Abstract
Objective
Hepatitis B virus (HBV) infection remains a serious public health problem, due in part to low vaccination rates among high-risk adults, many of whom decline vaccination because of barriers such as perceived inconvenience or discomfort. This study evaluates the efficacy of a self-prediction intervention to increase HBV vaccination rates among high-risk adults.
Method
Randomized controlled trial of 1175 adults recruited from three STD clinics in the United States over 28 months. Participants completed an audio-computer-assisted self-interview (A-CASI), which presented information about HBV infection and vaccination, and measured relevant beliefs, behaviors and demographics. Half of participants were assigned randomly to a "self-prediction" intervention, asking them to predict their future acceptance of HBV vaccination. The main outcome measure was subsequent vaccination behavior. Other measures included perceived barriers to HBV vaccination, measured prior to the intervention.
Results
There was a significant interaction between the intervention and vaccination barriers, indicating the effect of the intervention differed depending on perceived vaccination barriers. Among high-barriers patients, the intervention significantly increased vaccination acceptance. Among low-barrier patients, the intervention did not influence vaccination acceptance.
Conclusions
The self-prediction intervention significantly increased vaccination acceptance among "high-barriers" patients, who typically have very low vaccination rates. This brief intervention could be a useful tool in increasing vaccine uptake among high-barriers patients.
Keywords: HBV vaccination, Self-prediction, Mere Measurement, Temporal Construal
INTRODUCTION
Hepatitis B virus (HBV) infection is the world's tenth leading cause of death, resulting in between 500,000 and 1.2 million deaths a year worldwide (Levanchy, 2004) and 4,000–5,000 deaths a year in the United States (CDC 2006). An effective HBV vaccine has been available for more than two decades, and many countries are adopting routine infant immunization to decrease long-term HBV infection rates. However, even after routine infant immunization is initiated, unvaccinated young adults will continue to become infected with HBV over at least the next twenty years. HBV risk is highest for adults with multiple sex partners, men who have sex with men, and injection drug users (CDC 2008). Further, while HBV vaccination appears to confer protection for over 20 years (McMahon et al 2009), it is possible that a booster dose will be required to maintain immunity through adulthood. For these reasons, programs to increase vaccination rates among high-risk adults will be important for many years to come.
Unfortunately, achieving high rates of HBV immunization among high-risk adults has proven difficult (Baars et al., 2009; Koblin et al., 2007; Zimet et al., 2008). Even when HBV vaccination is offered for free, many high-risk adults refuse to be vaccinated, often due to perceived short-term vaccination barriers, such as perceived inconvenience (Rudy, et al., 2003).
Thus, it is important to find interventions to increase HBV vaccine acceptance among high-risk adults. Toward this end, this study explores the efficacy of a brief "self-prediction" intervention in increasing HBV vaccination among high-risk adults. In the following sections, we: 1) Review research on the effects of self-prediction on subsequent behavior; 2) Discuss the contradictory findings concerning the impact of self-prediction on health behaviors; 3) Propose a potential moderating variable (perceived short-term barriers) that may help explain these contradictory findings; 4) Present the results of a large, multi-year randomized trial, examining the interactive effects of self-prediction and perceived barriers on vaccination behavior; 5) Discuss implications of our findings for both future research and clinical practice.
THEORY AND HYPOTHESES
Several behavioral models commonly employed by health psychologists, including the Theory of Reasoned Action and Theory of Planned Behavior (see e.g., Sieverding, et al. 2010; Armitage 2005), posit that intention is a key antecedent to actual behavior. Hence, health psychologists often ask respondents to answer behavioral-intention questions. However, a growing body of research suggests that the very act of measuring intention can influence subsequent behavior (e.g., Sherman 1980; Morwitz, et al. 1993; Godin, et al. 2008).
In his seminal paper on this “mere measurement” phenomenon, Sherman (1980) conducted a series of experiments in which college students were randomly assigned to either a self-prediction condition (in which they were asked to predict how they would respond to a hypothetical request) or a control condition (not asked the self-prediction question). Later, all participants were actually confronted with the request situation. In each experiment, participants’ predictions exhibited a social-desirability bias; for example, 47.8% of self-prediction subjects said they would agree if asked to volunteer for the American Cancer Society (ACS), whereas only 4.2 of control subjects actually complied with such a request. Furthermore, Sherman found that self-prediction subjects tended to “live up” to their rosy self-predictions, and actually behave in a more socially desirable way. For example, 31.3% of self-prediction subjects actually ended up complying with a subsequent request to volunteer for the ACS, seven times the volunteer rate among subjects who had not made prior predictions.
Subsequent studies have found self-prediction to influence actual behavior in a variety of contexts, including voting behavior (Greenwald, et al. 1987), consumer purchase behavior (Morwitz, et al. 1993) and recycling behavior (Spangenberg, et al. 2003). Based on these findings, self-prediction would seem to be a promising intervention to increase adoption of important health protection behaviors. However, to date only a few studies have examined the effect of self-prediction on health-related behaviors, and these studies have yielded inconsistent findings. For example, Godin, et al. (2008) found that experienced blood donors who received a questionnaire that included behavioral-intention questions exhibited higher subsequent blood-donation behavior, compared to a control group who did not receive the questionnaire. However, a later study (Godin, et al. 2010) found that answering a behavioral-intent question did not increase blood donations among novice donors (though answering more specific “implementation-intention” questions did slightly increase donation behavior). Similarly inconsistent results have been obtained in the context of other health-related behaviors. For example, Williams, Block and Fitzsimons (2006) found that asking intention questions about illegal drug use seemed to increase respondents’ subsequent drug-use behavior, while McCambridge and Day (2008) found that asking people questions about alcohol abuse decreased their subsequent alcohol-abuse behavior (see also Sherman 2008).
Given these conflicting findings, it is important to identify contingency variables that can help explain the variability in how measuring intention affects actual health behavior. As stated eloquently by Godin, et al. (2008, p. 183): “Mere measurement interventions clearly hold the potential to become an important additional strategy for promoting public health. However, further research on the efficacy and boundary conditions of mere measurement effects is needed from health psychologists in order to realize this potential.”
One potential boundary variable is suggested by research on how people think differently about near-term vs. future behaviors. The next section discusses the potential relevance of this research in understanding how self-prediction influences later behavior.
Temporal Construal
According to research on "temporal construal," when people consider behaviors that will take place in the distant or hypothetical future, they focus on the desirability of the behavior's end state (e.g., its potential long-term benefits) and disregard the feasibility of the concrete steps required to achieve this end state (see, e.g., Liberman & Trope 1998; Trope & Liberman 2003). So, for example, if a professor is contemplating attending an international conference in the distant future, s/he is likely to focus on the abstract benefits of attendance (experiencing other cultures, expanding knowledge) and give less thought to the concrete details of getting there (e.g., scheduling air travel, arranging baby sitters, dealing with jet lag). Similarly, if a patient is asked to predict future acceptance of a vaccine, s/he is likely to focus more on the vaccine’s abstract health consequences (e.g., protecting oneself from a serious disease) and less on the concrete process of getting vaccinated (travelling to the clinic, sitting in the waiting room, getting a needle stick in the arm). However, when people are forced to make a choice requiring imminent action (e.g., “Would you like to get vaccinated today?”), they are more likely to focus on the concrete steps required to complete the action. Among people who find these concrete steps unattractive (e.g., those who fear needles, or have difficulties making clinic visits), such considerations may cause them to forego the behavior.
In summarizing this research, Benson (2002) states that "…when deciding on future courses of action we tend to overemphasize abstract, high-level goals and ignore the concrete, low-level steps needed to reach them…According to Trope and Liberman’s ‘construal level theory’...an activity will tend to look less attractive the closer it is when…the concrete details are less pleasant than the abstract goals.” This effect of time horizon on decision criteria has been found in a variety of behavioral contexts, including people's willingness to commit to immediate vs. delayed altruistic behaviors (e.g., Pronin, et al. 2008) and current vs. future retirement-savings (e.g., Leiser, et al. 2008).
Temporal construal theory suggests that the effects of self-prediction on subsequent health behavior (e.g., HBV vaccination) may be moderated by individual perceptions of the concrete steps involved in completing the behavior. Among those who find these steps unattractive (e.g., patients who particularly dislike needles or clinic visits) self-prediction may increase compliance, by diminishing the decisional weight given to these deterrents. However, among individuals for whom these concrete steps are already of little concern (e.g., those who don’t mind shots or clinic visits) self-prediction would be expected to have little effect.
Hypotheses
Based on temporal-construal theory, we expect that patients who are asked to form vaccination intentions will focus on long-term health considerations (e.g., perceived HBV vulnerability and vaccine benefits) rather than short-term barriers (e.g., vaccination discomfort or inconvenience). Thus, while perceived barriers are expected to strongly affect vaccine uptake among patients not asked intentions, they are expected to have little/no effect on vaccination intentions, or the subsequent behavior of patients asked intentions. Therefore, asking intentions will be most likely to increase vaccine uptake among high-barriers patients, by reducing the decisional weight they give to concerns (e.g., fear of needles) that would otherwise cause them to refuse vaccination. In summary, we hypothesize:
- H1: Perceived short-term vaccination barriers (e.g., discomfort and inconvenience)
-
Will have a strong effect on
- Vaccination behavior of patients not asked intentions
-
Will have little/no effect on
- Vaccination intentions
- Vaccination behavior among patients asked intentions
-
H2: Asking intention will have an interactive effect on subsequent behavior, moderated by perceived barriers. Specifically, asking intention will be most likely to increase vaccine uptake among patients with high perceived barriers to vaccination
METHOD
Overview
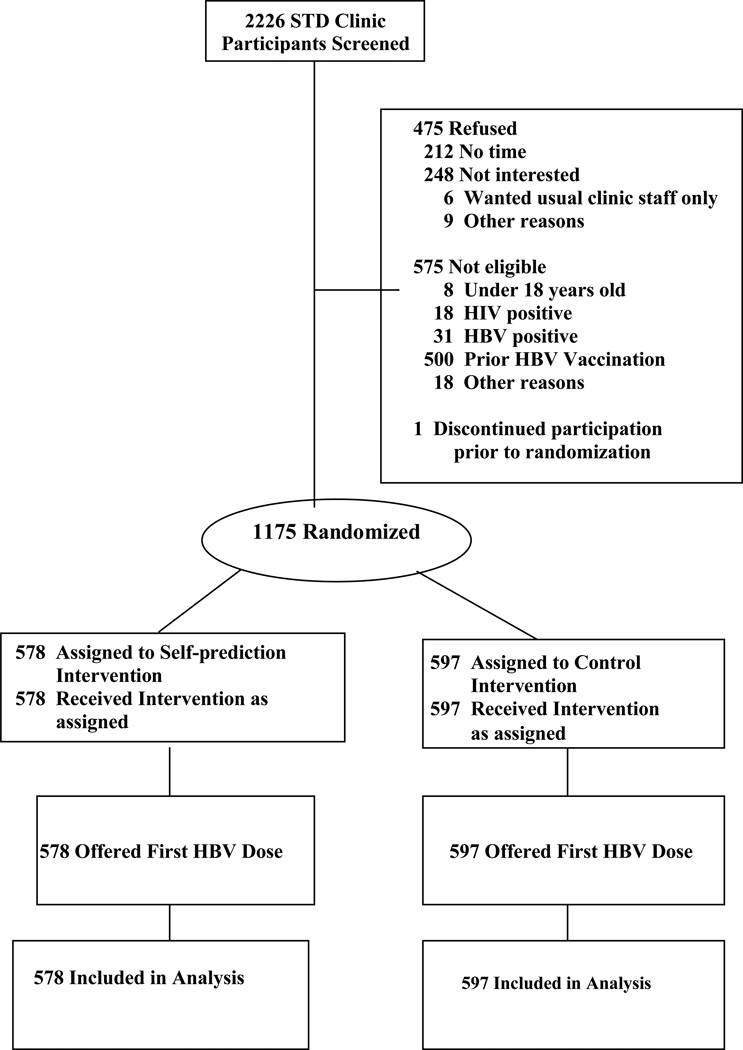
To test these hypotheses, we conducted a large-scale randomized controlled trial. A total of 1175 adult participants were recruited from the patient populations of three urban medical clinics over a period of 28 months, between December 2003 and April 2006. Following recruitment, each subject completed an audio computer-assisted self-interview (A-CASI), which included a detailed questionnaire concerning the target behavior (HBV vaccination) and related topics. Toward the end of the questionnaire, the A-CASI program randomly assigned participants to either a self-prediction condition (in which they were asked to state their intention to get vaccinated at some indefinite point in the future) or a control condition (with no intention questions). After completing the A-CASI, all participants were given the opportunity to receive the first vaccine dose, and their acceptance/rejection of this dose was recorded. Finally, the study tracked whether first-dose accepters returned for the second and third doses in the eight months following recruitment. The study flow is shown in Figure 1.
Figure 1.
Flow of Study Participants
Participants
Participants were adults attending clinics for diagnosis and treatment of sexually-transmitted diseases (STDs) who are therefore at high risk for HBV infection. A total of 1175 adult (age 18 and older) volunteers were recruited from the patient populations of three urban STD clinics, located in two large Midwestern cities, by two project managers (both of whom received extensive training in conducting clinical research and in the protocol of this specific study). Detailed sample demographics are reported in the Results section. Participants were recruited as part of a larger randomized trial examining the effects of brief interventions to increase HBV vaccine acceptance among high-risk adults. The interventions and questionnaire were administered via an audio computer-assisted self-interview (A-CASI) program, details of which are described below. Participants were compensated $20.00 for the time and effort involved in research participation; they were compensated only for completing the A-CASI, not for immunization. The study protocol was approved by the Institutional Review Boards at the investigators' university and the public health department that operated two of the clinics.
The inclusion criteria were age (18 years and older), no prior self-reported history of HBV immunization or infection, and ability to understand spoken and written English. While understanding of HBV vaccine acceptance among non-English speaking ethnic minorities is important, these populations were relatively small at all three study sites at the time of the study. Self-identified HIV-positive individuals were excluded from participation because they automatically receive HBV immunization as part of their HIV-related health care.
Intervention
Half of patients were assigned randomly to the self-prediction intervention, a series of five questions at the end of the A-CASI (see Figure 2) asking participants to state their future intention to receive the vaccination themselves and to recommend it to significant others. The remaining patients were assigned to a control condition that did not include the intention questions, but was otherwise identical to the intervention condition.
Figure 2.
Self-Prediction Intervention
Other than these five intention questions, there were no systematic differences in how the intervention and control subjects were treated in the clinic. The clinic personnel with whom participants interacted (the project manager and nurse practitioner) had no knowledge of which patients had been assigned to the self-prediction or control conditions. Furthermore, neither intervention nor control patients had any indication that their A-CASI questionnaire was different from that received by other participants.
Data Collection Procedure
After initial participant recruitment and screening, the project manager obtained written informed consent; seated the participant in a private setting in front of the computer; helped adjust headphones and screen angle; and started the A-CASI interviewing program. Next, the respondent completed several non-sensitive trial questions on the computer, with the project manager present to provide any additional instruction. After completing the trial questions, the participant began the self-interview. The project manager did not directly supervise the A-CASI interview, but was available to answer questions and resolve problems.
Over the past 10–15 years, there has been increasing use of A-CASI for collecting health-related self-reports (Romer, et al. 1994; Tourangeau and Smith 1996; Turner, et al. 1998). Advantages of A-CASI over written questionnaires include reduced data entry errors, decreased dependence on participant literacy (because of the audio presentation), increased reporting of sensitive behaviors, and greater protection of confidentiality because no written record exists.
The A-CASI program in this study contained the following sequence: First, participants received basic information about the hepatitis B virus and vaccine. Second, they completed demographic questions and reported on past HBV risk behaviors, including sexual and drug use behaviors. Third, they reported perceptions of any short-term discomfort associated with getting shots, and long-term risks of the HBV vaccine. Fourth, they received information on the health benefits of HBV vaccination. Fifth, they answered a series of health belief questions about HBV infection (including measures of perceived vulnerability to and severity of HBV infection, as well as perceived barriers to and benefits of HBV vaccination; specific questions are described in the Measures section). Finally, a randomization sub-program within the A-CASI program assigned half of participants to answer the self-prediction intervention questions (see Figure 2). On average, the A-CASI took subjects just under 18 minutes to complete.
Upon completion of the A-CASI, each participant was seen by the study's nurse practitioner for his/her STD visit, during which the participant was given an opportunity to receive his/her first HBV vaccine dose. If the patient agreed to vaccination, informed consent for HBV vaccination was obtained and the vaccine was administered by the nurse practitioner. The nurse practitioner documented vaccine acceptance/refusal, questions asked by the patient, reason for the clinic visit, and STD treatment or testing carried out and/or recommended.
Participants who received the first dose of HBV vaccine were scheduled for second and third doses, and asked to provide their address and phone numbers for contact purposes. Then approximately two weeks prior to each subsequent scheduled dose, patients received reminders of their scheduled appointments via both post card and telephone call.
Measures
Vaccination behavior
The primary dependent measure in this study was actual vaccination behavior. As noted earlier, participants' acceptance or rejection of the first vaccine dose was recorded by the nurse practitioner with whom the participant visited after completing the A-CASI; data on acceptance of subsequent vaccine doses was retrieved from the patients’ medical records by the study’s project manager. Both the nurse practitioners and the project manager were blinded to the patient’s experimental condition (i.e., self-prediction vs. control).
Perceived Short-term Barriers to Vaccination
Two scales assessed participants' perceived short-term barriers to getting the HBV vaccine. First, participants reported their agreement on a five-point Likert scale (5=Strongly Agree to 1=Strongly Disagree) with four statements measuring perceived discomfort associated with vaccination ("Getting shots can be scary; " "Shots are very painful;" "Needles don't bother me at all [reverse-scored, R];" "I am not afraid of shots[R]"). Participants' responses to the last two items were reverse scored, and then combined with scores on the first two items to form a mean scale ranging from 1 to 5, with a midpoint of 3. This mean scale had high internal consistency, with a coefficient alpha = .79. Second, participants reported their agreement (on the same five-point scale) with four statements measuring the perceived inconvenience of getting the HBV vaccine ("It would be hard for me to find time to get vaccinated for hepatitis B;" "It would be hard for me to get to a clinic or doctor 3 times to get completely vaccinated for hepatitis B;" "It would be hard for me to get transportation for more than one doctor's appointment to get vaccinated for hepatitis B;" "It would be easy for me to get to a clinic for the 3 shots of hepatitis B vaccine [R]"). Responses to the last item was reverse-scored, then combined with the other items to form a mean scale ranging from 1 to 5, with a midpoint of 3. This scale had a coefficient alpha = .85.
Perceived Long-Term Health Consequences of Vaccination
Two scales measured perceptions of the long-term health consequences of HBV infection and vaccination. Participants’ perceptions regarding their vulnerability to HBV infection were assessed by having them report their agreement/disagreement (on a five-point Likert scale) with three items ("I am worried about getting infected with the hepatitis B virus;" "The possibility of getting infected with hepatitis B virus concerns me;" "I don't worry about the possibility of getting infected with hepatitis B virus [R]"); a summed scale composed of these items had a coefficient alpha of .75. Participants’ perceptions regarding the protective benefits of HBV vaccination were assessed by having them report their agreement/disagree with three statements: “Getting vaccine shots against hepatitis B infection would be a good way to protect my health;” “One way for me to stay healthy would be to get the vaccine to prevent infection with hepatitis B;” and “The hepatitis B vaccine shots would not help me stay healthy [R].” Responses to these three items were combined to form a summed scale with coefficient alpha = .72.
RESULTS
Participants
A total of 2226 STD clinic patients were screened for potential participation in the study (see Figure 1). Of those screened, 575 were ruled ineligible (most due to prior HBV vaccination). Of the remaining 1651 eligible patients, 475 (28.8%) declined to participate (most due to reported lack of time or interest) and one patient discontinued participation prior to randomization. Thus, 1175 adult subjects were recruited into the study. This sample size provides >99% power to detect (with two-tailed alpha = .05) a true self-prediction/control difference in first-dose uptake of 60/40% in the total sample (n=1175); >99% power to detect an effect of this size among low-barriers patients (n=913); and 90.4% power to detect an effect this size among high-barriers patients (n=262).
Participants were 61% male; 82.7% African American (12.3% White, 5% mixed or other race); 48.3% between ages 20–29, 26.8% ages 30–39, 15.8% ages 40–49. By comparison, the total patient population of the three clinics (weighted by recruitment volume) was 62% male; 83.8% African American; with 45.4% ages 20–29, 23.8% ages 30–39, 10.0% ages 40–49. Only 2.4% of participants were Hispanic; 77% had at least a high school diploma or equivalent, 53.1% were employed, and 42.6% reported household incomes less than $10,000 per year.
Of the 1175 participants randomized, 578 were assigned to the self-prediction intervention, and 597 to the control condition. Table 1 compares these two groups on socio-demographic characteristics, self-reported HBV risk behaviors, and self-reported history of sexually-transmitted infections. As shown in Table 1, participants assigned to the self-prediction group did not differ from the control group on any of these variables.
Table 1.
Baseline Characteristics of the Self-Prediction and Control Conditions*
| Characteristics | Control Group (n=597) |
Self –Prediction Intervention (n=578) |
p** |
|---|---|---|---|
| Socio-demographics | |||
| Male | 360 (60.3) | 359 (62.1) | .525 |
| African-American | 491 (82.2) | 481 (83.2) | .709 |
| Hispanic | 10 (1.7) | 18 (3.1) | .316 |
| Employed | 320 (53.6) | 304 (52.6) | .349 |
| Income < $10K | 251 (42.0) | 250 (43.3) | .658 |
| Has raised children | 376 (63.0) | 348 (60.2) | .328 |
| High school diploma or more | 444 (74.4) | 455 (78.7) | .079 |
| Age (mean and standard deviation) | 31.8 (10.1) | 31 (9.6) | .175 |
| Self-Reported Risk Behaviors/Experiences | |||
| Used condom during last sexual encounter | 143 (24) | 168 (29.1) | .132 |
| Ever traded sex for money, drugs, food or shelter | 51 (8.5) | 60 (10.4) | .217 |
| Ever "shot up" drugs | 11 (1.8) | 5 (.9) | .148 |
| More than one sex partner, past 6 months | 362 (60.6) | 352 (60.9) | .926 |
| Ever received diagnosis for any sexually-transmitted disease | 442 (74.0) | 434 (75.1) | .530 |
Data represent Number (%) of participants, except age (mean and standard deviation)
All p values for chi-squared analysis, except age (p value for Student’s t statistic).
Effects of the Self-Prediction Intervention
Hypothesis 1 stated that a) among patients not asked to form intentions, short-term vaccination barriers would have a strong effect on vaccination behavior, but b) among patients asked to form intentions, short-term barriers would have little/no effect on either stated vaccination intention, or subsequent vaccination behavior. To test this hypothesis, we estimated three binary logistic regression models. The first model predicted actual first-dose vaccination acceptance (1=yes, 0=no) among patients not asked to form intentions. The second and third models predicted (respectively) stated intention to receive the first dose (1=yes, 0=no) and actual first-dose acceptance among patients asked to form intentions.
In all three models, the independent variables included both short-term barriers to receiving the HBV vaccination (the mean scales for perceived vaccination discomfort and perceived inconvenience) and long-term health considerations (the mean scales for perceived vulnerability to HBV infection and perceived health-protective benefits of HBV vaccination).
Table 2 summarizes the results of this analysis. As can be seen, among patients not asked to form vaccination intentions, vaccine acceptance is strongly influenced by both long-term health considerations (perceived vulnerability to HBV infection [Odds Ratio (OR)=1.55, p=.001] and protective benefits of the vaccine [OR= 3.07, p<.001]) and short-term barriers (perceived discomfort [OR= .676, p<.001] and inconvenience [OR= .670, p<.002]). However, among patients asked to form intentions, short-term barriers have no significant impact on either intentions or subsequent vaccination behavior. These results support Hypothesis 1.
Table 2.
Effect of Short-Term Barriers and Long-Term Health Considerations Among Patients Asked vs. Not Asked to Form Vaccination Intentions: Logistic Regression Analysis
| Patients NOT Asked Intention |
Patients ASKED Intention | ||||||
|---|---|---|---|---|---|---|---|
| First Dose Acceptance |
Stated Intention to Accept 1st Dose |
Actual 1st Dose Accepted |
|||||
| Independent Variables | Odds Ratio (95% CI) |
P value | Odds Ratio (95% CI) |
P value | Odds Ratio (95% CI) |
P value | |
| Short-Term Barriers | |||||||
| Perceived Discomfort | .68a(.56–.82) | <.001 | .89 (.72–1.1) | .29 | .97 (.81–1.17) | .76 | |
| Perceived Inconvenience | .67 (.52–.87) | .002 | .98 (.72–1.34) | .91 | 1.11 (.85–1.44) | .44 | |
| Long-Term Health Considerations | |||||||
| Perceived Vulnerability | 1.55b (1.19–2.0) | .001 | 2.41 (1.81–3.21) | <.001 | 1.77 (1.39–2.26) | <.001 | |
| Perceived Benefits | 3.07 (2.14–4.42) | <.001 | 3.69 (2.49–5.46) | <.001 | 2.29 (1.64–3.19) | <.001 | |
Note: Data from 1175 patients in urban STD clinics; 578 asked intention, 597 not asked intention.
Odds ratio of .68 indicates about a 32% decrease in the odds of first-dose acceptance for each one-unit increase in perceived vaccination discomfort.
Odds ratio of 1.55 indicates about a 55% increase in the odds of first-dose acceptance for each one-unit increase in perceived vulnerability to HBV infection.
Next, we examined whether asking intentions increased subsequent vaccination rates, either as a main effect within the entire sample, or (as predicted by H2) as an interactive effect, moderated by perceived barriers. A main effects analysis revealed that, in the total sample, patients asked to form intentions were slightly more likely to accept the first vaccine dose (55.2%) than patients not asked intentions (52.1%); however, this difference was not statistically significant (Χ2 (1) = 1.32, p=.29). We next examined whether (as posited by Hypothesis 2) self-prediction had an interactive effect on vaccination behavior, moderated by whether patients had high or low vaccination barriers. For the purposes of this analysis, participants were classified as having high vaccination barriers if the average of their scores on the two barriers subscales (perceived inconvenience and perceived discomfort) was above the scale midpoint of 3.0. Approximately one in four respondents (262 out of 1175) fell into this category. Next, we estimated a logistic regression model, in which the outcome variable was participants’ acceptance of the first HBV vaccine dose (1=accept first dose; 0=reject first dose), and the predictor variables were participants’ intervention condition (1=self-prediction; 0=control), perceived barriers (1=high; 0=low) and a multiplicative Intervention X Barriers interaction term. This analysis revealed a main effect of Barriers, in which high-barrier patients were less likely to accept the first dose than low-barrier patients (OR = 0.36; 95% confidence interval (CI): 0.24, 0.53; p<.001). The main effect of Intervention was not significant. However, as expected, there was a significant interaction between Intervention and Barriers (p<.001), indicating that the effect of the self-prediction intervention differed depending on the level of participants' perceived barriers to vaccination.
To better understand this interaction, we fit two additional logistic regression models, examining intervention effects within each Barrier group. As seen in Table 3, the intervention did not significantly affect vaccination acceptance among low-barriers patients (55.1% in intervention group vs. 57.6% in control; OR =0.90; 95% CI: 0.70, 1.17; p=.45). However, among high-barriers patients, the intervention significantly increased acceptance of the first vaccine dose (55.4% acceptance in intervention group vs. 32.6% in control; OR = 2.57; 95% CI: 1.56, 4.25; p<.001). Within the high-barriers group, patients seemed to exhibit what Sherman (1980, 2008) calls "self-erasing errors of prediction:" While only 32.6% of control (non-self-prediction) patients accepted the first vaccine dose, 61% of self-prediction patients said they would accept the first dose, and nearly as many (55.4%) actually did accept it (including 75.9% of those who had said "yes" to the first-dose intention question, and 23.5% of those who said had said "no.")
Table 3.
Effects of Self-Prediction Intervention on HBV Vaccine Acceptance among High-Barrier and Low-Barrier Patient
| High Barrier Patients (n=262) | Number (%) Accepting 1st Dose |
Odds Ratio (95%CI) |
P value |
|---|---|---|---|
| Self-Prediction Intervention (n=130) | 72 (55.4) | 2.57 (1.56, 4.25) | < .001 |
| Control (n=132) | 43 (32.6) | ||
| Low Barrier Patients (n=913) | |||
| Self-Prediction Intervention (n=448) | 247 (55.1) | .90 (.70, 1.17) | .45 |
| Control (n=465) | 268 (57.6) |
Thus, the self-prediction intervention increases acceptance of the first vaccine dose among patients with high perceived short-term barriers to vaccination. However, as noted earlier, a single dose of the HBV vaccine affords only partial disease protection. To receive the vaccine's full disease-protective benefit, consumers should receive multiple doses: a second dose one month after the first, and a third dose six months after the first. Thus, we next examined the impact of the self-prediction intervention on the total number of HBV vaccine doses participants received. As noted earlier, for each participant who received the first vaccine dose, the study recorded any subsequent HBV vaccine doses received over eight months following that participant's initial recruitment; i.e., the six-month period recommended for HBV vaccine-series completion, plus a two-month grace period. Since count data, such as number of vaccine doses, violate the distributional assumptions of conventional linear regression (Cameron & Trevidi 1998), the intervention's effect on total doses was analyzed using Poisson regression. The dependent variable in the Poisson regression model was number of vaccine doses received by the participant, a variable with potential values ranging from 0 to 3. The predictor variables were Intervention group (1=self-prediction; 0=control), vaccination Barriers (1=high; 0=low) and a multiplicative Intervention X Barriers interaction term.
The Poisson regression analysis revealed a significant main effect of Barriers, in which high-barrier patients received fewer total vaccine doses (M=.73) than low-barrier patients (M=1.01; Wald chi-square = 26.0, p<.001). The main effect of Intervention was not significant. However, there was again a significant interaction between the intervention and perceived vaccination barriers (Wald chi-square = 12.75, p<.001). To better understand this interaction, we fit two additional Poisson regression models to examine the intervention effects within each barrier group. These analyses revealed that, among high-barriers patients, the self-prediction intervention significantly increased the mean number of doses (M = .92 doses in intervention group vs. M = .54 doses in control group; Wald chi-square= 12.65; p< .001). However, among low-barriers patients, the intervention did not significantly affect number of doses (M = .98 doses in intervention group vs. M = 1.04 doses in control; Wald chi-square= .62; p=.43).
DISCUSSION
Hepatitis B infection remains a serious public health problem, in part due to low vaccination rates among high-risk adults. Even when HBV vaccination is provided for free, many resist vaccination because of short-term barriers, such as perceived inconvenience or discomfort. To address this problem, this study tested a brief intervention to increase vaccination rates among high-risk adults: simply asking participants to predict their future vaccination behavior. This intervention substantially increased vaccination rates among patients with high short-term vaccination barriers (who, in the absence of this intervention, have low vaccination acceptance rates). These findings are consistent with past research on temporal construal, which suggests that people asked to think about a future behavior tend to focus its abstract benefits, and disregard concrete barriers that might impede it.
The findings of this study may help deepen health psychologists’ understanding of how (and when) self-prediction influences future health behavior. Past research has shown that asking individuals to predict their future actions can influence a variety of non-health behaviors, ranging from volunteering to voting; however, research on the effects of self-prediction on health-related behavior has so far yielded contradictory results. This study suggests a boundary variable that may help explain these mixed findings: individuals’ perceptions of the concrete barriers to adopting the health behavior. Our findings suggest that self-prediction has little effect on patients with low vaccination barriers, but a substantial effect on patients with high barriers. Future research should examine whether perceived barriers moderate the effects of self-prediction on other health-related behaviors. In addition, future research should explore in greater depth the cognitive processes that may mediate the differential impact of self-prediction on the behavior of high-barriers vs. low-barriers patients (see, e.g., Muller, Judd and Yzerbyt 2005). Finally, future research should examine whether self-prediction effects are moderated, not only by individuals' short-term barriers to a specific health behavior, but also by their chronic tendencies to focus on short- vs. long-term consequences; e.g., by traits such as time orientation (e.g., Crockett, et al. 2009) or impulsiveness (e.g., Patton, et al. 1995).
The findings of this study also have potential implications for healthcare providers. At their most narrow level of application, the findings suggest that self-prediction may be a useful intervention in promoting HBV vaccination among high-risk adults, particularly those who normally resist vaccination because of perceived pain or inconvenience. The self-prediction intervention is quite brief, and could easily be integrated into existing patient questionnaires being employed in many clinics. However, it is important to note that while self-prediction strongly increased vaccine uptake among patients with high perceived vaccination barriers, it had no significant impact on patients with low barriers. This suggests that self-prediction might best be employed as a tailored intervention, in which patients identified as having high vaccination barriers (e.g., by earlier questionnaire responses) are asked to predict their future vaccination behavior, while low-barriers patients are not. As noted earlier, patient questionnaires are increasingly administered via computer survey software (e.g., CASI), whose branching-logic capabilities facilitate the use of such tailored interventions. Future research should explore the feasibility and efficacy of tailored use of self-prediction interventions.
More broadly, self-prediction holds promise for increasing patient utilization of other services that have long-term benefits but short-term barriers, including other vaccines, as well as other uncomfortable or inconvenient medical procedures. For example, the FDA has recently approved HPV vaccines, which could substantially reduce females' lifetime risk of cervical cancer (CDC 2010). However, these vaccines, like the one examined in our study, require three injections within the span of a few months. While surveys indicate generally positive attitudes toward the HPV vaccine among patients and caregivers, these positive attitudes are not translating into high vaccination rates, in part because some individuals balk at the prospect having to make three clinic visits and get three injections (e.g., Kahn, et al. 2008). Future research should examine the efficacy of self-prediction in increasing patient uptake of other products and procedures with high perceived inconvenience and discomfort, including intrusive medical procedures such as mammography, colonoscopy or HIV testing.
This study has several methodological strengths. First, it evaluates the efficacy of a behavioral intervention using a large-scale randomized controlled trial. Second, the brief intervention was administered very unobtrusively, so that patients in the intervention group had no way of knowing their A-CASI interview differed from that received by other patients, and study personnel were unaware of which patients were in the intervention group vs. control group. Third, the behavioral outcome of interest (vaccine acceptance or rejection) was directly observed and recorded in clinical records, without reliance on the accuracy of self-reports.
Any single study has limitations, and caution should be used in generalizing these findings to other settings and populations. As noted earlier, the participants in this study were patient volunteers in three STD clinics located in two American cities, and were primarily non-Hispanic African-Americans. Future research should test the efficacy of the self-prediction intervention in other populations, including other nationalities, non-urban communities, and high-HBV-risk adults who do not seek care in STD clinics.
More broadly, future research should test the efficacy of self-prediction in promoting other preventive behaviors. Many patients endorse prevention in the abstract, but find specific preventive behaviors, ranging from vaccinations to mammograms, less attractive as they consider the concrete steps required to complete them. How can one help patients overcome the short-term barriers that often keep them from performing such beneficial behaviors? In the long run, it may be possible to reduce the actual barriers; e.g., to make the procedures themselves more convenient, less painful and simpler (as has been seen, for example, in the evolution of blood-glucose-testing products for diabetics). However, in the short run, many such procedures retain inherent elements of inconvenience or discomfort. Shots and blood draws can be painful. Physical exams for prostate or breast cancer can be embarrassing. In increasing adoption of such preventive services, the best hope may be to reduce the impact that perceived short-term deterrents have on patients' behavior. The self-prediction intervention appears to hold promise for achieving that end. We encourage further research to test the usefulness of this intervention in promoting a broad range of health behaviors.
Acknowledgements
This study was funded by NIH grant R01 AI049644 (Dr. Zimet, PI)
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/hea.
REFERENCES
- Armitage CJ. Can the theory of planned behavior predict the maintenance of physical activity? Health Psychology. 2005;24(3):235–245. doi: 10.1037/0278-6133.24.3.235. [DOI] [PubMed] [Google Scholar]
- Baars JE, Boon BJF, Garretsen HF, van de Mheen D. Vaccination uptake and awareness of a free hepatitis B vaccination program among female commercial sex workers. Women's Health Issues. 2009;19:61–69. doi: 10.1016/j.whi.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Benson E. Gambling on the future you. Monitor on Psychology. 2002 Sept.33:48+. http://www.apa.org/monitor/sep02/gambling.aspx, downloaded 12-22-10.
- Cameron C, Trevidi P. Regression Analysis of Count Data. Cambridge, UK: Cambridge University Press; 1998. [Google Scholar]
- Centers for Disease Control and Prevention. Hepatitis B vaccination coverage among adults--United States, 2006. MMWR. 2006;55(18):509–511. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. What would happen if we stopped vaccinations. [Accessed Nov. 26, 2010]; http://www.cdc.gov/vaccines/vac-gen/whatifstop.htm.
- Centers for Disease Control and Prevention. Hepatitis B information for the public. [Accessed December 13, 2010];2008 http://www.cdc.gov/hepatitis/b/bfaq.htm.
- Centers for Disease Control and Prevention. FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices. Morbidity and Mortality Weekly Report. 2010;59:626–629. [PubMed]
- Crockett RA, Weinman J, Hankins M, Marteau T. Time orientation and health-related behaviour: Measurement in general population samples. Psychology and Health. 2009;24(3):333–350. doi: 10.1080/08870440701813030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin G, Sheeran P, Conner M, Germain M. Asking questions changes behavior: Mere measurement effects on frequency of blood donation. Health Psychology. 2008;27:179–184. doi: 10.1037/0278-6133.27.2.179. [DOI] [PubMed] [Google Scholar]
- Godin G, Sheeran P, Conner M, Delage G, Germain M, Belanger-Gravel A, Naccache H. Which survey questions change behavior? Randomized controlled trial of mere measurement interventions. Health Psychology. 2010;29:636–644. doi: 10.1037/a0021131. [DOI] [PubMed] [Google Scholar]
- Greenwald A, Carnot C, Beach R, Young B. Increased voting behavior by asking people if they expect to vote. Journal of Applied Psychology. 1987;72:315–318. [Google Scholar]
- Kahn J, Rosenthal S, Jin Y, Huang P, Namakydoust A, Zimet G. Rates of human papillomavirus vaccination, attitudes about vaccination, and human papillomavirus prevalence in young women. Obstetrics and Gynecology. 2008;111(5):1103–1110. doi: 10.1097/AOG.0b013e31817051fa. [DOI] [PubMed] [Google Scholar]
- Koblin BA, Xu G, Lucy D, Robertson V, Bonner S, Hoover DR, et al. Hepatitis B infection and vaccination among high-risk non-injection drug-using women: Baseline data from the UNITY Study. Sexually Transmitted Diseases. 2007;34(11):917–922. doi: 10.1097/OLQ.0b013e3180ca8f12. [DOI] [PubMed] [Google Scholar]
- Leiser D, Azar OH, Hadar L. Psychological construal of economic behavior. Journal of Economic Behavior. 2008;29(5):762–776. [Google Scholar]
- Levanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. Journal of Viral Hepatitis. 2004;11:97–107. doi: 10.1046/j.1365-2893.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- Liberman N, Trope Y. The role of feasibility and desirability considerations in near and distant future decisions: A test of temporal construal theory. Journal of Personality and Social Psychology. 1998;75(1):5–18. [Google Scholar]
- McCambridge J, Day M. Randomized controlled trial of the effects of completing alcohol use disorders identification test questionnaire on self-reported hazardous drinking. Addiction. 2008;103(2):241–248. doi: 10.1111/j.1360-0443.2007.02080.x. [DOI] [PubMed] [Google Scholar]
- McMahon JM, Dentinger CM, Bruden D, et al. Antibody levels and protection after hepatitis B vaccine: Results of a 22-year follow-up study and response to a booster dose. The Journal of Infectious Diseases. 2009;200:1390–1396. doi: 10.1086/606119. [DOI] [PubMed] [Google Scholar]
- Morwitz V, Johnson E, Schmittlein D. Does measuring intention change behavior? Journal of Consumer Research. 1993 June;20:46–61. [Google Scholar]
- Muller D, Judd CM, Yzerbyt VY. When moderation is mediated and mediation is moderated. Journal of Personality and Social Psychology. 2005;89(6):852–863. doi: 10.1037/0022-3514.89.6.852. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. Journal of Clinical Psychology. 1995;51(6):768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Pronin E, Olivola CY, Kennedy KA. Doing unto future selves as you would do unto others: Psychological distance and decision making. Personality and Social Psychology Bulletin. 2008;34(2):224–236. doi: 10.1177/0146167207310023. [DOI] [PubMed] [Google Scholar]
- Romer D, Black M, Ricardo I, et al. Social influences on the sexual behavior of youth at risk for HIV exposure. American Journal of Public Health. 1994;84:977–985. doi: 10.2105/ajph.84.6.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy ET, Detels R, Douglas W, Greenland S. Factors affecting hepatitis vaccination refusal at a sexually transmitted disease clinic among men who have sex with men. Sexually Transmitted Diseases. 2003;30:411–418. doi: 10.1097/00007435-200305000-00007. [DOI] [PubMed] [Google Scholar]
- Sherman SJ. On the self-erasing nature of errors of prediction. Journal of Personality and Social Psychology. 1980;39(2):211–221. [Google Scholar]
- Sherman SJ. Should we ask our children about sex, drugs and rock & roll? A different conclusion. Journal of Consumer Psychology. 2008;18:96–101. doi: 10.1016/j.jcps.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieverding M, Matterne U, Ciccarello L. What role do social norms play in the context of men's cancer screening intention and behavior? Application of an extended theory of planned behavior. Health Psychology. 2010;29(1):72–81. doi: 10.1037/a0016941. [DOI] [PubMed] [Google Scholar]
- Spangenberg E, Sprott D, Grohmann B, Smith R. Mass-Communicated prediction requests: Practical application and a cognitive dissonance explanation for self-prophecy. Journal of Marketing. 2003;67(July):47–62. [Google Scholar]
- Tourangeau R, Smith TW. Asking sensitive questions: The impact of data collection mode, question format, and question context. Public Opinion Quarterly. 1996;60:275–304. [Google Scholar]
- Trope Y, Liberman N. Temporal construal. Psychological Review. 2003;110:403–421. doi: 10.1037/0033-295x.110.3.403. [DOI] [PubMed] [Google Scholar]
- Turner CF, Ku L, Rogers SM, Lindberg LD, Pleck JH, Sonenstein FL. Adolescent sexual behavior, drug use, and violence: Increased reporting with computer survey technology. Science. 1998;280:867–873. doi: 10.1126/science.280.5365.867. [DOI] [PubMed] [Google Scholar]
- Williams P, Block L, Fitzsimons G. Simply asking questions about health behaviors increases both health and unhealthy behaviors. Social Influence. 2006;1:117–127. [Google Scholar]
- Zimet GD, Perkins SM, Winston Y, Kee R. Predictors of first and second dose acceptance of hepatitis B vaccine among STD clinic patients. International Journal of STD and AIDS. 2008;19:246–250. doi: 10.1258/ijsa.2007.007136. [DOI] [PubMed] [Google Scholar]