Abstract
Addition of LiOHMT (OHMT = O-2,6-dimesitylphenoxide) to W(O)(CH-t-Bu)(PMe2Ph)2Cl2 led to WO(CH-t-Bu)Cl(OHMT)(PMe2Ph) (4). Subsequent addition of Li(2,5-Me2C4H2N) to 4 yielded yellow W(O)(CH-t-Bu)(OHMT)(Me2Pyr)(PMe2Ph) (5). Compound 5 is a highly effective catalyst for the Z-selective coupling of selected terminal olefins (at 0.2% loading) to give product in >75% yield with >99% Z configuration. Addition of two equivalents of B(C6F5)3 to 5 led to catalyst activated at the oxo ligand by B(C6F5)3. 5.B(C6F5)3 is a highly active catalyst that produces thermodynamic products (~20% Z).
Early in the development of olefin metathesis catalysts that contain tungsten, it was shown that metathetically more active and reproducible systems were produced when tungsten oxo complexes were deliberately employed or were present as impurities in WCl6.1 The possibility that oxo alkylidene complexes, e.g., W(O)(CHR)X2 (where X is a chloride, alkoxide, etc.), are the true catalysts in at least some of the “classical” olefin metathesis systems became more likely when 1 (L = PMe3 and other phosphines) was prepared and isolated in good yield.2 Compound 1 was the first high oxidation state tungsten alkylidene complex that would both (i) metathesize terminal and internal olefins (in the presence of a trace of AlCl3) and (ii) produce a new alkylidene that could be observed as a consequence of olefin metathesis.
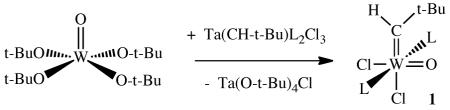 |
(1) |
By the time 1 was discovered, tantalum alkylidene complexes had been turned into functional olefin metathesis catalysts through use of alkoxides as ligands.3 Therefore, although some attempts were made to prepare a W(O)(CH-t-Bu)(OR)2 species from 1, no bisalkoxide species could be isolated. In view of the synthetic problems encountered upon attempted alkylation of oxo complexes, including removal of the oxo ligand entirely,1i and to protect alkylidenes against bimolecular decomposition, attention turned to the synthesis of imido alkylidene complexes of W and Mo, especially those containing a phenylimido ligand such as N(2,6-i-Pr2C6H3).4 Consequently, interest in oxo alkylidene complexes in the last 25 years has been sparce.5
The most recent development in Mo and W imido alkylidene chemistry has been monoaryloxide monopyrrolide (MAP) complexes.6 One of the most interesting discoveries is the ability of some MAP catalysts to promote Z selective metathesis reactions as a consequence of the presence of a relatively “large” aryloxide and “small” imido group.7 The preferred metal for Z selective couplings of terminal olefins at this time appears to be tungsten and the most successful aryloxide ligand has been O-2,6-(2,4,6-triisopropylphenyl)2C6H3 or OHIPT. (The more active molybdenum complexes8 appear to isomerize the Z product to E.) It has been proposed that the high steric demands of the OHIPT ligand force all metallacyclobutane substituents to one side of the metallacycle ring, and therefore allow only Z products to form. Since an oxo ligand is smaller than any NR ligand, the question arose as to whether MAP versions of tungsten oxo alkylidene complexes would be useful Z selective catalysts.
We chose to attempt to prepare W(O)(CH-t-Bu)(OHIPT)(Me2Pyr) (where Me2Pyr = 2,5-dimethylpyrrolide) from W(O)(CH-t-Bu)(PMe2Ph)2Cl2 (1a), hoping that both PMe2Ph ligands would dissociate from the metal in the crowded coordination sphere. The reaction between WO(CH-t-Bu)Cl2(PMe2Ph)2 and LiOHIPT in benzene at 22 °C for 14h led to isolation of off-white WO(CH-t-Bu)Cl(OHIPT)(PMe2Ph) (2) in 60% yield (equation 2). Two isomers of 2 are present in a 3:2 ratio according to 1H, 13C, and 31P NMR spectra. Both are syn alkylidenes on the basis of JCαH values for the alkylidene of 123 Hz (major isomer) and 117 Hz (minor isomer). The phosphine remains bound to tungsten on the NMR time scale (JPW = 420 Hz and 379 Hz, respectively) at 22 °C. An X-ray crystal structure (see Supporting Information) revealed a distorted square-pyramidal geometry with the neopentylidene ligand in the apical position and the phosphine ligand trans to chloride. The alkylidene was found to be disordered over syn and anti orientations in a 91:9 ratio. The other isomer of 2 could be (for example) one in which the OHIPT and Cl ligands (eq 2) have switched positions.
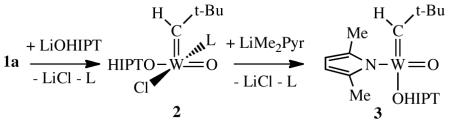 |
(2) |
Treatment of 2 with Li(Me2Pyr) in benzene at 60 °C for 16h led to formation of yellow W(O)(CH-t-Bu)(OHIPT)(Me2Pyr) (3) in 80% isolated yield. An X-ray structure of 3 showed it to have a pseudotetrahedral geometry, a syn alkylidene, and an η1-Me2Pyr ligand (Figure 1). We had considered the possibility that PMe2Ph was lost from the coordination sphere because the pyrrolide ligand was bound in an η5 fashion, thereby producing an 18 electron count at the metal. However, other steric factors alone appear to be sufficient to cause 14 electron 3 to be formed.
Figure 1.
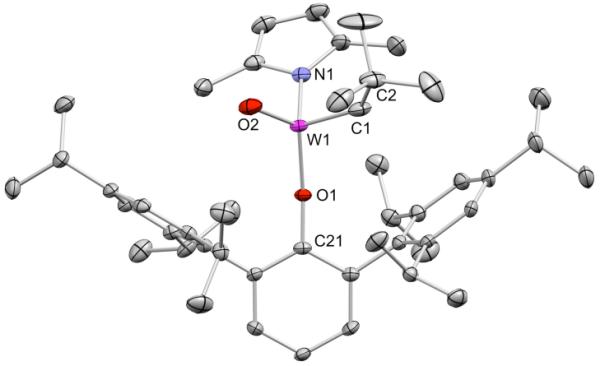
Thermal ellipsoid drawing (50% probability) of syn-W(O)(CH-t-Bu)(OHIPT)(Me2Pyr) (3). Hydrogen atoms have been omitted for clarity. Selected bond distances (Å) and angles (°): W1–C1 = 1.886(3), W1–O2 = 1.695(3), W1–O1= 1.868(2), W1–N1 = 2.001(2), W1–O1–C21 = 166.9(2), W1–C1–C2 = 136.7(3).
The analogous reaction between 1 and LiOHMT (OHMT = O-2,6-dimesitylphenoxide) in benzene at 22 °C for 3 h led to isolation of yellow WO(CH-t-Bu)Cl(OHMT)(PMe2Ph) (4) in 70% yield (equation 3). As with 2, the 1H NMR spectrum of the product contains two alkylidene doublet resonances that correspond to two isomers of 4 in a 87:13 ratio. The values of 1JCH (122 and 116 Hz) suggest that both isomers are syn alkylidenes. Addition of Li(Me2Pyr) to 4 in toluene at −30 °C followed by stirring the mixture at 22 °C for 10h led to yellow W(O)(CH-t-Bu)(OHMT)(Me2Pyr)(PMe2Ph) (5) in 70% isolated yield. An X-ray structure of 5 (Figure 2) showed it to be a square pyramid with the syn neopentylidene in the apical position and the phosphine bound trans to the pyrrolide.
Figure 2.
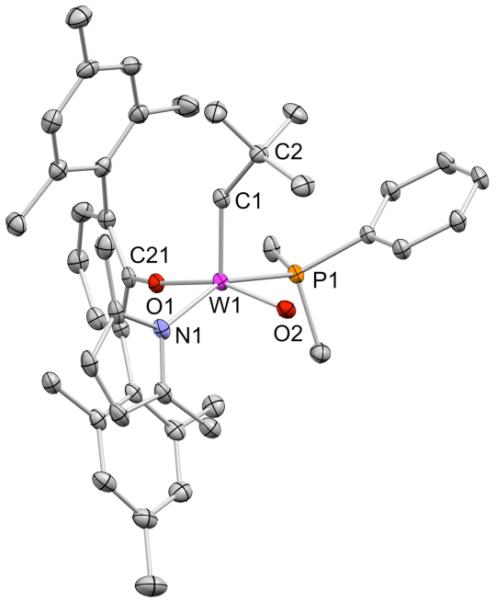
Thermal ellipsoid drawing (50% probability) of syn-W(O)(CH-t-Bu)(OHMT)(Me2Pyr)(PMe2Ph) (5). Hydrogen atoms have been omitted for clarity. Solvent molecules are not shown. Selected bond distances (Å) and angles (°): W1–C1 = 1.900(3), W1–O2 = 1.717(2), W1–O1 = 1.964(2), W1–N1 = 2.074(2), W1–P1 = 2.580(1), W1–O1–C21 = 159.8(2), W1–C1–C2 = 141.0(2).
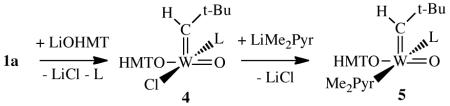 |
(3) |
The PMe2Ph ligand in 5 is partially dissociated at room temperature and rapidly exchanging on and off the metal. The alkylidene resonance is broad and its chemical shift is concentration dependent (8.57–9.14 ppm for 4 mM – 48 mM solutions in C6D6). Variable temperature 1H and 31P NMR studies of a 20 mM solution of 5 in CD2Cl2 showed that the phosphine is “bound” below −30 °C as indicated by a sharp 31P signal corresponding to the coordinated ligand (1.80 ppm, 1JPW = 289 Hz). On the basis of the chemical shift for free and coordinated phosphine the value of the equilibrium constant for phosphine dissociation can be estimated as 0.015 M at room temperature. This value corresponds to 57% dissociation of phosphine in a 20 mM solution of 5 in C6D6.
Both 3 and 5 react with ethylene to give an unsubstituted metallacyclobutane complex (and t-butylethylene) that has a square pyramidal structure (presumably with the oxo ligand in the apical position) on the basis of chemical shifts of metallacycle protons in the range 0.7–4.5 ppm.9 The reaction of 3 with ethylene is relatively slow and what we propose is an intermediate square pyramidal β-t-butylmetallacyclobutane complex can be observed before free t-butylethylene is formed. In the case of compound 5, a methylidene complex is formed in addition to the unsubstituted square pyramidal metallacycle. In both systems the unsubstituted metallacycles slowly decompose over a period of 24 h to unidentified products. A square pyramidal metallacyclobutane made from imido alkylidenes has been proposed to be further from the transition state for olefin loss than is the alternative TBP metallacycle.10a Extensive calculations have been performed on several high oxidation state metathesis systems that include metallacyclobutane complexes.10b,c
Both 3 and 5 serve as initiators for the polymerization of 5,6-dicarbomethoxynorbornadiene (DCMNBD). The polymerization of 50 equiv of DCMNBD is relatively slow (hours) with 3 and propagation is faster than initiation. The resulting polymer is >99% cis, 90% syndiotactic. The polymerization of 50 equiv of DCMNBD with 5 is relatively fast (minutes) and all initiator is consumed. The resulting polymer is >99% cis, 98% syndiotactic.11 These results suggest that the steric crowding in 3 is significantly greater than in 5 after phosphine is lost, and is in fact too great to allow formation of highly regular cis,syndiotactic-polyDCMNBD from 3.
Homocoupling of neat terminal olefins with 3 takes place slowly (hours) at room temperature. In contrast, 5 was found to be highly active and highly Z-selective (Table 1). A catalyst loading as low as 0.2 mol% yielded up to 86% conversion in 6 h for several of the six chosen substrates. No trans product could be observed in 1H NMR spectra of the Z products (see Supporting Information).
Table 1.
Conversions of Neat Terminal Olefins to Homocoupled >99% Z Metathesis Products Promoted by 5.a
| time | substrate/cat loading (mol%) | |||||
|---|---|---|---|---|---|---|
| S1/ 0.2 % |
S2/ 0.2 % |
S3/ 0.2 % |
S4/ 0.2 % |
S5/ 0.2 % |
S6/ 1 % |
|
| 10 min | 28% | 44% | 65% | - | 28% | - |
| 30 min | 39% | 67% | 75% | - | 39% | - |
| 1 h | 47% | 79% | 75% | 2% | 47% | 10% |
| 6 h | 66% | 86% | - | - | 73%b | - |
| 24 h | 72% | 88% | - | 11% | - | 59% |
S1 = 1-octene, S2 = allylbenzene, S3 = allylboronic acid pinacolate ester, S4 = allylSiMe3, S5 = 1-decene, S6 = Methyl-10-undecenoate.
The aliquot was taken after 7 h.
Only a small increase in conversion was found for >6 h reaction times, which suggests that the majority of the catalyst has decomposed at this stage. Decomposition of a catalyst prior to isomerization of the Z product to E can be a desirable feature of the coupling reaction. The reactions were run on a 200 mg scale in a closed vessel with a volume of ~20 mL. Homocoupling of 1-decene at 0.5 Torr did not show a significant increase in turnover compared to the reaction carried out under 1 atm of nitrogen. We ascribe the relatively low turnover in the case of allylTMS (S4) to steric issues, and in the case of methyl-10-undecenoate (S6, at 1% catalyst loading) to ester binding to W. These results should be compared with those obtained employing a W(N-3,5-Me2C6H3) catalyst system.7f
A long-standing question in classical olefin metathesis catalyst systems based on tungsten has been the role of a Lewis acid. One might expect that a Lewis acid in 5 could bind to the oxo ligand and thereby create a more electrophilic metal center and more reactive catalysts. Indeed we find that addition of Lewis acids to 5 significantly speeds up metathesis reactions. For example, addition of two equivalents of B(C6F5)3 to 5 resulted in a catalyst that converted 90% 1-octene to 7-tetradecene in one hour at 22 °C (0.2 mol% loading). However, the 7-tetradecene is only 20% Z. Since pure Z-7-tetradecene (in C6D6) is isomerized to a 78:22 mixture of E- and Z-tetradecene by 1 mol% 5 in the presence of 2 equiv of B(C6F5)3 in 15 minutes, any Z-7-tetradecene that is formed initially in the homocoupling reaction should be isomerized rapidly to a 4:1 E:Z mixture.
Addition of two equivalents of B(C6F5)3 to 5 led to formation of (Me2PhP)[B(C6F5)3] and 5.B(C6F5)3. The B(C6F5)3 in 5.B(C6F5)3 is labile at room temperature as demonstrated by a broadened alkylidene signal in the 1H NMR spectrum at 7.30 ppm. The 1H NMR spectrum of a 45 mM solution of 5.B(C6F5)3 at −60 °C shows a sharp alkylidene resonance at 7.06 ppm. An X-ray structure of 5.B(C6F5)3 showed that B(C6F5)3 is coordinated to the oxo ligand (Figure 3). The W1–O2–B1 unit is bent (W1–O2–B1 angle is 159.9(1)°). The W1–O2 distance is elongated (1.759(2) Å) relative to that in 5 (1.717(2) Å) or in 3 (1.695(3) Å) and is slightly shorter than in reported B(C6F5)3 adducts of tungsten oxo complexes.12 A relatively weak coordination of B(C6F5)3 to the oxo is also indicated by the B1–O2 bond length (1.571(3) Å), which is longer than in any B(C6F5)3 adducts of transition metal oxo complexes (1.484(3)–1.558(2) Å) in the literature. The average values of the C–B–C and O–B–C angles (112.6° and 106.1°, respectively) also suggest that B(C6F5)3 is relatively weakly coordinated.
Figure 3.
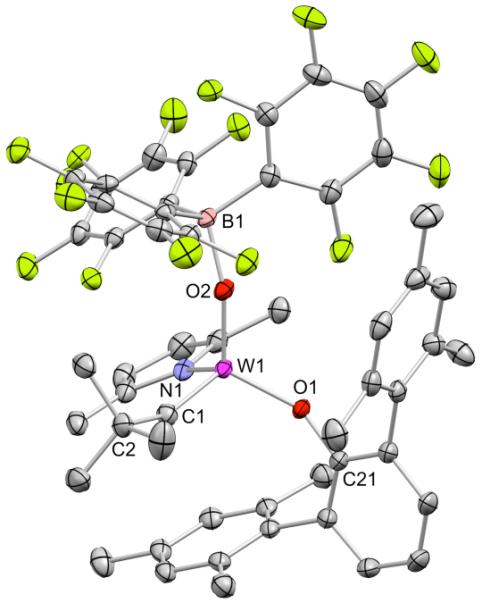
Thermal ellipsoid drawing (50% probability) of W(O)(B(C6F5)3)(CH-t-Bu)(OHMT)(Me2Pyr) (5.B(C6F5)3). Hydrogen atoms have been omitted for clarity. Selected bond distances (Å) and angles (°): W1–C1 = 1.868(2), W1–O2 = 1.759(2), W1–O1 = 1.860(2), W1–N1 = 1.968(2), B1–O2 = 1.571(3), W1–O1–C21 = 150.9(1), W1–C1–C2 = 155.4(2).
We conclude that tungsten oxo alkylidene complexes are effective Z-selective catalysts for the metathesis coupling of terminal olefins. We ascribe the selectivity to the small size of the oxo ligand relative to OHMT, the low rate of isomerization of the initial Z product relative to coupling of terminal olefins, and decomposition of the active catalyst under the conditions employed.
Supplementary Material
ACKNOWLEDGEMENT
We are grateful to the National Science Foundation (CHE-0841187 and CHE-1111133 to R.R.S.) and to the National Institutes of Health (Grant GM-59426 to R.R.S. and A.H.H.) for financial support. We thank the National Science Foundation for departmental X-ray diffraction instrumentation (CHE-0946721).
Footnotes
ASSOCIATED CONTENT
Supporting Information. Experimental details for all compounds, and crystal parameters, data acquisition parameters, and cif files for complexes 2, 3, 5, and 5.B(C6F5)3. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- (1).(a) Ivin KJ, Mol JC. Olefin Metathesis and Metathesis Polymerization. Academic Press; San Diego: 1997. [Google Scholar]; (b) Calderon N, Ofstead EA, Ward JP, Judy WA, Scott KW. J. Am. Chem. Soc. 1968;90:4133. [Google Scholar]; (c) Basset JM, Coudurier G, Praliaud H. J. Catal. 1974;34:152. [Google Scholar]; (d) Mocella MT, Rovner R, Muetterties EL. J. Am. Chem. Soc. 1976;98:4689. [Google Scholar]; (e) Burwell RL, Jr., Brenner A. J. Mol. Catal. 1976;1:77. [Google Scholar]; (f) Kress JRM, Russell MJM, Wesolek MG, Osborn JA. J. Chem. Soc. Chem. Comm. 1980;431 [Google Scholar]; (g) Muetterties EL, Band E. 1980;102:6572. [Google Scholar]; (h) Kress JRM, Wesolek MG, Le Ny J-P, Osborn JA. J. Chem. Soc. Chem. Comm. 1981;1039 [Google Scholar]; (i) Kress JRM, Wesolek MG, Osborn JA. J. Chem. Soc. Chem. Comm. 1982;514 [Google Scholar]
- (2).(a) Schrock RR, Rocklage SM, Wengrovius JH, Rupprecht G, Fellmann J. J. Molec. Catal. 1980;8:73. [Google Scholar]; (b) Churchill MR, Rheingold AL, Youngs WJ, Schrock RR, Wengrovius JH. J. Organometal. Chem. 1981;204:C17. [Google Scholar]; (c) Wengrovius JH, Schrock RR. Organometallics. 1982;1:148. [Google Scholar]; (d) Wengrovius JH, Schrock RR, Churchill MR, Missert JR, Youngs WJ. J. Am. Chem. Soc. 1980;102:4515. [Google Scholar]
- (3).(a) Rocklage SM, Fellmann JD, Rupprecht GA, Messerle LW, Schrock RR. J. Am. Chem. Soc. 1981;103:1440. [Google Scholar]; (b) Schrock RR. Polyhedron. 1995;14:3177. [Google Scholar]
- (4).(a) Schrock RR. Chem. Rev. Vol. 102. 2002. pp. 145–180. [DOI] [PubMed] [Google Scholar]; (b) Schrock RR. In: Reactions of Coordinated Ligands. Braterman PR, editor. Plenum; New York: 1986. p. 221. [Google Scholar]
- (5).(a) Bryan JC, Mayer JC. J. Am. Chem. Soc. 1990;112:2298. [Google Scholar]; (b) Blosch LL, Abboud K, Boncella JM. J. Am. Chem. Soc. 1991;113:7066. [Google Scholar]; (c) Ahn S, Mayr A. J. Am. Chem. Soc. 1996;118:7408. [Google Scholar]; (d) De la Mata FJ, Grubbs RH. Organometallics. 1996;15:577. [Google Scholar]; (e) O’Donoghue MB, Schrock RR, LaPointe AM, Davis WM. Organometallics. 1996;15:1334. [Google Scholar]; (f) Crane TW, White PS, Templeton JL. Organometallics. 1999;18:1897. [Google Scholar]
- (6).Schrock RR. Chem. Rev. 2009;109:3211. doi: 10.1021/cr800502p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).(a) Ibrahem I, Yu M, Schrock RR, Hoveyda AH. J. Am. Chem. Soc. 2009;131:3844. doi: 10.1021/ja900097n. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Flook MM, Jiang AJ, Schrock RR, Müller P, Hoveyda AH. J. Am. Chem. Soc. 2009;131:7962. doi: 10.1021/ja902738u. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Flook MM, Gerber LCH, Debelouchina GT, Schrock RR. Macromolecules. 2010;43:7515. doi: 10.1021/ma101375v. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Flook MM, Ng VWL, Schrock RR. J. Am. Chem. Soc. 2011;133:1784. doi: 10.1021/ja110949f. [DOI] [PubMed] [Google Scholar]; (e) Jiang AJ, Zhao Y, Schrock RR, Hoveyda AH. J. Am. Chem. Soc. 2009;131:16630. doi: 10.1021/ja908098t. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Marinescu SC, Schrock RR, Müller P, Takase MK, Hoveyda AH. Organometallics. 2011;30:1780. doi: 10.1021/om200150c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Marinescu SC, Levine DS, Zhao Y, Schrock RR, Hoveyda AH. J. Am. Chem. Soc. 2011;133:11512. doi: 10.1021/ja205002v. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Malcolmson SJ, Meek SJ, Sattely ES, Schrock RR, Hoveyda AH. Nature. 2008;456:933. doi: 10.1038/nature07594. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Meek SJ, O’Brien RV, Llaveria J, Schrock RR, Hoveyda AH. Nature. 2011;471:461. doi: 10.1038/nature09957. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Yu M, Wang C, Kyle AF, Jakubec P, Dixon DJ, Schrock RR, Hoveyda AH. Nature. 2011;479:88. doi: 10.1038/nature10563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Schrock RR, King AJ, Marinescu SC, Simpson JH, Müller P. Organometallics. 2010;29:5241. [Google Scholar]
- (9).Feldman J, Schrock RR. Prog. Inorg. Chem. 1991;39:1. [Google Scholar]
- (10).(a) Feldman J, Davis WM, Thomas JK, Schrock RR. Organometallics. 1990;9:2535. [Google Scholar]; (b) Poater A, Solans-Monfort X, Clot E, Copéret C, Eisenstein O. J. Am. Chem. Soc. 2007;129:8207. doi: 10.1021/ja070625y. [DOI] [PubMed] [Google Scholar]; (c) Solans-Monfort X, Copéret C, Eisenstein O. J. Am. Chem. Soc. 2010;132:7750. doi: 10.1021/ja101597s. [DOI] [PubMed] [Google Scholar]
- (11).Flook MM, Jiang AJ, Schrock RR, Müller P, Hoveyda AH. J. Am. Chem. Soc. 2009;131:7962. doi: 10.1021/ja902738u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).(a) Barrado G, Doerrer L, Green MLH, Leech MA. J. Chem. Soc., Dalton Trans. 1999;1061 [Google Scholar]; (b) Galsworthy JR, Green JC, Green MLH, Müller M. J. Chem. Soc., Dalton Trans. 1998;15 [Google Scholar]; (c) Wolff F, Choukroun R, Lorber C, Donnadieu B. Eur. J. Inorg. Chem. 2003;628 doi: 10.1021/ic034742f. [DOI] [PubMed] [Google Scholar]; (d) Sánchez-Nieves J, Royo P, Mosquera MEG. Eur. J. Inorg. Chem. 2006;127 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


