Abstract
Background
In several tumours the endogenous activity of histidine decarboxylase (HDC), the enzyme stimulating histamine synthesis, sustains the autocrine trophic effect of histamine on cancer progression. Cholangiocarcinoma is a biliary cancer with limited treatment options. Histamine interacts with four G-protein coupled receptors, H1–H4 histamine receptors (HRs).
Objective
To determine the effects of histamine stimulation and inhibition of histamine synthesis (by modulation of HDC) on cholangiocarcinoma growth.
Methods
In vitro studies were performed using multiple human cholangiocarcinoma lines. The expression levels of the histamine synthetic machinery and HRs were evaluated along with the effects of histamine stimulation and inhibition on cholangiocarcinoma proliferation. A xenograft tumour model was used to measure tumour volume after treatment with histamine or inhibition of histamine synthesis by manipulation of HDC. Vascular endothelial growth factor (VEGF) expression was measured in cholangiocarcinoma cells concomitant with the evaluation of the expression of CD31 in endothelial cells in the tumour microenvironment.
Results
Cholangiocarcinoma cells display (1) enhanced HDC and decreased monoamine oxidase B expression resulting in increased histamine secretion; and (2) increased expression of H1–H4 HRs. Inhibition of HDC and antagonising H1HR decreased histamine secretion in Mz-ChA-1 cells. Long-term treatment with histamine increased proliferation and VEGF expression in cholangiocarcinoma that was blocked by HDC inhibitor and the H1HR antagonist. In nude mice, histamine increased tumour growth (up to 25%) and VEGF expression whereas inhibition of histamine synthesis (by reduction of HDC) ablated the autocrine stimulation of histamine on tumour growth (~80%) and VEGF expression. No changes in angiogenesis (evaluated by changes in CD31 immunoreactivity) were detected in the in vivo treatment groups.
Conclusion
The novel concept that an autocrine loop (consisting of enhanced histamine synthesis by HDC) sustains cholangiocarcinoma growth is proposed. Drug targeting of HDC may be important for treatment of patients with cholangiocarcinoma.
INTRODUCTION
Cholangiocytes are the target cells in cholestatic liver diseases such as primary biliary cirrhosis, primary sclerosing cholangitis and cholangiocarcinoma which portray proliferation and loss of cholangiocytes.1 Cholangiocarcinoma arises from aberrant cholangiocyte hyperplasia caused by obstruction and inflammation of bile ducts.2,3 The diagnosis of cholangiocarcinoma is difficult and late, thus limiting the treatment options.2 Surgical resection is the only potential ‘cure’ for cholangiocarcinoma and increases long-term survivability in some patients, but is not always feasible.4 Cholangiocarcinoma is chemoresistant, limiting traditional pharmaceutical intervention.4 It is therefore imperative to elucidate the mechanisms of cholangiocarcinoma growth to develop effective treatments.
Histamine is formed after the decarboxylation of histidine by the converting enzyme histidine decarboxylase (HDC).5 HDC is found in almost all mammalian tissues including the CNS6 and fetal liver,7 and HDC expression is increased in tumorigenic sites.8,9 After release, histamine is stored or degraded by histamine-N-methyltransferase and monoamine oxidase B (MAO-B).5 Histamine interacts with four G protein-coupled receptors, H1–H4 histamine receptors (HRs),10 that exert differential actions on numerous G proteins.10 In the liver, activation of H1HR stimulates cholangiocyte growth whereas H3HR inhibits cholangiocyte hyperplasia in cholestatic rats.11
Histamine and HRs play a key role in tumorigenesis.12,13 For example, H3HR inhibits cholangiocarcinoma growth both in vitro and in vivo by downregulation of vascular endothelial growth factor (VEGF)-A/C expression,12 growth factors which modulate biliary function.14 A study has demonstrated that increased HDC activity and the growth of multiple cancers may be interrelated,15 and that the enzymes responsible for histamine synthesis are dysregulated in some cancers.15 The endogenous activity of HDC in tumour cells supports an autocrine regulatory mechanism in which histamine behaves like a growth factor.15 No data exist regarding the role of histamine and HDC in the autocrine modulation of cholangiocarcinoma growth. We performed studies to demonstrate that: (1) cholangiocarcinoma cells express higher levels of HDC and secrete greater amounts of histamine stimulating cholangiocarcinoma growth by autocrine mechanisms by upregulation of VEGF-A/C; and (2) pharmacological and molecular downregulation of HDC reduces cholangiocarcinoma growth.
METHODS
Materials
All reagents were obtained from Sigma Aldrich (St Louis, Missouri, USA) unless noted otherwise. Antibodies for immunoblots, immunohistochemistry and immunofluorescence were purchased from Santa Cruz Biotechnology (Santa Cruz, California, USA) unless indicated otherwise. Primers, plasmids and reagents for real-time PCR and shRNA transfection were obtained from SABiosciences (Fredrick, Maryland, USA). Histamine receptor antagonists for H1HR (terfenadine),11 H2HR (cimetidine)16 and H3/H4HR (thioperamide)17 were obtained from Tocris Bioscience (Ellisville, Missouri, USA). The mouse monoclonal antibody detecting endogenous levels of CD31 protein was purchased from Cell Signalling Technology (Boston, Massachusetts, USA).
Cell culture
Multiple intrahepatic and extrahepatic cholangiocarcinoma lines and non-malignant cholangiocytes were used. Mz-ChA-1 cells (from human gallbladder) were a gift from Dr J Fitz (University of Texas Southwestern, Dallas, Texas, USA).18,19 HuH-28 and TFK-1 cells (from human intrahepatic bile ducts)20 were obtained from Cancer Cell Repository, Tohoku University, Sendai, Japan.18 CCLP1, HuCC-T1 and SG231 cells (from intrahepatic bile ducts)21 were obtained from Dr A Demetris, University of Pittsburgh (Pittsburgh, Pennsylvania, USA).22–24 The non-malignant cholangiocyte line H69 was obtained from Dr G Gores, Mayo Clinic (Rochester, Minnesota, USA).25 The normal human intrahepatic cholangiocyte line (HIBEpiC) was obtained from ScienCell Research Laboratories (Carlsbad, California, USA).26
Expression of HDC and MAO-B and H1–H4 histamine receptors
Immunofluorescence
Cells were seeded on coverslips in a six-well plate (500 000 cells per well) and allowed to adhere overnight. Immunofluorescence was performed11 using specific antibodies against HDC and MAO-B (Abcam) and affinity purified IgG antibodies obtained (recognising H1–H4 HRs) from Alpha Diagnostic International, San Antonio, Texas, USA. Antibodies were diluted 1:50 in 1% BSA/PBST. Species-appropriate non-immune serum was used for negative controls. Images were visualised using an Olympus IX-71 confocal microscope (Tokyo, Japan).
Real-time PCR
The RT2 real-time PCR assay from SABiosciences was used.11 Specific primers designed against human HDC, MAO-B, H1–H4 HRs and glyceraldehyde 3-phosphate dehydrogenase (GAPDH, housekeeping)11 were purchased from (SABiosciences). Total RNA (1 µg) was extracted using the Qiagen RNeasy mini kit (Qiagen Inc, Valencia, California, USA) and, after amplification, a ΔΔCT (delta threshold cycle) analysis was done using H69 as control.26,27
Immunoblotting analysis
Immunoblotting analysis was performed in protein (10 µg) from whole cell lysates from non-malignant and cholangiocarcinoma cells.11 To verify equal protein loading we used β-actin.11 Band intensity was determined by scanning video densitometry using the phospho-imager Storm 860 (GE Healthcare, Piscataway, New Jersey, USA) and the ImageQuant TLV 2003.02 (Little Chalfont, Buckinghamshire, UK) software.
Human tissue array
Using the antibodies mentioned above, immunohistochemistry was performed in Accumax (US Biomax, Rockville, MD, USA) tissue arrays.26 Each tissue array contains 48 well-characterised human cholangiocarcinoma biopsy samples from a variety of tumour differentiation grades as well as four biopsies from non-malignant controls. Semi-quantitative analysis was performed by three independent observers in a blinded fashion using staining intensity (score 1–5) and abundance of positively stained cells (score 1–5). The staining index was calculated by multiplying the staining intensity by abundance.26
Histamine secretion
Histamine secretion was measured in conditioned media from non-malignant and cholangiocarcinoma cells and from Mz-ChA-1 cells treated with 0.2% BSA (basal), the HDC inhibitor or the antagonists for H1–H4 HRs by a commercially available ELISA (Cayman Chemical, Ann Arbor, Michigan, USA). Cells were trypsinised and the pellet was resuspended in Hank’s balanced salt solution (1×107 cells/ml). Cells were incubated for 6 h at 37°C28 and the amount of histamine released into the medium was assayed.
Evaluation of cholangiocarcinoma proliferation
Cells were seeded into 96-well plates and stimulated for up to 48 h with histamine (0–100 µM) to determine the appropriate dose. In separate experiments, we evaluated the effects of histamine (10 µM, a concentration used in other studies),15 histamine receptor antagonists and α-methyl-DL-histidine dihydrochloride (3 mM)29 on the growth of non-malignant and cholangiocarcinoma lines. Cell proliferation was evaluated by the CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega, Madison, Wiscosin, USA). Absorbance was measured at 490 nm on a microplate spectrophotometer (Versamax, Molecular Devices Sunnyvale, CA, USA).12 Data were expressed as the fold change of treated cells compared with vehicle-treated (0.2% BSA) cells.
Cells were cultured for 24 and 48 h and up to 14 days and treated daily with histamine (10 µM) or the HDC inhibitor (3 mM).29 After stimulation, cells were lysed and prepared for immunoblots.11 Proliferation was evaluated by immunoblots for proliferating cellular nuclear antigen (PCNA) and equal protein loading was verified by immunoblots for β-actin.11 Band intensity was determined by scanning video densitometry using the phospho-imager Storm 860 and the Image-Quant TL software Version 200.302.
Measurement of VEGF-A/C expression
Cells were cultured for up to 2 weeks and treated daily with histamine (10 µM) or α-methyl-DL-histidine dihydrochloride (3 mM). After treatment, cells were trypsinised and prepared for real-time PCR.11 Primers for human VEGF-A/C (from SABiosciences) were used.
In vivo studies
Eight-week-old male Balb/c nude mice weighing approximately 30 g (Taconic Farms Inc, Hudson, New York, USA) were kept in a temperature-controlled environment (20–22°C) with 12-h light/dark cycles with free access to drinking water and chow. Mz-ChA-1 cells (3×106) were suspended in extracellular matrix gel and injected subcutaneously into the flanks of these animals.12,30 Intraperitoneal injections were performed as follows: (1) four mice received 0.9% NaCl (100 µl); (2) four mice were treated with histamine (0.5 mg/kg in 100 µl NaCl)31; and (3) four mice were treated with α-methyl-DL-histidine dihydrochloride (150 mg/kg in 100 µl NaCl).32 Injections were performed every other day for 52 days after tumour establishment (day 10). Tumour parameters were measured twice a week using an electronic caliper and the volume was determined as follows: tumour volume (mm3) = 0.5×(length (mm)×width (mm)×height (mm)). After 52 days the mice were anaesthetised with sodium pentobarbital (50 mg/kg intraperitoneally) and the tissues were harvested. All experiments were conducted under the guidelines of the Scott & White and Texas A&M Health Science Center IACUC. Along with tumours, samples of heart, liver and kidney were collected to assess organ damage following H&E staining of paraffin-embedded sections. All analyses were performed in a coded fashion.
Effect of HDC shRNA transfection on cholangiocarcinoma growth
We performed experiments to demonstrate that inhibition of HDC expression (by stable transfection to knockdown the HDC gene) in Mz-ChA-1 cells blocks the proliferation of these cells in vitro and in vivo when implanted into the flanks of nude mice. SureSilencing shRNA (SABiosciences) plasmids for human HDC containing a marker for neomycin resistance were used for the generation and selection of stably transfected cells.33 After transfection and selection, real-time PCR and immunoblots for HDC expression were performed to determine the degree of knockdown.33 In Mz-HDC and the neomycin-negative (Mz-neg) transfected Mz-ChA-1 lines we measured (1) the amount of histamine secreted into the conditioned media; (2) gene expression of H1–H4 HRs and VEGF-A/C by real-time PCR; and (3) PCNA expression by immunoblots. Mz-HDC and Mz-neg cell lines were cultured and prepared for injection into the flanks of nude mice.12 Tumour measurements began after tumour establishment as described above. After 35 days the mice were anaesthetised with sodium pentobarbital (50 mg/kg intraperitoneally) and the tissues were harvested. All experiments were conducted under the guidelines of the Scott & White and Texas A&M Health Science Center IACUC.
Morphological analysis of tumour tissues
Tumour samples were fixed in 10% buffered formalin for 2–4 h and embedded in paraffin or in Tissue-Tek OCT compound and immediately frozen. Portions of tumours were lysed for protein detection by immunoblotting and prepared for RNA extraction to evaluate gene expression by real-time PCR for changes in PCNA, HDC and VEGF-A/C. To evaluate angiogenesis we determined, by immunohistochemistry,12 the expression of the glycoprotein found on endothelial cells, CD31, in tumour sections from nude mice treated with vehicle, histamine or the HDC inhibitor.34
Statistical analysis
Data are expressed as mean ± SEM. Differences between groups were analysed by the Student’s unpaired t test when two groups were analysed and by analysis of variance when more than two groups were analysed, followed by an appropriate post hoc test. A p value of <0.05 indicates statistical significance.
RESULTS
HDC and MAO-B are dysregulated in cholangiocarcinoma
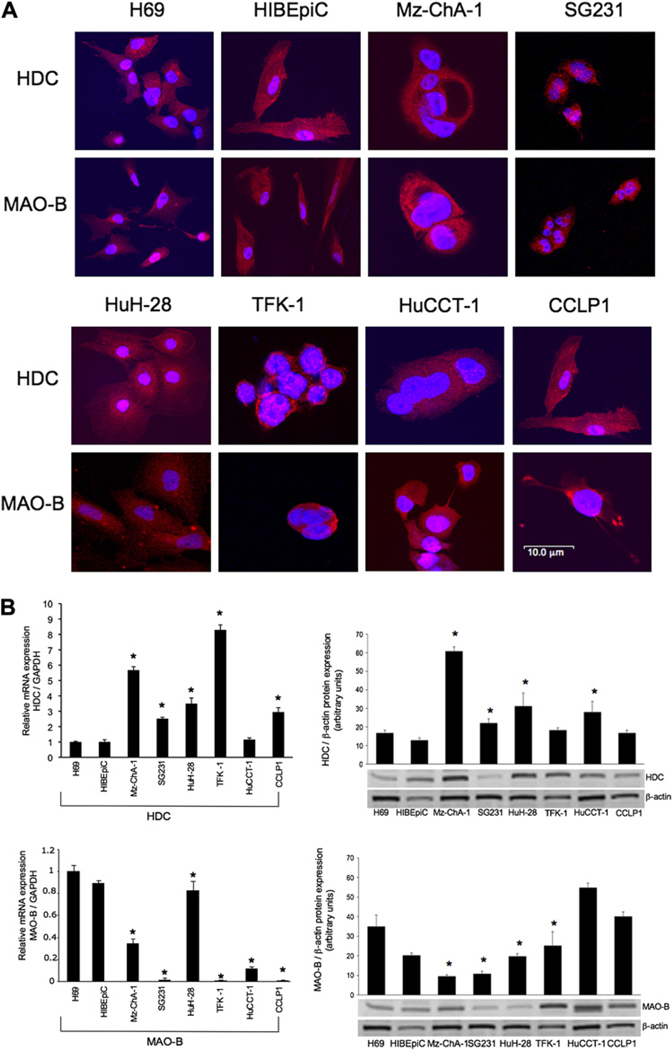
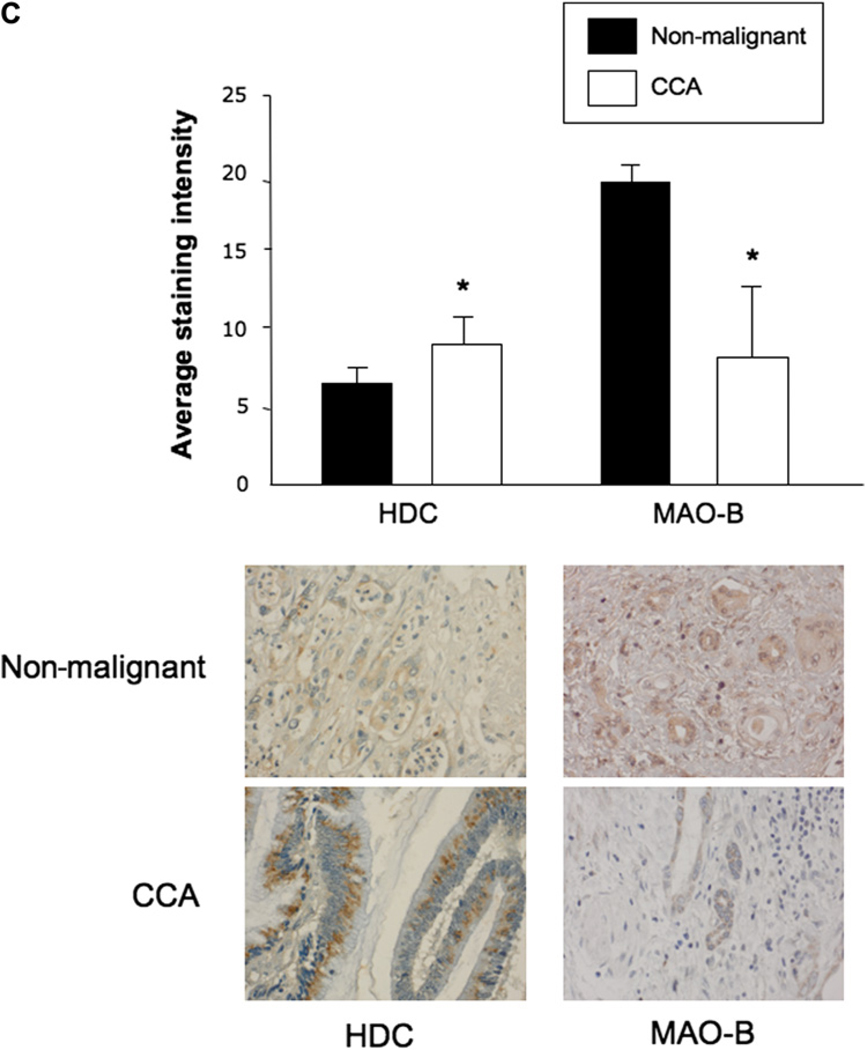
By immunofluorescence, all cell lines expressed HDC and MAO-B (figure 1A). Merged staining for nuclei (blue) and enzyme (red) is shown for each line. Negative controls showed no specific immunoreactivity (not shown). By real-time PCR and immunoblots (figure 1B), HDC was expressed at higher levels in several cholangiocarcinoma lines compared with non-malignant cholangiocytes. MAO-B levels were decreased compared with non-malignant cholangiocytes, suggesting that cholangiocarcinoma cells produce less of this enzyme which degrades histamine, enabling them to maintain higher levels of histamine (figure 1B). There was higher immunoreactivity for HDC and lower immunoreactivity for MAO-B in liver tumour biopsies compared with non-malignant controls (figure 1C).
Figure 1.
(A) By immunofluorescence, non-malignant cholangiocytes and cholangiocarcinoma cells express histidine decarboxylase (HDC) and monoamine oxidase B (MAO-B). Merged staining for nuclei (blue) and enzyme (red) is shown for each cell line. Bar=10 µm. (B) By both real-time PCR and immunoblots, HDC was expressed at higher levels in some of the cholangiocarcinoma lines (whereas MAO-B levels decreased) compared with non-malignant cholangiocytes. Data are mean±SEM of three experiments for real-time PCR and eight experiments for immunoblots (*p<0.05 vs the corresponding value of non-malignant cholangiocytes). (C) HDC immunoreactivity was increased in cholangiocarcinoma (CCA) samples whereas MAO-B immunoreactivity was significantly decreased compared with non-malignant samples (*p<0.05 vs non-malignant). Data are mean±SEM of 10 blinded evaluations of 10 randomly selected fields of three slides. Original magnification ×40.
Cholangiocarcinoma cells secrete more histamine than non-malignant cholangiocytes
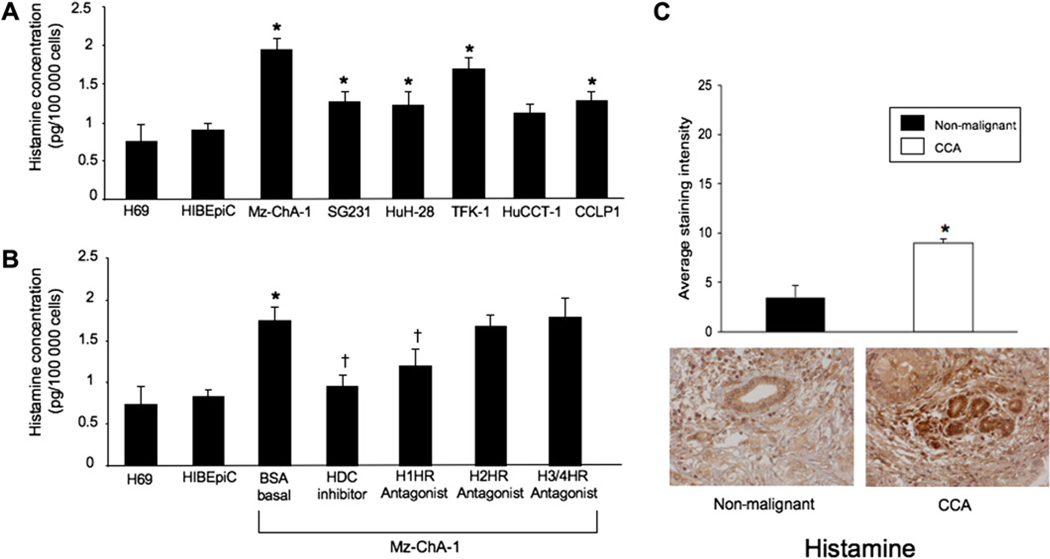
Mz-ChA-1 cells secrete almost twice as much histamine as non-malignant cells (figure 2A). When Mz-ChA-1 cells were treated with the HDC inhibitor alpha-methyl-DL-histidine dihydrochloride or the H1HR antagonist terfenadine, histamine secretion significantly decreased (figure 2B). The H2HR antagonist cimetidine and the H3/H4HR antagonist thioperamide did not alter histamine secretion from Mz-ChA-1 cells (figure 2B). The expression of histamine increased in cholangiocarcinoma biopsies compared with biopsies from non-malignant tissues (figure 2C).
Figure 2.
Histamine levels were evaluated by ELISA in the medium of non-malignant and cholangiocarcinoma (CCA) cell lines. (A) Histamine levels increased in all cholangiocarcinoma cell lines except HuCCT-1 compared with non-malignant cholangiocytes. (B) Mz-ChA-1 cells secreted almost twice as much histamine as non-malignant cells. In Mz-ChA-1 cells, histamine secretion was inhibited by the histidine decarboxylase (HDC) inhibitor and the H1 histamine receptor (H1HR) antagonist terfenadine but not the H2 or H3/4 HR antagonists (*p<0.05 vs H69; †p<0.05 vs bovine serum albumin (BSA)-treated Mz-ChA-1 cell). Data are mean±SEM of 12 experiments. (C) Immunohistochemistry in tissue array samples for histamine in human liver biopsies from healthy controls and patients with cholangiocarcinoma. Histamine immunoreactivity significantly increased in cholangiocarcinoma biopsy samples compared with non-malignant samples (*p<0.05 vs non-malignant cells). Data are mean±SEM of 10 blinded evaluations of 10 randomly selected fields of three slides.
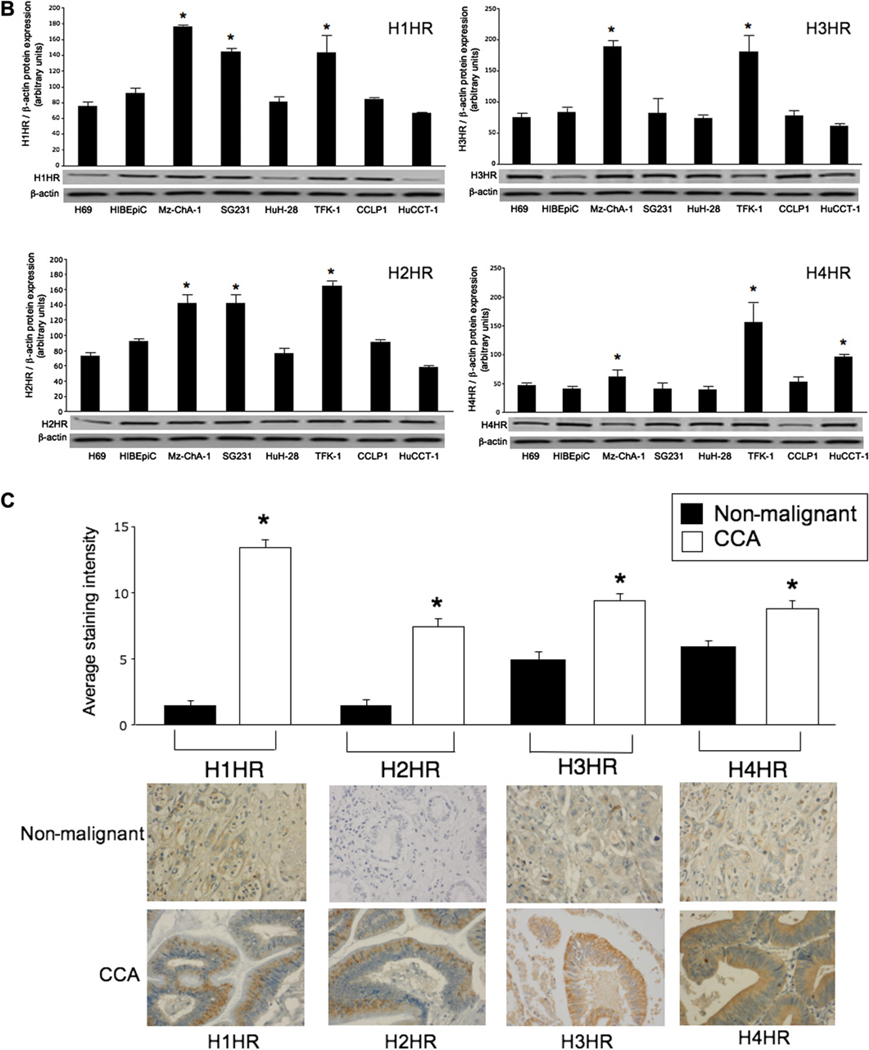
Histamine receptor expression is increased in cholangiocarcinoma
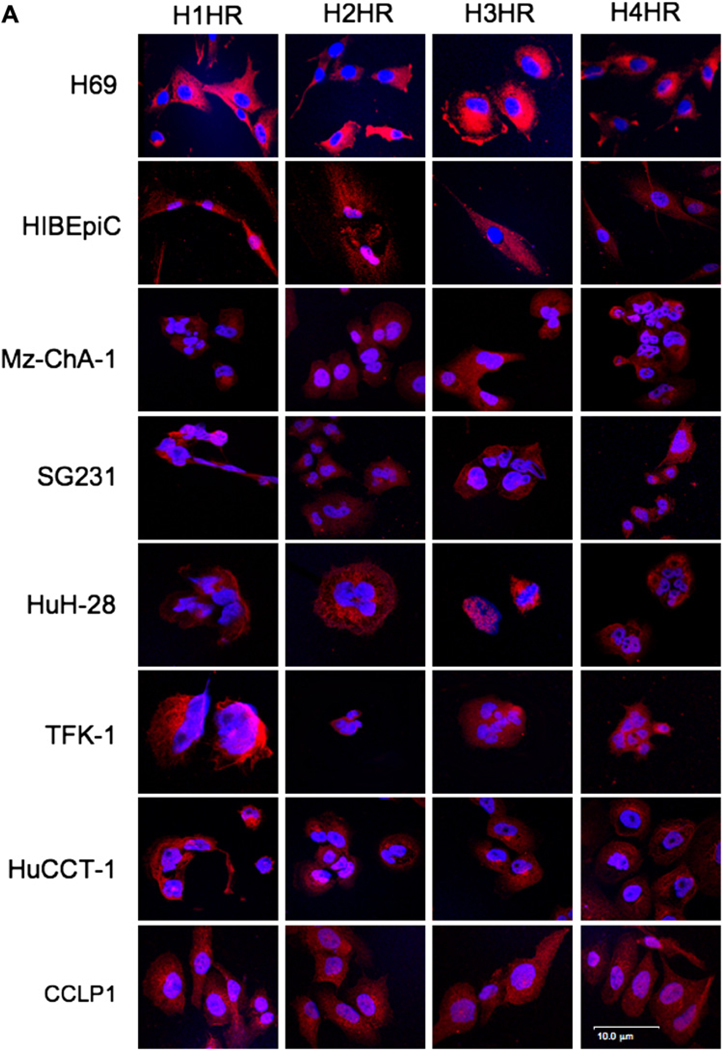
Non-malignant and cholangiocarcinoma cells express the four HRs H1–H4 (figure 3A). Merged staining for nuclei (blue) and receptor (red) is shown for each cell line (figure 3A). Negative controls showed no staining (not shown). By real-time PCR, non-malignant cholangiocytes and cholangiocarcinoma lines express the mRNA for H1–H4 HRs (not shown). By immunoblots, the protein expression of H1–H4 HRs was increased in multiple cholangiocarcinoma lines (including Mz-ChA-1) compared with non-malignant cholangiocytes (figure 3B). By tissue array analysis, the immunoreactivity for the HRs was upregulated in tumour liver biopsies compared with controls (figure 3C). The top rows in figure 3C are from non-malignant liver biopsies including hepatocytes and cholangiocytes. The bottom row is human cholangiocarcinoma containing neoplastic cholangiocytes.
Figure 3.
(A) By immunofluorescence, non-malignant and cholangiocarcinoma (CCA) cells were positive for four histamine receptors (HRs), H1–H4 HRs. Merged staining for nuclei (blue) and receptor (red) is shown for each cell line. Bar=10 µm. (B) By immunoblots, the protein expression of H1–H4 HRs was increased in multiple cholangiocarcinoma lines compared with normal cholangiocytes. Data are mean±SEM of six experiments (*p<0.05 vs corresponding values of non-malignant cells). (C) By tissue array analysis, the immunoreactivity for H1–H4 HRs increased in tumour liver biopsies compared with non-malignant controls (*p<0.05 vs non-malignant cells). Data are mean±SEM of 10 blinded evaluations of one randomly selected field of three slides.
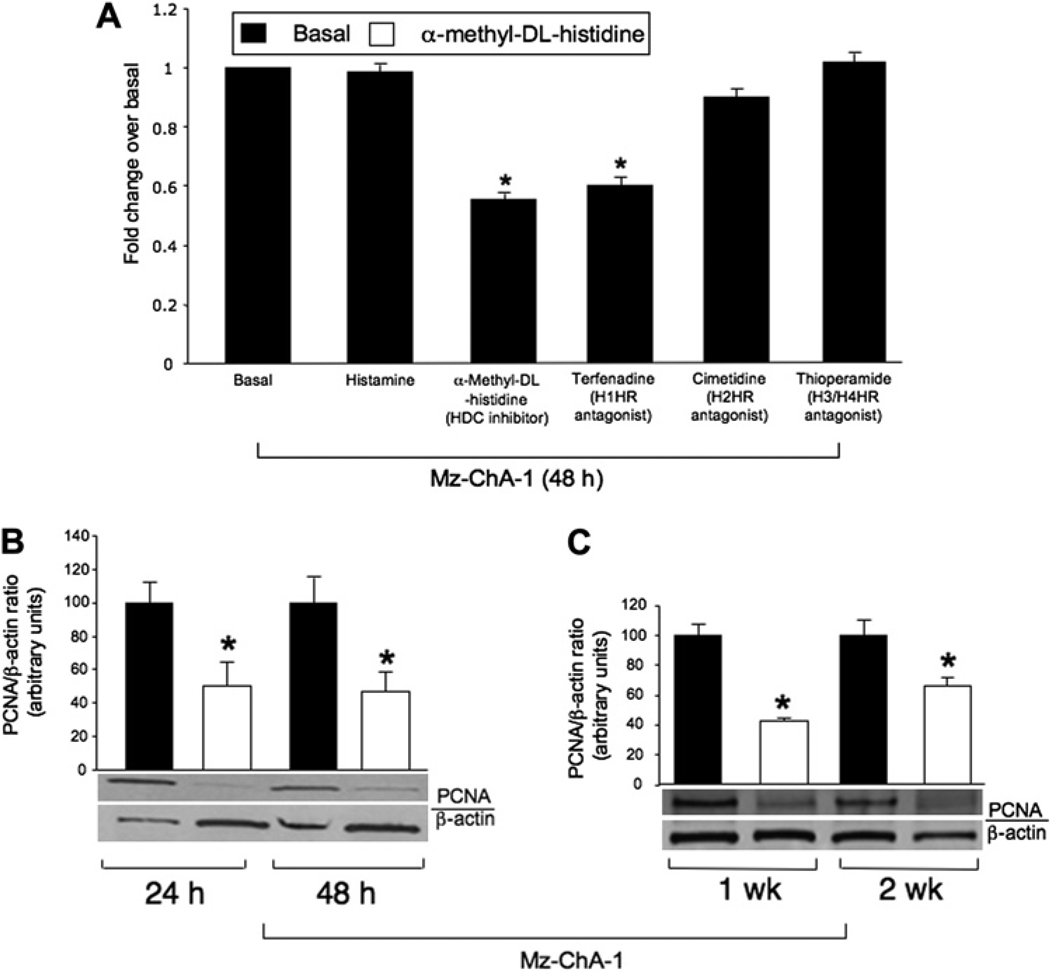
Short-term effects of histamine and the HDC inhibitor on non-malignant and cholangiocarcinoma growth
Histamine did not alter the proliferation (by MTS assay and PCNA immunoblots) of non-malignant and cholangiocarcinoma lines when these cells were treated for a short time (24 and 48 h) at doses of 10–100 µM (not shown). The finding that exogenous histamine has no short-term effect on cholangiocarcinoma growth is probably due to the fact that cholangiocarcinoma cells are overproducing histamine and therefore are not affected by additional histamine stimulation. Further experiments demonstrated that histamine (10 µM for 48 h, a concentration used in other studies)15 alone has no effect on the proliferation of Mz-ChA-1 cells, whereas both the HDC inhibitor and the H1HR antagonist terfenadine decreased Mz-ChA-1 proliferation (figure 4A). There were no effects on the growth of H69 cells after treatment with histamine, the HDC inhibitor or the antagonists of HRs (not shown). These results demonstrate that HDC and H1HR antagonists inhibit the growth of Mz-ChA-1 cells (but not non-malignant cells) by inhibition of histamine synthesis by blocking interaction with H1HR.
Figure 4.
(A) Short-term effect of histamine, the histidine decarboxylase (HDC) inhibitor and H1–H4 histamine receptor (HR) antagonists on the proliferation of cholangiocarcinoma cell lines. While histamine alone had no effect on cholangiocarcinoma growth, the HDC inhibitor α-methyl-D,L-histidine dihydrochloride and the H1HR antagonist terfenadine decreased cholangiocarcinoma growth. Neither the H2HR antagonist cimetidine nor the H3/H4 antagonist thioperamide affected cholangiocarcinoma growth. (B,C) Mz-ChA-1 cells were treated with the HDC inhibitor (3 mM) every day for up to 2 weeks before measuring cellular proliferation by proliferating cellular nuclear antigen (PCNA) immunoblots. Treatment with the HDC inhibitor reduced Mz-ChA-1 proliferation at all time points studied up to 2 weeks (*p<0.05 vs corresponding basal values). Data are mean±SEM of eight experiments.
Long-term effects of histamine stimulation on the proliferation of non-malignant and cholangiocarcinoma cells
Since short-term stimulation with histamine did not alter the growth of non-malignant cell lines (not shown) and cholangiocarcinoma cell lines (figure 4A), we studied the long-term effects of histamine and the HDC inhibitor on the growth of these cells. Histamine induced a significant (albeit modest) increase in the proliferation of non-malignant cholangiocytes after 1 and 2 weeks of treatment (see figure 1 in online supplement). The HDC inhibitor had no significant effects on the proliferation of non-malignant cholangiocytes at 24 h and up to 2 weeks (not shown).
Mz-ChA-1 cells were treated with histamine (10 µM) or the HDC inhibitor (3 mM) every day for up to 2 weeks and proliferation was measured. After long-term stimulation (1 and 2 weeks), histamine increased Mz-ChA-1 proliferation as demonstrated by enhanced PCNA protein expression (see figure 1 in online supplement). Importantly, treatment with the HDC inhibitor reduced Mz-ChA-1 proliferation at all the time points studied (figure 4B,C).
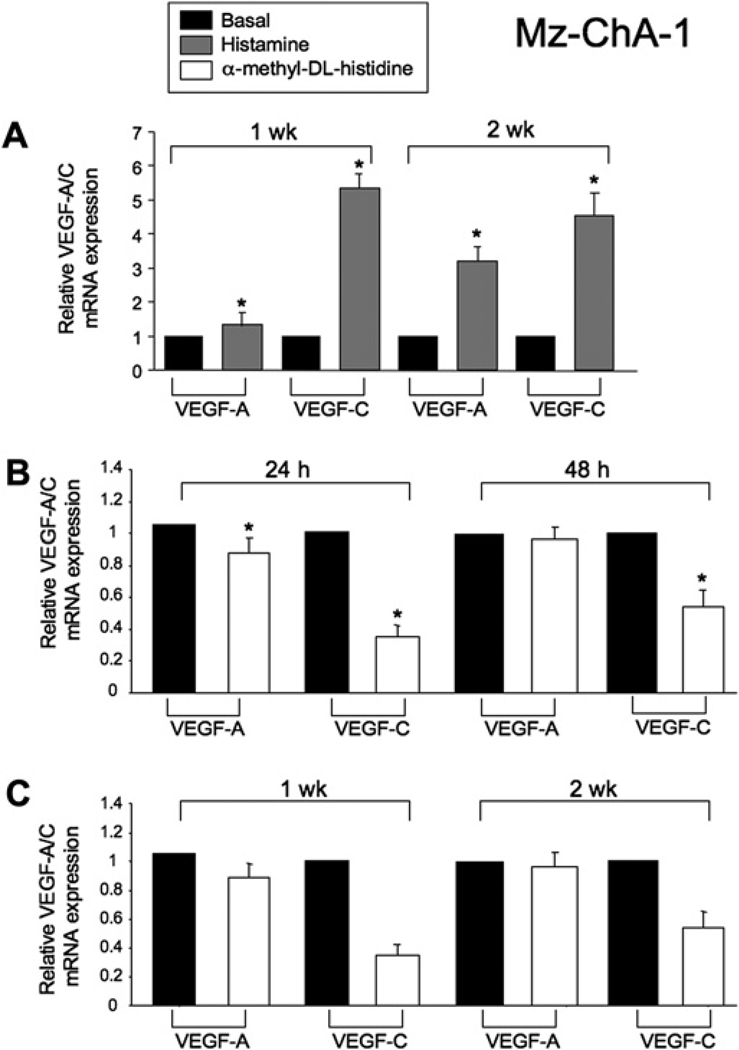
Effects of histamine and the HDC inhibitor on the expression of VEGF-A/C in cholangiocytes and cholangiocarcinoma cells
After treatment for 1 and 2 weeks, histamine increased VEGF-A/C gene expression in H69 cells (not shown). No changes in VEGF-A/C mRNA expression were seen in Mz-ChA-1 cells treated with histamine at 24 and 48 h (not shown). However, after stimulation of Mz-ChA-1 cells with histamine for 1 and 2 weeks there was increased expression of VEGF-A/C (figure 5A). No changes were found in the mRNA expression of VEGF-A/C in cholangiocytes after treatment with the HDC inhibitor at 24 and 48 h and up to 1 and 2 weeks (not shown). In Mz-ChA-1 cells stimulated with the HDC inhibitor there was a decrease in VEGF-A expression at 24 h but not at 48 h. VEGF-C expression was significantly decreased at both 24 and 48 h (figure 5B). The HDC inhibitor induced a decrease in VEGF-A mRNA expression at 1 week and VEGF-C mRNA expression at 1 and 2 weeks in Mz-ChA-1 cells (figure 5C).
Figure 5.
(A) After stimulation with histamine for 1 and 2 weeks there was a significant increase in the expression of vascular endothelial growth factor (VEGF)-A and VEGF-C. (B) In Mz-ChA-1 cells stimulated with the histidine decarboxylase (HDC) inhibitor there was a significant decrease in VEGF-A expression at 24 h but not at 48 h. VEGF-C expression was significantly decreased at both 24 and 48 h. (C) There was a significant decrease in VEGF-A mRNA expression at 1 week and VEGF-C mRNA expression at 1 and 2 weeks after treatment of Mz-ChA-1 cells with the HDC inhibitor. Data are mean±SEM of three experiments. *p<0.05 vs VEGF mRNA expression of basal-treated cells.
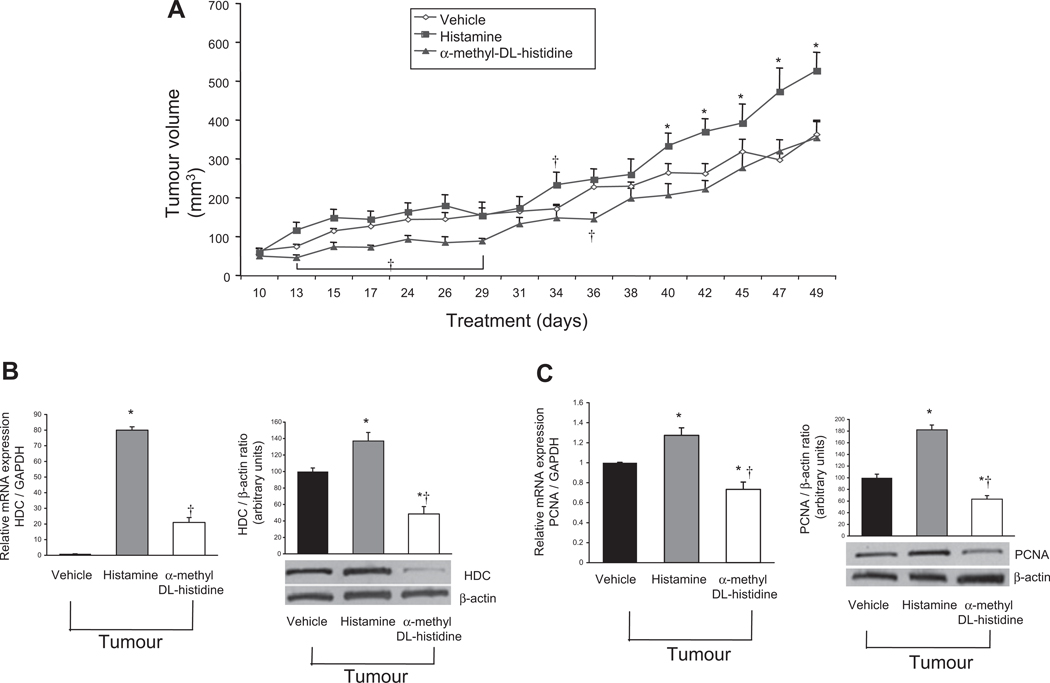
Effects of histamine stimulation and inhibition on tumour volume, proliferation and VEGF expression
In vivo, histamine increased tumour growth compared with vehicle and HDC inhibitor treatments. This effect was significant at random time points until day 40, where it continued to be significantly higher than vehicle-treated tumour growth for the duration of the experiment (figure 6A). Treatment with the HDC inhibitor decreased tumour volume compared with both vehicle and histamine treatment during early time points (days 15–29). This effect diminished over extended periods of time compared with vehicle alone, but tumour volume was still significantly lower than that of the histamine-treated group throughout the experiment (figure 6A). Treatment with histamine did not change the immunoreactivity of CD31 in tumour samples compared with mice treated with vehicle or the HDC inhibitor (see figure 2 in online supplement). The data show that there are no changes in angiogenesis of the tumour microenvironment after histamine treatment compared with mice treated with vehicle or the HDC inhibitor. We demonstrated that histamine increases HDC and PCNA expression compared with vehicle treatment (figure 6B,C). Treatment with the HDC inhibitor decreased protein but not mRNA expression of HDC and mRNA and protein expression of PCNA compared with both vehicle and histamine treatment (figure 6B,C). The data suggest that alpha-methyl-DL-histidine treatment increases protein degradation or instability of HDC (figure 6B). By real-time PCR, histamine treatment increased VEGF-A/C expression compared with vehicle-treated tumours (see figure 3 in online supplement). The mRNA levels of VEGF-A/C were lower in tumour samples from mice treated with the HDC inhibitor compared with histamine-treated mice (see figure 3 in online supplement).
Figure 6.
In vivo evaluation of xenograft tumour volume/growth over time after chronic histamine stimulation or inhibition. (A) Using two-way ANOVA analysis, histamine significantly increased tumour volume at days 13, 34 and 40–49 compared with vehicle (0.9% NaCl) and the histidine decarboxylase (HDC) inhibitor significantly decreased tumour volume at days 15–29, 36, 40, 42 and 45 compared with vehicle. Inhibition of HDC by α-methyl-DL-histidine significantly decreased tumour volume at all time points except day 10 compared with histamine-induced tumour volume. (B,C) Histamine increased HDC and proliferating cellular nuclear antigen (PCNA) expression compared with vehicle treatment. Treatment with the HDC inhibitor decreased protein but not mRNA expression of HDC and also decreased mRNA and protein expression of PCNA compared with both vehicle and histamine treatment. Data are mean±SEM of three experiments (for real-time PCR) and six experiments (for immunoblots). *p<0.05 HDC and PCNA expression vs vehicle treatment. †p<0.05 vs mRNA expression of histamine treatment.
Effects of knockdown of HDC on the growth of Mz-ChA-1 cells implanted in nude mice
Gene expression was reduced to approximately 50% of normal HDC expression and protein expression was ablated in the Mz-HDC transfected line compared with Mz-neg cells (see figure 4A in online supplement). There was decreased secretion of histamine into the conditioned medium of Mz-HDC compared with Mz-neg cells (see figure 4B in online supplement). In Mz-HDC cells there was decreased expression of H1–H4 HRs, PCNA and VEGF-A/C compared with Mz-neg cells (see figure 4C–E in online supplement). The data demonstrate that HDC directly regulates the expression of H1–H4 HRs and VEGF-A/C expression in cholangiocarcinoma. The decrease in the expression of the stimulatory HRs H1–H211 (Alpini, unpublished observations, 2011) following genetic loss of HDC probably depends on the lack of histamine secretion in cholangiocarcinoma. The downregulation of H4HR is not surprising as this receptor is inhibitory (similar to the H3HR)12 when activated so that, without endogenous histamine, the H4HR is unable to prevent proliferation of cholangiocarcinoma.
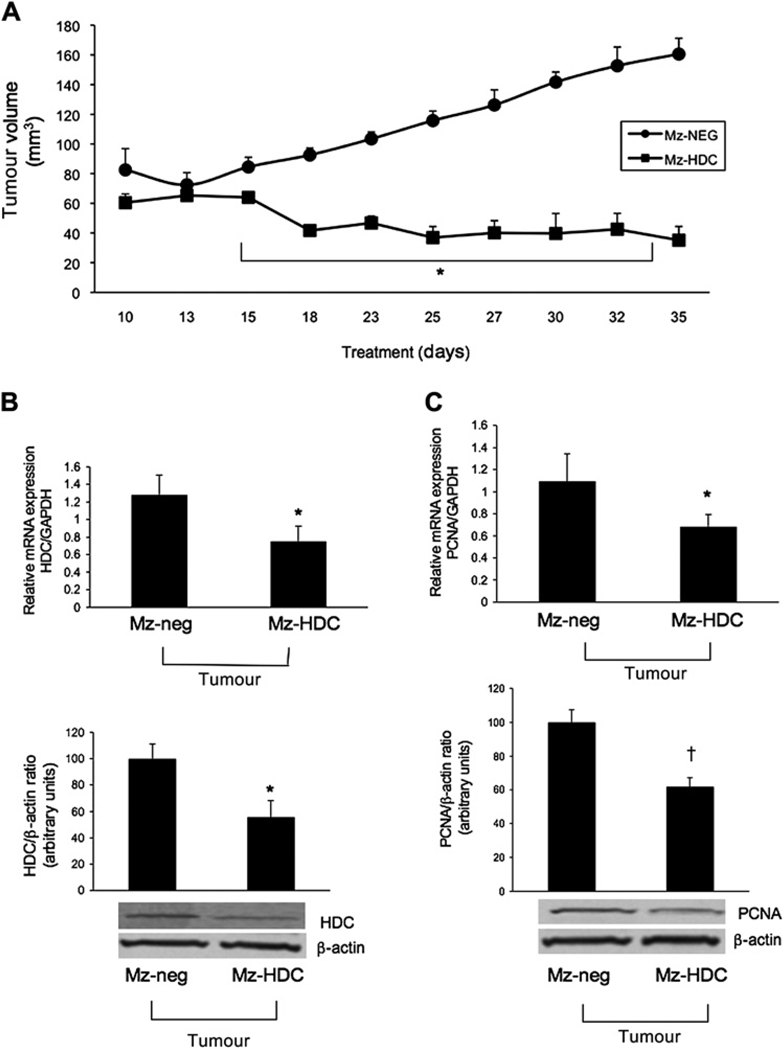
Figure 7A shows that, in the Mz-neg group, there was a significant and steady increase in tumour growth similar to the vehicle group shown in figure 6A, whereas the HDC knockdown group had little or no change in tumour growth in early time periods but at day 18 it decreased and remained at a similar level throughout the measurement period compared with the Mz-neg tumours. By real-time PCR and immunoblots we found a significant decrease in HDC and PCNA expression in tumours from Mz-HDC mice compared with Mz-neg tumours (figure 7B,C). There was decreased VEGF-A/C expression in RNA of tumours from Mz-HDC mice compared with samples from Mz-neg tumours (see figure 5 in online supplement).
Figure 7.
(A) Xenograft tumour growth over time after implantation of genetically modified Mz-ChA-1 cells. After 18 days the Mz-HDC tumours decreased in volume and remained similar throughout the measurement time compared with the Mz-neg tumours which continued steadily to increase in volume. By two-way ANOVA, tumour growth in Mz-HDC was significantly lower (p<0.001) than in Mz-neg at all time points except day 13. (B,C) By real-time PCR and immunoblots in RNA and protein samples from tumours extracted from both Mz-neg and Mz-HDC, a significant decrease was found in histidine decarboxylase (HDC) expression in Mz-HDC tumour cells compared with Mz-neg tumour cells and proliferating cellular nuclear antigen (PCNA) expression (*p<0.05 vs Mz-neg cells; †p<0.01 vs Mz-neg cells). Data are mean±SEM of three experiments (real-time PCR) and eight experiments (immunoblotting).
DISCUSSION
We have shown that increased synthesis of histamine (mediated by enhanced HDC expression) in cholangiocarcinoma increases the growth of this tumour by an autocrine mechanism and that pharmacological and molecular inhibition of HDC decreases cholangiocarcinoma proliferation in vitro and in vivo. Supporting an autocrine mechanism, we found that treatment with histamine did not change the expression of CD31 in tumour samples compared with mice treated with vehicle or the HDC inhibitor. Malignant cholangiocytes display enhanced HDC and decreased MAO-B expression resulting in increased histamine secretion, and also increased expression of H1–H4 HRs compared with non-malignant cholangiocytes. The over-secretion of histamine increased tumour growth and expression of VEGF-A/C by enhanced HDC expression. Inhibition of HDC decreased cholangiocarcinoma proliferation and VEGF expression both in vivo and in vitro. Increased cholangiocarcinoma growth (induced by enhanced HDC expression and histamine secretion) is ablated by the H1HR but not the H2HR antagonist. Since the H3HR agonist RAMH12 and the H4HR agonist clobenpropit35 inhibit cholangiocarcinoma growth, we propose that the progression of cholangiocarcinoma growth (by histamine) is due mainly to the activation of H1HR. The fact that the H1HR antagonists have detrimental effects on other organs including the heart36 strengthens the concept that selective blockage of HDC is an attractive strategy for treatment of cholangiocarcinoma.
Supporting our findings, the synthesis of dopamine and serotonin is dysregulated in cholangiocarcinoma, and blocking the rate-limiting enzymes responsible for the production of these amines decreases cholangiocarcinoma growth.27,37 In support of the expression pattern of H1–H4 HRs in cholangiocarcinoma, H3HRs are expressed by cholangiocytes and inhibit biliary hyperplasia17 whereas H1HRs increase the proliferation of cholangiocytes.11 In breast cancer cells, HDC is upregulated and identified as a key player in the progression of mammary carcinogenesis whereas the H4HR decreased breast cancer growth via cell cycle arrest and induction of cell death.9 Our current finding and the cited studies support the concept that an autocrine loop (consisting of enhanced histamine synthesis by HDC and enhanced H1HR expression) sustains cholangiocarcinoma growth. Our hypothesis is supported by the fact that (1) the HDC inhibitor and only the H1HR antagonist inhibited histamine production and growth in cholangiocarcinoma cells; and (2) HDC silencing in Mz-ChA-1 cells directly inhibits the expression of H1HRs in these cells. The fact that the HDC inhibitor decreases the growth of cholangiocarcinoma (but not normal cholangiocytes) strengthens the specificity of this enzyme as a key therapeutic approach for managing cholangiocarcinoma.
Histamine is a trophic factor in many cellular processes including tumorigenesis.9 A phenotypic profiling study found a positive correlation between histamine and tumour growth and angiogenesis in melanomas.38 In our study we show that, after chronic treatment, histamine behaves as a trophic autocrine factor increasing tumour growth and enhancing VEGF expression. Our in vitro work demonstrates a direct correlation between cholangiocarcinoma cell proliferation and increased/decreased VEGF expression mediated by changes in HDC expression. We have shown that there are no changes in angiogenesis in the tumour microenvironment after histamine treatment. These findings support the concept that histamine stimulates cholangiocarcinoma by autocrine mechanisms stimulating VEGF expression. Studies have shown that biliary hyperplasia in cholestatic rats precedes the proliferation of the peribiliary vascular plexus39 and is regulated by the VEGF synthesised by proliferating cholangiocytes by autocrine mechanisms.14
In vivo, we demonstrated that the loss of HDC by pharmacological or genetic modification inhibits tumour growth compared with histamine-treated mice or mice with normal levels of HDC. These studies show that histamine plays a critical role by increasing the progression of cholangiocarcinoma as well as the potential therapeutic value of blocking histamine production. Histamine is an autocrine growth factor in mammary adenocarcinoma where overexpression of HDC induces histamine release that changes H1HR and H2HR membrane density.40 Similar to our study, stimulation of H1 and H2 HRs induced a differential response in cAMP signalling and tumour cell proliferation.40 We demonstrated that the HRs induce a differential effect on cholangiocarcinoma growth; importantly, it appears that blocking the autocrine regulatory loop with the HDC inhibitor may be an efficient mode of action for inhibiting cholangiocarcinoma growth.
Treatment with antihistamines reduces inflammation after injury41,42 and decreases gastric acid secretion.43 These treatments target specific HRs and therefore do not completely ablate the effects induced by histamine. H2HR antagonists such as cimetidine and rantidine have been studied in mammary adenocarcinoma with beneficial results,40 and usage of the irreversible inhibitor of HDC monofluoro methylhistidine produces 100% remission of these tumours without changing endogenous histamine levels.44 After knockdown of HDC, tumour volume did not increase as aggressively as the tumours induced by the mock-transformed line but remained similar throughout most of the measurement time after an initial fall in tumour volume. However, our approach did not block whole body histamine synthesis. As a result, there may be an increase in inflammatory cells (eg, mast cells) activated during pathological responses producing histamine.45
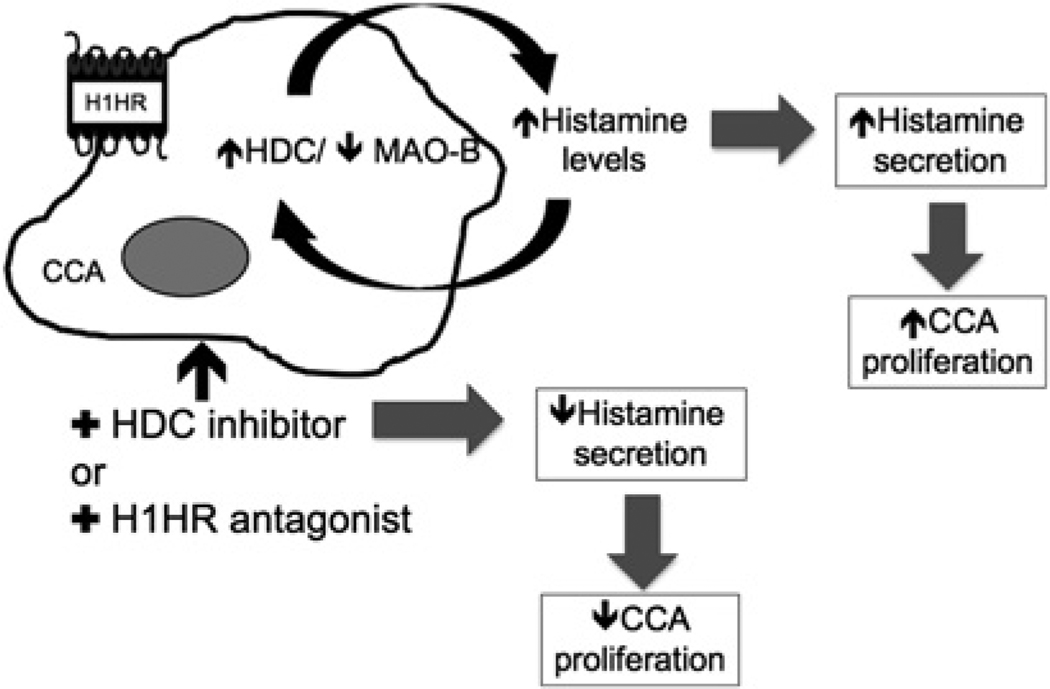
We propose that increased histamine release increases tumour growth whereas blocking HDC or inhibition of the H1HR decreases cholangiocarcinoma growth (figure 8). Our findings suggest that histamine biosynthesis and receptor upregulation play a role in cholangiocarcinoma, together these findings support the potential therapeutic value of blocking histamine synthesis (by targeting HDC) during cholangiocarcinogenesis.
Figure 8.
Schematic diagram of working model. Histamine secretion increases after histidine decarboxylase (HDC) expression is enhanced during cholangiocarcinogenesis. Increased HDC and histamine levels induce the growth of cholangiocarcinoma (CCA). Use of the HDC inhibitor or the H1 histamine receptor (H1HR) antagonist decreases histamine secretion levels and tumour growth.
Significance of this study.
What is already known about this subject?
-
▶
Histamine, via the H3 histamine receptor (H3HR), decreases cholangiocarcinoma growth via activation of protein kinase C α (PKCα) and downregulation of vascular endothelial growth factor (VEGF).
-
▶
Stimulation of the H4HR inhibits epithelial mesenchymal transition and cholangiocarcinoma metastasis.
What are the new findings?
-
▶
Histamine increases cholangiocarcinoma growth by an autocrine mechanism.
-
▶
Inhibition of the H1HR decreases cholangiocarcinoma growth.
-
▶
Inhibition of histamine synthesis (by blocking histidine decarboxylase (HDC)) significantly decreases human cholangiocarcinoma growth.
-
▶
Modulation of the autocrine loop (HDC → histamine → H1HR) may be important for the management of cholangiocarcinoma growth.
-
▶
No changes in angiogenesis were detected after in vivo treatment with histamine or the HDC inhibitor.
How might it impact clinical practice in the foreseeable future?
-
▶
Treatment with the HDC inhibitor may help to reduce histamine levels during the progression of cholangiocarcinoma.
-
▶
Pharmacological treatment with the HDC inhibitor and H1HR antagonists may aid in reducing histamine and VEGF levels during the progression of cholangiocarcinoma.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the Texas A&M Health Science Center Integrated Microscopy and Imaging Laboratory, Temple, Texas, USA for their assistance with the confocal microscopy and Bryan Moss (Medical Illustration, Scott & White, Graphic Services Department) for his assistance in the preparation of the figures.
Funding This work was supported by the Dr Nicholas C Hightower Centennial Chair of Gastroenterology from Scott & White, a VA Research Career Scientist Award, a VA Merit award and NIH grants DK58411 and DK76898 to GA and Federate Athenaeum funds from University of Rome “La Sapienza” to EG. This work was partially funded by a Scott & White Mentored Research Grant awarded to HF.
Footnotes
Competing interests None.
Contributors HF: overseeing the research project with GA, writing of manuscript and performed all the molecular studies. SDeM: helped with paper writing and molecular studies. JV: cell culture and transfections. PO: some of the immunohistochemical data and paper writing. MW: real-time PCR and some of the immunohistochemical data. EG: some of the immunohistochemical data and paper writing. TF: real-time PCR and immunoblots. JJG Jr: assisted with paper writing and discussions on project. ST: immunoblots. CJM: assisted with paper writing and discussions on project. GA: overseeing the research project.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Alpini G, Prall RT, LaRusso NF. The pathobiology of biliary epithelia. In: Arias IM, Boyer JL, Chisari FV, et al., editors. The Liver; Biology & Pathobiology. 4th edn. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. pp. 421–435. [Google Scholar]
- 2.Gores GJ. Cholangiocarcinoma: current concepts and insights. Hepatology. 2003;37:961–969. doi: 10.1053/jhep.2003.50200. [DOI] [PubMed] [Google Scholar]
- 3.Sirica AE. Cholangiocarcinoma: molecular targeting strategies for chemoprevention and therapy. Hepatology. 2005;41:5–15. doi: 10.1002/hep.20537. [DOI] [PubMed] [Google Scholar]
- 4.Aljiffry M, Walsh MJ, Molinari M. Advances in diagnosis, treatment and palliation of cholangiocarcinoma: 1990–2009. World J Gastroenterol. 2009;15:4240–4262. doi: 10.3748/wjg.15.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenthaler J, Guirard BM, Chang GW, et al. Purification and properties of histidine decarboxylase from lactobacillus 30a. Proc Natl Acad Sci U S A. 1965;54:152–158. doi: 10.1073/pnas.54.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dupre C, Lovett-Barron M, Pfaff DW, et al. Histaminergic responses by hypothalamic neurons that regulate lordosis and their modulation by estradiol. Proc Natl Acad Sci U S A. 2010;107:12311–12316. doi: 10.1073/pnas.1006049107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dartsch C, Chen D, Persson L. Multiple forms of rat stomach histidine decarboxylase may reflect posttranslational activation of the enzyme. Regul Pept. 1998;77:33–41. doi: 10.1016/s0167-0115(98)00045-7. [DOI] [PubMed] [Google Scholar]
- 8.Li Z, Liu J, Tang F, et al. Expression of non-mast cell histidine decarboxylase in tumor-associated microvessels in human esophageal squamous cell carcinomas. APMIS. 2008;116:1034–1042. doi: 10.1111/j.1600-0463.2008.01048.x. [DOI] [PubMed] [Google Scholar]
- 9.Medina V, Croci M, Crescenti E, et al. The role of histamine in human mammary carcinogenesis: H3 and H4 receptors as potential therapeutic targets for breast cancer treatment. Cancer Biol Ther. 2008;7:28–35. doi: 10.4161/cbt.7.1.5123. [DOI] [PubMed] [Google Scholar]
- 10.Repka-Ramirez MS. New concepts of histamine receptors and actions. Curr Allergy Asthma Rep. 2003;3:227–231. doi: 10.1007/s11882-003-0044-3. [DOI] [PubMed] [Google Scholar]
- 11.Francis H, Glaser S, DeMorrow S, et al. Small mouse cholangiocytes proliferate in response to H1 histamine receptor stimulation by activation of the IP3/CAMK I/CREB pathway. Am J Physiol Cell Physiol. 2008;295:C499–C513. doi: 10.1152/ajpcell.00369.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Francis H, Onori P, Gaudio E, et al. H3 histamine receptor-mediated activation of protein kinase C alpha inhibits the growth of cholangiocarcinoma in vitro and in vivo. Mol Cancer Res. 2009;7:1704–1713. doi: 10.1158/1541-7786.MCR-09-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lampiasi N, Azzolina A, Montalto G, et al. Histamine and spontaneously released mast cell granules affect the cell growth of human hepatocellular carcinoma cells. Exp Mol Med. 2007;39:284–294. doi: 10.1038/emm.2007.32. [DOI] [PubMed] [Google Scholar]
- 14.Gaudio E, Barbaro B, Alvaro D, et al. Vascular endothelial growth factor stimulates rat cholangiocyte proliferation via an autocrine mechanism. Gastroenterology. 2006;130:1270–1282. doi: 10.1053/j.gastro.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 15.Cianchi F, Vinci MC, Masini E. Histamine in cancer: the dual faces of the coin. Cancer Biol Ther. 2008;7:36–37. doi: 10.4161/cbt.7.1.5706. [DOI] [PubMed] [Google Scholar]
- 16.Schultheiss G, Hennig B, Schunack W, et al. Histamine-induced ion secretion across rat distal colon: involvement of histamine H1 and H2 receptors. Eur J Pharmacol. 2006;546:161–170. doi: 10.1016/j.ejphar.2006.07.047. [DOI] [PubMed] [Google Scholar]
- 17.Francis H, Franchitto A, Ueno Y, et al. H3 histamine receptor agonist inhibits biliary growth of BDL rats by downregulation of the cAMP-dependent PKA/ERK1/2/ELK-1 pathway. Lab Invest. 2007;87:473–487. doi: 10.1038/labinvest.3700533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanno N, Glaser S, Chowdhury U, et al. Gastrin inhibits cholangiocarcinoma growth through increased apoptosis by activation of Ca2+-dependent protein kinase C-alpha. J Hepatol. 2001;34:284–291. doi: 10.1016/s0168-8278(00)00025-8. [DOI] [PubMed] [Google Scholar]
- 19.Knuth A, Gabbert H, Dippold W, et al. Biliary adenocarcinoma. Characterisation of three new human tumor cell lines. J Hepatol. 1985;1:579–596. doi: 10.1016/s0168-8278(85)80002-7. [DOI] [PubMed] [Google Scholar]
- 20.Kusaka Y, Muraoka A, Tokiwa T, et al. Establishment and characterization of a human cholangiocellular carcinoma cell line. Hum Cell. 1988;1:92–94. [PubMed] [Google Scholar]
- 21.Shimizu Y, Demetris AJ, Gollin SM, et al. Two new human cholangiocarcinoma cell lines and their cytogenetics and responses to growth factors, hormones, cytokines or immunologic effector cells. Int J Cancer. 1992;52:252–260. doi: 10.1002/ijc.2910520217. [DOI] [PubMed] [Google Scholar]
- 22.Miyagiwa M, Ichida T, Tokiwa T, et al. A new human cholangiocellular carcinoma cell line (HuCC-T1) producing carbohydrate antigen 19/9 in serum-free medium. In Vitro Cell Dev Biol. 1989;25:503–510. doi: 10.1007/BF02623562. [DOI] [PubMed] [Google Scholar]
- 23.Wu T, Leng J, Han C, et al. The cyclooxygenase-2 inhibitor celecoxib blocks phosphorylation of Akt and induces apoptosis in human cholangiocarcinoma cells. Mol Cancer Ther. 2004;3:299–307. [PubMed] [Google Scholar]
- 24.Storto PD, Saidman SL, Demetris AJ, et al. Chromosomal breakpoints in cholangiocarcinoma cell lines. Genes Chromosomes Cancer. 1990;2:300–310. doi: 10.1002/gcc.2870020408. [DOI] [PubMed] [Google Scholar]
- 25.Grubman SA, Perrone RD, Lee DW, et al. Regulation of intracellular pH by immortalized human intrahepatic biliary epithelial cell lines. Am J Physiol Gastrointest Liver Physiol. 1994;266:G1060–G1070. doi: 10.1152/ajpgi.1994.266.6.G1060. [DOI] [PubMed] [Google Scholar]
- 26.Onori P, Wise C, Gaudio E, et al. Secretin inhibits cholangiocarcinoma growth via dysregulation of the cAMP-dependent signaling mechanisms of secretin receptor. Int J Cancer. 2010;127:43–54. doi: 10.1002/ijc.25028. [DOI] [PubMed] [Google Scholar]
- 27.Coufal M, Invernizzi P, Gaudio E, et al. Increased local dopamine secretion has growth promoting effects in cholangiocarcinoma. Int J Cancer. 2010;126:2112–2122. doi: 10.1002/ijc.24909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glaser S, DeMorrow S, Francis H, et al. Progesterone stimulates the proliferation of female and male cholangiocytes via autocrine/paracrine mechanisms. Am J Physiol Gastrointest Liver Physiol. 2008;295:G124–G136. doi: 10.1152/ajpgi.00536.2007. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Saxena SP, McNicol A, Brandes LJ, et al. A role for intracellular histamine in collagen-induced platelet aggregation. Blood. 1990;75:407–414. [PubMed] [Google Scholar]
- 30.Fava G, Marucci L, Glaser S, et al. Gamma-aminobutyric acid inhibits cholangiocarcinoma growth by cyclic AMP-dependent regulation of the protein kinase A/extracellular signal-regulated kinase 1/2 pathway. Cancer Res. 2005;65:11437–11446. doi: 10.1158/0008-5472.CAN-05-1470. [DOI] [PubMed] [Google Scholar]
- 31.Karavodin L, Jensen R, Sarno M, et al. Toxicology and toxicokinetics of acute and subchronic administration of histamine dihydrochloride in rats. Drug Chem Toxicol. 2003;26:35–49. doi: 10.1081/dct-120017556. [DOI] [PubMed] [Google Scholar]
- 32.Malmberg-Aiello P, Lamberti C, Ghelardini C, et al. Role of histamine in rodent antinociception. Br J Pharmacol. 1994;111:1269–1279. doi: 10.1111/j.1476-5381.1994.tb14883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeMorrow S, Francis H, Gaudio E, et al. The endocannabinoid anandamide inhibits cholangiocarcinoma growth via activation of the noncanonical Wnt signaling pathway. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1150–G1158. doi: 10.1152/ajpgi.90455.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miettinen M, Lindenmayer AE, Chaubal A. Endothelial cell markers CD31, CD34, and BNH9 antibody to H- and Y-antigens: evaluation of their specificity and sensitivity in the diagnosis of vascular tumors and comparison with von Willebrand factor. Mod Pathol. 1994;7:82–90. [PubMed] [Google Scholar]
- 35.Meng F, Alpini G, DeMorrow S, et al. Stimulation of the H4 histamine receptor decreases cholangiocarcinoma growth and invasion via integrin-dependent mechanisms. Hepatology. 2009;50:1071. [Google Scholar]
- 36.Taglialatela M, Castaldo P, Pannaccione A, et al. Cardiac ion channels and antihistamines: possible mechanisms of cardiotoxicity. Clin Exp Allergy. 1999;29 Suppl 3:182–189. doi: 10.1046/j.1365-2222.1999.0290s3182.x. [DOI] [PubMed] [Google Scholar]
- 37.Alpini G, Invernizzi P, Gaudio E, et al. Serotonin metabolism is dysregulated in cholangiocarcinoma, which has implications for tumor growth. Cancer Res. 2008;68:9184–9193. doi: 10.1158/0008-5472.CAN-08-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pos Z, Safrany G, Muller K, et al. Phenotypic profiling of engineered mouse melanomas with manipulated histamine production identifies histamine H2 receptor and rho-C as histamine-regulated melanoma progression markers. Cancer Res. 2005;65:4458–4466. doi: 10.1158/0008-5472.CAN-05-0011. [DOI] [PubMed] [Google Scholar]
- 39.Gaudio E, Onori P, Pannarale L, et al. Hepatic microcirculation and peribiliary plexus in experimental biliary cirrhosis: a morphological study. Gastroenterology. 1996;111:1118–1124. doi: 10.1016/s0016-5085(96)70081-1. [DOI] [PubMed] [Google Scholar]
- 40.Rivera ES, Cricco GP, Engel NI, et al. Histamine as an autocrine growth factor: an unusual role for a widespread mediator. Semin Cancer Biol. 2000;10:15–23. doi: 10.1006/scbi.2000.0303. [DOI] [PubMed] [Google Scholar]
- 41.Bairy KL, Rao CM, Ramesh KV, et al. Effects of antihistamines on wound healing. Indian J Exp Biol. 1991;29:398–399. [PubMed] [Google Scholar]
- 42.Han A, Maibach HI. Management of acute sunburn. Am J Clin Dermatol. 2004;5:39–47. doi: 10.2165/00128071-200405010-00006. [DOI] [PubMed] [Google Scholar]
- 43.Carella G. Antagonists of histamine H1 and H2 receptors: pharmacologic aspects and clinical significance. Clin Ter. 1984;110:375–379. [PubMed] [Google Scholar]
- 44.Cricco G, Engel N, Croci M, et al. Fluoromethylhistidine inhibits tumor growth without producing depletion of endogenous histamine. Inflamm Res. 1997;46 Suppl 1:S59–S60. [PubMed] [Google Scholar]
- 45.Francis H, Meininger CJ. A review of mast cells and liver disease: what have we learned? Dig Liver Dis. 2010;42:529–536. doi: 10.1016/j.dld.2010.02.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.