Abstract
Parkinson’s disease (PD) is the most common neurodegenerative movement disorder, affecting more than 1% of the population over age 60. The most common feature of PD is a resting tremor, though there are many systemic neurological effects, such as incontinence and sleep disorders. PD is histopathologically identified by the presence of Lewy bodies (LB), proteinaceous inclusions constituted primarily by α-synuclein. To date, there is no effective treatment to slow or stop disease progression. To help understand disease pathogenesis and identify potential therapeutic targets, many genetic mouse models have been developed. By far the most common of these models are the wildtype and mutant α-synuclein transgenic mice, because α-synuclein was the first protein shown to have a direct effect on PD pathogenesis and progression. There are many other gene-disrupted or -mutated models currently available, which are based on genetic anomalies identified in the human disease. In addition, there are also models which examine genes that may contribute to disease onset or progression but currently have no identified causative PD mutations. These genes are part of signaling pathways important for maintaining neuronal function in the nigrostriatal pathway. This review will summarize the most commonly used of the genetic mouse models currently available for PD research. We will examine how these models have expanded our understanding of PD pathogenesis and progression, as well as aided in identification of potential therapeutic targets in this disorder.
Introduction
Parkinson’s disease (PD) is a severe neurodegenerative movement disorder affecting more than 1% of the population over the age of 60 (NINDS Parkinson’s Disease Information Page). Originally described two centuries ago by James Parkinson as the “shaking palsy”, there is still no effective treatments to slow, stop, or reverse the effects of the disease. The hallmark symptom of PD is an involuntary, resting tremor due to neurodegeneration of dopaminergic neurons in the substantia nigra (SN). SN neurons project to the striatum and together these structures constitute the nigrostriatal pathway. Since its identification as a PD therapeutic, the most effective agent to treat involuntary tremor has been levodopa, or L-DOPA [1;2], the molecular precursor of dopamine (DA) which acts to replace endogenous DA that has been lost due to neurodegeneration. After more than 30 years, L-DOPA is still the first line of treatment given to patients to relieve tremor; however, L-DOPA merely masks the effects of the disease as pathology continues to progress until the extent of dopaminergic degeneration is so great that the drug no longer alleviates the symptoms. Aside from the cardinal resting tremor associated with PD, patients suffering from this disease may also experience additional manifestations of the disorder, including, but not limited to, incontinence, sleep disorder, olfactory disturbance, and cognitive impairment in later stages of the disease, which is reviewed elsewhere [3]. PD is part of a spectrum of disorders clinically classified as Parkinsonisms. Histopathologically, most Parkinsonisms involve aberrant accumulation of the protein α-synuclein into inclusions termed Lewy bodies (LB), which also contain a host of other proteins, many of which will be discussed in this review (Fig. 1). Whether these aggregates directly contribute to pathogenesis or disease progression is still debated; however, genetic duplications and triplications of the α-synuclein gene identified in human pedigrees have been shown to cause disease [4–6].
Figure 1. Findings characteristic of the spectrum of pathology found in patients with Parkinson’s disease.

A. In this hematoxylin and eosin stained section of the substantia nigra, a Lewy body (arrow) is evident in a neuromelanin containing neuron. B. Ubiquitin immunostaining of the cingulate gyrus highlights a cortical Lewy body (brown staining indicated by the arrow).
Before genetic models of PD were available, chemicals such as 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), rotenone, lipopolysaccharride (LPS), and 6-hydroxydopamine (6-OHDA), were used to induce dopaminergic neurodegeneration as models of PD and have been reviewed elsewhere [7–10]. MPTP causes an irreversible Parkinsonism resulting from selective loss of dopaminergic neurons of the SN, mediated through endogenous monoamine oxidase B (MAOB) which catalyzes the oxidation of MPTP to 1-methyl-4-phenylpyridinium (MPP+). MPP+ then binds and inhibits mitochondrial complex I and generates reactive oxygen species (ROS) [7]. Rotenone is a common ingredient of industrial pesticides and some epidemiological studies have shown a correlation between pesticide exposure and incidence of PD. Like MPP+, rotenone also causes mitochondrial complex I inhibition [8]. The bacterial endotoxin LPS models the inflammatory aspect of PD, causing glial activation (via binding of TLR-4 receptors) which in turn causes release of proinflammatory cytokines and free radicals with subsequent dopaminergic neurodegeneration [9]. DA can be readily oxidized in the normal cellular environment [11] leading to the formation of potentially cytotoxic adducts (reviewed in [12]). 6-OHDA has been used to model PD though a dual mechanisms of increasing ROS and quinines in the catacholaminergic system [10].
In more recent years, the use of knockout and transgenic mice have revolutionized the way we study human disease, enabling gene deletion, over expression, and mutation in vivo (reviewed in [13]). α-synuclein was the first gene implicated in PD pathology. Originally identified as the non-amyloid component (NAC) of Alzheimer’s disease plaques [14], α-synuclein was later found to be the predominant protein in LB inclusions in PD [15]. Since then, mutations in many genes, including PARK2 (encoding Parkin, a E3 ubiquitin ligase), DJ-1, and Leucine-rich repeat kinase 2 (LRRK2), have been linked to familial PD (Table 1). α-synuclein transgenic mice are the most commonly used genetic model of PD (Table 2); however, many additional models using identified, familial PD genes and other genes important in the nigrostriatal pathway have been created (Table 3). Here we summarize some of the genetic models that have contributed to our understanding of PD neuropathology and helped to reveal potential therapeutic targets for the disorder. We will examine genes and gene mutations that have been shown to be directly responsible for PD pathogenesis as well as those mouse models which have been shown to model specific aspects of PD pathology (summarized in Fig. 2). We will conclude with some of the ways that transgenic and knockout mice have aided in our overall understanding of PD pathogenesis and progression as well as some of the potential therapeutic targets that have been identified through the study of genetic mouse models of PD (illustrated in Fig. 3).
Table 1.
Genetic mutations identified in PD and their corresponding disease phenotypes
| Gene/Protein | Mutation | Age of Onset | Presence of LB/α-syn pathology | Dominant/Recessive | Motor Dysfunction | L-DOPA Response | Other Notable Pathologies | Ref |
|---|---|---|---|---|---|---|---|---|
| snca/α-synuclein | A53T | ~40 | severe and widespread | autosomal dominant | bradykinesia, rigidity, postural instability, ↓range of motion | yes | cognitive decline and dementia, rapid progression | [27;28] [139;140] |
| snca/α-synuclein | A30P | ~50 | severe and widespread | autosomal dominant | bradykinesia, rigidity, postural instability, ↓range of motion | yes | cognitive decline, glial aggregates | [27;29] [141] |
| snca/α-synuclein | E46K | severe and widespread | autosomal dominant | tremor, bradykinesia | unknown | late-onset dementia | [142;143] | |
| snca/α-synuclein | gene duplication | ~30’s–40s | severe and widespread | autosomal dominant | rigidity, bradykinesia, | yes | dementia, rapid progression | [4;6] [144;145] |
| snca/α-synuclein | gene triplication | ~30s | severe and widespread | autosomal dominant | resting tremor tremor, bradykinesia | glial inclusions | [5;146] | |
| park2/Parkin | many | before 40 | no LB but SN cell loss | autosomal recessive | bradykinesia, tremor, rigidity | yes | neurofibrillary tangles in some brain regions, gliosis | [147;148] |
| park6/PINK1 | various | ~30’s–40s | LBs with SN cell loss, LBs in brainstem | autosomal recessive | tremor, bradykinesia, rigidity, postural instability | yes | SN astrogliosis and microgliosis | [97;149] [150;151] |
| park7/DJ-1 | various | ~mid-30s | unknown but have nigral degeneration | autosomal recessive | resting tremor, postural tremor, bradykinesia | yes | psychiatric symptoms, slow progression | [105;152] |
| park8/LRRK2 | various | ~50 | nigral degeneration but no LBs or α-syn accumulation | autosomal dominant | bradykinesia, rigidity, resting tremor, postural instability | yes | slow progression, LB with α-syn pathology in rare cases | [153;154] |
| park9/ATP13A2 | various | juvenile | not reported | autosomal recessive | bradykinesia, rigidity, often no tremor | yes | cognitive decline and dementia | [155;156] |
| gba/glucosidase beta acid | various | depends on severity of mutation | severe and widespread | autosomal recessive | resting tremor, bradykinesia, postural instability | yes | cognitive decline, PD onset is secondary to primary Gaucher disease | [157;158] [159;160] |
Table 2.
α-synuclein mouse models of PD which explore generation of phenotypes relevant to PD pathogenesis and progression.
| Promoter | Gene/Protein Product |
Animal Model |
Protein Accumulation/ Aggregation |
Oxidative Stress/Altered Mitochondria |
Dopaminergic Cell Death |
Motor Phenotype | Dopamine Metabolism/ Homeostasis Alterations |
|---|---|---|---|---|---|---|---|
| human PDGF-β | snca wildtype α-synuclein | transgenic | widespread α-syn+ ubiquitin inclusions [16], ↑S129 phospho-α-syn [161]; ↑autophagic vacuoles + enlarged lysosomes [134] | neuron-to-glia α-syn transmission causes astroglial inclusion formation [131] | SN inclusions but no death [16]; ↓neurogenesis in adult hippocampus [162] | motor coordination impaired [16] | ↓striatal TH nerve terminals, ↓striatal TH levels and enzymatic activity [16] |
| α-synuclein- eGFP fusion protein | transgenic | prominent α-syn aggregates in neurons of neocortex and hippocampus [47] | unknown | not reported but lysosomal pathology was observed in frontal cortex and hippocampus [47] | not reported | not reported | |
| mouse Thy-1 | snca wildtype α-synuclein | transgenic | α-syn accumulation in several brain regions including SN [20], progressive olfactory impairments [34], ↑detergent-insoluble α-syn accumulation in neuronal cell bodies and neurites throughout brain [24] | not reported | no but α-syn- positive swollen neurites [37] | ↓movement, coordination impairments, sensorimotor deficits, fine motor skill deficits [33] | no, but ↑mGluR5 suggests excitotoxicity [35], ↓colonic motility [163] |
| snca wildtype, and A53T mutant α-synuclein | transgenic | neuronal α-synucleinopathy/Lewy pathology + neuromuscular denervation in brain stem and motor neurons [22], ↑S129 phospho-α-syn [164] | astrogliosis and microgliosis [22] | no transgene expression in SN [22] | progressive ↓motor performance [22] | not reported | |
| snca A30P α-synuclein | transgenic | hyperphospho- and proteinase K-resistant α-syn LB-like inclusions in several brain regions [38], insoluble ubiquitin aggregates, altered levels of proteasome subunits [165] | oxidized and nitrated α-synuclein [38], astrogliosis [165] | no SN or striatal α-syn pathology but denervation of neuromuscular junctions [38] | aged mice: weak extremities, abnormal tail posture leading to hind limb paralysis [38], ↓grip strength [165] | cognitive decline in aged mice [166] | |
| snca Y39C mutant α-synuclein | transgenic | widespread LB-like α-syn− and ubiquitin+ inclusions including SN, ↑S129 phospho-α-syn [167] | unknown | no [167] | ↓coordination also learning memory impairment [167] | no, however, pathology and cognitive decline suggest a model of diffuse LB disease [167] | |
| rat TH | snca wildtype α-synuclein | transgenic | no inclusions [17] | early microglial activation by α-syn precedes neuron loss [18] | ↓TH+ SN neurons with age [168] | no [168] | slight ↑DA transporter levels [17] |
| snca wildtype, A30P mutant, and A53T mutant α-synuclein | transgenic | ↑α-syn in TH+ cells but no inclusions [169] | not reported | no DA cell death through 1 year [169] | not reported | S129 phospho-α-syn decreases phospho-TH and TH activity [170] | |
| snca doubly- mutated A30P/A53T α-synuclein | transgenic | no inclusions, abnormal axons and terminals [17], ↓proteasome activity, ↓proteasome subunit levels [171] | early microgliosis, ↑pro-inflammatory molecules [172] | ↓TH+ SN neurons with age [168] | decreased motor activity and coordination in aged mice [17] | ↓striatal DA and metabolites with age [17] | |
| snca A53T mutant α-synuclein | transgenic | LB-like α-syn inclusions in TH+ SN neurons, ↑S129 α-syn phosphorylation [173] | not reported | no TH+ SN cell loss up to 1 year [173] | no [173] | not reported | |
| snca truncated 1– 130 α-synuclein | transgenic | LB-like α-syn inclusions [174] | no signs of gliosis; mitochondrial dysfunction unknown [174] | congenital ↓SN neuron number [174] | ↓spontaneous locomotor activity (L-DOPA responsive) [174] | ↓striatal DA and metabolites, impaired striatal axon terminals [174] | |
| mouse prion | snca wildtype α-synuclein | transgenic | ↑α-syn levels but no inclusions [19;23;175] | not reported | no [19;23;175] | no [19;23;175] | no [19;23;175] |
| snca A53T mutant α-synuclein | transgenic | widespread (including striatum) fibrillar and insoluble LB-like α-syn aggregates [23;175;176], some inclusions contained mitochondria [36], 14-3-3 and synphilin-1 proteins associated with α-syn inclusions [177] | oxidized α-syn oligomers, astrogliosis [23], swollen mitochondria and ↓regional complex IV activity [36], ↓complex I activity [43] | no SN inclusions or cell death, no cell death of spinal neurons [23], degeneration in brainstem and spinal cord with axonal swelling and motor neuron loss [19] | ↓grip strength and coordination, gait disturbances [175] motor dysfunction (bradykinesia, ataxia, dystonia) leading to paralysis and death [19;23] | aged mice: ↑striatal DA, ↓COMT transcript, ↑striatal DA D1 and D2 receptors, abnormal striatal synaptic plasticity [59], ↓DAT by 8 months [178] | |
| snca A30P α-synuclein | transgenic | α-syn accumulation in several brain regions including midbrain but less severe than A53T mice | no [19] | no, but low level neurodegeneration in brainstem and spinal cord with motor neuron loss; less severe than A53T mice [19] | no [19] | no change in TH or DAT levels; no change in striatal DA levels [19] | |
| CaMKIIα | snca wildtype α-synuclein | conditional over expression | α-syn accumulation in several brain regions including SN but aggregates not fibrillar or insoluble [179] | electron-dense mitochondrial inclusions [179] | TH+ SN neurodegeneration, hippocampal neurodegeneration [179] | impaired motor coordination and impaired memory retention [179] | ↓DA transporter binding sites and pre-synaptic terminals [179] |
| hamster prion | snca A30P α-synuclein | transgenic | ↑α-syn in several brain regions including SN but no LB-like inclusions [180] | gliosis associated with areas of ↑transgene expression [180] | no [180] | rigidity, dystonia, gait impairment, tremor [180] | no change in TH, DA or DAT [180] impaired hippocampal synaptic plasticity [181] |
| Cre-lox method | snca A30P α-synuclein | transgenic knock-in | not reported | not reported | not reported | age-dependent ↓motor coordination, ↓stride length, catalepsy [182] | ↓striatal DA and metabolite [182] |
| mouse TH, chicken beta actin or mouse prion | snca doubly- mutated A30P/A53T α-synuclein | transgenic | α-syn accumulation in several brain regions, including SN [183] | doubly-mutated α-syn under mouse TH or chicken beta actin promoter accumulation of abnormal mitochondria in glia [101] | no | no | not reported |
| rat TH on murine snca null background | snca truncated 1– 120 α-synuclein | knockout and transgenic | α-syn inclusions in SN and olfactory bulb [184] | microgliosis [184] | no cell loss despite pathological changes [184] | progressive ↓motor activity [184] | ↓striatal DA and metabolite levels [184] |
| conditional based on nestin-cre or ratTH-cre | snca truncated 1– 119 α-synuclein | transgenic | no SN α-syn inclusions observed [185] | unknown | no [185] | unknown | ↓striatal DA and metabolite levels [185] |
| astrocyte inducible based on GFAP promoter | snca A53T mutant α-synuclein | Tet-off inducible | α-syn accumulation in several brain regions including SN [186] | microgliosis [186] | loss of DA and motor neurons but not change in striatal neuron number [186] | ↓spontaneous locomotor activity and grip strength progressing to paralysis and death [186] | not reported |
Table 3.
Mouse models of PD based on familial PD gene mutations and/or the exhibition of an informative phenotype with respect to PD pathogenesis and/or progression
| Category | Gene | Gene Product |
Animal Model |
Protein Accumulation/ Aggregation |
Oxidative Stress/Altered Mitochondria |
Dopaminergic Cell Death |
Motor Phenotype |
Dopamine Metabolism/ Homeostasis Alterations |
|---|---|---|---|---|---|---|---|---|
| Proteasome Models | park2 knockout | Parkin | knockout | no [69;74;187;188] but ↑p53 mRNA and protein levels [189] ↑tau levels in geriatric mice but no inclusion formation [125] | mitochondrial dysfunction; ↑protein and lipid peroxidation [100], ↓mitochondria complex I activity [74], ↑abnormal glial mitochondria [101], ↑GSH may represent compensation to ↑oxidant stress [190] | no [68] but loss of TH+ catecholaminergic neurons in locus coeruleus [188], ↓ TH+ SN neurons in geriatric mice [125] | ↓coordination [68], ↓startle response [188] | ↑striatal DA, ↓striatal synaptic excitability [68], ↑midbrain DA levels, ↑striatal DA receptor binding [187], ↓norepinephrine [188], ↓striatal DAT and VMAT levels, [190] |
| park2 dominant negative mutant | Parkin | mutant transgenic | parkin substrate accumulation in SN TH+ neurons [191] | not reported | no [191] | ↓motor activity and ↓coordination responsive to L-DOPA treatment [191] | ↑striatal DA [191] | |
| park2 truncated mutant Q311X | Parkin | BAC transgenic | accumulation of proteinase K- resistant α-syn in SN [192] | 3-nitrotyrosine associated with accumulated α-syn [192] | age-dependent DA neuron degeneration in SN [192] | progressive ↓in motor activity [192] | ↓striatal DA and metabolite levels, loss of striatal DA nerve terminals [192] | |
| psmc1 conditional knockout in nigrostriatal pathway | 26S proteasome subunit | conditional knockout in SN and forebrain | intraneuronal inclusions containing α-syn, ubiquitin, mitochondria [64] | GFAP activation [64] | extensive loss of TH+ neurons and death by 3–4 months [64] | not reported | severe ↓striatal DA content [64] | |
| uchl1 I93M mutation | UCH-L1 | transgenic | ↑ SDS- insoluble UCH- L1 [67] | not reported | loss of DA SN neurons [67] | reduced locomotor activity [67] | ↓striatal DA [67] | |
| Dopamine Metabolism Models | drd2 knockout | DA D2 receptor | knockout | insoluble α-syn+ ubiquitin LB-like inclusions in TH+ SN neurons [78] | ↑oxidative stress, ↑lipid peroxidation [78] | loss of TH+ SN neurons [193] | bradykinesia, ↓locomotor activity, abnormal gait/posture [76] | abnormal synaptic plasticity [77], ↑striatal DA levels, DA terminal density and DA activity [193] |
| th knockout | TH | knockout | N/A | N/A | N/A | N/A | L-DOPA administration rescues perinatal lethality [79;80] | |
| maob wildtype, astrocyte inducible | MAOB | inducible | not reported | ↓mitochondria complex I activity, ↑oxidant levels, gliosis [85] | progressive degeneration of TH+ SN neurons [85] | ↓spontaneous locomotor activity [85] | reduced striatal DA [85] | |
| vmat2 hypomorphic allele | VMAT2 | 95% knockdown | α-syn accumulation in aged mice [91] | ↑cysteinyl DOPAC adducts, 3- nitrotyrosine formation [91] | TH+ SN cell neurodegeneration in aged mice [91] | progressive ↓motor coordination (L-DOPA responsive), ↓locomotor acitivity (L- DOPA responsive) [91] | ↓striatal DA [90], ↑DA turnover, progressive ↓striatal monoamines, ↓DAT levels [91] | |
| Mitochondria Models | park6 knockout | PINK1 | knockout | not reported | mitochondrial and respiratory deficits [99], enlarged mitochondria; age-dependent impairment of mitochondrial respiration [126] | no | progressive weight loss accompanied by ↓locomotion [99] | ↓striatal DA release but no change in striatal DA or metabolite levels, impaired corticostriatal plasticity rescued by L-DOPA [98], progressive ↓striatal DA with age [99] |
| park7 knockout | DJ-1 | knockout | no [194], ↑autophagic activity may prevent protein accumulation [129] | dysfunctional skeletal muscle mitochondria may explain motor deficit [128], no change in oxidatively modified proteins [194] altered mitochondrial morphology, ↑ROS [129] | no [194] | progressively ↓locomotion and grip strength; gait impairment [194;195] | ↑DA reuptake, ↑striatal DA [194] | |
| tfam conditional knockout in DA neurons | Tfam | conditional knockout in DA neurons | intraneuronal inclusions with mitochondrial components [109] | abnormal mitochondrial morphology [109] | DA nerve cell death [109] | tremor, rigidity (L-DOPA responsive) [109] | severely ↓nigrostriatal DA [109] | |
| Synphilin-1 Models | sncaip wildtype and R621C | synphilin-1 | transgenic | ubiquitin inclusions in cerebellum, synphilin-1 aggregation in various brain regions (SN, etc) [114] | mitochondrial swelling [114] | no but Purkinje cell degeneration [114] | ↓motor performance and motor skill learning [114] | ↑nigrostriatal DA levels [114] |
| sncaip wildtype | synphilin-1 | transgenic | synphilin-1 + ubiquitin insoluble aggregates [110] | unknown | no [110] | ↓motor function and step length [110] | unknown | |
| LRRK2 Models | park8 knockout | LRRK2 | knockout | no brain pathology but extensive α-syn and ubiquitin aggregates in kidneys with autophagy lysosome dysfunction [119] | inflammation and oxidative damage in kidneys [119] | no loss of TH+ SN neurons but apoptotic cell death in kidneys [119] | not reported | no change in striatal DA [119] |
| park8 R1441C | LRRK2 | knock-in | no [196] | no change in GFAP levels detected [196] | no [196] | no [196] | DA D2 receptor- mediated impairments, ↓DA neurotransmission [196] | |
| park8 wildtype | LRRK2 | BAC transgenic | no [118] | not reported | no [118] | hyperactive, ↑motor function [118] | ↑striatal DA release [118] | |
| park8 R1441G | LRRK2 | BAC transgenic | ↑phospho-tau, axonal pathology in nigrostriatal pathway [117] | no spinal gliosis [117] | loss of TH+ dendrites in SN and ↓SN neuron size [117] | progressive ↓locomotor activity (L- DOPA responsive) [117] | ↓DA release [117] | |
| park8 G2019S | LRRK2 | BAC transgenic | no [118] | not reported | no [118] | no [118] | progressive ↓striatal DA, DA release and uptake [118] | |
| Multi-gene Models | park8 wildtype/snca A53T, park8 G2019S/snca A53T and park8 kinase dead/snca A53T | LRRK2 + α-synuclein | LRRK2 transgenic + mutant α-syn transgenic | ↑somal and insoluble α-syn+ ubiquitin aggregates vs. A53T transgenic alone [137] | ↑gliosis, abnormal mitochondrial structure and function (vs. A53T alone) [137] | no but loss of striatal and cortical neurons (accelerated vs. A53T alone) [137] | not reported | not reported |
| park8 knockout/snca A53T | LRRK2 + α-synuclein | LRRK2 knockout + mutant α-syn transgenic | ↓ accumulation of α-syn oligomers (vs A53T alone) [137] | no significant gliosis (vs A53T alone) [137] | no but ↓neurodegeneration vs. A53T alone [137] | not reported | not reported | |
| park2 knockout/doubly mutated A30P/A53T α-synuclein | Parkin + α-synuclein mouse TH promoter | Parkin knockout + mutant α-syn transgenic | no [74] | ↑mitochondrial structural alterations and ↓complex I activity vs. non- transgenic mice [74] | no [74] | no [74] | not reported | |
| park2 knockout/snca A53T | Parkin +α-synuclein | Parkin knockout + mutant α-syn transgenic | not increased from A53T alone [72] | not reported | not increased from A53T alone [72] | not increased from A53T alone[72] | not increased from A53T alone [72] | |
| park2 knockout/doubly mutated A30P/A53T α-synuclein | Parkin + α-synuclein chicken beta actin promoter | Parkin knockout + mutant α-syn transgenic | no [74] | ↑mitochondrial structural alterations and ↓complex I activity vs. non- transgenic mice [74] | no [74] | no [74] | not reported | |
| park2 knockout/snca A30P | Parkin/α-synuclein | Parkin knockout + mutant α-syn transgenic | similar accumulation of insoluble S129 phospho-α-syn; ↓ubiquitin aggregation (vs. A30P alone) [197] | not reported | not reported | delayed onset of motor dysfunction (vs. A30P alone) [197] | not reported | |
| park2, park7, and park6 knockout | Parkin/DJ- 1/PINK1 | triple knockout | no [71] | unknown | no [71] | not reported | no [71] | |
| sncaip wildtype and snca A53T | synphilin- 1/α-synuclein | double transgenic | delayed synucleinopathy (vs. A53T alone) [138] | ↓astrogliosis (vs. A53T alone) [138] | no but ↓axonal degeneration (vs. A53T alone) [138] | ↓motor defects (vs. A53T alone) [138] | not reported |
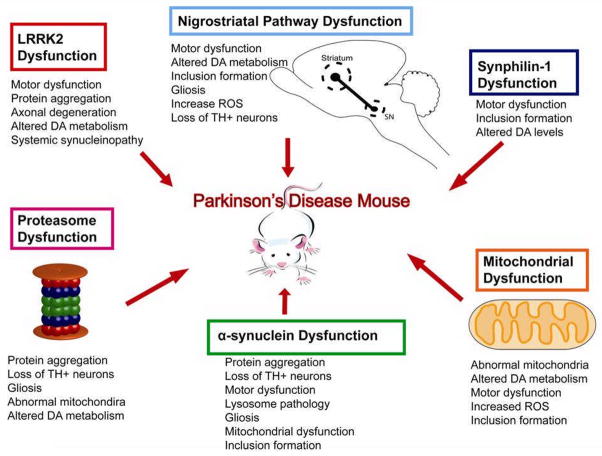
Figure 2. Schematic of PD mouse model-associated pathology.
Categories of genetic mouse models of PD which were discussed (α-synuclein dysfunction, proteasome dysfunction, mitochondrial dysfunction, nigrostriatal pathway dysfunction, LRRK2 dysfunction and synphilin-1 dysfunction) show PD-related pathologies associated with each category that result in the generation of PD mouse models.
Figure 3. Lessons from genetic mouse models of PD.
Mouse models of PD have shed light on some of the key proteins and cell signaling pathways which seem to contribute to disease pathogenesis, as well as helped us identify candidate therapeutic targets/compounds which may help to alleviate PD symptoms and possibly slow or halt the progression of neuropathology.
PD Models with α-synuclein Gene Modifications
Early genetic models of PD focused on α-synuclein (the snca gene) [16–24], including wildtype and autosomal dominant mutations in the protein which lead to early onset PD with a more aggressive progression and clinical pathology [25–29]. Mouse models carrying the human α-synuclein transgene exhibit diverse phenotypes, which is likely due in part to the insertion site of the gene within the host genome as well as the promoter utilized to drive transgene expression. Mouse models using wildtype α-synuclein have significantly improved our understanding of α-synuclein over expression-induced pathology. In addition to α-synuclein gene over expression, two autosomal dominant mutations in α-synuclein, A53T [28] and A30P [29], were found to lead to early onset autosomal dominant PD. To understand how α-synuclein mutations affect disease pathogenesis, models expressing mutant α-synuclein have been examined. We will discuss several α-synuclein mouse models utilizing various promoters to drive gene expression and how these models differ from one another with respect to PD phenotype.
One of the first developed PD mouse models expressed the human wildtype α-synuclein transgene under transcriptional control of the PDGF-β promoter [16]. These mice developed neuropathology by 2 months of age, evidenced by α-synuclein- and ubiquitin-positive inclusions in the neocortex, hippocampus and SN, resulting in motor dysfunction and nerve terminal degeneration in the basal ganglia. In contrast to protein aggregates found in PD, which are located almost exclusively in the cytoplasm, transgenic mice possessed both cytoplasmic and the nuclear inclusions, which were not fibrillar in nature like LBs. Tyrosine hydroxylase (TH) protein levels and enzymatic activity were significantly reduced, corresponding to observed phenomena in PD [16]. These mice have been subsequently used in multiple studies by other investigators as a platform to further characterize PD pathogenesis and progression as well as to identify potential therapeutic targets [30–32]. Using these mice, it was shown that calpain-cleaved α-synuclein preferentially forms aggregates, which were also identified in PD brains [30]. This model was also found to have progressive transcriptional dysregulation which preceded other pathological changes [31], which may also be an early pathological alteration in the human disease. Finally, stereotactic injection of beclin 1, a key regulator in the macroautophagy pathway, was shown to reduce neurodegeneration in this model of synucleinopathy [32], highlighting one potential method for therapeutic intervention.
A wildtype α-synuclein mouse utilizing the mouse Thy-1 promoter was shown to have widespread α-synuclein accumulation in the brain, including the SN [20]. Despite the lack of dopaminergic cell loss in these mice, they developed motor and coordination impairments as early as 2 months of age and by 6 months, sensorimotor deficits were detected [33]. Additionally, olfactory deficits, frequently observed in pre-clinical PD, were found in the Thy-1 mice [34]. These subtle and progressive phenotypes in response to α-synuclein accumulation make the Thy-1 mouse an attractive model for studying early pathological changes which may be occurring in PD as a result of increasing α-synuclein burden. Additionally, models displaying pre-clinical signs of PD may aid in the development of techniques to allow earlier diagnosis and therapeutic intervention. Use of this model identified an interaction between α-synuclein and the mGluR5 receptor, wherein α-synuclein causes over-activation of the receptor, implicating excitotoxicity as a potential pathogenic mechanism in PD [35]. Mice harboring the human A53T α-synuclein gene under the mouse Thy-1 promoter develop a severe synucleinopathy in the spinal cord and neuromuscular junctions with many features similar to human disease, including Lewy pathology and neurodegeneration with accompanying motor defects [22]. However, the observed motor dysfunction was in the absence of transgene expression and pathology in the SN dopaminergic neurons [22], suggesting there may be multiple mechanisms and cell types capable of inducing a Parkinson-like phenotype in mice.
Subsequently, two groups developed models utilizing the A53T transgene under the control of the mouse prion protein promoter. The first was a mouse which develops synucleinopathy progressing to a debilitating movement disorder [23]. Animals were healthy and without symptoms through 8 months of age, at which time some mice began presenting with grooming neglect and weight loss which rapidly progressed to paralysis of limbs and periods of hind limb freezing [23]. As in PD, signs of disease were delayed until late adulthood in these animals; however, once the phenotype manifested, the disease progression was rapid (animals were completely incapacitated by 10–21d after the first symptoms appeared), suggesting that mice are particularly vulnerable to α-synuclein-induced neuron loss in the spinal cord. Examination of brains from A53T transgenic mice showed massive α-synuclein pathology throughout the spinal cord, cerebellum, and cortex, where large detergent-insoluble aggregates of α-synuclein were found. Degeneration of motor neurons was apparent, explaining the paralytic phenotype but not its delayed onset or rapid progression. Interestingly, the wildtype α-synuclein transgenic mice in this study displayed no phenotype [23].
A second A53T transgenic mouse displayed a delayed-onset motor dysfunction rapidly progressing to death accompanied by pathological accumulation of α-synuclein in cerebellum and cortex [19]. Additionally, these mice were later found to develop mitochondrial degeneration (as seen by p53 co-localization, mitochondrial DNA damage, and enlarged, swollen mitochondria) concomitant with α-synuclein inclusion development [36]. Interestingly, the corresponding wildtype α-synuclein transgenic mice in this study were also without a remarkable phenotype [19]. SN α-synuclein accumulation and/or pathology was absent in both prion promoter A53T models, highlighting a key difference between these mice and PD pathology in humans.
The A30P α-synuclein transgenic mice tend to exhibit a milder disease phenotype than A53T mice. Both α-synuclein mutants more readily aggregate than wildtype α-synuclein, though A53T forms fibrils faster than A30P [26]. In contrast to the A53T mice, A30P mice from the same study expressing the human transgene under the control of the mouse prion protein promoter were free from any overt phenotype or pathology, even at 24 months of age, despite expressing higher protein levels of human A30P α-synuclein than the A53T transgenic mice. The A30P mice did show transgenic α-synuclein accumulation in the deep cerebellar nuclei but exhibited no accompanying ubiquitin accumulation or other discernable pathology. The accumulated α-synuclein seen in the A30P mice was diffuse and not primarily composed of detergent-insoluble aggregates as in the A53T mice, which could explain the lack of corresponding pathology in the A30P mice [19].
Another A30P human α-synuclein transgenic mouse model [24] showed that mice homozygous for the A30P transgene under control of the mouse Thy1 promoter accumulated α-synuclein inclusions occasionally positive for ubiquitin immunoreactivity [37]. Despite widespread transgene expression throughout the brain, neither the striatum nor SN contained any proteinase K-resistant α-synuclein [38]. Mice developed a progressive motor phenotype which manifested within the first year of life as an unsteady gait and weak hind limbs followed by abnormal posture and jerking of the tail which progressed to hunchbacked posture and hind limb paralysis. Astrogliosis was evident throughout the spinal cord and in areas of neuronal degeneration [38]. As in the A53T mouse model, the onset of pathology was slow, however, once evident, it quickly progressed. In a study on α-synuclein dysfunction and its relation to PD, the A30P human transgene was shown to reduce the normal interaction of α-synuclein with lipid rafts, an interaction which is necessary for the localization of α-synuclein to synapses [21]. The A53T and A30P α-synuclein models continue to be a valuable tool for investigating native functions of α-synuclein and how interrupting those functions may contribute to neurodegeneration.
Neuropathology in PD is most prominent in the SN, hence models with dopaminergic neuron-specific α-synuclein over expression were created. A mouse model expressing the wildtype human α-synuclein transgene under the control of the rat TH promoter showed no apparent nigrostriatal pathology and had no motor phenotype, despite the highest transgene expression being in the SN [17]. The only difference detected in this model from non transgenic animals was an increase in DA transporter levels, however, levels of DA and its corresponding metabolites were unaltered, suggesting the rodent nigrostriatal pathway may not be as vulnerable to α-synuclein accumulation-induced toxicity as the human brain. It is also possible that the lack of overt nigrostriatal pathology in this model may have been due to the relatively low-level transgene expression (human α-synuclein made up only one-third of total α-synuclein present in mouse striatum) [17]. Although there was no obvious SN dopaminergic neurodegeneration, significant microglial activation was found in the brains of α-synuclein transgenic mice as young as one month [18]. A large body of evidence implicates inflammation and concomitant microglial activation in perpetuating PD neuropathology [18;39–41]. α-synuclein over expressing mice exhibited increased TNFα expression by one month of age compared to age-matched controls, [18], suggesting this model may provide a platform for studying early inflammatory-mediated changes induced by increased α-synuclein levels in the brain.
A model in which the A53T α-synuclein was expressed under control of the rat TH promoter yielded a mouse which expressed the transgene solely in the soma, axons and terminals of the nigrostriatal system [17]. These mice exhibited age-dependent decreases in DA and its metabolites accompanied by impairments in motor coordination with abnormal axons and terminals [17]. Recently, these mice were found to develop mitochondrial dysfunction due to α-synuclein monomers and oligomers localizing to the inner mitochondrial membrane and inhibiting complex I, a phenomenon which had previously only been demonstrated in vitro [42]. α-synuclein over expression both in vitro and in vivo has been shown to cause complex I inhibition [43], and complex I inhibition in post-mortem PD brains has been recently shown to be α-synuclein-dependent [44]. Despite the appearance of abnormal nerve terminals, reduced DA level and age-dependent impairments in motor coordination, no dopaminergic cell loss was observed in these animals. Mentioned earlier, the wildtype α-synuclein transgenic mice from this study exhibited a far milder phenotype [17].
Aside from neurodegeneration and inflammation, there is evidence suggesting α-synuclein induces lysosomal pathology [45–49]. In fact, the autophagy lysosome pathway has been repeatedly shown to play a key role in α-synuclein degradation [50–54], reviewed in [55], including the degradation of α-synuclein aggregates [56;57]. A transgenic mouse model expressing human wildtype α-synuclein-eGFP fusion protein under the control of the PDGF-β promoter was shown to deleteriously affect the autophagy lysosome pathway [47]. The α-synuclein-eGFP fusion protein has a higher propensity to aggregate than native α-synuclein, which circumvents the difficulties of generating α-synuclein aggregation in murine models. Human α-synuclein aggregates were observed in the deep layers of the frontal, temporal and cingulate cortex, and these areas were accompanied by enlarged lysosomes which were subsequently fractionated and found to contain significant α-synuclein-eGFP protein aggregates [47]. Understanding how α-synuclein affects lysosomal function can help us to identify potential therapeutic targets in the autophagy lysosome pathway to improve lysosomal function and increase degradation efficiency.
To determine whether reducing α-synuclein burden is a strategy to combat PD, mice deficient in α-synuclein were generated. The α-synuclein knockout mouse, while having no severe phenotype, exhibits abnormalities in the nigrostriatal dopaminergic pathway in the form of increased DA release in response to either paired stimuli or elevated Ca2+ [58]. Incidentally, mice with mouse prion promoter-driven A53T α-synuclein were shown to have impaired DA neurotransmission and striatal synaptic plasticity, further supporting a role for α-synuclein in DA neurotransmission [59]. This sheds light on possible mechanisms for selective sensitivity of neurons of the nigrostriatal pathway seen in PD. Further evidence in support of the hypothesis that α-synuclein plays a role in PD pathology arises from the finding that α-synuclein knockout mice are resistant to mitochondrial toxin models of PD, including MPTP [60–62]. As our knowledge about α-synuclein function and relevant signaling pathways increases, it will likely expose more potential targets for neuroprotection and therapeutic intervention.
Non-α-Synuclein Genetic Models of PD
After the link between α-synuclein and PD was made, other genes purported to have a causative role in PD pathogenesis were identified. These genes function in multiple different pathways and are divided as follows: proteasome-related models, DA metabolism models, and mitochondrial-related models. The models for synphilin-1 and LRRK2 are discussed separately because of these proteins’ unique properties. The autophagy lysosome pathway, mentioned previously, affects many proteins and pathways and its relation to PD models will be discussed. For each model, we will consider its utility in PD research and whether it presents with α-synuclein pathology, as this phenomenon is common to nearly all types of PD.
Proteasome-Related Models of PD
One of the early candidates for α-synuclein degradation was the ubiquitin-proteasome system, first evidenced by pulse-chase studies for α-synuclein breakdown which were blocked after proteasome inhibition [63]. The 26S proteasome is responsible for degrading polyubiquitinated substrates [64]. The Psmc1 gene is an essential subunit of the 26S proteasome necessary for its assembly and activity. A conditional knockout model of Psmc1 in the SN, cortex, hippocampus, and striatum leads to neurodegeneration and accumulation of inclusions termed Lewy-like, which contained α-synuclein, ubiquitin, and mitochondria. Loss of TH-positive neurons was observed as well as increased GFAP activation [64]. This model recapitulates several key features of PD including protein aggregation (with α-synuclein) and degeneration of dopaminergic neurons and is a useful model system for investigating the contribution of the 26S proteasome to neuronal homeostasis and potential methods to perturb proteasome dysfunction with respect to neurodegeneration.
Ubiquitin carboxyl-terminal hydrolase L1 (UCH-L1) is a protein with dual function as both a ubiquitylating and de-ubiquitylating enzyme [65]. In PD, one study presents evidence for mutations in UCH-L1 causing familial PD [66]. In mice expressing mutant human UCH-L1 I93M, loss of dopaminergic neurons of the SN was observed as well as accompanying decrease in striatal DA content [67]. Degenerating dopaminergic neurons were observed that argyrophilic degenerating neurons which were absent in non-transgenic animals. Midbrains of transgenic mice also contained increased SDS-insoluble UCH-L1 [67], but not α-synuclein aggregates, suggesting that α-synuclein accumulation and/or aggregation is not a pre-requisite for PD-like pathology in this UCH-L1 mouse model.
Parkin is an E3 ubiquitin ligase and mutations in the gene are the most common cause of juvenile Parkinsonism; however, the corresponding mouse models with Parkin deficiency showed only a mild or no phenotype without any loss of dopaminergic neurons [68;69]. The mice were found to have increased extracellular DA content in the striatum and medium spiny neurons required increased currents to induce synaptic responses, therefore, the mice may have decreased synaptic excitability [68], and were later found to have impairments in long-term depression as well as long-term potentiation in the medium spiny neurons [70]. Interestingly, a mouse model deficient in three genes, parkin/DJ-1/PINK1, showed no pathological alterations in the nigrostriatal pathway, suggesting these genes may serve a protective rather than an essential role in the dopaminergic system [71]. Further, it was found that parkin deletion did not contribute to inclusion body formation in human A53T α-synuclein transgenic mice [72].
Another parkin-deficient model was found to have non-motor behavior abnormalities including reduced exploration and increased anxiety which was attributed to an alteration in DA metabolism as evidenced by increased homovanillic acid content [73]; however, these mice retained normal dopaminergic neuron counts [74]. Despite the inconspicuous phenotype of parkin deficient mice, parkin has been shown to be important for signaling in the cellular milieu. The autophagic degradation of damaged mitochondria (termed mitophagy) has been shown to be mediated by parkin co-localization [75]. The relationship between parkin and mitochondria is further discussed in the mitochondria section below. Models to further explore the mitochondrial-associated functions of parkin as it relates to oxidative stress and the accumulation of dysfunctional mitochondria could be useful.
Dopamine Metabolism Models of PD
Due to PD being a disease of the nigrostriatal pathway, there are multiple gene-disrupted models that focus on the dopaminergic signaling and metabolic pathways. Five types of DA receptors have been identified, D1–D5, falling into two categories: D1-like family (including D1 and D5 receptors) and D2-like family (including D2, D3, and D4 receptors). We will only examine the DA receptor D2 model, as it is the only one with an identified Parkinsonian phenotype. As PD is a disease of gradual DA depletion, targeting enzymes responsible for DA synthesis, processing, and homeostasis may give insights into PD pathogenesis or uncover new therapeutic targets. TH, monoamine oxidase B (MAO B) and vesicular monoamine transporter 2 (VMAT2) models have been created to explore these possibilities.
DA D2 receptor-deficient mice were observed to have motor dysfunction termed to be Parkinson-like which was defined by several tests of motor coordination [76]. D2 DA receptors are concentrated in the SN on dopaminergic nerve terminals where they act to inhibit feedback on DA release. D2-deficient mice were found to have normal reflexes, however, they possessed abnormal posture coupled with an abnormal gait. D2 knockout mice exhibited significantly less locomotion than wildtype mice as measured in the open-field test and also had a decreased latency to fall as measured by the rotarod. Additionally, D2-deficient mice exhibited cataleptic-like behavior in which they remained immobile significantly longer than heterozygous or wildtype mice [76;77]. Catalepsy is defined as a neurological condition which features muscle rigidity and fixed posture and is a common phenomenon of PD. Subsequently, D2 receptor knockout mice were found to have increased turnover of DA in the nigrostriatal pathway evidenced by the increased DOPAC/DA ratio compared to controls. Mutant mice exhibited increased oxidative stress as measured by lipid peroxidation levels using the MDA assay, which measures malondialdehyde, a common product of lipid peroxidation. D2−/− mice older than 18 months showed increased incidence of α-synuclein inclusions which were also positive for ubiquitin in the SN pars compacta and ventral tegmental area neurons.. There was no cell loss observed in the SN, but there was a reduction in DA terminals to the dorsal striatum in aged knockout mice. p62, a protein responsible for sequestering proteins destined for autophagic degradation, was significantly increased in D2 knockout mice compared to wildtype mice [78]. Macroautophagy (also known as simply autophagy) has been identified as a method of α-synuclein degradation as well as protein aggregate degradation. Increased p62 may be attributed to increased induction of autophagy or a backup in autophagic degradation, but these issues were not addressed in this study. The D2−/− mouse is a useful model for studying α-synuclein inclusion formation as well as its pathophysiological consequences and may provide a potential platform for studying novel therapeutics for movement disorder.
TH is the rate-limiting enzyme in catecholamine synthesis, which includes DA, adrenaline and noradrenaline, and the resting tremor that is the diagnostic hallmark of PD is due to dopaminergic cell death and the resulting TH insufficiency. When making a TH-knockout mouse model, it was found that TH was necessary for embryonic development and survival past the early neo-natal period [79;80]. The TH-deficient pups die during late embryonic development or very soon after birth, however, this phenotype can be completely rescued by administration of L-DOPA to pregnant females [79] or introduction of the human TH transgene into the murine TH-deficient mice through selective breeding [80]. L-DOPA is the metabolic precursor of all catecholamines and after several decades continues to be the primary treatment to alleviate PD symptoms. In TH-deficient mice, as long as L-DOPA is continually administered, the phenotype is completely rescued. Likewise, L-DOPA therapy was able to correct a Parkinsonian syndrome in infants found to have TH deficiency [81]. Throughout the pathogenesis of PD dopaminergic neurons are continually lost and so inevitably L-DOPA administration becomes insufficient to alleviate symptoms. DA deficient floxed stop (DDfs) mice with nonfunctional TH were created to further explore DA signaling [82]. Utilizing the Cre-lox system allowed restoration of functional TH through introduction of Cre recombinase. DDfs mice have severe hypoactivity with aphagia, and these phenotypes can be corrected via bilateral injection of Cre recombinase into the striatum [82]. These models may help in the development of novel methods of DA replacement in PD with respect to modulation of TH enzymatic activity.
Monoamine oxidase A and B (MAOA, MAOB) are both able to oxidize DA into 3,4-dihydroxyphenylacetic acid (DOPAC), therefore, genetic ablation of either MAOA or MAOB alone does not result in a Parkinsonian phenotype in mice [83;84]. However, an astrocyte-specific, inducible model of MAOB over expression revealed a PD-like pathology [85]. Currently, several FDA-approved drugs to treat PD are MAOB inhibitors and so increasing MAOB over endogenous levels would lead to an expected PD model. Upon induction of astrocytic MAOB, mice were found to have neurodegeneration specific to the dopaminergic neurons of the SN, accompanied by an increase in activated microglia. Decreased motor activity was also observed in MAOB over expressing animals [85]. This study provides evidence that accumulation of MAOB in the aging brain could be a causative factor in sporadic PD, and further examination of this model could prove useful in helping us understand more about the glia-neuron interactions in PD pathophysiology.
In addition to the monoamine oxidases, catechol-O-methyltransferase (COMT) inactivates DA by methylation and can also methylate the DA metabolite DOPAC to form HVA. To investigate whether COMT ablation can influence the development of a Parkinsonian phenotype, COMT-deficient male mice were generated. Male COMT−/− mice were found to have significantly increased levels of DA in the frontal cortex but not in the striatum or hypothalamus. Female mice deficient in COMT maintained normal DA levels throughout the brain, suggesting that female animals were more resistant to metabolic alterations induced by COMT deficiency. Both male and female COMT knockout mice exhibited significantly altered HVA/DOPAC ratios, but despite the alterations observed in DA metabolism, neither male nor female mice were found to have an overt Parkinsonian phenotype, though several non-Parkinsonian behavioral abnormalities were noted [86]. Like MAOB, the COMT protein may need to be over expressed rather than ablated in order to yield a Parkinsonian phenotype, but generation of a COMT over expressing mouse has not yet been reported.
A non-enzymatic protein that plays an important role in DA homeostasis is vesicular monoamine transporter 2 (VMAT2), which functions to sequester cytosolic DA for release at the synapse. Cytosolic DA can be become toxic through oxidation, and VMAT2 is thought to play a role in sequestering cytosolic DA into vesicles, thereby reducing its cytotoxic potential. A complete ablation of VMAT2, results in a mouse with early perinatal lethality [87–89]. Consequently, a transgenic mouse model was engineered to expresses significantly lower VMAT than wildtype mice, with a hypomorphic allele making VMAT2 levels biochemically undetectable [90] with a 95% reduction in VMAT2 expression compared to wildtype [91]. Mice with significantly reduced expression of VMAT2 exhibit several Parkinsonian phenotypes including significantly decreased levels of DA in the midbrain and striatum compared to controls as well as motor deficits manifested in the beam walking test, with homozygous low VMAT2 mice requiring significantly more time to cross the beam than either heterozygous or wildtype mice [90]. In a subsequent study, mice with very low VMAT2 levels were found to develop age-dependent dopaminergic neurodegeneration characterized by early appearance of oxidatively modified proteins and DA adducts followed by α-synuclein accumulation in the SN and ultimately, cell death [91]. Most recently, these mice were demonstrated to model many nonmotor symptoms of PD, including olfactory deficits, delayed gastric emptying, altered sleep latency, anxiety-like behavior, and age-dependent depression [92]. Loss of olfaction is one of the earliest PD symptoms reported, although the disease is not typically diagnosed at this stage due to nonspecificity, and the mechanisms are currently unclear [93;94]. Additionally, constipation is a common systemic manifestation in PD and causes great discomfort to patients. Sleep problems are often reported by PD patients, and anxiety and depression are common co-morbidities associated with PD.
These dopamine metabolism models have illustrated the importance of using genetic models in addition to those with corresponding mutations identified in human disease. Genes involved in dopamine homeostasis and metabolism play enormous roles in maintaining the integrity of the nigrostriatal system and their alteration may predispose to the development of PD-like pathology.
Mitochondrial Genetic Models of PD
Increased oxidative stress and mitochondrial dysfunction have long been recognized in neurodegenerative disease and likely play at least a partially causative role in their pathogenesis (reviewed in [95]). Being largely post-mitotic, neurons are particularly vulnerable to damage from reactive oxygen species (ROS) that accumulates in aging neurons. In post-mortem PD brains reduced complex I activity is a common feature and leads to increased ROS and decreased ATP, compromising neuronal function [96]. With regard to mitochondrial dysfunction, we will review mouse models of PINK1, DJ-1, and mitochondrial transcription factor A (TFAM).
Discovery of an autosomal recessive early-onset Parkinsonism caused by loss-of-function mutations in PTEN-induced putative kinase1 (PINK1) provided the first evidence of a primary mitochondrial dysfunction causing Parkinsonism [97]. PINK1 is a mitochondrial serine/threonine kinase which protects cells from apoptosis induced by oxidative stress. In patients with Parkinsonism due to PINK1 mutation, the disease presents with early onset, but its clinical progression is relatively mild, affecting the autonomic nervous system less than sporadic PD [97]. PINK1-deficient mice do not exhibit α-synuclein aggregates or loss of SN dopaminergic neurons [98;99]; however, PINK1−/− brain mitochondria had significantly reduced membrane potential [99]. PINK1 knockout mice exhibited reduced catecholamine release at the synapse [98], explaining lower striatal DA levels in PINK-deficient mice in the absence of neurodegeneration [99], as well as implicating a function for PINK1 in catecholamine homeostasis [98]. As mutant mice aged, progressive weight loss and spontaneous locomotor activity were observed, as well as deficits in pre-protein transport into the mitochondria. In light of these studies, PINK1 reveals a model for studying the contribution of mitochondrial dysfunction in PD pathogenesis, however, would not be sufficient for studying other aspects of PD, such as dopaminergic neuron loss, which is absent in this model.
Mentioned previously, the parkin mouse model of PD was initially disappointing due to its lack of a dramatic phenotype; however, upon closer examination, several parkin mouse models were found to have mitochondrial alternations and/or abnormalities. Parkin knockout mice were found to have compromised respiratory function with increased lipid peroxidation [100], decreased mitochondrial complex I activity [74], and abnormal morphology in glial mitochondria [101]. The interplay between parkin and mitochondria was further highlighted by the finding that parkin localization to mitochondria serves as a specific signal for degradation of damaged mitochondria via autophagy (mitophagy) [75]. In addition, PINK1 and parkin have been demonstrated to cooperate in regulating mitochondrial homeostasis in mammalian dopaminergic neurons [102], indicating these mouse models may be of use in identifying ways to abrogate mitochondrial dysfunction in PD.
DJ-1is widely expressed protein with a ThiJ domain, a motif implicated in RNA-protein interaction regulation, thiamine biosynthesis, Ras-related signal transduction, and having protease activity [103]. DJ-1 can be targeted to the mitochondria where it plays a protective role against increased ROS through its antioxidant properties [104]. Mutations in DJ-1 resulting in a loss of function cause a rare, autosomal recessive PD [105]. To understand how this occurs, mice deficient in DJ-1 were studied and found to have hypokinesia without loss of dopaminergic neurons. Upon closer examination, deficits in the nigrostriatal system were identified, including reduced evoked DA overflow in the striatum due to increased reuptake. Additionally, long term depression was absent in the medium spiny neurons. These deficiencies were traced back to D2 receptor-mediated functions, suggesting that DJ-1 plays a role in regulating the D2 DA receptor in vivo [106]. By modulating D2 receptor function, this model mildly recapitulates some observed phenomena from the previously mentioned D2−/− mouse. Immortalized mouse embryonic fibroblasts (MEFs) from DJ-1 knockout mice accumulated mitochondria with reduced respiration, increased ROS, and decreased mitochondria membrane potential. Clearance of the defective mitochondria via autophagy was also reduced [107], indicating loss of DJ-1 affects multiple pathways. Recently, the DJ-1 null mouse was shown to have fewer TH-positive neurons in the ventral tegmental area (VTA) than wildtype animals [108]. Despite being just adjacent to the SN, the dopaminergic neurons of the VTA are typically less affected in PD than those of the SN. DJ-1 deficient mice are also reported to have up regulated levels of mitochondrial respiratory enzymes, thought to be a compensatory response to the loss of DJ-1 antioxidant function [108]. Because the DJ-1 knockout mouse has a mild phenotype, it enables the study of a chronic mitochondrial dysfunction model and its evolution over time. It also provides a platform from which to study compensatory responses to the removal of a protein which acts to protect the mitochondria.
Although PINK1 and DJ-1 loss or mutation models have been shown to model certain aspects of PD, the question still remains whether a primary mitochondrial respiratory chain deficiency can lead to a Parkinsonian phenotype. To address this issue, a conditional knockout of mitochondrial transcription factor A (TFAM) specifically within dopaminergic neurons was generated and termed mitoPark mice [109]. TFAM is a protein essential for mitochondrial gene expression and maintenance. MitoPark mice developed an adult-onset, slow-progressing, Parkinsonian phenotype which included motor impairment, intraneuronal inclusions, and SN cell death [109]. Though α-synuclein accumulation was not a feature of this model, the animals were responsive to L-DOPA therapy, suggesting that this model has utility for identifying potential therapeutic targets for PD.
Synphilin-1 Models of PD
Synphilin-1 is a cytoplasmic protein which has been shown to interact with several PD-associated proteins including α-synuclein and parkin, though its exact function remains unclear [110]. Found most closely associated with synaptic vesicles, evidence has emerged that synphilin-1 may contribute to the formation of LBs through its interaction with α-synuclein [111]. Whether synphilin-1 mutations exist which lead to the development of PD is debated [112;113]. Identification of synphilin-1 as a component of LBs has sparked interest in determining whether synphilin-1 levels or mutations can contribute to PD pathogenesis. Transgenic mice over expressing synphilin-1 were generated to further investigate its physiological role in vivo [110]. While there was no difference in dopaminergic neurons of the SN between synphilin-1 mice and controls up to 8 months of age, synphilin-1 mice did exhibit a significant motor phenotype having a shorter latency to fall on either an accelerating rod or a rod with a fixed speed. Additionally, step length analyzed from serial 6 footprints of the transgenic mice was significantly shorter than the non-transgenics [110]. Supporting these results, a more recent study recapitulated the motor dysfunction in mice over expressing transgenes for synphilin-1 or a mutant R621C synphilin-1 [114]. Furthermore, they found increased levels of DA throughout the nigrostriatal system accompanied by ubiquitin-positive inclusions in the cerebellum with Purkinje cell degeneration [114]. It may be that synphilin-1 is more critical to cerebellar function than to nigrostriatal function, however the effect on DA levels suggests that synphinlin-1 may bind protein/s which regulate DA metabolism. The synphilin-1 models may be useful for further characterization of proteins important for DA homeostasis. To date, a synphilin-1-deficient mouse has not been characterized.
LRRK2 Models of PD
Leucine-rich repeat kinase 2 (LRRK2) mutations are the most commonly reported cause of familial PD. An enzyme with both a kinase and GTPase domain, LRRK2 resides primarily in the cytoplasm but has also been shown to associate with the outer mitochondrial membrane [115]. Recently, LRRK2 was shown to play an important role in modulating cytoskeletal stability, a function which may be disrupted in pathogenic LRRK2 mutations [116]; however, there is still much unknown about the physiological function of LRRK2 in vivo and how these functions may be important for PD pathogenesis. To investigate the effect of LRRK2 mutations on PD pathogenesis, we will discuss three different LRRK2 PD models.
A BAC transgenic mouse model expressing mutant LRRK2 R1441G was created after the discovery of the same missense mutation in a large family pedigree [117]. As the mutant transgenic mice aged, they developed progressive motor deficits which resulted in almost complete immobility by 10–12 months of age. Mutant mice developed axonal degeneration in the nigrostriatal pathway; however, dopaminergic cell numbers remained unchanged. LRRK2 R1441G mice had increased levels of both tau and hyperphosphorylated tau protein levels, also seen in many PD patients with LRRK2 mutations [117]. Overall, the LRRK2 R1441G mice are an excellent model for familial PD caused by LRRK mutations, recapitulating several key features of the human disease.
The most common PD-causing mutation in LRRK2 is G2019S, which leads to a gain of function mutant with enhanced kinase activity. In a BAC transgenic model in which mice over expressed either wildtype or G2019S LRRK2, mice with the wildtype transgene exhibited hyperactivity and enhanced motor performance in the open field test [118]. Mice with the wildtype LRRK2 transgene had increased striatal DA release compared to non-transgenic mice, whereas mice with the G2019S mutant transgene showed an age-dependent decline in striatal DA content and release which was below both the wildtype transgenic and non-transgenic mice. In spite of decreased DA levels in G2019S mice, there was no evidence of PD-associated pathology in any brain region, despite increased kinase activity in the brain [118]. This study suggests that LRRK2 plays a role in DA metabolism and/or homeostasis and that the G2019S mutation may inhibit that function. The model may represent a way of investigating early pathogenic events (i.e., DA dysregulation) thought to occur in PD.
To further investigate the physiological role of LRRK2, a knockout mouse model was generated [119]. Surprisingly, there were no alterations in DA or its metabolites nor was there any evidence for neuropathology anywhere in the brain. As it turns out, endogenous LRRK2 expression in the kidney is approximately six fold higher than in the brain. Kidneys from LRRK2−/− mice developed the classic PD pathophysiology including accumulation of high molecular weight triton-insoluble α-synuclein inclusions, increased ubiquitinated proteins, inflammatory responses, and oxidative damage [119]. In addition, loss of LRRK2 caused a significant increase in apoptotic cell death in the kidneys, concomitant with impairment of the autophagy lysosome pathway. Lifespan was not affected in the LRRK2−/− mice, despite the conspicuous kidney pathology [119]. Despite the absence of an obvious neuropathology, this model suggests involvement of LRRK2 in the regulation of α-synuclein homeostasis and perhaps a native function for LRRK2 in preventing oxidative stress and cell death and could be useful for identifying novel targets to offset pathology due to LRRK2 deficiency. This finding suggests it would be worthwhile to determine whether kidney or other systemic pathology outside the central nervous system exists in wildtype and mutant LRRK2 transgenic mice.
Conclusions
Lessons from PD Mouse Models: Translation
The use of transgenic and knockout mouse models of PD has helped us to better understand PD pathogenesis and progression as well as to identify potential therapeutic targets for PD. We will conclude this review with a brief discussion about some of the ways mouse models have broadened our understanding of possible mechanisms of selective toxicity in the nigrostriatal pathway and helped to reveal candidates for therapeutic intervention with respect to α-synuclein pathology, mitochondria, neuron-glial communication, and the autophagy lysosome pathway (summarized in Fig. 3).
Despite the many genetic mouse models of PD developed over the last decade (Tables 2 and 3), few of them present with progressive and striking nigrostriatal degeneration. The difference in lifespan between mice (~2 years) versus humans (~70 years) may be largely responsible for the resilience of murine SN compared to human. Glia and post-mitotic neurons in the murine brain have only to survive and thrive for a limited time, compared to human brains, where the cells must survive for many decades. Additionally, one of the defining gross morphological characteristics of human SN is the presence of neuromelanin, a dark pigment found in SN dopaminergic neurons. Neuromelanin accumulates throughout life and is composed primarily of oxidized DA and metals like iron and is thought to be a means of sequestering potentially toxic products from the cytosol [120]. During dopaminergic neuron degeneration, release of neuromelanin is thought to contribute to inflammation and microglial activation [121]. Injection of human neuromelanin extract into rodent SN causes dopaminergic neurodegeneration, inflammation, and microglial activation [121], suggesting that release of neuromelanin into the extracellular milieu may be one explanation for selective damage to the nigrostriatal pathway in PD. Additionally, the absence of neuromelanin in rodents may represent one explanation for enhanced resistance of murine SN neurons to Parkinsonian stimuli.
Despite α-synuclein being the first protein connected to PD etiology, much is still unknown about the mechanism/s of α-synuclein-induced neuropathology. Following the finding that cholesterol and its metabolites may contribute to α-synucleinopathy in vitro [122], two wildtype α-synuclein transgenic mouse models were treated with the cholesterol synthesis inhibitor lovastatin after the onset of significant motor deficits and α-synuclein accumulation [123]. Along with significantly reduced plasma cholesterol levels, lovastatin-treated mice showed marked reductions in α-synuclein accumulation, including significant reductions in oxidized α-synuclein, compared to control mice. The reduction of α-synuclein burden was concomitant with reduced neuropathology in both models [123]. As statins have been marketed as cholesterol-reducing agents for many years, they present an attractive candidate for adjuvant therapy for synucleinopathies, having demonstrated efficacy after the onset of pathology.
As discussed previously, mitochondrial damage and oxidative stress are considered a major source of pathology in PD and many mouse models have reflected this (see Table 1 and 2). We now know that α-synuclein itself can bind mitochondrial complex I and inhibit its activity [42;44], illustrating one way in which excess α-synuclein can aberrantly affect mitochondrial function. An A53T α-synuclein transgenic mouse [23] was found to have a significant increase in DJ-1 levels by 3 months of age [124], suggesting an attempt to preserve mitochondrial function and reduce oxidant burden. DJ-1 levels were no longer elevated in aged mice[124]; hence, promoting increased levels of DJ-1 may be a method of combating increasing oxidative stress in PD. Abnormal mitochondrial morphology with increased ROS has been reported in many PD mouse models including the parkin knockout [100;125], PINK1 knockout [99;126], and DJ-1knockout [127–129]. These models have all helped to uncover putative mitochondrial functions for these proteins, and despite the lack of conspicuous nigrostriatal pathology, their progressive mitochondrial dysfunction may represent an opportunity for studying pre-neurodegenerative events leading to PD development and subsequent cell death. Among the most important interactions in the brain is the neuron-glia relationship. It has been demonstrated that α-synuclein can cause inflammation leading to reactive gliosis both in vitro and in vivo [18;41;130]. Additionally, evidence for transmission of α-synuclein from neurons to glia resulting in subsequent formation of glial α-synuclein inclusions [131] in wildtype α-synuclein transgenic mice [16] has demonstrated a mechanism for both early and sustained inflammation in response to increased α-synuclein levels. Targeting and subduing activated glia, or preventing α-synuclein transfer, may represent a viable therapeutic approach to reverse or prevent further pathology in PD.
Finally, as alluded to earlier, the autophagy lysosome pathway has become increasingly relevant to research with respect to clearance of accumulated and/or aggregated proteins in neurodegenerative disorder, including PD. Lysosomes and autophagic pathways have both been demonstrated to be aberrantly altered in PD and PD models [45;46;49]. As both monomeric and aggregated α-synuclein have been shown to be susceptible to autophagic degradation [51;52], the autophagy lysosome pathway represents an attractive candidate for experimental therapeutics in PD. Indeed, several mouse models of PD have revealed the therapeutic potential of harnessing autophagy. In wildtype α-synuclein transgenic mice [16], targeted intracerebral administration of rapamycin (a pharmacologic agent which induces autophagy via inhibition of mTOR [132;133]) was able to reduce α-synuclein accumulation and corresponding neurodegeneration associated with the model [134]. α-synuclein levels in this mouse model were also reduced in response to beclin 1, an upstream initiator of autophagy, via targeted delivery of a beclin 1 lentivirus to cortex and hippocampus [32]. Taken together, these studies strongly suggest that multiple proteins in the autophagy pathway represent potential therapeutic targets for PD.
The Future of Genetic PD Modeling
As we continue to identify genes which cause or contribute to PD development, mouse models will continue to be created and characterized. These models help us to understand cause and effect with respect to genetic mutation and ablation; however, they cannot model the myriad of events which take place in sporadic PD. Only now are we beginning to understand some of the many molecules, proteins and signaling pathways involved in PD pathogenesis. Accumulating evidence suggests that many different factors are responsible for disease development in sporadic PD as well as the brain’s compensatory response to pathology. Applying multiple PD models in the same mouse allows us to uncover mechanisms which may exacerbate pathology as well as factors which may play a protective role against neurodegenerative insults. Compound models add an additional layer of complexity to disease pathogenesis and progression; however, they may also allow us to more closely model the complexity of the human disease. Several models have addressed the effects of combining α-synuclein transgenic mice (particularly the A53T α-synuclein transgene) with another transgenic or gene-disrupted model to examine effects of other genes on models of α-synucleinopathy. Additionally, chemical models of PD, such as MPTP and LPS, have been used in conjunction with a transgenic or gene-disrupted model to help understand what factors contribute to or alleviate pathology. A few examples of how pharmacological models have enhanced our understanding are briefly mentioned below.
Investigating mechanisms of α-synuclein-induced neuropathology revealed that the presence of the human α-synuclein transgene (either wildtype or A53T) on a murine α-synuclein-null background was able to markedly exacerbate pathology in response to the inflammagen LPS [135]. Targeted injection of LPS into the SN results in an inflammatory reaction which was unaltered in murine α-synuclein knockout mice compared to wildtype mice; however, when the knockout mice expressed the human α-synuclein transgene, the response to LPS included accumulation of insoluble, aggregated α-synuclein, which was often nitrated or oxidized, leading to SN dopaminergic neuron death [135]. This finding illustrated that while α-synuclein itself is not an oxidant, its presence during oxidative stress and inflammation mediates cell death. Mentioned previously, the inverse of this concept has also been demonstrated, as α-synuclein knockout mice have been shown to be resistant to pathology induced by mitochondrial toxins, including MPTP, which cause oxidative stress and neuroinflammation [60–62]. Additionally, though parkin-null mice did not present with a dramatic phenotype, they were found to be significantly more susceptible to SN neurodegeneration from systemic, long-term LPS injection [136], indicating parkin may be a target for protection from neuroinflammation.
As mentioned earlier, the endogenous function of LRRK2 has yet to be completely elucidated. To understand more closely how the presence or absence of LRRK2 may affect development of α-synucleinopathy, A53T α-synuclein mice were crossed with LRRK2-deficient mice or mice transgenic for wild type, G2019S, or kinase dead LRRK2 [137]. Mice with A53T α-synuclein and any one of the three LRRK2 transgenes exhibited increased α-synuclein aggregation, exacerbated gliosis, and abnormal mitochondrial structure and function as well an accelerated loss of striatal and cortical neurons compared to the A53T α-synuclein transgenic mice without a LRRK2 transgene. Importantly, the A53T α-synuclein mice which were LRRK2-deficient exhibited attenuated α-synuclein aggregation and delayed neurodegeneration compared to A53T α-synuclein mice alone [137]. This finding implies that LRRK2 enhances α-synuclein-induced neurodegeneration and that pharmacologic or genetic targeting of LRRK2 may represent a viable therapy for delaying α-synuclein-dependent cell death in PD. A similar study which generated mice transgenic for A53T α-synuclein and synphilin-1 found that increasing synphilin-1 expression delayed the onset of synucleinopathy, reduced reactive gliosis, and attenuated axonal degeneration in the A53T mouse [138]. This suggest that, synphilin-1 plays a protective role in α-synuclein-induced neurodegeneration, perhaps by acting as a chaperone and sequestering α-synuclein, thereby preventing pathologic interactions between α-synuclein and other cellular components. In this light, synphilin-1 could be considered a target for detoxifying α-synuclein.
Over the past 30 years, much effort has been made to generate and analyze mouse models for studying aspects of PD pathogenesis and progression. These models have provided insights into protein accumulation and mechanisms of toxicity which may be occurring in the human disease. PD is still thought to be largely a sporadic disease, however, the genetic mutations identified in familial PD have greatly contributed to our knowledge of how cell physiology may go awry and lead to disease development. Many different proteins and signaling pathways are aberrantly affected in PD. It is likely that early in PD pathogenesis, dysfunction occurs in a small subset of proteins or a specific pathway within the nigrostriatal system which then triggers a cascade of dysfunction resulting in global cell and tissue pathology. Different chemical and genetic models reflecting both early and late pathological changes, and the combination of various models will continue to enhance our understanding of pathologic events which occur during neurodegeneration, and help identification of potential therapeutic strategies.
Acknowledgments
We thank Drs. Steve Carroll and Victor Darley-Usmar for discussions and reading the review, Mr. Barry Bailey for assistance with tables and illustrations, and support from UAB start up fund, Michael J Fox Foundation, NIHR01-NS064090 and a VA merit award (J.Z.).
Abbreviations
- MPTP
1-methyl 4-phenyl 1,2,3,6-tetrahydropyridine
- MPP+
1-methyl-4-phenylpyridinium
- 6-OHDA
6-hydroxydopamine
- COMT
catechol-O-methyltransferase
- DA
dopamine
- DAT
dopamine transporter
- L-DOPA
levodopa
- LB
Lewy body
- LPS
lipopolysaccharride
- MAOB
monoamine oxidase B
- PD
Parkinson’s disease
- ROS
reactive oxygen species
- SN
substantia nigra
- TH
tyrosine hydroxylase
Reference List
- 1.Fehling C. Treatment of Parkinson’s syndrome with L-dopa.. A double blind study. Acta Neurol Scand. 1966;42:367–372. doi: 10.1111/j.1600-0404.1966.tb01188.x. [DOI] [PubMed] [Google Scholar]
- 2.Bruno A, Bruno SC. Effects of L-DOPA on pharmacological parkinsonism. Acta Psychiatr Scand. 1966;42:264–271. doi: 10.1111/j.1600-0447.1966.tb01931.x. [DOI] [PubMed] [Google Scholar]
- 3.Poewe W, Mahlknecht P. The clinical progression of Parkinson’s disease. Parkinsonism Relat Disord. 2009;15(Suppl 4):S28–S32. doi: 10.1016/S1353-8020(09)70831-4. [DOI] [PubMed] [Google Scholar]
- 4.Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, Waucquier N, Defebvre L, Amouyel P, Farrer M, Destee A. Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet. 2004;364:1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- 5.Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 6.Ibanez P, Bonnet AM, Debarges B, Lohmann E, Tison F, Pollak P, Agid Y, Durr A, Brice A. Causal relation between alpha-synuclein gene duplication and familial Parkinson’s disease. Lancet. 2004;364:1169–1171. doi: 10.1016/S0140-6736(04)17104-3. [DOI] [PubMed] [Google Scholar]
- 7.Przedborski S, Jackson-Lewis V, Naini AB, Jakowec M, Petzinger G, Miller R, Akram M. The parkinsonian toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a technical review of its utility and safety. J Neurochem. 2001;76:1265–1274. doi: 10.1046/j.1471-4159.2001.00183.x. [DOI] [PubMed] [Google Scholar]
- 8.Drechsel DA, Patel M. Role of reactive oxygen species in the neurotoxicity of environmental agents implicated in Parkinson’s disease. Free Radic Biol Med. 2008;44:1873–1886. doi: 10.1016/j.freeradbiomed.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dutta G, Zhang P, Liu B. The lipopolysaccharide Parkinson’s disease animal model: mechanistic studies and drug discovery. Fundam Clin Pharmacol. 2008;22:453–464. doi: 10.1111/j.1472-8206.2008.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bove J, Prou D, Perier C, Przedborski S. Toxin-induced models of Parkinson’s disease. NeuroRx. 2005;2:484–494. doi: 10.1602/neurorx.2.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adams RN, Murrill E, McCreery R, Blank L, Karolczak M. 6-Hydroxydopamine, a new oxidation mechanism. Eur J Pharmacol. 1972;17:287–292. doi: 10.1016/0014-2999(72)90172-0. [DOI] [PubMed] [Google Scholar]
- 12.Stokes AH, Hastings TG, Vrana KE. Cytotoxic and genotoxic potential of dopamine. J Neurosci Res. 1999;55:659–665. doi: 10.1002/(SICI)1097-4547(19990315)55:6<659::AID-JNR1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 13.Capecchi MR. Gene targeting in mice: functional analysis of the mammalian genome for the twenty-first century. Nat Rev Genet. 2005;6:507–512. doi: 10.1038/nrg1619. [DOI] [PubMed] [Google Scholar]
- 14.Jakes R, Spillantini MG, Goedert M. Identification of two distinct synucleins from human brain. FEBS Lett. 1994;345:27–32. doi: 10.1016/0014-5793(94)00395-5. [DOI] [PubMed] [Google Scholar]
- 15.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 16.Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, Sagara Y, Sisk A, Mucke L. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science. 2000;287:1265–1269. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- 17.Richfield EK, Thiruchelvam MJ, Cory-Slechta DA, Wuertzer C, Gainetdinov RR, Caron MG, Di Monte DA, Federoff HJ. Behavioral and neurochemical effects of wild-type and mutated human alpha-synuclein in transgenic mice. Exp Neurol. 2002;175:35–48. doi: 10.1006/exnr.2002.7882. [DOI] [PubMed] [Google Scholar]
- 18.Su X, Maguire-Zeiss KA, Giuliano R, Prifti L, Venkatesh K, Federoff HJ. Synuclein activates microglia in a model of Parkinson’s disease. Neurobiol Aging. 2008;29:1690–1701. doi: 10.1016/j.neurobiolaging.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee MK, Stirling W, Xu Y, Xu X, Qui D, Mandir AS, Dawson TM, Copeland NG, Jenkins NA, Price DL. Human alpha-synuclein-harboring familial Parkinson’s disease-linked Ala-53 --> Thr mutation causes neurodegenerative disease with alpha-synuclein aggregation in transgenic mice. Proc Natl Acad Sci U S A. 2002;99:8968–8973. doi: 10.1073/pnas.132197599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rockenstein E, Mallory M, Hashimoto M, Song D, Shults CW, Lang I, Masliah E. Differential neuropathological alterations in transgenic mice expressing alpha-synuclein from the platelet-derived growth factor and Thy-1 promoters. J Neurosci Res. 2002;68:568–578. doi: 10.1002/jnr.10231. [DOI] [PubMed] [Google Scholar]
- 21.Fortin DL, Troyer MD, Nakamura K, Kubo S, Anthony MD, Edwards RH. Lipid rafts mediate the synaptic localization of alpha-synuclein. J Neurosci. 2004;24:6715–6723. doi: 10.1523/JNEUROSCI.1594-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Putten H, Wiederhold KH, Probst A, Barbieri S, Mistl C, Danner S, Kauffmann S, Hofele K, Spooren WP, Ruegg MA, Lin S, Caroni P, Sommer B, Tolnay M, Bilbe G. Neuropathology in mice expressing human alpha-synuclein. J Neurosci. 2000;20:6021–6029. doi: 10.1523/JNEUROSCI.20-16-06021.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giasson BI, Duda JE, Quinn SM, Zhang B, Trojanowski JQ, Lee VM. Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron. 2002;34:521–533. doi: 10.1016/s0896-6273(02)00682-7. [DOI] [PubMed] [Google Scholar]
- 24.Kahle PJ, Neumann M, Ozmen L, Muller V, Jacobsen H, Schindzielorz A, Okochi M, Leimer U, van Der PH, Probst A, Kremmer E, Kretzschmar HA, Haass C. Subcellular localization of wild-type and Parkinson’s disease-associated mutant alpha -synuclein in human and transgenic mouse brain. J Neurosci. 2000;20:6365–6373. doi: 10.1523/JNEUROSCI.20-17-06365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jensen PH, Nielsen MS, Jakes R, Dotti CG, Goedert M. Binding of alpha-synuclein to brain vesicles is abolished by familial Parkinson’s disease mutation. J Biol Chem. 1998;273:26292–26294. doi: 10.1074/jbc.273.41.26292. [DOI] [PubMed] [Google Scholar]
- 26.Conway KA, Harper JD, Lansbury PT. Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat Med. 1998;4:1318–1320. doi: 10.1038/3311. [DOI] [PubMed] [Google Scholar]
- 27.Narhi L, Wood SJ, Steavenson S, Jiang Y, Wu GM, Anafi D, Kaufman SA, Martin F, Sitney K, Denis P, Louis JC, Wypych J, Biere AL, Citron M. Both familial Parkinson’s disease mutations accelerate alpha-synuclein aggregation. J Biol Chem. 1999;274:9843–9846. doi: 10.1074/jbc.274.14.9843. [DOI] [PubMed] [Google Scholar]
- 28.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di IG, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 29.Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 30.Dufty BM, Warner LR, Hou ST, Jiang SX, Gomez-Isla T, Leenhouts KM, Oxford JT, Feany MB, Masliah E, Rohn TT. Calpain-cleavage of alpha-synuclein: connecting proteolytic processing to disease-linked aggregation. Am J Pathol. 2007;170:1725–1738. doi: 10.2353/ajpath.2007.061232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yacoubian TA, Cantuti-Castelvetri I, Bouzou B, Asteris G, McLean PJ, Hyman BT, Standaert DG. Transcriptional dysregulation in a transgenic model of Parkinson disease. Neurobiol Dis. 2008;29:515–528. doi: 10.1016/j.nbd.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spencer B, Potkar R, Trejo M, Rockenstein E, Patrick C, Gindi R, Adame A, Wyss-Coray T, Masliah E. Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in alpha-synuclein models of Parkinson’s and Lewy body diseases. J Neurosci. 2009;29:13578–13588. doi: 10.1523/JNEUROSCI.4390-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fleming SM, Salcedo J, Fernagut PO, Rockenstein E, Masliah E, Levine MS, Chesselet MF. Early and progressive sensorimotor anomalies in mice overexpressing wild-type human alpha-synuclein. J Neurosci. 2004;24:9434–9440. doi: 10.1523/JNEUROSCI.3080-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fleming SM, Tetreault NA, Mulligan CK, Hutson CB, Masliah E, Chesselet MF. Olfactory deficits in mice overexpressing human wildtype alpha-synuclein. Eur J Neurosci. 2008;28:247–256. doi: 10.1111/j.1460-9568.2008.06346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Price DL, Rockenstein E, Ubhi K, Phung V, MacLean-Lewis N, Askay D, Cartier A, Spencer B, Patrick C, Desplats P, Ellisman MH, Masliah E. Alterations in mGluR5 expression and signaling in Lewy body disease and in transgenic models of alpha-synucleinopathy--implications for excitotoxicity. PLoS One. 2010;5:e14020. doi: 10.1371/journal.pone.0014020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Martin LJ, Pan Y, Price AC, Sterling W, Copeland NG, Jenkins NA, Price DL, Lee MK. Parkinson’s disease alpha-synuclein transgenic mice develop neuronal mitochondrial degeneration and cell death. J Neurosci. 2006;26:41–50. doi: 10.1523/JNEUROSCI.4308-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kahle PJ, Neumann M, Ozmen L, Muller V, Odoy S, Okamoto N, Jacobsen H, Iwatsubo T, Trojanowski JQ, Takahashi H, Wakabayashi K, Bogdanovic N, Riederer P, Kretzschmar HA, Haass C. Selective insolubility of alpha-synuclein in human Lewy body diseases is recapitulated in a transgenic mouse model. Am J Pathol. 2001;159:2215–2225. doi: 10.1016/s0002-9440(10)63072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neumann M, Kahle PJ, Giasson BI, Ozmen L, Borroni E, Spooren W, Muller V, Odoy S, Fujiwara H, Hasegawa M, Iwatsubo T, Trojanowski JQ, Kretzschmar HA, Haass C. Misfolded proteinase K-resistant hyperphosphorylated alpha-synuclein in aged transgenic mice with locomotor deterioration and in human alpha-synucleinopathies. J Clin Invest. 2002;110:1429–1439. doi: 10.1172/JCI15777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao HM, Jiang J, Wilson B, Zhang W, Hong JS, Liu B. Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson’s disease. J Neurochem. 2002;81:1285–1297. doi: 10.1046/j.1471-4159.2002.00928.x. [DOI] [PubMed] [Google Scholar]
- 40.Qin L, Liu Y, Wang T, Wei SJ, Block ML, Wilson B, Liu B, Hong JS. NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. J Biol Chem. 2004;279:1415–1421. doi: 10.1074/jbc.M307657200. [DOI] [PubMed] [Google Scholar]
- 41.Lee EJ, Woo MS, Moon PG, Baek MC, Choi IY, Kim WK, Junn E, Kim HS. Alpha-synuclein activates microglia by inducing the expressions of matrix metalloproteinases and the subsequent activation of protease-activated receptor-1. J Immunol. 2010;185:615–623. doi: 10.4049/jimmunol.0903480. [DOI] [PubMed] [Google Scholar]
- 42.Chinta SJ, Mallajosyula JK, Rane A, Andersen JK. Mitochondrial alpha-synuclein accumulation impairs complex I function in dopaminergic neurons and results in increased mitophagy in vivo. Neurosci Lett. 2010;486:235–239. doi: 10.1016/j.neulet.2010.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loeb V, Yakunin E, Saada A, Sharon R. The transgenic overexpression of alpha-synuclein and not its related pathology associates with complex I inhibition. J Biol Chem. 2010;285:7334–7343. doi: 10.1074/jbc.M109.061051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK. Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem. 2008;283:9089–9100. doi: 10.1074/jbc.M710012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meredith GE, Totterdell S, Petroske E, Santa CK, Callison RC, Jr, Lau YS. Lysosomal malfunction accompanies alpha-synuclein aggregation in a progressive mouse model of Parkinson’s disease. Brain Res. 2002;956:156–165. doi: 10.1016/s0006-8993(02)03514-x. [DOI] [PubMed] [Google Scholar]
- 46.Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305:1292–1295. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- 47.Rockenstein E, Schwach G, Ingolic E, Adame A, Crews L, Mante M, Pfragner R, Schreiner E, Windisch M, Masliah E. Lysosomal pathology associated with alpha-synuclein accumulation in transgenic models using an eGFP fusion protein. J Neurosci Res. 2005;80:247–259. doi: 10.1002/jnr.20446. [DOI] [PubMed] [Google Scholar]
- 48.Martinez-Vicente M, Talloczy Z, Kaushik S, Massey AC, Mazzulli J, Mosharov EV, Hodara R, Fredenburg R, Wu DC, Follenzi A, Dauer W, Przedborski S, Ischiropoulos H, Lansbury PT, Sulzer D, Cuervo AM. Dopamine-modified alpha-synuclein blocks chaperone-mediated autophagy. J Clin Invest. 2008;118:777–788. doi: 10.1172/JCI32806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dehay B, Bove J, Rodriguez-Muela N, Perier C, Recasens A, Boya P, Vila M. Pathogenic lysosomal depletion in Parkinson’s disease. J Neurosci. 2010;30:12535–12544. doi: 10.1523/JNEUROSCI.1920-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cullen V, Lindfors M, Ng J, Paetau A, Swinton E, Kolodziej P, Boston H, Saftig P, Woulfe J, Feany MB, Myllykangas L, Schlossmacher MG, Tyynela J. Cathepsin D expression level affects alpha-synuclein processing, aggregation, and toxicity in vivo. Mol Brain. 2009;2:5. doi: 10.1186/1756-6606-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee HJ, Khoshaghideh F, Patel S, Lee SJ. Clearance of alpha-synuclein oligomeric intermediates via the lysosomal degradation pathway. J Neurosci. 2004;24:1888–1896. doi: 10.1523/JNEUROSCI.3809-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vogiatzi T, Xilouri M, Vekrellis K, Stefanis L. Wild type alpha-synuclein is degraded by chaperone-mediated autophagy and macroautophagy in neuronal cells. J Biol Chem. 2008;283:23542–23556. doi: 10.1074/jbc.M801992200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mak SK, McCormack AL, Manning-Bog AB, Cuervo AM, Di Monte DA. Lysosomal degradation of alpha-synuclein in vivo. J Biol Chem. 2010;285:13621–13629. doi: 10.1074/jbc.M109.074617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qiao L, Hamamichi S, Caldwell KA, Caldwell GA, Yacoubian TA, Wilson S, Xie ZL, Speake LD, Parks R, Crabtree D, Liang Q, Crimmins S, Schneider L, Uchiyama Y, Iwatsubo T, Zhou Y, Peng L, Lu Y, Standaert DG, Walls KC, Shacka JJ, Roth KA, Zhang J. Lysosomal enzyme cathepsin D protects against alpha-synuclein aggregation and toxicity. Mol Brain. 2008;1:17. doi: 10.1186/1756-6606-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schneider L, Zhang J. Lysosomal function in macromolecular homeostasis and bioenergetics in Parkinson’s disease. Mol Neurodegener. 2010;5:14. doi: 10.1186/1750-1326-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wong ES, Tan JM, Soong WE, Hussein K, Nukina N, Dawson VL, Dawson TM, Cuervo AM, Lim KL. Autophagy-mediated clearance of aggresomes is not a universal phenomenon. Hum Mol Genet. 2008;17:2570–2582. doi: 10.1093/hmg/ddn157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu WH, Dorado B, Figueroa HY, Wang L, Planel E, Cookson MR, Clark LN, Duff KE. Metabolic activity determines efficacy of macroautophagic clearance of pathological oligomeric alpha-synuclein. Am J Pathol. 2009;175:736–747. doi: 10.2353/ajpath.2009.080928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 59.Kurz A, Double KL, Lastres-Becker I, Tozzi A, Tantucci M, Bockhart V, Bonin M, Garcia-Arencibia M, Nuber S, Schlaudraff F, Liss B, Fernandez-Ruiz J, Gerlach M, Wullner U, Luddens H, Calabresi P, Auburger G, Gispert S. A53T-alpha-synuclein overexpression impairs dopamine signaling and striatal synaptic plasticity in old mice. PLoS One. 2010;5:e11464. doi: 10.1371/journal.pone.0011464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Klivenyi P, Siwek D, Gardian G, Yang L, Starkov A, Cleren C, Ferrante RJ, Kowall NW, Abeliovich A, Beal MF. Mice lacking alpha-synuclein are resistant to mitochondrial toxins. Neurobiol Dis. 2006;21:541–548. doi: 10.1016/j.nbd.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 61.Dauer W, Kholodilov N, Vila M, Trillat AC, Goodchild R, Larsen KE, Staal R, Tieu K, Schmitz Y, Yuan CA, Rocha M, Jackson-Lewis V, Hersch S, Sulzer D, Przedborski S, Burke R, Hen R. Resistance of alpha -synuclein null mice to the parkinsonian neurotoxin MPTP. Proc Natl Acad Sci U S A. 2002;99:14524–14529. doi: 10.1073/pnas.172514599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Drolet RE, Behrouz B, Lookingland KJ, Goudreau JL. Mice lacking alpha-synuclein have an attenuated loss of striatal dopamine following prolonged chronic MPTP administration. Neurotoxicology. 2004;25:761–769. doi: 10.1016/j.neuro.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 63.Bennett MC, Bishop JF, Leng Y, Chock PB, Chase TN, Mouradian MM. Degradation of alpha-synuclein by proteasome. J Biol Chem. 1999;274:33855–33858. doi: 10.1074/jbc.274.48.33855. [DOI] [PubMed] [Google Scholar]
- 64.Bedford L, Hay D, Devoy A, Paine S, Powe DG, Seth R, Gray T, Topham I, Fone K, Rezvani N, Mee M, Soane T, Layfield R, Sheppard PW, Ebendal T, Usoskin D, Lowe J, Mayer RJ. Depletion of 26S proteasomes in mouse brain neurons causes neurodegeneration and Lewy-like inclusions resembling human pale bodies. J Neurosci. 2008;28:8189–8198. doi: 10.1523/JNEUROSCI.2218-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu Y, Fallon L, Lashuel HA, Liu Z, Lansbury PT., Jr The UCH-L1 gene encodes two opposing enzymatic activities that affect alpha-synuclein degradation and Parkinson’s disease susceptibility. Cell. 2002;111:209–218. doi: 10.1016/s0092-8674(02)01012-7. [DOI] [PubMed] [Google Scholar]
- 66.Leroy E, Boyer R, Polymeropoulos MH. Intron-exon structure of ubiquitin c-terminal hydrolase-L1. DNA Res. 1998;5:397–400. doi: 10.1093/dnares/5.6.397. [DOI] [PubMed] [Google Scholar]
- 67.Setsuie R, Wang YL, Mochizuki H, Osaka H, Hayakawa H, Ichihara N, Li H, Furuta A, Sano Y, Sun YJ, Kwon J, Kabuta T, Yoshimi K, Aoki S, Mizuno Y, Noda M, Wada K. Dopaminergic neuronal loss in transgenic mice expressing the Parkinson’s disease-associated UCH-L1 I93M mutant. Neurochem Int. 2007;50:119–129. doi: 10.1016/j.neuint.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 68.Goldberg MS, Fleming SM, Palacino JJ, Cepeda C, Lam HA, Bhatnagar A, Meloni EG, Wu N, Ackerson LC, Klapstein GJ, Gajendiran M, Roth BL, Chesselet MF, Maidment NT, Levine MS, Shen J. Parkin-deficient mice exhibit nigrostriatal deficits but not loss of dopaminergic neurons. J Biol Chem. 2003;278:43628–43635. doi: 10.1074/jbc.M308947200. [DOI] [PubMed] [Google Scholar]
- 69.Perez FA, Palmiter RD. Parkin-deficient mice are not a robust model of parkinsonism. Proc Natl Acad Sci U S A. 2005;102:2174–2179. doi: 10.1073/pnas.0409598102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kitada T, Pisani A, Karouani M, Haburcak M, Martella G, Tscherter A, Platania P, Wu B, Pothos EN, Shen J. Impaired dopamine release and synaptic plasticity in the striatum of parkin−/−mice. J Neurochem. 2009;110:613–621. doi: 10.1111/j.1471-4159.2009.06152.x. [DOI] [PubMed] [Google Scholar]
- 71.Kitada T, Tong Y, Gautier CA, Shen J. Absence of nigral degeneration in aged parkin/DJ-1/PINK1 triple knockout mice. J Neurochem. 2009;111:696–702. doi: 10.1111/j.1471-4159.2009.06350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Von CR, Thomas B, Andrabi SA, Lim KL, Savitt JM, Saffary R, Stirling W, Bruno K, Hess EJ, Lee MK, Dawson VL, Dawson TM. Inclusion body formation and neurodegeneration are parkin independent in a mouse model of alpha-synucleinopathy. J Neurosci. 2006;26:3685–3696. doi: 10.1523/JNEUROSCI.0414-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhu XR, Maskri L, Herold C, Bader V, Stichel CC, Gunturkun O, Lubbert H. Non-motor behavioural impairments in parkin-deficient mice. Eur J Neurosci. 2007;26:1902–1911. doi: 10.1111/j.1460-9568.2007.05812.x. [DOI] [PubMed] [Google Scholar]
- 74.Stichel CC, Zhu XR, Bader V, Linnartz B, Schmidt S, Lubbert H. Mono- and double-mutant mouse models of Parkinson’s disease display severe mitochondrial damage. Hum Mol Genet. 2007;16:2377–2393. doi: 10.1093/hmg/ddm083. [DOI] [PubMed] [Google Scholar]
- 75.Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baik JH, Picetti R, Saiardi A, Thiriet G, Dierich A, Depaulis A, Le MM, Borrelli E. Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature. 1995;377:424–428. doi: 10.1038/377424a0. [DOI] [PubMed] [Google Scholar]
- 77.Calabresi P, Saiardi A, Pisani A, Baik JH, Centonze D, Mercuri NB, Bernardi G, Borrelli E. Abnormal synaptic plasticity in the striatum of mice lacking dopamine D2 receptors. J Neurosci. 1997;17:4536–4544. doi: 10.1523/JNEUROSCI.17-12-04536.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tinsley RB, Bye CR, Parish CL, Tziotis-Vais A, George S, Culvenor JG, Li QX, Masters CL, Finkelstein DI, Horne MK. Dopamine D2 receptor knockout mice develop features of Parkinson disease. Ann Neurol. 2009;66:472–484. doi: 10.1002/ana.21716. [DOI] [PubMed] [Google Scholar]
- 79.Zhou QY, Quaife CJ, Palmiter RD. Targeted disruption of the tyrosine hydroxylase gene reveals that catecholamines are required for mouse fetal development. Nature. 1995;374:640–643. doi: 10.1038/374640a0. [DOI] [PubMed] [Google Scholar]
- 80.Kobayashi K, Morita S, Sawada H, Mizuguchi T, Yamada K, Nagatsu I, Hata T, Watanabe Y, Fujita K, Nagatsu T. Targeted disruption of the tyrosine hydroxylase locus results in severe catecholamine depletion and perinatal lethality in mice. J Biol Chem. 1995;270:27235–27243. doi: 10.1074/jbc.270.45.27235. [DOI] [PubMed] [Google Scholar]
- 81.de Rijk-Van Andel JF, Gabreels FJ, Geurtz B, Steenbergen-Spanjers GC, van Den Heuvel LP, Smeitink JA, Wevers RA. L-dopa-responsive infantile hypokinetic rigid parkinsonism due to tyrosine hydroxylase deficiency. Neurology. 2000;55:1926–1928. doi: 10.1212/wnl.55.12.1926. [DOI] [PubMed] [Google Scholar]
- 82.Hnasko TS, Perez FA, Scouras AD, Stoll EA, Gale SD, Luquet S, Phillips PE, Kremer EJ, Palmiter RD. Cre recombinase-mediated restoration of nigrostriatal dopamine in dopamine-deficient mice reverses hypophagia and bradykinesia. Proc Natl Acad Sci U S A. 2006;103:8858–8863. doi: 10.1073/pnas.0603081103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grimsby J, Toth M, Chen K, Kumazawa T, Klaidman L, Adams JD, Karoum F, Gal J, Shih JC. Increased stress response and beta-phenylethylamine in MAOB-deficient mice. Nat Genet. 1997;17:206–210. doi: 10.1038/ng1097-206. [DOI] [PubMed] [Google Scholar]
- 84.Cases O, Vitalis T, Seif I, De ME, Sotelo C, Gaspar P. Lack of barrels in the somatosensory cortex of monoamine oxidase A-deficient mice: role of a serotonin excess during the critical period. Neuron. 1996;16:297–307. doi: 10.1016/s0896-6273(00)80048-3. [DOI] [PubMed] [Google Scholar]
- 85.Mallajosyula JK, Kaur D, Chinta SJ, Rajagopalan S, Rane A, Nicholls DG, Di Monte DA, Macarthur H, Andersen JK. MAO-B elevation in mouse brain astrocytes results in Parkinson’s pathology. PLoS One. 2008;3:e1616. doi: 10.1371/journal.pone.0001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, Karayiorgou M. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci U S A. 1998;95:9991–9996. doi: 10.1073/pnas.95.17.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fon EA, Pothos EN, Sun BC, Killeen N, Sulzer D, Edwards RH. Vesicular transport regulates monoamine storage and release but is not essential for amphetamine action. Neuron. 1997;19:1271–1283. doi: 10.1016/s0896-6273(00)80418-3. [DOI] [PubMed] [Google Scholar]
- 88.Takahashi N, Miner LL, Sora I, Ujike H, Revay RS, Kostic V, Jackson-Lewis V, Przedborski S, Uhl GR. VMAT2 knockout mice: heterozygotes display reduced amphetamine-conditioned reward, enhanced amphetamine locomotion, and enhanced MPTP toxicity. Proc Natl Acad Sci U S A. 1997;94:9938–9943. doi: 10.1073/pnas.94.18.9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang YM, Gainetdinov RR, Fumagalli F, Xu F, Jones SR, Bock CB, Miller GW, Wightman RM, Caron MG. Knockout of the vesicular monoamine transporter 2 gene results in neonatal death and supersensitivity to cocaine and amphetamine. Neuron. 1997;19:1285–1296. doi: 10.1016/s0896-6273(00)80419-5. [DOI] [PubMed] [Google Scholar]
- 90.Mooslehner KA, Chan PM, Xu W, Liu L, Smadja C, Humby T, Allen ND, Wilkinson LS, Emson PC. Mice with very low expression of the vesicular monoamine transporter 2 gene survive into adulthood: potential mouse model for parkinsonism. Mol Cell Biol. 2001;21:5321–5331. doi: 10.1128/MCB.21.16.5321-5331.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Caudle WM, Richardson JR, Wang MZ, Taylor TN, Guillot TS, McCormack AL, Colebrooke RE, Di Monte DA, Emson PC, Miller GW. Reduced vesicular storage of dopamine causes progressive nigrostriatal neurodegeneration. J Neurosci. 2007;27:8138–8148. doi: 10.1523/JNEUROSCI.0319-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Taylor TN, Caudle WM, Shepherd KR, Noorian A, Jackson CR, Iuvone PM, Weinshenker D, Greene JG, Miller GW. Nonmotor symptoms of Parkinson’s disease revealed in an animal model with reduced monoamine storage capacity. J Neurosci. 2009;29:8103–8113. doi: 10.1523/JNEUROSCI.1495-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ferrer I. Neuropathology and neurochemistry of nonmotor symptoms in Parkinson’s disease. Parkinsons Dis. 2011;2011:708404. doi: 10.4061/2011/708404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Baba T, Takeda A, Kikuchi A, Nishio Y, Hosokai Y, Hirayama K, Hasegawa T, Sugeno N, Suzuki K, Mori E, Takahashi S, Fukuda H, Itoyama Y. Association of olfactory dysfunction and brain. Metabolism in Parkinson’s disease. Mov Disord. 2011;26:621–628. doi: 10.1002/mds.23602. [DOI] [PubMed] [Google Scholar]
- 95.Navarro A, Boveris A. Brain mitochondrial dysfunction and oxidative damage in Parkinson’s disease. J Bioenerg Biomembr. 2009;41:517–521. doi: 10.1007/s10863-009-9250-6. [DOI] [PubMed] [Google Scholar]
- 96.Parker WD, Jr, Parks JK, Swerdlow RH. Complex I deficiency in Parkinson’s disease frontal cortex. Brain Res. 2008;1189:215–218. doi: 10.1016/j.brainres.2007.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del TD, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, Gonzalez-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B, Auburger G, Wood NW. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 98.Kitada T, Pisani A, Porter DR, Yamaguchi H, Tscherter A, Martella G, Bonsi P, Zhang C, Pothos EN, Shen J. Impaired dopamine release and synaptic plasticity in the striatum of PINK1-deficient mice. Proc Natl Acad Sci U S A. 2007;104:11441–11446. doi: 10.1073/pnas.0702717104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gispert S, Ricciardi F, Kurz A, Azizov M, Hoepken HH, Becker D, Voos W, Leuner K, Muller WE, Kudin AP, Kunz WS, Zimmermann A, Roeper J, Wenzel D, Jendrach M, Garcia-Arencibia M, Fernandez-Ruiz J, Huber L, Rohrer H, Barrera M, Reichert AS, Rub U, Chen A, Nussbaum RL, Auburger G. Parkinson phenotype in aged PINK1-deficient mice is accompanied by progressive mitochondrial dysfunction in absence of neurodegeneration. PLoS One. 2009;4:e5777. doi: 10.1371/journal.pone.0005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Palacino JJ, Sagi D, Goldberg MS, Krauss S, Motz C, Wacker M, Klose J, Shen J. Mitochondrial dysfunction and oxidative damage in parkin-deficient mice. J Biol Chem. 2004;279:18614–18622. doi: 10.1074/jbc.M401135200. [DOI] [PubMed] [Google Scholar]
- 101.Schmidt S, Linnartz B, Mendritzki S, Sczepan T, Lubbert M, Stichel CC, Lubbert H. Genetic mouse models for Parkinson’s disease display severe pathology in glial cell mitochondria. Hum Mol Genet. 2011 doi: 10.1093/hmg/ddq564. [DOI] [PubMed] [Google Scholar]
- 102.Yu W, Sun Y, Guo S, Lu B. The PINK1/Parkin pathway regulates mitochondrial dynamics and function in mammalian hippocampal and dopaminergic neurons. Hum Mol Genet. 2011 doi: 10.1093/hmg/ddr235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nagakubo D, Taira T, Kitaura H, Ikeda M, Tamai K, Iguchi-Ariga SM, Ariga H. DJ-1, a novel oncogene which transforms mouse NIH3T3 cells in cooperation with ras. Biochem Biophys Res Commun. 1997;231:509–513. doi: 10.1006/bbrc.1997.6132. [DOI] [PubMed] [Google Scholar]
- 104.Martinat C, Shendelman S, Jonason A, Leete T, Beal MF, Yang L, Floss T, Abeliovich A. Sensitivity to oxidative stress in DJ-1-deficient dopamine neurons: an ES- derived cell model of primary Parkinsonism. PLoS Biol. 2004;2:e327. doi: 10.1371/journal.pbio.0020327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, van Dongen JW, Vanacore N, van Swieten JC, Brice A, Meco G, van Duijn CM, Oostra BA, Heutink P. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- 106.Goldberg MS, Pisani A, Haburcak M, Vortherms TA, Kitada T, Costa C, Tong Y, Martella G, Tscherter A, Martins A, Bernardi G, Roth BL, Pothos EN, Calabresi P, Shen J. Nigrostriatal dopaminergic deficits and hypokinesia caused by inactivation of the familial Parkinsonism-linked gene DJ-1. Neuron. 2005;45:489–496. doi: 10.1016/j.neuron.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 107.Krebiehl G, Ruckerbauer S, Burbulla LF, Kieper N, Maurer B, Waak J, Wolburg H, Gizatullina Z, Gellerich FN, Woitalla D, Riess O, Kahle PJ, Proikas-Cezanne T, Kruger R. Reduced basal autophagy and impaired mitochondrial dynamics due to loss of Parkinson’s disease-associated protein DJ-1. PLoS One. 2010;5:e9367. doi: 10.1371/journal.pone.0009367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pham TT, Giesert F, Rothig A, Floss T, Kallnik M, Weindl K, Holter SM, Ahting U, Prokisch H, Becker L, Klopstock T, Hrabe de AM, Beyer K, Gorner K, Kahle PJ, Vogt Weisenhorn DM, Wurst W. DJ-1-deficient mice show less TH-positive neurons in the ventral tegmental area and exhibit non-motoric behavioural impairments. Genes Brain Behav. 2010;9:305–317. doi: 10.1111/j.1601-183X.2009.00559.x. [DOI] [PubMed] [Google Scholar]
- 109.Ekstrand MI, Terzioglu M, Galter D, Zhu S, Hofstetter C, Lindqvist E, Thams S, Bergstrand A, Hansson FS, Trifunovic A, Hoffer B, Cullheim S, Mohammed AH, Olson L, Larsson NG. Progressive parkinsonism in mice with respiratory-chain-deficient dopamine neurons. Proc Natl Acad Sci U S A. 2007;104:1325–1330. doi: 10.1073/pnas.0605208103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jin HG, Yamashita H, Nakamura T, Fukuba H, Takahashi T, Hiji M, Kohriyama T, Matsumoto M. Synphilin-1 transgenic mice exhibit mild motor impairments. Neurosci Lett. 2008;445:12–17. doi: 10.1016/j.neulet.2008.08.073. [DOI] [PubMed] [Google Scholar]
- 111.Engelender S, Kaminsky Z, Guo X, Sharp AH, Amaravi RK, Kleiderlein JJ, Margolis RL, Troncoso JC, Lanahan AA, Worley PF, Dawson VL, Dawson TM, Ross CA. Synphilin-1 associates with alpha-synuclein and promotes the formation of cytosolic inclusions. Nat Genet. 1999;22:110–114. doi: 10.1038/8820. [DOI] [PubMed] [Google Scholar]
- 112.Marx FP, Holzmann C, Strauss KM, Li L, Eberhardt O, Gerhardt E, Cookson MR, Hernandez D, Farrer MJ, Kachergus J, Engelender S, Ross CA, Berger K, Schols L, Schulz JB, Riess O, Kruger R. Identification and functional characterization of a novel R621C mutation in the synphilin-1 gene in Parkinson’s disease. Hum Mol Genet. 2003;12:1223–1231. doi: 10.1093/hmg/ddg134. [DOI] [PubMed] [Google Scholar]
- 113.Myhre R, Klungland H, Farrer MJ, Aasly JO. Genetic association study of synphilin-1 in idiopathic Parkinson’s disease. BMC Med Genet. 2008;9:19. doi: 10.1186/1471-2350-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nuber S, Franck T, Wolburg H, Schumann U, Casadei N, Fischer K, Calaminus C, Pichler BJ, Chanarat S, Teismann P, Schulz JB, Luft AR, Tomiuk J, Wilbertz J, Bornemann A, Kruger R, Riess O. Transgenic overexpression of the alpha-synuclein interacting protein synphilin-1 leads to behavioral and neuropathological alterations in mice. Neurogenetics. 2010;11:107–120. doi: 10.1007/s10048-009-0212-2. [DOI] [PubMed] [Google Scholar]
- 115.Biskup S, Moore DJ, Celsi F, Higashi S, West AB, Andrabi SA, Kurkinen K, Yu SW, Savitt JM, Waldvogel HJ, Faull RL, Emson PC, Torp R, Ottersen OP, Dawson TM, Dawson VL. Localization of LRRK2 to membranous and vesicular structures in mammalian brain. Ann Neurol. 2006;60:557–569. doi: 10.1002/ana.21019. [DOI] [PubMed] [Google Scholar]
- 116.Gillardon F. Leucine-rich repeat kinase 2 phosphorylates brain tubulin-beta isoforms and modulates microtubule stability--a point of convergence in parkinsonian neurodegeneration? J Neurochem. 2009;110:1514–1522. doi: 10.1111/j.1471-4159.2009.06235.x. [DOI] [PubMed] [Google Scholar]
- 117.Li Y, Liu W, Oo TF, Wang L, Tang Y, Jackson-Lewis V, Zhou C, Geghman K, Bogdanov M, Przedborski S, Beal MF, Burke RE, Li C. Mutant LRRK2(R1441G) BAC transgenic mice recapitulate cardinal features of Parkinson’s disease. Nat Neurosci. 2009;12:826–828. doi: 10.1038/nn.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li X, Patel JC, Wang J, Avshalumov MV, Nicholson C, Buxbaum JD, Elder GA, Rice ME, Yue Z. Enhanced striatal dopamine transmission and motor performance with LRRK2 overexpression in mice is eliminated by familial Parkinson’s disease mutation G2019S. J Neurosci. 2010;30:1788–1797. doi: 10.1523/JNEUROSCI.5604-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tong Y, Yamaguchi H, Giaime E, Boyle S, Kopan R, Kelleher RJ, III, Shen J. Loss of leucine-rich repeat kinase 2 causes impairment of protein degradation pathways, accumulation of alpha-synuclein, and apoptotic cell death in aged mice. Proc Natl Acad Sci U S A. 2010;107:9879–9884. doi: 10.1073/pnas.1004676107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wakamatsu K, Fujikawa K, Zucca FA, Zecca L, Ito S. The structure of neuromelanin as studied by chemical degradative methods. J Neurochem. 2003;86:1015–1023. doi: 10.1046/j.1471-4159.2003.01917.x. [DOI] [PubMed] [Google Scholar]
- 121.Zecca L, Wilms H, Geick S, Claasen JH, Brandenburg LO, Holzknecht C, Panizza ML, Zucca FA, Deuschl G, Sievers J, Lucius R. Human neuromelanin induces neuroinflammation and neurodegeneration in the rat substantia nigra: implications for Parkinson’s disease. Acta Neuropathol. 2008;116:47–55. doi: 10.1007/s00401-008-0361-7. [DOI] [PubMed] [Google Scholar]
- 122.Bosco DA, Fowler DM, Zhang Q, Nieva J, Powers ET, Wentworth P, Jr, Lerner RA, Kelly JW. Elevated levels of oxidized cholesterol metabolites in Lewy body disease brains accelerate alpha-synuclein fibrilization. Nat Chem Biol. 2006;2:249–253. doi: 10.1038/nchembio782. [DOI] [PubMed] [Google Scholar]
- 123.Koob AO, Ubhi K, Paulsson JF, Kelly J, Rockenstein E, Mante M, Adame A, Masliah E. Lovastatin ameliorates alpha-synuclein accumulation and oxidation in transgenic mouse models of alpha-synucleinopathies. Exp Neurol. 2010;221:267–274. doi: 10.1016/j.expneurol.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.George S, Mok SS, Nurjono M, Ayton S, Finkelstein DI, Masters CL, Li QX, Culvenor JG. alpha-synuclein transgenic mice reveal compensatory increases in Parkinson’s disease-associated proteins DJ-1 and Parkin and have enhanced alpha-synuclein and PINK1 levels after rotenone treatment. J Mol Neurosci. 2010;42:243–254. doi: 10.1007/s12031-010-9378-1. [DOI] [PubMed] [Google Scholar]
- 125.Rodriguez-Navarro JA, Casarejos MJ, Menendez J, Solano RM, Rodal I, Gomez A, Yebenes JG, Mena MA. Mortality, oxidative stress and tau accumulation during ageing in parkin null mice. J Neurochem. 2007;103:98–114. doi: 10.1111/j.1471-4159.2007.04762.x. [DOI] [PubMed] [Google Scholar]
- 126.Gautier CA, Kitada T, Shen J. Loss of PINK1 causes mitochondrial functional defects and increased sensitivity to oxidative stress. Proc Natl Acad Sci U S A. 2008;105:11364–11369. doi: 10.1073/pnas.0802076105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kim RH, Smith PD, Aleyasin H, Hayley S, Mount MP, Pownall S, Wakeham A, You-Ten AJ, Kalia SK, Horne P, Westaway D, Lozano AM, Anisman H, Park DS, Mak TW. Hypersensitivity of DJ-1-deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) and oxidative stress. Proc Natl Acad Sci U S A. 2005;102:5215–5220. doi: 10.1073/pnas.0501282102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hao LY, Giasson BI, Bonini NM. DJ-1 is critical for mitochondrial function and rescues PINK1 loss of function. Proc Natl Acad Sci U S A. 2010;107:9747–9752. doi: 10.1073/pnas.0911175107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Irrcher I, Aleyasin H, Seifert EL, Hewitt SJ, Chhabra S, Phillips M, Lutz AK, Rousseaux MW, Bevilacqua L, Jahani-Asl A, Callaghan S, MacLaurin JG, Winklhofer KF, Rizzu P, Rippstein P, Kim RH, Chen CX, Fon EA, Slack RS, Harper ME, McBride HM, Mak TW, Park DS. Loss of the Parkinson’s disease-linked gene DJ-1 perturbs mitochondrial dynamics. Hum Mol Genet. 2010;19:3734–3746. doi: 10.1093/hmg/ddq288. [DOI] [PubMed] [Google Scholar]
- 130.Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhang W, Zhou Y, Hong JS, Zhang J. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J. 2005;19:533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- 131.Lee HJ, Suk JE, Patrick C, Bae EJ, Cho JH, Rho S, Hwang D, Masliah E, Lee SJ. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem. 2010;285:9262–9272. doi: 10.1074/jbc.M109.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shigemitsu K, Tsujishita Y, Hara K, Nanahoshi M, Avruch J, Yonezawa K. Regulation of translational effectors by amino acid and mammalian target of rapamycin signaling pathways. Possible involvement of autophagy in cultured hepatoma cells. J Biol Chem. 1999;274:1058–1065. doi: 10.1074/jbc.274.2.1058. [DOI] [PubMed] [Google Scholar]
- 133.Ueno T, Ishidoh K, Mineki R, Tanida I, Murayama K, Kadowaki M, Kominami E. Autolysosomal membrane-associated betaine homocysteine methyltransferase. Limited degradation fragment of a sequestered cytosolic enzyme monitoring autophagy. J Biol Chem. 1999;274:15222–15229. doi: 10.1074/jbc.274.21.15222. [DOI] [PubMed] [Google Scholar]
- 134.Crews L, Spencer B, Desplats P, Patrick C, Paulino A, Rockenstein E, Hansen L, Adame A, Galasko D, Masliah E. Selective molecular alterations in the autophagy pathway in patients with Lewy body disease and in models of alpha-synucleinopathy. PLoS One. 2010;5:e9313. doi: 10.1371/journal.pone.0009313. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 135.Gao HM, Kotzbauer PT, Uryu K, Leight S, Trojanowski JQ, Lee VM. Neuroinflammation and oxidation/nitration of alpha-synuclein linked to dopaminergic neurodegeneration. J Neurosci. 2008;28:7687–7698. doi: 10.1523/JNEUROSCI.0143-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Frank-Cannon TC, Tran T, Ruhn KA, Martinez TN, Hong J, Marvin M, Hartley M, Trevino I, O’Brien DE, Casey B, Goldberg MS, Tansey MG. Parkin deficiency increases vulnerability to inflammation-related nigral degeneration. J Neurosci. 2008;28:10825–10834. doi: 10.1523/JNEUROSCI.3001-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lin X, Parisiadou L, Gu XL, Wang L, Shim H, Sun L, Xie C, Long CX, Yang WJ, Ding J, Chen ZZ, Gallant PE, Tao-Cheng JH, Rudow G, Troncoso JC, Liu Z, Li Z, Cai H. Leucine-rich repeat kinase 2 regulates the progression of neuropathology induced by Parkinson’s-disease-related mutant alpha-synuclein. Neuron. 2009;64:807–827. doi: 10.1016/j.neuron.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Smith WW, Liu Z, Liang Y, Masuda N, Swing DA, Jenkins NA, Copeland NG, Troncoso JC, Pletnikov M, Dawson TM, Martin LJ, Moran TH, Lee MK, Borchelt DR, Ross CA. Synphilin-1 attenuates neuronal degeneration in the A53T alpha-synuclein transgenic mouse model. Hum Mol Genet. 2010;19:2087–2098. doi: 10.1093/hmg/ddq086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Spira PJ, Sharpe DM, Halliday G, Cavanagh J, Nicholson GA. Clinical and pathological features of a Parkinsonian syndrome in a family with an Ala53Thr alpha-synuclein mutation. Ann Neurol. 2001;49:313–319. [PubMed] [Google Scholar]
- 140.Puschmann A, Ross OA, Vilarino-Guell C, Lincoln SJ, Kachergus JM, Cobb SA, Lindquist SG, Nielsen JE, Wszolek ZK, Farrer M, Widner H, van WD, Hagerstrom D, Markopoulou K, Chase BA, Nilsson K, Reimer J, Nilsson C. A Swedish family with de novo alpha-synuclein A53T mutation: evidence for early cortical dysfunction. Parkinsonism Relat Disord. 2009;15:627–632. doi: 10.1016/j.parkreldis.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Seidel K, Schols L, Nuber S, Petrasch-Parwez E, Gierga K, Wszolek Z, Dickson D, Gai WP, Bornemann A, Riess O, Rami A, Den Dunnen WF, Deller T, Rub U, Kruger R. First appraisal of brain pathology owing to A30P mutant alpha-synuclein. Ann Neurol. 2010;67:684–689. doi: 10.1002/ana.21966. [DOI] [PubMed] [Google Scholar]
- 142.Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Gomez TE, del ST, Munoz DG, de Yebenes JG. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 143.Choi W, Zibaee S, Jakes R, Serpell LC, Davletov B, Crowther RA, Goedert M. Mutation E46K increases phospholipid binding and assembly into filaments of human alpha-synuclein. FEBS Lett. 2004;576:363–368. doi: 10.1016/j.febslet.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 144.Nishioka K, Hayashi S, Farrer MJ, Singleton AB, Yoshino H, Imai H, Kitami T, Sato K, Kuroda R, Tomiyama H, Mizoguchi K, Murata M, Toda T, Imoto I, Inazawa J, Mizuno Y, Hattori N. Clinical heterogeneity of alpha-synuclein gene duplication in Parkinson’s disease. Ann Neurol. 2006;59:298–309. doi: 10.1002/ana.20753. [DOI] [PubMed] [Google Scholar]
- 145.Fuchs J, Nilsson C, Kachergus J, Munz M, Larsson EM, Schule B, Langston JW, Middleton FA, Ross OA, Hulihan M, Gasser T, Farrer MJ. Phenotypic variation in a large Swedish pedigree due to SNCA duplication and triplication. Neurology. 2007;68:916–922. doi: 10.1212/01.wnl.0000254458.17630.c5. [DOI] [PubMed] [Google Scholar]
- 146.Farrer M, Kachergus J, Forno L, Lincoln S, Wang DS, Hulihan M, Maraganore D, Gwinn-Hardy K, Wszolek Z, Dickson D, Langston JW. Comparison of kindreds with parkinsonism and alpha-synuclein genomic multiplications. Ann Neurol. 2004;55:174–179. doi: 10.1002/ana.10846. [DOI] [PubMed] [Google Scholar]
- 147.Takahashi H, Ohama E, Suzuki S, Horikawa Y, Ishikawa A, Morita T, Tsuji S, Ikuta F. Familial juvenile parkinsonism: clinical and pathologic study in a family. Neurology. 1994;44:437–441. doi: 10.1212/wnl.44.3_part_1.437. [DOI] [PubMed] [Google Scholar]
- 148.Mori H, Kondo T, Yokochi M, Matsumine H, Nakagawa-Hattori Y, Miyake T, Suda K, Mizuno Y. Pathologic and biochemical studies of juvenile parkinsonism linked to chromosome 6q. Neurology. 1998;51:890–892. doi: 10.1212/wnl.51.3.890. [DOI] [PubMed] [Google Scholar]
- 149.Hatano Y, Sato K, Elibol B, Yoshino H, Yamamura Y, Bonifati V, Shinotoh H, Asahina M, Kobayashi S, Ng AR, Rosales RL, Hassin-Baer S, Shinar Y, Lu CS, Chang HC, Wu-Chou YH, Atac FB, Kobayashi T, Toda T, Mizuno Y, Hattori N. PARK6-linked autosomal recessive early-onset parkinsonism in Asian populations. Neurology. 2004;63:1482–1485. doi: 10.1212/01.wnl.0000142258.29304.fe. [DOI] [PubMed] [Google Scholar]
- 150.Bonifati V, Rohe CF, Breedveld GJ, Fabrizio E, De MM, Tassorelli C, Tavella A, Marconi R, Nicholl DJ, Chien HF, Fincati E, Abbruzzese G, Marini P, De GA, Horstink MW, Maat-Kievit JA, Sampaio C, Antonini A, Stocchi F, Montagna P, Toni V, Guidi M, Dalla LA, Tinazzi M, De PF, Fabbrini G, Goldwurm S, de KA, Barbosa E, Lopiano L, Martignoni E, Lamberti P, Vanacore N, Meco G, Oostra BA. Early-onset parkinsonism associated with PINK1 mutations: frequency, genotypes, and phenotypes. Neurology. 2005;65:87–95. doi: 10.1212/01.wnl.0000167546.39375.82. [DOI] [PubMed] [Google Scholar]
- 151.Samaranch L, Lorenzo-Betancor O, Arbelo JM, Ferrer I, Lorenzo E, Irigoyen J, Pastor MA, Marrero C, Isla C, Herrera-Henriquez J, Pastor P. PINK1-linked parkinsonism is associated with Lewy body pathology. Brain. 2010;133:1128–1142. doi: 10.1093/brain/awq051. [DOI] [PubMed] [Google Scholar]
- 152.van Duijn CM, Dekker MC, Bonifati V, Galjaard RJ, Houwing-Duistermaat JJ, Snijders PJ, Testers L, Breedveld GJ, Horstink M, Sandkuijl LA, van Swieten JC, Oostra BA, Heutink P. Park7, a novel locus for autosomal recessive early-onset parkinsonism, on chromosome 1p36. Am J Hum Genet. 2001;69:629–634. doi: 10.1086/322996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wszolek ZK, Pfeiffer B, Fulgham JR, Parisi JE, Thompson BM, Uitti RJ, Calne DB, Pfeiffer RF. Western Nebraska family (family D) with autosomal dominant parkinsonism. Neurology. 1995;45:502–505. doi: 10.1212/wnl.45.3.502. [DOI] [PubMed] [Google Scholar]
- 154.Hasegawa K, Kowa H. Autosomal dominant familial Parkinson disease: older onset of age, and good response to levodopa therapy. Eur Neurol. 1997;38(Suppl 1):39–43. doi: 10.1159/000113460. [DOI] [PubMed] [Google Scholar]
- 155.Najim al-Din AS, Wriekat A, Mubaidin A, Dasouki M, Hiari M. Pallido-pyramidal degeneration, supranuclear upgaze paresis and dementia: Kufor-Rakeb syndrome. Acta Neurol Scand. 1994;89:347–352. doi: 10.1111/j.1600-0404.1994.tb02645.x. [DOI] [PubMed] [Google Scholar]
- 156.Ramirez A, Heimbach A, Grundemann J, Stiller B, Hampshire D, Cid LP, Goebel I, Mubaidin AF, Wriekat AL, Roeper J, Al-Din A, Hillmer AM, Karsak M, Liss B, Woods CG, Behrens MI, Kubisch C. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat Genet. 2006;38:1184–1191. doi: 10.1038/ng1884. [DOI] [PubMed] [Google Scholar]
- 157.Goker-Alpan O, Schiffmann R, LaMarca ME, Nussbaum RL, McInerney-Leo A, Sidransky E. Parkinsonism among Gaucher disease carriers. J Med Genet. 2004;41:937–940. doi: 10.1136/jmg.2004.024455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Aharon-Peretz J, Rosenbaum H, Gershoni-Baruch R. Mutations in the glucocerebrosidase gene and Parkinson’s disease in Ashkenazi Jews. N Engl J Med. 2004;351:1972–1977. doi: 10.1056/NEJMoa033277. [DOI] [PubMed] [Google Scholar]
- 159.Neumann J, Bras J, Deas E, O’Sullivan SS, Parkkinen L, Lachmann RH, Li A, Holton J, Guerreiro R, Paudel R, Segarane B, Singleton A, Lees A, Hardy J, Houlden H, Revesz T, Wood NW. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson’s disease. Brain. 2009;132:1783–1794. doi: 10.1093/brain/awp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gan-Or Z, Giladi N, Orr-Urtreger A. Differential phenotype in Parkinson’s disease patients with severe versus mild GBA mutations. Brain. 2009;132:e125. doi: 10.1093/brain/awp161. [DOI] [PubMed] [Google Scholar]
- 161.Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, Iwatsubo T. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4:160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- 162.Crews L, Mizuno H, Desplats P, Rockenstein E, Adame A, Patrick C, Winner B, Winkler J, Masliah E. Alpha-synuclein alters Notch-1 expression and neurogenesis in mouse embryonic stem cells and in the hippocampus of transgenic mice. J Neurosci. 2008;28:4250–4260. doi: 10.1523/JNEUROSCI.0066-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wang L, Fleming SM, Chesselet MF, Tache Y. Abnormal colonic motility in mice overexpressing human wild-type alpha-synuclein. Neuroreport. 2008;19:873–876. doi: 10.1097/WNR.0b013e3282ffda5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Paleologou KE, Oueslati A, Shakked G, Rospigliosi CC, Kim HY, Lamberto GR, Fernandez CO, Schmid A, Chegini F, Gai WP, Chiappe D, Moniatte M, Schneider BL, Aebischer P, Eliezer D, Zweckstetter M, Masliah E, Lashuel HA. Phosphorylation at S87 is enhanced in synucleinopathies, inhibits alpha-synuclein oligomerization, and influences synuclein-membrane interactions. J Neurosci. 2010;30:3184–3198. doi: 10.1523/JNEUROSCI.5922-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gallardo G, Schluter OM, Sudhof TC. A molecular pathway of neurodegeneration linking alpha-synuclein to ApoE and Abeta peptides. Nat Neurosci. 2008;11:301–308. doi: 10.1038/nn2058. [DOI] [PubMed] [Google Scholar]
- 166.Freichel C, Neumann M, Ballard T, Muller V, Woolley M, Ozmen L, Borroni E, Kretzschmar HA, Haass C, Spooren W, Kahle PJ. Age-dependent cognitive decline and amygdala pathology in alpha-synuclein transgenic mice. Neurobiol Aging. 2007;28:1421–1435. doi: 10.1016/j.neurobiolaging.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 167.Zhou W, Freed CR. Tyrosine-to-cysteine modification of human alpha-synuclein enhances protein aggregation and cellular toxicity. J Biol Chem. 2004;279:10128–10135. doi: 10.1074/jbc.M307563200. [DOI] [PubMed] [Google Scholar]
- 168.Thiruchelvam MJ, Powers JM, Cory-Slechta DA, Richfield EK. Risk factors for dopaminergic neuron loss in human alpha-synuclein transgenic mice. Eur J Neurosci. 2004;19:845–854. doi: 10.1111/j.0953-816x.2004.03139.x. [DOI] [PubMed] [Google Scholar]
- 169.Matsuoka Y, Vila M, Lincoln S, McCormack A, Picciano M, LaFrancois J, Yu X, Dickson D, Langston WJ, McGowan E, Farrer M, Hardy J, Duff K, Przedborski S, Di Monte DA. Lack of nigral pathology in transgenic mice expressing human alpha-synuclein driven by the tyrosine hydroxylase promoter. Neurobiol Dis. 2001;8:535–539. doi: 10.1006/nbdi.2001.0392. [DOI] [PubMed] [Google Scholar]
- 170.Lou H, Montoya SE, Alerte TN, Wang J, Wu J, Peng X, Hong CS, Friedrich EE, Mader SA, Pedersen CJ, Marcus BS, McCormack AL, Di Monte DA, Daubner SC, Perez RG. Serine 129 phosphorylation reduces the ability of alpha-synuclein to regulate tyrosine hydroxylase and protein phosphatase 2A in vitro and in vivo. J Biol Chem. 2010;285:17648–17661. doi: 10.1074/jbc.M110.100867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chen L, Thiruchelvam MJ, Madura K, Richfield EK. Proteasome dysfunction in aged human alpha-synuclein transgenic mice. Neurobiol Dis. 2006;23:120–126. doi: 10.1016/j.nbd.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 172.Su X, Federoff HJ, Maguire-Zeiss KA. Mutant alpha-synuclein overexpression mediates early proinflammatory activity. Neurotox Res. 2009;16:238–254. doi: 10.1007/s12640-009-9053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wakamatsu M, Ishii A, Ukai Y, Sakagami J, Iwata S, Ono M, Matsumoto K, Nakamura A, Tada N, Kobayashi K, Iwatsubo T, Yoshimoto M. Accumulation of phosphorylated alpha-synuclein in dopaminergic neurons of transgenic mice that express human alpha-synuclein. J Neurosci Res. 2007;85:1819–1825. doi: 10.1002/jnr.21310. [DOI] [PubMed] [Google Scholar]
- 174.Wakamatsu M, Ishii A, Iwata S, Sakagami J, Ukai Y, Ono M, Kanbe D, Muramatsu S, Kobayashi K, Iwatsubo T, Yoshimoto M. Selective loss of nigral dopamine neurons induced by overexpression of truncated human alpha-synuclein in mice. Neurobiol Aging. 2008;29:574–585. doi: 10.1016/j.neurobiolaging.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 175.Gispert S, Del TD, Garrett L, Chen A, Bernard DJ, Hamm-Clement J, Korf HW, Deller T, Braak H, Auburger G, Nussbaum RL. Transgenic mice expressing mutant A53T human alpha-synuclein show neuronal dysfunction in the absence of aggregate formation. Mol Cell Neurosci. 2003;24:419–429. doi: 10.1016/s1044-7431(03)00198-2. [DOI] [PubMed] [Google Scholar]
- 176.Tsika E, Moysidou M, Guo J, Cushman M, Gannon P, Sandaltzopoulos R, Giasson BI, Krainc D, Ischiropoulos H, Mazzulli JR. Distinct region-specific alpha-synuclein oligomers in A53T transgenic mice: implications for neurodegeneration. J Neurosci. 2010;30:3409–3418. doi: 10.1523/JNEUROSCI.4977-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Shirakashi Y, Kawamoto Y, Tomimoto H, Takahashi R, Ihara M. alpha-Synuclein is colocalized with 14-3-3 and synphilin-1 in A53T transgenic mice. Acta Neuropathol. 2006;112:681–689. doi: 10.1007/s00401-006-0132-2. [DOI] [PubMed] [Google Scholar]
- 178.Graham DR, Sidhu A. Mice expressing the A53T mutant form of human alpha-synuclein exhibit hyperactivity and reduced anxiety-like behavior. J Neurosci Res. 2010;88:1777–1783. doi: 10.1002/jnr.22331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Nuber S, Petrasch-Parwez E, Winner B, Winkler J, von HS, Schmidt T, Boy J, Kuhn M, Nguyen HP, Teismann P, Schulz JB, Neumann M, Pichler BJ, Reischl G, Holzmann C, Schmitt I, Bornemann A, Kuhn W, Zimmermann F, Servadio A, Riess O. Neurodegeneration and motor dysfunction in a conditional model of Parkinson’s disease. J Neurosci. 2008;28:2471–2484. doi: 10.1523/JNEUROSCI.3040-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gomez-Isla T, Irizarry MC, Mariash A, Cheung B, Soto O, Schrump S, Sondel J, Kotilinek L, Day J, Schwarzschild MA, Cha JH, Newell K, Miller DW, Ueda K, Young AB, Hyman BT, Ashe KH. Motor dysfunction and gliosis with preserved dopaminergic markers in human alpha-synuclein A30P transgenic mice. Neurobiol Aging. 2003;24:245–258. doi: 10.1016/s0197-4580(02)00091-x. [DOI] [PubMed] [Google Scholar]
- 181.Steidl JV, Gomez-Isla T, Mariash A, Ashe KH, Boland LM. Altered short-term hippocampal synaptic plasticity in mutant alpha-synuclein transgenic mice. Neuroreport. 2003;14:219–223. doi: 10.1097/00001756-200302100-00012. [DOI] [PubMed] [Google Scholar]
- 182.Plaas M, Karis A, Innos J, Rebane E, Baekelandt V, Vaarmann A, Luuk H, Vasar E, Koks S. Alpha-synuclein A30P point-mutation generates age-dependent nigrostriatal deficiency in mice. J Physiol Pharmacol. 2008;59:205–216. [PubMed] [Google Scholar]
- 183.Maskri L, Zhu X, Fritzen S, Kuhn K, Ullmer C, Engels P, Andriske M, Stichel CC, Lubbert H. Influence of different promoters on the expression pattern of mutated human alpha-synuclein in transgenic mice. Neurodegener Dis. 2004;1:255–265. doi: 10.1159/000085064. [DOI] [PubMed] [Google Scholar]
- 184.Tofaris GK, Garcia RP, Humby T, Lambourne SL, O’Connell M, Ghetti B, Gossage H, Emson PC, Wilkinson LS, Goedert M, Spillantini MG. Pathological changes in dopaminergic nerve cells of the substantia nigra and olfactory bulb in mice transgenic for truncated human alpha-synuclein(1–120): implications for Lewy body disorders. J Neurosci. 2006;26:3942–3950. doi: 10.1523/JNEUROSCI.4965-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Daher JP, Ying M, Banerjee R, McDonald RS, Hahn MD, Yang L, Flint BM, Thomas B, Dawson VL, Dawson TM, Moore DJ. Conditional transgenic mice expressing C-terminally truncated human alpha-synuclein (alphaSyn119) exhibit reduced striatal dopamine without loss of nigrostriatal pathway dopaminergic neurons. Mol Neurodegener. 2009;4:34. doi: 10.1186/1750-1326-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gu XL, Long CX, Sun L, Xie C, Lin X, Cai H. Astrocytic expression of Parkinson’s disease-related A53T alpha-synuclein causes neurodegeneration in mice. Mol Brain. 2010;3:12. doi: 10.1186/1756-6606-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sato S, Chiba T, Nishiyama S, Kakiuchi T, Tsukada H, Hatano T, Fukuda T, Yasoshima Y, Kai N, Kobayashi K, Mizuno Y, Tanaka K, Hattori N. Decline of striatal dopamine release in parkin-deficient mice shown by ex vivo autoradiography. J Neurosci Res. 2006;84:1350–1357. doi: 10.1002/jnr.21032. [DOI] [PubMed] [Google Scholar]
- 188.Von CR, Thomas B, Savitt JM, Lim KL, Sasaki M, Hess EJ, Dawson VL, Dawson TM. Loss of locus coeruleus neurons and reduced startle in parkin null mice. Proc Natl Acad Sci U S A. 2004;101:10744–10749. doi: 10.1073/pnas.0401297101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.da Costa CA, Sunyach C, Giaime E, West A, Corti O, Brice A, Safe S, Abou-Sleiman PM, Wood NW, Takahashi H, Goldberg MS, Shen J, Checler F. Transcriptional repression of p53 by parkin and impairment by mutations associated with autosomal recessive juvenile Parkinson’s disease. Nat Cell Biol. 2009;11:1370–1375. doi: 10.1038/ncb1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Itier JM, Ibanez P, Mena MA, Abbas N, Cohen-Salmon C, Bohme GA, Laville M, Pratt J, Corti O, Pradier L, Ret G, Joubert C, Periquet M, Araujo F, Negroni J, Casarejos MJ, Canals S, Solano R, Serrano A, Gallego E, Sanchez M, Denefle P, Benavides J, Tremp G, Rooney TA, Brice A, Garcia de YJ. Parkin gene inactivation alters behaviour and dopamine neurotransmission in the mouse. Hum Mol Genet. 2003;12:2277–2291. doi: 10.1093/hmg/ddg239. [DOI] [PubMed] [Google Scholar]
- 191.Son JH, Kawamata H, Yoo MS, Kim DJ, Lee YK, Kim S, Dawson TM, Zhang H, Sulzer D, Yang L, Beal MF, Degiorgio LA, Chun HS, Baker H, Peng C. Neurotoxicity and behavioral deficits associated with Septin 5 accumulation in dopaminergic neurons. J Neurochem. 2005;94:1040–1053. doi: 10.1111/j.1471-4159.2005.03257.x. [DOI] [PubMed] [Google Scholar]
- 192.Lu XH, Fleming SM, Meurers B, Ackerson LC, Mortazavi F, Lo V, Hernandez D, Sulzer D, Jackson GR, Maidment NT, Chesselet MF, Yang XW. Bacterial artificial chromosome transgenic mice expressing a truncated mutant parkin exhibit age-dependent hypokinetic motor deficits, dopaminergic neuron degeneration, and accumulation of proteinase K-resistant alpha-synuclein. J Neurosci. 2009;29:1962–1976. doi: 10.1523/JNEUROSCI.5351-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Parish CL, Finkelstein DI, Drago J, Borrelli E, Horne MK. The role of dopamine receptors in regulating the size of axonal arbors. J Neurosci. 2001;21:5147–5157. doi: 10.1523/JNEUROSCI.21-14-05147.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Chen L, Cagniard B, Mathews T, Jones S, Koh HC, Ding Y, Carvey PM, Ling Z, Kang UJ, Zhuang X. Age-dependent motor deficits and dopaminergic dysfunction in DJ-1 null mice. J Biol Chem. 2005;280:21418–21426. doi: 10.1074/jbc.M413955200. [DOI] [PubMed] [Google Scholar]
- 195.Chandran JS, Lin X, Zapata A, Hoke A, Shimoji M, Moore SO, Galloway MP, Laird FM, Wong PC, Price DL, Bailey KR, Crawley JN, Shippenberg T, Cai H. Progressive behavioral deficits in DJ-1-deficient mice are associated with normal nigrostriatal function. Neurobiol Dis. 2008;29:505–514. doi: 10.1016/j.nbd.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tong Y, Pisani A, Martella G, Karouani M, Yamaguchi H, Pothos EN, Shen J. R1441C mutation in LRRK2 impairs dopaminergic neurotransmission in mice. Proc Natl Acad Sci U S A. 2009;106:14622–14627. doi: 10.1073/pnas.0906334106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Fournier M, Vitte J, Garrigue J, Langui D, Dullin JP, Saurini F, Hanoun N, Perez-Diaz F, Cornilleau F, Joubert C, Ardila-Osorio H, Traver S, Duchateau R, Goujet-Zalc C, Paleologou K, Lashuel HA, Haass C, Duyckaerts C, Cohen-Salmon C, Kahle PJ, Hamon M, Brice A, Corti O. Parkin deficiency delays motor decline and disease manifestation in a mouse model of synucleinopathy. PLoS One. 2009;4:e6629. doi: 10.1371/journal.pone.0006629. [DOI] [PMC free article] [PubMed] [Google Scholar]