Abstract
BACKGROUND
Endoscopic vein harvest (EVH) is the US standard of care for CABG but recent comparisons to open harvest suggest that conduit quality and outcomes may be compromised. To test the hypothesis that problems with EVH may relate to its learning curve and conduit quality, we analyzed the quality and early function of conduits procured by technicians with varying EVH experience.
METHODS
EVH was performed during CABG by “experienced” (>900 cases, n=55 patients) vs. “novice” (<100 cases, n=30 patients) technicians. Afterwards, conduits were and examined for vascular injury using optical coherence tomography (OCT), with segments identified as injured further examined for gene expression using a tissue injury array. Conduit diameter was measured intra- and postoperatively (day 5 and 6 months) using OCT and Computed-Tomography angiography.
RESULTS
EVH performed by novice harvesters resulted in increased number of discrete graft injuries and higher expression of tissue injury genes. Regression analysis revealed an association between shear stress and early dilation (positive remodeling) (R2 =0.48, p <0.01). Injured veins showed blunted positive remodeling at 5 days and a greater degree of late lumen loss at 6 months.
CONCLUSION
Under normal conditions, intraluminal shear stress leads vein grafts to develop positive remodeling over the first postoperative week. Injury to conduits, a frequent sequela of the learning curve for EVH, was a predictor of early graft failure, blunted positive remodeling and greater negative remodeling. Given the ongoing annual volume of EVH cases, rigorous monitoring of the learning curve represents an important and unrecognized public health issue.
Keywords: Endoscopy, Coronary Artery Bypass Surgery, Genetics, Endothelium
INTRODUCTION
Endoscopic vein harvesting (EVH) rapidly gained popularity over open harvest technique after initial studies showed dramatically improved wound outcomes and comparable patency during coronary artery bypass grafting (CABG)1–4. However, recent post-marketing data have suggested worse graft patency and poor clinical outcomes within the first year after CABG utilizing EVH compared to open harvest5–8. There is a tendency amongst harvesters inexperienced with this technique to more forcefully manipulate the vein in an effort to gain better endoscopic vision/exposure. Technique improves with experience and follows a “learning curve” phenomenon seen with other technically challenging procedures. A greater risk of endothelial injury within the saphenous vein graft (SVG) during the learning curve may help explain an associated risk of early graft thrombosis for patients undergoing EVH.
SVG can also develop a gradual loss of luminal caliber during the first 6–12 postoperative months due to negative or constrictive remodeling and neointimal hyperplasia9,10. Meticulous preservation of the surrounding tissue of the SVG with the so-called “no-touch” open harvesting technique avoids adventitial trauma and significantly improves SVG patency11,12. This suggests that adventitial injury during SVG procurement is a contributing factor to negative remodeling. Our lab has established the capability of optical coherence tomography (OCT) and CT angiography (CTA) as high-resolution tools to identify injury within the bypass conduit intraoperatively and monitor patency and lumen diameter after grafting. To test the hypothesis that problems with EVH may relate to its learning curve, we used these tools to analyze whether the degree of SVG injury after procurement the patency and vascular function of conduits.
MATERIAL AND METHODS
Subject Enrollment
Institutional Review Board approval was obtained at two institutions (protocol #H25350 and #H27266) to perform a prospective observational study of patients undergoing isolated CABG (clinicaltrials.gov: NCT00481806). Inclusion criteria for this subset analysis included those patients that underwent EVH during CABG. Exclusion criteria included the inability to obtain follow-up CT angiography due to creatinine >2.0 mg/dl or allergy to radiographic contrast, emergency cases, or prior bleeding diathesis. Demographics, preoperative risk factors and medications, and intraoperatively and postoperative data were prospectively imported into a relational database. All patients provided prospective informed consent.
Surgical Technique
All patients underwent off-pump CABG via median sternotomy by a single surgeon. All EVH procedures were performed concurrent to left internal mammary harvest by physician assistants (PA) using standardized technique (VasoView 6.0, Maquet Corp, Wayne, NJ). Heparin bolus was administered prior to CO2 insufflation at a pressure of 10 to 12 mm Hg within the perivenous tunnel created by the camera dissector. Division of branches was performed with bipolar electrocautery set at 20 watts. Proximal SV ligation was performed through a separate stab incision. At the study outset, we noted that the level of experience for those PA that performed EVH during this study separated into a novice group (n=5 PAs) with <100 prior total cases at a frequency of < 3 cases/month and expert group (n=2 PAs) with > 900 prior cases at the study outset and frequency of > 30 cases/month.
After grafting, blood flow was measured in each SVG using transit time ultrasound (Medistim, Inc, Oslo, Norway). Mean shear stress was calculated for each graft according to the Hagen-Poiseuille formula, Tw =4μQ/πr3, where Tw is shear stress in dynes/cm2, Q is the mean volumetric flow and μ, is the viscosity of blood, assumed to be 0.035 poise13. Luminal radius, r (cm), was measured using OCT imaging with the SVG distended to 100 mmHg pressure.
Intraoperative Image Acquisition and Analysis
After harvest, conduits were cannulated on a sterile back-table with a Y-adapter (Gateway TM Plus Y-Adapter.118″ I.D.) to allow flushing with a Plasma-Lyte solution (Baxter International, Inc, Deerfield, Ill) containing heparin, glyceryl trinitrate, and verapamil at a controlled pressure of 100mm Hg. A 1F OCT catheter (Image Wire, Light Lab Imaging, Westford, MA) was placed into the lumen of the SVG and images were acquired during a manual pullback at rate of 1mm/sec. Because the OCT wire emits a red light that was easily visualized through the vessel wall, the wire location can be identified in real time so that portions of the conduit identified as abnormal can be registered to the portions chosen (or excluded) from grafting.
A technician in the operating room acquired the OCT data, which was also used for intraoperative image-guided biopsy. Two separate technicians who were blinded to group assignment analyzed each OCT image independently after the operation. Conduit injury during procurement was categorized as isolated to the intima and minor when the abnormality was restricted to the ostium of branch points or severe when the intimal injury involved the luminal surface. Deep vessel injury was diagnosed by OCT when a separation of the intimal layer from the medial or adventitial layer was noted (with or without an intimal tear). Adventitial injury was diagnosed as discontinuity in the external elastic lamina. A composite injury score was created based on the sum total of all discrete injuries noted within each conduit.
Image guided biopsies were obtained from the affected segments, frozen in cutting compound (Tissue-Tek O.C.T., Redding, Calif) and sectioned at 5 μm. Specimens were stained with CD31 mAb (R&D System, Inc.) to determine endothelial integrity using previously described histochemical techniques14 or Verhoeff’s stain to assess vascular architecture. The external elastic lamina was identified as a thick layer of elastic fibers, immediately adjacent to the media. Adventitia was defined as the loose connective tissue external to the external elastic lamina.
Analysis of graft function
Cardiac CT angiography (CTA) was acquired via 64-MDCT scanner (Philips Medical Systems) on postoperative day 5 and interpreted by a thoracic radiologist using axial and reconstructed curved planar images. SVG patency was defined as any flow through the length of the graft regardless of the presence of stenosis15. Conduit diameter was calculated intraoperatively (by OCT) and on postoperative day 5 and 6 months (CTA) by obtaining the mean of three separate measurements taken from the proximal, mid and distal segments of the portion of conduit used for grafting. Positive and negative remodeling were defined as a postoperative mean SVG diameter that increased vs. decreased compared to the baseline measurement obtained by intraoperative OCT.
RNA Extraction and Reverse Transcription
Total RNA was extracted from image-guided biopsies of SVG using the miRNeasy Mini kit (Qiagen, Valencia, California). Genomic DNA was removed on column by treated with RNase-free DNase I (Qiagen, Valencia, California). The total RNA samples were quantified using Nanodrop spectrophotometry (Wilmington, Delaware), and the quality of each RNA sample was validated by measuring the A260/A280 ratio with Nanodrop. RNA integrity was measured byAgilent Bioanalyzer (Santa Clara, California). cDNA was synthesized using High Capacity RNA-to-cDNA Master Mix with No RT Control Kit (Applied Biosystems, Foster City, California).
qPCR array
Tissue stress response array was performed by ABI 7500 fast real time PCR system (Applied Biosystems, Foster City, California) according to manufacturer’s instructions (Qiagen, Lonza). Raw data were collected as cycle threshold (Ct) values that were then normalized using housekeeping genes as background. Sub-group analysis was done based on whether the vessel from which the biopsy was derived showed evidence of positive or negative remodeling and the severity of injury noted in other portions of the vessel by OCT. Regression analysis was also done to determine correlation between endpoints described above and expression levels of each gene studied.
Statistics
The primary endpoint of this trial was to establish a level of injury during procurement using EVH that was likely to have a significant effect on the vascular function of patent bypass conduits. Baseline and perioperative variables for patients assigned to the two groups were compared using Student’s t-test for continuous variables and χ2-test for categorical variables. Based the findings of previous studies of early vein remodeling, we included shear16, serum lipids17, endothelial integrity18 and gene expression of tissue injury markers as candidate predictors. Single and multivariate binary and logistic regressions were performed to determine predictive relationships among variables.
The accuracy of OCT-based diagnoses of injury was validated using histopathologic analysis of corresponding regions of interest based on OCT examinations and by determining interobserver agreement using the (Cohen’s) Kappa test. Statistical analysis was performed with SPSS 17.0 (Chicago, Illinois); significance was set at p<0.05.
RESULTS
EVH Experience
EVH, performed by either the novice group (n=30 SVG) or expert group (n=55 SVG), was completed in all cases without the need for open conversion. Conduits from the two groups had similar total length (33 vs. 34 cm, p=0.880), required similar harvesting times (32.4 vs. 31.8 minutes, p=0.640) and an equivalent number of repair sutures (0.5 vs. 0.7 per vein, p=0.750). Patients that were grafted with the SVG from these two groups had similar baseline characteristics, comorbidities and early and late postoperative outcomes (data not shown) with the exception of the incidence of unstable angina, which was greater in the expert group (68.09% vs. 26.67%, p<0.001). Notably, the rate of attrition was similar for SVG in the novice and expert groups (6.45% vs. 4.34% loss at 5 days, p=0.552).
Conduit injury
Intimal injury around the ostia of branch points (2.48 vs. 1.76 injuries/conduit, p=0.02) and disruption of the external elastic lamina (5.37 vs. 3.31 injuries/graft, p=0.014) were more frequent findings within SVG from the novice group. This resulted in a significantly higher composite injury score for the novice compared to the expert group (7.77(5.28) vs. 5.11(3.71) injuries/graft, p=0.009) as well as significantly higher numbers of adventitial injuries (5.37(4.33) vs. 3.31(3.13), p=0.014). Compared to a normal SVG where the adventitia constitutes the bulk of the wall, there was complete removal of a segment of adventitia extending for 7 cm along the most severely injured conduit from the novice group (figure 1). In less severe cases, adventitial injury included minor separations of the adventitia from the media, associated with a focal disruption in the external elastic lamina.
Figure 1. Representation of adventitial tear as seen on OCT imaging.
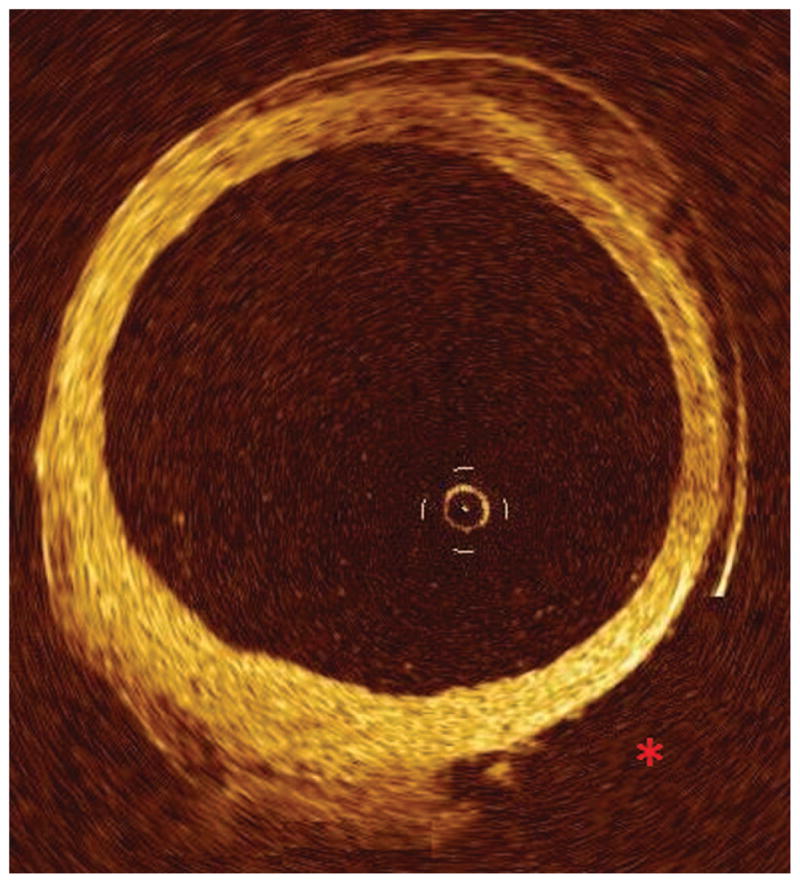
OCT imaging of SVG with significant adventitial tear (red “*”) with associated denudation of adventitia from the deeper vessel wall.
Regions of injury identified by OCT imaging showed an increased expression of several genes relevant to tissue stress. The composite vessel injury score showed a significant correlation to CXCL2 (R=0.748, p=0.053), and PDGFA (R=0.784, p=0.037). The degree of adventitial injury was significantly correlated to the expression of EGF and HSP90AA1, and ITGAV (R=0.766, p=0.045, R=0.828, p=0.042, and R=0.773, p=0.042, respectively). The degree of intimal injuries was significantly correlated with IL-1B (R=0.881, p=0.020) and IL-7 (R=0.855, p=0.030).
Validation of OCT Findings
There was strong interobserver agreement noted for the diagnosis of ostial branch injuries (κ=0.81), intimal injuries (κ=0.89), and deep vessel injuries (κ=0.82) using OCT. Histologic analyses of surplus segments of the SVG showed a significant correlation between the number of intimal abnormalities detected by OCT and percent endothelial integrity (R=−0.5, p=0.02). There was no difference in the degree of endothelial disruption found on histology in areas diagnosed by OCT as having either minor vs. severe intimal abnormalities.
Relationship between Shear and Early Dilation
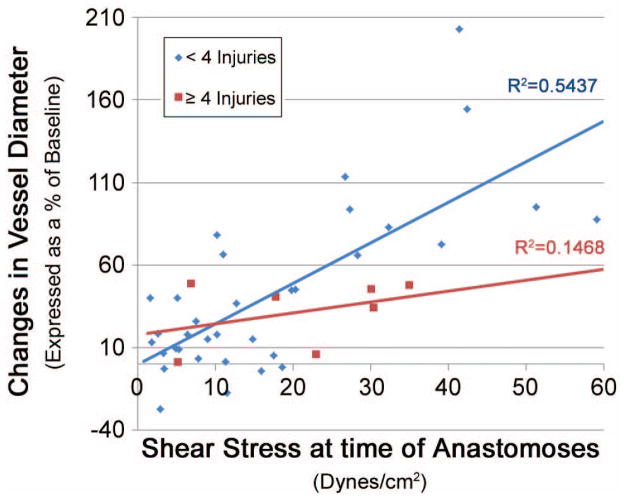
There was a strong relationship between the magnitude of shear force at the time of anastomoses and change in vessel diameter (positive remodeling) at 5 days (R2 =0.48, p<0.001) (figure 2).
Figure 2. Relationship between shear and early remodeling on Post-operative day 5.
Relationship between shear stress (dynes/cm2) after anastomoses and subsequent change in lumen diameter (expressed as a percentage of baseline, measured with intraoperative OCT) on post-operative day 5. Pearson’s R-value reached significance. Significance set at p < 0.05.
Relationship Between Graft Injury and Function
We demonstrated injury scores of ≥4 in SVG of 16 patients. The baseline clinical parameters of this group were similar the remaining group of patients that received SVG with <4 discrete injuries (Table 1). Serum lipids were similar between groups. After distension at 100mmHg using a venodilator solution, the baseline diameter of the SVG procured from both groups was similar (3.09(0.98) vs. 2.88 (0.72) mm, p=0.249). At 5 days after grafting, the percentage change in diameter was strongly associated with shear stress in the group with <4 injuries (R=0.686, p<0.001). However, this association between early positive remodeling and shear stress was no longer present in the group with SVG demonstrating ≥4 injuries (R=0.383, p=0.396). This association was also stronger in the expert harvester group vs. novice group (R=0.718, p<0.001, and R=0.531, p=0.028, respectively). Lumen loss at 6 months was greater in the group demonstrating ≥4 injuries, trending toward significance (Table 2).
Table 1.
Baseline Characteristics of Groups With and Without Severe SVG Injury
| Demographic | ≥4 Injuries (n=16) | < 4 Injuries (n=66) | P-Level |
|---|---|---|---|
| Age (years) | 67.1±11.4 | 65.2±10.9 | 0.542 |
| Male sex | 81.25% (13/16) | 83.33% (55/66) | 1.00 |
| Number of Diseased Vessels | 3.00±0.00 | 2.94±0.39 | 0.208 |
| BMI (kg/m2) | 29.7±6.7 | 30.0±6.1 | 0.819 |
| Hypertension | 81.25% (13/16) | 89.39% (59/66) | 0.401 |
| Diabetes mellitus | 43.75% (7/16) | 45.45% (30/66) | 1.00 |
| Dyslipidemia | 87.50% (14/16) | 78.78% (52/66) | 0.726 |
| Active Tobacco Smoking | 31.25% (5/16) | 28.78% (19/66) | 1.00 |
| Existing Renal Failure | 6.25% (1/16) | 1.52% (1/66) | 0.354 |
| Prior CVA | 6.25% (1/16) | 10.61% (7/66) | 1.00 |
| PVD | 6.25% (1/16) | 12.12% (8/66) | 0.681 |
| Family History of CAD | 25.00% (4/16) | 40.91% (27/66) | 0.269 |
| ASA | 100.00% (16/16) | 87.88% (58/66) | 0.344 |
| EF (<35%) | 31.25% (5/16) | 34.85% (23/66) | 1.00 |
| Unstable Angina | 25.00% (4/16) | 37.88% (25/66) | 0.054 |
ASA=On Active Aspirin Therapy; BMI=Body Mass Index; CAD=Coronary Artery disease; CVA=Prior Cerebral Vascular Accident/Stroke; EF=Ejection Fraction; HDL=High Density Lipoprotein; LDL=Low Density Lipoprotein; PVD=Peripheral Vascular Disease.
Table 2.
Comparison of SVG outcomes in groups stratified by injury
| Parameter | ≥4 Injuries (n=16) | < 4 Injuries (n=66) | P-level |
|---|---|---|---|
| Baseline Diameter (mm) | 2.77 ± 0.81 | 2.97 ± 0.84 | 0.41 |
| Δ Diameter vs. Shear (Pearson’s R2-value) | 0.147a | 0.544 | N/A |
| Mean Δ Diameter at 6 mo. (% of baseline) | −30.70 (5.79) | −12.4 (13.82) | 0.06 |
Table comparing outcomes in SVGs stratified by degree of injury. Each value is expressed as the mean (standard deviation) for each cohort unless otherwise specified. Significance was set at p < 0.05.
Correlation is Non-significant.
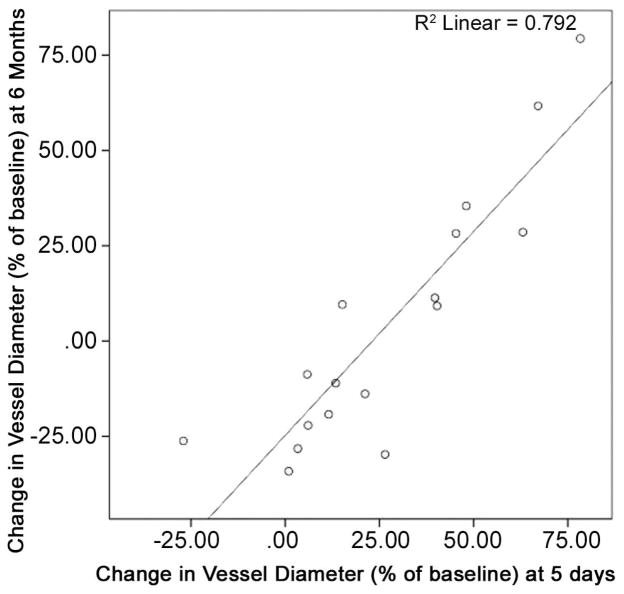
Predictors of SVG lumen diameter at 6 months included the degree of intimal injury noted intraoperatively (R=−0.744, p=0.041), the baseline SVG intimal thickness on histology (R =−0.58, P = 0.002), and the degree of positive remodeling on the post-operative day 5 (R=0.940, p=0.005) (Figure 3).
Figure 3. Relationship between early SVG remodeling and lumen diameter at 6 months.
Relationship between early ability of the vessel to dilate (POD5) and vessel diameter at 6-month follow up (measured via CT-angiogram). Pearson’s relationship achieved significance (significance set at p < 0.05).
COMMENT
Prior studies have demonstrated a variety of abnormalities within SVG harvested using EVH19 but have failed to address whether these histopathologic changes influence the performance of the vessel as a bypass graft. Our study utilized high resolution OCT and CTA imaging to identify graft injury within the grafted portion of the SVG and to follow serial changes in the diameter of the conduit postoperatively. This study design enabled a link to be established between severe vascular injury (i.e. ≥4 discrete injuries) and an abnormal positive remodeling of the graft over the first 5 days. SVG spared from injury showed a strong correlation between the calculated intraluminal shear and the degree of graft dilation measured on day 5 compared to baseline. In contrast, injured SVG showed little to no vasodilatory response to shear and showed a strong trend towards a higher risk of late lumen loss, or negative remodeling, at 6 months. Lumen loss within the first year post CABG is critical as SVGs with smaller lumen diameters are more prone to late graft failure20.
Early “positive” remodeling occurs in SVG when nitric oxide, prostacyclins and matrix metalloproteinases are produced locally in response to a chronic increase in intraluminal shear stress16,21. Animal and clinical studies demonstrate that positive remodeling requires intact endothelium18,22 and correlates with improved long-term SVG patency13. Impairment of this remodeling response has been attributed to trauma as well as biochemical and morphological changes within the graft16. SVG later develop wall thickening and stiffening in order to increase the elastic modulus of the graft in response to an increase in wall tension13,23. However, narrowing of the SVG lumen within the first year is predominantly caused by negative remodeling of the whole vessel rather than changes in thickness and stiffness10. Evidence suggests that adventitial injury and the subsequent the fibrotic scar response at the injured site are critical steps in the pathogenesis of negative remodeling24–26. We documented that the vessel and adventitial injury noted in our SVG were associated with increased expression of genes associated with tissue injury and remodeling response (Table 3), corroborating the importance of this finding.
Table 3.
Summary of the functions of genes found to be associated with SVG injury
| Gene | Function |
|---|---|
| PDGFA | Angiogenic Induces Neointimal Hyperplasia Possible role in vessel sclerosis |
|
| |
| EGF | Stimulates wound healing at endothelium SMC proliferation and invasion |
|
| |
| CXCL2 | Leukocyte recruitment / Chemotaxis Angiogenic |
|
| |
| ITGAV | Mediate cell-cell adhesive interactions Interaction with MMP1 (ITGA2) |
|
| |
| HSP90AA1 | Protective functions in context of cellular stress Positive modulator of eNOS |
|
| |
| IL-1B | Pro-inflammatory Mediates intimal hyperplasia |
|
| |
| IL-7 | Lymphangiogenesis |
Definition of abbreviations and alternative nomenclature for each gene/gene product. PDGFA – Platelet Derived Growth Factor subunit A, PDGF-A, PDGF-1; EGF=Epithelial Growth Factor; CXCL2 – Chemokine (C-X-C-motiff) Ligand 2, ITGAV= integrin alpha V, CD51; HSP90AA1= heat shock protein (HSP) 90kDa alpha (cytosolic) class A member 1, HSP 90-alpha; IL-1B=Interleukin 1-beta; IL-7=Interleukin 7; eNOS=endothelial Nitric Oxide Synthase; MMP – Matrix Metalloproteinase.
As the outer most layer of the vein, the adventitia is exposed to injury by EVH, particularly when novice technicians are performing initial blunt dissection step of this procedure. The ability of EVH to insure that the perivascular and adventitial tissues are fully preserved stands in stark contrast to the open “no touch” harvesting technique espoused by Souza and colleagues11,12. Their technique describes the procurement of full pedicle of fatty tissue completely encircling the vein with no actual dissection occurring on the vessel wall itself. With this as the “gold standard” for comparison, it is clear that minimizing this injury in order to procure vein conduits with normal postoperative function using EVH requires technicians to acquire a higher degree of technical mastery than previously estimated. Indeed, we confirm that technician inexperience increased the risk of abnormalities in the quality and function of conduits procured by EVH. Compared to the expert group, veins from the novice group had significantly more tears identified in the intima and deep vessel layers. In addition, the positive remodeling response induced by shear was significantly blunted in veins procured by notices compared to the experienced group. We have demonstrated that the degree of injury sustained by the graft affects graft function and early graft patency27. These findings provide support for our hypothesis that the EVH learning curve influences the quality and function of bypass conduits and warrant further study to more fully characterize the EVH learning curve.
The strengths of our study were that, unlike all prior analyses of the effect of EVH on conduit quality, our study quantified injury within the portion of conduit that was chosen for grafting. Previous studies have used sampling of discarded graft for histological and microscopic analysis. However, because only a small portion of vessel is used, and presumably one that is disproportionately poor in quality compared to the rest of the graft, such studies are limited in their ability to draw generalizable conclusions to the rest of the graft. In comparison, no differences were noted between our study groups using the standard “learning curve” metrics – time to complete EVH, number of sutures required for repair the harvested vein, and the need to convert to an open technique. Additionally, mRNA expression analysis provided evidence that the injury observed in SVG was associated with changes in the cellular biology of the vessel, and not simply an incidental finding.
Our study was limited by the small cohort size and lack of conventional angiography in order to confirm our perioperative OCT and CTA findings. Without this “gold standard”, we consider our work to be a “proof of concept” rather than an exhaustive validation of our protocol. However, defining the natural history and clinical importance of early SVG injury has remained elusive in large part due to the inconvenience of performing serial invasive angiography. Based on the variety of clinical advantages of serial imaging with OCT and CTA, future clinical research that incorporates this protocol may provide a renewed opportunity to expedite this overdue analysis.
In conclusion, intraluminal shear stress leads normal vein grafts to develop positive remodeling over the first postoperative week. Poor conduit quality, a sequela of the learning curve for EVH, was a predictor of early graft failure, blunted positive remodeling and greater negative remodeling. Given the ongoing annual volume of EVH cases, rigorous monitoring of the learning curve represents an important and under-recognized public health issue.
Discussion
13. Venous Grafts Procured During the Learning Curve for Endoscopic Veins Harvesting Show Compromised Vascular Remodeling. Paper presented by Soroosh Kiani, MA, Boston, MA. E mail: soroosh@bu.edu
Discussion by Keith B. Allen, M.D., Missouri E-mail: kallen2340@aol.com Dr. K. Allen (Kansas City, MO):
I congratulate you on your study. I’ve spent 15 years promoting the benefits of endoscopic vein harvesting with regard to decreasing wound complications. I am somewhat concerned, though, when I look at your data that even in the hands of an experienced harvester, you still have a composite injury score of over five. I am concerned that over the last 10 years when we saw an explosion in EVH use, it was primarily driven by the abandonment of some of the early EVH techniques where blunt dissection was not used in favor of the much easier technique that currently essentially involves blunt stripping of the vein. I think we need to be very cautious with some of the long-term data that is coming out showing the negative influence of endoscopic vein harvesting and look very carefully at the harvesting devices that we use.
Thank you.
MR. KIANI: Thank you for that comment.
13. Venous Grafts Procured During the Learning Curve for Endoscopic Veins Harvesting Show Compromised Vascular Remodeling. Paper presented by Soroosh Kiani, MA, Boston, MA. E mail: soroosh@bu.edu
Discussion by Michael Nowak, MD, Minnesota E mail: mnowak333@yahoo.com Dr. M. Nowak (Rochester, MN):
I just wanted to ask which harvesting device was used in your study.
13. Venous Grafts Procured During the Learning Curve for Endoscopic Veins Harvesting Show Compromised Vascular Remodeling. Response by Soroosh Kiani, MA, Boston, MA.
MR. KIANI: I will defer that to the primary surgeon, Dr. Poston; however, I believe it was VasoView.
DR. POSTON: VasoView.
13. Venous Grafts Procured During the Learning Curve for Endoscopic Veins Harvesting Show Compromised Vascular Remodeling. Paper presented by Soroosh Kiani, MA, Boston, MA. E mail: soroosh@bu.edu
Discussion by Leonard Baklarz, PA C, Maryland E mail: lbbaklarz@yahoo.com Mr. L. Baklarz (Bethesda, MD):
Thank you for the study. It is very important to me. I have been harvesting vein for about 14 years, teaching it for about 10, and it is important to note that the learning curve is significant. However, the technique which I have been performing I really do believe in, but we do have to focus on how do we shorten that learning curve, which is significant. The procedure is difficult to perform, as most people who have had their hands on it can attest to.
Some of the things I do have a concern with, we do know the shortfalls of the New England Journal of Medicine article, especially with randomizing to vein harvesting, and then there are two other articles that have been out, the Nova Scotia article, for one, and the recent one out of Anova Fairfax dealing with long term results in thousands of patients, which showed no difference between the two groups of open and endoscopic vein.
So how would you proceed forward with shortening the learning curve, and then what was your idea of the novice harvester, how many cases was that, and how would you change that?
13. Venous Grafts Procured During the Learning Curve for Endoscopic Veins Harvesting Show Compromised Vascular Remodeling. Response by Soroosh Kiani, MA, Boston, MA.
MR. KIANI: Thank you for that question. I will answer your second question first. The novice harvesters were harvesters that we defined at our institution, PAs that had less than 100 cases of experience, and the expert harvesters were those that had over 900 or close to 1,000.
In terms of shortening the learning curve, our study is a cross sectional analysis of what is happening with the learning curve - a snapshot in time. I think the first thing that would be interesting and useful to do would be to study the actual learning curve so as to be able to describe it further; perhaps through a longitudinal study that follows novice PAs through their learning curve and, therefore, be able to characterize it further before we made any specific recommendations about the specific pedagogy.
I do think that the one conclusion that we (at least at our institution) are able to draw is that, while conventional wisdom tells us that about 20 or 30 cases is enough to get a harvester through their novice phase, we showed it to be at least 100 cases. However, I think in order to really define this, we need to look at a larger number of harvesters throughout the course of their learning curve, perhaps utilizing a CUSUM analysis to characterize the outcomes.
Acknowledgments
This project was funded by National Institutes of Health (NIH) Grant #RO1 HL084020.
ABBREVIATIONS AND ACRONYMS
- CABG
Coronary Artery Bypass Surgery
- CT
Computed Tomography
- Ct
Cycle Threshold
- CTA
(Cardiac) Computed Tomography Angiogram
- EVH
Endoscopic Vein Harvest
- NIH
National Institutes of Health
- OCT
Optical Coherence Tomography
- PA
Physician’s Assistant
- PCR
Polymerase Chain Reaction
- SVG
Saphenous Vein Graft
Footnotes
MEETING PRESENTATION: STS 47th Annual Meeting, San Diego, CA. Jan 29 – Feb 2, 2011. Parallel Surgical Forum I: Adult Cardiac on Jan 31st.
DISCLOSURES: NONE.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Soroosh Kiani, University of Arizona School of Medicine, Division of Cardiac and Thoracic Surgery, 1501 N. Campbell Avenue, Tucson, AZ 85724-5071
Pranjal H. Desai, University of Arizona School of Medicine, Division of Cardiac and Thoracic Surgery, 1501 N. Campbell Avenue, Tucson, AZ 85724-5071
Nannan Thirumvalavan, Boston University School of Medicine, 80 East Concord Street, Boston, MA 02118
Dinesh John Kurian, Boston University School of Medicine, 80 East Concord Street, Boston, MA 02118
Mary Margaret Flynn, University of Virginia Health System, 1215 Lee Street, Charlottesville, VA 22908
XiaoQing Zhao, CVPath Institute, Inc., 19 Firstfield Road, Gaithersburg, MD 20878
Robert S. Poston, Email: rposton@surgery.arizona.edu, Jack G. Copeland Endowed Chair of Cardiovascular Surgery, Michael Drummond Distinguished Professor of Cardiovascular and Thoracic Surgery., Chief, Division of Cardiac and Thoracic Surgery, Co-Chairman, Sarver Heart Center, University of Arizona School of Medicine, 1501 N. Campbell Avenue, Tucson, AZ 85724-5071, Phone: 520-626-7951, Fax: 520-626-7991.
References
- 1.Yun KL, Wu Y, Aharonian V, et al. Randomized trial of endoscopic versus open vein harvest for coronary artery bypass grafting: Six-month patency rates. J Thorac Cardiovasc Surg. 2005 March 1;129(3):496–503. doi: 10.1016/j.jtcvs.2004.08.054. [DOI] [PubMed] [Google Scholar]
- 2.Bitondo JM, Daggett WM, Torchiana DF, et al. Endoscopic versus open saphenous vein harvest: a comparison of postoperative wound complications. The Annals of Thoracic Surgery. 2002;73(2):523–528. doi: 10.1016/s0003-4975(01)03334-3. [DOI] [PubMed] [Google Scholar]
- 3.Davis Z, Jacobs HK, Zhang M, Thomas C, Castellanos Y. Endoscopic vein harvest for coronary artery bypass grafting: technique and outcomes. The Journal of Thoracic and Cardiovascular Surgery. 1998;116(2):228–235. doi: 10.1016/s0022-5223(98)70121-7. [DOI] [PubMed] [Google Scholar]
- 4.Puskas JD, Wright CE, Miller PK, et al. A randomized trial of endoscopic versus open saphenous vein harvest in coronary bypass surgery. The Annals of Thoracic Surgery. 1999;68(4):1509–1512. doi: 10.1016/s0003-4975(99)00952-2. [DOI] [PubMed] [Google Scholar]
- 5.Shroyer AL, Grover FL, Hattler B, et al. On-Pump versus Off-Pump Coronary-Artery Bypass Surgery. New England Journal of Medicine. 2009;361(19):1827–1837. doi: 10.1056/NEJMoa0902905. [DOI] [PubMed] [Google Scholar]
- 6.Lopes RD, Hafley GE, Allen KB, et al. Endoscopic versus Open Vein-Graft Harvesting in Coronary-Artery Bypass Surgery. New England Journal of Medicine. 2009;361(3):235–244. doi: 10.1056/NEJMoa0900708. [DOI] [PubMed] [Google Scholar]
- 7.Brener SJ, Barr LA, Burchenal JEB, et al. Randomized, Placebo-Controlled Trial of Platelet Glycoprotein IIb/IIIa Blockade With Primary Angioplasty for Acute Myocardial Infarction. Circulation. 1998 August 25;98(8):734–741. doi: 10.1161/01.cir.98.8.734. [DOI] [PubMed] [Google Scholar]
- 8.Alexander JH, Hafley G, Harrington RA, et al. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA. 2005 Nov 16;294(19):2446–2454. doi: 10.1001/jama.294.19.2446. [DOI] [PubMed] [Google Scholar]
- 9.Kaneda H, Terashima M, Takahashi T, et al. Mechanisms of lumen narrowing of saphenous vein bypass grafts 12 months after implantation: an intravascular ultrasound study. Am Heart J. 2006 Mar;151(3):726–729. doi: 10.1016/j.ahj.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Lau GT, Ridley LJ, Bannon PG, et al. Lumen loss in the first year in saphenous vein grafts is predominantly a result of negative remodeling of the whole vessel rather than a result of changes in wall thickness. Circulation. 2006 Jul 4;114(1 Suppl):I435–440. doi: 10.1161/CIRCULATIONAHA.105.001008. [DOI] [PubMed] [Google Scholar]
- 11.Souza DSR, Dashwood MR, Tsui JCS, et al. Improved patency in vein grafts harvested with surrounding tissue: results of a randomized study using three harvesting techniques. The Annals of Thoracic Surgery. 2002;73(4):1189–1195. doi: 10.1016/s0003-4975(02)03425-2. [DOI] [PubMed] [Google Scholar]
- 12.Souza DSR, Johansson B, Bojö L, et al. Harvesting the saphenous vein with surrounding tissue for CABG provides long-term graft patency comparable to the left internal thoracic artery: Results of a randomized longitudinal trial. The Journal of Thoracic and Cardiovascular Surgery. 2006;132(2):373–373. doi: 10.1016/j.jtcvs.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Owens CD, Wake N, Jacot JG, et al. Early biomechanical changes in lower extremity vein grafts--distinct temporal phases of remodeling and wall stiffness. Journal of Vascular Surgery. 2006;44(4):740–746. doi: 10.1016/j.jvs.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Manchio JV, Gu J, Romar L, et al. Disruption of Graft Endothelium Correlates With Early Failure After Off-Pump Coronary Artery Bypass Surgery. Ann Thorac Surg. 2005 June 1;79(6):1991–1998. doi: 10.1016/j.athoracsur.2004.12.054. [DOI] [PubMed] [Google Scholar]
- 15.Frazier AA, Qureshi F, Read KM, Gilkeson RC, Poston RS, White CS. Coronary Artery Bypass Grafts: Assessment with Multidetector CT in the Early and Late Postoperative Settings1. Radiographics. 2005 July-August;25(4):881–896. doi: 10.1148/rg.254045151. [DOI] [PubMed] [Google Scholar]
- 16.Owens CD, Ho KJ, Conte MS. Lower extremity vein graft failure: a translational approach. Vascular Medicine. 2008;13(1):63–74. doi: 10.1177/1358863X07083432. [DOI] [PubMed] [Google Scholar]
- 17.RodÈs-Cabau J, Facta A, Larose E, et al. Predictors of Aorto-Saphenous Vein Bypass Narrowing Late After Coronary Artery Bypass Grafting. The American Journal of Cardiology. 2007;100(4):640–645. doi: 10.1016/j.amjcard.2007.03.080. [DOI] [PubMed] [Google Scholar]
- 18.Muto A, Model L, Ziegler K, Eghbalieh SD, Dardik A. Mechanisms of vein graft adaptation to the arterial circulation: insights into the neointimal algorithm and management strategies. Circ J. 2010 Aug;74(8):1501–1512. doi: 10.1253/circj.cj-10-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allen K, Cheng D, Cohn W, et al. Endoscopic Vascular Harvest in Coronary Artery Bypass Grafting Surgery: A Consensus Statement of the International Society of Minimally Invasive Cardiothoracic Surgery (ISMICS) 2005. Innovations:Technology and Techniques in Cardiothoracic and Vascular Surgery. 2005;1(2):51–60. doi: 10.1097/1001.gim.0000196315.0000132179.0000196382. [DOI] [PubMed] [Google Scholar]
- 20.Canos DA, Mintz GS, Berzingi CO, et al. Clinical, angiographic, and intravascular ultrasound characteristics of early saphenous vein graft failure. J Am Coll Cardiol. 2004 Jul 7;44(1):53–56. doi: 10.1016/j.jacc.2004.03.045. [DOI] [PubMed] [Google Scholar]
- 21.Vita JA, Holbrook M, Palmisano J, et al. Flow-Induced Arterial Remodeling Relates to Endothelial Function in the Human Forearm. Circulation. 2008 June 17;117(24):3126–3133. doi: 10.1161/CIRCULATIONAHA.108.778472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohta S, Komori K, Yonemitsu Y, Onohara T, Matsumoto T, Sugimachi K. Intraluminal gene transfer of endothelial cell-nitric oxide synthase suppresses intimal hyperplasia of vein grafts in cholesterol-fed rabbit: a limited biological effect as a result of the loss of medial smooth muscle cells. Surgery. 2002 Jun;131(6):644–653. doi: 10.1067/msy.2002.124878. [DOI] [PubMed] [Google Scholar]
- 23.Jacot JG, Abdullah I, Belkin M, et al. Early adaptation of human lower extremity vein grafts: wall stiffness changes accompany geometric remodeling. Journal of Vascular Surgery. 2004;39(3):547–555. doi: 10.1016/j.jvs.2003.09.045. [DOI] [PubMed] [Google Scholar]
- 24.Shi Y, Pieniek M, Fard A, O’Brien J, Mannion JD, Zalewski A. Adventitial Remodeling After Coronary Arterial Injury. Circulation. 1996 January 15;93(2):340–348. doi: 10.1161/01.cir.93.2.340. [DOI] [PubMed] [Google Scholar]
- 25.Josiah NW, Ron W, Spencer BK, Neal AS. The role of the adventitia in the arterial response to angioplasty: The effect of intravascular radiation. International journal of radiation oncology, biology, physics. 1996;36(4):789–796. doi: 10.1016/s0360-3016(96)00299-4. [DOI] [PubMed] [Google Scholar]
- 26.Wilensky RL, March KL, Gradus-Pizlo I, Sandusky G, Fineberg N, Hathaway DR. Vascular Injury, Repair, and Restenosis After Percutaneous Transluminal Angioplasty in the Atherosclerotic Rabbit. Circulation. 1995 November 15;92(10):2995–3005. doi: 10.1161/01.cir.92.10.2995. [DOI] [PubMed] [Google Scholar]
- 27.Desai P, Kiani S, Thiruvanthan N, et al. Impact of the Learning Curve for Endoscopic Vein Harvest on Conduit Quality and Early Graft Patency. Ann Thorac Surg. 2011 May 1;91(5):1385–1392. doi: 10.1016/j.athoracsur.2011.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]