Abstract
Susceptibility to autoimmune myocarditis has been associated with histamine release by mast cells during the innate immune response to Coxsackievirus B3 (CVB3) infection. To investigate the contribution of histamine H1 receptor (H1R) signaling to CVB3-induced myocarditis, we assessed susceptibility to the disease in C57BL/6J (B6) H1R−/− mice. No difference was observed in mortality between CVB3-infected B6 and H1R−/− mice. However, analysis of their hearts revealed a significant increase in myocarditis in H1R−/− mice that is not attributed to increased virus replication. Enhanced myocarditis susceptibility correlated with a significant expansion in pathogenic Th1 and Vγ4+γδ T cells in the periphery of these animals. Furthermore, an increase in regulatory T cells was observed, yet these cells were incapable of controlling myocarditis in H1R−/− mice. These data establish a critical role for histamine and H1R signaling in regulating T cell responses and susceptibility to CVB3-induced myocarditis in B6 mice.
Keywords: CVB3, autoimmune, myocarditis, histamine receptor, Th1, γδ T cells, Tregs, mice
1. Introduction
Myocarditis typically results from a microbial infection of the myocardium with either viruses, bacteria, or protozoa [1], and enteroviruses, including coxsackievirus B3 (CVB3), represent the major viral etiologic agents of this disease [2]. In both the clinical and experimental settings, myocarditis predominates in males and can result in acute, resolving, or chronic forms of the disease. Myocarditis frequently consists of a mononuclear cell infiltration of the myocardium and multiple pathogenic mechanisms contribute to cardiac injury, including direct virus infection, virus-specific immunity, and virus-induced autoimmunity [3].
CD8 T cells are the main contributors to autoimmune-mediated cardiac injury during CVB3 infection, which target cardiocytes through antigenic mimicry[4, 5]. The activation of the autoreactive CD8 T cells requires innate effector CD1d-restricted γδ T cells expressing the Vγ4 receptor[6]. Following CVB3 infection, CD1d is up-regulated on cardiac cells, which leads to the recruitment of Vγ4 cells into the heart[7]. Vγ4 cells produce high levels of proinflammatory cytokines, including IFN-γ, that effectively bias the virus-specific adaptive immune response to the pathogenic Th1 phenotype, which in turn leads to the activation of auto reactive CD8 effector T cells[6, 8, 9]. The CD8 T cells effectively kill uninfected cardiocytes resulting in cardiac injury. In addition to CD8 T cells, Vγ4 can selectively target and kill uninfected CD1d expressing cardiocytes and further contribute to cardiac injury[10]. Vγ4 cells also promote autoimmunity and inflammation by inhibiting regulatory T (Treg) cell activity. CD1d expressing Tregs are highly immunosuppressive compared to CD1d negative Tregs in the CVB3 myocarditis model. However, Vγ4 cells actively kill the CD1d+ Tregs in a CD1d and caspase dependent manner, thereby further facilitating autoimmune disease[11, 12].
In addition to γδ T cells, mast cells represent another innate effector cell type that has been implicated in the induction of myocarditis. Mast cells act as regulators of the innate and adaptive immune systems through the secretion of immune mediators, including histamine and an array of cytokines [13]. Studies have shown that elevated mast cell numbers, which undergo degranulation within 6 hours of CVB3 infection, correlate with enhanced myocarditis susceptibility [14, 15]. Furthermore, when using encephalomyocarditis virus (EMCV) as an inducer of myocarditis, mast cell deficient mice developed significantly less myocarditis than control littermates[16]. Treating mice after EMCV infection with histamine H1 receptor (H1R) antagonists substantially reduced myocarditis by inhibiting inflammatory cytokine expression[16, 17]. Therefore, histamine and H1R signaling may play a pivotal role in regulating the adaptive immune responses associated with viral myocarditis.
To investigate whether H1R signaling influences T cell responses and susceptibility to CVB3-induced myocarditis, we compared various disease parameters between infected wild-type C57BL/6J (B6) and H1R−/− mice. We show that the absence of H1R signaling increases susceptibility to CVB3-induced myocarditis and results in an increase in pathogenic CD4+IFN-γ+ and Vγ4+ peripheral T cells. Furthermore, infected H1R−/− mice also exhibit an increase in Tregs, which are clearly incapable of controlling disease. Therefore, as opposed to EMCV-induced myocarditis, H1R signaling helps protect CVB3-infected B6 mice from developing myocarditis by regulating T cell responses and autoimmunity associated with disease.
2. Materials and Methods
2.1. Mice
C57BL/6J(B6) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). B6.129P-Hrh1tm1Wat(H1R−/−) mice were generated in the vivarium of the Given Medical Building at the University of Vermont [18, 19]. H1R−/− mice were backcrossed onto the B6 background for more than 10 generations. Only male mice were used in the experiments. Male B6 mice 12 weeks of age or older were used in the histamine EIA experiments. Mice were maintained in the animal facility at the University of Vermont. The experimental procedures used in this study were approved by the Animal Care and Use Committee of the University of Vermont.
2.2. Virus and infection
The H3 variant of CVB3 was made from an infectious cDNA clone as previously described [20]. Mice were infected by intraperitoneal (i.p.) injection of 0.5 ml of PBS containing 100 PFU CVB3 and euthanized 7 days after infection. Controls were uninfected mice or PBS-injected mice that were euthanized at the same time as the infected animals.
2.3. Histamine EIA assay
Male B6 mice were injected i.p. with either PBS alone or 2, 20, 200 PFU CVB3 and euthanized 3 hours after injection. Histamine concentrations were assessed using an EIA histamine kit according to the manufacturer’s instructions (Beckman Coulter, Brea, CA). Briefly, spleens were homogenized in 10ul of 0.2N HClO4 per mg of tissue and neutralized using an equal volume of 1M Na2B4O7 (Sigma, St. Loius, MO). Supernatants were acylated and added to the histamine-coated plate plus conjugate and incubated overnight at 4°C. The wells were washed and the substrate was added for 30 minutes at RT while shaking. The reaction was stopped and the plate read at 405nm.
2.4. Evaluation of myocarditis
Myocarditis was quantified according to the method of Knowlton et al. [20]. Briefly, the heart is fixed in 10% formalin, paraffin embedded, ventricle sections taken at ~ 400 micron intervals and stained with hematoxylin and eosin. Sections are captured in transmitted light mode using a Nikon Eclipse 50i microscope fitted with a Nikon Digital Camera DXM1200F using a 4× objective lens and the images analyzed using the Meta Morph Series 6.1 program (Molecular Devices Co., Downingtown, PA). The area of the whole heart (square micrometers) is determined on a binary image with the areas of injury determined on the color image using the true color feature of the software. Final percent cardiac injury is calculated by dividing the area of injury by the total area of the heart.
2.5. Cardiac virus titers
Hearts are asceptically removed, weighed, homogenized and the cellular debris removed by centrifugation at 1500 × g for 15 min. The supernatants are diluted serially (10-fold) in RPMI 1640 with 5% FBS and incubated for 45 min on HeLa cell monolayers which are then overlaid with agar. After two days, HeLa cell monolayers are fixed with 10% formalin and stained with crystal violet. Plaques are counted and data expressed as plaques/g tissue.
2.6. Intracellular cytokine staining and flow cytometry
The details for the intracellular cytokine staining have been previously published [21]. Briefly, peripheral blood leukocytes (PBLs) were isolated from EDTA treated blood by centifugation on Histopaque (Sigma, St. Louis, MO). PBLs and red blood cell depleted splenocytes were cultured for 4 hrs in RPMI 1640 medium containing 10% FBS, antibiotics, 10 ng/ml phorbolmyristate acetate, 500 ng/ml ionomycin, and 10 µg/ml brefeldin A (Sigma, St. Louis, MO). The cells were washed, fixed with 2% paraformaldehyde, permeablized with 0.5% saponin in PBS containing 1% BSA and intracellularly stained with Alexa647-anti-IL-4 and PE-anti-IFN-γ and surface stained with PerCP-Cy5.5-anti-CD4. A second set of cells was stained using FITC-anti-Vγ4, PerCP-Cy5.5-anti-CD69, APC-Cy7-anti-CD8α, and Alexa647-anti-CD4 (BD Pharmingen, Franklin Lakes, NJ). FoxP3 labeling was performed as previously described using Alexa-647-anti-CD4, PerCP-Cy5.5-anti-CD25, FITC-anti-IL-10 and PE-anti-FoxP3[12]. The cell population was classified for cell size (forward scatter) and complexity (side scatter). At least 10,000 cells were evaluated. Positive staining was determined relative to isotype controls. The percentages of the different T cell subsets were obtained from the gated lymphocyte population using Flowjo Experiment-Based Analysis for flow cytometry (Tree Star Inc., Ashland, OR).
2.7. Statistics
Statistical analyses were performed using GraphPad Prism version 5.0c (GraphPad Software, San Diego, CA). The specific tests used are detailed in the figure legends. A p-value of ≤0.05 was considered significant.
3. Results and Discussion
3.1. CVB3 infection induces an innate histamine response in B6 mice
To date, the studies investigating the influence of histamine on CVB3-induced myocarditis have been performed using susceptible BALB/c mice. However, CVB3-infected B6 mice, although often thought of as myocarditis resistant, are also susceptible to myocarditis when inoculated with increased virus titers [22]. Therefore, to determine whether histamine and H1R signaling regulates CVB3-induced myocarditis susceptibility in B6 mice, we assessed whether infection induces a histamine response in these animals.
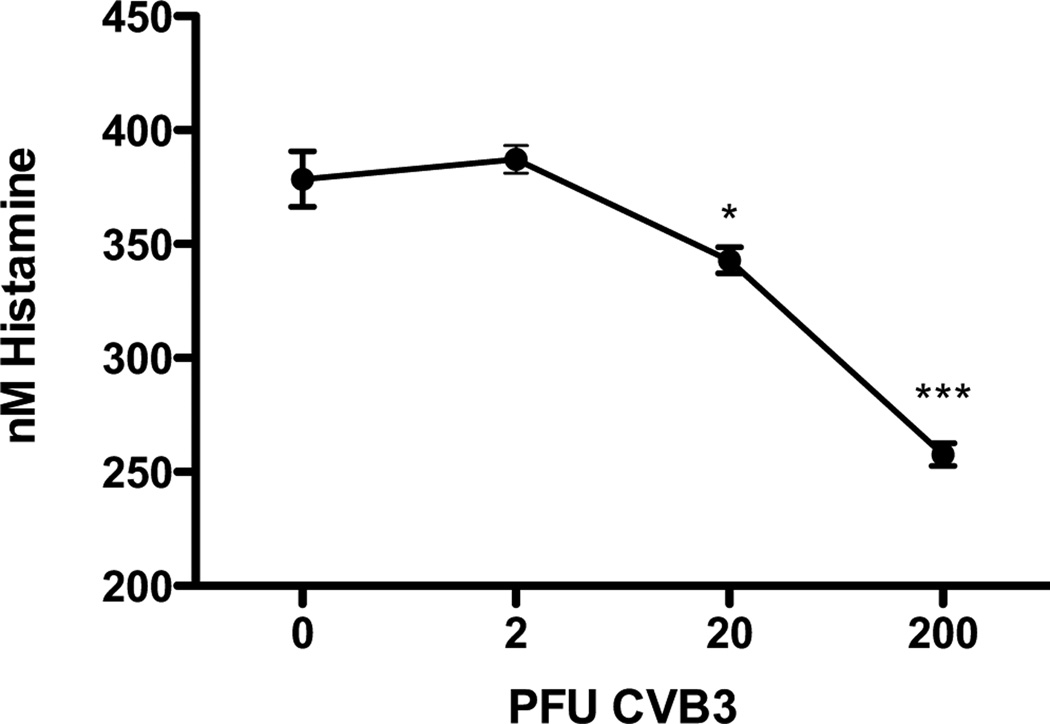
Our model of experimental myocarditis is induced in mice via i.p. injection of the virus making the resident immune cells within the spleen among the first to encounter the virus [23]. Therefore, to determine whether CVB3 can induce an innate histamine response, we measured histamine by EIA in the spleens of B6 mice 3 hours after i.p. injection of PBS alone or 2, 20, and 200 PFU CVB3. Importantly, this experiment revealed a significant decrease in splenic histamine that is dependent on the viral dose administered to the mice (Fig. 1). Although the mechanism behind the CVB3-induced decrease in histamine remains to be determined, it may be due to rapid splenic mast cell degranulation following infection resulting in the release of histamine into the periphery and/or the migration of these cells from the spleen in response to infection. These findings signify that CVB3 infection induces a rapid histamine response in B6 mice leading us to hypothesize that H1R signaling may contribute to disease susceptibility in this strain.
Figure 1.
CVB3 infection induces an innate histamine response in B6 mice. Mice were either PBS-injected or infected with 2, 20, or 200 PFU CVB3. Spleens were harvested 3 hours after injection and the histamine concentration measured by EIA. Data is representative of three separate experiments; n=7 mice per group; *, p<0.05, ***, p<0.005. The observed significance of differences was determined by One-way ANOVA followed by Dunnett’s Multiple Comparison Test.
3.2. H1R deficiency increases susceptibility to CVB3-induced myocarditis
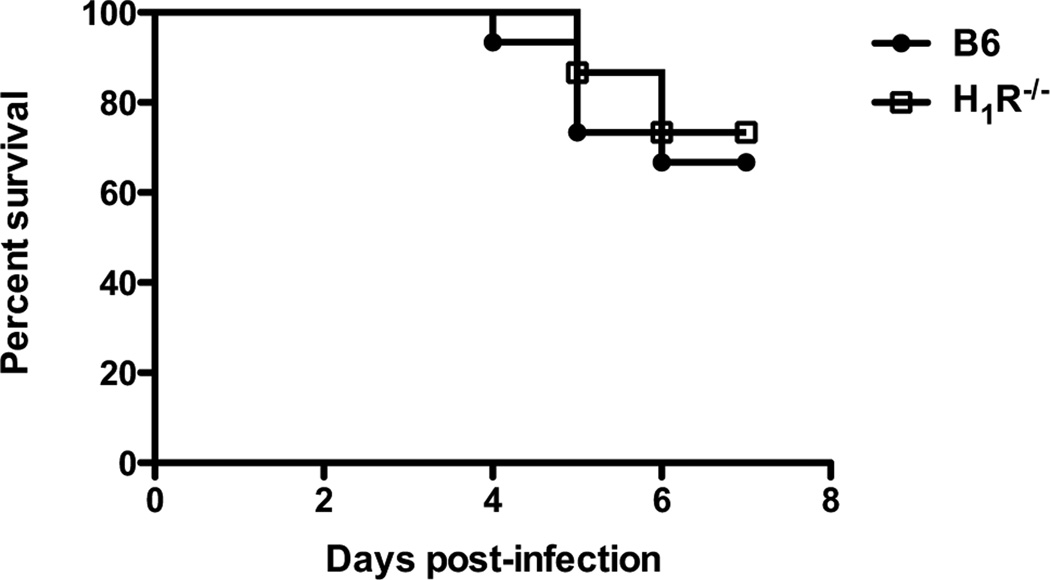
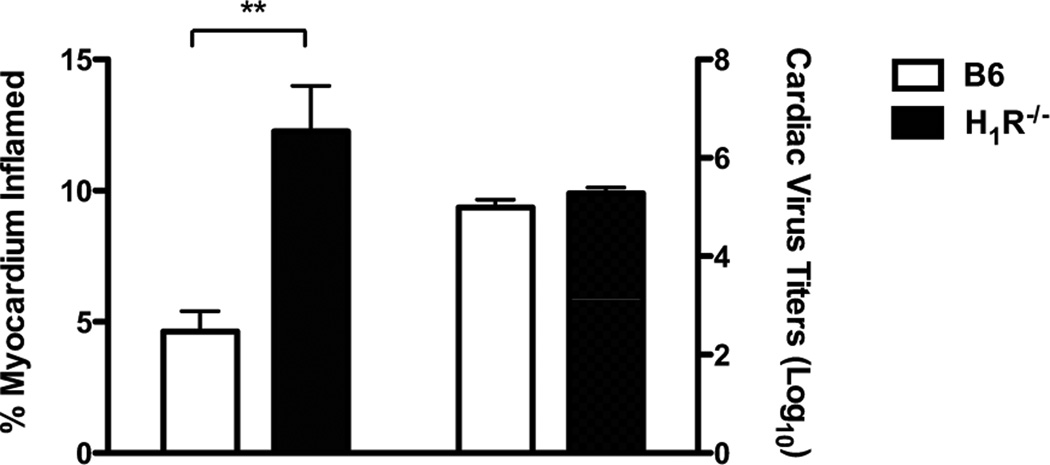
To assess the role of H1R signaling during CVB3-induced myocarditis, we utilized a traditional knockout approach using B6 mice. B6 and H1R−/− mice were infected with 100 PFU CVB3 and monitored for survival up to 7 days after infection. The surviving animals were then analyzed for myocarditis and cardiac virus titers. Although there was no difference in survival between B6 and H1R−/− mice (Fig. 2), histological analysis of the hearts from H1R−/− mice revealed a significant increase in myocarditis (p<0.01) (Fig. 3). This difference in disease is not due to an increase in virus replication, since the hearts from H1R−/− mice showed no difference in cardiac virus titers by plaque assay compared to controls (Fig. 3). Therefore, histamine signaling through the H1R assists in protecting B6 mice from CVB3-induced myocarditis.
Figure 2.
H1R signaling does not affect the survival of CVB3-infected B6 mice. Mice were infected with 100 PFU and monitored over 7 days for survival. n=15 mice per strain combined from 2 independent experiments; p=0.615 as determined by Log-rank (Mantel-Cox) test.
Figure 3.
H1R−/− mice are more susceptible to CVB3-induced myocarditis irrespective of virus replication. Surviving mice from Figure 2 were euthanized at day 7. Hearts were evaluated by image analysis to quantify the percent myocardium inflamed (left). Cardiac virus titers were determined by plaque assay (right). n ≥ 10 mice per strain; **, p<0.01. The observed significance of differences was determined by Student’s t-test.
Our results are in contrast to studies utilizing the EMCV-induced myocarditis model, where it has been shown that the treatment of DBA/2 mice with H1R antagonists significantly reduced myocarditis [16, 17]. Similarly, other differences in immune pathogenesis exist between the EMCV versus CVB3-induced myocarditis models. For example, CD1d dependent innate immunity is important in protecting mice from EMCV-induced disease but enhances myocarditis in the CVB3 model[10, 24]. These opposing results likely reflect viral specific differences underlying immunopathology and may reflect differences in the antiviral strategies used by the host during picornavirus infections. Therefore, our results accentuate the importance of determining the viral etiologic agent in patients with myocarditis before the regulation of histamine and/or H1R signaling could be used as therapeutic strategies.
3.3. H1R signaling regulates effector T cell responses during CVB3 infection
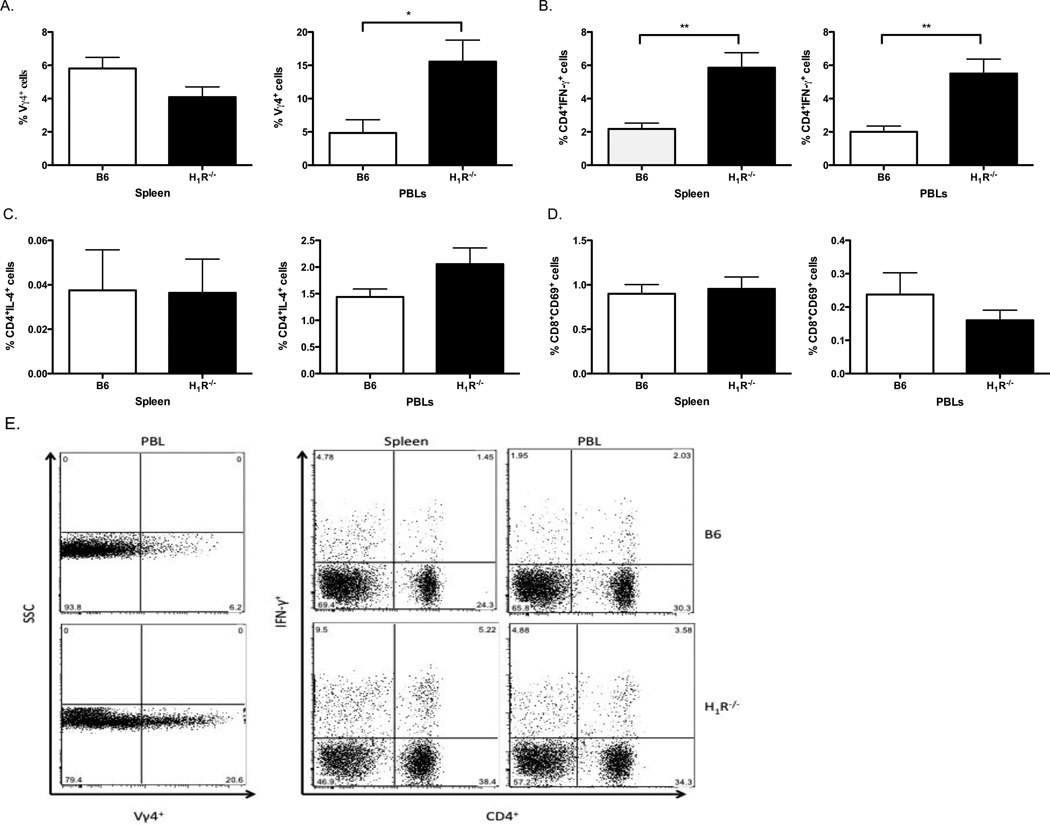
Susceptibility to CVB3-induced myocarditis is dependent on γδ and αβ T cell responses. γδ T cells are innate effector cells that regulate the developing adaptive immune response following viral infection. During CVB3 infection, Vγ4 expressing γδ T cells produce immunomodulatory cytokines, including IFN-γ, known to promote Th1 responses, which in turn induce the activation of autoimmune CD8 T cells[6, 8, 9]. To determine whether CVB3-induced H1R signaling regulated peripheral effector T cell populations, B6 and H1R−/− mice were infected with 100 PFU CVB3 and both splenocytes and PBLs were analyzed 7 days later by flow cytometry. In CVB3-infected H1R−/− mice, Vγ4+γδ T cells were significantly increased in the peripheral blood compared to infected B6 mice (Fig. 4A,E). Furthermore, these mice exhibited an increase in the percentage of pathogenic CD4+IFN-γ+ Th1 cells in the spleen and peripheral blood (p<0.01) (Fig. 4B,E). However, we did not observe a difference in the protective Th2 response, as H1R−/− mice possessed a similar percentage of CD4+IL-4+ PBLs as controls, and this population of cells was nearly undetectable in the spleen of both strains of mice (Fig. 4C). Lastly, no difference was detected in the percentage of activated CD8+T cells between CVB3-infected B6 and H1R−/− mice (Fig. 4D). Of note, the absence of H1R does not alter the T cell profiles of naïve mice indicating that these differences are a result of CVB3 infection (Data not shown).
Figure 4.
Myocarditis susceptibility correlates with an increase in pathogenic Vγ4+γδ T cells and Th1 cells in CVB3-infected H1R−/− mice. Splenocytes and PBLs from the mice in Figure 3 were stained with fluorescently labeled antibodies to detect the percentage of (A) Vγ4+γδ T cells, (B) Th1 effector T cells (CD4+IFN-γ+), (C) Th2 effector T cells (CD4+IL-4+), and (D) activated CD8 T cells (CD8+CD69+) by flow cytometry. (E) Representative dot plots of T cell subsets exhibiting statistical significance. n ≥ 10 mice per strain; *, p<0.05; **, p<0.005. The observed significance of differences was determined by Student’s t-test.
These data show that the absence of H1R signaling leads to an increased bias towards the Th1 phenotype in CVB3-infected H1R−/− mice. To our knowledge, this is the first report describing a role for H1R signaling during the differentiation of T helper cell responses following viral infection. Nonetheless, we have previously shown that H1R activation directly in CD4 T cells can lead to Th1 differentiation in experimental allergic encephalomyelitis (EAE), a Th1-mediated autoimmune model of multiple sclerosis[25]. However, as opposed to CVB3 infection, H1R−/− mice exhibit a decrease in IFN-γ producing Th1 cells and an immune bias towards the protective Th2 response in EAE. Therefore, either H1R signaling does not modulate the T helper cell response equivalently across different models of autoimmune and infectious diseases or H1R signaling in CD4 T cells is not responsible for the Th1 bias in CVB3-infected H1R−/− mice.
An alternative mechanism that may result in enhanced Th1 differentiation in CVB3-infected H1R−/− mice may be due to the increase observed in peripheral Vγ4 cells. We have previously shown that IFN-γ produced by Vγ4 cells is necessary for the differentiation of Th1 cells following CVB3-infection[8, 9]. Therefore, an increase in Vγ4 cells could lead to an increase in IFN-γ, thereby leading to an exaggerated Th1 bias in H1R−/− mice. Th1 cells contribute to myocarditis susceptibility by activating autoimmune CD8 T cells that cause cardiac injury. However, in CVB3-infected H1R−/− mice, the increased severity of myocarditis may not be due to CD8 T cells since there was no difference detected in their peripheral cellularity compared to infected B6 mice. This suggests that the increase in myocarditis may be due to the increase in Vγ4 cells themselves, as these cells are also known to contribute to cardiac injury through killing infected cardiocytes using Fas-dependent mechanisms[10]. Therefore, Vγ4 cells may be the primary contributor to the severity of myocarditis in infected H1R−/− mice by influencing Th1 polarity and cardiac injury. Little is known about the effect of histamine and H1R signaling on the γδ T cell population and further studies are needed to understand whether this may be a direct effect of H1R activation in γδ T cells or a downstream event.
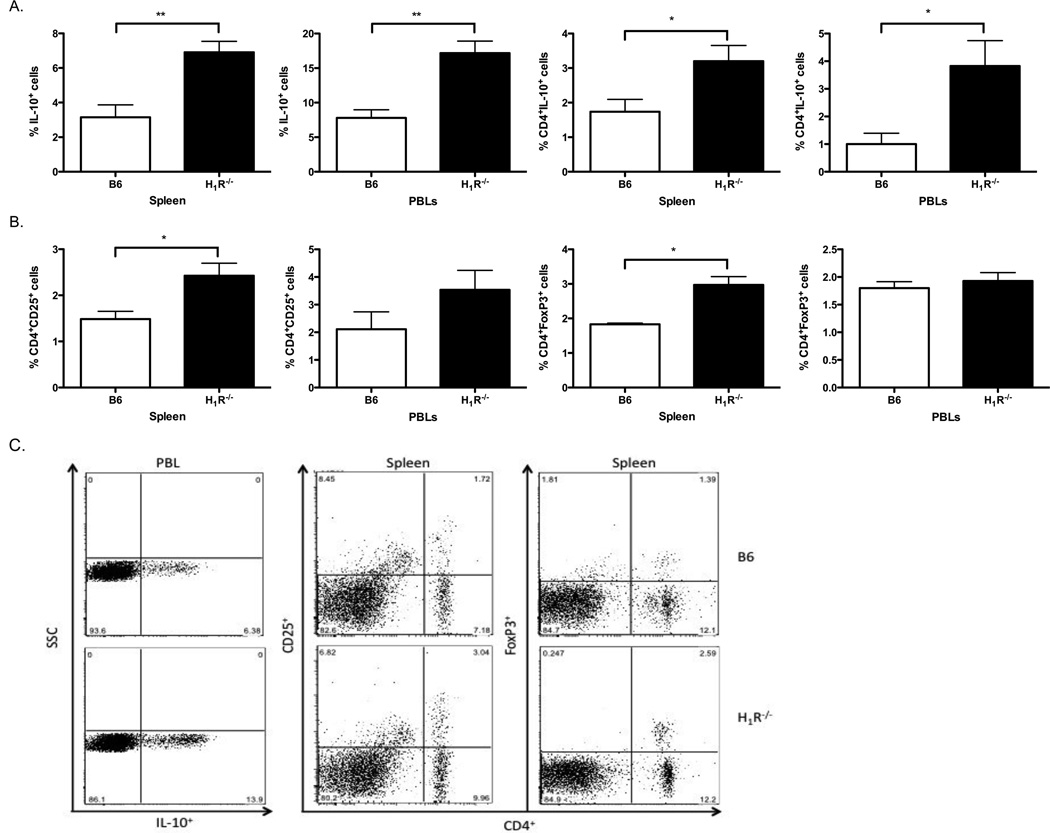
3.4. H1R signaling regulates Treg responses during CVB3 infection
It has previously been shown that Vγ4+γδ T cells can modulate Treg activity and further promote autoimmunity and inflammation following CVB3 infection and exposure to histamine has been reported to down regulate CD25 and Foxp3 expression on Treg cells [26]. To determine whether the absence of H1R signaling influenced Treg populations in CVB3-infected mice, we analyzed IL-10+ cells, as well as CD4+IL-10+, CD4+CD25+, and CD4+FoxP3+ splenocytes and PBLs by flow cytometry (Fig. 5). Interestingly, we found that CVB3-infected H1R−/− mice possess an overall increase in IL-10+ peripheral Tregs compared to infected B6 mice (Fig. 5A,C). Furthermore, we observed an increase in CD4+IL-10+, CD4+CD25+, and CD4+FoxP3+ in the spleen and an increase inCD4+IL-10+ in peripheral blood (Fig.5B,C).
Figure 5.
An increase inperipheral Tregs is incapable of controlling myocarditis in CVB3-infected H1R−/− mice. Splenocytes and PBLs from the mice in Figure 3 were analyzed for the percentage of Treg cells by staining for (A) all IL-10+ cells and CD4+IL-10+ cells, (B) CD4+CD25+ cells and CD4+FoxP3+ cells. (C) Representative dot plots for each class of Treg cells. n ≥ 10 mice per strain; *, p<0.05; **, p<0.01. The observed significance of differences was determined by Student’s t-test.
Although an increase in Tregs was observed in the periphery of CVB3-infected H1R−/− mice, these cells are clearly incapable of suppressing the effector T cell response. This may be due to the antagonizing role of Vγ4 cells on Treg activity in these animals, whereby the highly immunosuppressive CD1d+ Tregs are actively killed[11, 12]. Another possibility is that the Tregs cannot effectively outcompete the high number of effector T cells also present in CVB3-infected H1R−/− mice for antigen presentation. Lastly, these findings may result from the inability of the peripheral Tregs to home to the heart and prevent resident effector cells from causing cardiac injury. Despite the unknown mechanism, we can conclude that the suppressive activity of the peripheral Tregs is insufficient in preventing myocarditis in CVB3-infected H1R−/− mice.
4. Conclusion
H1R signaling plays a significant role in regulating T cell responses and susceptibility to myocarditis during CVB3 infection. While it was known that H1R signaling could influence the polarity of T helper cells, our mouse model revealed a previously uncharacterized link between H1R signaling and Vγ4 T cell responses to infection. Understanding the effector functions that are directly regulated by H1R signaling in CVB3-induced myocarditis may aid in delineating the role that the H1R plays in modulating T cell responses to infection. Lastly, the differing results obtained from our work with CVB3 and those obtained using the EMCV-induced myocarditis model emphasizes the importance of determining the viral etiologic agent of myocarditis when considering pharmacological targeting the H1R as a therapeutic strategy in the treatment of myocarditis. Therefore, these findings suggest that the routine use of anti-histamines by atopic patients has the potential, depending upon the viral etiologic agent, to significantly influence the clinical course of myocarditis.
Highlights.
H1R deficient mice are more susceptible to CVB3-induced myocarditis
Susceptibility correlated with expansion in pathogenic Th1 and Vγ4+γδ T cells
Tregs are incapable of controlling myocarditis in CVB3-infected H1R deficient mice
Acknowledgements
This work was supported by the National Institutes of Health Grants NS061014 (CT), NS069628 (CT), AI45666 (CT and SH), HL108371 (SH), and HL80594 (SH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Friman G, Wesslen L, Fohlman J, Karjalainen J, Rolf C. The epidemiology of infectious myocarditis, lymphocytic and dilated cardiomyopathy. Eur. Heart. J. 1995;16:36–41. doi: 10.1093/eurheartj/16.suppl_o.36. [DOI] [PubMed] [Google Scholar]
- 2.Kim KS, Hofling K, Carson SD, Chapman NM, Tracy S. The primary viruses of myocarditis. In: Cooper LT, editor. Myocarditis. New Jersey: Humana Press; 2003. [Google Scholar]
- 3.Gauntt C, Sakkinen P, Rose N, Huber S. Picornaviruses: Immunopathology and autoimmunity. In: Fujinmai MCaR., editor. Effects of microbes on the immune system. Philadelphia: Lippencott Williams & Wilkins; 2000. [Google Scholar]
- 4.Guthrie M, Lodge PA, Huber SA. Cardiac injury in myocarditis induced by Coxsackievirus group B, type 3 in Balb/c mice is mediated by Lyt 2 + cytolytic lymphocytes. Cellular immunology. 1984;88(2):558–567. doi: 10.1016/0008-8749(84)90188-6. [DOI] [PubMed] [Google Scholar]
- 5.Huber SA, Cunningham MW. Streptococcal M protein peptide with similarity to myosin induces CD4+ T cell-dependent myocarditis in MRL/++ mice and induces partial tolerance against coxsakieviral myocarditis. Journal of immunology. 1996;156(9):3528–3534. [PubMed] [Google Scholar]
- 6.Huber SA, Sartini D, Exley M. Vgamma4(+) T cells promote autoimmune CD8(+) cytolytic T-lymphocyte activation in coxsackievirus B3-induced myocarditis in mice: role for CD4(+) Th1 cells. Journal of virology. 2002;76(21):10785–10790. doi: 10.1128/JVI.76.21.10785-10790.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huber SA. Increased susceptibility of male BALB/c mice to coxsackievirus B3-induced myocarditis: role for CD1d. Medical microbiology and immunology. 2005;194(3):121–127. doi: 10.1007/s00430-004-0221-6. [DOI] [PubMed] [Google Scholar]
- 8.Huber SA, Mortensen A, Moulton G. Modulation of cytokine expression by CD4+ T cells during coxsackievirus B3 infections of BALB/c mice initiated by cells expressing the gamma delta + T-cell receptor. Journal of virology. 1996;70(5):3039–3044. doi: 10.1128/jvi.70.5.3039-3044.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huber SA, Graveline D, Born WK, O'Brien RL. Cytokine production by Vgamma(+)-T-cell subsets is an important factor determining CD4(+)-Th-cell phenotype and susceptibility of BALB/c mice to coxsackievirus B3-induced myocarditis. Journal of virology. 2001;75(13):5860–5869. doi: 10.1128/JVI.75.13.5860-5869.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huber S, Sartini D, Exley M. Role of CD1d in coxsackievirus B3-induced myocarditis. Journal of immunology. 2003;170(6):3147–3153. doi: 10.4049/jimmunol.170.6.3147. [DOI] [PubMed] [Google Scholar]
- 11.Huber SA. gammadelta T lymphocytes kill T regulatory cells through CD1d. Immunology. 2010;131(2):202–209. doi: 10.1111/j.1365-2567.2010.03292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huber SA. Depletion of gammadelta+ T cells increases CD4+ FoxP3 (T regulatory) cell response in coxsackievirus B3-induced myocarditis. Immunology. 2009;127(4):567–576. doi: 10.1111/j.1365-2567.2008.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marshall JS, King CA, McCurdy JD. Mast cell cytokine and chemokine responses to bacterial and viral infection. Curr. Pharm. Des. 2003;9:11–24. doi: 10.2174/1381612033392413. [DOI] [PubMed] [Google Scholar]
- 14.Fairweather D, Frisancho-Kiss S, Gatewood S, Njoku D, Steele R, Barrett M, Rose N. Mast cells and innate cytokines are associated with susceptibility to autoimmune heart disease following coxsackievirus B3 infection. Autoimmunity. 2004;37:131–145. doi: 10.1080/0891693042000196200. [DOI] [PubMed] [Google Scholar]
- 15.Afanasyeva M, Georgakopoulos D, Rose N. Autoimmune myocarditis: cellular mediators of cardiac dysfunction. Autoimmun. Rev. 2004;3:376–486. doi: 10.1016/j.autrev.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Higuchi H, Hara M, Yamamoto K, Miyamoto T, Kinoshita M, Yamada T, Uchiyama K, Matsumori A. Mast cells play a critical role in the pathogenesis of viral myocarditis. Circulation. 2008;118:363–372. doi: 10.1161/CIRCULATIONAHA.107.741595. [DOI] [PubMed] [Google Scholar]
- 17.Matsumori A, Yamamoto K, Shimada M. Cetirizine a histamine H1 receptor antagonist improves viral myocarditis. Journal of inflammation. 2010;7:39. doi: 10.1186/1476-9255-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banu Y, Watanabe T. Augmentation of antigen receptor-mediated responses by histamine H1 receptor signaling. J. Exp. Med. 1999;189:673–682. doi: 10.1084/jem.189.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobayashi T, Tonai S, Ishihara Y, Koga R, Okabe S, Watanabe T. Abnormal functional and morphological regulation of the gastric mucosa in histamine H2 receptor-deficient mice. J. Clin. Invest. 2000;105:1741–1749. doi: 10.1172/JCI9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knowlton KU, Jeon ES, Berkley N, Wessely R, Huber S. A mutation in the puff region of VP2 attenuates the myocarditis phenotype of an infectious cDNA of the Woddruff variant of Coxsackievirus B3. J. Immunol. 1996;149:2715–2721. doi: 10.1128/jvi.70.11.7811-7818.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huber S, Graveline D, Born W, O’Brien R. Cytokine production by Vγ+-T-Cell subset is an important factor determining CD4+-Th-cell phenotype and susceptibility of BALB/c mice to Coxsackievirus B3-induced myocarditis. J. Virol. 2001;75:5860–5869. doi: 10.1128/JVI.75.13.5860-5869.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huber SA, Sartini D. Roles of tumor necrosis factor alpha (TNF-alpha) and the p55 TNF receptor in CD1d induction and coxsackievirus B3-induced myocarditis. J. Virol. 2005;79:2659–2665. doi: 10.1128/JVI.79.5.2659-2665.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orlin T, Saldeen T. The lymphatic pathways from the peritoneal cavity: a lymphangiographic study in the rat. Cancer Res. 1964;24:1700–1711. [PubMed] [Google Scholar]
- 24.Ilyinskii PO, Wang R, Balk SP, Exley MA. CD1d mediates T-cell-dependent resistance to secondary infection with encephalomyocarditis virus (EMCV) in vitro and immune response to EMCV infection in vivo. J Virol. 2006;80(14):7146–7158. doi: 10.1128/JVI.02745-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noubade R, Milligan G, Zachary J, Blankenhorn EP, del Rio R, Rincon M, Teuscher C. Histamine receptor H1 is required for TCR-mediated p38 MAPK activation and optimal IFN-gamma production in mice. J. Clin. Invest. 2007;117:3507–3518. doi: 10.1172/JCI32792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forward NA, Furlong SJ, Yang Y, Lin TJ, Hoskin DW. Mast cells down-regulate CD4+CD25+ T regulatory cell suppressor function via histamine H1 receptor interaction. Journal of immunology. 2009;183(5):3014–3022. doi: 10.4049/jimmunol.0802509. [DOI] [PubMed] [Google Scholar]