Abstract
While 188Re has been used successfully in mice for tumor radiotherapy by MORF/cMORF pretargeting, previous radiolabeling of the 18 mer amine-derivatized cMORF with 90Y, a longer physical half life nuclide, was not very successful. After developing a method involving a pre-purification heating step during conjugation that increases labeling efficiency and label stability, the biodistribution of 90Y-DOTA-Bn-SCN-cMORF was measured in normal mice and in MORF-CC49 pretargeted mice that bear LS174T tumors. Absorbed radiation doses were then estimated and compared to those estimated for 188Re. The pharmacokinetics of the 90Y-DOTA-cMORF in normal mice and in the pretargeted nude mice was similar to that observed previously with 99mTc- and 188Re-MAG3-cMORFs. While the 90Y-DOTA-cMORF cleared rapidly from normal tissues, tumor clearance was very slow and tumor radioactivity accumulation was constant for at least 7 days such that the tumor/blood (T/B) ratio increased linearly from 6 to 25 over this period. Therefore by extrapolation, normal tissue toxicities following administration of therapeutic doses of 90Y may be comparable to that observed for 188Re in which the T/B increased from 5 to 20. In conclusion, radiolabeling of DOTA-cMORF with 90Y was improved by introducing a prepurification heating step during conjugation. The 90Y-DOTA-cMORF provided a similar T/B ratio and biodistribution to that of 188Re-MAG3-cMORF and retained well in the tumor pretargeted with MORF-CC49. Because of the longer physical half life, the T/NT absorbed radiation dose ratios were improved in most organs and especially in blood.
INTRODUCTION
The pretargeting using a pair of morpholino oligomers (MORF/cMORF) has been shown to be effective in preclinical studies.1, 2 A major advantage of the MORF/cMORF pretargeting approach is the ease with which the cMORF effector can be chemically modified, for example to change chelators or linkers for different radionuclides, without affecting the cMORF/MORF affinity critical to the pretargeting approach. Thus far, this effector has been radiolabeled with 99mTc and 111In for imaging and 188Re for radiotherapy.2–4 We now report on the radiolabeling of the cMORF with 90Y, biodistribution of 90Y-cMORF, and the estimated absorbed radiation dose improvements using 90Y-cMORF/MORF pretargeting compared to 188Re-cMORF/MORF pretargeting. Although 90Y and 188Re have similar average beta energies (0.935 vs 0.716 Mev respectively), under identical conditions (i.e. tumor retention and clearance from normal tissues) the longer physical half life of 90Y (64 h) compared to 188Re (17 h) will provide higher tumor to organ absorbed radiation dose ratios because a larger portion of the 90Y in circulation will be cleared without depositing its energy in the normal tissues.
Previous 90Y labeling using para-isothiocyanate benzyl DOTA (i.e. p-SCN-Bn-DOTA) as a chelator was judged to be not very successful due to low labeling efficiencies.5 A possible explanation for these marginal results may have been identified in a subsequent 111In labeling of cMORF using p-SCN-Bn-DTPA as a chelator, in which heat sensitive side conjugation products were observed.6 Because introduction of a heating step after conjugation reaction and before purification, after subsequent labeling, was found to greatly improve label stability in that study, a similar approach was applied in this investigation using p-SCN-Bn-DOTA.
After the successful 90Y labeling, the biodistribution of cMORF was measured and the absorbed radiation doses to normal organs and tumor pretargeted with MORF-CC49 were estimated and compared with those previously for 188Re-cMORF in the identical tumor model.3, 4. The dosages and timing determined to be optimal with 99mTc-cMORF 7 and used previously with 188Re-cMORF 4 were again used in this study, because the antibody is the same and the blood clearance among 99mTc, 188Re and 90Y labeled cMORFs was found to be similar. For the same reason and as explained below, the relationship between tumor accumulation of 90Y-cMORF and tumor size was assumed and also confirmed to be the same as that for 188Re-cMORF and for 99mTc-cMORF.
MATERIALS AND METHODS
The MORF (5’-TCTTCTACTTCACAACTA) and cMORF (5’-TAGTTGTGAAGTAGAAGA) were obtained from Gene-Tools (Philomath, OR) with a primary amine attached to the 3’ equivalent terminal via a 3-carbon linker. The p-SCN-Bn-DTPA or –DOTA was from Macrocyclics (Dallas, TX). The P4 resin (Bio-Gel P-4 Gel, medium) was from Bio-Rad Laboratories (Hercules, CA). The 111InCl3 and 90YCl3 was from Perkin Elmer Life Science Inc (Boston, MA). The CC49 antibody was conjugated with MORF as previously described.7, 8. All other chemicals were reagent grade and were used without purification.
The cMORF, MORF and antibody concentrations were determined by UV spectrophotometry. Size exclusion HPLC was used for the cMORF analysis. The HPLC system was equipped with a Superdex™ 75 column (optimal separation range: 1×102 to 7×103 Da; Amersham Pharmacia Biotech, Piscataway, NJ) and with both UV and radioactivity in-line detectors. The eluant was 0.10 M pH 7.2 phosphate buffer at a flow rate of 0.60 mL/min. Radioactivity recovery was routinely measured and was always greater than 90%.
90Y measurements
Because 90Y is a pure beta emitter, accurate quantitation of radioactivity in organs can be difficult under certain circumstances.9. In this investigation, we confirmed that radioactivity of liquid 90Y samples may be accurately measured in a NaI(Tl) well detector by using standards,9–11 provided that the volume was the same, the samples were in the same type of counting tube, and especially that the radioactivity was homogeneously distributed. As such, tissue samples were digested in the SOLVABLE solution (PerkinElmer, Waltham, MA) in 5-mL polystyrene tubes and the volume was adjusted to 2.5 mL before counting. The 90Y radioactivity value provided by the manufacturer was considered to be accurate and was used to calibrate the NaI(Tl) well counter.
DOTA conjugation and 90Y labeling of cMORF
In a previous study,6 after conjugation with p-SCN-Bn-DTPA, the cMORF was radiolabeled efficiently with 111In but the radiolabel was shown to be unstable to heating. Suspecting that the conjugation procedure had produced both stable and unstable conjugates, heating the reaction mixture before purification in the conjugation process yielded a product that provided both a high radiolabeling efficiency and a stable 111In label. The same approach was applied in this investigation to the conjugation of cMORF with p-SCN-Bn-DOTA. Thus 2.5 mg of p-SCN-Bn-DOTA was dissolved in 0.25 mL of a 0.5 M Na2CO3-NaHCO3 buffer at pH 9.8 and mixed with 2.3 mg of lyophilized 3’-amine derivatized cMORF. After an overnight incubation at room temperature, 1 mL of 0.25 M NH4OAc buffer at pH 5.2 was added and the conjugation solution was heated at 100 °C for about 3 h before loading onto a 1 × 50 cm open P4 column. The column was eluted with the 0.25 M NH4OAc buffer and three peak fractions of about 0.6 mL were pooled. The radiolabeling was achieved by adding 1–3 µL of 90YCl3 solution in 50 mM HCl to 10–30 µL of the purified DOTA-cMORF solution followed by heating at 100 °C for 10 min. All animal studies were performed with 90Y-DOTA-Bn-SCN-cMORF prepared in this fashion (90Y-cMORF).
In vitro stability of 90Y-cMORF
The 90Y-cMORF was tested for radiolabel stability in phosphate buffer, in saline, and in fresh mouse serum. Specifically, 60 µL of the 90Y-cMORF solution was added to 250 µL of each medium before incubation either at room temperature (phosphate buffer and saline) or 37 °C (mouse serum). An aliquot was removed for HPLC analysis at different times over 48 h. In addition, the 90Y-cMORF was radiolabeled at different specific activities up to 3.5 mCi/37 µg of cMORF in the final volume of 80 µL. The radiolabel stability was examined after 20 h at room temperature by HPLC for evidence of radiolysis.
Pharmacokinetics of 90Y-cMORF
Optimization of a pretargeting protocol requires information on pharmacokinetics.7, 12–15 Accordingly, the in vivo behavior of the 90Y labeled cMORF was again measured. Normal CD-1 mice (Charles River Labs, MA) in 5 groups (N=4) each received 1 µg (45 µCi) of 90Y-cMORF by a tail vein injection and were euthanized at 10 min, 0.5, 1, 3 and 6 h. Organs were harvested, digested, and counted as described above. The %ID and %ID/g were calculated against a standard of the injectate. All animal studies were performed with the approval of the UMMS Institutional Animal Care and Use Committee.
Subsequently, the dosage and timing parameters for pretargeting were selected following the guide lines described previously.15 Any convenient dosage of the pretargeting antibody may be selected that does not saturate the accessible tumor antigens and any pretargeting interval may be used that provides an acceptable antibody tumor/normal tissue ratio. Because the antibody and the mouse tumor model were the same, the antibody dosage and the pretargeting interval used in this study were identical to those previously used for 99mTc-cMORF and 188Re-cMORF.4, 7 The optimal dosage of the cMORF effector is related to antibody dosage, pretargeting interval and its own blood clearance curve. Because the blood clearance of 90Y-cMORF was shown to be sufficiently similar to those of 99mTc-cMORF and 188Re-cMORF, the optimal cMORF dosage determined previously was used again.
Twenty NIH Swiss Nude mice (Taconic Farms, Germantown, NY) each received 106 LS174T colon cancer cells in the left thigh. After 11 days, when the tumors were about 0.3 g, each mouse received via a tail vein 30 µg MORF-CC49 (MORFs per antibody = 0.68) and 48 h later 2.5 µg of 90Y-MORF (50 µCi). Thus the cMORF/MORF molar ratio was close to the optimal 3.1.4, 7 The biodistribution of the 90Y-cMORF was followed for 7 days. The target thigh containing the tumor and the contralateral thigh were excised at the same anatomical position. The skin on both thighs was removed, and the tumor weight was calculated by subtracting the weight of the normal thigh from that of the tumored thigh. After the bone and as much of the muscle tissues as possible were removed, the tumor was cut into pieces of 0.3–0.4 g, digested, and the radioactivity was measured in a NaI(Tl) well counter along with other organs as described above. Because the dosages and timing in this study are assumed to be optimal based on the above discussion, the tumor accumulations of the effector may therefore be assumed to be at their MPTAs (Maximum Percent Tumor Accumulations, defined as the percent tumor accumulation under the condition when the MORFs in tumor are just saturated by the cMORF).14, 15
Absorbed radiation dose estimates
The estimated absorbed radiation doses to normal organs and to tumor by pretargeting with 90Y-cMORF were compared with those by pretargeting with188Re-cMORF. The AUCs (area under the radioactivity-time curve, also known as cumulated radioactivity concentration) were calculated from the best fits to the %ID/g data with decay corrections, to estimate the absorbed radiation doses for the normal organs of interest. Using the self-absorbed model 16, 17 as in our previous 188Re studies,3, 4 the absorbed radiation dose in Rads was calculated by the expression 2.143* 0.935 (MeV)*AUC (µCi*h/g), where 2.143 is a unit conversion factor and 0.935 MeV is the average energy of the 90Y beta particles.
Estimating the absorbed radiation dose to tumor is more complicated, since tumor accumulation is strongly related to tumor size 18–20 and the tumor size is an unpredictable variable among these studies. Therefore a comparison of tumor dose for different effectors requires normalizing the tumor accumulation to a certain tumor size using its accumulation vs. size relationship under optimal dosage and timing (i.e. at the MPTA). The relationship for 99mTc-cMORF was previously measured accurately for a substantial number of animals and tumor sizes.18 Because the in vivo behavior of cMORF was found to be sufficiently independent of the radiolabels of 99mTc, 188Re and 90Y, the MPTA-tumor size relationship for 99mTc-cMORF was assumed to be the same for 188Re-cMORF and 90Y-cMORF. This assumption was confirmed by comparing the predicted and observed tumor accumulations (see below). In the absorbed radiation dose comparison between 90Y-cMORF and 188Re-cMORF, a tumor size of 0.5 g was selected, since most tumors in this and our previous studies fall roughly in the range between 0.25 to 1.0 g.
RESULTS
DOTA conjugation and 90Y labeling of cMORF
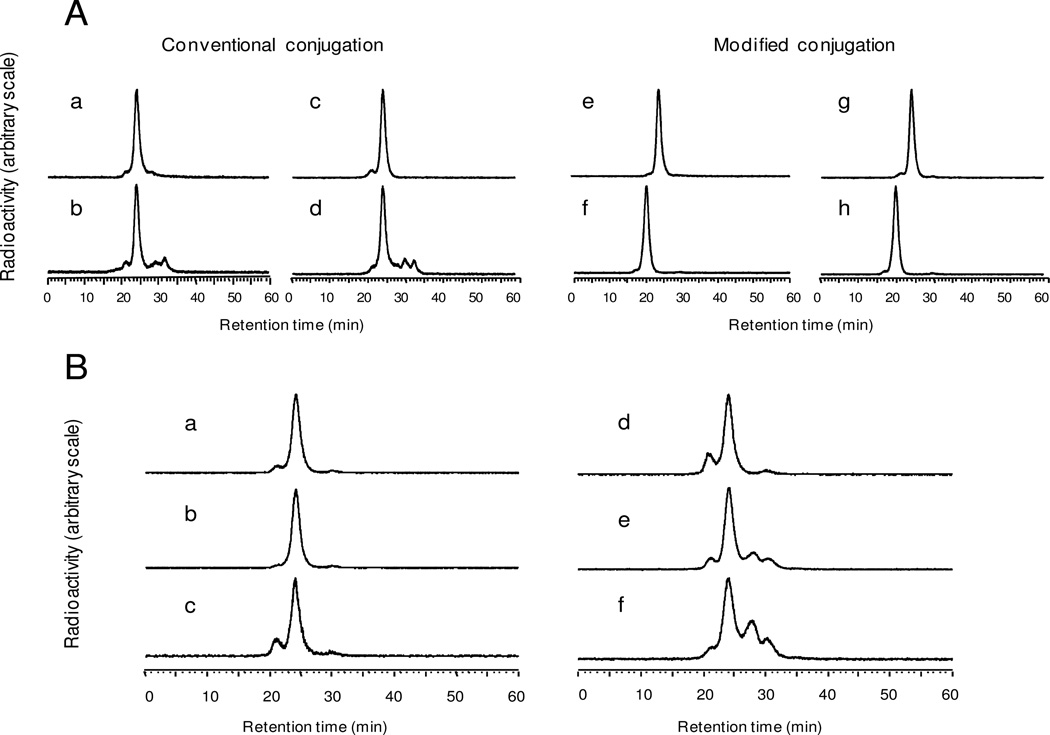
Similar to the conjugation with p-SCN-Bn-DTPA,6 conjugation of cMORF with p-SCN-Bn-DOTA without pre-purification heating yields a conjugate that provides a high 111In labeling efficiency (Fig 1A, a) but with label instability leading to partial decomposition after heating at 100°C for 1 h (Fig 1A, b). The equivalent results with 90Y are essentially identical (Fig 1A, c and d). However, after introducing a heating step after conjugation but before purification, the conjugation product can radiolabeled with either 111In or 90Y at high efficiency and the label becomes stable against heating (Fig 1A, e and g, respectively). The hybridization ability of the cMORF after conjugation and radiolabeling with either 111In or 90Y is preserved, as shown by the shift to higher molecular weight of the labeled cMORF peaks upon the addition of its MORF complement (Fig 1A, f and h, respectively).
Fig 1.
A: The HPLC radiochromatograms of 111In and 90Y labeled cMORFs after conjugating with p-SCN-Bn-DOTA without heating treatment (i.e. conventional) and radiolabeling with mild heating (a and c respectively) or heating at 100°C for 1 h (b and d respectively). Also presenting are the 111In and 90Y HPLC radiochromatograms of cMORF after conjugating with p-SCN-Bn-DOTA but after inclusion of the prepurification heating step during conjugation (i.e. modified) (e and g, respectively) as evidence of increased stability, and the corresponding radiochromatograms after addition of excess MORF (f and h respectively) as evidence of preserved hybridization affinity.
B: HPLC radiochromatograms of 90Y-cMORF (a) immediately before and (b) 48 h after incubation in serum at 37 °C and (c) 48 h after incubation in phosphate buffer at room temperature. Also presented are HPLC radiochromatgrams of 90Y-cMORF after 20 h of incubation at room temperature in the 80 µL of labeling solution at specific radioactivities of 63, 1430, and 3440 µCi/37 µg cMORF (d–f), showing decomposition most probably due to radiolysis.
In vitro stability of 90Y-cMORF
As shown in Fig 1B, b and c, after introducing the heating step into the conjugation procedure, the 90Y radiolabel on cMORF is also stable in phosphate buffer, saline, and serum at least for 48 h (the small peak at 21 min in c is due to self-association of cMORF at room temperature). Fig 1 B, d–f presents the radiochromatograms of 90Y-cMORF after 20 h of incubation at room temperature in the 80 µL labeling solution at values of specific radioactivity of 63, 1430, and 3440 µCi/37 µg. The increasing decomposition products are most probably the result of radiolysis. To avoid complications due to radiolysis, samples of 90Y-cMORF were used immediately after preparation.
Pharmacokinetics of 90Y-cMORF
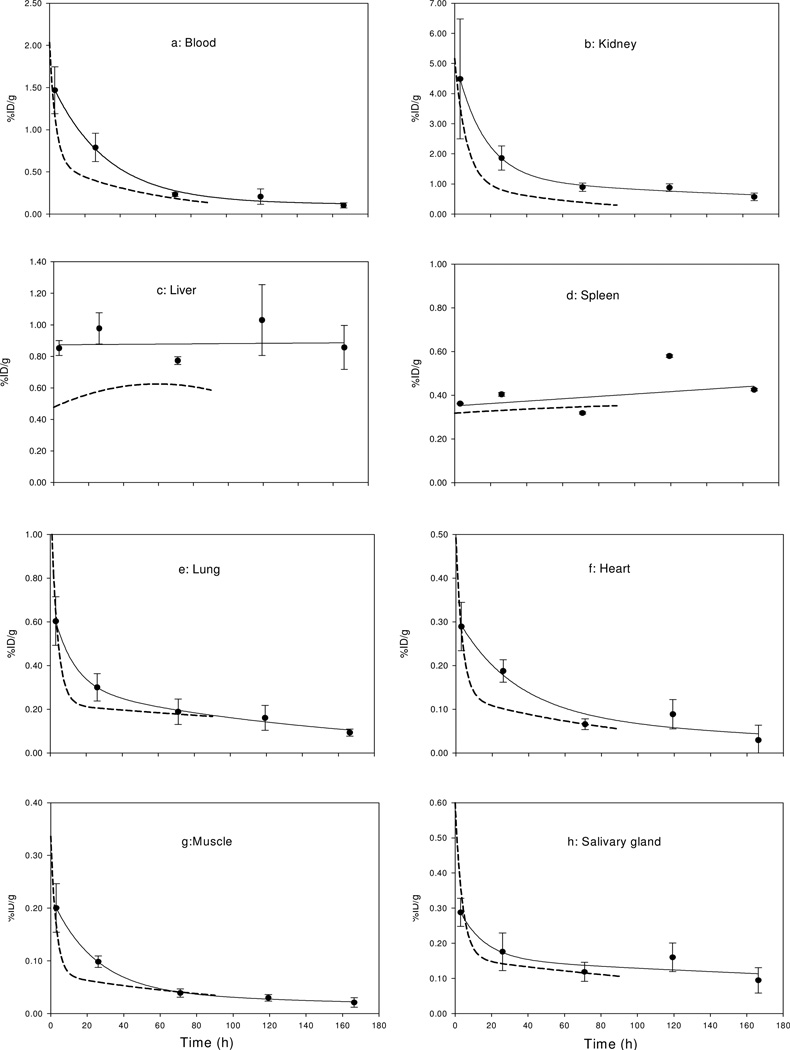
Table 1 presents the biodistributions at various times of 90Y-cMORF (prepared by the modified conjugation that includes the pre-purification heating) in normal mice and in tumored mice pretargeted with MORF-CC49. As shown by the data in normal mice, the 90Y-cMORF clears rapidly, similar to the 99mTc-cMORF and 188Re-cMORF. 21, 22 The blood and normal organ clearance is essentially completed by 3 h, although with slightly higher normal tissue levels.
Table 1.
Biodistribution (%ID/g and %ID/organ) of 90Y-cMORF in normal CD-1 mice (left) and in NIH Swiss nude mice bearing LS174T tumor xenograft and pretargeted with MORF-CC49 48 h earlier (right). Mean ± SD.
| In normal CD-1 mice | In pretargeted nude mice | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 10min | 30 min | 1 h | 3 h | 6 h | 3 h | 26 h | 71 h | 119 h | 166 h | |
| %ID/g | ||||||||||
| Liver | 0.92±0.07 | 0.68±0.14 | 0.58±0.04 | 0.56±0.10 | 0.58±0.07 | 0.85±0.05 | 0.98±0.10 | 0.77±0.02 | 1.03±0.22 | 0.86±0.14 |
| Heart | 1.12±0.11 | 0.25±0.05 | 0.16±0.05 | 0.14±0.07 | 0.07±0.01 | 0.29±0.06 | 0.19±0.03 | 0.07±0.01 | 0.09±0.03 | 0.03±0.03 |
| Kidney | 9.88±1.59 | 4.38±0.19 | 3.58±0.40 | 3.56±0.25 | 3.84±0.37 | 4.49±1.99 | 1.86±0.40 | 0.89±0.14 | 0.88±0.13 | 0.57±0.10 |
| Lung | 1.87±0.49 | 0.94±0.36 | 1.11±0.98 | 0.89±0.87 | 0.59±0.21 | 0.60±0.11 | 0.30±0.06 | 0.19±0.06 | 0.16±0.06 | 0.09±0.02 |
| Spleen | 0.57±0.06 | 0.24±0.02 | 0.18±0.02 | 0.24±0.07 | 0.19±0.02 | 0.36±0.03 | 0.40±0.13 | 0.32±0.05 | 0.58±0.04 | 0.43±0.10 |
| Muscle | 0.89±0.10 | 0.19±0.03 | 0.05±0.01 | 0.04±0.00 | 0.04±0.01 | 0.20±0.05 | 0.10±0.01 | 0.04±0.01 | 0.03±0.01 | 0.02±0.01 |
| Pancreas | 0.92±0.22 | 0.31±0.07 | 0.13±0.01 | 0.09±0.01 | 0.06±0.01 | 0.25±0.03 | 0.13±0.02 | 0.07±0.02 | 0.10±0.05 | 0.05±0.02 |
| Salivary | 1.09±0.38 | 0.26±0.04 | 0.12±0.02 | 0.08±0.01 | 0.10±0.02 | 0.29±0.04 | 0.18±0.05 | 0.12±0.03 | 0.16±0.04 | 0.09±0.04 |
| Blood | 4.27±0.46 | 0.94±0.13 | 0.41±0.03 | 0.16±0.01 | 0.10±0.01 | 1.47±0.28 | 0.79±0.17 | 0.23±0.02 | 0.21±0.09 | 0.10±0.03 |
| Tumor | --- | --- | --- | --- | --- | 7.15±2.19 | 5.21±1.98 | 2.89±1.07 | 3.95±2.07 | 2.43±1.08 |
| %ID/organ | ||||||||||
| Stomach | 0.29±0.05 | 0.07±0.01 | 0.04±0.01 | 0.05±0.05 | 0.02±0.00 | 0.10±0.05 | 0.11±0.04 | 0.03±0.01 | 0.03±0.01 | 0.02±0.00 |
| Sm. Int | 1.03±0.10 | 0.51±0.09 | 0.50±0.04 | 0.15±0.04 | 0.07±0.00 | 0.64±0.08 | 0.36±0.11 | 0.33±0.51 | 0.06±0.01 | 0.05±0.01 |
| Lg. Int. | 0.50±0.06 | 0.11±0.02 | 0.06±0.00 | 0.40±0.06 | 0.13±0.03 | 0.19±0.05 | 0.49±0.10 | 0.07±0.01 | 0.07±0.02 | 0.04±0.01 |
| Tumor | --- | --- | --- | --- | --- | 2.77±0.79 | 2.70±0.40 | 2.78±0.94 | 3.16±0.49 | 2.67±0.51 |
| Tumor weight (g) | --- | --- | --- | --- | --- | 0.46±0.30 | 0.56±0.19 | 1.00±0.26 | 1.01±0.56 | 0.90±0.34 |
Because of the longer half life of 90Y compared to 188Re, tumor retention and normal organ clearance could be followed for longer periods. As shown in Table 1, the tumor accumulation in %ID is fairly constant with time, while the tumor accumulation in %ID/g is decreasing due to tumor growth. As for normal organs, except in kidney, the background radioactivity is much lower than that in tumor and clears rapidly with time, similar to our earlier results with 188Re-cMORF.3, 4 Fig 2(a–h) reproduces the normal organ 90Y levels from 3 h to 7 days from the table. The solid lines represent the best fits and the dotted lines reproduced from the values for 188Re in our earlier study.4
Fig 2.
Pharmacokinetics of 90Y-cMORF in normal organs (a–h) of tumored mice pretargeted with MORF-CC49. The solid lines represent the best fits and the dashed lines represent the clearance curves of 188Re-cMORF from a previous pretargeting study (reference 4). Error bars represent one standard deviation.
The tumor accumulation vs. size relationship for 99mTc-cMORF determined earlier in the identical animal model was: MPTA (%ID/g) = 4.51*tumor weight (g)−0.66. 18 This relationship was assumed to hold true also for 90Y-cMORF and 188Re-cMORF, because the MPTA is proportional to the area under the blood clearance curve 13–15 and, as Table 2 presents, the blood clearance of the three effectors are sufficiently similar. This assumption is supported by the results presented in Table 1. From the Table, the average tumor size at 3 h was 0.46 ± 0.30 g and, based on the above relationship, the MPTA for 90Y-cMORF is calculated to be 7.53 %ID/g, therefore in close agreement with the measured 7.15 ± 2.19 %ID/g in Table 1. Previously, in the case of 188Re-cMORF, the tumor accumulation was 8.66±1.18 %ID/g for a tumor size of 0.36 g,3 also in close agreement with the predicted value of 8.76 %ID/g. These agreements provide confidence in the following calculations of absorbed radiation doses in tumor using normalized tumor accumulation.
Table 2.
Blood levels (%ID/g) of 99mTc-cMORF, 188Re-cMORF, and 90Y-cMORF in normal CD-1 mice at different times post injection. Mean ± SD.
Absorbed radiation dose estimate
The AUCs for organs were calculated from the best fits to the biodistribution data with decay correction. The AUC for tumor was based on a predicted tumor accumulation of 7.13 %ID/g for a 0.5 g LS174T tumor obtained from on the MPTA-tumor size relationship, assuming no tumor growth during the study. Both AUCs and the calculated absorbed radiation doses are listed in Table 3. Because of the higher accumulation and longer retention, the absorbed radiation dose to tumor by 90Y pretargeting is at least 5-fold higher than those for other organs. The tumor dose would be even higher in a cohort of animals with smaller tumors than 0.5 g. For comparison, historical data for 188Re pretargeting are also listed but again with the AUC and absorbed radiation dose for tumor size normalized to 0.5 g and calculated assuming no growth during therapy.
Table 3.
The AUCs (uCi*h/g) and absorbed radiation doses (rads) for the LS174T tumor and organs by cMORF/MORF pretargeting for the administration of 1 µCi of 90Y- or 188Re-cMORF.
| Tumor* | Blood | Kidney | Liver | Spleen | Lung | Heart | Muscle | Salivary gland |
Ref | |
|---|---|---|---|---|---|---|---|---|---|---|
| AUC(uCi*h/g) | ||||||||||
| 90Y | 6.53 | 0.46 | 1.36 | 0.81 | 0.37 | 0.22 | 0.11 | 0.06 | 0.12 | This study |
| 188Re | 1.75 | 0.47 | 0.58 | 0.31 | 0.18 | 0.23 | 0.12 | 0.05 | 0.20 | Ref 3 |
| 188Re | 1.75 | 0.15 | 0.40 | 0.15 | 0.08 | 0.08 | 0.04 | 0.02 | 0.05 | Ref 4 |
| Dose (Rad) | ||||||||||
| 90Y | 13.1 | 0.92 | 2.72 | 1.62 | 0.73 | 0.44 | 0.22 | 0.13 | 0.25 | This study |
| 188Re | 2.89 | 0.77 | 0.95 | 0.51 | 0.30 | 0.38 | 0.20 | 0.09 | 0.32 | Ref 3 |
| 188Re | 2.89 | 0.24 | 0.65 | 0.24 | 0.13 | 0.13 | 0.06 | 0.04 | 0.08 | Ref 4 |
The AUC and absorbed radiation dose for tumor are calculated based on the predicted accumulation of 7.13 %ID/g for a tumor of 0.5 g under the assumption of no growth.
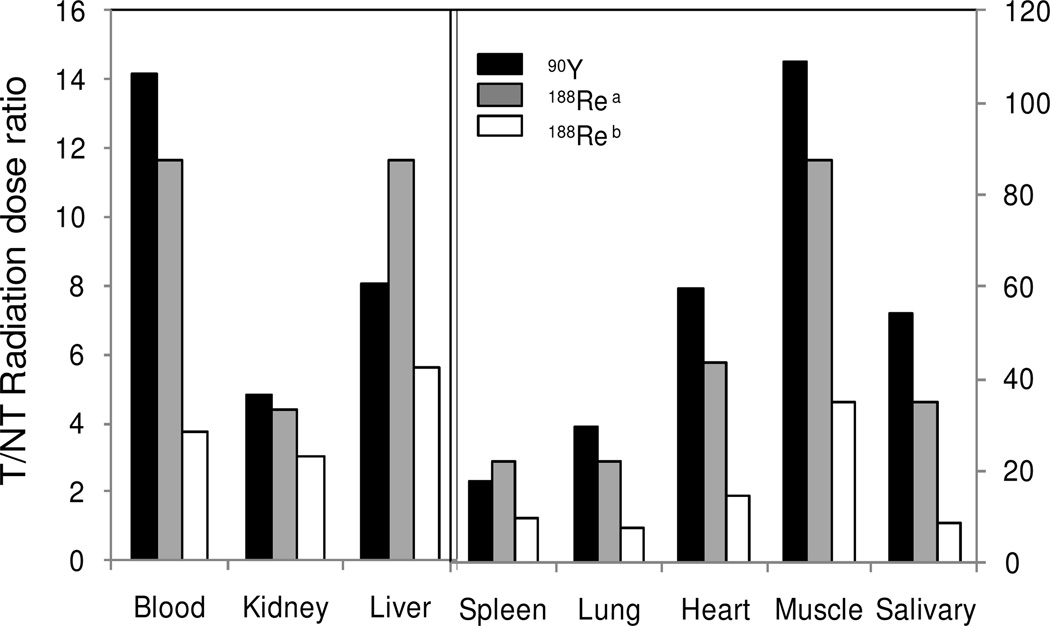
Fig 3 presents the T/NT AUC ratios (i.e. the absorbed radiation dose ratios or the therapeutic indexes 23) for 90Y pretargeting and two previous 188Re pretargeting studies in the same mouse model.3, 4 Although the normal tissue backgrounds are slightly higher for 90Y (Fig 2), the T/NT AUC ratios are not lower but higher due to its longer half life.
Fig 3.
Histograms presenting the T/NT dose ratios (i.e. the tumor therapeutic indexes) for 90Y and two previous 188Re pretargeting studies in the same mouse model. a: Ref 4; b: Ref 3.
DISCUSSION
Yttrium-90 is an attractive therapeutic radionuclide that has seen use in animal and patient studies while conjugated to antitumor antibodies. Of particular interest, 90Y has been the radionuclide of choice in several pretargeting studies using avidin/biotin or the bispecific antibody/hapten recognition system.17, 24–38 In the development of tumor radiotherapy approaches by MORF/cMORF pretargeting, we have previously employed 188Re as the therapeutic radionuclide.3, 4 We now consider 90Y as an alternative of longer physical half life that can potentially improve radiotherapy effectiveness. Accordingly, in this investigation a method was developed to label this nuclide to the cMORF effector and to evaluate the properties of the 90Y-cMORF in LS174T tumored NIH Swiss nude mice pretargeted with the MORF-CC49 antibody.
Although the radiolabeling of cMORF with 90Y via p-SCN-Bz-DOTA was expected to present few difficulties, we observed instabilities of the 90Y-cMORF, reminiscent of that observed previously when radiolabeling cMORF with 111In via p-SCN-Bz-DTPA.6 A search of the literature provided no mention of similar instabilities when these bifunctional chelators were used to label antibodies. In our earlier study of 111In-cMORF, we were able to resolve the label instability by including a prepurification heating step after conjugation. The same approach was applied in this investigation in connection with 90Y-cMORF labeling and proved to be successful.
Change in the effector or pretargeting antibody would require the dosage and timing of the pretargeting protocol to be optimized. However, as we have now demonstrated, the pharmacokinetic and pretargeting behavior of cMORF is generally not influenced by the nature of the chelators and radiolabels at least among 99mTc-MAG3-cMORF, 188Re-MAG3-cMORF and 90Y-DOTA-Bn-SCN-cMORF. Accordingly, optimization of the dosage and timing parameters for 90Y pretargeting became unnecessary since the values for 99mTc had been previously determined 7 and can be used again with confidence. For the same reason, the tumor accumulation-size relationship useful for comparing potential therapeutic effect was assumed and confirmed to be the same as that for 99mTc established previously.
With the tracer level pretargeting data, potential therapeutic improvement of 90Y as an alternative to 188Re was evaluated. This evaluation was facilitated by using identical MORF and cMORF sequences, identical CC49 antibody and identical LS174T tumor model to that used previously.7 Since the average beta energies of 90Y and 188Re and the pharmacokinetics of the labeled cMORFs are similar, the T/NT dose ratio improvement is mainly due to the longer physical half life.
CONCLUSION
Radiolabeling of DOTA-cMORF with 90Y was improved by introducing a prepurification heating step into the conjugation process. The T/B ratio and biodistribution of 90Y-DOTA-cMORF labeled in this fashion were found to be similar to that observed previously for 188Re-MAG3-cMORF. Nevertheless, because of the longer physical half life of 90Y, an improvement in therapeutic efficacy may be expected in future radiotherapy studies.
ACKNOWLEDGEMENTS
The authors are grateful to Dr Schlom (Laboratory of Tumor Immunology and Biology, Center for Cancer Research, NCI, NIH, Bethesda, MD) for providing the CC49 hybridoma. Financial support was in part from the National Institute of Health (CA94994 and DK082894).
Footnotes
No conflicts of interest are involved.
Supporting Information Available: None.
References
- 1.Liu G, Mang’era K, Liu N, Gupta S, Rusckowski M, Hnatowich DJ. Tumor pretargeting in mice using 99mTc-labeled morpholino, a DNA analog. J. Nucl. Med. 2002;43:384–391. [PubMed] [Google Scholar]
- 2.Liu G, Cheng D, Dou S, Chen X, Liang M, Pretorius PH, Rusckowski M, Hnatowich DJ. Replacing 99mTc with 111In Improves MORF/cMORF pretargeting by reducing intestinal accumulation. Mol. Imag. Biol. 2009;11:303–307. doi: 10.1007/s11307-009-0209-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu G, Dou S, Mardirossian G, He J, Zhang S, Liu X, Rusckowski M, Hnatowich DJ. Successful radiotherapy of tumor in pretargeted mice by 188Re-radiolabeled phosphorodiamidate morpholino oligomer, a synthetic DNA analogue. Clin. Cancer Res. 2006;12:4958–4964. doi: 10.1158/1078-0432.CCR-06-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu G, Dou S, Baker S, Akalin A, Cheng D, Chen L, Rusckowski M, Hnatowich DJ. A preclinical 188Re tumor therapeutic investigation using MORF/cMORF pretargeting and an antiTAG-72 antibody CC49. Cancer Biol. Ther. 2010;10:767–774. doi: 10.4161/cbt.10.8.12879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu CB, Liu GZ, Liu N, Zhang YM, He J, Rusckowski M, Hnatowich DJ. Radiolabeling morpholinos with 90Y, 111In, 188Re and 99mTc. Nucl. Med. Biol. 2003;30:207–214. doi: 10.1016/s0969-8051(02)00389-x. [DOI] [PubMed] [Google Scholar]
- 6.Liu G, Dou S, Liu Y, Liang M, Chen L, Cheng D, Greiner D, Rusckowski M, Hnatowich DJ. Unexpected side products in the conjugation of an amine-derivatized morpholino oligomer with p-isothiocyanate benzyl DTPA and their removal. Nucl. Med. Biol. 2011;38:159–163. doi: 10.1016/j.nucmedbio.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu G, Dou S, Pretorius PH, Liu X, Chen L, Rusckowski M, Hnatowich DJ. Tumor pretargeting in mice using MORF conjugated CC49 antibody and radiolabeled complimentary cMORF effector. Q. J. Nucl. Med. Mol. Imaging. 2010;54:333–340. [PMC free article] [PubMed] [Google Scholar]
- 8.He J, Liu G, Dou S, Gupta S, Rusckowski M, Hnatowich DJ. An improved method for covalently conjugating morpholino oligomers to antitumor antibodies. Bioconjug. Chem. 2007;18:983–988. doi: 10.1021/bc060208v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hnatowich DJ, Virzi F, Doherty PW. DTPA-coupled antibodies labeled with yttrium-90. J. Nucl. Med. 1985;26:503–509. [PubMed] [Google Scholar]
- 10.Djokić D, Janković D, Nikolić N. Preparation and in vivo evaluation of 90Y-mesodimercaptosuccinic acid (90Y-DMSA) for possible therapeutic use: comparison with 99mTc-DMSA. Cancer Biother. Radiopharm. 2009;24:129–136. doi: 10.1089/cbr.2008.0499. [DOI] [PubMed] [Google Scholar]
- 11.Siegel JA, Zimmerman BE, Kodimer K, Dell MA, Simon WE. Accurate dose calibrator activity measurement of 90Y-ibritumomab tiuxetan. J. Nucl. Med. 2004;45:450–454. [PubMed] [Google Scholar]
- 12.Sharkey RM, Karacay H, Richel H, McBride WJ, Rossi EA, Chang K, Yeldell D, Griffiths GL, Hansen HJ, Goldenberg DM. Optimizing bispecific antibody pretargeting for use in radioimmunotherapy. Clin. Cancer Res. 2003;9:3897S–913S. [PubMed] [Google Scholar]
- 13.Liu G, He J, Dou S, Gupta S, Rusckowski M, Hnatowich DJ. Further investigations of morpholino pretargeting in mice—establishing quantitative relations in tumor. Eur. J. Nucl. Med. Mol. Imaging. 2005;32:1115–1123. doi: 10.1007/s00259-005-1853-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu G, Dou S, He J, Liu X, Rusckowski M, Hnatowich DJ. Predicting the biodistribution of radiolabeled cMORF effector in MORF-pretargeted mice. Eur. J. Nucl. Med. Mol. Imaging. 2007;34:237–246. doi: 10.1007/s00259-006-0222-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu G, Hnatowich DJ. A semiempirical model of tumor pretargeting. Bioconjug. Chem. 2008;19:2095–2104. doi: 10.1021/bc8002748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gestin JF, Loussouarn A, Bardies M, Gautherot E, Gruaz-Guyon A, Saï-Maurel C, Barbet J, Curtet C, Chatal JF, Faivre-Chauvet A. Two-step targeting of xenografted colon carcinoma using a bispecific antibody and 188Re-labeled bivalent hapten: biodistribution and dosimetry studies. J. Nucl. Med. 2001;42:146–153. [PubMed] [Google Scholar]
- 17.Lubic SP, Goodwin DA, Meares CF, Song C, Osen M, Hays M. Biodistribution and dosimetry of pretargeted monoclonal antibody 2D12.5 and Y-Janus-DOTA in BALB/c mice with KHJJ mouse adenocarcinoma. J. Nucl. Med. 2001;42:670–678. [PubMed] [Google Scholar]
- 18.Liu G, Dou S, Liang M, Chen X, Rusckowski M, Hnatowich DJ. The ratio of maximum percent tumour accumulations of the pretargeting agent and the radiolabelled effector is independent of tumour size. Eur. J. Cancer. 2009;45:3098–3103. doi: 10.1016/j.ejca.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moshakis V, McIlhinney RAJ, Raghaven D, Neville AM. Localization of human tumour xenografts after i.v. administration of radiolabelled monoclonal antibodies. Br. J. Cancer. 1981;44:91–99. doi: 10.1038/bjc.1981.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siegel JA, Pawlyk DA, Lee RE, Sasso NL, Horowitz JA, Sharkey RM, Goldenberg DM. Tumor, red marrow, and organ dosimetry for 131I-labeled anti-carcinoembryonic antigen monoclonal antibody. Cancer Res. 1990;50:1039s–1042s. [PubMed] [Google Scholar]
- 21.Liu G, He J, Dou S, Gupta S, Vanderheyden JL, Rusckowski M, Hnatowich DJ. Pretargeting in tumored mice with radiolabeled morpholino oligomer showing low kidney uptake. Eur. J. Nucl. Med. Mol. Imaging. 2004;31:417–424. doi: 10.1007/s00259-003-1393-9. [DOI] [PubMed] [Google Scholar]
- 22.Liu G, Dou S, He J, Yin D, Gupta S, Zhang S, Wang Y, Rusckowski M, Hnatowich DJ. Radiolabeling of MAG3-morpholino oligomers with 188Re at high labeling efficiency and specific radioactivity for tumor pretargeting. Appl. Radiat. Isot. 2006;64:971–978. doi: 10.1016/j.apradiso.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeNardo SJ, Richman CM, Albrecht H, Burke PA, Natarajan A, Yuan A, Gregg JP, O'Donnell RT, DeNardo GL. Enhancement of the therapeutic index: from nonmyeloablative and myeloablative toward pretargeted radioimmunotherapy for metastatic prostate cancer. Clin. Cancer Res. 2005;11:7187s–7194s. doi: 10.1158/1078-0432.CCR-1004-0013. [DOI] [PubMed] [Google Scholar]
- 24.Goodwin DA, Meares CF, Watanabe N, McTigue M, Chaovapong W, Ransone CM, Renn O, Greiner DP, Kukis DL, Kronenberger SI. Pharmacokinetics of pretargeted monoclonal antibody 2D12.5 and 88Y-Janus-2-(p-nitrobenzyl)-1,4,7,10-tetraazacyclododecanetetraacetic acid (DOTA) in BALB/c mice with KHJJ mouse adenocarcinoma: a model for 90Y radioimmunotherapy. Cancer Res. 1994;54:5937–5946. [PubMed] [Google Scholar]
- 25.Chinol M, Paganelli G, Sudati F, Meares C, Fazio F. Biodistribution in tumour-bearing mice of two 90Y-labelled biotins using three-step tumour targeting. Nucl. Med. Commun. 1997;18:176–182. doi: 10.1097/00006231-199702000-00014. [DOI] [PubMed] [Google Scholar]
- 26.Paganelli G, Orecchia R, Jereczek-Fossa B, Grana C, Cremonesi M, De Braud F, Tradati N, Chinol M. Combined treatment of advanced oropharyngeal cancer with external radiotherapy and three-step radioimmunotherapy. Eur, J, Nucl, Med. 1998;25:1336–1339. doi: 10.1007/s002590050305. [DOI] [PubMed] [Google Scholar]
- 27.Goodwin DA, Meares CF, Osen M. Biological properties of biotin-chelate conjugates for pretargeted diagnosis and therapy with the avidin/biotin system. J. Nucl. Med. 1998;39:1813–1818. [PubMed] [Google Scholar]
- 28.DeNardo SJ, DeNardo GL, Brush J, Carter P. Phage library-derived human anti-TETA and anti-DOTA ScFv for pretargeting RIT. Hybridoma. 1999;18:13–21. doi: 10.1089/hyb.1999.18.13. [DOI] [PubMed] [Google Scholar]
- 29.Cremonesi M, Ferrari M, Chinol M, Stabin MG, Grana C, Prisco G, Robertson C, Tosi G, Paganelli G. Three-step radioimmunotherapy with yttrium-90 biotin: dosimetry and pharmacokinetics in cancer patients. Eur. J. Nucl. Med. 1999;26:110–120. doi: 10.1007/s002590050366. [DOI] [PubMed] [Google Scholar]
- 30.Axworthy DB, Reno JM, Hylarides MD, Mallett RW, Theodore LJ, Gustavson LM, Su F, Hobson LJ, Beaumier PL, Fritzberg AR. Cure of human carcinoma xenografts by a single dose of pretargeted yttrium-90 with negligible toxicity. Proc. Natl. Acad. Sci. U. S. A. 2000;97:1802–1807. doi: 10.1073/pnas.97.4.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Breitz HB, Weiden PL, Beaumier PL, Axworthy DB, Seiler C, Su FM, Graves S, Bryan K, Reno JM. Clinical optimization of pretargeted radioimmunotherapy with antibody-streptavidin conjugate and 90Y-DOTA-biotin. J. Nucl. Med. 2000;41:131–140. [PubMed] [Google Scholar]
- 32.Knox SJ, Goris ML, Tempero M, Weiden PL, Gentner L, Breitz H, Adams GP, Axworthy D, Gaffigan S, Bryan K, Fisher DR, Colcher D, Horak ID, Weiner LM. Phase II trial of yttrium-90-DOTA-biotin pretargeted by NR-LU-10 antibody/streptavidin in patients with metastatic colon cancer. Clin. Cancer Res. 2000;6:406–414. [PubMed] [Google Scholar]
- 33.Grana C, Chinol M, Robertson C, Mazzetta C, Bartolomei M, De Cicco C, Fiorenza M, Gatti M, Caliceti P, Paganelli G. Pretargeted adjuvant radioimmunotherapy with yttrium-90-biotin in malignant glioma patients: a pilot study. Br. J. Cancer. 2002;86:207–212. doi: 10.1038/sj.bjc.6600047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pagel JM, Hedin N, Subbiah K, Meyer D, Mallet R, Axworthy D, Theodore LJ, Wilbur DS, Matthews DC, Press OW. Comparison of anti-CD20 and anti-CD45 antibodies for conventional and pretargeted radioimmunotherapy of B-cell lymphomas. Blood. 2003;101:2340–2348. doi: 10.1182/blood-2002-03-0874. [DOI] [PubMed] [Google Scholar]
- 35.Zhang S, Xing J, Zhang Q, Song F, Li Y, Yang X, Chen Z. Optimal design of Ig 5' primers for construction of diverse phage antibody library established to select anti-HAb18GEF and anti-DOTA-Y Fabs for hepatoma pretargeting RIT. Front. Biosci. 2006;11:1733–1749. doi: 10.2741/1919. [DOI] [PubMed] [Google Scholar]
- 36.Urbano N, Papi S, Ginanneschi M, De Santis R, Pace S, Lindstedt R, Ferrari L, Choi S, Paganelli G, Chinol M. Evaluation of a new biotin-DOTA conjugate for pretargeted antibody-guided radioimmunotherapy (PAGRIT) Eur. J. Nucl. Med. Mol. Imaging. 2007;34:68–77. doi: 10.1007/s00259-006-0124-4. [DOI] [PubMed] [Google Scholar]
- 37.Karacay H, Sharkey RM, Gold DV, Ragland DR, McBride WJ, Rossi EA, Chang CH, Goldenberg DM. Pretargeted radioimmunotherapy of pancreatic cancer xenografts: TF10-90Y-IMP-288 alone and combined with gemcitabine. J. Nucl. Med. 2009;50:2008–2016. doi: 10.2967/jnumed.109.067686. [DOI] [PubMed] [Google Scholar]
- 38.Sharkey RM, Karacay H, Govindan SV, Goldenberg DM. Combination radioimmunotherapy and chemoimmunotherapy involving different or the same targets improves therapy of human pancreatic carcinoma xenograft models. Mol. Cancer Ther. 2011;10:1072–1081. doi: 10.1158/1535-7163.MCT-11-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]