Abstract
In the cerebellum, lamellar Bergmann glial (BG) appendages wrap tightly around almost every Purkinje cell dendritic spine. The function of this glial ensheathment of spines is not entirely understood. The development of ensheathment begins near the onset of synaptogenesis, when motility of both BG processes and dendritic spines are high. By the end of the synaptogenic period, ensheathment is complete and motility of the BG processes decreases, correlating with the decreased motility of dendritic spines. We therefore have hypothesized that ensheathment is intimately involved in capping synaptogenesis, possibly by stabilizing synapses. To test this hypothesis, we misexpressed GluR2 in an adenoviral vector in BG towards the end of the synaptogenic period, rendering the BG α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) Ca2+-impermeable and causing glial sheath retraction. We then measured the resulting spine motility, spine density and synapse number. Although we found that decreasing ensheathment at this time does not alter spine motility, we did find a significant increase in both synaptic pucta and dendritic spine density. These results indicate that consistent spine coverage by BG in the cerebellum is not necessary for stabilization of spine dynamics, but is very important in the regulation of synapse number.
Keywords: Synaptogenesis, Purkinje cell, cerebellum, GluR2
INTRODUCTION
Astroglia fill much of the space between neurons and link several central nervous system cell types, as they are in direct contact with each other, neurons, endothelial cells from capillaries and other glial cell types (Volterra and Meldolesi, 2005). Ultrastructural images show that astroglia have small processes that tightly wrap synapses from the post-synaptic side (Fig. 1B) (Peters and Kaiserman-Abramof, 1970; Spacek, 1985; Ventura and Harris, 1999; Grosche et al., 2002). The extent of the glial coverage and the percentage of synapses covered vary by region. For example, in the mature animal, approximately 57% of the synapses in the hippocampus (Ventura and Harris, 1999) and 29% of synapses in the neo-cortex (Spacek, 1985) are ensheathed by glial processes. Notably, almost all mature cerebellar excitatory synapses onto Purkinje cells are entirely covered by Bergmann glial (BG) processes (Spacek, 1985; Grosche et al., 1999). While the close apposition of the glial process to the synapse allows for easy interactions between the cells, the primary function of the ensheathment remains unclear.
Fig. 1. Transduction of Bergmann glia in the cerebellum with adenovirus.

(A) Injection of CMV-tdTomato adenovirus into cerebella of transgenic mice expressing GFP in all Purkinje cells results primarily in transduction of the Bergmann glia cells (red). (B) Interactions between dendritic spines (green) and labeled Bergmann glia (red) can be visualized. Bar = 250 μm in (A) and 4 μm in (B).
One potential function of glial ensheathment in the cerebellum is the regulation of synapses. Dendritic spines, small projections off of dendrites that provide the sites for 90% of excitatory synapses in the brain, are highly dynamic during the period of synaptogenesis, then stabilize shortly thereafter (Dunaevsky et al., 1999; Deng and Dunaevsky, 2005). What stops this motility remains unknown. Glial process dynamics follow a similar time course, and process outgrowth and ensheathment of synapses increase in the cerebellum coincident with the decrease of spine motility (Lippman et al., 2008). In the hippocampus, although glial coverage is less than that seen in the cerebellum, astrocytic contact with newly developing dendritic protrusions can both stabilize and promote maturation into spines (Haber et al., 2006; Nishida and Okabe, 2007).
Here, we set out to test the role of glial ensheathment of Purkinje cell dendritic spines in regulating dendritic spine motility and synapse number. To accomplish this, we have decreased glial ensheathment of spines at the end of the third postnatal week, when spines and BG processes are less dynamic and synapse ensheathment is almost complete (Lippman et al., 2008). We began by confirming and quantifying decreased ensheathment by misexpression of the GluR2 subunit in BGs, rendering the normally Ca2+ permeable BG AMPARs impermeable to Ca2+ (Iino et al., 2001). We then used this method to examine the effect of glial ensheathment on spine motility and synapse number. We found that although decreasing the level of ensheathment does not affect spine motility, it does increase the number of synapses and spine density.
METHODS
Animals
All experiments were performed using either C57/BL-6 mice from Charles River Laboratories or from our breeding facility, or the transgenic mouse line (Tg(Pcp2-cre)137Gsat) with enhanced green fluorescent protein-labeled Purkinje cells. The mice were kept on regular light/dark cycles throughout the procedures. All protocols were approved by the Brown University Institutional Animal Care and Use Committee.
Adenovirus production
The cytomegalvirus (CMV)–tdTomato and CMV–GluR2/tdTomato viral constructs were kindly provided by Sung Ok Yoon of Ohio State University. tdTomato and GluR2 were cloned into a shuttle vector (pAdTrack-CMV). This plasmid was recombined with an adenoviral backbone plasmid, pAdEasy-1. The Pac-1 linearized recombinant was then transfected into 293 cells. Virus was purified with Adeno-X Virus Mini Purification Kit (Clontech, Mountain View, CA). Transfection and functional expression of protein from these viral constructs were tested in HEK-293 cells, followed by western blotting.
Viral labeling of Bergmann glia in the intact animal
Postnatal day (P)22–23 mice were anesthetized with ketamine/dormitor cocktail and placed in a stereotaxic apparatus. After the animal was deeply anesthetized, a small incision was made on top of the head to expose the skull. A small hole was drilled above the vermis of cerebellum with a dental drill. A glass electrode backfilled with supernatant containing tdTomato and GluR2/tdTomato adenovirus was inserted into the anterior vermis at a depth of 100–700 μm, using stereotaxic equipment. Using a picospritzer, a volume of 0.2–0.4 μl was injected over 15 min into cerebellum. Following injections, skin was sutured. Pups were provided with food and water in their own cages. All pups were monitored regularly from the surgery until the day of imaging to ensure normal feeding and activity.
Assessing glial ensheathment of spines following adenoviral injection
ELECTRON MICROSCOPY
Mice were perfused with 2.5% glutaraldehyde, 2.5% paraformaldehyde, 0.1 M PB 2 days after injections. Brains were sectioned on a vibratome at 75 μm. Floating sections containing virally labeled tdTomato expressing cells were incubated in blocking solution to block nonspecific binding (10% NGS, 0.1% TritonX-100 in PBS) for 1 h and incubated in polyclonal anti-red fluorescent protein (RFP; Molecular Probes) in 1% NGS, 0.01% TritonX-100, overnight at 4°C. Sections were rinsed three times in PBS (20 min) and incubated in horse-radish peroxidase (HRP)-conjugated secondary antibody for 1 h at room temperature. Sections were exposed to diamino-benzidine (DAB, 0.15%) in Tris Buffer for 2–3 min. Thin sections were prepared and imaged with a Philips 410 transmission electron microscope at 10400× as described earlier (Lippman et al., 2008).
ENSHEATHMENT ANALYSIS
From each animal 10–13 fields containing RFP labeled BG were analyzed. For each synapse, we made two measurements: the circumference of the spine that is not in contact with the pre-synaptic terminal (S1) and the circumference of the spine that is in contact with a glial process (S2, Fig. 2C). We then calculated the fraction of spine covered as S2/S1.
Fig. 2. GluR2 expression in Bergmann glia causes reduced ensheathment of synapses.
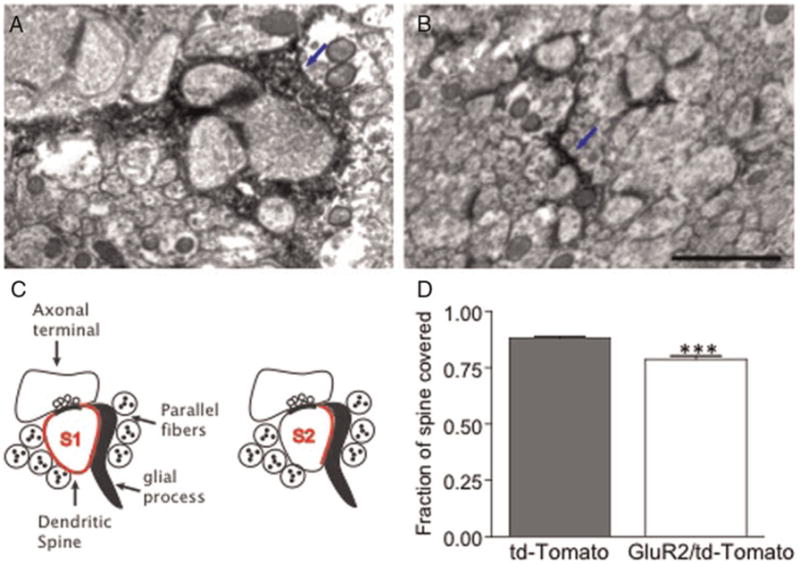
Electronmicrograph from P24 mouse cerebella injected with adenovirus containing (A) tdTomato alone or (B) GluR2–tdTomato. The tdTomato-expressing BG are identified by the presence of dark HRP reaction product, indicated with blue arrows here. (C) Schematic representation of measurement of glial ensheathment at the synapse. (D) The fraction of spine covered (as measured by S2/S1 ratio) significantly decreases with GluR2 expression in BG. Bar = 1 μm.
Assessing dendritic spine motility
ACUTE SLICES
After allowing 48–72 h for viral expression (Iino et al., 2001) P24–25 pups were anesthetized with ketamine/dormitor (70 mg kg−1, 0.5 mg kg−1, respectively) prior to rapid decapitation with sharp scissors. Brains were removed from the skulls immediately and the cerebellum was dissected out. Sagittal 300 μm cerebellar slices were prepared in ice cold artificial cerebral spinal fluid (ACSF) using a vibratome. To reduce drift during imaging, slices were placed on MF-Millipore membranes for 20 min in a 37°C, 5% CO2 incubator. Although changes in spines and glia occur immediately after slice preparation, they are transient and recover within 1–2 h (Fiala et al., 2003). We therefore allowed at least 1 h for recovery time before acute slice imaging experiments.
TIME-LAPSE IMAGING OF ACUTE SLICES
Imaging was conducted using a multiphoton laser-scanning microscope (Radiance 2000, BioRad coupled to a Nikon E-600-FN microscope). High-resolution imaging was performed with a long working distance, dipping objective 60×, N.A. 1. The membranes carrying the labeled slices were placed in the imaging chamber. Slices were perfused with oxygenated ACSF at 35–37°C. Slices were held in place using a platinum and nylon harp. The imaging chamber was kept at 35–37°C (Warner Instruments). Images were collected every 30 s for a period of 15 min at a digital zoom of 5 (yielding a pixel size of 0.08 × 0.08 μm). At each time point, three to seven focal planes 0.5 μm apart were collected. Although this volume included many complete spines and glial processes, we also collected an extended z-stack 5–10 μm deep before and after imaging to record the full extent of a dendritic shaft. In this way, we could determine whether structures that have appeared or disappeared during the time-lapse series are new structures or ones that entered or left the focal plane.
ANALYSIS OF DENDRITIC SPINE DYNAMICS
Images were only analyzed if they remained in focus throughout the imaging period, did not bleach and did not show signs of phototoxicity. Spine motility was quantified using a ‘motility index’ as previously described (Dunaevsky et al., 1999). In short, the motility index measures the overall displacement of a spine. We first measured the area of a spine at seven time points that differ the most from each other in a single time-lapse movie, then subtract the smallest area from the total projected or accumulated area and divided by the average area.
Analysis of synapse number
IMMUNOCYTOCHEMISTRY
P24–25 mice were perfused using 4% paraformaldehyde (2 days following adenoviral injection). Brains were removed and post-fixed overnight at 4°C in 4% paraformaldehyde, then sunk in 30% sucrose 0.1 M PB solution (1–2 days). The tissue was cast in optical cutting temperature compound and held at −80°C until it could be sectioned. At that time, the brains were equilibrated to −20°C and sectioned to 45 μm using a cryostat (Leica). Sections were kept in PBS, then floating sections were put in a blocking solution of 10% normal goat serum and 0.1% Triton X in PBS for 1 h at room temperature. Primary antibody VGluT1 (Chemicon, 1:5000) diluted in 1% normal goat serum and 0.1% Triton X in PBS were added to the sections and incubated either overnight at 4°C or 3 h at room temperature. Sections were rinsed in PBS and incubated in secondary antibody conjugated with Alexa Fluor 647 (Molecular Probes/Invitrogen, 1:500) for 1 h at room temperature. Sections were rinsed and mounted onto slides using VectaShield mounting medium for fluorescence (Vector Laboratories Inc., Burlingame, CA).
CONFOCAL IMAGING
Following immunohistochemistry, we imaged sections on a Zeiss LSM510 confocal microscope with a 63× (1.4 NA) oil objective at a digital zoom of 5, yielding a pixel size of 0.05 μm per pixel. Only areas with adenovirus-expressing BG (as visualized by tdTomato) were imaged.
PRE-SYNAPTIC PUNCTA ANALYSIS
Confocal images from sections immunostained for VGlut1 were separated by color, then converted to grayscale using Metamorph (Molecular Devices, Sunnyvale, CA). The VGlut1 labeling was put through a low-pass filter. In stacks of 9–11 consecutive z-planes, each 4 μm apart, we created two non-overlapping boxes of 250 × 350 and 250 × 300 pixels (rendering volumes of 600–875 μm3), and counted individual puncta within each box. Each punctum was counted only in the plane in which it first appeared, but only if the signal-to-noise ratio of the punctum exceeded 2.5 in at least one plane. Puncta that were very close together were determined to be separate if two intensity peaks were identified in a line scan passing through the puncta. To normalize for volume analyzed, we divided the number of puncta per box by the total volume of that box.
SPINE DENSITY ANALYSIS
Purkinje cell spines were identified by expression of GFP. The number of spines per 10 μm of dendrite was counted in sets of seven z-stacks in the same areas in which we measured pre-synaptic puncta.
Statistical analysis
Data were analyzed using SPSS. We checked for normality using the Shapiro–Wilk test. Data with normal distributions were analyzed using a t-test. Data without normal distributions were analyzed with the Wilcoxon signed-rank test to test for main effect in paired pre-treatment/post-treatment comparisons and the Mann–Whitney U test or Kruskal–Wallis test with a post-hoc Dunn’s multiple comparisons test to test for main effect in all other cases.
RESULTS
To test the role of ensheathment on spine dynamics and synapse number, we took advantage of a method described by Iino et al. (2001) in which misexpression of the AMPA receptor GluR2 subunit in BG cells, which normally only express AMPA receptors lacking the GluR2 subunit, causes the glial processes to retract. After verifying by western blot that our transcript was causing GluR2 expression (see supplementary figure 1 online), we injected GluR2 and tdTomato in an adenoviral vector into the cerebella of a transgenic line of mice, Purkinje cell protein 2 (PCP2) mice (Tg(Pcp2-cre)137Gsat), which express GFP under the PCP2 promoter. Although we used the ubiquitous CMV promoter, the adenovirus preferentially labels glial cells at this age allowing a targeted expression in BG (Fig. 1A). Following viral injection in these mice, we can examine Bergmann glial processes at the Purkinje cell synapse (Fig. 1B).
GluR2 misexpression in mature BG reduces ensheathment to immature levels
Because Iino et al. (2001) did not quantify the effect of GluR2 on glial process morphology, we first verified that expressing GluR2 in BGs caused glial sheath retraction by injecting adenoviral-GluR2/tdTomato into mouse cerebella, then viewing the synapses using transmission electron microscopy. We found the labeled areas by immunostaining sections with an antibody to RFP with an anti-HRP secondary antibody. To measure ensheathment, we compared the ratio of spine perimeter contacted by a glial process to spine perimeter not touching the pre-synaptic element (Fig. 2C; Lippman et al., 2008). We found a decrease in the level of glia-spine contact in the GluR2-adenovirus-injected mice (P < 0.0001, 0.76 ± 0.02 mean ± SEM, n = 49 frames, 408 synapses, four mice), as compared to controls (0.88 ± 0.01, n = 43 frames, 606 synapses; Fig. 2D). With this confirmation of decreased glial ensheathment of the synapse, we went on to use this method to determine if full ensheathment is required for the stabilization of dendritic spine motility.
Glial ensheathment of spines does not regulate spine dynamics
To determine whether ensheathment of spines by glial processes regulates dendritic spine motility, dendritic spines in cerebellar slices were imaged following viral injections of GluR2. We made acute slices from P24 PCP2 mice that had been injected 2–3 days prior with adenovirus containing either the tdTomato/GluR2 or tdTomato alone, then collected two-photon laser scanning time-lapse images. Purkinje cell dendritic spines in the areas with the highest concentration of tdTomato-labeled BG were imaged every 45 s for a period of 20 min. Contrary to our expectations, we found that the motility of dendritic spines in areas of GluR2–tdTomato labeled BG did not differ significantly from spines in areas containing BG that expressed tdTomato alone (P = 0.154, 0.82 ± 0.03 mean ± SEM, n = 89 spines in seven animals for tdTomato controls; 0.87 ± 0.03, n = 73 spines in five animals for GluR2–tdTomato; Fig. 3). This indicates that full, constant glial ensheathment of the spine is not necessary to maintain the decreased spine motility seen at the end of synaptogenesis.
Fig. 3. Decreased ensheathment by GluR2 expression in BG does not affect dendritic spine motility.

Time-lapse images of Purkinje cell dendritic spines in acute slices from P24 mouse cerebella injected with adenovirus containing (A) tdTomato alone or (B) GluR2–tdTomato show similar levels of motility, as quantified in C. In each time-lapse image, an example of a stable spine is highlighted by a red arrow and an example of a more motile spine is highlighted by a yellow arrow. Time is indicated in minutes. Bar = 4 μm.
GluR2 misexpression in BG causes an increase in synapse number and spine density
If BG sheaths and/or Ca2+ permeability of the BG AMPARs play a role in synapse formation or maintenance, we would expect to see a change in synaptic number when we alter GluR2 expression in BG. Therefore, we next asked whether GluR2 misexpression and the subsequent sheath retraction could alter synapse number. We immunostained sections from tdTomato- and tdTomato/GluR2-adenovirus-injected P24 mice with the pre-synaptic marker VGluT1 (Fig. 4) and counted synaptic puncta and dendritic spines. We found a 28.6% increase in VGluT1 puncta in sections with BG mis-expressing GluR2 (P = 0.013, 14.1 ± 0.9 and 18.1 ± 1.2 puncta μm−3, mean ± SEM for tdTomato-injected and tdTomato–GluR2-injected mice, respectively, n = 6; Fig. 4C–E). In addition, we measured dendritic spine density in a subset of these same sections and saw a similar 24% increase (P = 0.004, 3.2 ± 0.2 and 4.0 ± 0.2 spines μm−1, mean ± SEM, for tdTomato-injected (n = 4) and tdTomato–GluR2-injected mice (n = 5), respectively; Fig. 4F–H). These results indicate that full synapses (pre-synaptic terminals and post-synaptic spines) may either form or be maintained more readily if the glial sheath does not fully surround the spine. This suggests a role for glial ensheathment and glial sheath Ca2+ permeability in capping synaptogenesis through synapse maintenance in vivo in the cerebellum.
Fig. 4. Decreased ensheathment by GluR2 expression in BG results in increased density of synapses.

Confocal images of BG (red) expressing tdTomato–GluR2 in sections of transgenic mice expressing GFP in PC (green), immunostained with the pre-synaptic marker VGluT1 (blue) in low (A) and high (B) magnification. Pre-synaptic terminals are identified with VGluT1 labeled puncta in cerebella injected with control tdTomato (C) or with tdTomato–GluR2 (D) expressing virus. Decreasing the level of glial ensheathment with GluR2 results in increase of the number of VGluT1 puncta (E). Dendritic spine density was measured in cerebella injected with control tdTomato (F) or with tdTomato–GluR2 (G) expressing virus. Decreasing the level of glial ensheathment with GluR2 results in increase of the density of dendritic spines (H). Bar: 40 μm in A, 6 μm in B–D and 5 μm in F and G.
DISCUSSION
We have previously described the developmental and molecular regulation of BG process growth and dynamics and showed that BG processes increase in length and complexity, and decrease in motility, over the course of synaptogenesis. Here, we examined the functional implications of BG ensheathment of synapses. We found that although reduced glial ensheathment of the synapse by GluR2 misexpression in glia did not alter dendritic spine motility in P24 mice, it did result in an increase in the density of pre-synaptic terminals and dendritic spines.
We have previously shown that decreased BG process motility, increased process complexity and spine ensheathment correlate with the end of synaptogenesis. In the cerebellum, although dendritic spine motility is developmentally regulated, dendritic spines are not stabilized through contact with a pre-synaptic element (Dunaevsky et al., 2001; Deng and Dunaevsky, 2005), although this is not the case in the hippocampus (Korkotian and Segal, 2001). Although glial ensheathment of spines was a good candidate for regulation of the decreased dendritic spine dynamics seen at the end of synaptogenesis, in the cerebellum, it does not seem to be involved in this process. This is in contrast to studies in the hippocampus demonstrating that astrocytes seem to promote stabilization and maturation of newly formed dendritic protrusions (Haber et al., 2006; Nishida and Okabe, 2007). In contrast with the cerebellum, in which most synapses onto PCs spines are fully ensheathed, in the mature hippocampus, only about half of the synapses onto spines are ensheathed, with a wide range of coverage levels (Peters and Kaiserman-Abramof, 1970; Spacek, 1985; Fiala and Harris, 2001). Considering this vast difference in glial coverage between the cerebellum and the hippocampus, it might not be surprising that glial ensheathment of dendritic spines does not seem to play the same role in regulating spine motility in these brain regions.
Although glial misexpression of GluR2 and decreased ensheathment did not affect rapid spine motility, it did result in a higher number of synapses than in controls. This indicates that, in the cerebellum, although complete synaptic coverage by glia is not essential for stabilizing rapid dendritic spine movement, full ensheathment may inhibit further synaptogenesis and/or induce synaptic pruning. Therefore, ensheathment by glial processes with Ca2+-permeable AMPARs may still play a role in stabilizing the number of synapses, although other factors may be in place to keep the spine still. Importantly, even the relatively small change of 14% glial coverage was enough to elicit a difference in synapse number, underscoring the important regulatory differences that may occur between the cerebellum and areas with less dense glial coverage of synapses. It remains unclear whether the increase in synapses we describe here is due to a physical interaction with the synapse (i.e. the glial sheath is taking up space that, when then made available following glial sheath retraction, will host a synapse) or whether it is a downstream effect of a pathway involving Ca2+ flux through GluR2-lacking AMPARs.
Our results described here, together with our previous study in which expressing a dominant-negative Rac1 in BG similarly decreased ensheathment and increased pre-synaptic puncta (Lippman et al., 2008), could point to Rac1 and AMPARs as being part of the same pathway that results in actin regulation in glia. A good candidate may be the mTor pathway, which is the primary pathway dysregulated in tuberosclerosis (Bourgeron, 2009) and has been implicated in regulating spine density (Kumar et al., 2005). In astrocytes, mTor can regulate actin through a rac1-dependent mechanism (Sandsmark et al., 2007). In addition, a recent study has shown that mTor phosphorylation in cultured Bergmann glia is regulated by glutamate in a dose-dependent and Ca2+-dependent manner (Zepeda et al., 2009). As we are likely impairing both glutamate signaling and Ca2+ entry through AMPA receptors by misexpressing GluR2, we may be disrupting multiple aspects of this pathway by altering GluR2 and Rac1.
Why should GluR2 misexpression and glial sheath retraction affect synapse formation but not spine motility? One possibility is that spine motility increases immediately as the processes begin retract, before we begin to image, mediating the increased number we see, but in a time frame that we miss by waiting 2–3 days for full expression. Also, it is possible that while the rapid motility seen early in synaptogenesis and which we measured here are not affected by reduced glial ensheathment, spine turnover and formation are affected. Spine turnover and formation might occur at a different time scale and could be more relevant to the final number of synapses.
It is also possible that, at this developmental stage, motility is not as crucial to synapse formation as it is earlier in development. Early in synaptogenesis, when their potential contacts may be far away, spines may need to be more dynamic to locate contacts, but later in synaptogenesis, the potential partners are all in place so motility may become less crucial to finding a contact. Although it decreases dramatically, spine motility does not come to a complete halt even in Purkinje cells of P24 mice (Dunaevsky et al., 1999). Perhaps at the end of synaptogenesis, the spine simply needs to sample its surroundings less before it successfully contacts a terminal.
Yet another possibility is that the amount of glial contact with the spine that remained after GluR2 misexpression was enough to stabilize the spine. Developmentally regulated signaling molecules in BG could be responsible for the effects we see and may require a larger decrease in ensheathment to disengage. A potential candidate interaction that could be at work here is the Eph–ephrin interactions, as it has been indicated in neuron–glial communication resulting in changes in spine shape and number in the hippocampus (Murai et al., 2003; Nestor et al., 2007; Nishida and Okabe, 2007). Perhaps both increased ensheathment and increased production of a signaling molecule such as ephrins work in concert to inhibit spine motility, and by only decreasing some of the contact we are still allowing enough of a BG/spine interaction to occur to keep the spine from moving, but not to keep new synapses from forming.
If decreased ensheathment is increasing synapse formation (rather than decreasing pruning) without altering spine motility it is possible that these ‘new’ synapses are actually synapses that had been pruned back earlier in synaptogenesis and therefore form readily with even the slightest retraction of the glial processes from the spine. Whether these are entirely new synapses, reformation of previous synapses or un-pruned synapses, the increase in synapse number suggests that, in the cerebellum, complete synaptic coverage by glia may serve as a cap for synaptogenesis. In this model, reducing ensheathment may revert the cells to a less mature state that supports formation of synapses. Consistent with our findings, removal of glial ensheathment by over-expressing the GluR2 subunit in BG results in residual multiple climbing fiber innervation of PCs (Iino et al., 2001). If glial ensheathment is a major force in driving synaptogenesis to a close, studying BG process regulation, and therefore regulation of ensheathment, could open a door for potential targets in synaptogenesis and synaptic plasticity research. In addition, synaptic ensheathment could provide therapeutic targets for the many disorders characterized by dysfunctional synapse formation.
Supplementary Material
Acknowledgments
We thank Sung Ok Yoon for assistance with making the GluR2 adenovirus construct. NINDS (1R01NS057667)
Footnotes
Statement of interest
None
The supplementary material referred to in this article can be found online at journals.cambridge.org/ngb.
References
- Bourgeron T. A synaptic trek to autism. Current Opinion in Neurobiology. 2009;19:231–234. doi: 10.1016/j.conb.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Deng J, Dunaevsky A. Dynamics of dendritic spines and their afferent terminals: spines are more motile than presynaptic boutons. Developmental Biology. 2005;277:366–377. doi: 10.1016/j.ydbio.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Dunaevsky A, Tashiro A, Majewska A, Mason C, Yuste R. Developmental regulation of spine motility in the mammalian central nervous system. Proceedings of the National Academy of Sciences of the USA. 1999;96:13438–13443. doi: 10.1073/pnas.96.23.13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunaevsky A, Blazeski R, Yuste R, Mason C. Spine motility with synaptic contact. Nature Neuroscience. 2001;4:685–686. doi: 10.1038/89460. [DOI] [PubMed] [Google Scholar]
- Fiala JC, Harris KM. Extending unbiased stereology of brain ultrastructure to three-dimensional volumes. Journal of American Medical Information Association. 2001;8:1–16. doi: 10.1136/jamia.2001.0080001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala JC, Kirov SA, Feinberg MD, Petrak LJ, George P, Goddard CA, et al. Timing of neuronal and glial ultrastructure disruption during brain slice preparation and recovery in vitro. Journal of Comparative Neurology. 2003;465:90–103. doi: 10.1002/cne.10825. [DOI] [PubMed] [Google Scholar]
- Grosche J, Matyash V, Moller T, Verkhratsky A, Reichenbach A, Kettenmann H. Microdomains for neuron–glia interaction: parallel fiber signaling to Bergmann glial cells. Nature Neuroscience. 1999;2:139–143. doi: 10.1038/5692. [DOI] [PubMed] [Google Scholar]
- Grosche J, Kettenmann H, Reichenbach A. Bergmann glial cells form distinct morphological structures to interact with cerebellar neurons. Journal of Neuroscience Research. 2002;68:138–149. doi: 10.1002/jnr.10197. [DOI] [PubMed] [Google Scholar]
- Haber M, Zhou L, Murai KK. Cooperative astrocyte and dendritic spine dynamics at hippocampal excitatory synapses. Journal of Neuroscience. 2006;26:8881–8891. doi: 10.1523/JNEUROSCI.1302-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M, Goto K, Kakegawa W, Okado H, Sudo M, Ishiuchi S, et al. Glia-synapse interaction through Ca2+-permeable AMPA receptors in Bergmann glia. Science. 2001;292:926–929. doi: 10.1126/science.1058827. [DOI] [PubMed] [Google Scholar]
- Korkotian E, Segal M. Regulation of dendritic spine motility in cultured hippocampal neurons. Journal of Neuroscience. 2001;21:6115–6124. doi: 10.1523/JNEUROSCI.21-16-06115.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Zhang MX, Swank MW, Kunz J, Wu GY. Regulation of dendritic morphogenesis by Ras-PI3 K-Akt-mTOR and Ras-MAPK signaling pathways. Journal of Neuroscience. 2005;25:11288–11299. doi: 10.1523/JNEUROSCI.2284-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippman JJ, Lordkipanidze T, Buell ME, Yoon SO, Dunaevsky A. Morphogenesis and regulation of Bergmann glial processes during Purkinje cell dendritic spine ensheathment and synaptogenesis. Glia. 2008;56:1463–1477. doi: 10.1002/glia.20712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murai KK, Nguyen LN, Irie F, Yamaguchi Y, Pasquale EB. Control of hippocampal dendritic spine morphology through ephrin-A3/EphA4 signaling. Nature Neuroscience. 2003;6:153–160. doi: 10.1038/nn994. [DOI] [PubMed] [Google Scholar]
- Nestor MW, Mok LP, Tulapurkar ME, Thompson SM. Plasticity of neuron–glial interactions mediated by astrocytic EphARs. Journal of Neuroscience. 2007;27:12817–12828. doi: 10.1523/JNEUROSCI.2442-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida H, Okabe S. Direct astrocytic contacts regulate local maturation of dendritic spines. Journal of Neuroscience. 2007;27:331–340. doi: 10.1523/JNEUROSCI.4466-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Kaiserman-Abramof IR. The small pyramidal neuron of the rat cerebral cortex. The perikaryon, dendrites and spines. American Journal of Anatomy. 1970;127:321–355. doi: 10.1002/aja.1001270402. [DOI] [PubMed] [Google Scholar]
- Sandsmark DK, Zhang H, Hegedus B, Pelletier CL, Weber JD, Gutmann DH. Nucleophosmin mediates mammalian target of rapamycin-dependent actin cytoskeleton dynamics and proliferation in neurofibromin-deficient astrocytes. Cancer Research. 2007;67:4790–4799. doi: 10.1158/0008-5472.CAN-06-4470. [DOI] [PubMed] [Google Scholar]
- Spacek J. Three-dimensional analysis of dendritic spines. III. Glial sheath. Anatomy and Embryology. 1985;171:245–252. doi: 10.1007/BF00341419. [DOI] [PubMed] [Google Scholar]
- Ventura R, Harris KM. Three-dimensional relationships between hippocampal synapses and astrocytes. Journal of Neuroscience. 1999;19:6897–6906. doi: 10.1523/JNEUROSCI.19-16-06897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nature Reviews. Neuroscience. 2005;6:626–640. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- Zepeda RC, Barrera I, Castelan F, Suarez-Pozos E, Melgarejo Y, Gonzalez-Mejia E, et al. Glutamate-dependent phosphorylation of the mammalian target of rapamycin (mTOR) in Bergmann glial cells. Neurochemistry International. 2009;55:282–287. doi: 10.1016/j.neuint.2009.03.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


