Abstract
Purpose
To measure the peripapillary retinal nerve fiber layer (RNFL) thickness using spectral-domain OCT (SD-OCT) in patients with retinitis pigmentosa.
Methods
Fifty eyes of 30 patients with retinitis pigmentosa underwent a complete ocular examination, including best-corrected visual acuity (BCVA) using a Snellen chart, slit-lamp biomicroscopic examination, and Goldmann applanation intraocular pressure (IOP) measurement. Dilated fundus examination was done by using both direct and indirect ophthalmoscopy. In addition, all patients underwent peripapillary RNFL thickness measurements by using an OPKO SD-OCT.
Results
The mean (±SD) age of the study cohort was 45.8 (16.3) years. Eighteen of the 50 eyes (36%) showed a thinning of the peripapillary RNFL in one or more quadrants and 21 of the 50 eyes (42%) showed a thickening of the peripapillary RNFL in one or more quadrants. Four eyes (8%) showed both thinning and thickening of the peripapillary RNFL thickness. The overall circumferential RNFL thickness of the 14 eyes that showed only thinning in at least one quadrant was 78.78 µm. For the 17 eyes that showed only thickening in at least one quadrant, the RNFL thickness was 119.69 µm. The values of the eyes with thinning and the eyes with thickening were significantly different from normal (t=6.31, p<0.01 for thickening; t=3.62, p<0.01 for thinning).
Conclusion
By using SD-OCT testing, we demonstrated in the current study that the peripapillary RNFL thickness in RP patients can be either decreased or increased as well as within normal limits. Assessment of the RNFL seems prudent in these patients, particularly for identifying notable degrees of RNFL thinning, in those being considered for future therapeutic trials.
Keywords: retinitis pigmentosa, spectral-domain OCT, peripapillary retinal nerve fiber layer thickness measurements, retinal nerve fiber layer abnormalities
Introduction
Retinitis pigmentosa (RP) is a genetically heterogeneous disorder with an incidence of about 1 in 3500 individuals.1 Affected individuals suffer from progressive degeneration of the photoreceptors, eventually resulting in severe visual impairment. The disease is characterized by bone spicule-like pigmentary deposits and reduced or absent electroretinographic (ERG) amplitudes.2 Although the disorder is known to primarily affect the outer retinal layers (the photoreceptors and retinal pigment epithelium), assessment of the structural changes of the inner retinal layers is particularly important when evaluating patients with RP. Therapeutic trials aiming to restore outer retinal structure and function require intact inner retinal elements to possibly improve vision in these patients.
Previous histopathologic and morphometric studies in RP patients have shown that inner retinal cells degenerate along with the progression of photoreceptor cell death, possibly due to either transneuronal damage or vascular compromise.3,4 Additionally, Newman and co-workers5 reported ophthalmoscopically evident defects of the nerve fiber layer in a series of patients with hereditary retinal dystrophies including patients with RP.
The introduction of optical coherence tomography (OCT) in the last decade added another useful tool for clinicians to evaluate the structural integrity of the outer and inner retinal layers in vivo. Previously published studies have evaluated the peripapillary retinal nerve fiber layer (RNFL) by means of OCT in RP patients but the reported results vary significantly. Using time-domain OCT (TD-OCT) in a previous study from our group,6 peripapillary RNFL measurements showed thinning in 40% of the study population while some RP patients showed thickening. Subsequently, another study from our unit that used newer technology, Fourier-domain OCT (FD-OCT), to evaluate peripapillary RNFL thickness abnormalities, showed thinning in 38% of the eyes and thickening in almost 22% of the studied eyes.7
However, in a more recent study by Hood et al,8 the authors reported that RNFL thickness in RP patients was significantly greater than normal when the inner retinal layers were evaluated and delineated manually after exporting the acquired raw images from a FD-OCT. Additionally, an OCT study by Oishi et al,9 that evaluated the average RNFL thickness in RP patients, found no significant differences in thickness in comparison to their control subjects.
The purpose of the present study was to measure the peripapillary RNFL using a new spectral-domain OCT (SD-OCT) in RP patients to further address the existing discrepancy in the literature concerning the RNFL layer thickness in these patients.
Materials and Methods
The study was conducted in the Department of Ophthalmology at the University of Illinois at Chicago. It was approved by an institutional review board and was performed in accordance with the tenets of the Declaration of Helsinki.
Thirty patients with RP were included after written informed consent was obtained. The diagnosis of RP was based on a history of nyctalopia, constriction of peripheral visual fields on kinetic perimetry, reduction of predominantly rod, but also cone electroretinogram amplitudes, and the presence of characteristic pigmentary changes in the retinal periphery. Patients were enrolled either during a follow-up visit or after invitation via telephone so as to include a representative sample from the various degrees (spectrum) of disease severity. Of the 30 patients included, seven had been tested for peripapillary RNFL with FD-OCT in a previous study from our group and their results were compared with the findings from the current study.
Exclusion criteria included a history of glaucoma or raised intraocular pressure (IOP) of more than 21 mmHg, best corrected visual acuity (BCVA) worse than 20/100, inability to hold steady fixation, diabetic retinopathy, optic nerve head drusen, refractive error of more than ± 6 diopter (D) sphere or ± 2D cylinder, evidence of cystoid macular edema (CME), the presence of an epiretinal membrane or significant media opacities sufficient to hinder OCT examination.
All patients underwent a complete ocular examination, including measurement of BCVA using the Snellen chart, slit lamp biomicroscopic examinations, and IOP measurements using Goldmann applanation tonometry. Fundus examination was performed after dilation with 2.5% phenylephrine and 1% tropicamide using both direct and indirect ophthalmoscopy. Optic disc pallor was graded by three of the authors (AA, MAG, GAF) as normal to mild, mild to moderate, or moderate to severe (the grading scores were consistent among the three authors).
SD-OCT Testing
All patients included in our study underwent peripapillary RNFL measurements using the OPKO SD-OCT scanning laser ophthalmoscope (SLO) device (OPKO Instrumentations, Miami, FL). This instrument is a combination of a confocal SLO and SD OCT. An optic nerve tracking software, which utilizes the major blood vessels around the optic nerve, is used to locate the position of the RNFL scan in relationship to the optic nerve. The system acquires 27,000 A-scans per second with an axial resolution of 5 µm. A circular OCT scan around the optic disc with a diameter of 3.4 mm is obtained and the system continuously captures the circular OCT image around the optic disc (three consecutive OCT images of the RNFL are captured in each acquisition and averaged with less than a 5% thickness variation).
The RNFL exam protocol was used for the peripapillary RNFL measurements. The parameters calculated by the OPKO software and evaluated in this study included: the average peripapillary RNFL thickness (360 degrees), and the RNFL thickness of each separate quadrant (temporal, superior, nasal, and inferior quadrants). Three independent consecutive measurements were initially acquired by the operator and these data were only used if the scans were reproducible (at least two out of the three scans showed consistent results) within either a normal or abnormal range. In cases where the results from the first three scans were inconsistent, measurements were repeated (three additional scans were acquired).
Data Analysis
The peripapillary total circumferential RNFL thickness was considered abnormally thin if its value was less than the 5th percentile of the age-related normal values and thicker if it was more than the 95th percentile. Abnormal thinning or thickening of the RNFL in at least one quadrant, consistently in at least 2/3 of the RNFL OCT scans, was considered significant and therefore abnormal.
The instrument calculated the average RNFL thickness ± standard deviation (SD) in all 4 individual quadrants and compared these values with normative data provided by the manufacturer that was corrected for age and was retrieved from 245 eyes of 123 visually normal subjects aged 19 to 85 years old (mean [SD] was 43.1[14.9] years). Statistically significant differences in the mean peripapillary total circumferential RNFL thickness between our patient cohort and that from normative control data was analyzed using the two-tailed t-test. Additionally, linear regression was used to evaluate the influence of age on the overall peripapillary RNFL thickness measurements.
Results
Fifty eyes of 30 patients with RP were included in the current study (26 right and 24 left eyes). Of the 30 patients, we excluded 10 eyes from 10 patients from the analysis due to an inability to obtain consistent and reproducible scans. Our study cohort included 23 female and 7 male subjects. The average age was 45.8 ± 16.3 years (range: 15–73 years). Nineteen patients were Caucasian, 6 were African-American, 3 were Hispanic, and 2 were Asian. The genetic subtypes of our study population were as follows: 4 cases were classified as autosomal dominant, 10 cases as autosomal recessive, 14 cases as isolated, 1 could not be characterized (the patient was adopted), and 1 case of Usher syndrome.
The average best-corrected visual acuity in the right eyes was 0.18 log MAR (range: −0.14 to 0.58), while the average best-corrected visual acuity in the left eyes was 0.17 log MAR (range: −0.16 to 0.46). The range of IOP for all patients was from 9 to 19 mmHg (mean: 14.2 mmHg).
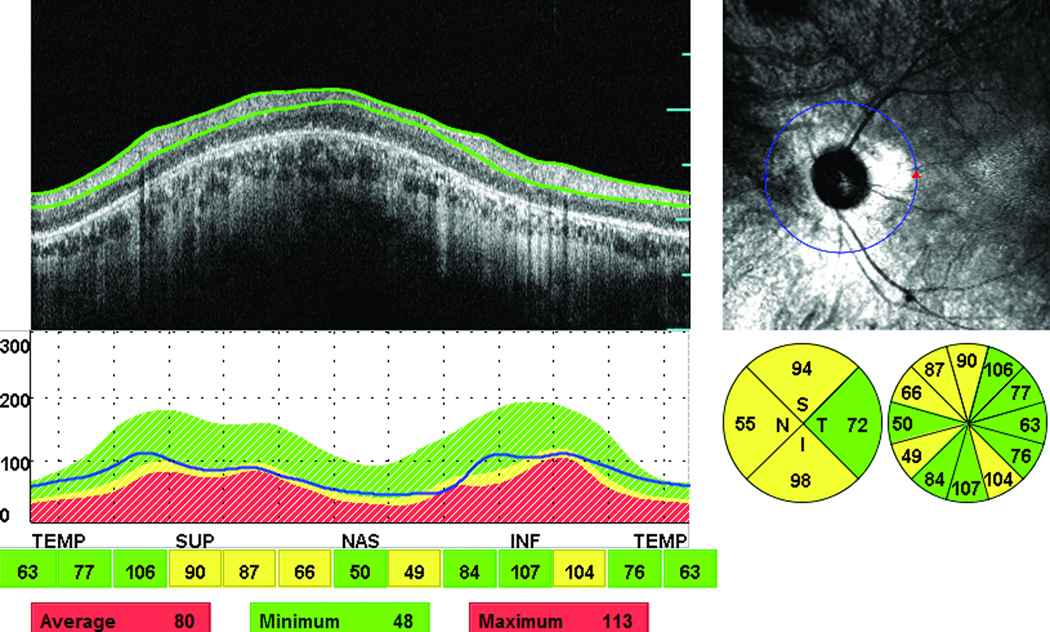
Thinning of the peripapillary RNFL in one or more quadrants was observed in 18 of the 50 eyes (36%) studied (characteristic clinical example in Figure 1). Four eyes (8%) showed both thinning and thickening of the peripapillary RNFL thickness in at least one quadrant (Figure 2). In eyes that showed only thinning, the RNFL was on average 42.9 (±10.1) µm thinner than normal. Of the 18 eyes that showed thinning of the RNFL, 10 eyes showed thinning in three quadrants, 2 eyes in two quadrants, and 6 eyes in one quadrant (there was a total of 40 abnormally thin quadrants). Regarding the distribution for the abnormal thinning of the RNFL, this was most commonly observed in the inferior quadrant (16 eyes) followed by the nasal quadrant in (13 eyes) and the superior quadrant (11 eyes). No eyes showed thinning of the temporal quadrant. Comparison of the ophthalmoscopic appearance of the optic disc with the findings from the peripapillary RNFL measurements in the 14 eyes that only showed thinned quadrants observed on the OCT scans showed that thinning of the RNFL was accompanied by normal-mild pallor in 5 eyes, mild to moderate pallor in 6 eyes, and moderate to severe pallor in 3 eyes.
Figure 1.
Abnormal thinning of the peripapillary RNFL in 3 out of 4 quadrants in the left eye of an affected male (55-year-old) with isolated RP. The green color indicates normal RNFL thickness and the yellow a thin RNFL (outside 95% confidence limit of normal). The figure also shows a cross-sectional retinal infrared (IR) image sampled along a 3.4 mm circle centered to optic disc, an OCT scan, and RNFL thickness graph.
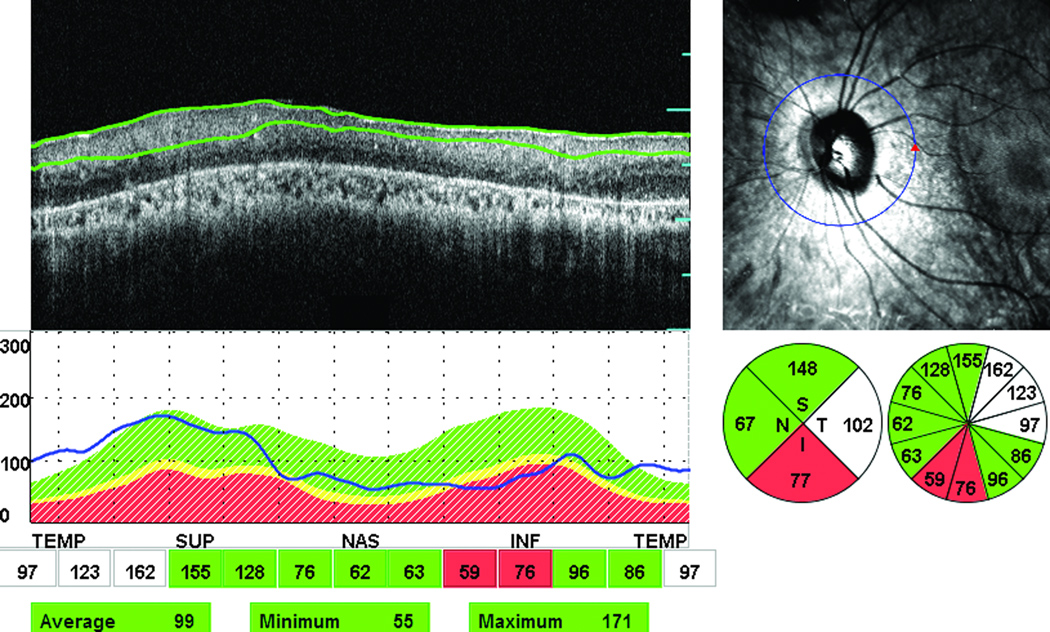
Figure 2.
Abnormal thinning and thickening of the peripapillary RNFL in 2 out of 4 quadrants (inferior and temporal quadrants, respectively) in the left eye of an affected female (64-year-old) with autosomal recessive RP. The green color indicates normal RNFL thickness, red a thin RNFL (outside 99% confidence limit of normal), and white indicates a thick RNFL The figure also includes one infrared image for the optic nerve, OCT scan, and RNFL thickness graph in all quadrants.
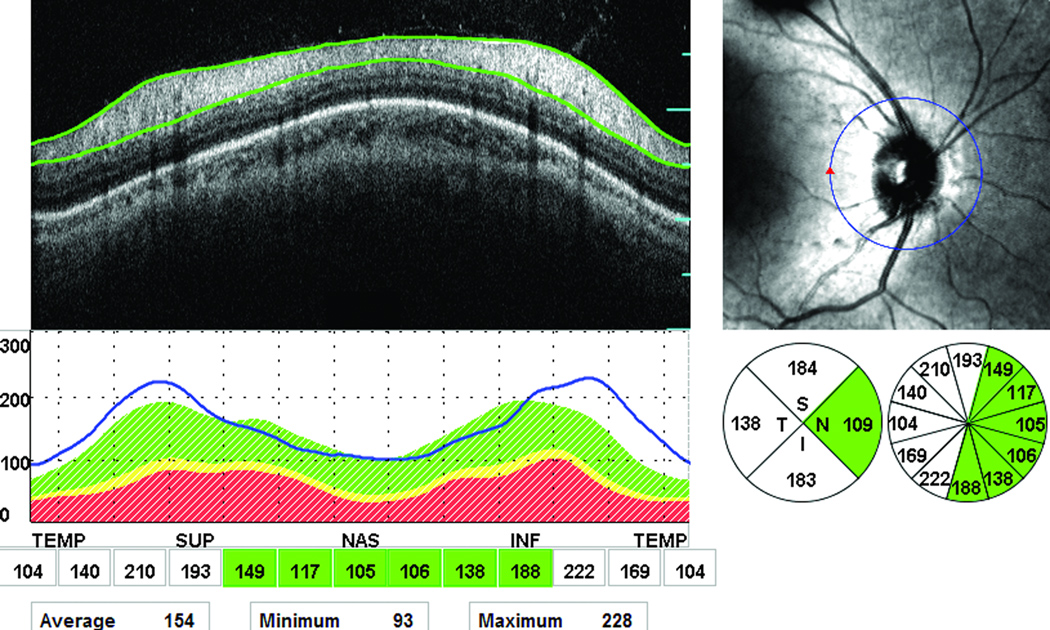
Thickening of the peripapillary RNFL in one or more quadrants was observed in 21 of the 50 eyes (42%) (characteristic example in Figure 3). Four eyes (8%) showed both thinning and thickening of the peripapillary RNFL thickness. For the quadrants in which thickening was observed, the RNFL measurements were on average 37.7 (±12.9) µm thicker than normal. Among these 21 eyes, 18 eyes showed abnormal thickening in one quadrant and 3 eyes in three quadrants (there was a total of 27 abnormally thick quadrants). The distribution of the abnormally thick quadrants was as follows: 20 temporal quadrants, 4 superior quadrants, and 3 inferior quadrants. No eyes showed RNFL thickening in the nasal quadrant. Comparison of the ophthalmoscopic appearance of the optic disc with the findings from the peripapillary RNFL measurements in the 17 eyes that showed only RNFL thickening on the OCT scans revealed that thickening of the RNFL was accompanied by normal to mild pallor in 9 eyes, mild to moderate pallor in 7 eyes and by moderate to severe pallor in 1 eye.
Figure 3.
Abnormal thickening of the peripapillary RNFL in 3 out of 4 quadrants in the right eye of an affected female (15-year-old) with autosomal recessive RP. The green color indicates normal RNFL thickness and the white indicates a thick RNFL. The OCT scan, infrared image of optic nerve, and a graph for the RNFL thickness are also shown in the figure.
Additional analysis was performed on the eyes that showed either only thickening or only thinning of the RNFL in at least one quadrant. The overall circumferential RNFL thickness of the 14 eyes that showed only thinning in at least one quadrant was 78.78 µm. For the 17 eyes that showed only thickening in at least one quadrant, the RNFL thickness was 119.69 µm. The RNFL thickness values of the eyes with thinning and the eyes with thickening were significantly different from normal (t=6.31, p<0.01 for thickening; t=3.62, p<0.01 for thinning).
We determined that the overall average total circumferential RNFL thickness in the 50 eyes of our 30 patients, including quadrants with either increased or decreased RNFL thickness, was 100.1 µm (SD of 18.8 µm). For the normative data, the average RNFL thickness was 105.2 µm (SD±10.8). There was no statistically significant difference in the overall average circumferential RNFL thickness when compared to the normative data (t = 0.77, p= 0.45), which might be expected given that the patients had both thickening and thinning of the RNFL.
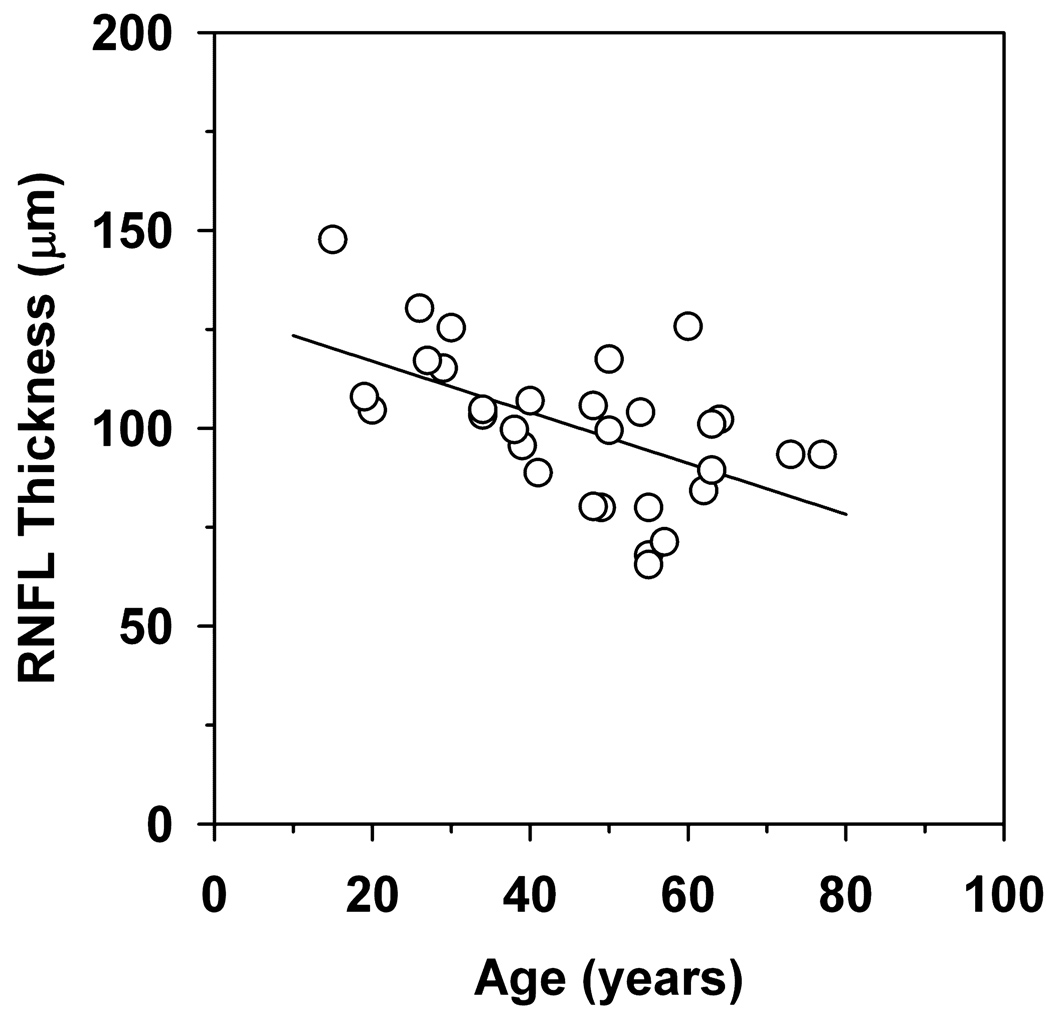
The effect of age on the overall RNFL thickness in the RP patients is shown in Figure 4. In this plot, overall RNFL thickness is plotted as a function of age for each patient. The data are fit with a linear regression line that has a slope of −0.65, which is significantly different from zero (t=−3.52, p<0.01). This finding indicates that there is an approximate 6.5 µm loss of RNFL thickness per decade of life.
Figure 4.
RNFL thickness as a function of age. The solid line is a linear regression line fit to the data, as described in the text.
Discussion
The peripapillary RNFL thickness in RP patients was evaluated in the current study using SD-OCT. Thirty percent of the eyes studied did not show any abnormal quadrants, 36% showed at least one abnormally thin quadrant in at least one eye, and 42% showed at least one abnormally thick quadrant in at least one eye. Thinning of the RNFL was most commonly observed in the inferior quadrant followed by the nasal quadrant, while thickening of the RNFL was most commonly seen in the temporal quadrant followed by an approximately equal number of superior and inferior quadrants. In our current cohort of RP patients, there was no apparent trend between the RNFL thickness and the ophthalmoscopic appearance of optic nerve pallor.
The findings of the current study are similar to previous studies from our group (although the criteria for analysis were not identical). However, thinning was found in 40% of the RP patients when a TD-OCT was used to assess the peripapillary RNFL and thickening was found in another 40% of the patients studied (thickening was found mostly in the temporal quadrant in that study).6 In addition, another TD-OCT study (Sobasi et al. IOVS 2009; 50: ARVO E-abstract 6272) reported thinning in almost 30% and thickening in 37% of the RP patients. Furthermore, when FD-OCT was used to evaluate RNFL defects in RP patients in another study from our group, 38% of the eyes showed thinning of the RNFL, while thickening was observed in 22%.7
Despite the fact that more eyes in our study showed at least one abnormally thick quadrant in comparison with the eyes that showed at least one abnormally thin quadrant, the total number of abnormally thinned quadrants exceeded that of the abnormally thick quadrants. The findings of the peripapillary RNFL measurements in seven patients of the current study who participated in a previous study evaluating the RNFL defects in RP patients by FD-OCT showed notable consistency. Six out of these seven patients showed consistent results either in an abnormal or normal thickness range in three of the four quadrants, while 1 patient showed consistency in 2 of the 4 quadrants in both studies.
Although the percentages of thinning and thickening of the RNFL in RP patients were somewhat different in the three studies from our group (using different instrumentation), in all three studies we were able to identify thinning as well as thickening of the RNFL in our patients with RP. Differences in the RNFL measurements obtained by different devices have been reported in previous studies.10–17 The use of different devices may account, at least in part, for the slight differences in the percentage of thinning of the RNFL found in the three studies from our group.
Improvements in OCT technology have resulted in a greater speed of image acquisition, better image resolution, and a reduction of motion artifacts, which permits a denser sampling of the tissue and better definition of the individual retinal layers.10 SD OCT technology provides a reliable means for the evaluation of the RNFL thickness. Of note, the signal to noise ratio in our study was 7 or greater in all cases. The device used in the current study also allows for manual correction of the contour lines of the retinal layers in cases where the signal is partially reduced due to vitreous floaters (this feature was used when necessary).
Hood et al8 evaluated the RNFL in 30 patients with RP using a different approach. Raw OCT images acquired by FD-OCT were exported and the thickness of RNFL was measured with a manual segmentation procedure aided by a computer program. The authors reported that the thickness of the RNFL was significantly greater than normal in their RP patients and suggested that measuring the retinal ganglion cell layer with FD-OCT possibly provides a better index of ganglion cell integrity than RNFL measurements in these patients. It is unclear why thickening was only observed in this study but we cannot exclude the possibility that their study cohort differed from ours (perhaps in disease severity or genetic subtypes). Additionally, the normative data and the age of the studied cohorts may have differed between the current study and that of Hood et al8.
In summary, by SD-OCT testing, the current study demonstrated that the peripapillary RNFL thickness in RP patients can be either decreased, increased, or within normal limits. Furthermore, thinning and thickening can occur within a single eye. Assessment of the RNFL by using OCT exam seems prudent in these patients, particularly for these being considered for future therapeutic trials.
Acknowledgements
Foundation Fighting Blindness, Owing Mills, Maryland, and Grant Healthcare Foundation, Chicago, Illinois (GAF); NIH core grant EY01792; NIH research grant EY019510 (JJM) and an unrestricted departmental grant from Research to Prevent Blindness.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Proprietary interests: None
REFERENCES
- 1.Blacharski PA. Fundus flavimaculatus. In: Newsome DA, editor. Retinal dystrophies and Degenerations. New York: Raven Press; 1988. pp. 135–159. [Google Scholar]
- 2.Pagon RA. Retinitis Pigmentosa. Surv Ophthalmol. 1998;33(3):137–177. doi: 10.1016/0039-6257(88)90085-9. [DOI] [PubMed] [Google Scholar]
- 3.Stone JL, Barlow WE, Humayun MS, de Juan E, Jr, Milam AH. Morphometric analysis of macular photoreceptors and ganglion cells in retinas with retinitis pigmentosa. Arch Ophthalmol. 1992;110:1634–1639. doi: 10.1001/archopht.1992.01080230134038. [DOI] [PubMed] [Google Scholar]
- 4.Li ZY, Possin DE, Milam AH. Histopathology of bone spicule pigmentation in retinitis pigmentosa. Ophthalmology. 1995;102(5):805–816. doi: 10.1016/s0161-6420(95)30953-0. [DOI] [PubMed] [Google Scholar]
- 5.Newman NM, Stevens RA, Heckenlively JR. Nerve fibre layer loss in diseases of the outer retinal layer. Br J Ophthalmol. 1987;71:21–26. doi: 10.1136/bjo.71.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walia S, Fishman GA, Edward DP, Lindeman M. Retinal Nerve Fiber Layer Defects in RP Patients. Invest Ophthalmol Vis Sci. 2007;48:4748–4752. doi: 10.1167/iovs.07-0404. [DOI] [PubMed] [Google Scholar]
- 7.Walia S, Fishman GA. Retinal Nerve Fiber Layer Analysis in RP Patients using Fourier-Domain OCT. Invest Ophthalmol Vis Sci. 2008;49:3525–3528. doi: 10.1167/iovs.08-1842. [DOI] [PubMed] [Google Scholar]
- 8.Hood DC, Lin CE, Lazow MA, Locke KG, Zhang X, Birch DG. Thickness of receptor and post-receptor retinal layers in patients with retinitis pigmentosa measured with frequency-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2009;50:2328–2336. doi: 10.1167/iovs.08-2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oishi A, Otani A, Sasahara M, Kurimoto M, Nakamura H, Kojima H, et al. Retinal nerve fiber layer thickness in patients with retinitis pigmentosa. Eye. 2009;23:561–566. doi: 10.1038/eye.2008.63. [DOI] [PubMed] [Google Scholar]
- 10.Sakata LM, DeLeon-Ortega J, Sakata V, Girkin CA. Optical coherence tomography of the retina and optic nerve (review) Clin Exp Ophthalmol. 2009;37:90–99. doi: 10.1111/j.1442-9071.2009.02015.x. [DOI] [PubMed] [Google Scholar]
- 11.Blumenthal EZ, Parikh RS, Pe’er J. Retinal nerve fibre layer imaging compared with histological measurements in a human eye. Eye. 2009;23:171–175. doi: 10.1038/sj.eye.6702942. [DOI] [PubMed] [Google Scholar]
- 12.Schuman JS. Spectral domain optical coherence tomography for glaucoma. Trans Am Ophthalmol Soc. 2008;106:426–458. [PMC free article] [PubMed] [Google Scholar]
- 13.Knight OJ, Chang RT, Feuer WJ, Budenz DL. Comparison of retinal nerve fiber layer measurements using time domain and spectral domain optical coherent tomography. Ophthalmology. 2009;116:1271–1277. doi: 10.1016/j.ophtha.2008.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sung KR, Kim DY, Park SB, Kook MS. Comparison of retinal nerve fiber layer thickness measured by Cirrus HD and Stratus optical coherence tomography. Ophthalmology. 2009;116:1264–1270. doi: 10.1016/j.ophtha.2008.12.045. [DOI] [PubMed] [Google Scholar]
- 15.Vizzeri G, Weinreb RN, Gonzalez-Garcia AO, Bowd C, Medeiros FA, Sample PA, et al. Agreement between spectral-domain and time-domain OCT for measuring RNFL thickness. Br J Ophthalmol. 2009;93:775–781. doi: 10.1136/bjo.2008.150698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sehi M, Grewal DS, Sheets CW, Greenfield DS. Diagnostic ability of Fourier-Domain vs time-Domain optical coherence tomography for glaucoma detection. Am J Ophthalmol. 2009;148:597–605. doi: 10.1016/j.ajo.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hood DC, Raza AS, Kay KY, Sandler SF, Xin D, Ritch R, et al. A comparison of retinal nerve fiber layer (RNFL) thickness obtained with frequency and time domain optical coherence tomography (OCT) Opt Express. 2009;17:3997–4003. doi: 10.1364/oe.17.003997. [DOI] [PMC free article] [PubMed] [Google Scholar]