Abstract
Rheumatoid arthritis develops in association with a defect in peripheral CD4+ T cell homeostasis. T cell lymphopenia has also been shown to be a barrier to CD4+ T cell clonal anergy induction. We, therefore, explored the relationship between clonal anergy induction and the avoidance of autoimmune arthritis by tracking the fate of glucose-6-phosphate isomerase (GPI)-reactive CD4+ T cells in the setting of selective T cell lymphopenia. CD4+ T cell recognition of self GPI peptide/MHCII complexes in normal murine hosts did not lead to arthritis, and instead caused those T cells to develop a Folate receptor 4 (FR4)hi CD73hi anergic phenotype. In contrast, hosts selectively depleted of polyclonal Foxp3+ CD4+ T regulatory cells could not make GPI-specific CD4+ T cells anergic, and failed to control arthritis. This suggests that autoimmune arthritis develops in the setting of lymphopenia when Foxp3+ CD4+ T regulatory cells are insufficient to functionally inactivate all autoreactive CD4+ T cells that encounter self Ag.
Introduction
Rheumatoid arthritis (RA)3 is a prevalent and debilitating autoimmune disease characterized by chronic inflammation and eventual destruction of the synovial joints (1). Although the pathogenesis of RA remains unknown, CD4+ T cell- and B cell-mediated autoimmunity directed against citrulline-modified proteins in genetically predisposed individuals is tightly associated with the uncontrolled activation of innate immune cells (e.g., neutrophils, mast cells, synoviocytes, osteoclasts) and the elaboration of cytokines (e.g., TNF-α, IL-1, IL-6, IL-17a) that promote synovial tissue infiltration, inflammation, and damage. Successful management of RA disease activity and progression currently relies on immunomodulatory drug therapies. However, chronic use of these agents is associated with an increased risk of serious infection and/or malignancy as a consequence of generalized immunosuppression. Therefore, therapeutic strategies designed to reinstitute immunological tolerance to RA-related self Ag could offer the possibility of improved long-term safety, as well as greater or more durable efficacy. Unfortunately, our inadequate knowledge regarding the control of normal immune self tolerance slows the development of such therapeutics.
Patients with RA have a primary immune abnormality that is manifested by accelerated T cell ‘aging’, perhaps as a direct result of defective thymic output or abnormal peripheral T cell homeostasis, and this may predispose them to the development of autoimmune arthritis (2). Other primary and acquired immunodeficiencies that lead to peripheral T cell lymphopenia can also be associated with autoimmune disease manifestations (3). In many animal models of autoimmunity, lymphopenia has been shown to be an important contributing factor to disease development (4–6). Experiments in the non-obese diabetic NOD mouse model of type I diabetes mellitus have, in particular, implicated T cell lymphopenia in the spontaneous loss of immunological tolerance to pancreatic islet cell Ag (7). Often, an adoptive transfer of polyclonal CD4+ T cells into lymphopenic hosts can restore normal peripheral self tolerance and prevent the development of immunopathology (8, 9).
Decreased numbers and/or function of Foxp3+ CD4+ T regulatory cells may relate lymphopenia to the development of systemic autoimmune diseases such as RA. Reduced function of synovial CD25+ CD4+ T regulatory cells has been implicated in the pathogenesis of RA, and the elimination of CD25+ Foxp3+ CD4+ T regulatory cells worsens disease in mouse models of inflammatory arthritis, including the K/BxN system of autoimmunity directed against the self Ag glucose-6-phosphate isomerase (GPI) (4, 6, 10–14). Human CD4+ T cells with a CD25+ Foxp3+ T regulatory cell phenotype appear to control autoreactivity in the peripheral immune system, based on their ability to protect against the development of the IPEX syndrome (immune dysfunction, polyendocrinopathy, enteropathy, X-linked inheritance) (15, 16). Similarly, mutation of the Foxp3 gene in mice leads to multi-organ immune cell infiltration and autoimmunity as a consequence of defective CD4+ T regulatory cell generation and function (17–19). Taken together, these observations support the hypotheses that the maintenance of a normal peripheral CD25+ Foxp3+ CD4+ T regulatory cell compartment and the suppression of CD4+ T cells having autoreactive Ag-receptor (TCR) specificities are essential for the avoidance of autoimmune arthritis.
We previously demonstrated that following a partial reconstitution of the CD4+ T cell compartment in lymphopenic hosts, CD25+ Foxp3+ CD4+ T regulatory cells play an important role in promoting Ag-specific tolerance within CD4+ T cells through the induction of clonal anergy. In the absence of infection or adjuvant, naïve CD4+ T cells recognizing an experimental Ag for the first time lost their capacity to produce IL-2 when T regulatory cells were present, whereas anergy could not be induced in the absence of T regulatory cells (9). To further explore the relationships between the homeostasis of the CD4+ T regulatory cell compartment, the induction of clonal anergy to an arthritogenic self Ag, and the avoidance of autoimmune arthritis, we have taken advantage of GPI-specific KRN TCR-transgenic (TCR-Tg) CD4+ T cells and their adoptive transfer into either wildtype (WT) or lymphopenic TCRα−/− hosts that naturally express GPI/I-Ag7 complexes. Using Foxp3DTR gene knock-in CD4+ T cells that have been made deficient for Foxp3+ T regulatory cells with Diphtheria toxin (DT), our results now demonstrate that lymphopenic hosts are prone to the development of autoimmune arthritis because they lack the polyclonal CD25+ Foxp3+ CD4+ T regulatory cells necessary to induce anergy in pathogenic CD4+ T cells that recognize self Ag for the first time in the peripheral immune system. Furthermore, the adoptive transfer of CD25+ Foxp3+ CD4+ T regulatory cells into lymphopenic hosts restores high-level expression of two novel anergy markers Folate receptor 4 (FR4) and CD73 on the autoreactive Foxp3− CD4+ T cells, reduces the potential for autoreactive CD4+ T cells to undergo clonal expansion and effector cell differentiation, and limits the capacity of these autoreactive T cells to break natural immunological tolerance within the GPI-reactive B cell repertoire.
Materials and Methods
Mice
B6.g7 (H-2g7 congenic) mice as well as B6 strain KRN mice that express a TCR transgene specific for glucose-6-phosphate isomerase (GPI)/I-Ag7 were gifts from Drs. Diane Mathis and Christophe Benoist (Harvard Medical School, Boston, MA) and the Institut de Génétique et de Biologie Moléculaire et Cellulaire (Strasbourg, France) (20). B6 mice were purchased from Charles River Breeding Laboratories under a contract from the National Cancer Institute (Frederick, MD). B6 TCRα−/− (B6.129S2-Tcratm1Mom/J), Rag1−/− (B6.129S7-Rag1tm1Mom/J), and CD45.1+ (B6.SJL-Ptprca Pep3b/BoyJ) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). The breeding of CD45.1+ KRN, Rag1−/− KRN, B6×B6.g7, TCRα−/− B6.g7, and TCRα−/− B6×B6.g7 mice was carried out in our own colonies. B6 Foxp3DTR gene knock-in mice were a kind gift from Dr. Alexander Rudensky (Memorial Sloan-Kettering Cancer Center, New York, NY) and were bred to homozygosity on the B6×B6.g7 F1 background (19). All experiments used age and sex matched controls. Experiments were reviewed and approved by the University of Minnesota Institutional Animal Care and Use Committee.
In vivo adoptive transfer and NK cell depletion
NK cells were depleted from WT and TCRα−/− B6×B6.g7 recipients of B6 strain KRN T cells using anti-asialo GM1 Ab (Wako Chemicals USA, Inc., Richmond, VA) at a dose of 25 μg/mouse i.v. injection on days -1, 4, 9 and 14 relative to the day of KRN CD4+ T cell adoptive transfer. Donor CD45.1+ KRN spleen and lymph node cells were enriched for naïve CD44− CD25− CD4+ T cells by negative selection using biotin-conjugated anti-CD25 (PC61.5) and anti-CD44 (IM7) from eBioscience (San Diego, CA), together with a biotin-conjugated antibody cocktail for CD4+ T cell isolation and an LS column (Miltenyi Biotec Inc., Auburn, CA). Purified naïve CD4+ T cells were adoptively transferred to recipients by tail-vein injection. In some experiments, the T cells were labeled with CFSE prior to adoptive transfer, and the average cell division rate for CFSE-labeled KRN CD4+ T cell populations was determined as previously described (21).
T regulatory cell transfer and Foxp3+ T cell ablation
Spleens and lymph nodes from either WT or Foxp3DTR B6×B6.g7 mice were harvested, and polyclonal CD4+ T cells were purified by negative selection using the CD4+ T cell isolation kit and an LS column (Miltenyi). To deplete Foxp3–expressing cells from reconstituting polyclonal Foxp3DTR CD4+ T cell populations, Diphtheria toxin (DT) (List Biological, Campbell, CA) was injected i.p. at a concentration of 50 μg/kg body weight on days 8, 10, and 12 after CD4+ T cell reconstitution (19). In some experiments, donor Foxp3DTR B6×B6.g7 spleen and lymph nodes cells were stained with fluorochrome-labeled anti-CD4 and anti-CD25 Ab prior to physical cell sorting to purify CD25− GFP− CD4+ and CD25+ GFP+ CD4+ populations using a FACSAria flow cytometer (BD Biosciences, San Jose, CA).
Flow cytometric analysis
For the analysis of CD4+ T cells in adoptive transfer recipients, spleen and lymph node cells were stained with phycoerythrin (PE)-conjugated Ab to CD45.1 (A20; eBioscience), and then KRN T cells were re-isolated by positive selection using anti-PE magnetic beads and an LS column (Miltenyi). All T cells were then also stained with eBioscience antibiodies allophycocyanin (APC)-eFluor780-conjugated anti-B220 (RA3-6B2), anti-CD11b (MI-70), anti-CD11c (N418), as well as anti-F4/80 (BM8; from Invitrogen Corp., Carlsbad, CA), for use as “dump channel” reagents. Additional eBioscience antibodies studied: APC-conjugated anti-CD27 (LG.759) or CD25 (PC61.5), Pacific Blue- or eFluor 450-conjugated anti-CD4 (RM4-5), PE-indotricarbocyanine (Cy7)-conjugated anti-FR4 (eBio12A5), Alexa Fluor 700-conjugated anti-CD44 (IM7), peridinin chlorophyll protein-cyanine 5.5-conjugated anti-CCR7 (4B12) and biotin-conjugated anti-CD73 (TY/11.8). Secondary staining for biotin-conjugated antibodies was preformed with streptavidin-conjugated APC. In some experiments, previously surface stained cells were treated with Foxp3 Fixation/Permeabilization Concentrate and Diluent (eBioscience) and stained with APC-conjugated anti-Foxp3 (FJK-16s).
For intracellular cytokine staining, T cells were incubated for 3 hrs at 37°C in RPMI medium 1640 + 10% FCS in the presence of 50 ng/ml PMA (Sigma-Aldrich, Saint Louis, MO), 1 μM ionomycin (EMD Chemicals, Gibbstown, NJ) and with 10 μg/ml Brefeldin A (Sigma-Aldrich) for the final 2 hours. Cells were then stained for surface markers as described above. Intracellular staining was preformed using the Cytofix/Cytoperm kit (BD PharMingen) per the manufacturer’s instructions with APC-conjugated IL-2 (JESS-5H4); PE-Cy7-conjugated anti-IFN-γ (XMG1.2); and eFluor450 anti-TNF-α (MP6-XT22).
For B cell staining, bulk lymphocytes were stained with eBioscience reagents APC-eFluor780-conjugated anti-CD4 (RM4-5), anti-CD8α (53-6.7), anti-CD11c (N418), anti-Gr-1(RB6-8C5), as well as anti-F4/80 (BM8; from Invitrogen Corp.). Pacific Blue or eFluor450-conjugated anti-B220 (RA3-6B2) (eBioscience) was also studied. Intracellular staining was preformed using the Cytofix/Cytoperm kit (BD PharMingen, San Diego, CA) per manufacturer’s instructions with Pacific Orange-conjugated F(ab′)2 fragment of goat anti-mouse immunoglobulin heavy and light chain (P31585; Molecular Probes) and biotin-conjugated recombinant mouse GPI (kindly provided by Dr. Haochu Huang, University of Chicago, Chicago, IL) (23). All cells were analyzed using a BD LSR II flow cytometry (BD Biosciences). Data were analyzed with FlowJo software (TreeStar, Ashland, OR).
Reverse transcriptase quantitative real time qPCR
CD45.1+ CD4+ KRN T cells were recovered from primary adoptive transfer hosts by a combination of CD45.1 MACS microbead positive selection and BD FACSAria (BD Biosciences) flow cytometric cell sorting. Purified KRN T cells were then lysed and homogenized using QIAshredder and total RNA extracted using an RNeasy Micro or Mini Kit (Qiagen, Valencia, CA). cDNA was produced using the qScript cDNA SuperMix (Quanta BioSciences, Inc., Gaithersburg, MD) and examined for gene expression using real-time quantitative PCR (qPCR) (Applied Biosystems 7000 Real-Time PCR system (Life Technologies Corporation, Carlsbad, CA) or Cepheid Smart Thermocycler (Cepheid, Sunnyvale, CA)) and PerfeCTa SYBR Green FastMix (Quanta BioSciences, Inc.). All qPCR primers sets (Suppl. Fig. 4) were designed to utilize identical qPCR settings.
H&E staining
For histological analysis, ankles and feet were dissected, frozen in O.T.C. medium (Sakura Finetek U.S.A., Inc., Torrance, CA), cryo-sectioned to a thickness of 10 μm and stained with hematoxylin and eosin.
Arthritis scoring
Ankle thickness was measured with a Quick-Mini Series 700 comparator (Mitutoyo U.S.A., Aurora, IL) and was reported as the percent change in ankle thickness from day 0. Arthritis severity was also assigned a score 0–3 for each paw based on swelling and erythema, resulting in a maximum Arthritis Clinical Index score of 12 for each mouse (22).
Anti-GPI IgG1 antibody measurement
Serum was isolated from recipient mice on specified days, and measured for anti-GPI IgG1 antibodies by ELISA using recombinant mouse GPI together with IgG1-specific anti-mouse Ig reagents.
Statistical analysis
Mean Arthritis Clinical Index scores were compared using the Mann-Whitney U test. Other tests of significance shown represent the results of an unpaired one-tailed Student’s t test. R2 values shown were calculated using log-transformed data.
Results
Induction of autoimmune arthritis in T-cell deficient hosts by an adoptive transfer of GPI-reactive KRN CD4+ T cells
To explore the role of the clonal anergy tolerance mechanism in protection from autoimmune disease, we made use of B6 KRN TCR-Tg mice whose CD4+ T cells recognize the self-peptide GPI282–294 bound to the MHC class II molecule I-Ag7 (20, 23, 24). KRN CD4+ T cells are known to cause autoimmune arthritis when transferred into I-Ag7–expressing T cell-deficient hosts, whereas wildtype recipients remain free of disease (25). For our experiments, 104 naïve GPI-specific B6 strain KRN CD4+ T cells were adoptively transferred into I-Ag7–expressing WT or TCRα−/− B6×B6.g7 mice. B6×B6.g7 recipient mice (or B6 controls) were then monitored for development of systemic inflammatory disease, including arthritis and weight loss.
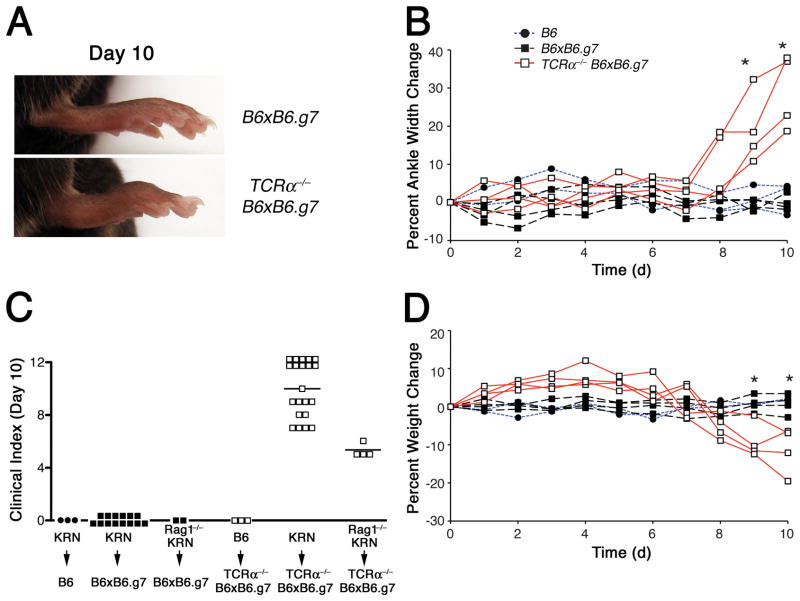
In preliminary experiments, we determined that an adoptive transfer of 104 CD25− CD44lo CD4+ T cells was sufficient to park about 103 naïve T cells in recipient mice ((21), and data not shown). This small number of naïve GPI-reactive T cells caused no disease in either B6 or B6×B6.g7 WT recipients, consistent with a maintenance of peripheral self tolerance to GPI/I-Ag7 complexes in the WT B6×B6.g7 mice (Fig. 1A–C). In contrast, T cell lymphopenic TCRα−/− B6×B6.g7 recipients of KRN CD4+ T cells developed swelling and erythema involving the proximal synovial joints of all four paws beginning on about day 8 after adoptive transfer and progressing to maximal disease by day 12 to 14 (Fig. 1A–C, and data not shown). Arthritis signs in these paws were accompanied by loss of articular cartilage, intense periarticular and bone marrow immune cell infiltration (both mononuclear and polymorphonuclear), marrow fat cell replacement, and thinning/erosion of marginal cortical bone tissue (Supplemental Fig. 1, and data not shown). Beginning on day 7 after adoptive transfer, TCRα−/− B6×B6.g7 hosts also lost significant body weight (Fig. 1D). The development of disease in these lymphopenic mice was entirely dependent on the GPI-specific KRN transgenic TCR, as 104 naïve polyclonal B6 CD4+ T cells demonstrated no ability to promote arthritis in TCRα−/− B6×B6.g7 hosts (Fig. 1C). Furthermore, the recognition of GPI/I-Ag7 complexes by the KRN transgenic TCR appeared to be sufficient for T cell activation and arthritis induction, as KRN T cells on the Rag1−/− background that lacked endogenous TCR gene rearrangements also caused joint disease (albeit with slower kinetics; data not shown) when transferred into the T cell-lymphopenic mice.
Figure 1.
Naïve KRN CD4+ T cells cause autoimmune arthritis in T cell lymphopenic hosts. Host mice were given 104 TCR-transgenic KRN CD4+ T cells on day 0 and were then monitored for signs of arthritis and weight loss. A, Representative rear paws of B6×B6.g7 (top) and TCRα−/− B6×B6.g7 (bottom) at day 10 after adoptive transfer. B, Arthritis, as assessed by change in measured ankle thickness (mm) of individual B6 (●), WT B6×B6.g7 (■), and TCRα−/− B6×B6.g7 (□) adoptive transfer recipient mice. C, Clinical disease scores of individual B6, B6×B6.g7, and TCRα−/− B6×B6.g7 hosts 10 days after adoptive transfer of either non-transgenic B6, KRN, or Rag1−/− KRN CD4+ T cells, with median score indicated by the bars. D, Daily weight measurements in individual mice, expressed as change in body weight (gm). Statistically significant differences (P < 0.05; Student’s t test) between the WT B6×B6.g7 and TCRα−/− B6×B6.g7 groups are indicated by an asterisk.
Autoimmune arthritis develops in association with a breakdown in natural B cell tolerance to GPI
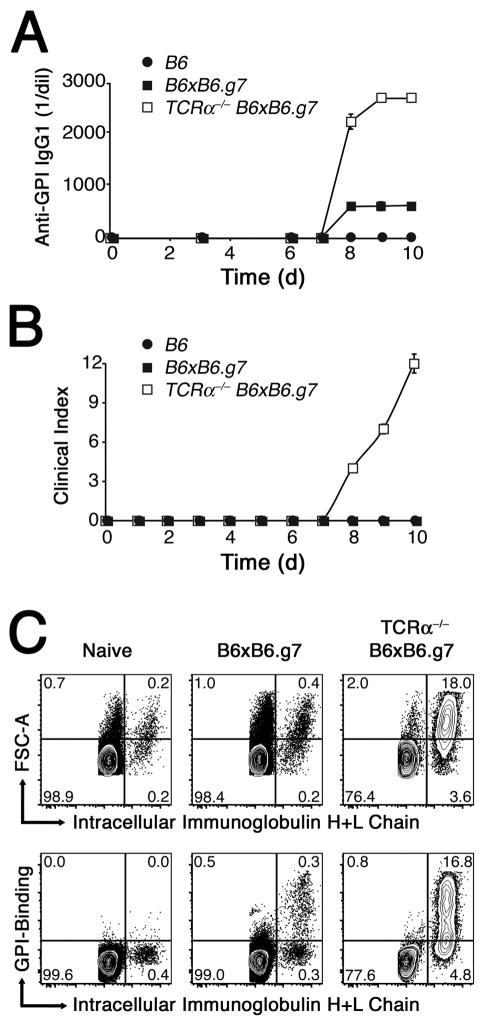
To assess autoreactivity to GPI in the B cell compartment during the induction of arthritis, serum samples were taken from animals at various times after the adoptive transfer and examined for the presence of anti-GPI antibodies of the IgG1 isotype by ELISA. Interestingly, both normal WT and TCRα−/− B6×B6.g7 hosts began to produce some anti-GPI IgG1 by 8 days after the KRN CD4+ T cell adoptive transfer, whereas control B6 recipients produced none (Fig. 2A). Beyond day 8, TCRα−/− B6×B6.g7 hosts eventually accumulated much higher levels of anti-GPI IgG1 Ab compared to wildtype recipients. Note that the time at which anti-GPI IgG1 was first detected in lymphopenic hosts coincided with their onset of arthritis (Fig. 2A,B). In contrast, the anti-GPI IgG1 autoantibody response observed in WT B6×B6.g7 hosts never appeared sufficient to elicit joint inflammation.
Figure 2.
KRN CD4+ T cells cause an increase in the amount of anti-GPI antibody and numbers of GPI specific B plasmablasts in hosts expressing the I-Ag7 allele. A, Mean anti-GPI IgG1 titers ±SEM were determined by ELISA for B6 (●), WT B6×B6.g7 (■), and TCRα−/− B6×B6.g7 (□) animals. B, Mean disease activity scores in the same experiment. C, Polyclonal spleen and lymph node B cells either from normal naïve mice (left), or from day 10 KRN T cell adoptive transfer recipient WT B6×B6.g7 (middle) and TCRα−/− B6×B6.g7 (right) mice, stained for intracellular immunoglobulin H+L chain accumulation and GPI-binding capacity. Plots and tracings are representative of multiple mice (> 6 animals per group).
Autoreactive, anti-GPI IgG1 Ab–producing B cells were investigated by flow cytometry using biotinylated GPI molecules and streptavidin-conjugated allophycocyanin (SA-APC) (Supplemental Fig. 2). A greatly expanded population of GPI-specific FSChi IgH+Lhi B220lo CD38− GL7− IgG1-switched plasmablasts was observed at day 10 in arthritic TCRα−/− B6×B6.g7 hosts (Fig. 2C, and data not shown). In contrast, only a modest number of GPI-specific plasmablasts appeared in WT B6×B6.g7 recipients. I-Ag7–expressing mice that received no KRN CD4+ T cells, or animals lacking the I-Ag7 allele, had no detectable anti-GPI Ab or GPI-specific plasmablasts, further demonstrating that the GPI-specific CD4+ T cells and GPI/I-Ag7 complexes were important to the breakdown of immunological tolerance to GPI and the induction of arthritis in this model system, as previously described (23).
KRN CD4+ T cells develop an anergic phenotype during self Ag recognition in the normal peripheral immune system
The failure of naïve KRN CD4+ T cells to completely break tolerance to GPI in the B cell compartment of WT mice led us to hypothesize that T cell clonal anergy develops before these T cells can expand to a effector population size that is sufficient to support autoreactive B cell growth and differentiation. It has previously been shown that naïve CD4+ T cell recognition of soluble peptide Ag in normal mice is suboptimal in the absence of infection or adjuvant, and is limited by the development of clonal anergy (26–28). On the other hand, anergy induction in lymphopenic hosts can be defective due to the absence of CD25+ Foxp3+ T regulatory cells (9, 29). Therefore, we investigated naïve CD45.1+ KRN CD4+ T cell clonal expansion and phenotypic change in WT and TCRα−/− B6×B6.g7 hosts. For these experiments, lymph node and spleen cells were isolated from CD45.2+ recipient mice, and then the donor-derived CD45.1+ KRN CD4+ T cells were enriched by positive selection and analyzed by multiparameter flow cytometry (Supplemental Fig. 3).
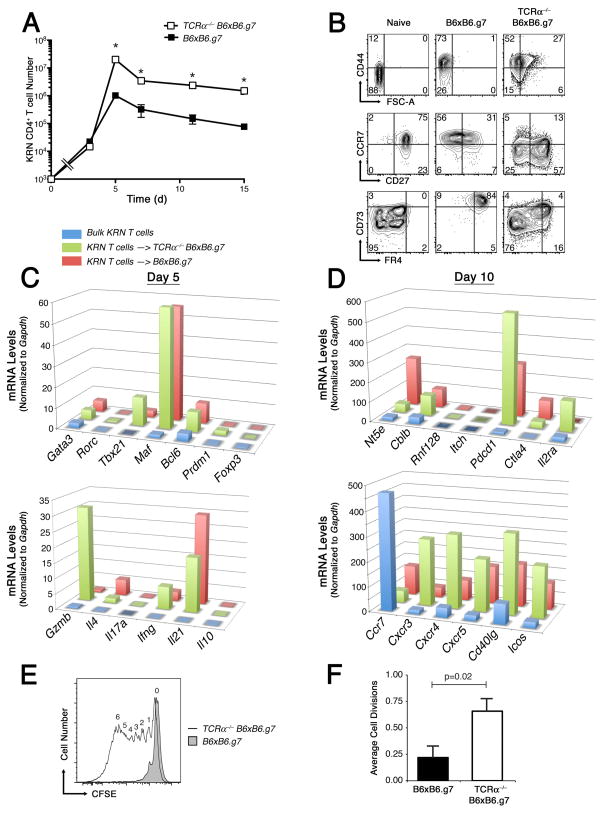
Naïve KRN CD4+ T cells were observed to proliferate to significantly higher numbers in TCRα−/− T cell lymphopenic hosts, and maintained this difference from WT recipients throughout a slow clonal contraction phase that occurred beyond day 5 (Fig. 3A). This enhanced KRN T cell clonal expansion in the lymphopenic hosts appeared to relate at least in part to an increased rate of cell cycle progression, based on the degree of CFSE dye dilution observed for KRN T cells on day 3 of the response (data not shown). By day 10, the expanded KRN CD4+ T cell populations demonstrated an antigen-experienced phenotype (CD44+) in both hosts (Fig. 3B). However, in the TCRα−/− B6×B6.g7 mice, KRN CD4+ T cells maintained higher forward scatter profile, suggesting that they were still responding to self pMHCII presentation with growth. Most GPI-reactive KRN CD4+ T cells in T cell lymphopenic hosts were also found to have dimmed their CCR7 expression, and some additionally lost CD27, consistent with intense and prolonged/repeated TCR stimulation and progressive effector cell differentiation (30). In contrast, KRN CD4+ T cells in WT hosts frequently dimmed their expression of CD27, yet maintained a high level CCR7 expression. These GPI-specific KRN CD4+ T cells in tolerant hosts were also observed to express high levels of the FR4 and CD73 surface markers, whereas KRN CD4+ T cells in T cell lymphopenic hosts expressed either an intermediate (int) or low level of both FR4 and CD73, often in a bimodal fashion.
Figure 3.
KRN CD4+ T cell number, phenotype, and function following adoptive transfer into WT or TCRα−/− B6×B6.g7 hosts. A, Mean ±SEM KRN CD4+ T cell number in combined spleen and lymph nodes from WT B6×B6.g7 (■) and TCRα−/− B6×B6.g7 (□) recipients. The open square at T = 0 represents historical post-adoptive transfer cell count; asterisks indicate significant differences between groups (P < 0.05). B, KRN CD4+ T cell FSc, CD44, CCR7, CD27, FR4 and CD73 expression at day 10. Plots are representative of greater than 6 animals per group. C,D, Purified KRN T cell mRNA expression levels on days 5 (C) and 10 (D) after adoptive transfer into either WT (red) or TCRα−/− (green) B6×B6.g7 hosts. Freshly isolated naïve KRN T cells are also shown as a control (blue). E, Purified KRN CD4+ T cells were exposed for 7 days to GPI/I-Ag7 in WT (shaded tracing) or TCRα−/− (open tracing) B6×B6.g7 hosts, recovered and labeled with CFSE, and then examined for CFSE dye–dilution 3 d after adoptive transfer into B6×B6.g7 secondary hosts. Digits above the tracing indicate the number of times a cell has divided. F, Average cell division rate ±SEM (n = 7) for groups as indicated. P values based on Student’s t test.
Coordinate expression of both FR4 and CD73 was previously shown to occur on CD4+ T regulatory cells (31, 32). However, KRN CD4+ T cells in WT tolerant hosts did not differentiate down a T regulatory cell pathway, as Foxp3, Il2ra, and Il10 mRNA expression levels remained very low in purified KRN T cells (Fig. 3C, D). Instead, a modest up-regulation of Gata3 and Tbx21 gene expression was observed in these T cells, with Gata3>Tbx21. Consistent with this, mRNA extracted from this population of KRN CD4+ T cells in healthy WT hosts also contained Il4 >Ifng mRNA. In contrast to these results, KRN CD4+ T effector cells in T cell-deficient hosts expressed Tbx21>Gata3 and Ifng>Il4 mRNA as well as high levels of Gzmb and Cxcr3. Thus, in addition to differences in clonal expansion, the KRN CD4+ T cells in normal WT hosts appeared to divert their differentiation to a distinct helper phenotype more reminiscent of Th2, whereas KRN T cells in TCRα−/− B6×B6.g7 mice destined to become arthritic expressed genes in a more Th1-like pattern. Although Th17 differentiation and IL-17a production have been implicated in the development of GPI-dependent autoimmune arthritis in mice (33), we were unable to detect significant levels of either Rorc or Il17a mRNA at any time point in our adoptive transfer experiments, and only infrequent IL-17a–producing KRN CD4+ T cells were observed by flow cytometry (Fig. 3C, and data not shown).
GPI autoantibody-dependent arthritis in mice has also been shown to depend on IL-4 production by CD4+ T helper cells (34); therefore, the expression of Gata3 and Il4 may have been essential to the differentiation of KRN CD4+ T effector cells capable of interacting with tolerant GPI-specific B cells and inducing the production of anti-GPI autoantibody. In both WT and TCRα−/− B6×B6.g7 hosts the KRN CD4+ T cells expressed similar amounts of Bcl6 and Maf mRNAs, two gene regulators known to be important to the differentiation of T follicular helper (Tfh) cells and the induction of Il21 gene transcription, as shown in figure 3C (35–37). Nevertheless, the KRN T cells in TCRα−/− B6×B6.g7 mice expressed more of the Cxcr4, Cxcr5, Cd40lg, and Icos mRNAs that are important to Tfh function (Fig. 3C,D).
Based on all of these results, we speculated that the proliferative arrest and subsequent failure of KRN CD4+ T cells to fully differentiate down Th1 or Tfh pathways in normal hosts was the result of clonal anergy induction during their encounter with self Ag. Perhaps consistent with this hypothesis, KRN T cells recovered from healthy WT mice expressed higher amounts of Ctla4 and Rnf128 (GRAIL) mRNA; nevertheless, two other genes thought to be important to the development or maintenance of clonal anergy (Cblb and Pdcd1) were actually expressed at higher levels in chronically activated KRN CD4+ T cells from arthritic TCRα−/− mice. To more formally test the question of whether KRN CD4+ T cells became anergic in normal hosts, T cells were recovered from either WT or TCRα−/− B6×B6.g7 mice, CFSE-labeled, and then examined for their capacity to divide following re-exposure to GPI/I-Ag7 complexes in WT B6×B6.g7 hosts. KRN T cells recovered from the TCRα−/− B6×B6.g7 hosts were found capable of undergoing multiple rounds of cell division in response to GPI/I-Ag7 complexes within the secondary host, whereas KRN T cells from tolerant WT hosts only rarely divided more than once, and this difference was significant (p = 0.02; Fig. 3E,F). Thus, naïve self Ag-specific T cells could be shown to develop a proliferative unresponsiveness to self pMHCII complexes in normal hosts, and this likely contributed to their premature termination of clonal expansion and effector cell differentiation. Furthermore, these data suggested that high-level FR4 and CD73 co-expression marked self Ag-specific CD4+ T cells that had lost proliferative responsiveness, whereas the FR4− CD73− phenotype predicted sustained responsiveness to self Ag.
Partial reconstitution of TCRα−/− B6×B6.g7 hosts with polyclonal CD4+ T cells promotes clonal anergy induction and protects against autoimmune arthritis
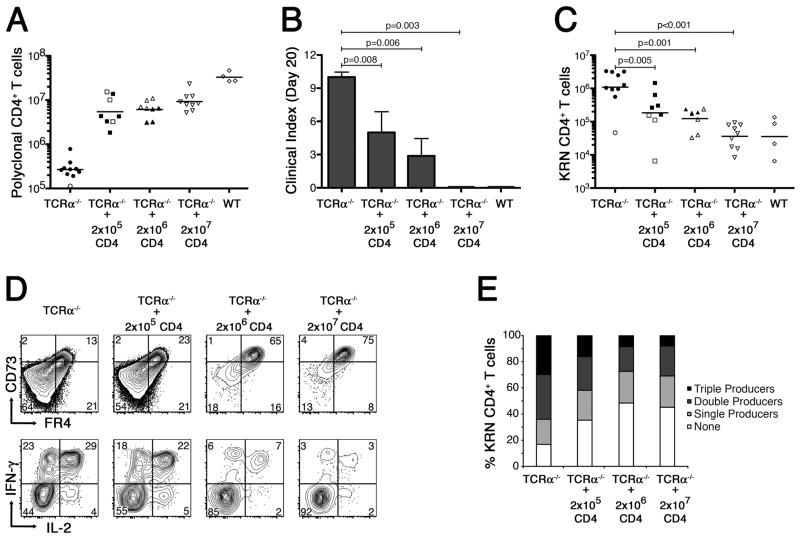
The partial reconstitution of lymphopenic mice with Ag-nonspecific CD4+ T cells was previously shown to inhibit Ag-dependent CD4+ T cell clonal expansion in the absence of adjuvant or infection, and to promote the development of clonal anergy (9). Therefore, we wished to test whether reconstitution of TCRα−/− B6×B6.g7 hosts with bulk polyclonal CD4+ T cells could prevent the exaggerated clonal expansion and differentiation of adoptively transferred naïve GPI-reactive CD4+ T cells, and protect the mice from autoimmune arthritis. TCRα−/− B6×B6.g7 hosts were initially given varying numbers of B6×B6.g7 CD4+ T cells, followed 10 d later by an adoptive transfer of 104 naïve KRN CD4+ T cells to elicit the onset of autoimmune arthritis. Lymphopenic hosts reconstituted with 2 × 107 CD4+ T cells were found to be fully protected from arthritis induction; however, transfer of 2 × 105 or 2 × 106 polyclonal CD4+ T cells proved insufficient to prevent the development of at least a modest degree of arthritis in some animals (Fig. 4A,B). Additional study of reconstituted hosts showed that the KRN CD4+ T cell clonal expansion was inversely correlated with the number of polyclonal CD4+ T cells transferred into these mice (Fig. 4C). FR4 and CD73 expression on the GPI-specific CD4+ T cells also increased in proportion to the number of polyclonal CD4+ T cells used in the reconstitution, suggesting that CD4+ T cells are sufficient to blunt the growth and differentiation of GPI-specific T cells and restore clonal anergy induction in TCRα−/− B6×B6.g7 mice (Fig. 4D). Consistent with this, KRN CD4+ T cells recovered from animals reconstituted at high CD4+ T cell number and then stimulated ex vivo with PMA plus ionomycin, less frequently produced IL-2, IFN-γ, and/or TNF-α (Fig. 4D, and data not shown). Using a Boolean gating strategy as previously described (38), we also found that the KRN CD4+ T cells from TCRα−/− B6×B6.g7 hosts had a large number of triple cytokine-producers, whereas KRN CD4+ T cells in partially reconstituted hosts had a larger percentage of cells that produced no cytokines (Fig. 4E). These experiments confirmed that the development of autoimmune arthritis can be associated with the differentiation of self Ag-specific CD4+ T cells into cytokine triple-producers. Furthermore, protection from arthritis could be achieved in T cell-deficient hosts through partial reconstitution of the CD4+ T cell polyclonal repertoire.
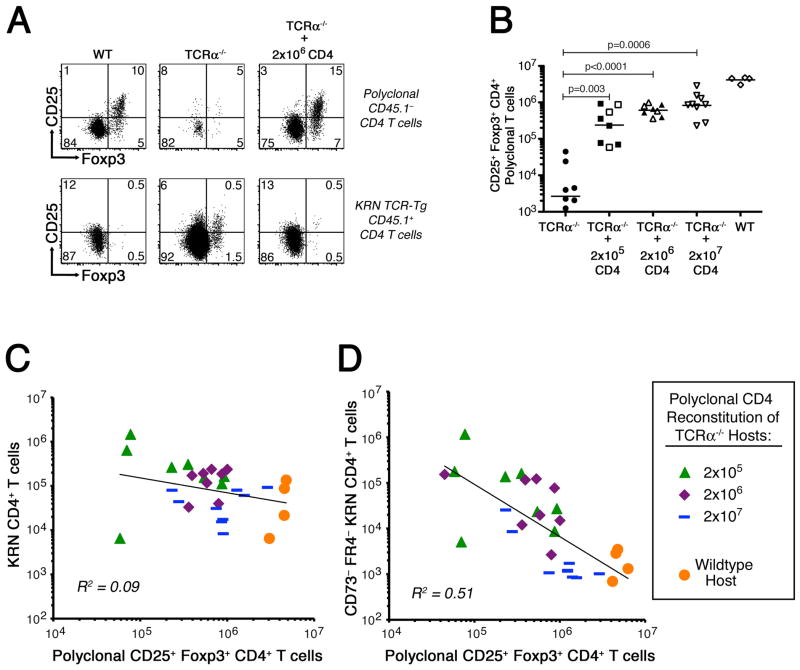
Figure 4.
Partial reconstitution of TCRα−/− B6×B6.g7 mice with polyclonal Foxp3+ CD4+ T cells prevents autoimmune arthritis by inducing clonal anergy in the GPI-reactive CD4+ T cells. A, Polyclonal CD4+ T cell counts in TCRα−/− B6×B6.g7 mice on day 20 after partial reconstitution, as indicated. Counts from tolerant WT B6.B6.g7 mice are also shown for comparison. B, Mean clinical score in each experimental group ±SEM. P values are based on the Mann-Whitney U test. C, Absolute number of KRN CD4+ T cells recovered from host animals following partial polyclonal CD4+ T cell reconstitution, as in panel A. D, In a separate experiment, FR4− and CD73− levels (top) were measured on KRN CD4+ T cells recovered from partially reconstituted TCRα−/− B6×B6.g7 mice (as indicated) 10 d after the KRN adoptive transfer. Flow cytometric measurement of IFNγ and IL-2 intracellular accumulation was also performed (bottom) on ex vivo PMA plus ionomycin stimulated KRN CD4+ T cells. E, Percentage of KRN CD4+ T cells recovered from various hosts (as in panel D) capable of synthesizing IL-2, IFNγ, and/or TNFα in various combinations. In panels A and C, filled symbols represent animals developing at least one arthritic joint; open symbols are animals that remained free of disease. Bars indicate the mean cell count, and P values are as indicated, using Student’s t test.
Reconstitution of a polyclonal CD25+ Foxp3+ CD4+ T regulatory cell repertoire is associated with decreased KRN CD4+ T cell differentiation to a FR4− CD73− phenotype
In a number of animal models, polyclonal CD25+ CD4+ T regulatory cells have been shown to support the induction and maintenance of peripheral self-tolerance and prevent the development of autoimmune disease (8, 19, 39, 40). Our own experiments have also previously demonstrated a role for CD25+ Foxp3+ CD4+ T regulatory cells in the induction of clonal anergy in vivo (9). Consequently, we thought that the CD25+ Foxp3+ CD4+ T regulatory cells within the reconstituting polyclonal CD4+ T cell population might be most important for limiting the expansion and differentiation of FR4− CD73− KRN effector CD4+ T cells, and for promoting anergy in the GPI-specific T cells.
The partial reconstitution of TCRα−/− B6×B6.g7 mice with an adoptive transfer of bulk polyclonal CD4+ T cells led to a restoration of the CD25+ Foxp3+ CD4+ T regulatory cell compartment and protection from arthritis (Fig. 4B and 5A,B). This reconstitution was not accompanied by any preferential differentiation of the KRN CD4+ T cells to a Foxp3+ T regulatory cell phenotype, despite their high level FR4 and CD73 expression (Fig. 4D and 5A). Interestingly, the total number of KRN CD4+ T cells recovered from normal and reconstituted mice showed no correlation with the number of polyclonal CD25+ Foxp3+ CD4+ T regulatory cells present (Fig. 5C); however, the number of non-tolerant/effector phenotype FR4− CD73− KRN CD4+ T cells was observed to correlate inversely with the number of CD25+ Foxp3+ CD4+ T regulatory cells generated (Fig. 5D). Thus, polyclonal CD4+ T cells in these reconstituted mice did not induce the KRN naïve CD4+ T cells to take on a CD25+ Foxp3+ T regulatory phenotype. Rather, CD25+ Foxp3+ CD4+ T regulatory cells within the polyclonal CD4+ T cell compartment appeared to suppress the expansion and differentiation of GPI-specific FR4− CD73− CD4+ T effector cells that cause arthritis.
Figure 5.
Polyclonal CD25+ Foxp3+ CD4+ T regulatory cells inhibit the accumulation of FR4− CD73− GPI-specific CD4+ T effector cells. A, Identification of endogenous (top) and KRN (bottom) CD25+ Foxp3+ CD4+ T regulatory cells following partial polyclonal CD4+ T cell reconstitution of TCRα−/− B6×B6.g7 mice. B, Absolute number of endogenous Foxp3+ CD4+ T regulatory cells identified in partially reconstituted hosts. C,D, Correlation between endogenous Foxp3+ T regulatory cells and either total KRN CD4+ T cell number (C) or FR4− CD73− KRN CD4+ T cell number (D). In panel B, filled symbols represent animals developing at least one arthritic joint; open symbols are animals that remained free of disease. Bars indicate the mean cell count, and P values are as indicated, using Student’s t test.
Purified CD25+ Foxp3+ CD4+ T regulatory cells protect T cell lymphopenic animals from severe autoimmune arthritis
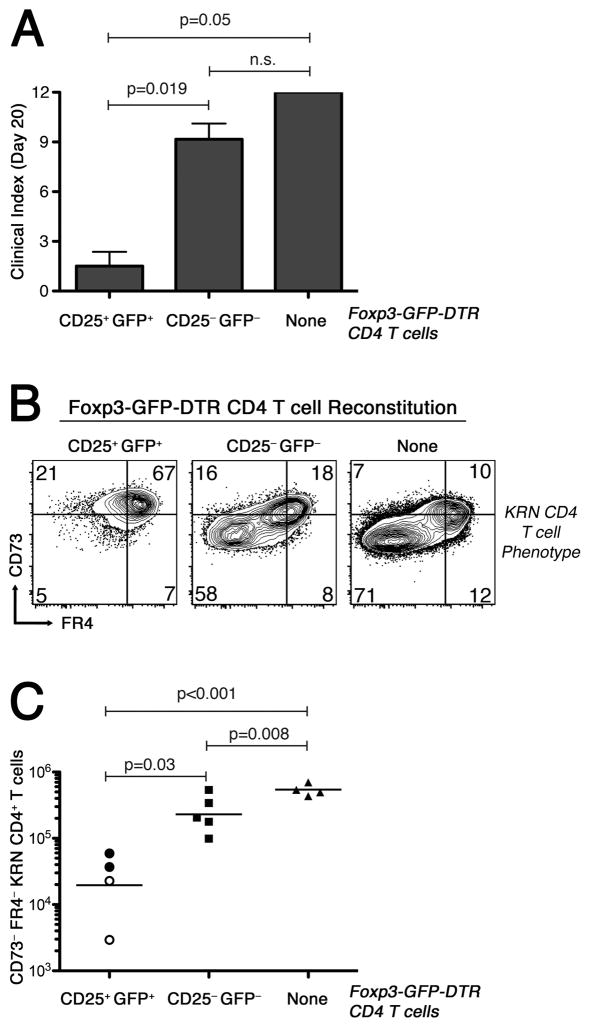
To explore the hypothesis that CD25+ Foxp3+ CD4+ T regulatory cells control the number, phenotype, and function of KRN CD4+ T cells in WT B6×B6.g7 mice, we made use of B6×B6.g7-strain Foxp3DTR mice whose Foxp3+ T cells all express GFP together with a diphtheria toxin receptor (DTR). Foxp3DTR mice were used to sort CD25+ GFP+ and CD25− GFP− polyclonal CD4+ T cells, and then the sorted populations (106 cells) were transferred into TCRα−/− B6×B6.g7 animals (19). We observed that mice reconstituted with purified CD25+ GFP+ CD4+ T cells had significantly reduced clinical scores as well as preferential differentiation of the KRN CD4+ T cells to a FR4hi CD73hi anergic phenotype (Fig. 6A,B). Furthermore, the absolute number of FR4− CD73− effectors among the GPI-reactive CD4+ T cells was significantly reduced (Fig. 6C). In contrast, hosts that received CD25− GFP− polyclonal CD4+ T cells behaved much like the T cell lymphopenic controls.
Figure 6.
Lymphopenic hosts reconstituted with CD25+ FoxP3+ CD4+ T cells are protected from autoimmune arthritis. On day 0, lymphopenic TCRα−/− B6×B6.g7 animals were given 106 purified CD25+ Foxp3+ or CD25− Foxp3− CD4+ polyclonal T cells from syngeneic Foxp3DTR (Foxp3-GFP-DTR) B6×B6.g7 donors, or were given no cells, as indicated in the panels. On day 10, all mice received 104 KRN CD4+ T cells. Arthritis severity measurements and T cell analysis were performed on day 20. A, Average arthritis clinical score ±SEM. P values based on the Mann-Whitney U test. B, KRN CD4+ T cell expression of FR4 and CD73 in representative animals. C, KRN FR4− CD73− CD4+ T cell absolute number. Bars represent the mean cell number, with filled symbols indicating arthritic animals, and open symbols indicating animals free of disease. P values using Student’s t test.
Polyclonal CD25+ Foxp3+ CD4+ T regulatory cells are necessary to promote the induction of clonal anergy, to achieve protection against the development of autoimmune arthritis
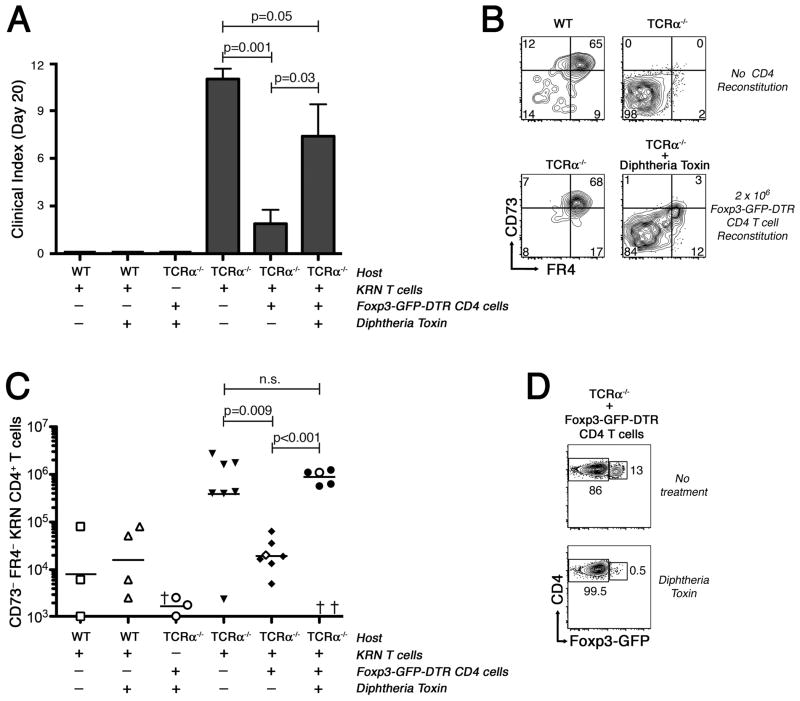
In a final series of experiments, we sought to formally test the hypothesis that polyclonal CD25+ Foxp3+ CD4+ T regulatory cells are necessary to allow for the induction of clonal anergy in self Ag-specific naïve CD4+ T cells, to prevent the accumulation of FR4− CD73− autoreactive effector T cells, and to protect against arthritis development. Use of B6×B6.g7-strain Foxp3DTR mice allows for the specific ablation of Foxp3+ T regulatory cells from within the polyclonal CD4+ T cell compartment in vivo using Diphtheria toxin (DT). To carry out these experiments, TCRα−/− B6×B6.g7 hosts were reconstituted with 106 bulk CD4+ T cells from B6×B6.g7 Foxp3DTR donor mice, with DT administered on days 8, 10, and 12 to deplete Foxp3+ CD4+ T regulatory cells from the reconstituting population. To evaluate the effect on arthritis, naïve KRN CD4+ T cells were adoptively transferred into the mice at day 10, and then mice were monitored throughout the remainder of the experiment for evidence of arthritis, with the final analysis of KRN CD4+ T cells performed at day 20.
Remarkably, nearly all TCRα−/− B6×B6.g7 mice reconstituted with polyclonal Foxp3DTR CD4+ T cells, and subsequently treated with DT to eliminate Foxp3–expressing CD4+ T regulatory cells, developed an autoimmune arthritis with a severity similar to that seen in the non-reconstituted TCRα−/− B6×B6.g7 hosts (Fig. 7A). KRN CD4+ T cells in these T regulatory cell-depleted hosts also failed to develop the anergic FR4hi CD73hi phenotype (Fig. 7B). Rather, DT-treated mice accumulated to high number the highly differentiated FR4− and CD73− subpopulation of KRN CD4+ T cells shown to be associated with autoimmune arthritis development (Fig. 7C). As expected, CD4+ T cell-reconstituted TCRα−/− B6×B6.g7 mice not given DT suppressed the clonal expansion of FR4− CD73− CD4+ KRN T cells and instead drove these GPI-specific T cells into an anergic (FR4hi CD73hi) state, with the eventual result being development of only the mildest signs of arthritis (Fig. 7A,B).
Figure 7.
Ablation of polyclonal CD4+ CD25+ Foxp3+ regulatory T cells prevents clonal anergy development and leads to autoimmune arthritis. Hosts were either WT B6×B6.g7 or lymphopenic TCRα−/− B6×B6.g7 as indicated in the panels. On day 0, some of the lymphopenic animals received 106 polyclonal CD4+ T cells from syngeneic Foxp3DTR B6×B6.g7 donors. Diphtheria toxin (DT) was administered to some mice on days 8, 10, and 12. On day 10, 104 KRN CD45.1+ CD4+ T cells were adoptively transferred into most mice. Arthritis severity measurements and T cell analysis were performed on day 20. A, Average arthritis clinical score ±SEM in experimental groups as indicated. Significance determined by Mann-Whitney U test. B, Representative KRN CD4+ T cell FR4 and CD73 expression. C, KRN FR4− CD73−CD4+ T cell absolute numbers. Bars represent the mean cell number, with filled symbols indicating arthritic animals, and open symbols indicating animals free of disease. P values using Student’s t test. D, Foxp3+ GFP+ CD4+ T cells in Foxp3DTR-reconstituted TCRα−/−B6×B6.g7 mice, either with or without DT treatment.
Flow cytometry confirmed the efficacy of the Foxp3+ CD4+ T cell depletion (Fig. 7D). Note that we did observe some deaths (n = 3) in Foxp3DTR CD4+ T cell–reconstituted and DT-treated hosts, presumably as consequence of unregulated autoimmunity from within the remaining polyclonal CD4+ T cell repertoire (19). However, the worsening of arthritis in reconstituted and DT-treated mice cannot be simply explained by such a non-specific loss of immunological tolerance, since reconstituted and DT-treated hosts that were not given KRN CD4+ T cells failed to show signs of arthritis. DT itself also had no capacity to directly enhance the responsiveness of the KRN CD4+ T cells to GPI, as exacerbation of arthritis with DT required the presence of the polyclonal Foxp3DTR CD4+ T cells, and did not occur in the normal B6×B6.g7 hosts. Thus, the arthritis–inducing effects of DT in these experiments were tightly coupled to the ablation of polyclonal CD25+ Foxp3+ CD4+ T regulatory cells, the resultant inhibition of clonal anergy induction in the naïve KRN CD4+ T cells, and the pathological outgrowth of the FR4− CD73− subset of antigen-experienced, GPI-specific CD4+ T cells that cause autoimmune arthritis.
Discussion
These data provide important evidence that the clonal anergy peripheral self tolerance mechanism can serve as the basis for protection against the development of severe autoimmune arthritis. We note that Singh et al. (41) previously showed a role for in vivo anergy (their ‘adaptive tolerance’) to modulate the induction of chronic arthritis by CD4+ T cells responding to a transgenic self Ag: membrane–targeted pigeon cytochrome C (PCC). Similar to our model system, their adoptive transfer of PCC-specific 5C.C7 CD4+ T cells into lymphopenic CD3ε−/− PCC transgenic mice led to a deregulated clonal expansion response, the production of multiple autoantibodies (e.g., anti-dsDNA, -histone, and -GPI), and the development of a destructive chronic polyarthropathy, whereas adoptive transfer into normal transgenic hosts led to only an abortive clonal expansion response and no disease. In contrast to our KRN CD4+ T cells responding to GPI/I-Ag7 complexes in lymphopenic TCRα−/− mice (as reported here), their 5C.C7 T cells became desensitized to self Ag in both the lymphopenic and T cell replete hosts. Nevertheless, the peak 5C.C7 CD4+ T cell number was observed to be reduced by more than 10-fold in the normal transgenic recipients because of a slowed rate of cell cycle progression and an apparent decrease in survival time. Therefore, their studies as well as those of others suggest that in vivo anergy can be an effective barrier to autoimmune disease only if self-reactive CD4+ T cell numbers are held below a threshold frequency (28, 42). Likewise, it is now clear that in the absence of a normal peripheral T cell compartment, self Ag-reactive CD4+ T cell numbers can rise above this threshold despite any adaptation to constant TCR signaling and can retain the capacity to cause immunopathology (41, 43).
In lymphopenic hosts, GPI-specific CD4+ T cells expanded and differentiated into two separate Ag-experienced subpopulations in association with arthritis development—one FR4int CD73int and one FR4− CD73−. Our preliminary experiments suggest that the FR4− CD73− subpopulation in lymphopenic mice is a Tbx21 (T-bet)–expressing T effector cell population with a high capacity to proliferate and produce IL-2, TNF-α, and IFN-γ upon re-stimulation. The FR4int CD73int population appears similar to CXCR5–expressing T follicular helper cells, has lost much of its proliferative capacity, and does not demonstrate the same commitment to an inflammatory cytokine production (R.J.M., unpublished observation). IFN-γ has been shown unnecessary for the development of spontaneous arthritis in K/BxN mice; therefore, it seems unlikely that the inability of anergic KRN CD4+ T cells to produce IFN-γ is important to the avoidance of arthritis in our system (33). Nevertheless, loss of some other Tbx21-dependent T effector cell activity may contribute to protection from arthritis in the normal hosts. Of note, IL-4 production by GPI-reactive T cells has previously been implicated in the breakdown of B cell tolerance and production of anti-GPI IgG1 autoantibody in K/BxN mice (34). We, too, have observed an induction of Il4 mRNA synthesis in our system, even in anergic KRN CD4+ T cells responding to GPI/I-Ag7 complexes in normal mice. KRN CD4+ T cell production of IL-4 may indeed be important to the activation of previously tolerant GPI-specific B cells in both normal and lymphopenic hosts, but it clearly is not sufficient for arthritis development.
In our experimental system, a relatively modest yet significant amount of anti-GPI IgG1 autoantibody could be found even in the healthy, tolerant mice that had been given 104 naïve GPI-specific CD4+ T cells. Nevertheless, these animals demonstrated no signs of arthritis or weight loss. Perhaps the numbers of GPI-reactive T cells rose sufficiently quickly to allow for at least some collaborative interaction with the GPI-specific B cells that promotes their growth and differentiation prior to T cell anergy induction. Alternatively, anergic KRN CD4+ T cells that survive in normal hosts may retain the capacity to promote anti-GPI B cell responses, but their low frequency does not threaten the continued maintenance of self tolerance and avoidance of immunopathology. For either case, we would predict that CD4+ T cell expression of Gata3, Maf, Bcl6, Il4, Il21, Cxcr5, Cd40lg, and Icos is important to this B cell helper activity. Our previous investigation of chicken ovalbumin (OVA)-dependent immunity indicated that OVA-specific CD4+ T cells induced into clonal anergy in vivo retain the capacity to stimulate anti-OVA IgG2a class switching and antibody production, despite their continued inability to produce IL-2 (28). Unlike the results shown here for KRN CD4+ T cells induced into anergy in normal B6×B6.g7 recipients, anergic OVA-specific Th1-like T cells never effectively reduced their IFN-γ production and also remained capable of responding in a delayed-type hypersensitivity assay. Nonetheless, the continuous recognition of GPI/I-Ag7 complexes in normal hosts (and in the absence of infection or adjuvant) may not be sufficient to induce the differentiation of KRN CD4+ T cells to an IFN-γ–producing effector cell phenotype, even though it was sufficient to induce the expression of nuclear factors thought important to differentiation down Th2 and Tfh pathways. Perhaps the low frequency anergic GPI-specific CD4+ T cell population that remains in normal mice retains some capacity to stimulate anti-GPI B cells to undergo clonal expansion and IgG1 class switching; however, arthritis development requires additional T effector cell differentiation events that either fail to occur or are disrupted by the development of clonal anergy.
We now show that KRN CD4+ T cell proliferative responsiveness to continuous GPI/I-Ag7 complex presentation eventually wanes in association with an up-regulation of FR4 and CD73. Re-challenge experiments confirmed that these T cells underwent a proliferative arrest and lost (or failed to gain) much of their capacity to produce IL-2, TNF-α, and IFN-γ. Therefore, high-level co-expression of FR4 and CD73 (in the absence of Foxp3) appears to mark autoreactive CD4+ T cells that have entered an anergic state. FR4 and CD73 have previously been shown to be highly expressed on Foxp3+ CD4+ T regulatory cells, and we also confirmed this in our own study (data not shown) (31, 32, 44). CD73 is an ecto-5′-nucleotidase (encoded by Nt5e) responsible for extracellular conversion of 5′-AMP to adenosine. CD73 is thought to catalyze adenosine receptor- and cAMP-mediated anti-proliferative effects at sites of intense cell injury or ischemia, where adenosine nucleotide release into the extracellular space is prevalent (44, 45). Interestingly, CD73 ecto-5′-nucleotidase enzyme activity mediates at least part of the anti-arthritic effects of methotrexate and sulfasalazine (46–48).
The pharmacologic effects of methotrexate also relate to folate metabolism. Through its inhibition of dihydrofolate reductase, methotrexate blocks the regeneration of tetrahydrofolate cofactors responsible for the synthesis of DNA and other purines important to T cell proliferation and survival (48, 49). FR4 mediates the transport of folic acid into T cells, and it has been suggested that metabolically active T regulatory cells are highly dependent on this vitamin to maintain cellular health (32). Although experiments shown here do not test the role of either molecule in the development or maintenance of CD4+ T cell anergy, the up-regulation of both FR4 and CD73 reinforces the notion that the continued survival of anergic CD4+ T cells in a non-proliferative state is actively reinforced. Whether FR4 and CD73 expression also provide these anergic GPI-reactive Foxp3− CD4+ T cells with some suppressive or regulatory capacity in vivo remains unknown.
The mechanisms by which polyclonal Foxp3+ CD25+ CD4+ T regulatory cells might control the clonal expansion of GPI/MHCII-specific CD4+ T cells and promote their acquisition of a FR4hi CD73hi anergic phenotype, remain unclear. It is formally possible that GPI/MHCII-induced T effector cell differentiation and/or survival are inhibited by T regulatory cells, and only the least responsive (FR4hi CD73hi) KRN T cells are allowed to persist in the normal hosts. This seems unlikely, since we also observed that by day 3 after naïve KRN CD4+ T cell adoptive transfer, clonal expansion and survival were similar in the WT and TCRα−/− B6×B6.g7 hosts, yet about 50% of the GPI/MHCII-specific T cells in the WT mice (versus ~5% in the lymphopenic recipients) had already developed a FR4hi CD73hi anergic phenotype (data not shown). Therefore, we favor the model that Foxp3+ T regulatory cells drive self-reactive CD4+ T cells into an anergic state that eventually aborts their clonal expansion. Consistent with this notion, CD25+ CD4+ T regulatory cells have been shown to induce anergy in CD25− CD4+ T cells undergoing 4–5 days of in vitro stimulation with CD3 mAb and APC (50). Interestingly, IL-10 and TGF-β secretion by these CD25+ T regulatory cells was unnecessary for in vitro anergy induction. Nevertheless, a number of other molecules expressed by Foxp3+ T regulatory cells (e.g., CTLA4, CD73, CD39, LAG3, CD25) have been implicated in the control of CD4+ T cell proliferation and resistance to autoimmune disease, and may have acted here to promote in vivo clonal anergy either through direct effects on the KRN T cells or via counter-regulation of APC costimulatory molecule expression (51).
What might be the relevance of clonal anergy induction to patients with RA? The development of RA has previously been shown to be associated with a defect in peripheral T cell homeostasis (2). The exaggerated T cell homeostatic proliferative rate observed in RA patients is reminiscent of NOD mice, where the development of insulitis and pancreatic islet cell destruction is associated with mild T cell lymphopenia, enhanced T cell homeostatic proliferation, and increased T cell turnover rate (7). Young K/BxN mice also demonstrate reduced peripheral CD4+ T cell numbers prior to their onset of disease, and an infusion of syngeneic polyclonal CD4+ T cells reduces their drive for homeostatic T cell proliferation and protects them from arthritis (52). Our data indicate that polyclonal CD25+ Foxp3+ CD4+ T regulatory cells protect lymphopenic individuals from autoimmune arthritis by inducing clonal anergy in their GPI-specific CD4+ T cells. Consistent with our results, the elimination of all Foxp3-dependent T regulatory cell function in K/BxN mice also leads to significantly increased numbers of GPI-reactive CD4+ T cells and GPI-specific antibody forming cells, as well as the accelerated production of anti-GPI IgG1 autoantibody (4, 14). Thus, lymphopenia together with its attendant reduction in the number of CD25+ Foxp3+ CD4+ T regulatory cells is itself a risk factor for deregulated T helper cell proliferation and differentiation, as well as resistance to T cell clonal anergy and apoptosis (53).
Patients with chronic inflammatory arthritis have relatively normal percentages of CD25+ CD4+ T cells in the peripheral blood and, in fact, have increased percentages within their affected synovial joints (11, 54, 55). Although such CD25+ CD4+ T cells can effectively suppress in vitro T cell proliferation assays, there is now considerable evidence to suggest that they have reduced function, particularly with regard to their capacity to inhibit CD4+ T helper cell differentiation and effector cytokine generation (10, 54). TNF-α accumulation within the inflamed RA joint appears, in part, to be responsible for reduced CD25+ CD4+ T regulatory cell Foxp3 expression and IL-10 production in patients (10, 56). In addition, cytokines produced by activated CD25−CD4+ T cells and/or synoviocytes present within the affected joint (e.g., IL-7, IL-15, TNF-α) may make CD25− CD4+ responder T cells resistant to the suppressive effects of CD25+ CD4+ T regulatory cells (54). Therefore, it is conceivable that a primary defect in CD25+ Foxp3+ CD4+ T regulatory cell function underlies the loss of self tolerance that causes RA. Alternatively, decreased T regulatory cell function may be a consequence of poorly controlled systemic inflammation at the onset of disease triggering, thus leading to a failure of CD4+ T cell anergy induction. Regardless, therapeutic strategies designed to restore the full functional capacity of CD25+ Foxp3+ CD4+ T regulatory cells in RA patients have the potential to reinstitute immunological tolerance to self Ag within the pathogenic CD4+ T cell repertoire through the induction of clonal anergy.
Supplementary Material
Acknowledgments
We wish to thank Jennifer Hebert for her assistance in the initial analysis of GPI antibody production. We are also grateful to Matthew Mescher and Kristin Hogquist for their insightful comments regarding our manuscript. Finally, we appreciate our ongoing discussions with Marion Pepper regarding the nature of T helper cell function in the setting of chronic antigen stimulation.
Footnotes
Grant Support: This study was supported by grants from the Lupus Foundation of Minnesota, the American College of Rheumatology-REF, and the NIH (P01 AI35296, R01 AI80764).
Abbreviations: DT, diphtheria toxin; DTR, diphtheria toxin receptor; FR4, Folate receptor 4; GPI, glucose-6-phosphate isomerase; Int, intermediate; PCC, pigeon cytochrome c; qPCR, real-time quantitative PCR; RA, rheumatoid arthritis; TCR-Tg, TCR-transgenic; WT, wildtype.
References
- 1.Cope AP. T cells in rheumatoid arthritis. Arthritis Res Ther. 2008;10(Suppl 1):S1. doi: 10.1186/ar2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koetz K, Bryl E, Spickschen K, O’Fallon WM, Goronzy JJ, Weyand CM. T cell homeostasis in patients with rheumatoid arthritis. Proc Natl Acad Sci USA. 2000;97:9203–9208. doi: 10.1073/pnas.97.16.9203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arkwright PD, Abinun M, Cant AJ. Autoimmunity in human primary immunodeficiency diseases. Blood. 2002;99:2694–2702. doi: 10.1182/blood.v99.8.2694. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen LT, Jacobs J, Mathis D, Benoist C. Where FoxP3-dependent regulatory T cells impinge on the development of inflammatory arthritis. Arthritis Rheum. 2007;56:509–520. doi: 10.1002/art.22272. [DOI] [PubMed] [Google Scholar]
- 5.Kang SM, Jang E, Paik DJ, Jang YJ, Youn J. CD4(+)CD25(+) regulatory T cells selectively diminish systemic autoreactivity in arthritic K/BxN mice. Mol Cells. 2008;25:64–69. [PubMed] [Google Scholar]
- 6.Monte K, Wilson C, Shih FF. Increased number and function of Foxp3 regulatory T cells during experimental arthritis. Arthritis Rheum. 2008;58:3730–3741. doi: 10.1002/art.24048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King C, Ilic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117:265–277. doi: 10.1016/s0092-8674(04)00335-6. [DOI] [PubMed] [Google Scholar]
- 8.Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int Immunol. 1993;5:1461–1471. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- 9.Vanasek TL, Nandiwada SL, Jenkins MK, Mueller DL. CD25+Foxp3+ regulatory T cells facilitate CD4+ T cell clonal anergy induction during the recovery from lymphopenia. J Immunol. 2006;176:5880–5889. doi: 10.4049/jimmunol.176.10.5880. [DOI] [PubMed] [Google Scholar]
- 10.Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, Mauri C. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004;200:277–285. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Amelsfort JM, Jacobs KM, Bijlsma JW, Lafeber FP, Taams LS. CD4(+)CD25(+) regulatory T cells in rheumatoid arthritis: differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum. 2004;50:2775–2785. doi: 10.1002/art.20499. [DOI] [PubMed] [Google Scholar]
- 12.van Amelsfort JM, van Roon JA, Noordegraaf M, Jacobs KM, Bijlsma JW, Lafeber FP, Taams LS. Proinflammatory mediator-induced reversal of CD4+, CD25+ regulatory T cell-mediated suppression in rheumatoid arthritis. Arthritis Rheum. 2007;56:732–742. doi: 10.1002/art.22414. [DOI] [PubMed] [Google Scholar]
- 13.Oh S, Rankin AL, Caton AJ. CD4+CD25+regulatory T cells in autoimmune arthritis. Immunol Rev. 2009;233:97–111. doi: 10.1111/j.0105-2896.2009.00848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jang E, Cho WS, Cho ML, Park HJ, Oh HJ, Kang SM, Paik DJ, Youn J. Foxp3+ regulatory T cells control humoral autoimmunity by suppressing the development of long-lived plasma cells. J Immunol. 2011;186:1546–1553. doi: 10.4049/jimmunol.1002942. [DOI] [PubMed] [Google Scholar]
- 15.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 16.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, Bricarelli FD, Byrne G, McEuen M, Proll S, Appleby M, Brunkow ME. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 17.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 18.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 19.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 20.Kouskoff V, Korganow AS, Duchatelle V, Degott C, Benoist C, Mathis D. Organ-specific disease provoked by systemic autoimmunity. Cell. 1996;87:811–822. doi: 10.1016/s0092-8674(00)81989-3. [DOI] [PubMed] [Google Scholar]
- 21.Yarke CA, Dalheimer SL, Zhang N, Catron DM, Jenkins MK, Mueller DL. Proliferating CD4+ T Cells undergo immediate growth arrest upon cessation of TCR signaling in vivo. J Immunol. 2008;180:156–162. doi: 10.4049/jimmunol.180.1.156. [DOI] [PubMed] [Google Scholar]
- 22.Binstadt BA, Patel PR, Alencar H, Nigrovic PA, Lee DM, Mahmood U, Weissleder R, Mathis D, Benoist C. Particularities of the vasculature can promote the organ specificity of autoimmune attack. Nat Immunol. 2006;7:284–292. doi: 10.1038/ni1306. [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto I, Staub A, Benoist C, Mathis D. Arthritis provoked by linked T and B cell recognition of a glycolytic enzyme. Science. 1999;286:1732–1735. doi: 10.1126/science.286.5445.1732. [DOI] [PubMed] [Google Scholar]
- 24.Basu D, Horvath S, Matsumoto I, Fremont DH, Allen PM. Molecular basis for recognition of an arthritic peptide and a foreign epitope on distinct MHC molecules by a single TCR. J Immunol. 2000;164:5788–5796. doi: 10.4049/jimmunol.164.11.5788. [DOI] [PubMed] [Google Scholar]
- 25.Korganow AS, Ji H, Mangialaio S, Duchatelle V, Pelanda R, Martin T, Degott C, Kikutani H, Rajewsky K, Pasquali JL, Benoist C, Mathis D. From systemic T cell self-reactivity to organ-specific autoimmune disease via immunoglobulins. Immunity. 1999;10:451–461. doi: 10.1016/s1074-7613(00)80045-x. [DOI] [PubMed] [Google Scholar]
- 26.Kearney ER, Pape KA, Loh DY, Jenkins MK. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity. 1994;1:327–339. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 27.Perez VL, Van Parijs L, Biuckians A, Zheng XX, Strom TB, Abbas AK. Induction of peripheral T cell tolerance in vivo requires CTLA-4 engagement. Immunity. 1997;6:411–417. doi: 10.1016/s1074-7613(00)80284-8. [DOI] [PubMed] [Google Scholar]
- 28.Malvey EN, Jenkins MK, Mueller DL. Peripheral immune tolerance blocks clonal expansion, but fails to prevent the differentiation of Th1 cells. J Immunol. 1998;161:2168–2177. [PubMed] [Google Scholar]
- 29.Wu Z, Bensinger SJ, Zhang J, Chen C, Yuan X, Huang X, Markmann JF, Kassaee A, Rosengard BR, Hancock WW, Sayegh MH, Turka LA. Homeostatic proliferation is a barrier to transplantation tolerance. Nat Med. 2004;10:87–92. doi: 10.1038/nm965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pepper M, Linehan JL, Pagan AJ, Zell T, Dileepan T, Cleary PP, Jenkins MK. Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nat Immunol. 2010;11:83–89. doi: 10.1038/ni.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kobie JJ, Shah PR, Yang L, Rebhahn JA, Fowell DJ, Mosmann TR. T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5′-adenosine monophosphate to adenosine. J Immunol. 2006;177:6780–6786. doi: 10.4049/jimmunol.177.10.6780. [DOI] [PubMed] [Google Scholar]
- 32.Yamaguchi T, Hirota K, Nagahama K, Ohkawa K, Takahashi T, Nomura T, Sakaguchi S. Control of immune responses by antigen-specific regulatory T Cells expressing the folate receptor. Immunity. 2007;27:145–159. doi: 10.1016/j.immuni.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 33.Wu HJ, I, Ivanov I, Darce J, Hattori K, Shima T, Umesaki Y, Littman DR, Benoist C, Mathis D. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohmura K, Nguyen LT, Locksley RM, Mathis D, Benoist C. Interleukin-4 can be a key positive regulator of inflammatory arthritis. Arthritis Rheum. 2005;52:1866–1875. doi: 10.1002/art.21104. [DOI] [PubMed] [Google Scholar]
- 35.Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL, Srivastava M, Linterman M, Zheng L, Simpson N, Ellyard JI, Parish IA, Ma CS, Li QJ, Parish CR, Mackay CR, Vinuesa CG. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31:457–468. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho IC, Sharpe AH, Kuchroo VK. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat Immunol. 2009;10:167–175. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, Verma NK, Smyth MJ, Rigby RJ, Vinuesa CG. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med. 2010;207:353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Darrah PA, Patel DT, De Luca PM, Lindsay RWB, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, Roederer M, Seder RA. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:8. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 39.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 40.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor Foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 41.Singh NJ, Chen C, Schwartz RH. The impact of T cell intrinsic antigen adaptation on peripheral immune tolerance. PLoS Biol. 2006;4:e340. doi: 10.1371/journal.pbio.0040340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adler AJ, Marsh DW, Yochum GS, Guzzo JL, Nigam A, Nelson WG, Pardoll DM. CD4+ T cell tolerance to parenchymal self-antigens requires presentation by bone marrow-derived antigen-presenting cells. J Exp Med. 1998;187:1555–1564. doi: 10.1084/jem.187.10.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knoechel B, Lohr J, Kahn E, Abbas AK. Cutting Edge: The link between lymphocyte deficiency and autoimmunity: roles of endogenous T and B lymphocytes in tolerance. J Immunol. 2005;175:21–26. doi: 10.4049/jimmunol.175.1.21. [DOI] [PubMed] [Google Scholar]
- 44.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zarek PE, Huang CT, Lutz ER, Kowalski J, Horton MR, Linden J, Drake CG, Powell JD. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood. 2008;111:251–259. doi: 10.1182/blood-2007-03-081646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morabito L, Montesinos MC, Schreibman DM, Balter L, Thompson LF, Resta R, Carlin G, Huie MA, Cronstein BN. Methotrexate and sulfasalazine promote adenosine release by a mechanism that requires ecto-5′-nucleotidase-mediated conversion of adenine nucleotides. J Clin Invest. 1998;101:295–300. doi: 10.1172/JCI1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Montesinos MC, Takedachi M, Thompson LF, Wilder TF, Fernandez P, Cronstein BN. The antiinflammatory mechanism of methotrexate depends on extracellular conversion of adenine nucleotides to adenosine by ecto-5′-nucleotidase: findings in a study of ecto-5′-nucleotidase gene-deficient mice. Arthritis Rheum. 2007;56:1440–1445. doi: 10.1002/art.22643. [DOI] [PubMed] [Google Scholar]
- 48.Wessels JA, Huizinga TW, Guchelaar HJ. Recent insights in the pharmacological actions of methotrexate in the treatment of rheumatoid arthritis. Rheumatology. 2008;47:249–255. doi: 10.1093/rheumatology/kem279. [DOI] [PubMed] [Google Scholar]
- 49.Zhao R, I, Goldman D. Resistance to antifolates. Oncogene. 2003;22:7431–7457. doi: 10.1038/sj.onc.1206946. [DOI] [PubMed] [Google Scholar]
- 50.Qiao M, Thornton AM, Shevach EM. CD4+ CD25+ [corrected] regulatory T cells render naive CD4+ CD25− T cells anergic and suppressive. Immunology. 2007;120:447–455. doi: 10.1111/j.1365-2567.2007.02544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 52.Jang E, Kim HR, Cho SH, Paik DJ, Kim JM, Lee SK, Youn J. Prevention of spontaneous arthritis by inhibiting homeostatic expansion of autoreactive CD4+ T cells in the K/BxN mouse model. Arthritis Rheum. 2006;54:492–498. doi: 10.1002/art.21567. [DOI] [PubMed] [Google Scholar]
- 53.Shen SQ, Ding Y, Tadokoro CE, Olivares-Villagomez D, Camps-Ramirez M, de Lafaille MAC, Lafaille JJ. Control of homeostatic proliferation by regulatory T cells. J Clin Invest. 2005;115:3517–3526. doi: 10.1172/JCI25463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ruprecht CR, Gattorno M, Ferlito F, Gregorio A, Martini A, Lanzavecchia A, Sallusto F. Coexpression of CD25 and CD27 identifies FoxP3+ regulatory T cells in inflamed synovia. J Exp Med. 2005;201:1793–1803. doi: 10.1084/jem.20050085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mottonen M, Heikkinen J, Mustonen L, Isomaki P, Luukkainen R, Lassila O. CD4+ CD25+ T cells with the phenotypic and functional characteristics of regulatory T cells are enriched in the synovial fluid of patients with rheumatoid arthritis. Clin Exp Immunol. 2005;140:360–367. doi: 10.1111/j.1365-2249.2005.02754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Valencia X, Stephens G, Goldbach-Mansky R, Wilson M, Shevach EM, Lipsky PE. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood. 2006;108:253–261. doi: 10.1182/blood-2005-11-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.