Abstract
Purpose of the study
To describe a new method of CPR that optimizes vital organ perfusion pressures and carotid blood flow. We tested the hypothesis that a combination of high dose sodium nitroprusside (SNP) as well as non-invasive devices and techniques known independently to enhance circulation would significantly improve carotid blood flow (CBF) and return of spontaneous circulation (ROSC) rates in a porcine model of cardiac arrest.
Methods
15 Isofluorane anesthetized pigs (30±1Kg), after 6 minutes of untreated ventricular fibrillation, were subsequently randomized to receive either 15 minutes of standard CPR (S-CPR) (8 animals) or 5 minute epochs of S-CPR followed by active compression-decompression (ACD) +inspiratory impedance threshold device (ITD) CPR followed by ACD+ITD+abdominal binding (AB) with 1mg of SNP administered at minutes 2, 7, 12 of CPR (7 animals). Primary endpoints were CBF and ROSC rates. ANOVA and Fisher’s exact test were used for comparisons.
Results/Conclusion
There was significant improvement in the hemodynamic parameters in the SNP animals. ROSC was achieved in 7/7 animals that received SNP and in 2/8 in the S-CPR (p=0.007). CBF and end tidal CO2 (ETCO2) were significantly higher in the ACD+ITD+AB+SNP (SNPeCPR) animals during CPR. Bolus doses of SNP, when used in conjunction with ACD+ITD+AB CPR, significantly improve CBF and ROSC rates compared to S-CPR.
Introduction
Cardiopulmonary resuscitation (CPR) rates have remained disappointingly low over the past half-century with only minimal improvements in neurologically intact survival.1 Even when optimally performed, blood flow generated by manual chest compressions is, at best, less than 25% of normal.2 Moreover, drugs such as epinephrine and other vasoconstrictors have been found to increase blood pressure but not significantly improve long-term outcomes.3 In fact, there is evidence that vasoconstrictors may actually be counterproductive as they perpetuate further ischemia and reduce microcirculatory flow.4–5
We sought to develop a new method of CPR that significantly enhances forward blood flow, and distributes it predominantly to the brain and heart. Such an approach is likely not possible with a single drug or device. However, recently developed non-invasive devices and techniques that increase perfusion to the heart and brain during CPR could be used as a mechanical platform to support the use of pharmacological vasodilator therapy.
We simultaneously employed simple, currently available, mechanical adjuncts together with high dose of SNP in an effort to augment forward blood flow, and optimize vital organ perfusion pressures. This new method of CPR, termed SNPeCPR, contains three fundamental components: 1) ACD CPR with an ITD which increases vital organ blood flow by actively increasing venous return and by lowering right atrial pressure4–5, 2) SNP to optimize forward blood flow as well as oxygen delivery by decreasing vascular resistance6 and 3) lower abdominal binding to actively decrease descending aortic blood flow and redistribute it to the thorax and brain by diminishing the circulatory distribution volume.
Experiments were designed to determine whether: 1) ACD+ITD and manual AB act synergistically to increase carotid blood flow and perfusion pressures to the heart and brain and 2) if the addition of large intravenous doses of SNP further improves carotid blood flow by decreasing systemic vascular resistance without affecting central aortic pressure.
Methods
The study was approved by the Institutional Animal Care Committee of the Minneapolis Medical Research Foundation of Hennepin County Medical Center. All animal care was compliant with the National Research Council’s 1996 Guidelines for the Care and Use of Laboratory Animals. All studies were performed by a qualified, experienced research team in Yorkshire female farm-bred pigs weighing 30 ± 1.5kg. A certified and licensed veterinarian assured the protocols were performed in accordance with the aforementioned Guidelines.
Our protocol was designed as a proof of concept hemodynamic evaluation of the synergistic effects of ACD+ITD CPR with abdominal binding in addition to SNP.
Preparatory Phase
The anesthesia, surgical, preparation, data monitoring, and recording procedures used in this study are previously described.7 Briefly, we employed aseptic surgical conditions, using initial sedation with intramuscular Ketamine (7 mL of 100 mg/mL, Ketaset, Fort Dodge Animal Health, Fort Dodge, Iowa) followed by inhaled Isoflurane at a dose of 0.8 to 1.2 %. While spontaneously breathing but sedated, each pig was intubated with a size 7.0 endotracheal tube. While sedated and mechanically ventilated, a burr hole was made half way between the left eyebrow and the posterior bony prominence of the skull in all the animals. A micromanometer-tipped catheter (Mikro-Tip Transducer, Millar Instruments Houston, Texas) was placed through the burr hole to enable real-time recording of intracranial pressure. The animal’s temperature was carefully maintained at 37.5 ± 0.5°C, with a warming blanket (Bair Hugger, Augustine Medical, Eden Prairie, Minnesota). The animals were placed supine and unilateral femoral artery cannulation was performed. Central aortic blood pressure was recorded continuously with a micromanometer-tipped (Mikro-Tip Transducer, Millar Instruments, Houston, Texas) catheter placed at the beginning of the descending thoracic aorta. A second Millar catheter was inserted in the right atrium via the right external jugular vein. All animals received an intravenous heparin bolus (100 units/kg). The left common carotid artery was then surgically exposed and an ultrasound flow probe (Transonic 420 series multichannel, Transonic Systems, Ithaca, New York) placed to quantify carotid blood flow (ml/min). The animals were then ventilated with room air, using a volume-control ventilator (Narcomed, Telford, Pennsylvania), with a tidal volume of 10 ml/kg and a respiratory rate adjusted to continually maintain a PaCO2 of 40 mm Hg and PaO2 of 80 mm Hg (blood oxygen saturation >95%), as measured from arterial blood (Gem 3000, Instrumentation Laboratory, Lexington, Massachusetts). Airway pressure was measured continuously with a micromanometer-tipped catheter positioned 2 cm above the carina. Surface electrocardiographic tracings were continuously recorded. All data were recorded with a digital recording system (Superscope II version 1.295, GW Instruments, Somerville, Massachusetts). End tidal CO2, tidal volume, minute ventilation, and blood oxygen saturation were continuously measured with a respiratory monitor (CO2SMO Plus, Novametrix Medical Systems, Wallingford, Connecticut). Right atrial pressure was adjusted from 2–4 mmHg with saline infusion as needed before induction of VF.
Measurements and Recording
Aortic pressure, right atrial pressure, intracranial pressure, ETCO2, and carotid blood flow were continuously recorded. Coronary perfusion pressure (CPP) during CPR was calculated from the mean arithmetic difference between right-atrial pressure and aortic pressure during the decompression phase. Cerebral perfusion pressure was calculated from the difference between the mean values of aortic pressure and intracranial pressure based on the recorded pressure waveforms. Ultrasound-derived carotid blood flow was reported in ml/sec.
Experimental Protocol
Upon completion of the surgical preparation, when oxygen saturation was greater than 95% and ETCO2 was stable between 35–42 mm Hg for five minutes, VF was induced by delivering direct current via a temporary pacing wire (Daig Division, St Jude Medical, Minnetonka, Minnesota) positioned in the right ventricle. The ventilator was disconnected from the endotracheal tube. After 6 minutes of untreated ventricular fibrillation, closed-chest standard CPR (standard compression mode) was performed with a pneumatically driven automatic piston device (Pneumatic Compression Controller, Ambu International, Glostrup, Denmark) as previously described.8 With this device, decompressions can selectively be performed in either active or passive mode. Uninterrupted chest compressions at a rate of 100 compressions/min, with a 50% duty cycle and a compression depth of 25% of the anterior-posterior chest diameter were provided. During CPR, asynchronous positive-pressure ventilations were delivered to simulate Advanced Life Support with a manual resuscitator bag (Smart Bag, O2 Systems, Toronto, Ontario, Canada). The fraction of inspired oxygen was 1.0, the tidal volume was maintained at ~10ml/kg and the respiratory rate was 10 breaths/min.
Fifteen pigs were used. After 6 minutes of untreated VF, seven pigs progressed through three 5-minute epochs of CPR for a total of 15 minutes. Standard (S-) CPR for 5 minutes was followed by ACD CPR with the addition of an inspiratory impedance threshold device (ITD) (ResQPOD® Advanced Circulatory Systems, Roseville, Minnesota) for 5 minutes. At the beginning of the last 5-minute epoch, manual abdominal binding was added to provide approximately 40 lbs of force (upper body weight of one investigator leaning forward at a 45 degree angle as measured on a scale) to the lower half of the abdomen (ACD+ITD+AB). Measurements have been made in our laboratory during cardiac arrest and CPR using a pigtail catheter above and below the binding site. By applying 40 lbs of force there is elimination of blood flow to the distal aorta. Long femoral arterial sheaths had to be used to obtain blood gasses. Over the course of this 15-minute protocol when CPR was delivered, at minutes 2, 7, and 12 of CPR animals received 1 mg of intravenous (IV) SNP delivered into the external jugular vein. (Figure 1) Eight other control animals received 15 minutes of standard CPR alone. At the end of the 15 minutes, external, biphasic, DC 150J shocks were delivered to achieve ROSC. No epinephrine was given at any point in the intervention group until stable sinus rhythm was established. The control animals received 0.5mg IV epinephrine one minute before defibrillation. If animals did not achieve ROSC within an additional 10 minutes, resuscitation efforts were terminated. Resuscitated animals were observed for 60 minutes.
Figure 1. Protocol timelines.
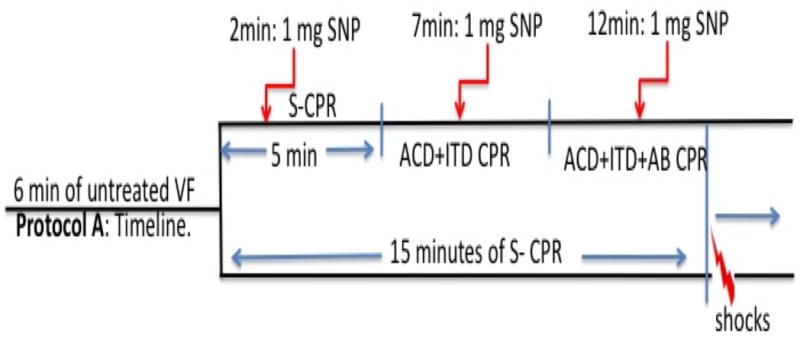
ROSC: return of spontaneous circulation. S-CPR: standard CPR, ACD+ITD: active compression decompression CPR, AB: abdominal binding, SNP: sodium nitroprusside.
The primary endpoints were carotid blood flow and ROSC rates. Secondary endpoints were the number of DC shocks required to achieve ROSC, coronary and cerebral perfusion pressure, and ETCO2.
Statistical analysis
Values were expressed as mean ± standard deviation. Baseline data were compared using the t-test. Hemodynamics and blood gases during CPR were analyzed with two way ANOVA. A 2-tailed Fischer exact test was used to compare ROSC rates. A p-value of <0.05 was considered statistically significant.
Results
Baseline characteristics between all the animals that did and did not receive SNP are presented in Table 1. Sodium nitroprusside did not increase carotid blood flow when it was given during S-CPR. It did not significantly change the central aortic pressure or vital organ perfusion pressures.
Table 1. Baseline characteristics.
Baseline blood gasses of the control S-CPR and animals that received the sequential interventions and sodium nitroprusside.
| Baseline | No SNP CPR | SNPeCPR | P-value |
|---|---|---|---|
| pH | 7.4±0.05 | 7.43±0.05 | 0.6 |
| PaCO2 | 42±3 | 43±3 | 0.8 |
| PaO2 | 195±45 | 187±39 | 0.4 |
| ETCO2 | 37±4 | 36±6 | 0.6 |
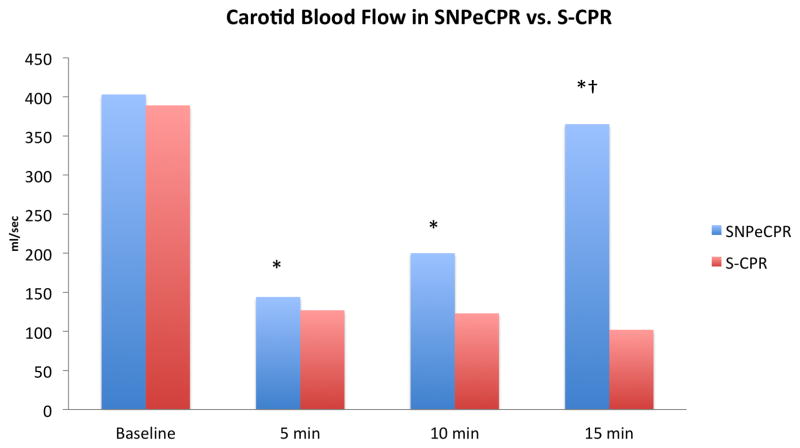
With the addition of ACD+ITD, and subsequently abdominal binding, there was a progressive improvement in cerebral and coronary perfusion pressure. (Table 2) Administration of intravenous SNP did not have a significant effect on cerebral or coronary perfusion pressures, but did significantly improve carotid blood flow. When SNP was administered while ACD+ITD+AB CPR (SNPeCPR) was performed, carotid flow reached baseline physiologic pre-arrest levels. In addition, systolic, diastolic, coronary, and cerebral pressures increased over time in the device/drug group (p<0.05 for all parameters in intra-group comparison). By contrast, in the S-CPR group, key hemodynamic parameters did not change considerably over time, and were significantly lower than the combined SNPeCPR group (p<0.05 for all parameters for inter-group comparison). (Table 2)(Figure 2) All animals (7/7) in the sequential interventions with SNP group achieved ROSC with a single, biphasic, 150J shock compared with 2/8 in the STD CPR control group, p=0.007. Only one animal in the SNP group required epinephrine post-ROSC for hemodynamic support.
Table 2. Protocol A: Hemodynamic Parameters and Return of Spontaneous Circulation (ROSC).
Mean ± SD. All pressures in mmHg and carotid blood flow in ml/min.
| Base line | 2 min STD | 5 min STD+SNP | 7 min ACD+ITD | 10 min ACD+ITD+SNP | 12 min ACD+ITD+AB | 15 min ACD+ITD+AB+SNP | No of shocks to ROSC | ROSC to 60m | ||
|---|---|---|---|---|---|---|---|---|---|---|
| SNP CPR | SBP | 118±14 | 52±6 | 49±12 | 66±14* | 70±16†* | 91±18* | 85±17†* | 1±0.5† | 7/7 (100%)† |
| DBP | 85±15 | 18±4 | 19.6±2 | 26±6 | 28±9†* | 40±10* | 38.8±9†* | |||
| RA | 2±1 | 6±2 | 5±2 | 4±3 | 4±2 | 12±6* | 9±6 | |||
| CPP | 83±15 | 12±3 | 16±2 | 24±5* | 26±6†* | 29±7* | 28.5±7†* | |||
| CerPP | 81±16 | 13±5 | 14±2 | 18±8* | 18±8†* | 25±9* | 23±7†* | |||
| ICP | 18±2 | 21.5±3 | 21±4 | 23±4 | 23±5 | 33.5±8* | 33±6† | |||
| CBF | 403±70 | 141±50* | 127±32* | 164±70* | 200±81* | 270±132* | 365±141*† | |||
| S-CPR | Base line | 5 min S-CPR | 10 min S-CPR | 15 min S-CPR | ||||||
| SBP | 100±20 | 52±6 | 42±8 | 38±8 | 4±3 | 2/8 (25%) | ||||
| DBP | 78±12 | 20±3 | 16±5 | 16±2 | ||||||
| RA | 2±2 | 3±2 | 2±2 | 4±3 | ||||||
| CPP | 76±11 | 17±3 | 14±3 | 12±3 | ||||||
| CerPP | 66±6 | 14±4 | 9±2 | 7±2 | ||||||
| ICP | 15±3 | 21±3 | 20±3 | 20±3 | ||||||
| CBF | 389±82 | 144±45 | 123±52 | 102±42 | ||||||
means p<0.05 within the same group;
means p<0.05 between groups.
SBP: systolic blood pressure; DBP: diastolic blood pressure; RAP: right atrial pressure; CPP: coronary perfusion pressure; CerPP: cerebral perfusion pressure; ICP: intracranial pressure: CBF: carotid blood flow. STD: Standard CPR; SNP: sodium nitroprusside; ACD: active compression decompression CPR; ITD: inspiratory impedance threshold device; AB: abdominal binding.
Figure 2. Carotid Blood Flow in S-CPR versus SNPeCPR.
Values are shown as means. * means p<0.05 within the same group; † means p<0.05 between groups.
Discussion
Recognizing the inadequacies of S-CPR, this study was designed to combine recent advances in our understanding of the pathophysiology of cardiac arrest with mechanical and pharmacological means to improve perfusion pressures to the heart and brain while increasing forward blood flow. Similar to the treatment of severe acute heart failure,6,11 we hypothesized that administration of large boluses of SNP to provide afterload reduction to thoracic compressions would be lifesaving if co-administered with a means to maintain adequate coronary and cerebral perfusion pressures. Thus, this investigation utilized the combination of ACD+ITD CPR with abdominal binding to provide sufficiently high levels of vital organ perfusion pressure to support the concurrent use of SNP. Building upon this mechanical CPR platform, these results demonstrate, for the first time, that administration of SNP is feasible and leads to improved hemodynamics, carotid blood flow, and resuscitation rates without adverse effects in a classic model of porcine cardiac arrest.
It appears from our findings that a method that actively enhances venous return and decreases right atrial and intracranial pressures (ACD CPR+ ITD) can eliminate the potential negative effects of abdominal binding which by itself can raise the same pressures, and decrease coronary and cerebral perfusion pressure when combined with S-CPR.4,12–15 ACD CPR+ITD and abdominal binding act synergistically when used with SNP, as hypothesized, to significantly increase vital organ perfusion pressures. Previously, abdominal binding was used with standard CPR and simultaneous compression and ventilation CPR and was not found to be beneficial.16 For this reason we did not examine combination of S-CPR +AB in the present study.14,17
It is also very important to note that SNP administration during S-CPR does not decrease central aortic pressure and during prolonged CPR. Conversely, when added on a superior mechanical CPR platform it significantly increases carotid blood flow and ETCO2 compared to S-CPR. Although not proven in our study, the increase of carotid blood flow and ETCO2 in conjunction with higher coronary and cerebral perfusion pressures support the contention that SNPeCPR actually increases vital organ blood flow compared to S-CPR.
Our initial study has several limitations. First, we cannot comment on the dosing. It is possible that a higher initial dose could accelerate its effects and improve flow earlier. A dosing study is underway and preliminary data suggest that larger doses are tolerated and can be more effective. Second, we did not directly measure blood flow to the vital organs or microcirculation. The higher ICP observed in the present study raises the possibility that carotid blood flow may not reflect true cerebral blood flow, nonetheless, we have demonstrated that cerebral recovery is improved with SNPeCPR compared with S-CPR or eCPR alone.18 A large study is underway to directly measure blood flow with this new method. Third, we did not use epinephrine every 5 minutes in the S-CPR group and therefore the results on survival are not translatable to clinical practice. We chose to use this control in order to assess the temporal natural progression of S-CPR hemodynamics. Finally the increase in carotid blood flow observed in our study could represent unwanted blood flow to the external carotid. The microsphere study will shed more light to this issue.
Conclusion
The mechanical components of SNPeCPR (ACD CPR +ITD and abdominal binding) act synergistically to significantly improve vital organ perfusion pressures and carotid blood flow. SNPeCPR as a whole significantly improves vital organ perfusion pressures and carotid blood flow compared to S-CPR.
Footnotes
Conflict of interest: No conflicts of interest are present in this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nichol G, Thomas E, Callaway CW, et al. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008;300:1423–31. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen TJ, Tucker KJ, Lurie KG, et al. Active compression-decompression. A new method of cardiopulmonary resuscitation. Cardiopulmonary Resuscitation Working Group. JAMA. 1992;267:2916–23. doi: 10.1001/jama.267.21.2916. [DOI] [PubMed] [Google Scholar]
- 3.Olasveengen TM, Sunde K, Brunborg C, Thowsen J, Steen PA, Wik Ls. Intravenous drug administration during out-of-hospital cardiac arrest: a randomized trial. JAMA. 2009;302:2222–9. doi: 10.1001/jama.2009.1729. [DOI] [PubMed] [Google Scholar]
- 4.Lurie KG, Coffeen P, Shultz J, McKnite S, Detloff B, Mulligan K. Improving active compression-decompression cardiopulmonary resuscitation with an inspiratory impedance valve. Circulation. 1995;91:1629–32. doi: 10.1161/01.cir.91.6.1629. [DOI] [PubMed] [Google Scholar]
- 5.Aufderheide TP, Frascone RJ, Wayne MA, et al. Comparative Effectiveness of an Impedance Threshold Device and Active Compression Decompression CPR versus Standard CPR for Treatment of Out-of Hospital Cardiac Arrest. The Lancet. 2011 In press. [Google Scholar]
- 6.Cohn JN, Burke LP. Nitroprusside. Ann Intern Med. 1979;91:752–7. doi: 10.7326/0003-4819-91-5-752. [DOI] [PubMed] [Google Scholar]
- 7.Yannopoulos D, Matsuura T, McKnite S, et al. No assisted ventilation cardiopulmonary resuscitation and 24-hour neurological outcomes in a porcine model of cardiac arrest. Crit Care Med. 2010;38:254–60. doi: 10.1097/CCM.0b013e3181b42f6c. [DOI] [PubMed] [Google Scholar]
- 8.Shultz JJ, Coffeen P, Sweeney M, et al. Evaluation of standard and active compression-decompression CPR in an acute human model of ventricular fibrillation. Circulation. 1994;89:684–93. doi: 10.1161/01.cir.89.2.684. [DOI] [PubMed] [Google Scholar]
- 9.2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2005;112:IV1–203. doi: 10.1161/CIRCULATIONAHA.105.166550. [DOI] [PubMed] [Google Scholar]
- 10.Indik JH, Donnerstein RL, Hilwig RW, et al. The influence of myocardial substrate on ventricular fibrillation waveform: a swine model of acute and postmyocardial infarction. Crit Care Med. 2008;36:2136–42. doi: 10.1097/CCM.0b013e31817d798c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guiha NH, Limas CJ, Franciosa JA, Cohn JN. Treatment of refractory heart failure with sodium nitroprusside. Circulation. 1972;46:105. doi: 10.1056/NEJM197409192911201. [DOI] [PubMed] [Google Scholar]
- 12.Lurie KG, Zielinski T, McKnite S, Aufderheide T, Voelckel W. Use of an inspiratory impedance valve improves neurologically intact survival in a porcine model of ventricular fibrillation. Circulation. 2002;105:124–9. doi: 10.1161/hc0102.101391. [DOI] [PubMed] [Google Scholar]
- 13.Lurie KG, Voelckel WG, Zielinski T, et al. Improving standard cardiopulmonary resuscitation with an inspiratory impedance threshold valve in a porcine model of cardiac arrest. Anesth Analg. 2001;93:649–55. doi: 10.1097/00000539-200109000-00024. [DOI] [PubMed] [Google Scholar]
- 14.Niemann JT, Rosborough JP, Ung S, Criley JM. Hemodynamic effects of continuous abdominal binding during cardiac arrest and resuscitation. Am J Cardiol. 1984;53:269–74. doi: 10.1016/0002-9149(84)90438-7. [DOI] [PubMed] [Google Scholar]
- 15.Yannopoulos D, McKnite SH, Metzger A, Lurie KG. Intrathoracic pressure regulation for intracranial pressure management in normovolemic and hypovolemic pigs. Crit Care Med. 2006;34:S495–500. doi: 10.1097/01.CCM.0000246082.10422.7E. [DOI] [PubMed] [Google Scholar]
- 16.Sanders AB, Ewy GA, Alferness CA, Taft T, Zimmerman M. Failure of one method of simultaneous chest compression, ventilation, and abdominal binding during CPR. Crit Care Med. 1982;10:509–13. doi: 10.1097/00003246-198208000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Niemann JT, Rosborough JP, Criley JM. Continuous external counterpressure during closed-chest resuscitation: a critical appraisal of the military antishock trouser garment and abdominal binder. Circulation. 1986;74:IV102–7. [PubMed] [Google Scholar]
- 18.Yannopoulos D, Matsura T, Schultz J, Rudser K, Halperin H, Lurie KG. Sodium nitroprusside enhanced cardiopulmonary resuscitation improves survival with good neurological function in a porcine model of prolonged cardiac arrest. Crit Care Med. 2011;39:1269–1274. doi: 10.1097/CCM.0b013e31820ed8a6. [DOI] [PMC free article] [PubMed] [Google Scholar]