligned along the long axis of a tendon. Tenocytes produce extracellular matrix (ECM), such as collagen, fibronectin, and proteoglycans, to maintain tendon homeostasis and repair injured tendons. Recently, a new type of tendon cells, termed tendon stem cells (TSCs), has been identified in humans, rabbits, mice, and rats (Fig. 1) [17–20]. By virtue of their ability to self-renew (or produce new, identical TSCs) and differentiate into tenocytes, TSCs play a crucial role in tendon maintenance and repair. Moreover, recent studies suggest that TSCs may also be responsible for the development of tendinopathy by undergoing aberrant differentiation (or non-tenocyte differentiation) due to excessive mechanical loading conditions [21, 22].
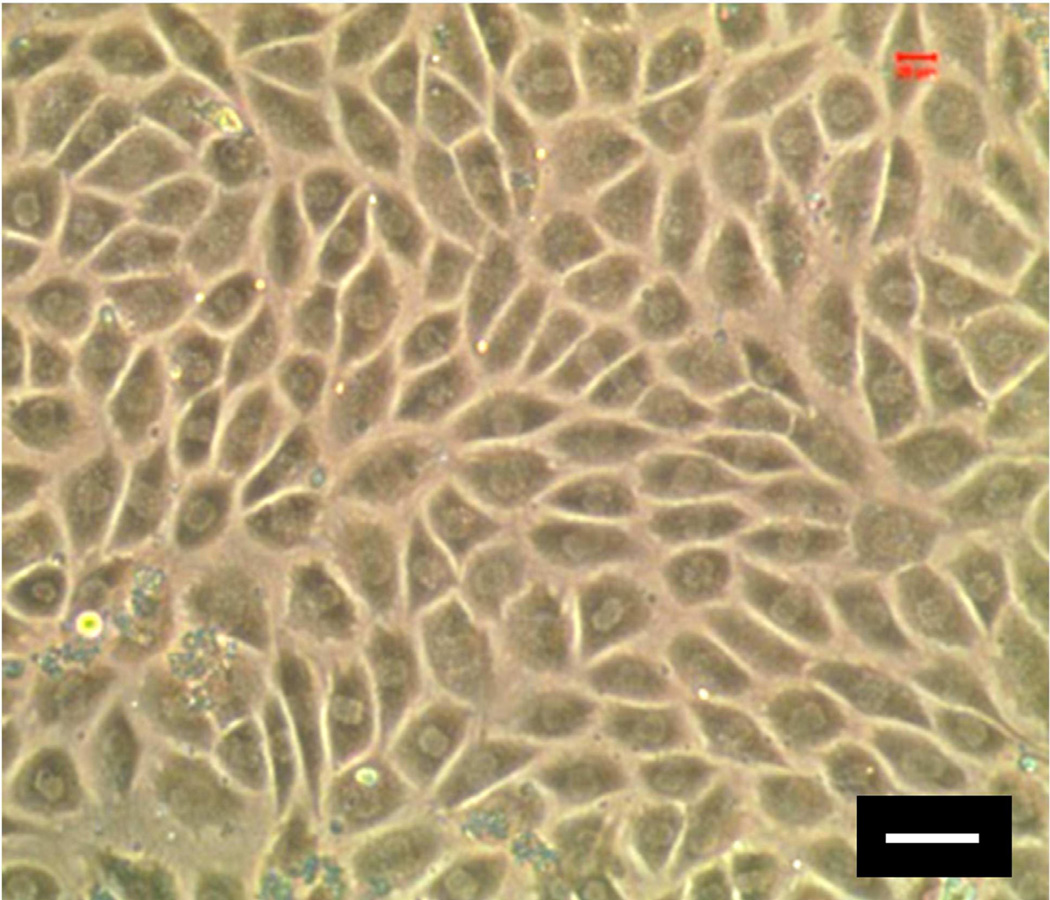
Fig. 1.
Human tendon stem cells (hTSCs). As seen, hTSCs in culture exhibit a cobble-stone shape, whereas tenocytes have a highly elongated shape (not shown). For detailed analysis of the differential properties of TSCs and tenocytes, see [18]. Because TSCs are tendon-specific stem cells, they may be the optimal cell source in restoring normal structure and function to injured tendons. (Bar: 50 µm).
The unique structure and composition of tendons afford them the characteristic mechanical behavior, which is reflected by a typical stress-strain curve consisting of four regions [8]. The first region is the toe region in the stress-strain curve when the strain of the tendon is below 2%. This region represents the "stretching-out" of crimped tendon fibrils due to mechanical loading on the tendon. Depending on the type and location of the tendon, this wavy fibril pattern results in different initial mechanical properties due to the varying angle and length of “crimping”. Following the toe region, there is a linear region in which the strain is less than 4%, i.e., the physiological upper limit of strain in tendons. In this region, the collagen fibrils orient themselves in the direction of tensile mechanical load. The slope of this region is the Young’s modulus of the tendon, which represents tendon stiffness. When the strain is above 4%, microscopic tearing of tendon fibers occurs, resulting in micro-tear failure of the tendon. When the strain further increases beyond 8–10%, macroscopic tearing of tendon fibers ensues, eventually leading to tendon rupture.
As expected from their different functions, different types of tendons differ greatly in their mechanical properties. For example, the Young’s modulus of the human patellar tendon is 660 ± 266MPa (mean ±SD) [23]; in contrast, the human tibialis anterior (TA) tendon has a Young's modulus of about 1200 MPa [24]. Moreover, aging significantly affects the mechanical properties of tendons: the Young’s modulus of human patellar tendons from young donors (29–50 years old) was 660±266 MPa, whereas that of the same tendons from old donors (64–93 years old) was 504±222 MPa [23].
In addition to their high stiffness, tendons are also viscoelastic, meaning that their mechanical behavior depends on the rate of mechanical strain. Viscoelasticity makes tendons more deformable at low strain rates but less deformable at high strain rates. Therefore, tendons at low strain rates tend to absorb more mechanical energy, but are less effective in carrying mechanical loads. At high strain rates, on the other hand, tendons become stiffer and more effective in transmitting large muscular loads to bone [8]. The viscoelasticity of tendons likely results from collagenous proteins, water, and the interactions between collagens and proteoglycans [25, 26].
III. Mechano-responses of Tendons
Physiological mechano-responses
Tendons are constantly subjected to mechanical loads in vivo. Appropriate mechanical loads at physiological levels are usually beneficial to tendons in terms of enhancing the mechanical properties of the tendon. For example, 4 weeks of exercise markedly improved the tensile strength of the peroneus brevis tendon in rabbits [27]. Also, compared to sedentary controls, the digital extensors of exercised swines became stronger following 12 months of training [28]. However, depending on the tendon type and load level, the effects of exercise varies. In a study of digital flexor tendons of miniature swines subjected to 12 months of running, it was found that training improved only the strength of the tendon insertion site but not much the tendon substance [29]. Finally, adequate mechanical loading may help reverse the deteriorating mechanical properties (e.g., decreased Young’s modulus) of aging tendons [30].
Mechanical loads also induce biochemical changes in tendons. In a human subject study, microdialysis was used to analyze the interstitial concentrations of both procollagen type I C-terminal propeptide (PICP) and type I collagen degradation product (ICTP), the measures of collagen synthesis and degradation, respectively [31]. It was found that PICP concentration in the peritendinous region of Achilles' tendons increased about seven times in response to a 4-week physical training period and remained elevated throughout the 11 weeks of total training period. In contrast, ICTP level was elevated only transiently in the tissue with training. In the plasma, however, PICP remained unchanged whereas the concentration of ICTP declined by 17 % in response to training. Therefore, exercise increased the turnover of type I collagen in the Achilles’ tendons. While at early stages of training both collagen synthesis and degradation increased, at later stages the anabolic processes dominated and resulted in net increase of collagen in tendons.
In a more recent study, the effects of mechanical loads on tendon cells have been investigated on a mouse treadmill running model [32]. In mice subjected to intensive treadmill running, a large number of myofibroblasts were present in the patellar tendons compared to control mice with cage activities. Myofibroblasts are known to be activated fibroblasts and are involved in repair and remodeling of injured tissues [33, 34]; therefore, the presence of myofibroblasts in the tendon following treadmill running indicates that they actively repair and remodel tendon tissue that may have been micro-injured due to demanding mechanical loads on the tendon.
Mechanical loads also may improve the healing of acutely injured tendons. In healing flexor tendons, for instance, early mobilization increases their tensile strength and stimulates restoration of the gliding surface while reducing intrasynovial adhesions [35–38]. Also, during repair of canine flexor digitorum profundus tendons, passive-motion rehabilitation, which produces moderate excursion (2 mm) of the tendon at low force levels, inhibits adhesion formation and promotes healing [39, 40]. Based on the beneficial mechano-effects on tendons, a series of eight therapeutic exercises of progressive intensities and range of motion has been suggested as a postoperative rehabilitation protocol to facilitate tendon healing and to allow more complete recovery of the digital range of motion [35].
Pathological mechano-responses
While appropriate mechanical loads have anabolic effects on tendons, strengthening the tendons and improving healing quality of injured tendons, excessive mechanical loads result in tendon injuries. Such non-traumatic tendon injuries, either due to "overloading" or "overuse", are commonly referred to as tendinopathy [41]. In a study using a rat treadmill running model, overuse of tendons was induced by an intensive running regimen for 4, 8, or 16 weeks [42]. Compared to the control group, which had only cage activities, the supraspinatus tendons of the running group showed increased cellularity and loss of normal collagen fiber organization, characteristics often seen in human tendinopathy. In addition, the mechanical properties of the tendons, including elastic modulus and maximum stress at failure, markedly declined in response to tendon overuse.
In addition to altering structural and mechanical properties of tendons, excessive mechanical loading on tendons also causes the production of high levels of PGE2 [43], a known inflammatory mediator present in injured tissues [44, 45]. Intensive mouse treadmill running has resulted in high levels of PGE2 production, which may be detrimental to tendons as they caused differentiation of TSCs into non-tenocytes, potentially leading to the formation of non-tendinous tissues in the tendon [22]. Over-production of leukotrienes, including LTB4, after repetitive mechanical load has also been found in anesthetized rats subjected to mechanical trauma [46]. Presence of abundant leukotrienes subsequently induces neutrophil infiltration and activation, resulting in tissue edema as seen in tendons with tendinopathy [47].
On the other hand, insufficient mechanical loads also have adverse effects on tendons. Disuse- or immobilization-caused stress deprivation within tendon tissues led to dramatic changes in tendon cell shape, cell number, and collagen fiber alignment, and eventually caused tendon degeneration [48]. Without a certain amount of mechanical load placed on them, tendons became atrophied and lost total weight, stiffness, as well as tensile strength [49–52]. In adult canines, for example, the expression of collagen types I and III in flexor tendons significantly decreased after their forepaws were suspended for 6 weeks [52]. Expression of other matrix components including collagen type II, aggrecan, decorin, and fibronectin was also reduced in the flexor tendons of suspended limbs after 6 weeks. The effect of mechanical stress deprivation through limb suspension was also seen in the expression of various matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) [52]. In general, tendon disuse leads to decrease in anabolic activities but increase in catabolic activities of the tendon matrix. Such stress deprivation-initiated metabolic alterations are likely responsible for certain degenerative changes in tendons [53].
Mechano-responses of tendon fibroblasts
It is clear that the mechano-responses of tendons, both anabolic and catabolic, are due to the activities of tendon cells in response to various mechanical loading conditions. As a dominant cell type in tendons, tendon fibroblasts (or tenocytes) are certainly responsible for changes in tendons by altering ECM gene and protein expression [8, 54]. With the development of many in vitro cell loading systems, a spectrum of mechano-responses of tenocytes and their molecular mechanisms have been extensively investigated in the past two decades [8, 55–57]. Considering the uniaxial loading conditions on patellar and Achilles tendons, our group designed an in vitro system that can mimic the cell alignment and shape and the repetitive uniaxial stretching conditions of tendon fibroblasts in vivo [45]. Using this system, human tendon fibroblasts were found to increase in proliferation as well as gene expression and protein production of type I collagen in a stretching magnitude-dependent manner. In addition, depending on stretching magnitude, cyclic stretching increased gene expression and production of type I collagen [58]. Finally, cyclic stretching increased expression of cyclooxygenase-2 (COX-2) and the production of PGE2 and LTB4 in human tendon fibroblasts in a stretching magnitude-dependent manner [45, 59]. Similar results in terms of PGE2 production were also seen in human finger flexor tenocytes under cyclic stretching [44, 60]. Since PGE2 is a known inflammatory mediator in tendons and can decrease proliferation and collagen production in human tendon fibroblasts, PGE2 production at high levels in vivo may contribute to the development of tendinopathy [61].
Besides the direct effects on tendon fibroblasts through mechanical loading, there are also interactions between mechanical loading and cytokines, and the outcome of such interactions is stretching magnitude-dependent. For example, a 4% cyclic uniaxial stretching of human tendon fibroblasts decreased the COX-2 and MMP-1 gene expression and the PGE2 production that were stimulated by IL 1β. However, an 8% stretching further increased expression of these genes and PGE2 production to boost the effects of IL-1β treatment. This implies that repetitive mechanical stretching has two opposite effects — it is anti-inflammatory at small magnitudes, whereas it becomes pro-inflammatory at large magnitudes. It is likely that such anti- or pro-inflammatory effects on tendon fibroblasts also involve the nuclear factor-κB (NF-κB) pathway [62]. Therefore, this pathway may be manipulated pharmaceutically in combination with appropriate rehabilitation regimens to manage tissue inflammation in clinical settings.
Mechano-responses of TSCs
While fibroblasts represent the majority of resident cells in tendons, recently a number of studies have identified TSCs in human and animal tendon tissues [17–20]. TSCs have common characteristics of adult stem cells, including self-renewal, clonogenicity, and multipotency. Once transplanted in vivo, TSCs can generate tendon-like tissues [17]. By default, TSCs differentiate into tenocytes under normal conditions [18]. By virtue of their ability to self-renew and differentiate into tenocytes, TSCs play a critical role in tendon maintenance and repair.
Like tendon fibroblasts, TSCs are subjected to mechanical loading in vivo. Therefore, effects of mechanical loading on TSCs have been investigated on both in vivo and in vitro models. Using treadmill running as a means to apply mechanical loads to mouse tendons, it was found that mechanical loading increased the number of TSCs in both patellar and Achilles tendons, although the level of increase differed by tendon type [63]. In addition, the production of collagen in TSCs derived from the tendons of the running group was significantly higher compared to that of TSCs from the control group with cage activities. Findings from this study suggest that the anabolic effect of running on tendons may at least partially come from its stimulating capability on TSC proliferation as well as TSC-related cellular production of collagen. In an in vitro study, mechanical loading promoted cellular proliferation in TSC cultures; moreover, mechanically loaded cells underwent loading magnitude-dependent differentiation: at small mechanical stretching (4%), TSCs differentiated into tenocytes, whereas at large mechanical stretching (8%), TSCs differentiated into non-tenocytes (adipocytes, chondrocytes, and osteocytes) in addition to tenocytes [21]. Such non-tenocyte differentiation may explain the histopathological features of tendinopathy at later stages, including lipid deposition, proteoglycan accumulation, and calcification. Thus, it seems likely that TSCs play a major role in the development of tendinopathy by undergoing aberrant, non-tenocyte differentiation under excessive mechanical loading conditions.
Also using mouse treadmill running as an experimental model, a further study found that when tendons were subjected to repetitive, strenuous mechanical loading in vivo, high levels of PGE2 were produced [22]. This finding confirmed the results of previous in vitro studies, which showed that mechanical loading on tendon fibroblasts increased their PGE2 production [44, 45]. Moreover, this study further showed that high levels of PGE2 decreased proliferation of TSCs and induced TSCs to differentiate into adipocytes and osteocytes [22]. Taken together, these findings suggest that intensive mechanical loading placed on tendons may cause tendinopathy by two parallel, PGE2-mediated mechanisms: a) reducing the number of TSCs, and b) inducing TSCs to differentiate into non-tenocytes.
IV. Concluding Remarks
Because of their unique structure and composition, tendons possess characteristic biomechanical properties: they are mechanically strong and viscoelastic, enabling them to store mechanical energy and transmit large muscular forces to bone effectively.
Moreover, tendons are live tissues that are mechano-responsive. This means that they have the capacity to adapt themselves to altered mechanical loading conditions (Table 1). Previous studies have shown that appropriate mechanical loads are beneficial to tendons by enhancing their anabolic processes, including synthesis of matrix proteins (e.g. collagen). Excessive mechanical loads, on the other hand, are detrimental to tendons by promoting catabolic processes such as matrix degradation. Disuse or immobilization of tendons also causes catabolic effects on tendons. Additionally, appropriate mechanical loading is required for improving repair of injured tendons such as reducing adhesions in flexor tendon injuries. Thus, an appropriate range of mechanical loads on tendons is necessary to promote tendon homeostasis and repair.
Table 1.
The differential effects of mechanical loading on tendons.
| Mechanical Load Level |
Effects on tendon |
|---|---|
| Low |
|
| Moderate |
|
| Excessive |
|
Mechano-responses of tendons are due to the cells present in tendons. Tendon fibroblasts, being relatively abundant, are surely responsible for the maintenance and repair of tendons. Another recently identified type of tendon cells is tendon stem cells (TSCs). While relatively low in number, TSCs play a critical role in tendon physiology because of their ability to self-renew and differentiate into tenocytes to replenish lost tenocytes due to regular turn-over and injury to tendons. Moreover, recent studies suggest that TSCs may also be responsible for the development of tendinopathy, or degenerative changes in tendons, by undergoing non-tenocyte differentiation in response to excessive mechanical loading (Fig. 2) [21, 64].
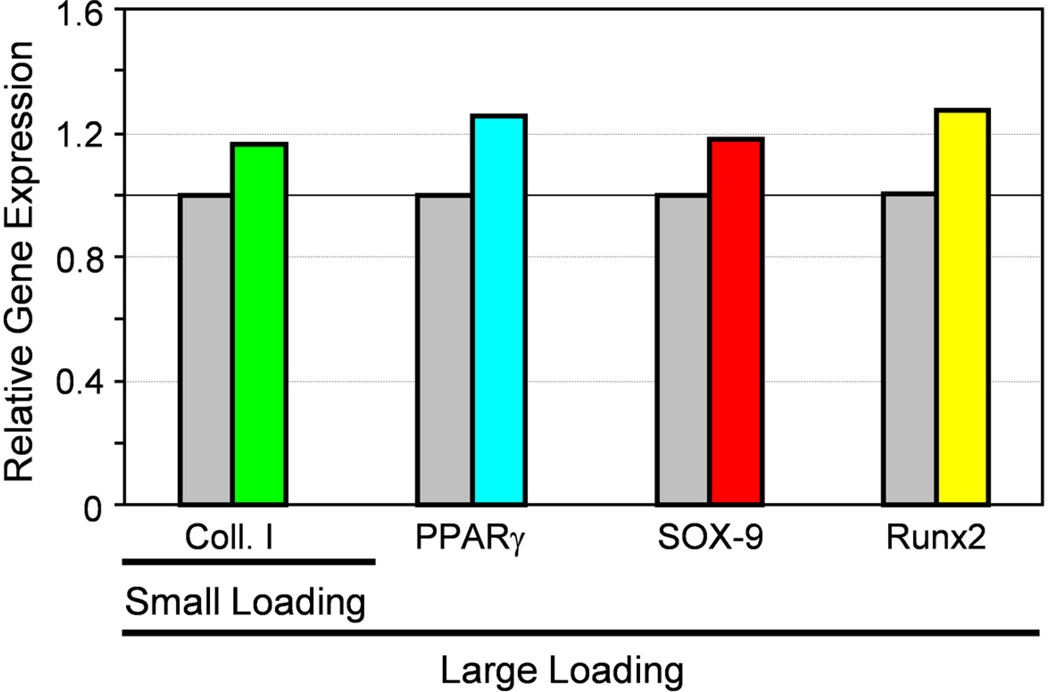
Fig. 2.
Mechano-response of TSCs depends on mechanical loading conditions. Application of small mechanical loading to TSCs induces the cells to differentiate into tenocytes only, as marked by significant upregulation of the collagen type I gene but no changes in those non-tenocyte related genes (PPARβ, SOX-9, and Runx2). However, application of large mechanical loading leads to non-tenocyte differentiation in addition to tenocyte differentiation, as evidenced by upregulation of the genes PPARβ (a marker for adipocytes), SOX-9 (a marker for chondrocytes), and Runx2 (a marker for osteocytes). This mechano-adaptive response to different mechanical loading conditions by TSCs could explain why appropriate exercise is beneficial to tendons in terms of their homeostasis and repair, whereas excessive mechanical loading is detrimental in terms of causing chronic tendon injury, or tendinopathy. The gray bars represent baseline expression levels of the genes, i.e., without mechanical loading. The color bars represent relative levels of gene expression in cells subjected to mechanical loads compared to those in unloaded cells. Note that relative gene expression levels are presented for illustration only (Adapted with permission from Fig. 3 in [64]).
Therefore, mechanical loading and TSCs may be two important factors in the maintenance of functionally normal tendons and in the repair or regeneration of injured tendons. Functional tissue engineering approaches, which combine these two factors, may prove promising in improving the repair of injured tendons. In addition, it is known that adult stem cells like TSCs need appropriate "niches" for normal function [17]. Therefore, scaffolding materials that mimic tendon structure and composition may also be of paramount importance for improving tendon healing. Additionally, growth factors are known to play a vital role in tissue healing. In this regard, platelet-rich plasma (PRP), which contains nearly all growth factors needed for tissue healing, may offer hope in that the major tendon healing problems, namely, scar tissue formation and tendon adhesions, may be mitigated by using PRP as an ideal growth factor source [64–66]. A case in point is that by combining use of collagen scaffolds, PRP can promote healing of injured anterior cruciate ligaments (ACLs) [67, 68], which are known not to heal spontaneously.
Acknowledgements
NIH funding support (AR049921 and AR049921S2) to this work is gratefully acknowledged (JHW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Praemer A, Furner S, Rice D. Musculoskeletal Condition in the United States. Parke Ridge, IL: American Academy of Orthopaedic Surgeons; 1999. [Google Scholar]
- 2.Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73(10):1507–1525. [PubMed] [Google Scholar]
- 3.Almekinders LC, Temple JD. Etiology, diagnosis, and treatment of tendonitis: an analysis of the literature. Med Sci Sports Exerc. 1998;30(8):1183–1190. doi: 10.1097/00005768-199808000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Wang JH, Iosifidis MI, Fu FH. Biomechanical basis for tendinopathy. Clin Orthop Relat Res. 2006;443:320–332. doi: 10.1097/01.blo.0000195927.81845.46. [DOI] [PubMed] [Google Scholar]
- 5.Katzel EB, Wolenski M, Loiselle AE, Basile P, Flick LM, Langstein HN, Hilton MJ, Awad HA, Hammert WC, O'Keefe RJ. Impact of Smad3 loss of function on scarring and adhesion formation during tendon healing. J Orthop Res. 2011;29(5):684–693. doi: 10.1002/jor.21235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong JK, Lui YH, Kapacee Z, Kadler KE, Ferguson MW, McGrouther DA. The cellular biology of flexor tendon adhesion formation: an old problem in a new paradigm. Am J Pathol. 2009;175(5):1938–1951. doi: 10.2353/ajpath.2009.090380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Renstrom P, Woo SL-Y. Tendinopathy in athletes. Hong Kong: Blackwell Publishing; 2007. [Google Scholar]
- 8.Wang JH. Mechanobiology of tendon. J Biomech. 2006;39(9):1563–1582. doi: 10.1016/j.jbiomech.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Woo SL, Fisher MB, Feola AJ. Contribution of biomechanics to management of ligament and tendon injuries. Mol Cell Biomech. 2008;5(1):49–68. [PubMed] [Google Scholar]
- 10.Woo SL, Thomas M, Chan Saw SS. Contribution of biomechanics, orthopaedics and rehabilitation: the past present and future. Surgeon. 2004;2(3):125–136. doi: 10.1016/s1479-666x(04)80072-6. [DOI] [PubMed] [Google Scholar]
- 11.Tanzer ML. Cross-linking of collagen. Science. 1973;180(86):561–566. doi: 10.1126/science.180.4086.561. [DOI] [PubMed] [Google Scholar]
- 12.Birk DE, Mayne R. Localization of collagen types I, III and V during tendon development. Changes in collagen types I and III are correlated with changes in fibril diameter. Eur J Cell Biol. 1997;72(4):352–361. [PubMed] [Google Scholar]
- 13.Liu SH, Yang RS, al-Shaikh R, Lane JM. Collagen in tendon, ligament, and bone healing. A current review. Clin Orthop Relat Res. 1995;(318):265–278. [PubMed] [Google Scholar]
- 14.Niyibizi C, Visconti CS, Kavalkovich K, Woo SL. Collagens in an adult bovine medial collateral ligament: immunofluorescence localization by confocal microscopy reveals that type XIV collagen predominates at the ligament-bone junction. Matrix Biol. 1995;14(9):743–751. doi: 10.1016/s0945-053x(05)80017-4. [DOI] [PubMed] [Google Scholar]
- 15.Vogel KG, Heinegard D. Characterization of proteoglycans from adult bovine tendon. J Biol Chem. 1985;260(16):9298–9306. [PubMed] [Google Scholar]
- 16.Pins GD, Christiansen DL, Patel R, Silver FH. Self-assembly of collagen fibers. Influence of fibrillar alignment and decorin on mechanical properties. Biophys J. 1997;73(4):2164–2172. doi: 10.1016/S0006-3495(97)78247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, Li L, Leet AI, Seo BM, Zhang L, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13(10):1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, Wang JH. Characterization of differential properties of rabbit tendon stem cells and tenocytes. BMC Musculoskelet Disord. 2010;11:10. doi: 10.1186/1471-2474-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Z, Akinbiyi T, Xu L, Ramcharan M, Leong DJ, Ros SJ, Colvin AC, Schaffler MB, Majeska RJ, Flatow EL, et al. Tendon-derived stem/progenitor cell aging: defective self-renewal and altered fate. Aging Cell. 2010;9(5):911–915. doi: 10.1111/j.1474-9726.2010.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rui YF, Lui PP, Li G, Fu SC, Lee YW, Chan KM. Isolation and characterization of multipotent rat tendon-derived stem cells. Tissue Eng Part A. 2010;16(5):1549–1558. doi: 10.1089/ten.TEA.2009.0529. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J, Wang JH. Mechanobiological response of tendon stem cells: implications of tendon homeostasis and pathogenesis of tendinopathy. J Orthop Res. 2010;28(5):639–643. doi: 10.1002/jor.21046. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J, Wang JH. Production of PGE(2) increases in tendons subjected to repetitive mechanical loading and induces differentiation of tendon stem cells into non-tenocytes. J Orthop Res. 2010;28(2):198–203. doi: 10.1002/jor.20962. [DOI] [PubMed] [Google Scholar]
- 23.Johnson GA, Tramaglini DM, Levine RE, Ohno K, Choi NY, Woo SL. Tensile and viscoelastic properties of human patellar tendon. J Orthop Res. 1994;12(6):796–803. doi: 10.1002/jor.1100120607. [DOI] [PubMed] [Google Scholar]
- 24.Maganaris CN, Paul JP. In vivo human tendon mechanical properties. J Physiol. 1999;521(Pt 1):307–313. doi: 10.1111/j.1469-7793.1999.00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Purslow PP, Wess TJ, Hukins DW. Collagen orientation and molecular spacing during creep and stress-relaxation in soft connective tissues. J Exp Biol. 1998;201(Pt 1):135–142. doi: 10.1242/jeb.201.1.135. [DOI] [PubMed] [Google Scholar]
- 26.Elliott DM, Robinson PS, Gimbel JA, Sarver JJ, Abboud JA, Iozzo RV, Soslowsky LJ. Effect of altered matrix proteins on quasilinear viscoelastic properties in transgenic mouse tail tendons. Ann Biomed Eng. 2003;31(5):599–605. doi: 10.1114/1.1567282. [DOI] [PubMed] [Google Scholar]
- 27.Viidik A. The effect of training on the tensile strength of isolated rabbit tendons. Scand J Plast Reconstr Surg. 1967;1(2):141–147. doi: 10.3109/02844316709022844. [DOI] [PubMed] [Google Scholar]
- 28.Woo SL, Ritter MA, Amiel D, Sanders TM, Gomez MA, Kuei SC, Garfin SR, Akeson WH. The biomechanical and biochemical properties of swine tendons--long term effects of exercise on the digital extensors. Connect Tissue Res. 1980;7(3):177–183. doi: 10.3109/03008208009152109. [DOI] [PubMed] [Google Scholar]
- 29.Woo SL, Gomez MA, Amiel D, Ritter MA, Gelberman RH, Akeson WH. The effects of exercise on the biomechanical and biochemical properties of swine digital flexor tendons. J Biomech Eng. 1981;103(1):51–56. doi: 10.1115/1.3138246. [DOI] [PubMed] [Google Scholar]
- 30.Narici MV, Maganaris CN. Adaptability of elderly human muscles and tendons to increased loading. J Anat. 2006;208(4):433–443. doi: 10.1111/j.1469-7580.2006.00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langberg H, Rosendal L, Kjaer M. Training-induced changes in peritendinous type I collagen turnover determined by microdialysis in humans. J Physiol. 2001;534(Pt 1):297–302. doi: 10.1111/j.1469-7793.2001.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szczodry M, Zhang J, Lim C, Davitt HL, Yeager T, Fu FH, Wang JH. Treadmill running exercise results in the presence of numerous myofibroblasts in mouse patellar tendons. J Orthop Res. 2009;27(10):1373–1378. doi: 10.1002/jor.20878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell. 2001;12(9):2730–2741. doi: 10.1091/mbc.12.9.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3(5):349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 35.Groth GN. Pyramid of progressive force exercises to the injured flexor tendon. J Hand Ther. 2004;17(1):31–42. doi: 10.1197/j.jht.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 36.Manske PR, Gelberman RH, Vande Berg JS, Lesker PA. Intrinsic flexor-tendon repair. A morphological study in vitro. J Bone Joint Surg Am. 1984;66(3):385–396. [PubMed] [Google Scholar]
- 37.Zhao C, Amadio PC, Zobitz ME, An KN. Gliding characteristics of tendon repair in canine flexor digitorum profundus tendons. J Orthop Res. 2001;19(4):580–586. doi: 10.1016/S0736-0266(00)00055-3. [DOI] [PubMed] [Google Scholar]
- 38.Khanna A, Gougoulias N, Maffulli N. Modalities in prevention of flexor tendon adhesion in the hand: what have we achieved so far? Acta Orthop Belg. 2009;75(4):433–444. [PubMed] [Google Scholar]
- 39.Silva MJ, Brodt MD, Boyer MI, Morris TS, Dinopoulos H, Amiel D, Gelberman RH. Effects of increased in vivo excursion on digital range of motion and tendon strength following flexor tendon repair. J Orthop Res. 1999;17(5):777–783. doi: 10.1002/jor.1100170524. [DOI] [PubMed] [Google Scholar]
- 40.Silva MJ, Boyer MI, Gelberman RH. Recent progress in flexor tendon healing. J Orthop Sci. 2002;7(4):508–514. doi: 10.1007/s007760200090. [DOI] [PubMed] [Google Scholar]
- 41.Khan KM, Cook JL, Bonar F, Harcourt P, Astrom M. Histopathology of common tendinopathies. Update and implications for clinical management. Sports Med. 1999;27(6):393–408. doi: 10.2165/00007256-199927060-00004. [DOI] [PubMed] [Google Scholar]
- 42.Soslowsky LJ, Thomopoulos S, Tun S, Flanagan CL, Keefer CC, Mastaw J, Carpenter JE. Overuse activity injures the supraspinatus tendon in an animal model: a histologic and biomechanical study. J Shoulder Elbow Surg. 2000;9(2):79–84. [PubMed] [Google Scholar]
- 43.Langberg H, Skovgaard D, Karamouzis M, Bulow J, Kjaer M. Metabolism and inflammatory mediators in the peritendinous space measured by microdialysis during intermittent isometric exercise in humans. J Physiol. 1999;515(Pt 3):919–927. doi: 10.1111/j.1469-7793.1999.919ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Almekinders LC, Banes AJ, Ballenger CA. Effects of repetitive motion on human fibroblasts. Medicine & Science in Sports & Exercise. 1993;25(5):603–607. [PubMed] [Google Scholar]
- 45.Wang JH, Jia F, Yang G, Yang S, Campbell BH, Stone D, Woo SL. Cyclic mechanical stretching of human tendon fibroblasts increases the production of prostaglandin E2 and levels of cyclooxygenase expression: a novel in vitro model study. Connect Tissue Res. 2003;44(3–4):128–133. doi: 10.1080/03008200390223909. [DOI] [PubMed] [Google Scholar]
- 46.Denzlinger C, Rapp S, Hagmann W, Keppler D. Leukotrienes as mediators in tissue trauma. Science. 1985;230(4723):330–332. doi: 10.1126/science.4048937. [DOI] [PubMed] [Google Scholar]
- 47.Denzlinger C. Biology and pathophysiology of leukotrienes. Crit Rev Oncol Hematol. 1996;23(3):167–223. doi: 10.1016/1040-8428(96)00205-3. [DOI] [PubMed] [Google Scholar]
- 48.Hannafin JA, Arnoczky SP, Hoonjan A, Torzilli PA. Effect of stress deprivation and cyclic tensile loading on the material and morphologic properties of canine flexor digitorum profundus tendon: an in vitro study. J Orthop Res. 1995;13(6):907–914. doi: 10.1002/jor.1100130615. [DOI] [PubMed] [Google Scholar]
- 49.Amiel D, Woo SL, Harwood FL, Akeson WH. The effect of immobilization on collagen turnover in connective tissue: a biochemical-biomechanical correlation. Acta Orthop Scand. 1982;53(3):325–332. doi: 10.3109/17453678208992224. [DOI] [PubMed] [Google Scholar]
- 50.Noyes FR. Functional properties of knee ligaments and alterations induced by immobilization: a correlative biomechanical and histological study in primates. Clin Orthop. 1977;(123):210–242. [PubMed] [Google Scholar]
- 51.Nakagawa Y, Totsuka M, Sato T, Fukuda Y, Hirota K. Effect of disuse on the ultrastructure of the achilles tendon in rats. Eur J Appl Physiol Occup Physiol. 1989;59(3):239–242. doi: 10.1007/BF02386194. [DOI] [PubMed] [Google Scholar]
- 52.Sun YL, Thoreson AR, Cha SS, Zhao C, An KN, Amadio PC. Temporal response of canine flexor tendon to limb suspension. J Appl Physiol. 2010;109(6):1762–1768. doi: 10.1152/japplphysiol.00051.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yasuda K, Hayashi K. Changes in biomechanical properties of tendons and ligaments from joint disuse. Osteoarthritis Cartilage. 1999;7(1):122–129. doi: 10.1053/joca.1998.0167. [DOI] [PubMed] [Google Scholar]
- 54.Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84(2):649–698. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- 55.Banes AJ, Tsuzaki M, Yamamoto J, Fischer T, Brigman B, Brown T, Miller L. Mechanoreception at the cellular level: the detection, interpretation, and diversity of responses to mechanical signals. Biochem Cell Biol. 1995;73(7–8):349–365. doi: 10.1139/o95-043. [DOI] [PubMed] [Google Scholar]
- 56.Arnoczky SP, Tian T, Lavagnino M, Gardner K, Schuler P, Morse P. Activation of stress-activated protein kinases (SAPK) in tendon cells following cyclic strain: the effects of strain frequency, strain magnitude, and cytosolic calcium. J Orthop Res. 2002;20(5):947–952. doi: 10.1016/S0736-0266(02)00038-4. [DOI] [PubMed] [Google Scholar]
- 57.Devkota AC, Weinhold PS. Mechanical response of tendon subsequent to ramp loading to varying strain limits. Clin Biomech (Bristol, Avon) 2003;18(10):969–974. doi: 10.1016/s0268-0033(03)00168-2. [DOI] [PubMed] [Google Scholar]
- 58.Yang G, Crawford RC, Wang JH. Proliferation and collagen production of human patellar tendon fibroblasts in response to cyclic uniaxial stretching in serum-free conditions. J Biomech. 2004;37(10):1543–1550. doi: 10.1016/j.jbiomech.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 59.Li Z, Yang G, Khan M, Stone D, Woo SL, Wang JH. Inflammatory response of human tendon fibroblasts to cyclic mechanical stretching. Am J Sports Med. 2004;32(2):435–440. doi: 10.1177/0095399703258680. [DOI] [PubMed] [Google Scholar]
- 60.Almekinders LC, Baynes AJ, Bracey LW. An in vitro investigation into the effects of repetitive motion and nonsteroidal antiinflammatory medication on human tendon fibroblasts. American Journal of Sports Medicine. 1995;23(1):119–123. doi: 10.1177/036354659502300120. [DOI] [PubMed] [Google Scholar]
- 61.Cilli F, Khan M, Fu F, Wang JH. Prostaglandin E2 affects proliferation and collagen synthesis by human patellar tendon fibroblasts. Clin J Sport Med. 2004;14(4):232–236. doi: 10.1097/00042752-200407000-00006. [DOI] [PubMed] [Google Scholar]
- 62.Agarwal S, Long P, Seyedain A, Piesco N, Shree A, Gassner R. A central role for the nuclear factor-kappaB pathway in anti-inflammatory and proinflammatory actions of mechanical strain. FASEB J. 2003;17(8):899–901. doi: 10.1096/fj.02-0901fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang J, Pan T, Liu Y, Wang JH. Mouse treadmill running enhances tendons by expanding the pool of tendon stem cells (TSCs) and TSC-related cellular production of collagen. J Orthop Res. 2010;28(9):1178–1183. doi: 10.1002/jor.21123. [DOI] [PubMed] [Google Scholar]
- 64.Wang JH-C. Tendon stem cells and platelet-rich plasma. Chin J Sports Med. 2011;30(1):70–78. [Google Scholar]
- 65.Kang YH, Jeon SH, Park JY, Chung JH, Choung YH, Choung HW, Kim ES, Choung PH. Platelet-rich fibrin is a Bioscaffold and reservoir of growth factors for tissue regeneration. Tissue Eng Part A. 2010;17(3–4):349–359. doi: 10.1089/ten.TEA.2010.0327. [DOI] [PubMed] [Google Scholar]
- 66.Zhang J, Wang JH. Platelet-rich plasma releasate promotes differentiation of tendon stem cells into active tenocytes. Am J Sports Med. 38(12):2477–2486. doi: 10.1177/0363546510376750. [DOI] [PubMed] [Google Scholar]
- 67.Murray MM, Spindler KP, Abreu E, Muller JA, Nedder A, Kelly M, Frino J, Zurakowski D, Valenza M, Snyder BD, et al. Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J Orthop Res. 2007;25(1):81–91. doi: 10.1002/jor.20282. [DOI] [PubMed] [Google Scholar]
- 68.Murray MM, Spindler KP, Devin C, Snyder BS, Muller J, Takahashi M, Ballard P, Nanney LB, Zurakowski D. Use of a collagen-platelet rich plasma scaffold to stimulate healing of a central defect in the canine ACL. J Orthop Res. 2006;24(4):820–830. doi: 10.1002/jor.20073. [DOI] [PubMed] [Google Scholar]